Abstract
Mucus forms a protective hydrogel layer over the intestinal epithelium, presenting a selective and robust barrier to the uptake of particulates and microbe invasion. Disease can alter mucus production and composition, thus potentially modifying mucosal barrier properties. Hirschsprung’s disease (HD) is a developmental abnormality of the nervous system often complicated by intestinal infection. An investigation of colonic mucus barrier properties in an HD animal model, endothelin receptor B mutant mice, revealed significantly reduced microsphere (passive) and microbe (active) transport rates (7-fold and 3.6-fold, respectively, in proximal colonic mucus) relative to wild-type. Transport differences were evident in both the ganglionic and aganglionic colon segments, in agreement with the risk of Hirschsprung’s disease-associated enterocolitis after surgery to remove aganglionic colon segments. The development of therapies aimed at altering colonic mucus barrier properties could be explored towards preventing the onset of enterocolitis in Hirschsprung’s disease.
Keywords: mucus, Hirschsprung’s disease, nanoparticle diffusion, microbe transport, intestine
1. Introduction
Mucus, a highly complex hydrogel composed of mucin glycoproteins with a pore size reported to be approximately 10–500 nm,[1] provides a barrier against particulate materials and biological pathogens. The barrier properties of mucus are a combined result of physical effects, such as size exclusion and hydrodynamic drag, and intermolecular interactions between diffusing entities and mucus components.[1–5] The significance of the mucus barrier in controlling access to the underlying epithelium raises the question of whether mucosal structural and/or chemical changes occur in disease states, potentially contributing to pathogen invasion.
Hirschsprung’s disease (HD), also known as intestinal aganglionosis, is a developmental abnormality of the enteric nervous system that affects 1 in 5,000 live births.[6, 7] HD is characterized by the lack of ganglion cells along a variable length of the distal intestine, resulting in the absence of peristalsis in the aganglionic segment and dilation of the colon proximally.[6, 7] HD is associated with the development of Hirschsprung’s-associated enterocolitis (HAEC), a severe inflammation of the intestinal mucosa that presents with abdominal distension, diarrhea, and fever and can progress to sepsis and death.[6, 7] HAEC, the leading cause of death in HD, occurs in up to 50% of untreated HD cases, and the risk persists even after surgical removal of the aganglionic intestine, suggesting that the abnormality associated with HAEC extends beyond the region of aganglionosis.[6]
It is thought that HD leads to changes in colonic microbiota, innate immunity, and epithelial barrier properties,[8] any of which may contribute to the risk of developing enterocolitis. Although these theories are being investigated, little attention has been focused on the potential contributory role of mucus barrier properties in this disease. Recent insight into changes in HD mucosal properties has been gained by investigating mucus turnover rate, mucin gene expression, and histological structure.[8–13] These studies have shown that HD is associated with lowered mucus turnover rate, lowered mucin concentrations, abnormal mucin ratios (neutral mucins:acidic-sulphomucins),[13] and differences in mucin producing goblet cell size and proliferation compared to the healthy state.[14] These results therefore support differences in mucus quantity and composition in HD, but do not specifically test potential changes in mucus barrier properties that may play a role in microbe invasion and in the development of enterocolitis.
Given the important role of mucus in protecting underlying epithelia from microbial invasion, we hypothesize that the inherent barrier properties of colonic mucus are altered in HD. To test this hypothesis, transport of passively diffusing entities (polystyrene microspheres) and actively moving microbes (Escherichia coli) through colonic mucus in an HD animal model were investigated. In this work, live imaging and multiple particle tracking (MPT),[15–21] a powerful technique enabling non-disruptive investigation of transport across the mucosal layer, was employed. Particle and microbe trajectories were used to calculate time-averaged mean squared displacements (MSDs) and average velocities to quantitatively characterize transport across mucus layers. Results supported differences in colonic mucus barriers between HD and wild-type (WT) animals. A deeper understanding of the mechanisms underlying mucus barrier differences could lead to therapeutic treatments to modulate mucus barrier properties and potentially prevent the onset of HAEC.
