Abstract
Access to diverse PET tracers for preclinical and clinical research remains a major obstacle to research in cancer and other diseases research. The prohibitive cost and limited availability of tracers could be alleviated by microfluidic radiosynthesis technologies combined with high-yield microscale radiosynthetic method. In this report, we demonstrate the multistep synthesis of 3′-deoxy-3′-[18F]fluorothymidine ([18F]FLT) with high yield on an electrowetting on dielectric (EWOD) microfluidic radiosynthesizer, previously developed in our group. We have identified and established several parameters that are most critical in the microscale radiosynthesis such as the reaction time, reagent concentration, and molar ratios, to successfully synthesize [18F]FLT in this compact platform.
Methods
[18F]FLT was synthesized from the 3-N-Boc-1-[5-O-(4,4′-dimethoxytrityl)-3-O-nosyl-2-deoxy-β-d-lyxofuranosyl] thymine precursor on the EWOD chip starting from the first solvent exchange and [18F]fluoride ion activation step to the final deprotection step. The fluorination reaction was performed in a mixture of thexyl alcohol and DMSO. The crude product after deprotection was collected from the chip and purified on a custom-made solid phase extraction (SPE) cartridge and subjected to quality control testing. The purified [18F]FLT was suitable for microPET studies in multiple nude mice xenografted with the A431 carcinoma cell line.
Results
[18F]FLT was successfully synthesized on the EWOD microdevice coupled with an off-chip SPE purification with a decayed-corrected radiochemical yield of 63±5% (n=5) and passed all of the quality control test required by the United States Pharmacopeia for radiotracers to be injected into humans. We have successfully demonstrated the synthesis of several batches of [18F]FLT on EWOD starting with ∼ 333 MBq of radioactivity and obtained up to 52 MBq (non-decay corrected) of [18F]FLT upon cartridge purification. The specific activity of two representative preparations of [18F]FLT synthesized on the EWOD chip were measured to be 1800 and 2400 GBq/μmol.
Conclusion
The EWOD microchip and optimized synthesis method in combination represent an effective platform for synthesizing [18F]FLT with high yield and of good quality for imaging. This compact platform, with configurable synthesis steps, could potentially form the basis of a stand-alone system that decouples PET probe production from the cyclotron and specialized radiochemistry facilities and increases diversity and flexibility in probe production.
Keywords: [18F]FLT, microfluidic chip, radiosynthesis, high specific activity
Introduction
Positron emission tomography (PET) is an extremely sensitive molecular imaging technique, capable of measuring in vivo metabolism, that is increasingly being used in clinical practice and research to diagnose and study a wide range of diseases including cancer, Alzheimer's disease, and Parkinson's disease (1-3). However, due to the difficulties and challenges involved in PET probe production, (4) the majority of PET imaging studies are limited to 2-[18F]fluoro-2-deoxy-D-glucose ([18F]FDG), a glucose analog used to quantify glucose metabolism. Other PET probes that are currently used in clinical trials and research settings, such as 3′-deoxy-3′-[18F]fluorothymidine ([18F]FLT), [18F]fluoromisonidazole, [18F]fluoroethylcholine, 6-[18F]fluoro-3,4-dihydroxy-L-phenylanaline, and many others, are only available at high cost and with limited availability from specialized research laboratories (5). Thus, there is a critical need to develop a new, affordable radiosynthesizer technology coupled with reliable radiosynthetic methodologies that could empower researchers and clinicians to synthesize probes of interest on-demand (at the imaging site) at low cost to address the diversity of biological events being studied via PET imaging. The new technology platform should produce probes such as [18F]FLT with a high radiochemical yield and a final product that can be purified without the need for additional equipment, for example via a simple cartridge purification, similar to the synthesis of [18F]FDG (6).
