Abstract
Purpose
Although studies suggest that higher consumption of added sugars is associated with cardiovascular risk factors in adolescents, none have adjusted for measurement errors or examined its association with the risk of dyslipidemia.
Methods
We analyzed data of 4,047 adolescents aged 12–19 years from the 2005–2010 National Health and Nutrition Examination Survey, a nationally representative, cross-sectional survey. We estimated the usual percentage of calories (%kcal) from added sugars using up to two 24-hour dietary recalls and the National Cancer Institute method to account for measurement error.
Results
The average usual %kcal from added sugars was 16.0%. Most adolescents (88.0%) had usual intake of ≥10% of total energy, and 5.5% had usual intake of ≥25% of total energy. After adjustment for potential confounders, usual %kcal from added sugars was inversely associated with high-density lipoprotein (HDL) and positively associated with triglycerides (TGs), TG-to-HDL ratio, and total cholesterol (TC) to HDL ratio. Comparing the lowest and highest quintiles of intake, HDLs were 49.5 (95% confidence interval [CI], 47.4–51.6) and 46.4 mg/dL(95% CI, 45.2–47.6; p = .009), TGs were 85.6 (95% CI, 75.5–95.6) and 101.2 mg/dL(95% CI, 88.7–113.8; p = .037), TG to HDL ratios were 2.28 (95% CI, 1.84–2.70) and 2.73 (95% CI, 2.11–3.32; p = .017), and TC to HDL ratios were 3.41 (95% CI, 3.03–3.79) and 3.70 (95% CI, 3.24–4.15; p = .028), respectively. Comparing the highest and lowest quintiles of intake, adjusted odds ratio of dyslipidemia was 1.41 (95% CI, 1.01–1.95). The patterns were consistent across sex, race/ethnicity, and body mass index subgroups. No association was found for TC, low-density lipoprotein, and non-HDL cholesterol.
Conclusions
Most U.S. adolescents consumed more added sugars than recommended for heart health. Usual intake of added sugars was significantly associated with several measures of lipid profiles.
Keywords: Usual percentage of calories, Added sugars, Lipid profiles, Dyslipidemia
Dyslipidemia is a modifiable risk factor for cardiovascular disease, which is a leading cause of morbidity and mortality among the U.S. adults. The dramatic increase in the prevalence of childhood obesity since the 1980s has coincided with an increased prevalence of dyslipidemia among children and adolescents [1]. Studies have shown that dyslipidemia in childhood is a strong predictor of the disorder in adulthood [2–4], and there is also a strong association between adverse lipid profiles and the early onset of atherosclerosis in children and young adults [5,6]. Risk factors and risk behaviors that accelerate the development of atherosclerosis begin in childhood, such as unhealthy dietary habits and physical inactivity [1]. Reductions in risk factors and risk behaviors delay the progression toward clinical disease [1], which indicates the importance of early prevention to reduce cardiovascular disease risk later in life.
Eating a healthy diet, including limiting intake of added sugars, plays an important role in lowering the risk of cardiovascular disease. Although the consumption of added sugars in the United States has decreased among all age groups recently, adolescents continue to be the highest consumers [7]. Studies among adults have consistently shown an association between the high consumption of added sugars—especially sugar-sweetened beverages (SSBs) and weight gain, obesity, type 2 diabetes, and increased cardiovascular disease risk, including lipid disorders [8–10].
A few population-based studies also have examined the association of added sugars with lipid profiles among adolescents [11–14]. Welsh et al. [12] examined added sugars from one 24-hour dietary recall from the National Health and Nutrition Examination Survey (NHANES), 1999–2004, and found a statistically significant correlation between added sugars and high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, and triglycerides (TGs) among 2,157 U.S. adolescents aged 12–18 years, after adjusting for covariates. Ambrosini et al. [11] reported a prospective association between SSBs and increased TG in both boys and girls aged 14–17 years, independent of body mass index (BMI) and an association with HDL cholesterol in boys among 1,433 adolescent offspring from the Western Australia Pregnancy Cohort Study. Kell et al. [14] used the average of added sugars from two 24-hour dietary recalls and found significant association between the higher consumption of added sugars and elevated TG among 322 children aged 7–12 years. In a 10-year cohort study of 2,379 non-Hispanic, Caucasian, and African-American girls aged 9 years and 10 years at baseline, Lee et al. [15] used multiple 3-day food records and reported that low added sugars consumption (0%–<10% of total energy) compared with high added sugars consumption (≥10% of total energy) was associated with a .26 mg/dL greater annual increase in HDL levels. However, these studies were limited by the use of intake estimates on the basis of a single or the average of two 24-hour dietary recalls without explicitly taking into account individual day-to-day variability in consumption, which represents a type of measurement error. This intraindividual variation generally leads to the biased estimates of the nutrient–health outcome association [16,17]. Although one study [11] used food frequency questionnaire which can be interpreted as assessing a person’s long-term intake, the number of foods reported in food frequency questionnaire is usually very limited, and therefore, these data might not capture complete added sugars intake. Furthermore, no studies have examined the association between added sugars intake and the risk of dyslipidemia among adolescents. In the present study, we used the methods developed by the National Cancer Institute (NCI) that estimate the usual intake of added sugars from up to two 24-hour dietary recalls, which adjusted for the measurement error due to the intraindividual variations in intake. We examined the associations between the estimated usual intake of added sugars and lipid profiles and risk of dyslipidemia among the U.S. adolescents using 2005–2010 data from the NHANES, a nationally representative, cross-sectional survey.
