Abstract
Purpose
In an era of increasing prostate cancer incidence and earlier detection, the assessment of clinical significance of prostate cancer is critical. Minimally invasive therapies are increasingly being investigated in localized prostate cancer.
Methods and results
In this review, we discuss the current status of magnetic resonance imaging targeted fusion prostate biopsy and focal therapy for prostate cancer, its rationale, and techniques.
Conclusion
Focal therapy offers a promising outlook for prostate cancer treatment, with the goal of effectively achieving cancer control while minimizing morbidity. Long term studies are needed.
Keywords: Ablation, Focal therapy, Fusion prostate biopsy, MRI, MRI-targeted prostate biopsy, Prostate cancer
1. Rationale for focal therapy for prostate cancer
With the widespread use of prostate-specific antigen (PSA) screening and increasing life-expectancy, more men are being diagnosed with localized, low-risk, low-grade prostate cancer.1 These patients can be managed with definitive therapy, including radical prostatectomy (RP) or radiation therapy (RT). However, these radical therapies are associated with significant complication risks and side effects, which may be unsuitable for or undesired by the patient with low-risk prostate cancer. In an era of increasing prostate cancer incidence and stage migration toward earlier disease, appropriate management of the disease requires assessment of the risk of clinical significance of the disease. Minimally invasive therapies are increasingly being investigated as an alternative.
Prostate cancer is relatively slow growing, with doubling times for local tumors estimated at 2–4 years. Some prostate cancers prove to be so small, low-grade, and noninvasive that they appear to pose little risk to the patient, and are considered indolent. A recent review suggests that 49% of men undergoing RP have pathological features in the RP specimen consistent with an insignificant or indolent cancer (organ-confined cancer < 0.5 mL, no Gleason Grade 4 or 5 component).2
Up to 33% of patients on active surveillance (AS) eventually fall out of surveillance and undergo definitive treatment after 2–5 years because of initial understaging or disease progression.3 Seventy-three percent of patients initially enrolled in AS who undergo RP have a significant cancer on RP specimens.4 Other downsides of AS include the mental and emotional burden and anxieties associated with untreated cancer. Therefore, AS is an option for only a select group of men.
In order to cure and control localized prostate cancer, the concept of focal therapy has emerged. Focal therapy is the middle ground between AS and radical therapy, offering much less morbidity with cancer control. Focal destruction of cancer, with preservation of the surrounding organ, has already been used widely in the oncological treatment of kidney, liver, breast, and brain.
The concept of focal therapy is relevant for prostate cancer in a number of ways. First of all, there is strong evidence that the vast majority of metastases find their origin in the same prostate cancer cell clone, derived from the same lesion called the index lesion.5,6 Histopathological features of the index lesion predict the clinical behavior of the entire gland despite multiple synchronous tumors in >90% of patients.7,8 While prostate cancer is typically multifocal with clonal heterogeneity of prostate cancer within the gland, not all tumors within a single gland have the potential for lethality. Historically, the threshold for clinically significant disease, capable of metastatic progression, has been set at 0.5 mL, with some Gleason grade component ≥ 4.7,8 It has been shown that in >80% patients with an index lesion of cancer, the aggregate volume of secondary tumors is < 0.5 mL.7,8 Since most metastatic cancers originate from a single clonal cancer cell, it would be reasonable and effective to identify and target this potentially lethal lesion with focal therapy. Thus, selective treatment of clinically significant disease, with acceptance of residual, insignificant disease may serve as a meaningful treatment paradigm. To date, limited clinical data exist regarding outcomes of focal therapy.9
2. Candidate selection/risk strata
The selection of patients is a critical element of the challenges of focal therapy adoption and use. Patient candidate selection should ultimately be based on the intent of focal therapy. In those patients in whom focal therapy is utilized for cure, the disease should be low risk and low volume in a targetable area of the prostate. The ideal patient would be one with low-stage, low-risk prostate cancer that could be completely eradicated.
Focal therapy can be used with the intent of disease control. A therapy to control cancer would prolong the natural history of prostate cancer and delay the morbidity of radical treatment. In this situation, focal therapy would treat the dominant lesion or index lesion. In doing so, focal therapy could prolong the period of surveillance, and mitigate the uncertainties and anxieties of pure AS.10
Lastly, focal therapy could be utilized as a part of a multimodal treatment approach in the high-risk patient who would likely fail single-modality therapy, but avoid the morbidities associated with radical treatment. Use of focal therapy for noncurative intent has yet to be validated and studied.10
Up to now, most trials have included only low-risk patients under the premise that men with low-risk disease are at little risk of systemic relapse, and thus, local disease control can be a measure of treatment efficacy.11 As focal targeting methods develop, there is a stronger impetus to treat men who are at risk of disease-related mortality, as they may be the ones to benefit the most. In treating only low-risk patients, one can argue that the benefit of therapy may never be proven, as these patients would have fared well on surveillance anyway. However, most focal therapy trials include low-risk patients due to the known risk of 30–40% upgrading of surgical pathology from biopsy pathology. At this time, it is not clear if Gleason 7 (3 + 4) with small proportion of 4 has a similar favorable outcome as Gleason 6. Gleason 7 (3 + 4) has an intermediate risk of relapse, and therefore gives focal therapy the opportunity to treat and prevent prostate cancer relapse. The heterogeneity in biological behavior of Gleason 7 tumors has been shown. Gleason score 4 + 3 tumors had an increased risk of progression (compared to Gleason 3 + 4 tumors) independent of stage and margin status, and were predictive of metastatic disease (as opposed to Gleason 3 + 4 tumors).12 In addition, Gleason 4 + 3 tumors were more strongly associated with extraprostatic extension and upgrading on surgical specimens than Gleason 3 + 4 tumors were.13 Gleason 7 (4 + 3) tumors have a similar risk of relapse as Gleason 8 (4 + 4) tumors.
