Abstract
Working memory capacity reflects a core ability of the individual that affects performance on many cognitive tasks. Recent work has suggested that an important covariate of memory capacity is attentional control, and specifically that low-capacity individuals are more susceptible to attentional capture by distractors than high-capacity individuals are, with the latter being able to resist capture. Here, we tested an alternative account according to which all individuals are equally susceptible to attentional capture, but high-capacity individuals recover more quickly than low-capacity individuals. Using psychophysical and electrophysiological methods, we measured recovery time from attentional capture. In two experiments, we found that high- and low-capacity individuals showed equivalent attentional capture effects in the initial moments following capture, but that low-capacity individuals took much longer to recover than high-capacity individuals did. These results suggest that the poor attentional control associated with low capacity is due to slow disengagement from distractors.
Keywords: attentional capture, working memory capacity, individual differences
Working memory (WM) is a capacity-limited cognitive resource that represents a small amount of information in an on-line state. Numerous studies have suggested that visual WM capacity is generally limited to an average of about three to four simple objects (Alvarez & Cavanagh, 2004; Awh, Barton, & Vogel, 2007; Luck & Vogel, 1997; Pashler, 1988; Sperling, 1960; Vogel & Machizawa, 2004; Xu & Chun, 2006), and that there are stable and substantial differences across individuals in WM capacity (Awh et al., 2007; Rouder et al., 2008). These individual differences in WM capacity have been shown to be strongly predictive of performance on aptitude measures, such as fluid intelligence (Cowan et al., 2005; Cowan, Fristoe, Elliott, Brunner, & Saults, 2006; Cusack, Lehmann, Veldsman, & Mitchell, 2009; Engle, Tuholski, Laughlin, & Conway, 1999; Fukuda, Vogel, Mayr, & Awh, 2010). Consequently, it is important to carefully characterize the nature of differences in WM capacity.
Many researchers have suggested that individual differences in WM capacity reflect poor attentional control over the use of WM resources (Bleckley, Durso, Crutchfield, Engle, & Khanna, 2003; Kane, Bleckley, Conway, & Engle, 2001; McNab & Klingberg, 2008; Vogel, McCollough, & Machizawa, 2005). For example, Vogel et al. (2005) provided evidence that low-capacity individuals were much poorer at keeping irrelevant items from being stored in WM than their high-capacity counterparts, who were highly efficient at excluding task-irrelevant information. More recently, we extended these results by showing that high-capacity individuals appear to be able to resist capture of spatial attention by irrelevant items, whereas low-capacity individuals are more prone to reorient spatial attention toward such items (Fukuda & Vogel, 2009). This susceptibility to attentional capture at an early stage likely results in later WM filtering problems for low-capacity individuals and is consistent with results from Kane et al. (2001) indicating that high-capacity individuals are better than low-capacity individuals at overriding prepotent eye movements toward distractors in antisaccade tasks.
Although the research just summarized suggests that low-capacity individuals are more susceptible to attentional capture than high-capacity participants are, an alternative account is that all individuals have equal susceptibility to attentional capture but that people vary in how long it takes them to recover from attentional capture (e.g., Theeuwes, Atchely, & Kramer, 2000). That is, high-capacity subjects may not be able to override the capture of attention, but instead may recover from it more quickly than low-capacity subjects do. Thus, it is plausible that previous work suggesting that low-capacity individuals are more susceptible to attentional capture may have not had sufficient temporal sensitivity to measure the rapid recovery of high-capacity individuals. Here, we report two experiments that used new procedures that allowed us to distinguish predictions of the susceptibility and recovery-time accounts for the relationship between WM capacity and attentional capture.
Experiment 1
The first experiment tested the susceptibility and recovery-time accounts of attentional capture by measuring the time course of recovering from attentional capture. Specifically, participants performed a visual search task in which they viewed a brief array of four colored Landolt Cs that were presented within placeholders and reported the orientation of the single item with the target color. On some trials, at varying intervals prior to the onset of the visual search array, a task-irrelevant colored box was briefly presented flanking one of the placeholders. There were three types of trials, randomly intermixed: relevant-flanker capture trials, in which the task-irrelevant box matched the color of the target for the search array (Folk, Remington, & Johnston, 1992; Folk, Remington, & Wright, 1994); irrelevant-flanker capture trials, in which the box did not match the target color (Theeuwes, 1994; Theeuwes & Godijn, 2002); and no-flanker trials, in which the irrelevant box (flanker) was not presented. This task follows the general logic of many attentional capture paradigms in that performance on the visual search task will likely be lower following the task-irrelevant box than in the no-flanker trials (i.e., a capture cost is expected).
