SUMMARY
Congenital CMV infection is a leading cause of childhood disability. Many children born with congenital CMV infection are asymptomatic or have nonspecific symptoms and therefore are typically not diagnosed. A strategy of newborn CMV screening could allow for early detection and intervention to improve clinical outcomes. Interventions might include antiviral drugs or nonpharmaceutical therapies such as speech-language therapy or cochlear implants. Using published data from developed countries, we analyzed existing evidence of potential benefit that could result from newborn CMV screening. We first estimated the numbers of children with the most important CMV-related disabilities (i.e. hearing loss, cognitive deficit, and vision impairment), including the age at which the disabilities occur. Then, for each of the disabilities, we examined the existing evidence for the effectiveness of various interventions. We concluded that there is good evidence of potential benefit from nonpharmaceutical interventions for children with delayed hearing loss that occurs by 9 months of age. Similarly, we concluded that there is fair evidence of potential benefit from antiviral therapy for children with hearing loss at birth and from nonpharmaceutical interventions for children with delayed hearing loss occurring between 9 and 24 months of age and for children with CMV-related cognitive deficits. We found poor evidence of potential benefit for children with delayed hearing loss occurring after 24 months of age and for children with vision impairment. Overall, we estimated that in the United States, several thousand children with congenital CMV could benefit each year from newborn CMV screening, early detection, and interventions. Copyright © 2014 John Wiley & Sons, Ltd.
INTRODUCTION
Congenital CMV is an important public health problem in pediatric populations (Table 1) [1]. It is a leading cause of childhood hearing loss, cognitive deficit, and vision impairment [2,3]. The number of children with congenital CMV-related disabilities is similar to or greater than the number with better-known conditions such as Down syndrome or spina bifida [4]. The economic burden caused by congenital CMV is substantial, as many affected children require significant ongoing care and special therapeutic and educational services [5]. This has led to calls for improved strategies to reduce the burden of congenital CMV [6–8], including earlier identification through maternal or newborn screening [9–11], vaccines [12,13], behavioral interventions [1,14–16], treatment for infected pregnant women [17,18], and treatment of affected infants [19–21]. The objective of this article is to address the strategy of newborn CMV screening in developed countries, reviewing the existing evidence for potential benefit.
Table 1.
Estimates of children with congenital CMV-related disability: examples from Australia, England and Wales, and the United States
| Annual number of: | Australia (population ~22 million) | England and Wales (Population ~50 million) | United States (Population ~307 million) |
|---|---|---|---|
| Live births | 296,600c | 709,000d | 4,248,000e |
| Congenital CMV infections (0.6%)a | 1,780 | 4,254 | 25,488 |
| Symptomatic at birth (12.8%)a | 228 | 544 | 3,262 |
| Symptomatic at birth who have or develop disability (50%)b | 114 | 272 | 1,631 |
| Asymptomatic at birth (87.2%)a | 1,552 | 3,710 | 22,226 |
| Asymptomatic at birth who have or develop disability (13.5%)b | 210 | 501 | 3,001 |
| Total with congenital CMV-related disabilities | 324 | 773 | 4,632 |
Diagnosis and classification of congenital CMV
Diagnosis of congenital CMV infection based on clinical signs or symptoms alone is not definitive. Rather, a definitive diagnosis requires the detection in urine, saliva, or blood of CMV via viral culture or of CMV DNA via nucleic acid testing (NAT) within the first 2–3 weeks after birth [22,23]. Detection of CMV after this age could be due to postnatal infection, which has not been associated with birth defects or developmental disabilities [24]. Therefore, if hearing loss or cognitive deficit becomes apparent at a later age, a post hoc diagnosis of congenital CMV infection generally cannot be made.
Children with congenital CMV infection may be symptomatic or asymptomatic at birth. The diagnostic criteria and definition of symptomatic congenital CMV infection vary in the literature despite attempts at standardization [25], partly because the clinical signs and symptoms (e.g. small for gestational age, petechiae, hemolytic anemia, splenomegaly, hepatomegaly, jaundice, pneumonia, microcephaly, and seizures) are not pathognomonic for congenital CMV infection and have significant variability ranging from minimal damage to fetal death [26]. For the purposes of this article, we classify children as symptomatic if they have acute symptoms present at birth. We also describe children who have CNS sequelae according to those clinical sequelae at their appearance, but we classify them as symptomatic or asymptomatic depending on the presence or absence of acute symptoms at birth. Literature definitions of symptomatic congenital CMV frequently include many children whose symptoms are sufficiently nonspecific that they generally do not prompt the physician to order a CMV test that could lead to a diagnosis of congenital CMV infection. Therefore, in the absence of universal newborn CMV screening, not only asymptomatic infections but also many symptomatic infections go undiagnosed [3].
Newborn CMV screening
Whether screening for a given condition should become an established health practice requires weighing the benefits versus the harms and is usually determined on the basis of a number of criteria, such as those described by Wilson and Jungner, and others [27–29]. Congenital CMV infection already meets many screening criteria. For example, it is an important public health problem [1] whose incidence is similar to the combined incidence of all metabolic or endocrine disorders in the current US core screening panel [9,30]; there is a presymptomatic or early symptomatic stage [31]; the test would generally be acceptable to the population [32], and much is known of the natural history of the condition [22]. Furthermore, there is reason to believe that suitable screening technology, such as CMV DNA testing from dried blood spots (DBS), saliva, or urine, will be available soon [33–38]. On the other hand, potential harms may include increased parental stress or altered parent–child relationships from a false positive or true positive screening result (approximately three fourths of truly positive children never develop sequelae) [39], inappropriate antiviral treatment, or added costs from unnecessary medical visits or tests [40]. One criterion that merits greater examination, however, is whether screening can lead to beneficial interventions.
Potential benefits of newborn CMV screening
Congenital CMV would be atypical as a newborn screening condition, in that making a definitive diagnosis requires testing during the newborn period and cannot generally be accomplished at a later age. The exception is when stored biological specimens from the newborn period, such as DBS, are available for testing at a later time. However, such specimens are often stored for a limited period under less-than-ideal conditions, and accessing them has unresolved ethical difficulties in some populations [41]. Therefore, a key benefit of newborn CMV screening is that it could ensure a definitive diagnosis, which otherwise would be precluded for the majority of infected children.
Several immediate benefits may then follow. A diagnostic odyssey [42–44] could be avoided for children with congenital CMV infection who are born with nonspecific symptoms or who are asymptomatic at birth but who subsequently develop disabilities, preventing some of the difficulties introduced by multiple tests that may have limited diagnostic utility [45] (Box 1). Substantial cost savings could be created by avoiding unnecessary diagnostic tests, hospital admissions, and therapies that might otherwise occur in the quest to diagnose other diseases. Parents would have more confidence that symptoms were not the result of genetic causes, clarifying the child's prognosis and the parents’ future reproductive decision-making. Furthermore, a definitive diagnosis of congenital CMV infection could reduce the considerable stress and anxiety caused by an uncertain diagnosis [44]. Conversely, it is possible that additional diagnostic tests, particularly of the CNS, will be undertaken in some CMV-infected infants who ultimately do not have neurological disease at birth. We have not included this in the analysis as the costs and benefits of such investigation cannot be easily quantified, while acknowledging that this will occur in some infants who have congenital CMV infection but no clinical disease.
Though the immediate benefits mentioned above are plausible and perhaps likely to occur, they are difficult to measure, and therefore, only limited data exist to support them. Furthermore, they are less directly related to the child's health and typically are excluded from assessments of the value of newborn screening for other conditions. As a result, we focused our attention on benefits that directly impact the child's health, are more easily measurable, and for which there are more existing data. Specifically, in this review, we assessed the benefits that might accrue from early detection and intervention for children who are asymptomatic at birth or whose nonspecific symptoms do not lead to a CMV test and definitive diagnosis. Possible interventions might include antiviral drugs or nonpharmaceutical therapies such as speech-language therapy, occupational therapy, physical therapy, cochlear implants, and/or special education services.
METHODS
We categorized measurable potential benefits according to the most common disabilities associated with congenital CMV infection: hearing loss, cognitive deficit (defined as in Dollard et al. [46] as intellectual disability or developmental delay), and vision impairment. For each disability, we evaluated the evidence of benefit for early detection and intervention with nonpharmaceutical interventions or pharmaceutical treatments. For the former, studies have not specifically measured outcomes in children known to have congenital CMV infection but have measured outcomes in children with disabilities that can be caused by congenital CMV infection.