Candidate selection relies heavily on accurate patient identification and risk stratification. Risk stratification can be used to assess the chance of unfavorable pathology, poor oncological outcome, biochemical recurrence, and survival. Low-risk category patients have a low risk of short-term cancer mortality. The D'Amico classification is the most common classification used to stratify the risk of biochemical recurrence after radical treatment.14 The percentage of Gleason 4 tumors is sharply correlated with outcome. Stamey et al suggested that ≤20% of Gleason 4/5 tumors on biopsy (which is correlated to the same percentage of Gleason 4/5 tumor in RP specimens) represents the lower-risk subset of those harboring a Gleason 4 pattern.15
3. Limitations of standard systematic biopsy
Transrectal ultrasound (TRUS)-guided biopsy using a 12-core sampling scheme is the standard approach for prostate cancer diagnosis.16 Performing TRUS biopsy for focal therapy selection is felt to be inadequate due to the risk of underestimating disease risk, volume, and focality.17 It has been shown that if a 12-core biopsy shows unilateral disease, there is a 75% chance of a tumor on the contralateral side.18 Focal therapy selection and planning requires accurate assessment of these parameters.
The success of focal therapy clearly depends on the ability to detect the extent and laterality of prostate cancer and then accurately target it. There is no consensus currently on patient selection protocols for focal therapy. The reason for this is twofold. So far, there has been a lack of adequate biopsy techniques that can accurately detect prostate cancer lesions, and also a lack of imaging modalities to complement inadequate biopsies. Detection relies upon reduction of sampling error through the number of samples taken and the location of the samples in the prostate.19,20 In men with negative biopsies, repeat biopsy is often used up to five or six times before detection – sampling error is overcome through increased sampling. This approach of random sampling leads to three intrinsic errors: (1) underdetection by missing a potentially lethal cancer; (2) overdetection by identifying a small nonlethal cancer; and (3) misclassification by identifying an apparent low-risk cancer in someone with high-risk disease. Even extended TRUS-guided saturation biopsy appears to be inadequate in the proper selection of patients for focal therapy.21 Transperineal (TP) biopsy with three-dimensional (3D) mapping was thought to improve on cancer localization, as samples are taken every 5 mm throughout the volume of the prostate using a brachytherapy template grid under TRUS guidance. However, >61% of patients diagnosed with unilateral cancer on TP biopsy were found to have bilateral disease, and 27% were upstaged in Gleason score.22,23 Moreover, TP biopsy has fallen out of favor due to time demands, need for anesthesia, and cost.
Biopsy sampling error may be better addressed through localization of the cancer region by imaging than through simply increasing sampling. To achieve this goal, fusion biopsy has evolved as the standard for accurate maximal fusion of disease foci, according to a consensus panel.24
4. MRI-targeted fusion biopsy
The evolution of MRI to multiparametric MRI (MP MRI) is an important innovation for focal therapy in prostate cancer. A typical MP MRI includes T1-weighted sequences with dynamic contrast enhancement (DCE) sequence, T2-weighted sequences, and diffusion-weighted imaging (DWI) sequences performed by torso phased-array coils.25 MP MRI is the best noninvasive imaging test for the visualization of cancer foci in prostate. While MP-MRI may not detect all foci of disease in the prostate, it appears to better detect clinically significant foci based upon Gleason score and cancer volume.26 For significant lesions, as defined previously, sensitivity and specificity of MP MRI are up to 90%.27 In one study, sensitivity, specificity, negative predictive value, and accuracy for peripheral zone cancer detection at biopsy were, respectively, 100, 51.4, 100 and 66.7%.28 In a series of 83 patients studied by multiparametric imaging (T2 + DWI + DCE) at 1.5 T before biopsy, MRI was associated with a high sensitivity, specificity, and accuracy for detection of prostate cancer of 95%, 74%, and 86%, respectively.29 MP MRI, as a 3D technique, can determine prostate cancer foci location within the gland and volume/shape of the tumor and can be used to target lesions.
MRI–ultrasound fusion technology has recently allowed targeted biopsies to cancer-suspicious regions noted on MRI. The Artemis spatial tracking and computerized biopsy system functions to record the position of biopsy cores within a 3D template reconstruction of the prostate. Computer software allows fusion of the patient's MRI with real-time ultrasound while performing the Artemis biopsy, allowing targeting of the abnormal region on MRI during Artemis biopsy.