Recently, our group and others (7-11) have investigated microfluidic technology platforms as a means of achieving on-demand radiosynthesis of diverse PET probes (12). Microfluidic devices (throughout this manuscript, macroscale synthesis refers to any reaction performed with conventional radiochemistry apparatus, typically in vials at volumes above 250 μL, while microscale synthesis refers to reactions performed in microfluidic chips where at least one dimension of the reaction volume is on the order of hundreds of microns or less. In the EWOD microfluidic platform, the reaction volume is typically in the range of 1-10 μL) that integrate many laboratory functions on a single chip, also known as lab-on-chip, can automate repetitive laboratory tasks, and enable users to perform hazardous reactions on chip in a safer manner (13, 14). Of particular importance for PET probe synthesis using short-lived radioisotopes, microfluidic reactors enable radiosyntheses to be completed in a shorter time, minimize dilution of the radioisotopes to speed up reaction kinetics (note that only nmol to μmol amounts are typically produced), simplify purification due to the increased reaction selectivity, use smaller amounts of reagents, and have the potential to eliminate the high cost of infrastructure such as hot cells needed in a typical radiopharmacy facility (4, 15). Our group has developed an all-electronic (i.e., no fluidic systems external to the chip) microfluidic radiosynthesizer based on the electrowetting-on-dielectric (EWOD) principle (16) and successfully demonstrated reliable synthesis of [18F]FDG (12). EWOD is an exemplary microfluidic platform for performing batch radiosynthesis, where a finite volume of liquid can be manipulated sequentially by applying electrical potential, without the need of moving parts such as pumps and valves. This work focuses on the development of a high yielding and reliable microscale radiochemistry method for the synthesis of a useful tracer with currently limited availability, namely [18F]FLT, a radiolabeled analog of thymidine, on the EWOD chip. Demonstration of this 2-step synthesis in a reliable fashion on the EWOD chip (Fig. 1), combined with previous results (12, 17), suggests the capability of this platform to perform diverse syntheses and perhaps form the basis of a compact, benchtop device for producing diverse probes on demand.
Figure 1.
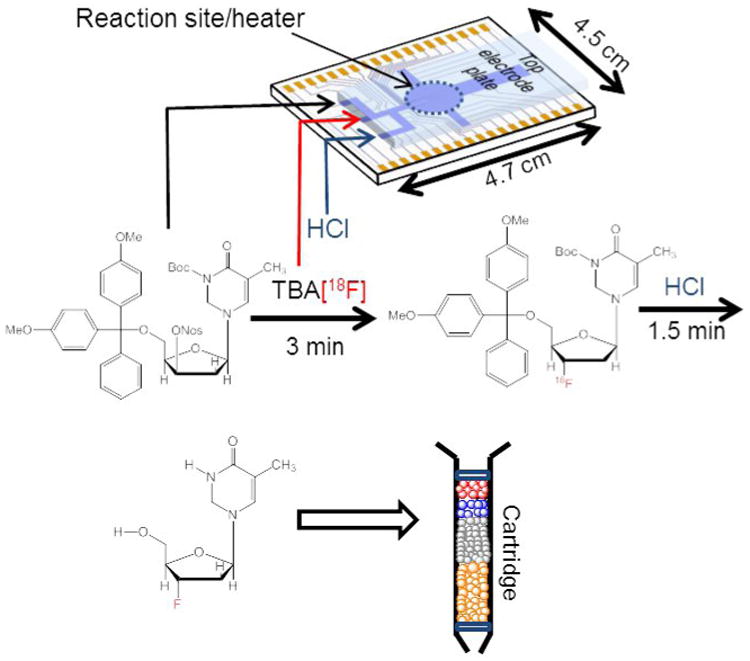
Overall workflow of radiosynthesis of [18F]FLT on EWOD chip followed by cartridge purification to produce an injectable dose of [18F]FLT for mice imaging. Synthetic scheme of the radiosynthesis of [18F]FLT using a mixture of thexyl alcohol and DMSO in the fluorination reaction. The crude [18F]FLT product was extracted and purified via a simple cartridge purification to yield ∼ 52 MBq (non-decay corrected) of [18F]FLT.
Materials and Methods
Reagents
Tetrabutylammonium bicarbonate (TBAHCO3), 2,3-dimethyl-2-butanol, HCl, anhydrous acetonitrile (99.8%), anhydrous dimethyl sulfoxide (DMSO, 99.9%), hexanes, ethyl acetate, ethanol, and methanol were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO). 3-N-Boc-5′-O-dimethoxytrityl-3′-O-nosyl-thymidine (DMTr-Boc-nosyl precursor), 5′-O-dimethoxytrityl-3′-O-nosyl-thymidine (DMTr-nosyl precursor) and the 3-fluoro-3-deoxythymidine (FLT) standard compound were purchased from Advanced Biochemical Compounds (ABX) (Radeberg, Germany). Ion retardation resin (AG11 A8) and cation exchange (AG-50W-X4) were purchased from BioRad Laboratories (Hercules, CA). 2′,3′-Didehydro-3′-deoxy-thymidine reference standard was purchased from Tokyo Chemical Industry (Japan) and used as received. Neutral alumina (particle size 50–300 μm), C-18 (particle size 55–105 μm), and the Oasis Hydrophilic Lipophilic Balanced HLB resin were purchased from Waters corporation (Milford, MA).