Subjects and Methods
Study participants
Study participants included the U.S. adolescents aged 12–19 years who participated in the 2005–2010 NHANES. The NHANES uses a complex, stratified, multistage probability cluster sampling, cross-sectional design to collect health and nutritional data from a representative sample of the noninstitutionalized U.S. population. The design and operation of the NHANES has been described previously [18–20]. There were 4,536 NHANES participants aged 12e19 years who provided at least one 24-hour dietary recall. The dietary recalls were completed by the adolescents, rather than a proxy, and were included in this study if the recall was deemed reliable and complete [21]. A reliable recall was determined if all relevant dietary recall variables associated with the 24-hour dietary recall contain a value. For the dietary intake and lipid profile study, we excluded participants who had missing total cholesterol (TC) data (n = 447) or were pregnant (n = 42), leaving 4,047 adolescents for the analysis. For the association study, we further excluded adolescents who were on restricted diets, such as low-sugar, low-calorie, or low-carbohydrates (n = 282) or whose total calorie intake was <500 or >5,000 kcal/day (n = 113) because restricted diets or extreme calorie intakes might not represent the adolescents’ usual dietary intakes. Because they would be more likely to have underlying health conditions, such as digestive or eating disorders or thyroid disease, we excluded underweight participants (defined as BMI <5th percentile based on the CDC 2000 growth charts; n = 106). Finally, we excluded participants with missing covariate data (n = 213), yielding a sample of 3,333 participants for analyses. Study protocols for the NHANES were approved by the National Center for Health Statistics Ethics Review Board. Signed informed consent was obtained from all participants or their parent or guardian.
Lipid profiles
Outcome variables include levels of TC, HDL cholesterol, non-HDL cholesterol, LDL cholesterol, TG, TG-to-HDL cholesterol ratio, and TC-to-HDL cholesterol ratio. The standardized laboratory procedures to measure lipid levels have been described elsewhere [22]. Values for LDL cholesterol were calculated using the Friedewald calculation for TG levels ≤400 mg/dL:
LDL cholesterol = TC − HDL cholesterol − (TG/5)[23].
Non-HDL cholesterol was calculated as TC – HDL cholesterol. Because the TG level can increase up to 20%–30% after a meal, which would throw off the equation, LDL cholesterol, TG, and TG-to-HDL cholesterol ratio analyses were limited to those who had fasted 8 hours or more before the physical examination. Dyslipidemia was defined according to the National Cholesterol Education Program Expert Panel on Cholesterol Levels in Children as meeting one or more of the following criteria: TC ≥200 mg/dL, TG ≥130 mg/dL, LDL ≥130 mg/dL, or HDL <40 mg/dL [1]. All lipid measurements were standardized through the Lipid Standardization Program maintained by the Centers for Disease Control and Prevention in collaboration with the National Heart, Lung, and Blood Institute [24].
Covariates
Race/ethnicity was categorized into non-Hispanic white, non-Hispanic black, Mexican-American, or other. BMI was categorized on the basis of the CDC 2000 growth chart sex- and age-specific percentiles for adolescents aged 12–19 years. Normal BMI was defined as 5th to <85th percentile among adolescents. Overweight was defined as BMI values from 85th and <95th percentile and obesity as BMI ≥95th percentile among adolescents. Parental educational attainment was categorized as less than high school, high school, some college, and college or above. Active smokers were defined as those with a serum cotinine >10 ng/mL [25].
The 2010 Healthy Eating Index (HEI) scores were based on a 12-component index: total fruit, whole fruit, total vegetables, grains and beans, whole grains, dairy, total protein foods, seafood and plant proteins, fatty acids, refined grains, sodium, and empty calories, with total scores ranging from 0 to 100 and a higher score indicating a healthier diet. The HEI-2010 scores were calculated on the basis of the first 24-hour dietary recall. Because of changes in physical activity questions, the 2008 Physical Activity Guidelines for Americans recommendation for adolescents to avoid inactivity was used as a basic assessment of activity [26]. Physical activity status (active or inactive) was based on whether participants reported engaging in at least 10 minutes of moderate- or vigorous-intensity activity per week.
Estimating usual percentage of calories from added sugars
Data on nutrient content of foods eaten, including added sugars and total calories, were assessed using up to two 24-hour dietary recalls. The first recall was administered in person, followed by a second recall administered via phone 3–10 days later. Nutrient values were assigned to foods using the U.S. Department of Agriculture Food and Nutrient Database for Diet Studies [27]. To obtain information on added sugars for each food, we merged individual food files from the NHANES 2005–2010 with the Food Patterns Equivalents Database [28]. A detailed description of the database and estimates of added sugars from foods is published elsewhere [28].
Added sugars refer to sugars and syrups that are added to foods during preparation, processing, or at the table and which are not naturally occurring, as are the sugars found in fruits and milk products. Estimates of total added sugars were given in teaspoons for each participant, and we converted those to grams by calculating 4 g/teaspoon; we calculated calories from added sugars by multiplying 4 kcal/g. Finally, we calculated the percentage of calories (%kcal) from added sugars by dividing the calories from added sugars by the total calories. The calculations were done separately for the first and second 24-hour dietary recalls.
We used a method developed by the NCI to estimate the usual %kcal from added sugars and total calories, accounting for between- and within-person variation in intake [29]. This method allowed us to estimate the distribution of the usual %kcal from added sugars in our study population [16] and to examine the nutrient–disease association corrected for measurement error, also known as the regression calibration, a statistical method for adjusting point and interval estimates of effect from the regression models for bias due to measurement error [30,31]. The NCI method requires that at least some respondents have multiple days of nutrient values to estimate the between- and within-person variations [16,17]. The estimate of usual intake distribution was adjusted for age in years, sex, race/ethnicity, the first- or second-day dietary recalls (all participants had a first-day and 89.6% had a second-day dietary recall), and the day of the week when 24-hour recall was collected (weekday [Monday–Thursday] vs. weekend [Friday–Sunday]). For the association analysis, we also included BMI status, parental educational attainment, smoking status, physical activity, the 2010 HEI scores (excluding the added sugars component), and total calories to estimate the % kcal from added sugars [32]. Fay’s adjustment method was used to calculate the balanced repeated replication weights using PROC SURVEYREG in SAS (SAS Institute, Cary, NC).