Contemporary series suggest that 63% of men with elevated PSA would have an abnormality suspicious for PC on MP MRI. Targeted biopsies of these areas would lead to cancer identification in ∼65% of those men.30–32 While overall cancer detection rates are lower with targeted than systematic biopsy, the majority of cancers missed on targeted biopsy are deemed clinically insignificant (likely nonlethal) as measured by current pathological classification methods.30,31,33,34 MRI-targeted biopsy results in a 42% clinically significant cancer detection rate.30 Haffner et al studied extended systematic biopsies and MRI-targeted biopsies in the same patient in their study group. Targeted biopsies detected 16% more Grade 4–5 cases and better quantified the cancer than did extended systematic biopsies, with cancer lengths of 5.56 mm versus 4.70 mm (P = 0.002).31 In a recent study by Sonn et al35 of patients with negative prior biopsies or with prostate cancer on AS, the addition of MRI–US fusion targeted biopsies to systematic biopsies increased the rate of diagnosis of all cancers, as well as Gleason ≥7 cancer. Thirty-eight percent of men with Gleason ≥7 cancer had disease detected only via targeted biopsies of lesions identified on MRI.35 In men with clinically suspected prostate cancer, a biopsy using MRI to inform the sampling was associated with a 42% clinically significant cancer detection rate.30 Fusion biopsy is more accurate than transrectal biopsy, with a higher cancer detection rate of 55% (vs. 24–40% in standard 12-core biopsy) and more upgrading of the Gleason score in up to 32% of cases, with increased detection of clinically significant higher Gleason score cancers.36,37
5. Cryotherapy
The initial experience in focal cryoablation was reported by Onik and colleagues.38 In their study, nine men with unilateral prostate cancer on biopsy underwent cryoablation with preservation of the neurovascular bundle on the contralateral unaffected side. With a mean follow up of 3 years, all men had stable PSAs and all six men who underwent repeat biopsies were negative for pathological recurrence. Seven of nine men were potent.
Several other clinical studies have investigated the use of focal cryotherapy since Onik's initial study.39–41 While a majority of trials utilized a hemiablative approach, optimal cryoprobe placement has yet to be determined (Fig. 1). Computer-based technologists have strived to develop and improve cryoprobe placement to maximize destruction of targeted tissue while sparing adjacent noncancerous tissue42 (Figs. 2 and 3).
Fig. 1.
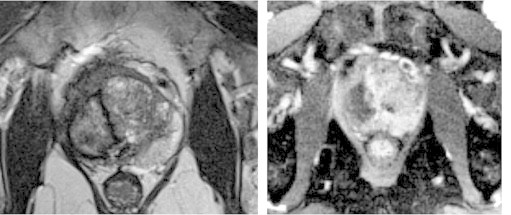
Hemi-cryoablation 3T magnetic resonance imaging. T2-weighted image showing atrophic right lobe after hemi-cryoablation. Dynamic contrast enhancement image (right) showing nonenhancing cavity in lobe.
Fig. 2.
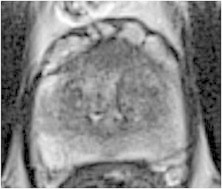
Pre-cryoablation 3T magnetic resonance imaging: anterior prostate tumor.
Fig. 3.
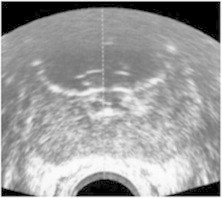
Post-cryoablation ultrasound: image depicting cryoablation cavity in anterior zone.
There is a lack of consensus on how recurrence is defined after cryotherapy, and no accepted definition of PSA failure after primary therapy, making data on outcomes hard to interpret. Among the existing literature, it is also difficult to ascertain whether these patients had true recurrence from missed treatment versus cancer that was originally missed on staging and biopsy. Lambert et al reported a 12% biochemical recurrence rate (defined as PSA nadir > 50%) with 43% with biopsy-proven recurrence on repeat biopsy. Bahn et al had a low 7% rate of biochemical recurrence with only 1/25 (4%) men having evidence of cancer when undergoing repeat biopsy.40 Truesdale et al43 reported a biochemical failure rate of 27.3% according to the Phoenix definition of PSA nadir +2 and a 46% positive rebiopsy rate among cases with suspicion for recurrence. Most of these recurrences (70–93%) occurred in the untreated contralateral side, which may indicate more a failure of initial staging, rather than the treatment.
The largest published experience and outcomes with focal cryotherapy comes from the Cryo On-Line Data (COLD) registry. In its latest update, of 1160 patients that had been treated with focal cryoablation, the biochemical recurrence-free rate (ASTRO definition of three consecutive PSA rises after post-treatment nadir) at 3 years was 75.7%. Prostate biopsy was performed in 14.1%, and positive in 26.3% of these patients, which comprised only 3.7% (43/1160) of all treated patients.44
Older trials and reviews reflect a combination of older and newer cryosystems, making the data also difficult to interpret or apply to current methods. From the COLD registry, urinary continence (defined as use of 0 pads) was 98.4%, and maintenance of spontaneous erections was 58.1%. Urinary retention and fistula rates were both low, with prolonged urinary retention (>30 days) occurring in six (1.1%) patients, and rectourethral fistula observed in one (0.1%) patient.44
Focal cryoablation is increasingly used for selected patients with prostate cancer, with a 10-fold increase in use from 1999 to 2005 based on the COLD registry. Oncological efficacy in the most recent COLD series update appears similar to that of whole-gland cryoablation.44
6. High-intensity focused ultrasound
The initial study demonstrating high-intensity focused ultrasound (HIFU) success in treating prostate cancer was published in 1995.45 HIFU works by ablating tissue via US-guided application of mechanical and thermal energy. The two mechanisms of tissue damage are by the conversion of mechanical energy into heat and inertial cavitation.