Parameters and assumptions
The parameters we estimated are shown in Table 2. We used these parameters to compute the numbers of children with disabilities in Figures 1–3. Rather than reinvent the wheel, we based many of the parameter estimates on a recent comprehensive literature review by Dollard et al. [46], which used systematic search criteria and explicit inclusion/exclusion criteria. In particular, studies were only included if their populations had been identified through universal screening at birth (i.e. no patients referred to the study because of symptomatic congenital CMV), if they screened at least 800 children in order to identify congenitally infected children, if they were from high-income countries, and if they used viral culture or PCR detection methods. We also included relevant studies published since the Dollard review (2007–2012), which we identified using the same systematic search criteria and inclusion/exclusion criteria (e.g. screened populations only) [46]. To estimate specific parameters for hearing loss, cognitive deficit, and vision impairment, we manually reviewed studies that fit the original search criteria and included all those that reported follow-up outcome data for these sequelae (Table 2). As most of the available outcome data were from the United States, we computed estimates for each of the three types of disability based on live births in the United States, but where necessary, we derived parameters using studies from other developed countries where CMV epidemiology is similar to that of the United States.
Table 2.
Calculation of estimates related to the epidemiology of congenital CMV-related hearing loss, cognitive deficit, and vision impairment in the United States
| Estimate | Calculation | Rationale | References |
|---|---|---|---|
| Figures 1 – 3 | |||
| Live births per year | 4,248,000 | US national vital statistics report | [106] |
| Birth prevalence of congenital CMV infection | 93/17662 + 810/117986 + 74/14021 + 66/21272 + 4/2841 = 1047/173782 → 0.6% | Summary result from studies of screened populations | [33,35,46,107,108] |
| Percentage of children born without congenital CMV infection | 100% – 0.6% = 99.4% | ||
| Percentage of infected children who were symptomatic at birth | 103/810 + 4/74 + 15/66 + 0/4 = 122/954 → 12.8% | Summary result from studies of screened populations | [33,46,107,108] |
| Percentage of infected children who were asymptomatic at birth | 100% – 12.8% = 87.2% | ||
| Figure 1 | |||
| Percentage of symptomatic children likely to be diagnosed clinically with congenital CMV infection | 25% (maximum of 3.8%, 12.5%, 15.7%, 25.0%) | Highest estimate from studies of symptomatic cases identified through surveillance divided by expected number of symptomatic cases | [3,47–49] |
| Percentage of symptomatic children unlikely to be diagnosed clinically with congenital CMV infection | 100% – 25% = 75% | ||
| Percentage of symptomatic children with hearing loss at birth | 5/9 + 12/53 = 17/62 → 27.4% | Hearing loss occurring in first 3 months. Determined or extrapolated from data in figures and/or text | [31,109] |
| Percentage of symptomatic children with delayed hearing loss occurring by 9 months of age | 0/9 + 2/53 = 2/62 → 3.2% | Hearing loss occurring between months 3 and 9. Determined or extrapolated from data in figures and/or text | [31,109] |
| Percentage of symptomatic children with delayed hearing loss occurring between 9 and 24 months of age | 0/9 + 2/53 = 2/62 → 3.2% | Hearing loss occurring between months 9 and 24. Determined or extrapolated from data in figures and/or text | [31,109] |
| Percentage of symptomatic children with delayed hearing loss occurring between 24 and 72 months of age | 0/9 + 3/53 = 3/62 → 4.8% | Hearing loss occurring between months 24 and 72. Determined or extrapolated from data in figures and/or text | [31,109] |
| Percentage of symptomatic children with no hearing loss | 100% – 27.4% – 3.2% – 3.2% – 4.8% = 61.4% | No hearing loss developed by 72 months | |
| Percentage of asymptomatic children with hearing loss at birth | 9/59 + 13/335 = 22/394 → 5.6% | Hearing loss occurring in first 3 months. Determined or extrapolated from data in figures and/or text | [31,109] |
| Percentage of asymptomatic children with delayed hearing loss occurring by 9 months of age | 1/59 + 3/335 = 4/394 → 1% | Hearing loss occurring between months 3 and 9. Determined or extrapolated from data in figures and/or text | [31,109] |
| Percentage of asymptomatic children with delayed hearing loss occurring between 9 and 24 months of age | 0/59 + 3/335 = 3/394 → 0.8% | Hearing loss occurring between months 9 and 24. Determined or extrapolated from data in figures and/or text | [31,109] |
| Percentage of asymptomatic children with delayed hearing loss occurring between 24 and 72 months of age | 1/59 + 18/335 = 19/394 → 4.8% | Hearing loss occurring between months 24 and 72. Determined or extrapolated from data in figures and/or text | [31,109] |
| Percentage of asymptomatic children with no hearing loss | 100% – 5.6% – 1% – 0.8% – 4.8% = 87.8% | No hearing loss developed by 72 months | |
| Figure 2 | |||
| Percentage of children with cognitive deficits (intellectual disability or developmental delay) | 9/22 → 41% | Summary result from studies of CMV-screened populations that assessed cognitive deficit | [46] |
| Percentage of symptomatic children with cognitive deficits who would likely be diagnosed clinically with congenital CMV infection | 4/7 → 57.1% | Summary result from studies of CMV-screened populations that assessed cognitive deficit. Assumed that children with >1 sequelae would be diagnosed clinically | [46] |
| Percentage of symptomatic children with cognitive deficits who would not likely be diagnosed clinically with congenital CMV infection | 3/7 → 42.9% | Summary result from studies of CMV-screened populations that assessed cognitive deficit. Assumed that children with oonly ne sequela would not be diagnosed clinically | [46] |
| Percentage of asymptomatic children with cognitive deficits | 12/261 + 4/49 + 6/159 = 22/469 = 4.7% | Summary results plus results from two later publications that assessed cognitive deficits among asymptomatic children | [46,70,89] |
| Percentage of asymptomatic children with no cognitive deficits | 100% – 4.7% = 95.3% | ||
| Figure 3 | |||
| Percentage of symptomatic children with vision impairment | 4/41 + 0/22 = 4/63 → 6.3% | Results from CMV-screened populations where vision impairment was assessed | [50,110] |
| Percentage of symptomatic children with no vision impairment | 100% – 6.3% = 93.7% | ||
| Percentage of asymptomatic children with vision impairment | 0/54 + 14/332 + 2/83 + 0/44 = 16/513 → 3.1% | Results from studies that assessed vision impairment | [50,88,90,111] |
| Percentage of asymptomatic children with no vision impairment | 100% – 3.1% = 96.9% |
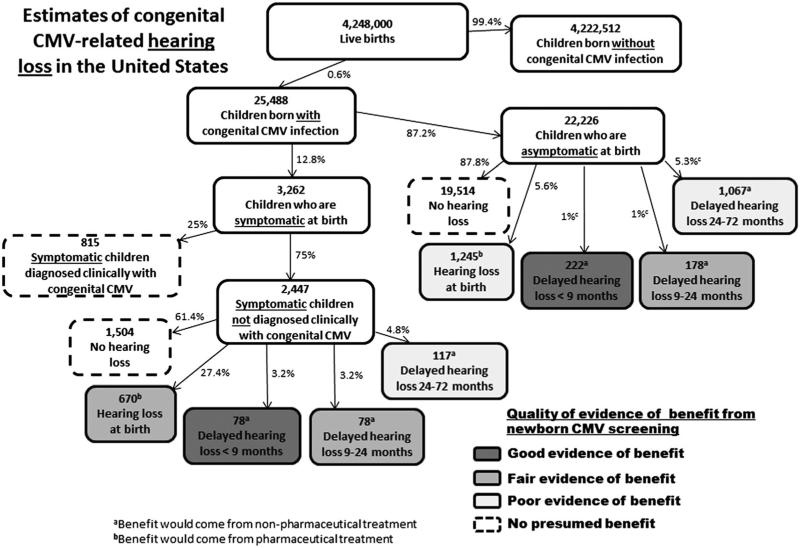
Figure 1.
Estimates of USA annual congenital CMV-related hearing loss, including numbers of children with hearing loss who would potentially benefit from newborn CMV screening. Boxes denoting children who would potentially benefit are shaded from lighter to darker according to a subjective rating of the increasing strength of evidence. For some, the benefit would be due to pharmaceutical treatment (i.e. those with hearing loss at birth); whereas, for the others the benefit would be due to nonpharmaceutical interventions. Because children can experience multiple disabilities, the number of children in Figures 1–3 who would potentially benefit from screening cannot necessarily be added together
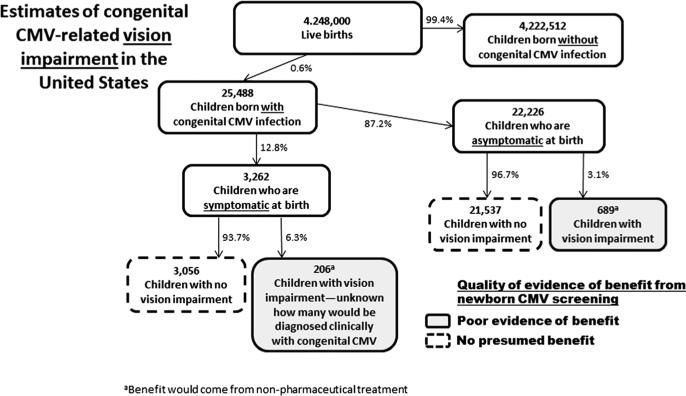
Figure 3.