2. Experimental Section
2.1 Preparation of Microspheres
Particle solutions were prepared using 200 nm carboxylate-modified yellow-green fluorescent microspheres (FluoSpheres®, Invitrogen Molecular Probes, Carlsbad, CA). Particle suspensions (2% solids in distilled water with 2 mM azide) were diluted in maleate buffer (pH 6.5) containing 100 mM Trizma, 10 mM CaCl2, 65 mM NaCl, 40 mM NaOH and 3 mM NaN3 for a final concentration of 0.0025% wt/vol. All chemicals were purchased from Sigma Aldrich, St. Louis, MO.
2.2 Preparation of Microbes
A green fluorescent protein (GFP) expressing strain Escherichia Coli ASV (E. coli) was cultured by dilution (1:1,000) of overnight cultures in 12–25 mL Luria-Bertani (LB) broth for ~2.5 h with aeration (250 rpm) at 37 °C. Overnight cultures were made by dilution of thawed cells from a 20% glycerol stock (−80 °C) and incubation in LB medium with aeration for 16 to 20 h.
2.3 Collection and Preparation of Colonic Tissue
Colon segments were excised from 11–20 day-old Endothelin receptor B mutant (Ednrb−/−) mice obtained from Jackson Labs (Ednrb−/−tm1Ywa, stock #003295) and from their wild-type (WT) littermates. Experiments were approved by the Institutional Animal Care and Use Committee for Massachusetts General Hospital (MGH IACUC OLAW Assurance number: A3596-01, Protocol number: 2009N000239). The most distal and proximal sections (~1 cm) of colon were extracted and placed on a glass slide prepared by attaching silicone isolators (1.6 mm depth and 13 mm diameter, Grace Bio-Labs) (Figure 1). The distal colon represents the aganglionic segment, while the proximal colon contains ganglion cells. Tissues were opened along the antimesenteric border such that they laid flat on the slide with mucosal surface facing up, and 5 μL of diluted carboxylate-modified microsphere solution was carefully added onto the mucus surface to prevent any perturbation in mucus structure. Samples were incubated in a dark, humid environment for 90 min at room temperature before video microscopy. For microbial studies, 5 μL of microbe solution was added onto the mucus surface and incubated for 15 min at room temperature prior to microscopy.
Figure 1.
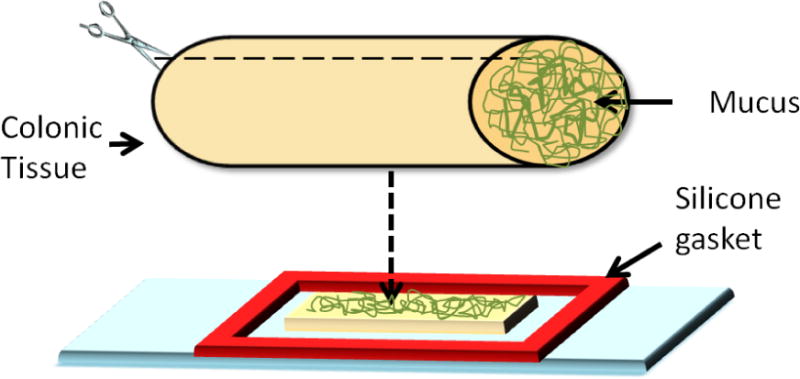
Schematic representation of colonic tissue preparation with undisturbed mucus layer for video microscopy.