The majority of published results using HIFU have investigated its efficacy as a whole-gland treatment (Fig. 4). Ganzer and colleagues recently reported 14-year follow-up data on oncological and functional outcomes in 538 men. The biochemical disease-free rate at 5 years was 81% and at 61% at 10 years.46 Previous studies have cited biochemical disease-free rate ranging from 45% to 84% at 5 years and 69% at 7 years, using ASTRO or Phoenix criteria.47 In the Ganzer study, metastatic disease was reported in 0.4–6% of low- and intermediate-risk patients, and 15.4% in high-risk patients. prostate-cancer-specific death occurred in 18 (3.3%) patients.46
Fig. 4.
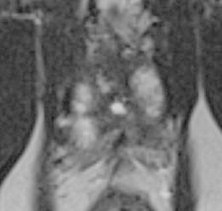
Post-HIFU 3T magnetic resonance imaging: atrophic prostate total gland post-HIFU. HIFU, high-intensity focused ultrasound.
Based on recent reviews, the most commonly encountered morbidities after whole-gland therapy include impotence (44%), urinary incontinence (8%), urinary retention (5.3%), chronic perineal pain (3.4%), and rectourethral fistula (1%).48 Other common complications include stress urinary incontinence (1–28%), urinary tract infection (0–58%), urethral/bladder neck stenosis or strictures (1–31%). For the Ablatherm HIFU device, the rate of complications has been significantly reduced over the years, due to technical improvements. The rate of urinary retention was <10% and of rectourethral fistula was 0–3%.49
To date, not many studies have used HIFU used as focal therapy. Muto and colleagues compared 70 patients undergoing whole-gland HIFU to 29 with unilateral disease undergoing focal HIFU. At 12 months, there was an 82% negative biopsy rate, with focal treatment not appearing to compromise cancer control. Urinary symptoms did not differ significantly.50
El Fegoun and colleagues51 reported results of focal HIFU hemiablation performed on 12 patients. Median follow-up was 10 years. Recurrence-free survival was 90% at 5 years, and 38% at 10 years. Five patients had salvage therapy with repeat HIFU (n = 1) or hormonal therapy (n = 4) and there were no metastases. Complications included one case of urinary retention and two patients with urinary tract infections.51 Another study by Ahmed et al9 reported results of HIFU hemiablation in 20 patients with unilateral cancer. On follow-up biopsy of the treated side at 6 months, 89% of men had negative biopsy. At 12 months follow-up, 95% of men reported erections sufficient for intercourse and 90% of men were pad and leak free.9
7. Other approaches
While cryoablation and HIFU are currently the two modalities with the most long-standing experience in focal therapy, there are various other treatment strategies currently under investigation. Focal laser ablation (FLA) is a recent technique that uses laser energy to ablate MRI visible lesions. The advantage of this approach is that it can be done with real-time monitoring via MRI, allowing the surgeon to ensure completeness of treatment as well as avoid vital structures in order to minimize morbidity (Figs. 5 and 6). A Phase I trial of 12 patients reported by Lindner et al showed that 50% of the cohort had no evidence of disease after FLA, while 67% of them had no evidence of disease only at the site of ablation. One patient with residual disease at the ablation site underwent RP without complications. There were no changes in erectile function or voiding symptoms.52 Another recent study by Oto et al53 examined nine patients who underwent FLA. Immediate contrast-enhanced post-treatment MRI showed a hypovascular defect in eight patients. Average International Prostate Symptom Score (IPSS) and Sexual Health Inventory for Men (SHIM) scores did not change significantly. MRI-guided biopsy of the ablation zone showed no cancer in seven patients (78%) and Gleason Grade 6 cancer in two (22%). Self-resolving perineal abrasion and focal paresthesia of the glans penis each occurred in one patient.53
Fig. 5.
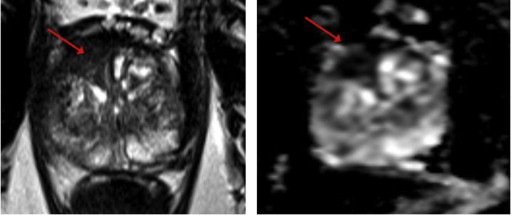
Candidate for focal laser ablation 3T magnetic resonance imaging. Preoperative T2-weighted image (left) and apparent diffusion coefficient map (right) showing right anterior TZ tumor.
Fig. 6.