Statistical analyses
Data on characteristics were expressed as means and standard errors for continuous variables or as percentages and standard errors for categorical variables and were compared by sex. At test was used to perform comparisons between sex groups for continuous variables. The χ2 test was used for categorical variables. We used multivariable linear regression to examine the association between participants’ usual %kcal from added sugars (per 5.0% increase) and lipid profiles. Because usual %kcal from added sugars had an approximately linear relationship, done graphically, to lipid profiles, we calculated the 10th, 30th, 50th, 70th, and 90th percentile distribution of the estimated usual in-takes.Using the parameters from the linear regression models, we estimated the adjusted mean lipids levels of the 10th, 30th, 50th, 70th, and 90th percentile distribution of the estimated usual intakes. We interpreted these adjusted means as the middle value of each quintile (Q1, Q2, Q3, Q4, and Q5) [33].
For risk of dyslipidemia, we used multivariable logistic regression analysis to estimate adjusted odds ratio (OR) comparing Q5, Q4, Q3, and Q2 with the lowest quartile (Q1) using the similar approach as the linear regression models. For both linear and logistic regression models, covariates included were age in years, sex, race/ethnicity, BMI status, parental educational attainment, smoking status, physical activity, the 2010 HEI scores (excluding added sugars component), and total calories. The weight for the first-day dietary recall was used for the association study. For the analyses of TG, LDL cholesterol, and the ratio of TG to HDL cholesterol, the fasting weight was used. We tested the interaction between the usual %kcal from added sugars and age, sex, race/ethnicity, and BMI status by including the interaction terms in the regression models. SUDAAN 11 (RTI International, Research Triangle Park, NC) was used to take the complex sampling design into account. All tests were two sided, and a p value of <.05 was considered statistically significant.
Results
Table 1 presents the characteristics of adolescents by sex. There were no differences in age, race/ethnicity, BMI status, or parental educational attainment by sex. Compared with their female counterparts, male adolescents were more likely to be active smokers, to be physically active, to have lower overall diet quality, to have higher prevalence of dyslipidemia, to have higher daily intake of added sugars and total calories, and to have marginally higher usual %kcal from added sugars. Overall, the study participants’ average estimated usual intakes of added sugars, total calories, and usual %kcal from added sugars were 87.4 g/day, 2,166 kcal/day, and 16.0% respectively. The prevalence of adolescents who had usual %kcal from added sugars ≥10% was 88.0% and ≥25% was 5.5%.
Table 1.
Characteristics of adolescents aged 12–19 years by sex, the NHANES 2005–2010a
| Characteristics | Overall, % (SE) | Male (n = 2,120), % (SE) | Female (n = 1,927), % (SE) | p value |
|---|---|---|---|---|
| Age, years | 15.6 (.06) | 15.6 (.08) | 15.5 (.10) | .179 |
| Race/ethnicity | .252 | |||
| Non-Hispanic white | 61.1 (2.20) | 61.5 (2.42) | 60.7 (2.36) | |
| Non-Hispanic black | 14.7 (1.33) | 13.9 (1.23) | 15.5 (1.68) | |
| Mexican-American | 12.5 (1.27) | 12.8 (1.23) | 12.2 (1.43) | |
| Other | 11.7 (1.40) | 11.8 (1.64) | 11.7 (1.43) | |
| BMI statusb | .550 | |||
| Normal | 65.0 (1.03) | 64.2 (1.75) | 65.9 (1.74) | |
| Overweight/obese | 35.0 (1.03) | 35.8 (1.75) | 34.1 (1.74) | |
| Parental educational attainmentc | .219 | |||
| Less than high school | 14.7 (.92) | 15.3 (1.17) | 14.1 (1.03) | |
| High school | 21.3 (1.39) | 19.6 (1.39) | 23.1 (1.92) | |
| Some college | 34.5 (1.55) | 35.2 (1.96) | 33.7 (2.12) | |
| College or above | 29.5 (2.25) | 29.8 (2.33) | 29.1 (2.84) | |
| Physically actived | <.001 | |||
| Inactive | 17.2 (.85) | 12.0 (1.13) | 22.7 (1.41) | |
| Active | 82.8 (.85) | 88.0 (1.13) | 77.3 (1.41) | |
| Smoker statuse | <.001 | |||
| Active | 14.8 (1.05) | 19.0 (1.43) | 10.5 (1.17) | |
| Nonactive | 85.2 (1.05) | 81.0 (1.43) | 89.5 (1.17) | |
| 2010 HEI scoref | 50.2 (.33) | 49.6 (.41) | 51.0 (.51) | .035 |
| TC (mg/dL) | 160.0 (1.00) | 157.5 (1.55) | 162.7 (1.06) | .005 |
| TG (mg/dL) | 90.8 (2.60) | 93.3 (3.69) | 88.2 (3.13) | .266 |
| LDL (mg/dL) | 90.4 (1.54) | 88.6 (2.23) | 92.3 (1.48) | .110 |
| HDL (mg/dL) | 51.0 (.37) | 48.5 (.47) | 53.5 (.44) | <.001 |
| Dyslipidemiag | 27.6 (1.14) | 32.4 (1.59) | 22.5 (1.96) | .001 |
| TC ≥200 mg/dL | 9.6 (.83) | 9.6 (1.26) | 9.6 (1.07) | .988 |
| TG ≥130 mg/dL | 7.3 (1.07) | 7.8 (1.34) | 6.9 (1.34) | .588 |
| LDL ≥130 mg/dL | 3.8 (.61) | 3.4 (.74) | 4.2 (.88) | .494 |
| HDL <40 mg/dL | 16.5 (.91) | 22.1 (1.43) | 10.6 (1.25) | <.001 |
| Mean usual intake of added sugars (g/day)h | 87.4 (2.61) | 100.5 (3.47) | 73.8 (2.09) | <.001 |
| Mean usual percentage of calories from added sugarsh | 16.0 (.35) | 16.4 (.46) | 15.6 (.38) | .090 |
| Mean usual total calories (kcal/day)h | 2,166 (35.1) | 2,472 (45.9) | 1,846 (38.7) | <.001 |
| Percent of participants with 10% or more calories from added sugarsh | 88.0 (2.40) | 89.4 (2.30) | 86.7 (2.70) | .223 |
| Percent of participants with ≥25% calories from added sugarsh | 5.5 (1.30) | 6.4 (1.60) | 4.7 (1.10) | .191 |
BMI = body mass index; HDL = high-density lipoprotein; HEI = Healthy Eating Index; LDL = low-density lipoprotein; NHANES = National Health and Nutrition Examination Survey; SE = standard error; TC = total cholesterol; TG = triglycerides.