The susceptibility and recovery-time hypotheses make distinct predictions in this task. According to the susceptibility hypothesis, high-capacity individuals should show smaller capture costs than low-capacity individuals irrespective of the interval between the flanker and the search array. Alternatively, the recovery-time hypothesis predicts that high- and low-capacity individuals will show equivalent amounts of capture interference at the shortest interval between the task-irrelevant flanker and the search array, but that high-capacity individuals will show faster recovery from capture than low-capacity subjects do as the interval becomes longer.
Method
Participants
Thirty undergraduate students at the University of Oregon volunteered for participation in return for credits offered in introductory psychology classes.
Stimuli and procedure
After providing informed consent, participants first performed a WM memory task. This was a change-detection task with colored squares (e.g., Awh et al., 2007; Fukuda & Vogel, 2009). Participants were presented with arrays of four, six, or eight colored squares for 150 ms (memory array); the squares disappeared for 900 ms (retention interval), after which one colored square (test probe) was presented at the location of one of the items from the memory array. Participants made an unspeeded button press to indicate whether the color of the test probe was the same as or different from the color of the original memory item in that location (equal probability of same and different trials). Forty trials were presented for each array size. Each individual’s accuracy for each array size was transformed into a K estimate following a standard formula (Cowan, 2001), and these three values were then averaged into a single WM capacity estimate.
Before participants performed the capture task described in the introduction to this experiment, we titrated the duration of the search array for each subject so that each subject’s performance was approximately 75% correct in the no-flanker condition. To do so, we had participants perform a visual search task in which they viewed four Landolt Cs (0.8° × 0.8°) and identified the orientation of the one that was in a target color (red for half the subjects and green for the other half). The three distractors were drawn in colors different from the target color (e.g., blue, magenta, or green when the target was red). The items in the search array appeared within placeholders (1.6° × 1.6°) that were present throughout the duration of each trial. Shortly following the onset of the search array, a multi-colored pattern mask was presented at each placeholder location. At this point, participants were asked to report if the gap in the target item was on the top, right, left, or bottom, recording their answer by pressing one of the four arrow keys on a keyboard. If the response was correct, the duration of the search array for the next trial was shortened by 10%. However, if the response was incorrect, the duration was extended by 30%. Thus, the duration of the visual search array was titrated so that each individual could perform the no-flanker condition with approximately 75% accuracy. Each participant performed three blocks of 60 trials, and the search-array durations for the last 20 trials in the three blocks were averaged to estimate the baseline search-array duration for the following capture task.
After completing the staircase procedure, each participant performed an onset-capture variation of the same visual search task that used the search-array duration estimated by the staircase procedure. The primary difference in this version of the task was that prior to the onset of the search array on a third of the trials, a task-irrelevant box (flanker; 0.8° × 0.8°) was presented for 50 ms at a position that flanked the position of one of the placeholders (but never the position of the target stimulus). On the other trials, no flanker was presented. On flanker-present trials, there were four possible stimulus onset asynchronies (SOAs) between the flanker and the search array: 50 ms, 150 ms, 250 ms, and 350 ms. The flanker was drawn in either the target color (relevant flanker) or a distractor color (irrelevant flanker), with equal probability (see Fig. 1a for an illustration of the task). Each participant performed eight blocks of 160 trials, with all conditions randomly intermixed within blocks.
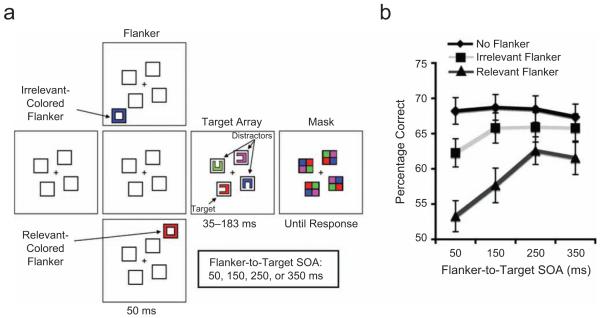
Fig. 1.