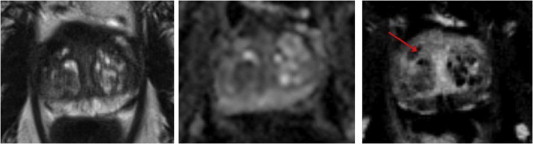
Focal laser ablation 3T MRI: same patient after laser ablation. T2-weighted image (left), apparent diffusion coefficient map (middle), and subtracted DCE (right). Lesion no longer seen. Small cavity on DCE (arrow). DCE, dynamic contrast enhancement.
Bipolar radiofrequency ablation (RFA) is another technique under investigation that can be performed under TRUS guidance.54–56 The ultrasound images and probe-driving mechanism template are mated to allow accurate position of the probe so as to precisely target the regions of the prostate that were mapped during the biopsy. A specially designed driver mechanism is used to position the probe to within 0.5 mm of the desired location. Appropriate insertions of bipolar RFA probes are designed in the planning process to target the selected regions. Although the FDA has approved bipolar RFA for the treatment of prostate cancer, no trials have as yet reported its outcomes.56
8. Follow-up of patients for assessment of efficacy
The best method to follow patients after focal therapy is controversial. The current methods generally use follow-up PSA, MP MRI, and/or biopsy in some combination. In utilizing biopsy as a follow-up parameter, the rigor of the follow-up should be the same as the selection biopsy in order to determine treatment efficacy.
The follow-up of focal therapy using MRI is possible, especially using the DCE sequence as the treated lesion/region no longer enhances57 (Figs. 7 and 8). The optimal timing of such imaging depends upon the goal, with immediate imaging at 1 week best demonstrating the zone of treatment effect and delayed imaging at 6 months demonstrating residual regions of cancer left untreated.
Fig. 7.
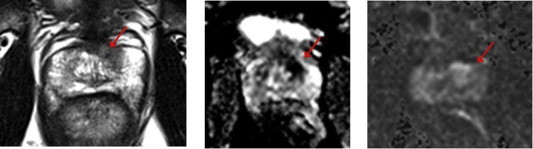
Candidate for focal cryoablation 3T magnetic resonance imaging. Preoperative T2-weighted image (left), apparent diffusion coefficient map (middle), and high B on diffusion-weighted imaging (right) show left anterior transition zone (TZ) lesion.
Fig. 8.
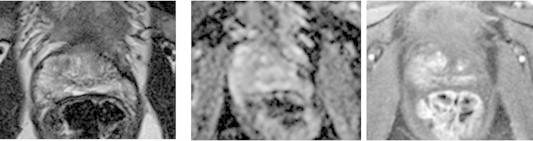
Focal cryoablation 3T magnetic resonance imaging. T2-weighted image (left), apparent diffusion coefficient map (middle), and dynamic contrast-enhanced image (right) in same patient from prior slide, following focal cryoablation. Atrophy of this region with decreased enhancement.
The relative amount of tissue ablated varies greatly in focal therapy, due to differences in volume, location and Gleason score. Thus, PSA levels may not be reflective, predictable, or comparable across patients. The ideal PSA nadir is also undefined. It has been shown that PSA decreases by 30–60% after focal therapy.40,50,58,59 In a study using focal HIFU, the PSA decreased by 80% at 6 months.9 The mean PSA after focal therapy is 2–3 ng/dL.10 Many groups have used the ASTRO criteria to define biochemical recurrence after focal therapy, to account for variability in PSA due to benign prostatic hyperplasia, inflammation, and residual disease. The Phoenix definition is thought to be more specific for recurrence in patients who have had definitive radiation treatment.10
9. Conclusion
Focal therapy has the potential to offer an array of treatments that stand midway between AS and radical therapy for patients with low-to intermediate-risk disease. Future randomized studies in focal therapy must critically evaluate candidate selection criteria and robustly answer questions regarding outcomes and follow-up monitoring. Focal therapy offers a promising outlook for the future in the treatment of prostate cancer, with the goal of effectively achieving cancer control while minimizing morbidity.