2.4 Real-time Multiple Particle and Microbe Tracking
Videos of particle diffusion and microbe transport were obtained using an Olympus DP70 digital color camera (Olympus, Center Valley, PA) mounted on an inverted Olympus IX51 microscope with attached X-Cite 120 fluorescence illumination system (EXFO, Mississauga, Ontario, Canada). Twenty second videos were captured using Olympus DP imaging software, and ParticleTracker ImageJ plugin was used to generate particle trajectories.[22] Videos were captured within the mucus as confirmed by focusing at different planes between the top and bottom focal planes containing mucus (as indicated by diffusing nanoparticles and diffuse mucus autofluorescence). Particle coordinates were used to calculate time-averaged mean squared displacement (<MSD>) and effective diffusivities (Deff):
| (1) |
| (2) |
where x(t) and y(t) represent the particle coordinates at a given time, and τ is the time scale. Particle interaction with the mucus constituents was further investigated by fitting particle <MSD> vs. time scale:
| (3) |
where α is the anomalous exponent indicating particle motion obstruction and D0 is the time independent diffusion coefficient.[20] Effective diffusivities were used to estimate the fraction of particles predicted to penetrate intestinal mucus layers of given thickness using a numerical integration of Fick’s second law:
| (4) |
where C is the concentration of particles, t is time and x is position. Boundary conditions included assumptions of a constant concentration at the mucus surface and initial absence of particles within the mucus layer.[23, 24] Individual bacteria trajectories and velocities were obtained using a modified version of the interactive data language (IDL) software colloid-tracking algorithm from Crocker and Grier.[25, 26] This algorithm was edited to account for elongated particles (bacteria) as opposed to spheroids.[25]
2.5 Statistical Analysis
Three tissue segments were analyzed for each condition (passive and active transport, WT, and Ednrb−/− tissue). More specifically, the proximal and distal sections of three WT and three Ednrb−/− mice were used for passive transport and another six mice were used to analyze active transport. In the passive case n ≥ 100 particles were analyzed per tissue section (total sections=12) and in the active case n ≥ 90 microbes per section (total sections=12). A one tailed, unequal variance Student’s t-test was used to evaluate statistical significance with p < 0.05 as significant.
3. Results
3.1 Passive Transport in Colonic Mucus
Particle mobility in mucus was assessed to explore the barrier properties of colonic mucus in both diseased (Ednrb−/−) and WT mice. Aganglionic distal segments and normally innervated proximal segments from mutant mice were compared to similar sections from WT mice.
3.1.1 Colonic Mucus Barrier Differences in Healthy and Diseased State Tissue
Results indicate that Ednrb−/− mucus provided a greater barrier to particle transport than WT mucus, with this difference being markedly more prominent in the proximal relative to the distal colon. Particles diffusing within WT proximal colonic mucus displayed less confined trajectories that probed greater distances during 20 s analyses than particles in Ednrb−/− proximal colonic mucus (Figure 2A). The transport rates of particles in mucus were quantified by their time scale dependent <MSD>. At a time scale of 10 s, the <MSD> values of WT were 7-fold higher compared to Ednrb−/− (Figure 2B–C). As expected, transport in mucus was subdiffusive (α < 1) due to hindrance of particle transport by the mucus gel.[20] Average α for WT and Ednrb−/− were 0.43 and 0.34, respectively at τ = 10 s, indicating a less obstructive barrier in WT mucus relative to Ednrb−/−. Particle mobility was also investigated in the distal section of WT and Ednrb−/− mouse colon. Trajectory profiles indicated slightly less confined motion in WT as compared to Ednrb−/− (Figure 2A). At a time scale of 10 seconds particles in WT were 1.5-fold faster than particles in Ednrb−/− (Figure 2B–C). This was supported by α values of 0.63 and 0.57 in WT and Ednrb−/−, respectively at τ = 10 s.
Figure 2.