Stimuli and results from Experiment 1. The schematic (a) illustrates the visual search task. On one third of the trials, a flanker (a task-irrelevant box in either the target color or a distractor color) was presented before the search array; on the other trials, no flanker was presented. The stimulus onset asynchrony (SOA) between the flanker and search array varied across trials. The duration of the search array was titrated for each subject. The task was to report the orientation of the Landolt C that had been presented in the target color. The graph (b) shows percentage correct as a function of trial type and SOA. Chance performance is 25%. Error bars represent standard errors of the mean.
Isolating contingent effects from stimulus-driven effects
When a flanker in this task is in the target color (relevant flanker), the capture cost is a combination of two potentially separable effects: attentional capture by the onset of an object (stimulus-driven capture) and attentional capture by the target color (contingent capture). To isolate the contingent capture effect from the stimulus-driven effect, we took the difference between irrelevant-flanker trials and relevant-flanker trials as the contingent capture effect.
Results and discussion
WM task
The mean WM capacity estimate was 2.4 (SD = 0.82). The range of the estimates was from 1.0 to 4.2, which is comparable to findings of previous experiments using this paradigm (Awh et al., 2007; Vogel et al., 2005).
Visual search task: staircase procedure
The baseline search-array durations (M = 98 ms, SD = 46 ms) ranged from 35 ms to 183 ms. There was a weak, but nonsignificant, correlation between this estimate and the WM capacity estimate (r = −.28, p > .1).
Visual search task: flanker capture
In the no-flanker condition, the mean accuracy of target identification was 68% (see Fig. 1b). A repeated measures analysis of variance (ANOVA) was conducted to examine the effect of the relevant and irrelevant flankers on accuracy. First, there was a main effect of flanker type (relevant, irrelevant, or no flanker), F(1, 31) = 82.2, p < .001, with relevant flankers producing larger capture costs than irrelevant flankers. Second, there also was a significant interaction between flanker type and SOA, F(3, 93) = 3.0, p < .05. Whereas irrelevant flankers induced a significant capture cost only at the 50-ms SOA (p < .001), relevant flankers induced significant capture costs at all SOAs (ps < .001). These results suggest that stimulus-driven attentional capture is as short-lived as 150 ms and that contingent attentional capture lasts as long as 350 ms.
Individual differences in attentional capture
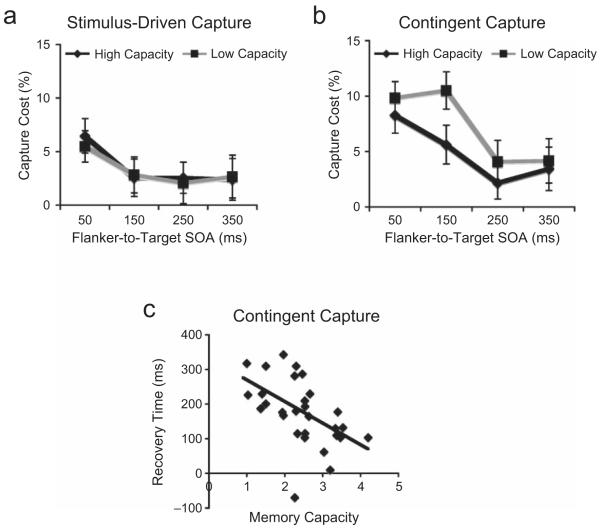
A median split on the WM capacity estimates was used to divide participants into high-capacity (M = 3.1, SD = 0.6) and low-capacity (M = 1.5, SD = 0.6) groups. Figure 2 shows the capture costs for each group as a function of SOA. For stimulus-driven capture (accuracy on no-flanker trials minus accuracy on irrelevant-flanker trials; Fig. 2a), the two groups showed equivalent performance at each delay, and there was no main effect of group, F(1, 30) = .28, p > .8). However, for contingent capture trials (Fig. 2b), the two groups showed equivalent decrements at the shortest SOA (p > .6), but diverged substantially at the 150-ms SOA. Although capture costs decreased substantially at the 150-ms SOA in the high-capacity group, a large capture cost persisted at this SOA in the low-capacity group (p < .05). The difference in capture costs between groups was no longer significant at the 250-ms and 350-ms SOAs (ps > .5).
Fig. 2.