Estimates of US annual congenital CMV-related vision impairment, including numbers of children with vision impairment who would potentially benefit from newborn CMV screening. Boxes denoting children who would potentially benefit are shaded from lighter to darker according to a subjective rating of the increasing strength of evidence. Because children can experience multiple disabilities, the number of children in Figures 1–3 who would potentially benefit from screening cannot necessarily be added together
Several parameter estimates (Table 2) require additional explanation. Children born with symptomatic congenital CMV who would be tested for CMV because of their presenting clinical signs or symptoms are referred to herein as “diagnosed clinically.” We presumed these children would derive no additional benefit from newborn CMV screening, except perhaps for the very limited benefit of a more immediate diagnosis. We estimated the proportion of symptomatic children diagnosed clinically by using studies that identified children through active surveillance in well-defined populations in the absence of newborn CMV screening. For each study, we divided the observed number of symptomatic congenital CMV cases by the expected number of symptomatic cases derived using the appropriate parameters in Table 2. The proportions were 3.8% [47], 12.5% [3], 15.7% [48], and 25.0% [49]. Active clinical surveillance misses some symptomatic cases correctly diagnosed by health care providers; hence, we assigned our parameter the highest percentage (i.e. 25%) in order to be conservative. We also made an alternative calculation of this parameter for comparison, in which we assumed that children who had only one CMV-associated symptom (e.g. Apgar score <7, small for gestational age, and petechiae) would not be diagnosed clinically with congenital CMV infection in the absence of screening, whereas those with more than one CMV-associated symptom would be diagnosed clinically [50]. This calculation from a screened population showed that 13% of newborns with symptomatic congenital CMV had more than one symptom. Using health insurance claims data as another source of comparison, we found that few newborns (0–10%) who have CMV-associated symptoms are tested for CMV [51], and therefore, few would be diagnosed.
For our estimate of the percentage of children born with symptomatic congenital CMV infection and who have cognitive deficits, we assumed that only those with additional CNS sequelae such as hearing loss or motor disability would be diagnosed clinically, whereas those with isolated cognitive deficits would not. We made this assumption because isolated cognitive deficit is not typically diagnosed until months or years after birth [52], at which time it is usually too late for a definitive diagnosis of congenital CMV infection.
Finally, for the various groups of children whose outcomes might be affected by newborn CMV screening, we categorized the quality of evidence for potential benefit as good, fair, or poor, where good included consistent evidence from well-designed studies; fair included evidence limited by the number, quality, or consistency of the individual studies; and poor included evidence limited by the number or power of studies, flaws in their design or conduct, or gaps in the chain of evidence [53].
RESULTS
Hearing loss
The epidemiology of CMV-related hearing loss is shown in Figure 1. On the basis of the parameter values calculated in Table 2, we estimated that each year in the United States, approximately 25,000 children are born with congenital CMV infection. We divided the children into groups based on whether they were symptomatic at birth and diagnosed clinically (i.e. because their signs and symptoms led to a CMV test), were symptomatic at birth and not diagnosed clinically, or were asymptomatic at birth. We further divided the latter two groups into their various hearing-related outcomes. Hearing loss can occur in any of these groups, sometimes being present at birth and other times developing over months or years.
Nonpharmaceutical interventions
Aside from the indirect potential benefits mentioned earlier (avoidance of diagnostic odyssey, parental peace of mind, physician awareness of diagnosis, etc.), we concluded that children with hearing loss at birth would derive no additional direct benefit from newborn CMV screening and nonpharmaceutical interventions, because these children would presumably be detected through universal newborn hearing screening. Because newborn hearing screening misses some children born with hearing loss, it is possible that having a congenital CMV diagnosis would lead to higher clinical suspicion and more careful monitoring for hearing loss, thus increasing opportunities for early intervention. However, we did not include this as a direct benefit because there are insufficient data to quantify it.
For children with delayed congenital CMV-associated hearing loss that occurs by 9 months of age, we concluded that there is good evidence of benefit from newborn CMV screening (Figure 1). In a landmark study assessing the benefit of universal newborn hearing screening, Kennedy et al. [54] demonstrated that children diagnosed with hearing loss by 9 months of age were significantly more likely to develop better receptive and expressive language than children diagnosed after 9 months of age.
We next considered children whose delayed hearing loss occurs between 9 and 24 months of age because they would, on average, still be diagnosed earlier with newborn CMV screening than they would in its absence. Prior to universal newborn hearing screening, the average age at diagnosis of hearing loss was approximately 24–30 months [55,56]. For these children, we concluded that newborn CMV screening has fair evidence of benefit from nonpharmaceutical intervention because a number of studies (summarized by Nelson et al. [57]) found evidence of better receptive and expressive language among children whose hearing loss was identified earlier rather than later, but these studies had more methodological limitations than Kennedy et al. [54]. The nonpharmaceutical interventions varied across the studies, but they all could be classified as early intervention services, which may include speech-language therapy, occupational therapy, assistive technology devices, and so on.
Finally, we considered the children with congenital CMV infection whose delayed hearing loss occurs between 24 and 72 months of age (Figure 1) [31]. If they were screened for congenital CMV at birth, they might receive closer clinical follow-up and an earlier diagnosis of hearing loss; although moderate to profound hearing loss is frequently recognized and treated before school age, mild or unilateral hearing loss can remain undiagnosed for years [58]. An earlier diagnosis of hearing loss may be beneficial even among children between 24 and 72 months of age, because the many benefits derived from cochlear implants are greater for children with a shorter time from hearing loss until implantation [59–63]. Therefore, we concluded that children whose onset of hearing loss happens between 24 and 72 months of age may receive limited benefit, but the quality of evidence remains poor.
Pharmaceutical interventions
Treatment with IV ganciclovir was shown in a randomized controlled trial (RCT) to reduce the progression of hearing loss in children with symptomatic congenital CMV who have CNS manifestations [20]. However, the subset of symptomatic children who are diagnosed clinically and who meet the trial eligibility requirements (e.g. CNS manifestations) may benefit from pharmaceutical treatment, but newborn screening would not provide an added benefit because they are already identified in the absence of screening. The subset of symptomatic newborns who are not diagnosed clinically but who have hearing loss may also benefit from pharmaceutical treatment because they fit the trial criteria (e.g. clinically apparent disease and hearing deficit). For them, newborn screening would provide a diagnosis that would make them potential candidates for such treatment, and thus, we concluded that they have fair evidence of benefit from newborn CMV screening. Newborns who are asymptomatic at birth but who have hearing loss might benefit from pharmaceutical treatment, but the benefit is less certain because this category of children was excluded from the trial. Ongoing trials of valganciclovir are looking for benefit in this subgroup (clinicaltrials.gov, study identifiers NCT02005822 and NCT01649869), but for the time being, we concluded that they have poor evidence of benefit from newborn screening.
Cognitive deficits
Estimates of the frequency of cognitive deficit (i.e. intellectual disability or developmental delay) among children with congenital CMV infection are shown in Figure 2. Congenital CMV-related cognitive deficits most likely have a natural history that is similar to other neurodevelopmental disorders where the condition is present at birth, but recognition by providers and parents is often delayed until the first or second year of life. Frequently, a confirmatory diagnosis may not occur until the child is even older [52,64]. Among children with symptomatic congenital CMV who have cognitive deficits, some will have severe manifestations at birth that would likely lead to a CMV test and subsequent diagnosis. However, some are likely to be diagnosed only through newborn CMV screening.
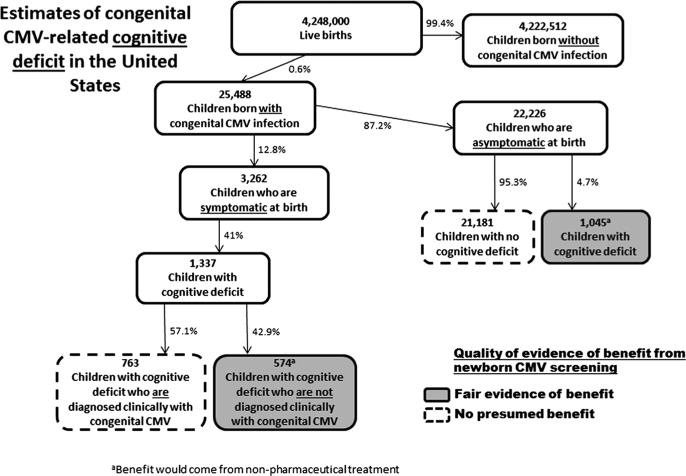
Figure 2.