Sample size is unweighted. Pregnant women and individuals who had missing TC data were excluded.
BMI was categorized on the basis of the CDC 2000 growth chart sex- and age-specific percentiles for adolescents aged 12–19 years. Normal BMI was defined as 5th to <85th percentile among adolescents. Overweight was defined as BMI 85th to <95th percentile, and obesity as BMI ≥95th percentile among adolescents.
Parental educational attainment was categorized as less than high school, high school, some college, and college or above.
Adolescents were classified as physically inactive if participants reported engaging in <10 minutes of moderate- and/or vigorous-intensity activity per week.
Active smoker was defined as serum cotinine >10 ng/mL
The 2010 HEI scores, a 12-component index: total fruits, whole fruit, total vegetables, grains and beans, whole grains, dairy, total protein foods, seafood and plant proteins, fatty acids, refined grains, sodium, and empty calories, with total scores ranging from 0 to 100 and a higher score indicating a healthier diet.
Dyslipidemia was defined as TC ≥200 mg/dL, LDL cholesterol ≥130 mg/dL, TG ≥130 mg/dL, or HDL cholesterol <40 mg/dL
The differences between males and females were tested using z tests.
After adjustment for potential confounders, the usual %kcal from added sugars was inversely associated with HDL cholesterol, with a decrease of 1.09 mg/dL for every 5.0% increase in % kcal (95% confidence interval [CI], .29–1.90 mg/dL). The usual % kcal from added sugars was positively associated with TG, the ratio of TG to HDL cholesterol, and the ratio of TC to HDL cholesterol, with an increase of 5.92 (95% CI, .38–11.46), .19 (95% CI, .04–34), and .10 mg/dL (95% CI, .01 – 19), respectively, for every 5.0% increase in %kcal from added sugars (Table 2). TC, LDL cholesterol, and non-HDL cholesterol were not significantly associated with usual %kcal from added sugars (Table 2). The insignificant association for LDL might be due to smaller sample size.
Table 2.
Association between usual percentage of calories from added sugars and lipid profiles among adolescents aged 12–19 years, the NHANES 2005–2010a
| Characteristic | β Coefficient (95% CI)b | p value |
|---|---|---|
| HDL cholesterol (n = 3,333) | ||
| Adjusted for age, sex, and race/ethnicity only | − 1.62 (−2.45 to −.80) | <.001 |
| Fully adjusted modelc | − 1.09 (−1.90 to −.29) | .009 |
| TG (n = 1,512) | ||
| Adjusted for age, sex, and race/ethnicity only | 7.82 (2.92–12.71) | .002 |
| Fully adjusted modelc | 5.92 (.38–11.46) | .037 |
| TC (n = 3,333) | ||
| Adjusted for age, sex, and race/ethnicity only | .38 (−2.35 to 3.11) | .781 |
| Fully adjusted modelc | .78 (−1.89 to 3.46) | .600 |
| LDL cholesterol (n = 1,510) | ||
| Adjusted for age, sex, and race/ethnicity only | 1.47 (−1.52 to 4.46) | .327 |
| Fully adjusted modelc | 1.49 (−1.53 to 4.50) | .326 |
| Non-HDL cholesterol (n = 3,333) | ||
| Adjusted for age, sex, and race/ethnicity only | 2.00 (−1.04 to 5.05) | .192 |
| Fully adjusted modelc | 1.88 (−.99 to 4.74) | .194 |
| TC to HDL cholesterol ratio (n = 3,333) | ||
| Adjusted for age, sex, and race/ethnicity only | .13 (.03–.23) | .011 |
| Fully adjusted modelc | .10 (.01–.19) | .028 |
| TG to HDL cholesterol ratio (n = 1,512) | ||
| Adjusted for age, sex, and race/ethnicity only | .25 (.11–.38) | <.001 |
| Fully adjusted modelc | .19 (.04–.34) | .017 |
CI = confidence interval; HDL = high-density lipoprotein; LDL = low-density lipoprotein; NHANES = National Health and Nutrition Examination Survey; TC = total cholesterol; TG = triglycerides.