Results from Experiment 1: (a) stimulus-driven capture cost and (b) contingent capture cost as a function of stimulus onset asynchrony (SOA) and working memory capacity and (c) correlation between recovery time for contingent capture (time point at which capture cost declined to 5%) and working memory capacity. Stimulus-driven capture cost was calculated as accuracy on no-flanker trials minus accuracy on irrelevant-flanker trials, and contingent capture cost was calculated as accuracy on irrelevant-flanker trials minus accuracy on relevant-flanker trials.
We also calculated the correlation between WM capacity and capture cost for each SOA. Stimulus-driven capture did not correlate significantly with WM capacity at any SOA: rs = 03, .00, −.01, and .13 for the 50-ms, 150-ms, 250-ms, and 350-ms SOAs, respectively (ps > .15 ). For contingent capture, although there was no relationship with WM capacity at the 50-ms SOA (r = −.04, p > .9), a significant negative correlation emerged at the 150-ms SOA (r = −56, p < .01); there was no correlation at the longer SOAs (rs = −.17 and −.19, respectively, ps > .6). Thus, a continuous analysis yielded the same pattern of results as our median-split analysis.
Recovery time
To estimate recovery time from attentional capture for each subject, we modeled (linear derivation) the flanker-to-target SOA at which the capture effect had declined to 5% for each type of capture. Two participants never reached this threshold at any SOA and were removed from the analysis. This analysis indicated that recovery time from stimulus-driven capture (M = 85 ms) was significantly faster than recovery time from contingent capture (M = 169 ms; p < .01). Although recovery time did not correlate with individual WM capacity in the case of stimulus-driven capture (p > .9), recovery time from contingent capture showed a strong negative relationship with WM capacity (r = −.51, p < .01; Fig. 2c); low-capacity individuals took much longer to recover from contingent capture than high-capacity individuals did.
Correlations between stimulus-driven and contingent capture
We calculated the correlation between the magnitude of the contingent capture cost and the stimulus-driven capture cost. Although split-half reliability estimates were acceptable (both > .6), we found no relationship between these two effects (r = −.2, n.s.). Furthermore, recovery-time estimates for the two types of capture also showed no relationship (r = −.17, n.s.). Thus, the results suggest that these two forms of capture may not reflect the operation of a single mechanism.
Experiment 2
The first experiment provided initial evidence that high- and low-capacity individuals show equal susceptibility to attentional capture, but that low-capacity individuals take longer to recover from contingent capture than high-capacity individuals do. Initially, these results seem to contradict our own recent work suggesting that high-capacity individuals are capable of overriding attentional capture (Fukuda & Vogel, 2009). In that study, we used an event-related potential (ERP) procedure to measure whether participants had involuntarily reoriented spatial attention away from a cued target and toward the positions of distractors. We had subjects perform a cued target discrimination task in which they identified a target that was surrounded by similar distractors. Shortly following this display, we flashed a task-irrelevant probe either at the target position or at one of the distractor positions so that we could measure the early visual-evoked components (P1 and N1) in order to determine where attention was allocated following the display.
We first tested voluntary orientation of attention and found that high- and low-capacity individuals showed equivalently large P1 and N1 attentional responses to targets. We then tested whether onset of the target array triggered a reorienting of attention away from the cued location by measuring the ERP response to the task-irrelevant probe that followed the target array. If individuals held their attention exclusively on the target locations, we would expect large P1 and N1 attentional responses to probes at target locations and negligible responses to probes at distractor locations. However, if the distractors captured attention away from the target location, we would expect measurable P1 and N1 attentional responses to probes at distractor locations. We found that although high-capacity individuals showed large P1 and N1 attentional responses to target probes and negligible attentional responses to distractor probes, low-capacity individuals showed equivalent attentional responses to target and distractor probes. From this pattern of results, we concluded that the high-capacity group was able to resist capture and the low-capacity group involuntarily reallocated attention to include the distractors. However, in that study, we used a fixed timing of 100 ms between the onset of the target array and the onset of the probe. In light of the recovery-time estimates of Experiment 1, it is quite plausible that the high-capacity group had already begun to recover from attentional capture in the 100 ms before the probe was presented, and that an earlier probe onset might have detected attentional capture in the high-capacity subjects.