Estimates of US annual congenital CMV-related cognitive deficit, including numbers of children with cognitive deficit who would potentially benefit from newborn CMV screening. Boxes denoting children who would potentially benefit are shaded from lighter to darker according to a subjective rating of the increasing strength of evidence. Because children can experience multiple disabilities, the number of children in Figures 1–3 who would potentially benefit from screening cannot necessarily be added together
The occurrence of cognitive deficits is clearly higher among children with symptomatic congenital CMV infection (Figure 2) than in the general population of children. It is less clear whether children born with asymptomatic congenital CMV infection have a higher prevalence of cognitive deficits than the general population of children. On the basis of a combined population of 469 asymptomatic children, we found a prevalence of cognitive deficit of 4.7% (Table 2). A recent summary estimate of cognitive deficit among the general population was only slightly lower, at 3.8% [65], but this would include some children with congenital CMV-induced deficits. Some studies have reported a moderately lower mean IQ among children with asymptomatic congenital CMV infection compared with controls but only among children younger than 6 years of age [66–70]. Studies of children older than 6 years have not found significant differences in mean IQ [67,68,71–73].
Nonpharmaceutical interventions
Children with CMV-associated cognitive deficits (albeit unknown at birth) who screen positive for congenital CMV infection could be considered to be at risk for poor developmental outcomes. Some studies indicate that children with an identified at-risk condition or a clinical diagnosis have an earlier age of first concern from parents or caregivers, an earlier development of an Individual Family Service Program, and an earlier receipt of early intervention services [74–77]. Other studies indicate that children with risk factors for cognitive deficits are more likely to be screened for developmental delays and that screened children are more likely to be referred for and receive early intervention services [76,78]. Importantly, children with cognitive deficits who receive early intervention services have better outcomes than similar children who do not receive such services [79–84]. On the basis of these studies, we concluded that there is fair evidence of benefit related to newborn CMV screening for children with CMV-associated cognitive benefits.
Pharmaceutical interventions
In the same RCT of children with symptomatic congenital CMV and CNS involvement [20], those who received 6 weeks of iv ganciclovir treatment had better developmental outcomes than children who received no treatment [85]. It is unlikely, however, that newborn CMV screening would lead to added benefit for these children because they most likely would be diagnosed because of their clinical presentation. For the children who would only be detected early if newborn screening were in place (i.e. children with cognitive deficit who are asymptomatic or who have nonspecific symptoms), no evidence is available to assess whether they would experience improved developmental outcomes because of antiviral drug treatment.
Vision impairment
Although less common than hearing loss or cognitive deficit, vision impairment is another significant disability that can be caused by congenital CMV infection. Vision impairment may be present at birth or it may occasionally be delayed [86,87]. Vision impairment typically occurs among children who are symptomatic at birth, but in some studies, it has been reported in children who were asymptomatic at birth [86,88–90]. Estimates of the epidemiology of congenital CMV-related vision impairment are shown in Figure 3.
Nonpharmaceutical interventions
Although CMV-related blindness is likely to be identified early, partial vision impairment may be less apparent. Such vision impairment may not be detected because approximately one third of children aged 3–5 years do not receive vision testing [91,92]. For children whose vision impairment is diagnosed, there is some evidence that developmental outcomes can be improved through training of visual functions [93,94]. Therefore, if newborn CMV screening leads to better follow-up and more vision screening, it could also lead to early intervention for vision impairment and thus contribute to improved outcomes among these children. Because of insufficient data, we concluded that the evidence for newborn CMV screening leading to better outcomes for children with CMV-related vision impairment remains poor (Figure 3).
Pharmaceutical interventions
Little evidence is available on the effect of antiviral treatment on vision impairment. The previously cited RCT did not measure vision impairment as an endpoint [20], and even if it had, the enrolled children would have been diagnosed with congenital CMV in the absence of screening. One case report found that intravitreal injections of ganciclovir led to regression of ocular disease in a child with congenital CMV infection [95].
DISCUSSION
On the basis of available evidence, we conclude that each year in the United States as many as several thousand children with congenital CMV could benefit from newborn CMV screening, early detection, and intervention. These analyses may apply to other developed countries as well, suggesting that many more thousands of infected children could benefit worldwide. None of the benefits of newborn CMV screening will occur without adequate follow-up for early detection and intervention. Therefore, newborn screening represents the potential for benefit that can accrue as a result of integration of screening for CMV into the newborn screening program.
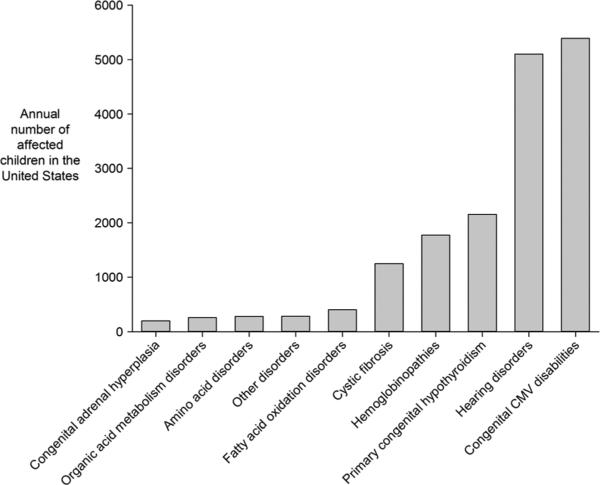
To provide some perspective, each year in the United States, approximately 6400 children with potential adverse health conditions are detected through the newborn DBS screening program [30], and approximately 5100 children with hearing loss are detected through newborn hearing screening programs (Figure 4) [96]. The type and magnitude of the benefit that can result for these children is variable, ranging from the prevention of death or cognitive deficit to the improvement of developmental or educational outcomes. The potential screening benefits for congenital CMV would not include the prevention of death or severe disability but would be similar to the types of benefits associated with the newborn hearing screening program, with improved outcomes through early detection and intervention.
Figure 4.
Annual cases for conditions that make up the core US newborn screening panel [30], along with estimates of annual US cases of congenital CMV-related disabilities. There is some overlap among the children with hearing loss and the children with congenital CMV-related disabilities. However, children in these two groups may not be strictly comparable because newborn hearing screening typically uses >40 dB as a cutoff for hearing loss, whereas the congenital CMV literature sometimes uses a cutoff as low as >20 dB
The potential benefits of newborn CMV screening would be maximized by monitoring and screening children identified with congenital CMV infection in order to detect hearing loss, cognitive deficit, and vision impairment as early as possible. Some practical guidelines for a follow-up and monitoring schedule have been proposed and would include confirmation of screening results, tests to aid the prognosis (e.g. CMV viral load), regular audiologic, neurodevelopmental, and ophthalmologic assessments, as well as provision of family support [97]. The US Joint Committee on Infant Hearing also provides specific guidance for follow-up of children with risk conditions such as congenital CMV infection [98].
The evidence of potential benefit for newborn CMV screening is limited by the scarcity of data to generate estimates for some parameters. As a result, we were unable to generate a precise estimate of potential benefit, and the numbers we provide should be considered approximate and provisional until more data become available. Furthermore, the numbers of children with each disability who might benefit from screening cannot be added together, because some children are affected by multiple disabilities [99]. In addition, not every child will benefit from the interventions, because neither the nonpharmaceutical nor the pharmaceutical interventions are 100% effective. For example, in the RCT, ganciclovir did not lead to improved hearing, although it did appear to limit hearing deterioration, and its efficacy remains controversial because nearly 60% of the trial participants did not have evaluable outcomes. Nevertheless, on the basis of our analyses, we conclude that newborn CMV screening has the potential to provide a meaningful benefit to at least as many children as are already helped by existing newborn screening programs for some other conditions.
Another limitation of our analyses was the subjective grading of the potential benefits. We based our grading on a scale used previously by the US Preventive Services Task Force that seemed to best fit this type of evidence review [53]. The grading was complicated by the different medical conditions we evaluated, the different types of evidence, the different ways of linking the evidence, and the absence of intervention studies in children known to have congenital CMV. Therefore, we presented our subjective judgment of the totality of the evidence, while providing readers with the supporting data on which we based these judgments.