Sample size is unweighted. Pregnant women and individuals who had missing TC data or reporting being on a sugar-free/low-sugar diet, on a low-fat/low-cholesterol diet, on a diabetic diet, or on a weight-loss/low-calorie/low-carbohydrate/high-protein diet, with total calorie intake <500 or >5,000 kcal/day, being underweight, and with missing covariates data were excluded. The weight for the first day 24-hour dietary recall was used for the analyses of TC, HDL cholesterol, non-HDL cholesterol, and the ratio of TC to HDL cholesterol. Fasting weight was used for the analyses of TG, LDL cholesterol, and the ratio of TG to HDL cholesterol.
β Coefficients for usual percentage of calories from added sugars represent the changes in lipid levels associated with each 5.0% change in intake.
Adjusted for age, sex, race/ethnicity, body mass index, parental educational attainment, smoking status, physical activity, and 2010 healthy eating scores excluding the sugar component.
The patterns of association were largely consistent across sex, race/ethnicity, and BMI (normal vs. overweight/obese) subgroups. In non-Hispanic whites, every 5.0% increase in usual intake was associated with 1.70 mg/dL decrease in HDL. The corresponding decreases of HDL were .14, .13, and .03 mg/dL respectively, for non-Hispanic blacks, Mexican-Americans, and other race/ethnicity group. However, the p value for the interaction between the usual intake and race/ethnicity was not significant (p = .077; Supplementary Table 1).
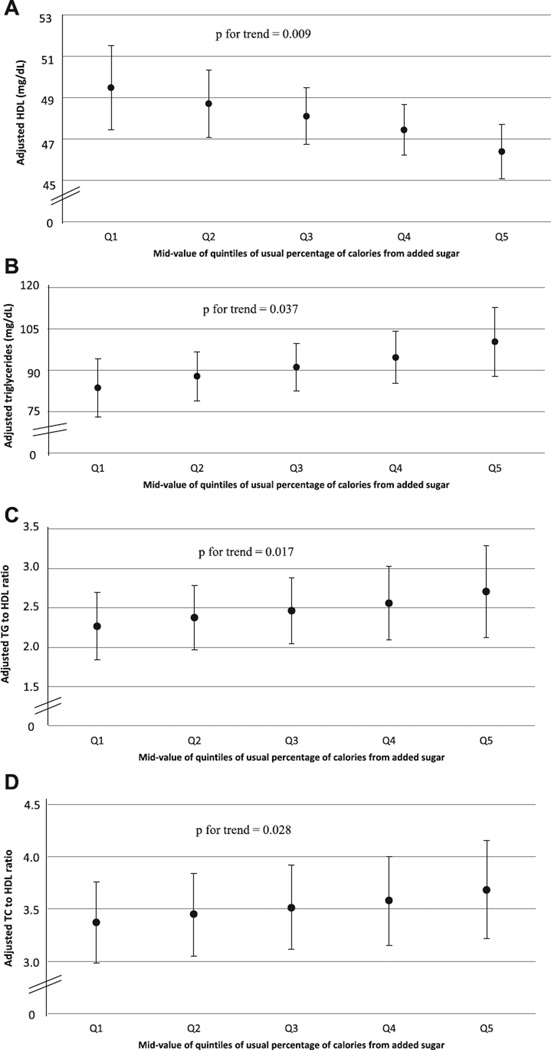
For the lowest and highest quintiles of added sugars intake, HDL cholesterol was 49.5 (95% CI, 47.4–51.6) and 46.4 mg/dL (95% CI, 45.2–47.6), respectively (p for trend = .009) (Figure 1A); TGs were 85.6 (95% CI, 75.5–95.6) and 101.2 mg/dL (95% CI, 88.7–113.8), respectively (p for trend = .037; Figure 1B); ratios of TG to HDL cholesterol were 2.28 (95% CI, 1.84–2.70) and 2.73 (95% CI, 2.11–3.32), respectively (p for trend = .017; Figure 1C); and ratios of TC to HDL cholesterol were 3.41 (95% CI, 3.03–3.79) and 3.70 (95% CI, 3.24–4.15), respectively (p for trend = .028; Figure 1D).
Figure 1. Adjusted high-density lipoprotein (HDL) cholesterol (A), triglycerides (TG) (B), TG-to-HDL cholesterol ratio (C), and TC-to-HDL cholesterol ratio (D) (95% confidence interval) by midvalue of quintile of usual percentage of calories from added sugars among adolescents aged 12–19 years who were not on a restricted diet, NHANES 2005–2010.
The adjusted OR for risk of dyslipidemia comparing adolescents in the highest versus those in the lowest quintile of %kcal of added sugars was 1.41 (95% CI, 1.01–1.95; Table 3). Adjusted OR was 1.20 (95% CI, 1.01–1.44) for every 5% increase in the usual % kcal of added sugars. The associations were largely consistent across gender, race/ethnicity, and BMI groups (normal vs. overweight/obese; data not shown).
Table 3.
Adjusted OR of dyslipidemia according to usual percentage of calories from added sugars among the U.S. adolescents aged 12–19 years, the NHANES 2005e2010a
| Characteristic | Midvalue of quintiles of usual percentage of calories from added sugar |
p valueb | |||||
|---|---|---|---|---|---|---|---|
| Q1, 9.5% | Q2, 13.9% | Q3, 15.8% | Q4, 17.6% | Q5, 22.8% | |||
| Dyslipidemia | 802 | ||||||
| Range/usual percent | 5.4%–35.5% | 5.4%–<12.8% | 12.8%–<14.9% | 14.9%–<16.6% | 16.6%–<18.8% | 18.8%–35.5% | |
| OR adjusted for age, sex, and race/ethnicity Only | 1.00 | 1.14 (1.04–1.24) | 1.25 (1.07–1.45) | 1.37 (1.11–1.69) | 1.59 (1.16–2.18) | .005 | |
| Fully adjusted ORc | 1.00 | 1.10 (1.00–1.21) | 1.18 (1.01–1.38) | 1.26 (1.01–1.57) | 1.41 (1.01–1.95) | .042 | |
NHANES = National Health and Nutrition Examination Survey; OR = odds ratio; Q = quintile.