In Experiment 2, we used the same ERP procedure as in this earlier study (Fukuda & Vogel, 2009), with the exception that we varied the timing of the probe onset (50 ms, 100 ms, or 200 ms). In addition, prior to the ERP task, each participant performed the same visual search task we used to measure attentional capture in Experiment 1. Thus, Experiment 2 had two primary goals. First, we tested whether we could provide converging evidence for the recovery-time hypothesis by using a variation of our ERP attentional capture procedure. Second, we examined whether the recovery-time estimate, calculated as in Experiment 1, was predictive of the attentional capture effects measured by our ERP procedure. If the two procedures tap into the same general construct of attentional recovery time, we would expect them to yield estimates that are strongly predictive of one another.
Method
Participants
Twenty-two students at the University of Oregon participated and were paid $10 per hour.
Stimuli and procedure
The procedure began with the same WM task that was used in Experiment 1. After completing the WM task, participants performed the same behavioral attentional capture (visual search) procedure as in Experiment 1. The only difference was that on flanker-present trials, the flanker was always drawn in the relevant (target) color.
In the last phase of the experiment, participants performed a cued target identification task while we recorded ERPs (as in Fukuda & Vogel, 2009). Subjects held central fixation throughout each trial. A trial began with a cue array containing two diamond-like shapes (each 1.6° × 1.6°), one with a green dot and one with a red dot (0.3° × 0.3°); this array was presented for 500 ms (Fig. 3a). The four corners of each diamond represented the four possible target locations in the corresponding hemifield. Half of the participants were instructed that the position of the green dot would indicate where the target item would be presented in the subsequent target array. The other half of the participants were told that the red dot was the cue for the target location. Two hundred milliseconds following the offset of the cue, we presented the target array, which was composed of two Landolt Cs (1° × 1°) in each hemifield; within the cued hemifield, one of these stimuli was at the cued position (target) and the other was at one of the uncued positions (distractor). The target array was presented for 50 ms, and participants were asked to press a button to report where the gap was on the target. On two thirds of the trials, a task-irrelevant white square (probe) was presented in each hemifield for 50 ms; this probe array was separated from the target array by one of three possible SOAs (50 ms, 100 ms, or 200 ms). The probe in the cued hemifield appeared at the target location or the distractor location with equal probability. The remaining third of the trials contained no probe during this 50-ms period; they allowed us to measure the ERP response without the presence of probes.
Fig. 3.
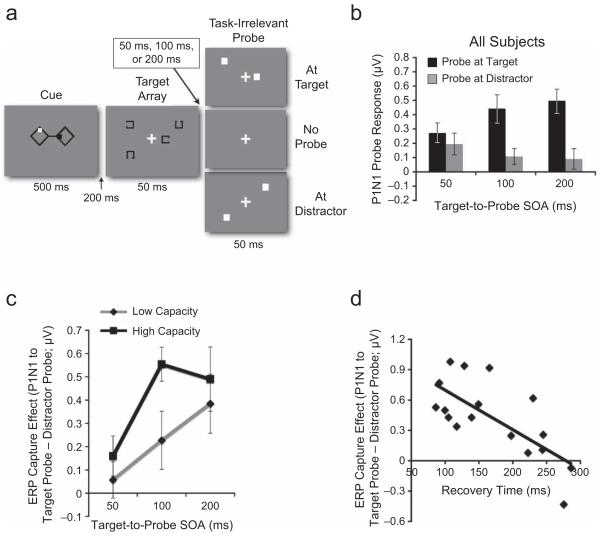
Stimuli and event-related potential (ERP) results from Experiment 2. The schematic (a) illustrates the cued target identification task. Participants were instructed to pay attention to the location of either the red dot (shown here in white) or the green dot (shown here in black). Then, a target at the cued location was briefly presented along with one distractor in the same hemifield. Participants reported where the gap was on the target item. Next, in two thirds of the trials, a task-irrelevant probe was presented either at the target location or at the distractor location. The stimulus onset asynchrony (SOA) between the target array and the probe array was variable. In the remainder of the trials, no probe was presented. So that the stimulus presentation would be balanced, the same number of stimuli shown in the cued hemifield were presented on the uncued side in the target and probe arrays. The bar graph (b) shows mean amplitude of the P1N1 response to the probe as a function of the probe’s position and the target-to-probe SOA. Larger responses indicate increased levels of spatial attention at the position of the probe. The graph in (c) shows the difference in amplitude between responses to probes at target positions and responses to probes at distractor positions (ERP capture effect) as a function of SOA and working memory capacity. Note that more positive values indicate a larger attention response to the target position than to the distractor position; values at or near zero indicate equivalent attention responses at these positions (i.e., attentional capture). In (b) and (c), error bars represent standard errors of the mean. The graph in (d) shows the correlation between the ERP capture effect at the 100-ms SOA and the recovery-time estimate derived from the visual search task.