References
- 1.Stamey T.A., Caldwell M., McNeal J.E., Nolley R., Hemenez M., Downs J. The prostate specific antigen era in the United States is over for prostate cancer: what happened in the last 20 years? J Urol. 2004;172:1297–1301. doi: 10.1097/01.ju.0000139993.51181.5d. [DOI] [PubMed] [Google Scholar]
- 2.Steyerberg E.W., Roobol M.J., Kattan M.W., van der Kwast T.H., de Koning H.J., Schroder F.H. Prediction of indolent prostate cancer: validation and updating of a prognostic nomogram. J Urol. 2007;177:107–112. doi: 10.1016/j.juro.2006.08.068. [DOI] [PubMed] [Google Scholar]
- 3.Klotz L., Zhang L., Lam A., Nam R., Mamedov A., Loblaw A. Clinical results of long-term follow-up of a large, active surveillance cohort with localized prostate cancer. J Clin Oncol. 2010;28:126–131. doi: 10.1200/JCO.2009.24.2180. [DOI] [PubMed] [Google Scholar]
- 4.Duffield A.S., Lee T.K., Miyamoto H., Carter H.B., Epstein J.I. Radical prostatectomy findings in patients in whom active surveillance of prostate cancer fails. J Urol. 2009;182:2274–2278. doi: 10.1016/j.juro.2009.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu W., Laitinen S., Khan S. Copy number analysis indicates monoclonal origin of lethal metastatic prostate cancer. Nat Med. 2009;15:559–565. doi: 10.1038/nm.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmed H.U. The index lesion and the origin of prostate cancer. N Engl J Med. 2009;361:1704–1706. doi: 10.1056/NEJMcibr0905562. [DOI] [PubMed] [Google Scholar]
- 7.Villers A., McNeal J.E., Freiha F.S., Stamey T.A. Multiple cancers in the prostate. Morphologic features of clinically recognized versus incidental tumors. Cancer. 1992;70:2313–2318. doi: 10.1002/1097-0142(19921101)70:9<2313::aid-cncr2820700917>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 8.Wise A.M., Stamey T.A., McNeal J.E., Clayton J.L. Morphologic and clinical significance of multifocal prostate cancers in radical prostatectomy specimens. Urology. 2002;60:264–269. doi: 10.1016/s0090-4295(02)01728-4. [DOI] [PubMed] [Google Scholar]
- 9.Ahmed H.U., Freeman A., Kirkham A. Focal therapy for localized prostate cancer: a phase I/II trial. J Urol. 2011;185:1246–1254. doi: 10.1016/j.juro.2010.11.079. [DOI] [PubMed] [Google Scholar]
- 10.Ahmed H.U. Wiley-Blackwell; Chichester: 2012. Focal Therapy in Prostate cancer. [Google Scholar]
- 11.Taneja S.S., Mason M. Candidate selection for prostate cancer focal therapy. J Endourol. 2010;24:835–841. doi: 10.1089/end.2010.0006. [DOI] [PubMed] [Google Scholar]
- 12.Chan T.Y., Partin A.W., Walsh P.C., Epstein J.I. Prognostic significance of Gleason score 3+4 versus Gleason score 4+3 tumor at radical prostatectomy. Urology. 2000;56:823–827. doi: 10.1016/s0090-4295(00)00753-6. [DOI] [PubMed] [Google Scholar]
- 13.Makarov D.V., Trock B.J., Humphreys E.B. Updated nomogram to predict pathologic stage of prostate cancer given prostate-specific antigen level, clinical stage, and biopsy Gleason score (partin tables) based on cases from 2000 to 2005. Urology. 2007;69:1095–1101. doi: 10.1016/j.urology.2007.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D'Amico A.V., Whittington R., Malkowicz S.B. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. J Am Med Assoc. 1998;280:969–974. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 15.Stamey T.A., McNeal J.E., Yemoto C.M., Sigal B.M., Johnstone I.M. Biological determinants of cancer progression in men with prostate cancer. J Am Med Assoc. 1999;281:1395–1400. doi: 10.1001/jama.281.15.1395. [DOI] [PubMed] [Google Scholar]
- 16.Thompson I., Thrasher J.B., Aus G. Guideline for the management of clinically localized prostate cancer: 2007 update. J Urol. 2007;177:2106–2131. doi: 10.1016/j.juro.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Taneja S.S., Tareen B. Targeting prostate cancer for focal destruction: can we find it? Cancer. 2008;113:1500–1501. doi: 10.1002/cncr.23780. [DOI] [PubMed] [Google Scholar]
- 18.Polascik T.J., Mayes J.M., Sun L., Madden J.F., Moul J.W., Mouraviev V. Pathologic stage T2a and T2b prostate cancer in the recent prostate-specific antigen era: implications for unilateral ablative therapy. Prostate. 2008;68:1380–1386. doi: 10.1002/pros.20804. [DOI] [PubMed] [Google Scholar]
- 19.Boccon-Gibod L.M., de Longchamps N.B., Toublanc M., Boccon-Gibod L.A., Ravery V. Prostate saturation biopsy in the reevaluation of microfocal prostate cancer. J Urol. 2006;176:961–963. doi: 10.1016/j.juro.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 20.Moussa A.S., Meshref A., Schoenfield L. Importance of additional “extreme” anterior apical needle biopsies in the initial detection of prostate cancer. Urology. 2010;75:1034–1039. doi: 10.1016/j.urology.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 21.Mian B.M., Lehr D.J., Moore C.K. Role of prostate biopsy schemes in accurate prediction of Gleason scores. Urology. 2006;67:379–383. doi: 10.1016/j.urology.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 22.Onik G., Barzell W. Transperineal 3D mapping biopsy of the prostate: an essential tool in selecting patients for focal prostate cancer therapy. Urol Oncol. 2008;26:506–510. doi: 10.1016/j.urolonc.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 23.Onik G., Miessau M., Bostwick D.G. Three-dimensional prostate mapping biopsy has a potentially significant impact on prostate cancer management. J Clin Oncol. 2009;27:4321–4326. doi: 10.1200/JCO.2008.20.3497. [DOI] [PubMed] [Google Scholar]