Pregnant women and individuals who had missing TC data or reporting being on a sugar-free/low-sugar diet, on a low-fat/low-cholesterol diet, on a diabetic diet, or on a weight-loss/low-calorie/low-carbohydrate/high-protein diet, with total calorie intake <500 or >5,000 kcal/day, being underweight, and with missing covariates data were excluded.
p Value for testing significant association between the usual percentage of calories from added sugars and dyslipidemia based on the Satterthwaite F test; all tests are two tailed.
Adjusted for age, sex, race/ethnicity, body mass index, parental educational attainment, smoking status, physical activity, and 2010 healthy eating scores excluding the sugar component.
In the sensitivity analysis, we repeated analysis by including 319 participants who were underweight or had missing covariates. The results showed a similar pattern of association between the usual %kcal of added sugars and lipid profiles and risk for dyslipidemia as that of the main analyses (Supplementary Table 2 and 3).
Discussion
In this large, nationally representative sample of the U.S. adolescents, we found that the average usual %kcal from added sugars was 16.0%. The overwhelming majority (~ 88%) of the U.S. adolescents consumed ≥10% of calories from added sugars and 5.5% consumed ≥25% of calories from added sugars. Higher %kcal from added sugars was inversely associated with HDL cholesterol and positively associated with TG, the ratio of TG to HDL cholesterol, the ratio of TC to HDL cholesterol, and the risk of dyslipidemia. After adjustment for demographics and other characteristics, the pattern of association was consistent across sex, race/ethnicity, and BMI categories. When participants who were underweight or had missing baseline covariates were included, the associations remain largely unchanged.
Although the recommendations for added sugars consumption vary by the organizations, for example, the Institute of Medicine recommends that added sugars make up <25% of total calories, World Health Organization recommends <10% of total calories, and the American Heart Association advises that the daily caloric intake from added sugars be <100 calories for most women and <150 calories for most men, the general idea that added sugars consumption should be limited is universal. The 2010 Dietary Guidelines for Americans encourage limiting total intake of discretionary calories, which include added sugars and solid fats, to 5%–15% of daily caloric intake. Our results suggested that several adverse measures of lipids and risk of dyslipidemia were significantly higher when comparing adolescents who consumed >22.8% calories from added sugars (highest quintile) with those who consumed <9.5% of calories from added sugars (lowest quintile).
On average, SSBs and sweetened fruit drinks have been found to contribute about half of total added sugars consumed [28,34], and many studies suggest that high consumption of SSBs is associated with obesity, type 2 diabetes, and cardiovascular disease in adults [8–10]. However, few studies have examined this association for total added sugars among adolescents, particularly for lipid profiles and risk of dyslipidemia [11–14]. Our findings about the associations between the usual %kcal from added sugars and HDL cholesterol and TG are consistent with results from the previous studies [11–14]. However, none of the previously mentioned studies used a statistical approach to take into account the day-to-day variability in consumption of added sugars, which may have resulted in a weakened association. It is hard to compare our results with those from previous studies because different categorization on the %kcal from added sugars was used. For example, Welsh et al. [12] used six categories (<10%, 10%–<15%, 15%–<20%, 20%–<25%, 25%–<30%, and ≥30%) of kcal from added sugar, and Lee et al. [15] reported the results by two categories (0%–<10% vs. ≥10% of total energy).
Several potential mechanisms have been proposed to explain the effect of added sugars on lipid profiles. A likely mechanism by which excess consumption of added sugars may increase TG concentrations is through stimulation of hepatic de novo lipogenesis [9,35–37]. Being readily available for absorption and being rapidly metabolized, added sugars induce sharp increases in glucose and insulin concentrations [10]. Other studies suggested that fructose, one type of the added sugars, increases hepatic TG synthesis and secretion of very LDL cholesterol and also reduces lipoprotein lipase activity at the adipocyte, which decreases the rate of peripheral lipid clearance [9,38]. Fructose can also produce advanced glycation end products, which can trigger oxidative damage, cause changes in the arterial wall structure, and facilitate LDL disposition [37].
Our study has major strengths. To our knowledge, this is the first study to assess the association between usual %kcal from added sugars and lipid profiles and risk of dyslipidemia in a large nationally representative sample of the U.S. adolescents. We used a measurement error model to estimate usual %kcal from added sugars and total calories from up to two 24-hour dietary recalls, while accounting for variation in intake within individuals.
When interpreting our results, a number of issues should be considered. First, we used 24-hour dietary recalls to estimate participants’ usual %kcal from added sugars. The actual usual intake may be underestimated or overestimated. A previous validation study using 24-hour dietary recalls suggested that energy intake may be underestimated by as much as 11% [39]. Second, puberty may influence the lipid profiles of adolescents. However, information on puberty was not collected during the NHANES 2005–2010 cycles. Third, our first 24-hour dietary was conducted in person and the second recall was collected via telephone interview that may introduce inconsistencies in the estimation of food quantity and food ingredients, such as lower estimates of total calories. However, previous research had found 24-hour recalls obtained by telephone interview to be as effective as those obtained in person [40]. Fourth, questions regarding physical activity differed between the NHANES 2005–2006 and 2007–2010 cycles. To minimize the influence of this inconsistency in physical activity measures, we defined physically inactive as participating in <10 minutes of moderate and/or vigorous activity per week. Fifth, in our multivariable models, we adjusted for a set of covariates that were available in the NHANES for adolescents, but the associations in our study may be due to other unobserved confounding variables, residual confounding, or other dietary variables. Finally, our study was cross sectional; thus, the associations between usual %kcal from added sugars and lipid profiles should be interpreted with caution.