We did not attempt to address targeted newborn CMV screening, although such an approach might be worth pursuing. For example, rather than screen all newborns for CMV, screening could be limited to those children having particular risk factors, such as being small for gestational age or having failed their initial hearing screen. Targeted CMV screening would benefit fewer children, but it may also have fewer negative effects and could possibly be more cost-effective than universal screening.
Our review clearly highlights some deficiencies in research on congenital CMV. Relatively few studies have been carried out on longitudinal outcomes of congenital CMV infection, undoubtedly because of the substantial resources required to screen tens of thousands of children and then follow hundreds of them for years. In addition, few treatment options exist for infected mothers or children, and no effective vaccine is imminent. The high disease burden that we chronicled suggests the importance of better epidemiology and better treatment and prevention options.
Finally, we examined only the evidence of benefit and did not attempt to provide a complete accounting of the value of newborn CMV screening. Such analyses can be found elsewhere [8,9,100]. As with all medical screening programs, potential benefits need to be weighed against potential harms [101]. The costs of newborn CMV screening need to be evaluated, and appropriate screening tests need to be available. Because newborn CMV screening might be considered a public health service rather than public health emergency [102], prenatal or postnatal counseling and consent may be more ethical than mandatory screening [103,104]. Nevertheless, the evidence for potential benefits to thousands of children each year in the United States alone should lead to careful consideration of newborn CMV screening.
Box 1. Case example of diagnostic odyssey.
Ms. SI was born preterm (34 weeks gestation), with weight <10th centile, and breast-fed normally, with normal neonatal hearing screening. She attained her developmental milestones until 4 months of age when she presented in winter with severe, bilateral pneumonitis, and minor hepatosplenomegaly. She was admitted via emergency to intensive care (CICU), was ventilated, and treated with antibiotics for community acquired pneumonia and oseltamivir for possible influenza. The diagnosis was made from chlamydia IgM seropositivity and possible influenza from single high titer serology. Her respiratory status improved over the 8-day CICU and ward admission, and she was discharged 2 weeks following admission. At home, Ms. SI was feeding poorly, and failed to gain weight, with continuing minor hepatosplenomegaly and occasional lack of response to some auditory stimuli. She was readmitted at 5 months of age with deteriorating respiratory status, required continuous positive airway pressure support, and investigations showed chlamydia IgG+/IgM+, mycoplasma IgM+, and nucleic acid tests (NAT) negative for influenza A/B, RSV, parainfluenza 1/2/3, and adenovirus; she was restarted on antibiotics. Over the following week, she required intubation and ventilation by high frequency oscillation. She developed bilateral pneumothoraces requiring intercostal drainage and intravenous support via subclavian lines. Additional testing demonstrated CMV NAT positivity on bronchial washings and CMV IgG+/IgM+. She received intravenous ganciclovir for a total of 6 weeks and CMV immunoglobulin on two occasions. Ms. SI's Guthrie (neonatal blood screening) card was CMV NAT positive, diagnostic of congenital CMV.
The lack of initial diagnosis of CMV pneumonitis resulted in inappropriate antibiotic therapy; missed opportunities for early antiviral therapy; subsequent development of chronic lung disease, most likely on the basis of untreated congenital CMV; and on final discharge, she required omeprazole and prokinetic doses of erythromycin for reflux and increased work of breathing. She could not be assessed for hearing loss without anesthesia, because of the late diagnosis, as she had two attempted brainstem auditory evoked response (BAER) tests without sedation, which were unsuccessful because of her intolerance of the procedure. Sedated BAER testing later was abnormal, showing normal tympanograms bilaterally, bilaterally absent transient evoked otoacoustic emissions consistent with poor outer hair cell function, and air conduction BAER showing moderate hearing loss in the left ear. She had no enhanced speech therapy initially, although hearing loss was subsequently proven. By 6 years of age, she had moderate left hearing loss, mild neurodevelopmental delay, improving respiratory function, and was otherwise well. Her parents had never heard of CMV prior to her diagnosis, and they were diagnosed with clinical anxiety during counseling for how to care for a child with chronic lung disease.
Abbreviations used
- NAT
nucleic acid testing
- DBS
dried blood spots
- RCT
randomized controlled trial
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
CONFLICTS OF INTEREST The authors have no competing interest.
REFERENCES
- 1.Cannon MJ, Davis KF. Washing our hands of the congenital cytomegalovirus disease epidemic. BMC Public Health. 2005;5:70. doi: 10.1186/1471-2458-5-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Griffiths PD, Walter S. Cytomegalovirus. Current Opinion in Infectious Diseases. 2005;18:241–245. doi: 10.1097/01.qco.0000168385.39390.1b. [DOI] [PubMed] [Google Scholar]
- 3.McMullan BJ, Palasanthiran P, Jones CA, et al. Congenital cytomegalovirus—time to diagnosis, management and clinical sequelae in Australia: opportunities for earlier identification. Medical Journal of Australia. 2011;194:625–629. doi: 10.5694/j.1326-5377.2011.tb03144.x. [DOI] [PubMed] [Google Scholar]
- 4.Cannon MJ. Congenital cytomegalovirus (CMV) epidemiology and awareness. Journal of Clinical Virology. 2009;46S:S6–S10. doi: 10.1016/j.jcv.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 5.Institute of Medicine Committee to Study Priorities for Vaccine D . Vaccines for the 21st Century: A Tool for Decision Making. National Academy Press; Washington D. C.: 2000. [Google Scholar]
- 6.Yow MD, Demmler GJ. Congenital cytomegalovirus disease—20 years is long enough. New England Journal of Medicine. 1992;326:702–703. doi: 10.1056/NEJM199203053261010. [DOI] [PubMed] [Google Scholar]
- 7.Demmler-Harrison GJ. Congenital cytomegalovirus: public health action towards awareness, prevention, and treatment. Journal of Clinical Virology. 2009;46:S1–S5. doi: 10.1016/j.jcv.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 8.de Vries JJC, Vossen A, Kroes ACM, van der Zeijst BAM. Implementing neonatal screening for congenital cytomegalovirus: addressing the deafness of policy makers. Reviews in Medical Virology. 2011;21:54–61. doi: 10.1002/rmv.679. [DOI] [PubMed] [Google Scholar]
- 9.Grosse SD, Dollard S, Ross DS, Cannon M. Newborn screening for congenital cytomegalovirus: options for hospital-based and public health programs. Journal of Clinical Virology. 2009;46:S32–S36. doi: 10.1016/j.jcv.2009.08.019. [DOI] [PubMed] [Google Scholar]
- 10.Revello MG, Gerna G. Diagnosis and management of human cytomegalovirus infection in the mother, fetus, and newborn infant. Clinical Microbiology Reviews. 2002;15:680–715. doi: 10.1128/CMR.15.4.680-715.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lazzarotto T. The best practices for screening, monitoring, and diagnosis of cytomegalovirus disease, part II. Clinical Microbiology Newsletter. 2010;32:9–15. [Google Scholar]
- 12.Pass RF, Zhang C, Evans A, et al. Vaccine prevention of maternal cytomegalovirus infection. New England Journal of Medicine. 2009;360:1191–1199. doi: 10.1056/NEJMoa0804749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sung H, Schleiss MR. Update on the current status of cytomegalovirus vaccines. Expert Review of Vaccines. 2010;9:1303–1314. doi: 10.1586/erv.10.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Picone O, Vauloup-Fellous C, Cordier AG, et al. A 2-year study on cytomegalovirus infection during pregnancy in a French hospital. BJOG: An International Journal of Obstetrics & Gynaecology. 2009;116:818–823. doi: 10.1111/j.1471-0528.2009.02139.x. [DOI] [PubMed] [Google Scholar]