Electroencephalogram recording
ERPs were recorded from 22 electrodes using our standard recording and analysis procedures, including rejection of trials contaminated by blinks or large (> 1°) eye movements (e.g., Fukuda & Vogel, 2009). Trials containing ocular artifacts, movement artifacts, or amplifier saturation were excluded from the averaged ERP waveforms. Two participants for whom more than 20% of trials were rejected in at least one condition were excluded from the analysis.
ERP measure of attentional capture
As in our previous study (Fukuda & Vogel, 2009), we isolated the ERP response to probes by subtracting response on probe-absent trials from response on probe-present trials; this calculation minimizes the overlapping activity evoked by the preceding target array. The amplitude of the P1N1 response to the probe was measured as the difference in mean amplitude between contralateral activity and ipsilateral activity from 100 ms to 200 ms after the onset of the probe array. P1N1 was measured by averaging across three posterior pairs of lateral electrode sites (PO3, PO4; OL, OR; and T5, T6). To measure the ERP attentional capture effect, we subtracted the response to probes at distractor positions from the response to probes at target positions (i.e., after subtracting response on probe-absent trials). Thus, large residual P1N1 amplitudes reflect exclusive attention to the target position, whereas smaller P1N1 amplitudes reflect attention that is distributed between the target and distractor positions (i.e., a capture effect).
Results
WM task
The mean WM capacity estimate was 2.8 (SD = 0.90), with a range from 1.2 to 4.5. Participants were divided into high-capacity (M = 3.5, SD = 0.6) and low-capacity (M = 2.1, SD = 0.5) groups by a median split.
Behavioral results
Replicating the results of Experiment 1 (see Fig. 1b), we found that as SOA increased, the capture cost declined monotonically, F(1, 19) = 8.75, p < .01. Moreover, we again found a significant negative correlation between contingent capture cost and WM capacity only at the 150-ms SOA (r = −.66, p < .01). Further, when we modeled each individual’s recovery time, we again found a negative correlation between recovery time and memory capacity (r = −.62, p < .01).
ERP results
We conducted a repeated measures ANOVA on the mean amplitude of the P1N1 responses to the probe and found a main effect of probe location, F(1, 19) = 47.2, p < .001, with probes at target positions showing larger P1N1 attentional responses than probes at distractor locations did. There also was a significant interaction between probe location and SOA, F(2, 38) = 3.83, p < .05. The P1N1 response at target locations increased from the 50-ms SOA to the 100-ms SOA, whereas the P1N1 response at distractor locations decreased from the 50-ms SOA to the 100-ms SOA (Fig. 3b).
To evaluate the susceptibility hypothesis, we calculated the difference in P1N1 attentional responses to target probe and distractor probes (i.e., P1N1 capture effect) and submitted these values to a repeated measures ANOVA with factors of WM capacity group and SOA. This analysis revealed a main effect of WM capacity group, F(1, 18) = 8.33, p < .01, with low-capacity individuals showing larger ERP capture effects (i.e., smaller differences between target and distractor responses) than high-capacity individuals. More important, there was a significant interaction between capacity group and SOA, F(1, 18) = 4.89, p < .05. At the 50-ms SOA, high- and low-capacity individuals showed equivalent P1N1 capture effect (p > .1; Fig. 3c). However, at the 100-ms SOA, the low-capacity group still showed a substantial capture effect (p >.1), whereas the high-capacity group had already recovered attention to the target (P1N1 to target probe > P1N1 to distractor probe, p <.01). By the 200-ms SOA, the low-capacity group had recovered to the level of the high-capacity group (P1N1 to target probe > P1N1 to distractor probe, ps < .02). Furthermore, we measured the correlation between the P1N1 capture effect and WM capacity separately for each SOA. A positive correlation (r = .49, p < .05) was observed only at the 100-ms SOA, and not at the 50-ms and 200-ms SOAs (both rs < .2, ps > .5).
We estimated recovery time from the behavioral results and calculated the correlation between this estimate and the ERP capture effects at each SOA. As expected, a significant correlation (r = −.70, p < .01; Fig. 3d) was observed only at the 100-ms SOA; at that SOA, as estimated recovery time became longer, ERP capture effects became larger.