Our results indicate that the overwhelming majority of the U.S. adolescents consume more added sugars than recommended for heart health. Increased intake of added sugar is associated with several lipid measures and risk of dyslipidemia.
Supplementary Material
IMPLICATIONS AND CONTRIBUTION.
Studies examining the association between added sugars intake and lipid profiles among adolescents have not taken in to account the measurement error. We used the method developed by the National Cancer Institute and found usual intake of added sugars was significantly associated with several measures of lipid profiles and risk of dyslipidemia among adolescents.
Acknowledgments
An early version of this work was presented as oral presentation at the 2014 American Heart Association Scientific Session for Cardiovascular Disease, Epidemiology and Prevention, Nutrition, Physical Activity and Metabolism.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to report.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Supplementary Data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.jadohealth.2014.12.001.
References
- 1.Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents National Heart, Lung, and Blood Institute. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: Summary report. Pediatrics. 2011 Dec;128(Suppl 5):S213–S256. doi: 10.1542/peds.2009-2107C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lauer RM, Clarke WR. Use of cholesterol measurements in childhood for the prediction of adult hypercholesterolemia. The Muscatine Study. JAMA. 1990;264:3034–3038. [PubMed] [Google Scholar]
- 3.Morrison JA, Glueck CJ, Horn PS, et al. Pediatric triglycerides predict cardiovascular disease events in the fourth to fifth decade of life. Metabolism. 2009;58:1277–1284. doi: 10.1016/j.metabol.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Webber LS, Srinivasan SR, Wattigney WA, et al. Tracking of serum lipids and lipoproteins from childhood to adulthood. The Bogalusa Heart Study. Am J Epidemiol. 1991;133:884–899. doi: 10.1093/oxfordjournals.aje.a115968. [DOI] [PubMed] [Google Scholar]
- 5.A preliminary report from the Pathobiological Determinants of Atherosclerosis in Youth (PDAY) Research Group. Relationship of atherosclerosis in young men to serum lipoprotein cholesterol concentrations and smoking. JAMA. 1990;264:3018–3024. doi: 10.1001/jama.1990.03450230054029. [DOI] [PubMed] [Google Scholar]
- 6.McGill HC, Jr, McMahan CA, Malcom GT, et al. Effects of serum lipoproteins and smoking on atherosclerosis in young men and women. The PDAY Research Group. Pathobiological Determinants of Atherosclerosis in Youth. Arterioscler Thromb Vasc Biol. 1997;17:95–106. doi: 10.1161/01.atv.17.1.95. [DOI] [PubMed] [Google Scholar]
- 7.Welsh JA, Sharma AJ, Grellinger L, et al. Consumption of added sugars is decreasing in the United States. Am J Clin Nutr. 2011;94:726–734. doi: 10.3945/ajcn.111.018366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elliott SS, Keim NL, Stern JS, et al. Fructose, weight gain, and the insulin resistance syndrome. Am J Clin Nutr. 2002;76:911–922. doi: 10.1093/ajcn/76.5.911. [DOI] [PubMed] [Google Scholar]
- 9.Fried SK, Rao SP. Sugars, hypertriglyceridemia, and cardiovascular disease. Am J Clin Nutr. 2003;78:873S–880S. doi: 10.1093/ajcn/78.4.873S. [DOI] [PubMed] [Google Scholar]
- 10.Tappy L, Lê KA, Tran C, et al. Fructose and metabolic diseases: New findings, new questions. Nutrition. 2010;26:1044–1049. doi: 10.1016/j.nut.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 11.Ambrosini GL, Oddy WH, Huang RC, et al. Prospective associations between sugar-sweetened beverage intakes and cardiometabolic risk factors in adolescents. Am J Clin Nutr. 2013;98:327–334. doi: 10.3945/ajcn.112.051383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Welsh JA, Sharma A, Cunningham SA, et al. Consumption of added sugars and indicators of cardiovascular disease risk among U.S. adolescents. Circulation. 2011;123:249–257. doi: 10.1161/CIRCULATIONAHA.110.972166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kosova EC, Auinger P, Bremer AA. The relationships between sugar-sweetened beverage intake and cardiometabolic markers in young children. J Acad Nutr Diet. 2013;113:219–227. doi: 10.1016/j.jand.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kell KP, Cardel MI, Bohan Brown MM, et al. Added sugars in the diet are positively associated with diastolic blood pressure and triglycerides in children. Am J Clin Nutr. 2014;100:46–52. doi: 10.3945/ajcn.113.076505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee AK, Binongo JN, Chowdhury R, et al. Consumption of less than 10% of total energy from added sugars is associated with increasing HDL in females during adolescence: A longitudinal analysis. J Am Heart Assoc. 2014;3:e000615. doi: 10.1161/JAHA.113.000615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tooze JA, Midthune D, Dodd KW, et al. A new statistical method for estimating the usual intake of episodically consumed foods with application to their distribution. J Am Diet Assoc. 2006;106:1575–1587. doi: 10.1016/j.jada.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Cancer Institute. [Accessed July 7, 2014];Usual dietary intakes: The NCI method. Available at: http://riskfactor.cancer.gov/diet/usualintakes/method.html.