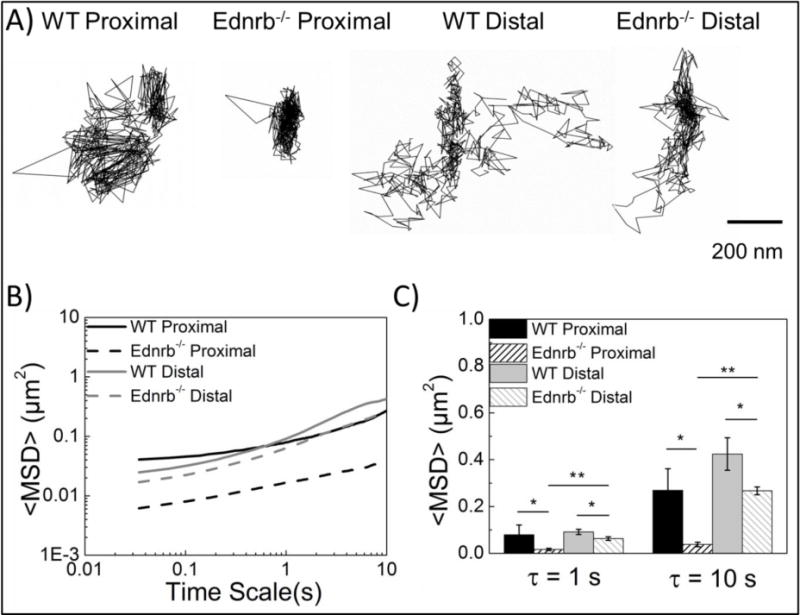
Transport behaviors of carboxylate-modified microspheres in excised colonic tissue. A) Representative 20 s trajectories of carboxylate-modified particles in WT proximal, Ednrb−/− proximal, WT distal, and Ednrb−/− distal tissue. B) Ensemble averaged mean-squared displacement (<MSD>) in WT and Ednrb−/− mucus over 10 s. C) Ensemble <MSD> averages at τ = 1 s & 10 s. Error bars denote standard errors based on three independent experiments, with n ≥ 100 microspheres for each experiment. * p<0.05, ** p<0.01
3.1.2 Colonic Mucus Barrier Differences in Proximal and Distal Tissue
Mucus in proximal colon sections of Ednrb−/− mice provided a more robust barrier to particulate transport than mucus in distal colon sections, while there was no significant difference between proximal and distal mucus barrier properties in WT mice. Specifically, mucus in the distal colon sections displayed effective diffusivities 1.33-fold and 3.41-fold higher than in the proximal section of WT and Ednrb−/−, respectively. Particle penetration across mucus thicknesses representative of those found in the colon was estimated over a time frame on the order of mucus turnover rate (1 hour)[27] (Figure 3). Approximately 11 %, 17 %, and 8 % of 200 nm particles are predicted to penetrate a normal rat colonic mucus layer (average thickness 50 μm[28]) in WT proximal, WT distal, and Ednrb−/− distal colon, respectively, while particles in the Ednrb−/− proximal colon were predicted to be unable to penetrate this mucus thickness in one hour (0.02 % of particles penetrating).
Figure 3.
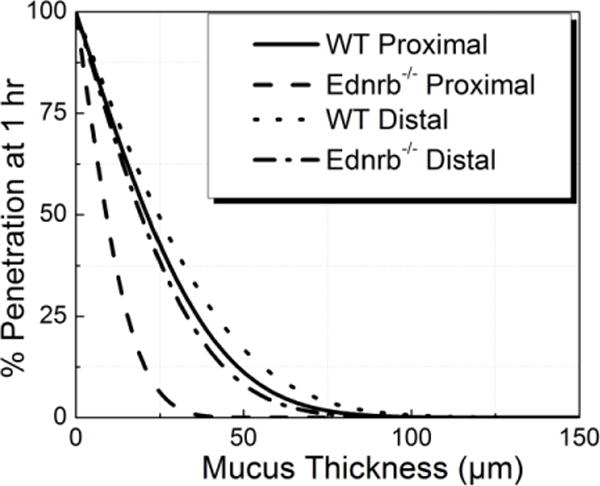
Predicted percentage of particle penetration across mucus thickness after 1 hr.
3.2 Active Transport in Colonic Mucus
Mucus plays a critical role in selective binding to prevent microbe penetration to the underlying epithelium.[4, 5, 29] In this investigation, the barrier properties of colonic mucus with respect to Escherichia Coli transport in Ednrb−/− and WT mouse colonic mucus were studied using real time microbe tracking.
Microbes in WT proximal colonic mucus were less hindered and more mobile than microbes in Ednrb−/− proximal colonic mucus (Figure 4A–B). The speed of microbes in WT proximal mucus ranged between 0.6 to 12 μm/s (Figure 4C) with an average speed of 6.41 μm/s (Figure 5); while the speed of E. coli in Ednrb−/− was significantly lower, varying between 0.5 to 2.6 μm/s with an average speed of 1.78 μm/s.
Figure 4.