- 24.Muller B.G., Futterer J.J., Gupta R.T. The role of magnetic resonance imaging in focal therapy for prostate cancer: recommendations from a consensus panel. BJU Int. 2014 Feb;113(2):218–227. doi: 10.1111/bju.12243. [Epub 2013 Nov 13] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dickinson L., Ahmed H.U., Allen C. Magnetic resonance imaging for the detection, localisation, and characterisation of prostate cancer: recommendations from a European consensus meeting. Eur Urol. 2011;59:477–494. doi: 10.1016/j.eururo.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 26.Villers A., Lemaitre L., Haffner J., Puech P. Current status of MRI for the diagnosis, staging and prognosis of prostate cancer: implications for focal therapy and active surveillance. Curr Opin Urol. 2009;19:274–282. doi: 10.1097/MOU.0b013e328329a2ed. [DOI] [PubMed] [Google Scholar]
- 27.Kirkham A.P., Emberton M., Allen C. How good is MRI at detecting and characterising cancer within the prostate? Eur Urol. 2006;50:1163–1174. doi: 10.1016/j.eururo.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 28.Cirillo S., Petracchini M., Della Monica P. Value of endorectal MRI and MRS in patients with elevated prostate-specific antigen levels and previous negative biopsies to localize peripheral zone tumours. Clin Radiol. 2008;63:871–879. doi: 10.1016/j.crad.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 29.Tanimoto A., Nakashima J., Kohno H., Shinmoto H., Kuribayashi S. Prostate cancer screening: the clinical value of diffusion-weighted imaging and dynamic MR imaging in combination with T2-weighted imaging. J Magn Reson Imaging. 2007;25:146–152. doi: 10.1002/jmri.20793. [DOI] [PubMed] [Google Scholar]
- 30.Moore C.M., Robertson N.L., Arsanious N. Image-guided prostate biopsy using magnetic resonance imaging-derived targets: a systematic review. Eur Urol. 2013;63:125–140. doi: 10.1016/j.eururo.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 31.Haffner J., Lemaitre L., Puech P. Role of magnetic resonance imaging before initial biopsy: comparison of magnetic resonance imaging-targeted and systematic biopsy for significant prostate cancer detection. BJU Int. 2011;108:E171–E178. doi: 10.1111/j.1464-410X.2011.10112.x. [DOI] [PubMed] [Google Scholar]
- 32.Park B.K., Park J.W., Park S.Y. Prospective evaluation of 3-T MRI performed before initial transrectal ultrasound-guided prostate biopsy in patients with high prostate-specific antigen and no previous biopsy. AJR Am J Roentgenol. 2011;197:W876–W881. doi: 10.2214/AJR.11.6829. [DOI] [PubMed] [Google Scholar]
- 33.Haas G.P., Delongchamps N.B., Jones R.F. Needle biopsies on autopsy prostates: sensitivity of cancer detection based on true prevalence. J Natl Cancer Inst. 2007;99:1484–1489. doi: 10.1093/jnci/djm153. [DOI] [PubMed] [Google Scholar]
- 34.Epstein J.I., Walsh P.C., Carmichael M., Brendler C.B. Pathologic and clinical findings to predict tumor extent of nonpalpable (stage T1c) prostate cancer. J Am Med Assoc. 1994;271:368–374. [PubMed] [Google Scholar]
- 35.Sonn G.A., Natarajan S., Margolis D.J. Targeted biopsy in the detection of prostate cancer using an office based magnetic resonance ultrasound fusion device. J Urol. 2013;189:86–91. doi: 10.1016/j.juro.2012.08.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rastinehad A.R., Turkbey B., Salami S.S. Improving detection of clinically significant prostate cancer: MRI/TRUS fusion-guided prostate biopsy. J Urol. 2013;191:1749–1754. doi: 10.1016/j.juro.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Siddiqui M.M., Rais-Bahrami S., Truong H. Magnetic resonance imaging/ultrasound-fusion biopsy significantly upgrades prostate cancer versus systematic 12-core transrectal ultrasound biopsy. Eur Urol. 2013;64:713–719. doi: 10.1016/j.eururo.2013.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Onik G., Narayan P., Vaughan D., Dineen M., Brunelle R. Focal “nerve-sparing” cryosurgery for treatment of primary prostate cancer: a new approach to preserving potency. Urology. 2002;60:109–114. doi: 10.1016/s0090-4295(02)01643-6. [DOI] [PubMed] [Google Scholar]
- 39.Ellis D.S., Manny T.B., Jr., Rewcastle J.C. Focal cryosurgery followed by penile rehabilitation as primary treatment for localized prostate cancer: initial results. Urology. 2007;70:9–15. doi: 10.1016/j.urology.2007.07.036. [DOI] [PubMed] [Google Scholar]