- 15.Vauloup-Fellous C, Picone O, Cordier A-G, et al. Does hygiene counseling have an impact on the rate of CMV primary infection during pregnancy? Results of a 3-year prospective study in a French hospital. Journal of Clinical Virology. 2009;46S:S49–S53. doi: 10.1016/j.jcv.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 16.Adler SP, Finney JW, Manganello AM, Best AM. Prevention of child-to-mother transmission of cytomegalovirus by changing behaviors: a randomized controlled trial. The Pediatric Infectious Disease Journal. 1996;15:240–246. doi: 10.1097/00006454-199603000-00013. [DOI] [PubMed] [Google Scholar]
- 17.Jacquemard F, Yamamoto M, Costa JM, et al. Maternal administration of valaciclovir in symptomatic intrauterine cytomegalovirus infection. BJOG. 2007;114:1113–1121. doi: 10.1111/j.1471-0528.2007.01308.x. [DOI] [PubMed] [Google Scholar]
- 18.Nigro G, Adler SP, La Torre R, Best AM. Passive immunization during pregnancy for congenital cytomegalovirus infection. New England Journal of Medicine. 2005;353:1350–1362. doi: 10.1056/NEJMoa043337. [DOI] [PubMed] [Google Scholar]
- 19.Kimberlin DW, Acosta EP, Sanchez PJ, et al. Pharmacokinetic and pharmacodynamic assessment of oral valganciclovir in the treatment of symptomatic congenital cytomegalovirus disease. Journal of Infectious Diseases. 2008;197:836–845. doi: 10.1086/528376. [DOI] [PubMed] [Google Scholar]
- 20.Kimberlin DW, Lin CY, Sanchez PJ, et al. Effect of ganciclovir therapy on hearing in symptomatic congenital cytomegalovirus disease involving the central nervous system: a randomized, controlled trial. Journal of Pediatrics. 2003;143:16–25. doi: 10.1016/s0022-3476(03)00192-6. [DOI] [PubMed] [Google Scholar]
- 21.Whitley RJ, Cloud G, Gruber W, et al. Ganciclovir treatment of symptomatic congenital cytomegalovirus infection: results of a phase II study. National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. Journal of Infectious Diseases. 1997;175:1080–1086. doi: 10.1086/516445. [DOI] [PubMed] [Google Scholar]
- 22.Mocarski ES, Jr., Shenk T, Pass RF. Cytomegaloviruses. In: Knipe DM, Howley PM, editors. Fields’ Virology. 5th edn Lippincott Williams & Wilkins; Philadelphia: 2007. pp. 2702–2772. [Google Scholar]
- 23.Stagno S, Remington JS, Klein JO. Cytomegalovirus. In: Remington JS, Klein JO, editors. Infectious diseases of the fetus and newborn infant. W.B. Saunders Company; Philadelphia: 2001. pp. 389–424. [Google Scholar]
- 24.Schleiss MR. Role of breast milk in acquisition of cytomegalovirus infection: recent advances. Current Opinion in Pediatrics. 2006;18:48–52. doi: 10.1097/01.mop.0000192520.48411.fa. [DOI] [PubMed] [Google Scholar]
- 25.Istas AS, Demmler GJ, Dobbins JG, Stewart JA. Surveillance for congenital cytomegalovirus disease: a report from the National Congenital Cytomegalovirus Disease Registry. Clinical Infectious Diseases. 1995;20:665–670. doi: 10.1093/clinids/20.3.665. [DOI] [PubMed] [Google Scholar]
- 26.Iwasenko JM, Howard J, Arbuckle S, et al. Human cytomegalovirus infection is detected frequently in stillbirths and is associated with fetal thrombotic vasculopathy. Journal of Infectious Diseases. 2011;203:1526–1533. doi: 10.1093/infdis/jir121. [DOI] [PubMed] [Google Scholar]
- 27.Wilson JMG, Jungner G. Principles and practice of mass screening for disease. Boletin De La Oficina Sanitaria Panamericana. 1968;65:281–393. [PubMed] [Google Scholar]
- 28.Calonge N, Green NS, Rinaldo P, et al. Committee report: method for evaluating conditions nominated for population-based screening of newborns and children. Genetics in Medicine. 2010;12:153–159. doi: 10.1097/GIM.0b013e3181d2af04. [DOI] [PubMed] [Google Scholar]
- 29.Watson MS, Mann MY, Lloyd-Puryear MA, Rinaldo P, Howell RR. American College of Medical Genetics Newborn Screening Expert Group. Newborn screening: toward a uniform screening panel and system —executive summary. (Vol. 117, pg S296–307). Pediatrics. 2006;117:851–851. doi: 10.1542/peds.2005-2633I. [DOI] [PubMed] [Google Scholar]
- 30.Centers for Disease C, Prevention Impact of expanded newborn screening—United States, 2006. MMWR. Morbidity and Mortality Weekly Report. 2008;57:1012–1015. [PubMed] [Google Scholar]
- 31.Fowler KB, Dahle AJ, Boppana SB, Pass RF. Newborn hearing screening: will children with hearing loss caused by congenital cytomegalovirus infection be missed? Journal of Pediatrics. 1999;135:60–64. doi: 10.1016/s0022-3476(99)70328-8. [DOI] [PubMed] [Google Scholar]
- 32.Din ES, Brown CJ, Grosse SD, et al. Attitudes toward newborn screening for cytomegalovirus infection. Pediatrics. 2011;128:e1434–1442. doi: 10.1542/peds.2011-1444. [DOI] [PubMed] [Google Scholar]
- 33.Koyano S, Inoue N, Oka A, et al. Screening for congenital cytomegalovirus infection using newborn urine samples collected on filter paper: feasibility and outcomes from a multicentre study. BMJ Open. 2011;1:e000118. doi: 10.1136/bmjopen-2011-000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boppana SB, Ross SA, Novak Z, et al. Dried blood spot real-time polymerase chain reaction assays to screen newborns for congenital cytomegalovirus infection. JAMA. 2010;303:1375–1382. doi: 10.1001/jama.2010.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boppana SB, Ross SA, Shimamura M, et al. Saliva polymerase-chain-reaction assay for cytomegalovirus screening in newborns. New England Journal of Medicine. 2011;364:2111–2118. doi: 10.1056/NEJMoa1006561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kharrazi M, Hyde T, Young S, Amin MM, Cannon MJ, Dollard SC. Use of screening dried blood spots for estimation of prevalence, risk factors, and birth outcomes of congenital cytomegalovirus infection. Journal of Pediatrics. 2010;157:191–197. doi: 10.1016/j.jpeds.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 37.Barbi M, Binda S, Caroppo S, Primache V. Neonatal screening for congenital cytomegalovirus infection and hearing loss. Journal of Clinical Virology. 2006;35:206–209. doi: 10.1016/j.jcv.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 38.Howard J, Hall B, Brennan LE, et al. Utility of newborn screening cards for detecting CMV infection in cases of stillbirth. Journal of Clinical Virology. 2009;44:215–218. doi: 10.1016/j.jcv.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 39.Gurian EA, Kinnamon DD, Henry JJ, Waisbren SE. Expanded newborn screening for biochemical disorders: the effect of a false-positive result. Pediatrics. 2006;117:1915–1921. doi: 10.1542/peds.2005-2294. [DOI] [PubMed] [Google Scholar]
- 40.Woolf SH, Harris R. The harms of screening: new attention to an old concern. JAMA. 2012;307:565–566. doi: 10.1001/jama.2012.100. [DOI] [PubMed] [Google Scholar]
- 41.Douglas CMW, van El CG, Faulkner A, Cornel MC. Governing biological material at the intersection of care and research: the use of dried blood spots for biobanking. Croatian Medical Journal. 2012;53:390–397. doi: 10.3325/cmj.2012.53.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bailey DB., Jr. Newborn screening for fragile X syndrome. Mental Retardation and Developmental Disabilities Research Reviews. 2004;10:3–10. doi: 10.1002/mrdd.20002. [DOI] [PubMed] [Google Scholar]
- 43.Harrell H. Currents in contemporary ethics the role of parents in expanded newborn screening. The Journal of Law, Medicine & Ethics. 2009;37:846–851. doi: 10.1111/j.1748-720X.2009.00456.x. [DOI] [PubMed] [Google Scholar]
- 44.Knott M, Leonard H, Downs J. The diagnostic odyssey to Rett syndrome: the experience of an Australian family. American Journal of Medical Genetics Part A. 2012;158A:10–12. doi: 10.1002/ajmg.a.34372. [DOI] [PubMed] [Google Scholar]
- 45.Busson L, Van den Wijngaert S, Dahma H, et al. Evaluation of 10 serological assays for diagnosing Mycoplasma pneumoniae infection. Diagnostic Microbiology and Infectious Disease. 2013;76:133–137. doi: 10.1016/j.diagmicrobio.2013.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dollard SC, Grosse SD, Ross DS. New estimates of the prevalence of neurological and sensory sequelae and mortality associated with congenital cytomegalovirus infection. Reviews in Medical Virology. 2007;17:355–363. doi: 10.1002/rmv.544. [DOI] [PubMed] [Google Scholar]