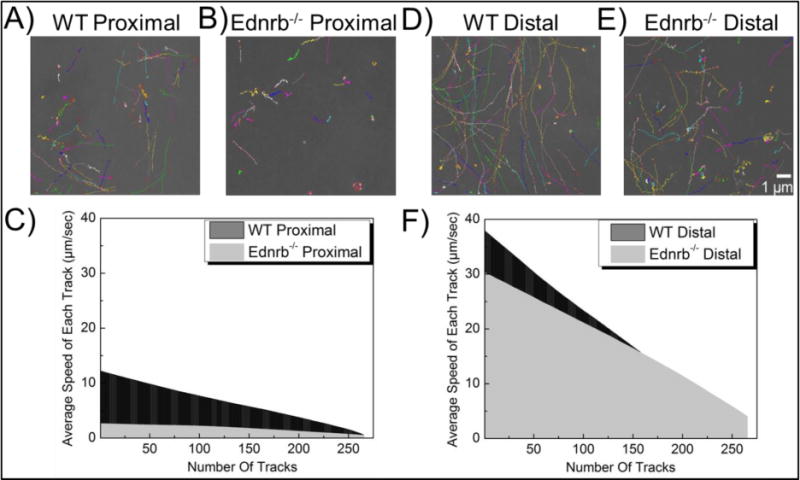
Transport of E. Coli ASV in mouse colonic mucus. Representative 20 s trajectories in proximal (A–B) and distal (D–E) colonic mucus of WT (A, D) and Ednrb−/− (B, E) mice. Average speed of individual microbes in mucus of proximal (C) and distal (F) colon.
Figure 5.

Transport of E. Coli ASV in mouse colonic mucus. Average speeds were compared in WT and Ednrb−/− mutants in both proximal and distal colonic mucus. Data represent the ensemble average, and error bars denote standard errors based on three independent experiments, with n ≥ 90 microbes for each experiment. *p < 0.05, **p < 0.01
In the distal colon sections, microbes had similar average speed in both the Ednrb−/− and WT mucus (Figure 4D). Average microbe speed in the Ednrb−/− distal section was 19.11 μm/s (Figure 5), ranging between 0.8 to 37.8 μm/s (Figure 4F) compared to the WT distal section that had an average speed of 17.74 μm/s, ranging between 4.1 to 30.2 μm/s. Interestingly, transport of microbes was significantly different in proximal vs distal sections of both WT and Ednrb−/− colon. Average speeds of microbes in Ednrb−/− and WT distal colonic mucus are more than 10- and 3-fold higher, respectively, than microbes in proximal colonic mucus (Figure 5).
4. Discussion
Enterocolitis is a serious complication of Hirschsprung’s disease with a high incidence both before and after surgery to remove the aganglionic colon.[6] While its cause remains unknown, abnormalities of the functional barrier properties of the colonic mucus layer could play a role; however, barrier properties of mucus in HD have not previously been directly measured. In this investigation, barrier properties of mucus on intact excised colonic tissue from WT and Ednrb−/− mice were investigated by analyzing passive and active transport of microparticles and microbes. Our major findings show there are significant differences in active and passive transport between Ednrb−/− and WT and between anatomical positions (i.e. proximal vs. distal). The altered transport indicates that there are structural and/or chemical changes in the mucus lining in HD, and motivate exploration of therapeutic treatments to impact mucus barrier properties to possibly impact the onset of enterocolitis.
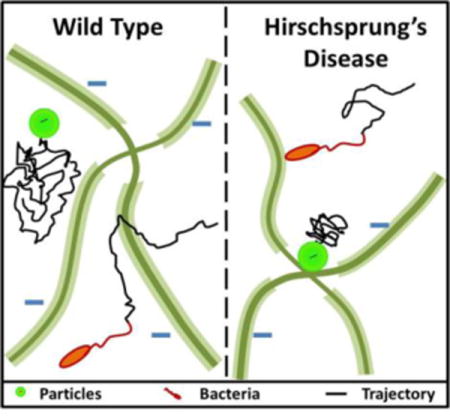
In this work, video analysis of passive transport indicated that mucus barrier properties are significantly altered, resulting in 7-fold and 1.5-fold lower <MSD> values in Ednrb−/− proximal and distal sections, respectively, compared to WT mice (Figure 2). Reduced mucus turnover has been seen in both aganglionic and ganglionic bowel of HD patients, indicating that the mucus defensive barrier is inherently altered throughout the colon of HD patients.[11] The observed differences in passive diffusion between WT and Ednrb−/− could also be related to differences in MUC2 concentration and density of negatively charged glycoproteins in mucus.[9, 30–33] Mucins, such as MUC2, the main gel-forming intestinal mucin, have different oligosaccharide side chains which impart their neutral or acidic nature (i.e., sulfomucins or sialomucins).[34] An increased ratio of neutral mucins:acidic mucins, as seen in HD,[13] would correspond to a relative negative charge decrease, which could diminish electrostatic repulsive forces between the microspheres and mucin fibers (Figure 6), leading to slower particle diffusion and reduced predicted penetration in Ednrb−/− mucus as compared to WT (Figure 3). When comparing proximal to distal colon, there was notably slower particle transport (3.41-fold) proximally for Ednrb−/− but not WT mucus (1.33-fold) (Figure 2C). This could be due to bulk mucosal damage in the distal colon of the HD model animals.