- 18.Centers for Disease Control and Prevention. National Health and. [Accessed July 7, 2014];Nutrition Examination Survey, 2005–2006. Available at: http://wwwn.cdc.gov/nchs/nhanes/search/nhanes05_06.aspx.
- 19.Centers for Disease Control and Prevention. [Accessed July 7, 2014];National Health and Nutrition Examination Survey, 2007–2008. Available at: http://wwwn.cdc.gov/nchs/nhanes/search/nhanes07_08.aspx.
- 20.Centers for Disease Control and Prevention. [Accessed July 7, 2014];National Health and Nutrition Examination Survey, 2009–2010. Available at: http://wwwn.cdc.gov/nchs/nhanes/search/nhanes09_10.aspx.
- 21.Centers for Disease Control and Prevention. [Accessed December 31, 2014];National Health and Nutrition Examination Survey; 2009–2010 Data Documentation, Codebook, and Frequencies. Available at: http://wwwn.cdc.gov/nchs/nhanes/2009-2010/DR1IFF_F.htm.
- 22.Centers for Disease Control and Prevention, National Center for Health Statistics. [Accessed July 7, 2014];Natl Health Nutr Examination Surv Data. Available at: http://www.cdc.gov/NCHS/nhanes.htm.
- 23.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 24.The Centers for Disease Control and Prevention Lipid Standardization Program. [Accessed July 7, 2014];Laboratory quality assurance and standardization programs. 2012 Available at: http://www.cdc.gov/labstandards/lsp_faq.html;
- 25.Hukkanen J, Jacob 3rd P, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. 2005;57:79–115. doi: 10.1124/pr.57.1.3. [DOI] [PubMed] [Google Scholar]
- 26.United States Department of Health and Human Services. [Accessed July 7, 2014]; Available at: http://www.health.gov/paguidelines.
- 27.USDA Food and Nutrient Database for Dietary Studies. Beltsville, MD: Agricultural Research Service, Food surveys research group; [Accessed July 7, 2014]. Available at: www.ars.usda.gov/Services/docs.htm?docid=12089. [Google Scholar]
- 28.United States Department of Agriculture Agricultural Research Service. [Accessed July 7, 2014]; Availabe at: http://www.ars.usda.gov/Services/docs.htm?docid=23869.
- 29.Guenther PM, Kott PS, Carriquiry AL. Development of an approach for estimating usual nutrient intake distributions at the population level. J Nutr. 1997;127:1106–1112. doi: 10.1093/jn/127.6.1106. [DOI] [PubMed] [Google Scholar]
- 30.Kipnis V, Midthune D, Buckman DW, et al. Modeling data with excess zeros and measurement error: Application to evaluating relationships between episodically consumed foods and health outcomes. Biometrics. 2009;65:1003–1010. doi: 10.1111/j.1541-0420.2009.01223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosner B, Spiegelman D, Willett WC. Correction of logistic regression relative risk estimates and confidence intervals for random within-person measurement error. Am J Epidemiol. 1992;136:1400–1413. doi: 10.1093/oxfordjournals.aje.a116453. [DOI] [PubMed] [Google Scholar]
- 32.Joseph ML, Carriquiry A. A measurement error approach to assess the association between dietary diversity, nutrient intake, and mean probability of adequacy. J Nutr. 2010;140:2094S–2101S. doi: 10.3945/jn.110.123588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tooze JA, Kipnis V, Buckman DW, et al. A mixed-effects model approach for estimating the distribution of usual intake of nutrients: The NCI method. Stat Med. 2010;29:2857–2868. doi: 10.1002/sim.4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Drewnowski A, Rehm CD. Consumption of added sugars among U.S. children and adults by food purchase location and food source. Am J Clin Nutr. 2014;100:901–907. doi: 10.3945/ajcn.114.089458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hellerstein MK. De novo lipogenesis in humans: Metabolic and regulatory aspects. Eur J Clin Nutr. 1999;53(Suppl 1):S53–S65. doi: 10.1038/sj.ejcn.1600744. [DOI] [PubMed] [Google Scholar]
- 36.Parks EJ, Hellerstein MK. Carbohydrate-induced hypertriacylglycerolemia: Historical perspective and review of biological mechanisms. Am J Clin Nutr. 2000;71:412–433. doi: 10.1093/ajcn/71.2.412. [DOI] [PubMed] [Google Scholar]
- 37.Prasad A, Bekker P, Tsimikas S. Advanced glycation end products and diabetic cardiovascular disease. Cardiol Rev. 2012;20:177–183. doi: 10.1097/CRD.0b013e318244e57c. [DOI] [PubMed] [Google Scholar]
- 38.Chong MF, Fielding BA, Frayn KN. Mechanisms for the acute effect of fructose on postprandial lipemia. Am J Clin Nutr. 2007;85:1511–1520. doi: 10.1093/ajcn/85.6.1511. [DOI] [PubMed] [Google Scholar]
- 39.Espeland MA, Kumanyika S, Wilson AC, et al. Statistical issues in analyzing 24-Hour dietary recall and 24-hour urine collection data for sodium and potassium intakes. Am J Epidemiol. 2001;153:996–1006. doi: 10.1093/aje/153.10.996. [DOI] [PubMed] [Google Scholar]
- 40.Tran KM, Johnson RK, Soultanakis RP, In-person vs, et al. telephone-administered multiple-pass 24-hour recalls in women: Validation with doubly labeled water. J Am Diet Assoc. 2000;100:777–783. doi: 10.1016/S0002-8223(00)00227-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.