- 47.Morita M, Morishima T, Yamazaki T, Chiba S, Kawana T. Clinical survey of congenital cytomegalovirus infection in Japan. Acta Paediatrica Japonica. 1998;40:432–436. doi: 10.1111/j.1442-200x.1998.tb01963.x. [DOI] [PubMed] [Google Scholar]
- 48.Townsend CL, Peckham CS, Tookey PA. Surveillance of congenital cytomegalovirus in the UK and Ireland. Archives of Disease in Childhood. Fetal and Neonatal. 2011;96:F398–F403. doi: 10.1136/adc.2010.199901. [DOI] [PubMed] [Google Scholar]
- 49.Buchheit J, Marshall GS, Rabalais GP, Dobbins GJ. Congenital cytomegalovirus disease in the Louisville area: a significant public health problem. Journal of the Kentucky Medical Association. 1994;92:411–415. [PubMed] [Google Scholar]
- 50.Ahlfors K, Ivarsson SA, Harris S. Report on a long-term study of maternal and congenital cytomegalovirus infection in Sweden. Review of prospective studies available in the literature. Scandinavian Journal of Infectious Diseases. 1999;31:443–457. doi: 10.1080/00365549950163969. [DOI] [PubMed] [Google Scholar]
- 51.Leung J, Cannon MJ, Grosse SD, Bialek SR. Laboratory testing and diagnostic coding for cytomegalovirus among privately insured infants in the United States: a retrospective study using administrative claims data. BMC Pediatrics. 2013;13:90–90. doi: 10.1186/1471-2431-13-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boyle CA, Murphy CC. Neurodevelopmental disabilities. In: Nelson LM, Tanner CM, Van Den Eeden SK, McGuire VM, editors. Neuroepidemiology: From Principles to Practice. Oxford University Press; Oxford: 2004. pp. 360–383. [Google Scholar]
- 53.Harris RP, Helfand M, Woolf SH, et al. Current methods of the US Preventive Services Task Force—a review of the process. American Journal of Preventive Medicine. 2001;20:21–35. doi: 10.1016/s0749-3797(01)00261-6. [DOI] [PubMed] [Google Scholar]
- 54.Kennedy CR, McCann DC, Campbell MJ, et al. Language ability after early detection of permanent childhood hearing impairment. New England Journal of Medicine. 2006;354:2131–2141. doi: 10.1056/NEJMoa054915. [DOI] [PubMed] [Google Scholar]
- 55.Drews CD, Yeargin-Allsopp M, Murphy CC, Decoufle P. Hearing impairment among 10-year-old children: metropolitan Atlanta, 1985 through 1987. American Journal of Public Health 1994. 84:1164–1166. doi: 10.2105/ajph.84.7.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Harrison M, Roush J. Age of suspicion, identification, and intervention for infants and young children with hearing loss: a national study. Ear and Hearing. 1996;17:55–62. doi: 10.1097/00003446-199602000-00007. [DOI] [PubMed] [Google Scholar]
- 57.Nelson HD, Bougatsos C, Nygren P. Universal newborn hearing screening: systematic review to update the 2001 US Preventive Services Task Force recommendation. Pediatrics 2008. 122:E266–E276. doi: 10.1542/peds.2007-1422. [DOI] [PubMed] [Google Scholar]
- 58.Smith RJ, Bale JF, Jr., White KR. Sensori-neural hearing loss in children. Lancet. 2005;365:879–890. doi: 10.1016/S0140-6736(05)71047-3. [DOI] [PubMed] [Google Scholar]
- 59.Stacey PC, Fortnum HA, Barton GR, Summerfield AQ. Hearing-impaired children in the United Kingdom, I: auditory performance, communication skills, educational achievements, quality of life, and cochlear implantation. Ear Hearing. 2006;27:161–186. doi: 10.1097/01.aud.0000202353.37567.b4. [DOI] [PubMed] [Google Scholar]
- 60.Nicholas JG, Geers AE. Will they catch up? The role of age at cochlear implantation in the spoken language development of children with severe to profound hearing loss. Journal of Speech, Language, and Hearing Research. 2007;50:1048–1062. doi: 10.1044/1092-4388(2007/073). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Niparko JK, Tobey EA, Thal DJ, et al. Spoken language development in children following cochlear implantation. JAMA. 2010;303:1498–1506. doi: 10.1001/jama.2010.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Geers AE. Speech, language, and reading skills after early cochlear implantation. Archives of Otolaryngology - Head and Neck Surgery. 2004;130:634–638. doi: 10.1001/archotol.130.5.634. [DOI] [PubMed] [Google Scholar]
- 63.Kileny PR, Zwolan TA, Ashbaugh C. The influence of age at implantation on performance with a cochlear implant in children. Otology & Neurotology. 2001;22:42–46. doi: 10.1097/00129492-200101000-00008. [DOI] [PubMed] [Google Scholar]
- 64.Kylat RI, Kelly EN, Ford-Jones EL. Clinical findings and adverse outcome in neonates with symptomatic congenital cytomegalo-virus (SCCMV) infection. European Journal of Pediatrics. 2006;165:773–778. doi: 10.1007/s00431-006-0172-6. [DOI] [PubMed] [Google Scholar]
- 65.Leonard H, Wen XY. The epidemiology of mental retardation: challenges and opportunities in the new millennium. Mental Retardation and Developmental Disabilities Research Reviews. 2002;8:117–134. doi: 10.1002/mrdd.10031. [DOI] [PubMed] [Google Scholar]
- 66.Fowler KB, Pass RF, Boppana S, Britt WJ. Neurodevelopmental deficits in school-aged children who have asymptomatic congenital cytomegalovirus infection. Paediatric and Perinatal Epidemiology. 1999;13:A19–20. [Google Scholar]
- 67.Kashden J, Frison S, Fowler K, Pass RF, Boll TJ. Intellectual assessment of children with asymptomatic congenital cytomegalovirus infection. Journal of Developmental and Behavioral Pediatrics. 1998;19:254–259. doi: 10.1097/00004703-199808000-00003. [DOI] [PubMed] [Google Scholar]
- 68.Temple RO, Pass RF, Boll TJ. Neuropsychological functioning in patients with asymptomatic congenital cytomegalovirus infection. Journal of Developmental and Behavioral Pediatrics. 2000;21:417–422. doi: 10.1097/00004703-200012000-00003. [DOI] [PubMed] [Google Scholar]
- 69.Williamson WD, Percy AK, Yow MD, et al. Asymptomatic congenital cytomegalovirus infection. Audiologic, neuroradiologic, and neurodevelopmental abnormalities during the first year. American Journal of Diseases of Children. 1990;144:1365–1368. doi: 10.1001/archpedi.1990.02150360091031. [DOI] [PubMed] [Google Scholar]
- 70.Zhang XW, Li F, Yu XW, Shi XW, Shi J, Zhang JP. Physical and intellectual development in children with asymptomatic congenital cytomegalovirus infection: a longitudinal cohort study in Qinba mountain area, China. Journal of Clinical Virology. 2007;40:180–185. doi: 10.1016/j.jcv.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 71.Cazacu AC, Chung S, Greisser C, et al. Neurodevelopmental outcome at elementary school age of children born with asymptomatic congenital cytomegalovirus infection. Pediatric Research. 2004;55:319A–319A. [Google Scholar]
- 72.Conboy TJ, Pass RF, Stagno S, et al. Intellectual development in school-aged children with asymptomatic congenital cytomegalovirus infection. Pediatrics. 1986;77:801–806. [PubMed] [Google Scholar]
- 73.Shan RB, Wang XL, Fu P. Growth and development of infants with asymptomatic congenital cytomegalovirus infection. Yonsei Medical Journal. 2009;50:667–671. doi: 10.3349/ymj.2009.50.5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lipkin PH, Daniel J, Desch LW, et al. Role of the medical home in family-centered early intervention services. Pediatrics. 2007;120:1153–1158. doi: 10.1542/peds.2007-2638. [DOI] [PubMed] [Google Scholar]
- 75.Hix-Small H, Marks K, Squires J, Nickel R. Impact of implementing developmental screening at 12 and 24 months in a pediatric practice. Pediatrics. 2007;120:381–389. doi: 10.1542/peds.2006-3583. [DOI] [PubMed] [Google Scholar]
- 76.Schonwald A, Huntington N, Chan E, Risko W, Bridgemohan C. Routine developmental screening implemented in urban primary care settings: more evidence of feasibility and effectiveness. Pediatrics. 2009;123:660–668. doi: 10.1542/peds.2007-2798. [DOI] [PubMed] [Google Scholar]
- 77.King TM, Tandon SD, Macias MM, et al. Implementing developmental screening and referrals: lessons learned from a national project. Pediatrics. 2010;125:350–360. doi: 10.1542/peds.2009-0388. [DOI] [PubMed] [Google Scholar]