Figure 6.
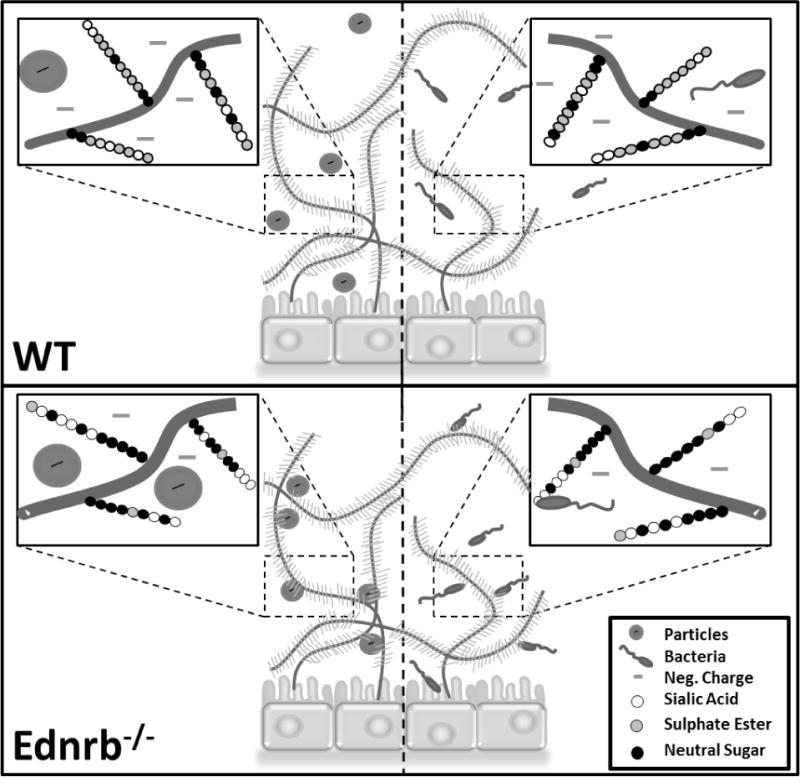
Schematic representation of particle (Left side) and bacteria (Right side) interactions with mucus gel in WT and Ednrb−/− colon. The higher density of negatively charged groups in WT vs. Ednrb−/− mucus could reduce interactions between particles and mucins, resulting in less hindered motion. Microbes may have increased adherence in mutant colon due to increased sialomucin binding.
Analysis of active transport revealed differences in average microbe speeds, especially in the proximal sections where transport was 3.6-fold higher in WT colonic mucus. These differences could again be related to mucin composition. In addition to influencing charge, each subtype of acidic mucins plays an important role in preventing microbe invasion. Sulphomucins aid in the prevention of mucin breakdown by an inherent resistance to bacterial mucin-degrading enzymes.[29] Alternatively, sialic acids form the terminal ends of oligosaccharides to act as a decoy food source, binding microbes to prevent bacterial penetration toward the underlying epithelia. Thus, changes in mucin subtype concentrations could alter mucus interactions with microbes and microbe penetration. A lowered concentration of sulphomucins reported to be associated with HD would result in a reduced resistance to bacterial mucin-degrading enzymes and potential increase of bacterial penetration and onset of enterocolitis.[29, 34] However, HD patients also display heightened sialomucin secretion,[33] especially in the proximal colon,[34] suggesting an increased degree of bacterial adhesion to mucus. Lower average microbe speeds in the Ednrb−/− proximal section compared to WT could be due to increased sialomucin binding (Figure 6). A reduced quantity of MUC2 (as measured in HD patient stool[32]) and bulk mucosal damage could result in comparable numbers of binding sites, and thus similar microbe transport, in the healthy and HD distal colon.