- 40.Lambert E.H., Bolte K., Masson P., Katz A.E. Focal cryosurgery: encouraging health outcomes for unifocal prostate cancer. Urology. 2007;69:1117–1120. doi: 10.1016/j.urology.2007.02.047. [DOI] [PubMed] [Google Scholar]
- 41.Bahn D.K., Silverman P., Lee Sr F., Badalament R., Bahn E.D., Rewcastle J.C. Focal prostate cryoablation: initial results show cancer control and potency preservation. J Endourol. 2006;20:688–692. doi: 10.1089/end.2006.20.688. [DOI] [PubMed] [Google Scholar]
- 42.Mouraviev V., Johansen T.E., Polascik T.J. Contemporary results of focal therapy for prostate cancer using cryoablation. J Endourol. 2010;24:827–834. doi: 10.1089/end.2009.0546. [DOI] [PubMed] [Google Scholar]
- 43.Truesdale M.D., Cheetham P.J., Hruby G.W. An evaluation of patient selection criteria on predicting progression-free survival after primary focal unilateral nerve-sparing cryoablation for prostate cancer: recommendations for follow up. Cancer J. 2010;16:544–549. doi: 10.1097/PPO.0b013e3181f84639. [DOI] [PubMed] [Google Scholar]
- 44.Ward J.F., Jones J.S. Focal cryotherapy for localized prostate cancer: a report from the national cryo on-line database (COLD) registry. BJU Int. 2012;109:1648–1654. doi: 10.1111/j.1464-410X.2011.10578.x. [DOI] [PubMed] [Google Scholar]
- 45.Madersbacher S., Pedevilla M., Vingers L., Susani M., Marberger M. Effect of high-intensity focused ultrasound on human prostate cancer in vivo. Cancer Res. 1995;55:3346–3351. [PubMed] [Google Scholar]
- 46.Ganzer R., Fritsche H.M., Brandtner A. Fourteen-year oncological and functional outcomes of high-intensity focused ultrasound in localized prostate cancer. BJU Int. 2013;112:322–329. doi: 10.1111/j.1464-410X.2012.11715.x. [DOI] [PubMed] [Google Scholar]
- 47.Warmuth M., Johansson T., Mad P. Systematic review of the efficacy and safety of high-intensity focussed ultrasound for the primary and salvage treatment of prostate cancer. Eur Urol. 2010;58:803–815. doi: 10.1016/j.eururo.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 48.Lukka H., Waldron T., Chin J. High-intensity focused ultrasound for prostate cancer: a systematic review. Clin Oncol R Coll Radiol. 2011;23:117–127. doi: 10.1016/j.clon.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 49.Rebillard X., Soulie M., Chartier-Kastler E. High-intensity focused ultrasound in prostate cancer; a systematic literature review of the French Association of Urology. BJU Int. 2008;101:1205–1213. doi: 10.1111/j.1464-410X.2008.07504.x. [DOI] [PubMed] [Google Scholar]
- 50.Muto S., Yoshii T., Saito K., Kamiyama Y., Ide H., Horie S. Focal therapy with high-intensity-focused ultrasound in the treatment of localized prostate cancer. Jpn J Clin Oncol. 2008;38:192–199. doi: 10.1093/jjco/hym173. [DOI] [PubMed] [Google Scholar]
- 51.El Fegoun A.B., Barret E., Prapotnich D. Focal therapy with high-intensity focused ultrasound for prostate cancer in the elderly. A feasibility study with 10 years follow-up. Int Braz J Urol. 2011;37:213–219. doi: 10.1590/s1677-55382011000200008. [DOI] [PubMed] [Google Scholar]
- 52.Lindner U., Weersink R.A., Haider M.A. Image guided photothermal focal therapy for localized prostate cancer: phase I trial. J Urol. 2009;182:1371–1377. doi: 10.1016/j.juro.2009.06.035. [DOI] [PubMed] [Google Scholar]
- 53.Oto A., Sethi I., Karczmar G. MR imaging-guided focal laser ablation for prostate cancer: phase I trial. Radiology. 2013;267:932–940. doi: 10.1148/radiol.13121652. [DOI] [PubMed] [Google Scholar]
- 54.Hu B., Chen L., Li J., Huang J. Contrast-enhanced ultrasonography evaluation of radiofrequency ablation of the prostate: a canine model. J Endourol. 2010;24:89–93. doi: 10.1089/end.2009.0191. [DOI] [PubMed] [Google Scholar]
- 55.Chen Y.Y., Hossack T., Woo H. Long-term results of bipolar radiofrequency needle ablation of the prostate for lower urinary tract symptoms. J Endourol. 2011;25:837–840. doi: 10.1089/end.2010.0563. [DOI] [PubMed] [Google Scholar]
- 56.Richstone L., Ziegelbaum M., Okeke Z. Ablation of bull prostate using novel bipolar radiofrequency ablation probe. J Endourol. 2009;23:11–16. doi: 10.1089/end.2008.0105. [DOI] [PubMed] [Google Scholar]
- 57.Kirkham A.P., Emberton M., Hoh I.M., Illing R.O., Freeman A.A., Allen C. MR imaging of prostate after treatment with high-intensity focused ultrasound. Radiology. 2008;246:833–844. doi: 10.1148/radiol.2463062080. [DOI] [PubMed] [Google Scholar]
- 58.Babaian R.J., Donnelly B., Bahn D. Best practice statement on cryosurgery for the treatment of localized prostate cancer. J Urol. 2008;180:1993–2004. doi: 10.1016/j.juro.2008.07.108. [DOI] [PubMed] [Google Scholar]
- 59.Onik G., Vaughan D., Lotenfoe R., Dineen M., Brady J. The “male lumpectomy”: focal therapy for prostate cancer using cryoablation results in 48 patients with at least 2-year follow-up. Urol Oncol. 2008;26:500–505. doi: 10.1016/j.urolonc.2008.03.004. [DOI] [PubMed] [Google Scholar]