General Discussion
The primary goal of this study was to evaluate whether the relationship between individual differences in WM capacity and attentional capture is the consequence of differences in the susceptibility to capture or is due to how long it takes individuals to recover from capture. Using two distinct approaches (behavior and ERPs), we found strong evidence that high- and low-capacity individuals show equivalent capture effects within the first 50 ms of the capturing event, but that high-capacity subjects are able to recover attention to the target much more quickly than low-capacity subjects are. Thus, these results strongly favor the recovery-time hypothesis. In addition, the finding that behavioral recovery-time estimates were strongly predictive of the early visually evoked ERP attention effects in a separate task suggests that recovery time may reflect a general construct that is a critical factor in individual differences in cognitive ability.
Disengagement time versus reengagement time?
When attention is captured, there are two factors that affect how long recovery takes: the time to disengage from the capturing distractor and the time to reengage the target. Although the design of Experiment 2 does not allow us to distinguish between these two alternatives, we feel that the results of Experiment 1 favor a disengagement interpretation because stimulus-driven and contingent capture placed equivalent demands on reengaging the target following capture. If the low-capacity subjects had had difficulties reengaging or rescaling attention to the target following capture, we would have expected that they would have shown a deficit when the capture was purely stimulus driven, yet they showed no such deficit. Furthermore, disengagement time can better explain the results of a previous study (Vogel et al., 2005) in which subjects with low WM capacity were less able than high-capacity subjects to keep distractors from being stored in WM along with targets; if the low-capacity subjects were too slow to disengage attention from the distractors after their initial onset, this would have made the distractors difficult to exclude from WM.
Broader implications for capture
These results also have implications regarding the basic mechanisms underlying attentional capture, which have been actively debated for many years. First, our results reinforce the proposal that capture is both fast and obligatory for all observers. Second, these results are consistent with the proposal by Theeuwes and his colleagues (Theeuwes, 2010; Theeuwes et al., 2000) that contingent capture has a longer duration than stimulus-driven capture, as our estimates of recovery time for contingent capture were nearly double those for stimulus-driven capture. Finally, these results also inform the basic debate regarding whether stimulus-driven and contingent capture effects reflect different capture mechanisms or a single mechanism (Folk et al., 1992; Theeuwes et al., 2000). Our finding of no correlation between contingent capture and stimulus-driven capture effects is evidence against the single-mechanism view, which predicts that they should covary. However, further work will be necessary to provide a stronger case for this particular claim.
Acknowledgments
Funding
This work was supported through a grant from the National Institute of Mental Health to E.K.V.
Footnotes
Declaration of Conflicting Interests
The authors declared that they had no conflicts of interest with respect to their authorship or the publication of this article.
References
- Alvarez GA, Cavanagh P. The capacity of visual short-term memory is set both by visual information load and by number of objects. Psychological Science. 2004;15:106–111. doi: 10.1111/j.0963-7214.2004.01502006.x. [DOI] [PubMed] [Google Scholar]
- Awh E, Barton B, Vogel EK. Visual working memory represents a fixed number of items regardless of complexity. Psychological Science. 2007;18:622–628. doi: 10.1111/j.1467-9280.2007.01949.x. [DOI] [PubMed] [Google Scholar]
- Bleckley MK, Durso FT, Crutchfield JM, Engle RW, Khanna MM. Individual differences in working memory capacity predict visual attention allocation. Psychonomic Bulletin & Review. 2003;10:884–889. doi: 10.3758/bf03196548. [DOI] [PubMed] [Google Scholar]
- Cowan N. The magical number 4 in short-term memory: A reconsideration of mental storage capacity [Target article and discussion] Behavioral & Brain Sciences. 2001;24:87–185. doi: 10.1017/s0140525x01003922. [DOI] [PubMed] [Google Scholar]