The decrease in passive and active transport (7-fold and 3.6-fold respectively) in the Ednrb−/− compared to WT proximal colon is important due to the fact that intestinal aganglionosis is limited to the most distal portions of the colon, while the proximal colon appears to have a normal enteric nervous system.[7] The fact that the risk of enterocolitis persists in human HD after removal of the aganglionic segment suggests that the mucosal abnormality extends beyond the aganglionic segment. Our findings suggest that despite the presence of normal-appearing ganglion cells proximally,[7] the mucosa of the proximal colon exhibits abnormalities compared to WT, in agreement with the ongoing risk of HAEC after surgery.
5. Conclusions
Mucus barrier properties are altered in an animal model of Hirschprung’s disease, impacting both passive diffusion of particulate material and active microbe transport. These results support exploration of the direct role that the abnormal mucus barrier properties may play in the onset of enterocolitis in patients with Hirschsprung’s disease to motivate potential therapies.
Acknowledgments
We thank Sarah Alyce Miller for her valuable contributions to tissue extractions. The authors also thank Professor Slava Epstein, Paul Miller, and David Stancyk for culturing and providing GFP expressing E. Coli ASV bacteria. We gratefully acknowledge financial support from National Institutes of Health Grant R21EB015750.
Contributor Information
Dr. Hasan M. Yildiz, Department of Chemical Engineering, Northeastern University, Boston, MA 02115, USA.
Taylor L. Carlson, Department of Chemical Engineering, Northeastern University, Boston, MA 02115, USA.
Dr. Allan M. Goldstein, Department of Pediatric Surgery, Massachusetts General Hospital, Boston, MA 02114, USA
Prof. Rebecca L. Carrier, Department of Chemical Engineering, Northeastern University, Boston, MA 02115, USA.
References
- 1.Cone RA. Adv Drug Deliver Rev. 2009;61:2. doi: 10.1016/j.addr.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 2.Crater JS, Carrier RL. Macromol Biosci. 2010;10:12. doi: 10.1002/mabi.201000137. [DOI] [PubMed] [Google Scholar]
- 3.Scaldaferri F, Pizzoferrato M, Gerardi V, Lopetuso L, Gasbarrini A. J Clin Gastroenterol. 2012;46:9. doi: 10.1097/MCG.0b013e31826ae849. [DOI] [PubMed] [Google Scholar]
- 4.Hansson GC. Curr Opin Microbiol. 2012;15:1. doi: 10.1016/j.mib.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johansson MEV, Gustafsson JK, Holmén-Larsson J, Jabbar KS, Xia L, Xu H, Ghishan FK, Carvalho FA, Gewirtz AT, Sjövall H, Hansson GC. Gut. 2013;63:2. doi: 10.1136/gutjnl-2012-303207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burkardt DDC, Graham JM, Short SS, Frykman PK. Clin Pediatr (Phila) 2013;53:1. doi: 10.1177/0009922813500846. [DOI] [PubMed] [Google Scholar]
- 7.Robertson K, Mason I, Hall S. Gut. 1997;41:4. doi: 10.1136/gut.41.4.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murphy F, Puri P. Pediatr Surg Int. 2005;21:10. doi: 10.1007/s00383-005-1551-1. [DOI] [PubMed] [Google Scholar]
- 9.Teitelbaum DH, Caniano DA, Qualman SJ. J Pediatr Surg. 1989;24:12. doi: 10.1016/s0022-3468(89)80566-4. [DOI] [PubMed] [Google Scholar]
- 10.Aslam A, Spicer RD, Corfield AP. J Pediatr Surg. 1997;32:8. doi: 10.1016/s0022-3468(97)90683-7. [DOI] [PubMed] [Google Scholar]