- 78.Dawson G, Rogers S, Munson J, et al. Randomized, controlled trial of an intervention for toddlers with autism: the early start Denver model. Pediatrics. 2010;125:E17–E23. doi: 10.1542/peds.2009-0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Del Giudice E, Titomanlio L, Brogna G, et al. Early intervention for children with Down syndrome in southern Italy—the role of parent-implemented developmental training. Infants Young Children. 2006;19:50–58. [Google Scholar]
- 80.Fey ME, Warren SF, Brady N, et al. Early effects of responsivity education/prelinguistic milieu teaching for children with developmental delays and their parents. Journal of Speech, Language, and Hearing Research. 2006;49:526–547. doi: 10.1044/1092-4388(2006/039). [DOI] [PubMed] [Google Scholar]
- 81.Guralnick MJ. Effectiveness of early intervention for vulnerable children: a developmental perspective. American Journal on Mental Retardation. 1998;102:319–345. doi: 10.1352/0895-8017(1998)102<0319:eoeifv>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 82.Tang MH, Lin CK, Lin WH, Chen CH, Tsai SW, Chang YY. The effect of adding a home program to weekly institutional-based therapy for children with undefined developmental delay: a pilot randomized clinical trial. Journal of the Chinese Medical Association. 2011;74:259–266. doi: 10.1016/j.jcma.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 83.Thomaidis L, Kaderoglou E, Stefou M, Damianou S, Bakoula C. Does early intervention work? A controlled trial. Infants Young Children. 2000;12:17–22. [Google Scholar]
- 84.van der Schuit M, Segers E, van Balkom H, Verhoeven L. Early language intervention for children with intellectual disabilities: a neurocognitive perspective. Research in Developmental Disabilities. 2011;32:705–712. doi: 10.1016/j.ridd.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 85.Oliver SE, Cloud GA, Sanchez PJ, et al. Neurodevelopmental outcomes following ganciclovir therapy in symptomatic congenital cytomegalovirus infections involving the central nervous system. Journal of Clinical Virology. 2009;46:S22–S26. doi: 10.1016/j.jcv.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Boppana S, Amos C, Britt W, Stagno S, Alford C, Pass R. Late onset and reactivation of chorioretinitis in children with congenital cytomegalovirus infection. The Pediatric Infectious Disease Journal. 1994;13:1139–1142. doi: 10.1097/00006454-199412000-00012. [DOI] [PubMed] [Google Scholar]
- 87.Brubaker JW, Bale JF, Jr., Ampofo K, Dries DC. Congenital cytomegalovirus infection: progressive postnatal chorioretinitis. Journal of Pediatric Ophthalmology and Strabismus. 2009;46:249–251. doi: 10.3928/01913913-20090706-16. [DOI] [PubMed] [Google Scholar]
- 88.Anderson KS, Amos CS, Boppana S, Pass R. Ocular abnormalities in congenital cytomegalovirus infection. Journal of the American Optometric Association. 1996;67:273–278. [PubMed] [Google Scholar]
- 89.Britt WJ. Cytomegalovirus. In: Remington JS, Klein JO, Wilson CB, Nizet V, Maldonado YA, editors. Infectious Disease of the Fetus and Newborn Infant. 7th edn Elsevier Saunders; Philadelphia: 2011. pp. 706–755. [Google Scholar]
- 90.Coats DK, Demmler GJ, Paysse EA, Du LT, Libby C. Ophthalmologic findings in children with congenital cytomegalovirus infection. Journal of AAPOS. 2000;4:110–116. doi: 10.1067/mpa.2000.103870. [DOI] [PubMed] [Google Scholar]
- 91.Wasserman RC, Croft CA, Brotherton SE. Preschool vision screening in pediatric practice: a study from the pediatric research in office settings (PROS) network. Pediatrics. 1992;89:834–838. [PubMed] [Google Scholar]
- 92.Kemper AR, Wallace DK, Patel N, Crews JE. Preschool vision testing by health providers in the United States: FINDINGS from the 2006–2007 Medical Expenditure Panel Survey. Journal of AAPOS 2011. 15:480–483. doi: 10.1016/j.jaapos.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 93.Sonksen PM, Petrie A, Drew KJ. Promotion of visual development of severely visually-impaired babies: evaluation of a developmentally based program. Developmental Medicine and Child Neurology. 1991;33:320–335. doi: 10.1111/j.1469-8749.1991.tb14883.x. [DOI] [PubMed] [Google Scholar]
- 94.Vervloed MPJ, Janssen N, Knoors H. Visual rehabilitation of children with visual impairments. Journal of Developmental and Behavioral Pediatrics. 2006;27:493–506. doi: 10.1097/00004703-200612000-00008. [DOI] [PubMed] [Google Scholar]
- 95.Lalezary M, Recchia FM, Kim SJ. Treatment of congenital cytomegalovirus retinitis with intravitreous ganciclovir. Archives of Ophthalmology. 2012;130:525–527. doi: 10.1001/archophthalmol.2011.1615. [DOI] [PubMed] [Google Scholar]
- 96.Centers for Disease C, Prevention CDC grand rounds: newborn screening and improved outcomes. MMWR. Morbidity and Mortality Weekly Report. 2012;61:390–393. [PubMed] [Google Scholar]
- 97.Kadambari S, Williams EJ, Luck S, Griffiths PD, Sharland M. Evidence based management guidelines for the detection and treatment of congenital CMV. Early Human Development. 2011;87:723–728. doi: 10.1016/j.earlhumdev.2011.08.021. [DOI] [PubMed] [Google Scholar]
- 98.Joint Comm Infant H. Clarification for year 2007 JCIH position statement. 2007 [Google Scholar]
- 99.Sharon B, Schleiss MR. Congenital cytomegalovirus infection: an unrecognized epidemic. Infection Medicine. 2007;24:402–415. [Google Scholar]
- 100.Dollard SC, Schleiss MR, Grosse SD. Public health and laboratory considerations regarding newborn screening for congenital cytomegalovirus. Journal of Inherited Metabolic Disease. 2010;33:S249–S254. doi: 10.1007/s10545-010-9125-3. [DOI] [PubMed] [Google Scholar]
- 101.Roser D, Nielsen HV, Petersen E, Saugmann-Jensen P, Norgaard-Pedersen PB. Congenital toxoplasmosis-a report on the Danish neonatal screening programme 1999–2007. Journal of Inherited Metabolic Disease 2010. 33:S241–S247. doi: 10.1007/s10545-010-9124-4. [DOI] [PubMed] [Google Scholar]
- 102.Grosse SD, Boyle CA, Kenneson A, Khoury MJ, Wilfond BS. From public health emergency to public health service: the implications of evolving criteria for newborn screening panels. Pediatrics. 2006;117:923–929. doi: 10.1542/peds.2005-0553. [DOI] [PubMed] [Google Scholar]
- 103.Ross LF. Mandatory versus voluntary consent for newborn screening? Kennedy Institute of Ethics Journal. 2010;20:299–328. [PubMed] [Google Scholar]
- 104.Moyer VA, Calonge N, Teutsch SM, Botkin JR, Force USPST Expanding newborn screening: process, polity, and priorities. Hastings Center Report. 2008;38:32–39. doi: 10.1353/hcr.0.0011. [DOI] [PubMed] [Google Scholar]
- 105.Office for National Statistics Review of the national statistician on births and patterns of family building in England and Wales. 2008 [Google Scholar]
- 106.Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Mathews TJ, Osterman MJK. Births: final data for 2008. National vital statistics reports: from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System 2010. 59:3–71. [PubMed] [Google Scholar]
- 107.Foulon I, Naessens A, Foulon W, Casteels A, Gordts F. A 10-year prospective study of sensorineural hearing loss in children with congenital cytomegalovirus infection. Journal of Pediatrics. 2008;153:84–88. doi: 10.1016/j.jpeds.2007.12.049. [DOI] [PubMed] [Google Scholar]
- 108.Paradiz KR, Seme K, Puklavec E, Paro-Panjan D, Poljak M. Prevalence of congenital cytomegalovirus infection in Slovenia: a study on 2841 newborns. Journal of Medical Virology. 2012;84:109–115. doi: 10.1002/jmv.22230. [DOI] [PubMed] [Google Scholar]
- 109.Foulon I, Naessens A, Faron G, Foulon W, Jansen AC, Gordts F. Hearing thresholds in children with a congenital CMV infection: a prospective study. International Journal of Pediatric Otorhinolaryngology. 2012;76:712–717. doi: 10.1016/j.ijporl.2012.02.026. [DOI] [PubMed] [Google Scholar]
- 110.Boppana SB, Fowler KB, Britt WJ, Stagno S, Pass RF. Symptomatic congenital cytomegalovirus infection in infants born to mothers with preexisting immunity to cytomegalovirus. Pediatrics. 1999;104:55–60. doi: 10.1542/peds.104.1.55. [DOI] [PubMed] [Google Scholar]
- 111.Saigal S, Lunyk O, Larke RP, Chernesky MA. The outcome in children with congenital cytomegalovirus infection. A longitudinal follow-up study. American Journal of Diseases of Children. 1982;136:896–901. doi: 10.1001/archpedi.1982.03970460026006. [DOI] [PubMed] [Google Scholar]