- Cowan N, Elliott EM, Saults JS, Morey CC, Mattox S, Hismjatullina A, Conway ARA. On the capacity of attention: Its estimation and its role in working memory and cognitive aptitudes. Cognitive Psychology. 2005;51:42–100. doi: 10.1016/j.cogpsych.2004.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan N, Fristoe NM, Elliott EM, Brunner RP, Saults JS. Scope of attention, control of attention, and intelligence in children and adults. Memory & Cognition. 2006;34:1754–1768. doi: 10.3758/bf03195936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusack R, Lehmann M, Veldsman M, Mitchell DJ. Encoding strategy and not visual working memory capacity correlates with intelligence. Psychonomic Bulletin & Review. 2009;16:641–647. doi: 10.3758/PBR.16.4.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engle RW, Tuholski SW, Laughlin JE, Conway AR. Working memory, short-term memory, and general fluid intelligence: A latent-variable approach. Journal of Experimental Psychology: General. 1999;128:309–331. doi: 10.1037//0096-3445.128.3.309. [DOI] [PubMed] [Google Scholar]
- Folk CL, Remington RW, Johnston JC. Involuntary covert orienting is contingent on attentional control settings. Journal of Experimental Psychology: Human Perception and Performance. 1992;18:1030–1044. [PubMed] [Google Scholar]
- Folk CL, Remington RW, Wright JH. The structure of attentional control: Contingent attentional capture by apparent motion, abrupt onset, and color. Journal of Experimental Psychology: Human Perception and Performance. 1994;20:317–329. doi: 10.1037//0096-1523.20.2.317. [DOI] [PubMed] [Google Scholar]
- Fukuda K, Vogel EK. Human variation in overriding attentional capture. Journal of Neuroscience. 2009;29:8726–8733. doi: 10.1523/JNEUROSCI.2145-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda K, Vogel E, Mayr U, Awh E. Quantity, not quality: The relationship between fluid intelligence and working memory capacity. Psychonomic Bulletin & Review. 2010;17:673–679. doi: 10.3758/17.5.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane MJ, Bleckley MK, Conway AR, Engle RW. A controlled-attention view of working-memory capacity. Journal of Experimental Psychology: General. 2001;130:169–183. doi: 10.1037//0096-3445.130.2.169. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Vogel EK. The capacity of visual working memory for features and conjunctions. Nature. 1997;390:279–281. doi: 10.1038/36846. [DOI] [PubMed] [Google Scholar]
- McNab F, Klingberg T. Prefrontal cortex and basal ganglia control access to working memory. Nature Neuroscience. 2008;11:103–107. doi: 10.1038/nn2024. [DOI] [PubMed] [Google Scholar]
- Pashler H. Familiarity and visual change detection. Perception & Psychophysics. 1988;44:369–378. doi: 10.3758/bf03210419. [DOI] [PubMed] [Google Scholar]
- Rouder JN, Morey RD, Cowan N, Zwilling CE, Morey CC, Pratte MS. An assessment of fixed-capacity models of visual working memory. Proceedings of the National Academy of Sciences, USA. 2008;105:5975–5979. doi: 10.1073/pnas.0711295105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling G. The information available in brief visual presentations. Psychological Monographs: General and Applied. 1960;74 (Whole No. 498) [Google Scholar]
- Theeuwes J. Stimulus-driven capture and attentional set: Selective search for color and visual abrupt onsets. Journal of Experimental Psychology: Human Perception and Performance. 1994;20:799–806. doi: 10.1037//0096-1523.20.4.799. [DOI] [PubMed] [Google Scholar]
- Theeuwes J. Top-down and bottom-up control of visual selection. Acta Psychologica. 2010;135:77–99. doi: 10.1016/j.actpsy.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Theeuwes J, Atchely P, Kramer AF. On the time course of top-down and bottom-up control of visual attention. In: Driver J, editor. Attention and performance XVIII: Control of cognitive processes. MIT Press; Cambridge, MA: 2000. pp. 105–124. [Google Scholar]
- Theeuwes J, Godijn R. Irrelevant singletons capture attention: Evidence from inhibition of return. Perception & Psychophysics. 2002;64:764–770. doi: 10.3758/bf03194743. [DOI] [PubMed] [Google Scholar]
- Vogel EK, Machizawa MG. Neural activity predicts individual differences in visual working memory capacity. Nature. 2004;428:748–751. doi: 10.1038/nature02447. [DOI] [PubMed] [Google Scholar]
- Vogel EK, McCollough AW, Machizawa MG. Neural measures reveal individual differences in controlling access to working memory. Nature. 2005;438:500–503. doi: 10.1038/nature04171. [DOI] [PubMed] [Google Scholar]
- Xu Y, Chun MM. Dissociable neural mechanisms supporting visual short-term memory for objects. Nature. 2006;440:91–95. doi: 10.1038/nature04262. [DOI] [PubMed] [Google Scholar]