- 11.Aslam A, Spicer RD, Corfield AP. J Pediatr Surg. 1998;33:1. doi: 10.1016/s0022-3468(98)90372-4. [DOI] [PubMed] [Google Scholar]
- 12.Aslam A, Spicer RD, Corfield AP. J Pediatr Surg. 1999;34:2. doi: 10.1016/s0022-3468(99)90202-6. [DOI] [PubMed] [Google Scholar]
- 13.Corfield AP, Myerscough N, Longman R, Sylvester P, Arul S, Pignatelli M. Gut. 2000;47:4. doi: 10.1136/gut.47.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Puri P, Holschneider AM. Hirschsprung’s Disease and Allied Disorders. Berlin, Germany: Springer; 2008. [Google Scholar]
- 15.Dawson M, Wirtz D, Hanes J. J Biol Chem. 2003;278:50. doi: 10.1074/jbc.M309026200. [DOI] [PubMed] [Google Scholar]
- 16.Dawson M, Krauland E, Wirtz D, Hanes J. Biotechnol Prog. 2004;20:3. doi: 10.1021/bp0342553. [DOI] [PubMed] [Google Scholar]
- 17.Wang YY, Lai SK, Suk JS, Pace A, Cone R, Hanes J. Angew Chem Int Ed. 2008;47:50. doi: 10.1002/anie.200803526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lai SK, O’Hanlon DE, Harrold S, Man ST, Wang YY, Cone R, Hanes J. PNAS. 2007;104:5. doi: 10.1073/pnas.0608611104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suh J, Wirtz D, Hanes J. PNAS. 2003;100:7. doi: 10.1073/pnas.0636277100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suh J, Dawson M, Hanes J. Adv Drug Deliver Rev. 2005;57:1. doi: 10.1016/j.addr.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 21.Suk JS, Suh J, Lai SK, Hanes J. Exp Biol Med. 2007;232:3. [PubMed] [Google Scholar]
- 22.Sbalzarini IF, Koumoutsakos P. J Struct Biol. 2005;151:2. doi: 10.1016/j.jsb.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 23.Jachak A, Hida K, Suk J, Markovic N, Biswal S, Breysse P, Hanes J. Nanotoxicology. 2011;6:6. doi: 10.3109/17435390.2011.598244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang B, Lai S, Wang Y, Suk J, Yang M, Zeitlin P, Boyle M, Fu J, Hanes J. PNAS. 2009;106:46. doi: 10.1073/pnas.0905998106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gibiansky ML, Conrad JC, Jin F, Gordon VD, Motto DA, Mathewson MA, Stopka WG, Zelasko DC, Shrout JD, Wong GCL. Science. 2010;330:6001. doi: 10.1126/science.1194238. [DOI] [PubMed] [Google Scholar]
- 26.Pontier-Bres R, Prodon F, Munro P, Rampal P, Lemichez E, Peyron JF, Czerucka D. PLoS One. 2012;7:3. doi: 10.1371/journal.pone.0033796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lehr CM, Junginger HE, Tukker JJ. Int J Pharm. 1991;70:3. [Google Scholar]
- 28.Schreiber O, Petersson J, Waldén T, Ahl D, Sandler S, Phillipson M, Holm L. PLoS One. 2013;8:8. doi: 10.1371/journal.pone.0071843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Derrien M, van Passel MWJ, van de Bovenkamp JHB, Schipper R, de Vos W, Dekker J. Gut Microbes. 2010;1:4. doi: 10.4161/gmic.1.4.12778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanders NN, De Smedt SC, Demeester J. J Pharm Sci. 2000;89:7. doi: 10.1002/1520-6017(200007)89:7<835::AID-JPS1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 31.Fujimoto T, Miyano T. Pediatr Surg Int. 1994;9:4. [Google Scholar]
- 32.Mattar AF, Coran AG, Teitelbaum DH. J Pediatr Surg. 2003;38:3. doi: 10.1053/jpsu.2003.50071. [DOI] [PubMed] [Google Scholar]
- 33.Fujimoto T. J Pediatr Surg. 1988;23:3. doi: 10.1016/s0022-3468(88)80730-9. [DOI] [PubMed] [Google Scholar]
- 34.Speare M., MD University of London. 2011 Jan; [Google Scholar]