No-carrier-added [18F]fluoride ion was obtained from the UCLA Crump Institute for Molecular Imaging Cyclotron Facility by irradiation of 97% 18O-enriched water with an 11 MeV proton beam using an RDS-111 cyclotron (Siemens Medical Solution, Knoxville, TN). Radioactivity was measured using a calibrated ion chamber (CRC-15R, Capintec Inc., Ramsey, NJ). A radioactive thin layer chromatography scanner (MiniGITA star, Raytest USA, Inc, Wilmington, NC) was used to analyze fluorination efficiency. Samples of the crude fluorination product were spotted on silica TLC plates and developed in a mixture of ethanol and ethyl acetate (50:50 v/v). Analytical high performance liquid chromatography (HPLC) was carried out using a Phenomenex Luna (Torrance, CA) reversed-phase C-18 column (250 × 4.6 mm). Elution was performed at constant flow rate of 1 mL/min with water:ethanol (90:10 v/v) at and the UV absorbance was measured at 265 nm. Pyrogeneticity testing was performed using the Charles River Endosafe-PTS Portable Testing System (California, USA).
EWOD Chip Fabrication and Operation
Similar to our previous report (12), the EWOD chip was constructed from two parallel plates: the base plate and the cover plate. In comparison to our previous chip design, two additional reagent loading sites and inlet pathways were added to increase the flexibility for multi-step radiochemistry (Fig. 2). Additional details of EWOD chip fabrication and operation can be found in the supplemental information.
Figure 2.
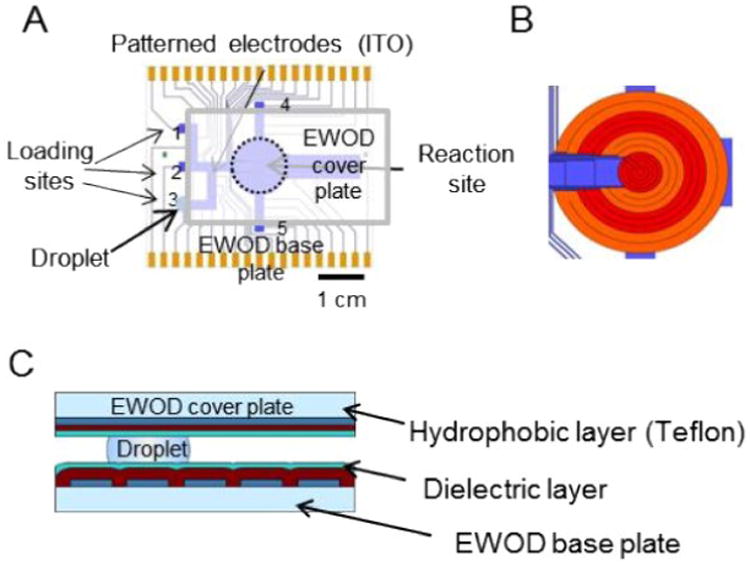
(A) EWOD chip base plate design with a reaction site and multiple loading sites. A reagent droplet (blue circle), sandwiched between the EWOD device plate and the cover plate, is shown at loading site 3. (B) Detail of multifunctional electrowetting, heating, and temperature sensing electrodes. (C) Cross-sectional view of EWOD chip with a sandwiched droplet.
Radiosynthesis of [18F]FLT on the EWOD chip
Stock solution of TBAHCO3 (5 μL, 75 mM) was mixed with the no-carrier-added [18F]fluoride (20 μL; ∼740 MBq) solution to form the [18F]TBAF complex. Three droplets (2 μL each) of the [18F]TBAF complex were pipetted to loading site 1 and then transported to the reaction site by EWOD actuation, followed by the addition of a 3 μL droplet of MeCN. The [18F]TBAF complex was heated to 105 °C and held at 105 °C for 1 min to evaporate the solvent. Subsequently, one cycle of azeotropic distillation was performed by adding a 9 uL MeCN droplet via loading site 1 to the dried residue and heating to 105 °C for 1 min. Upon completion of this drying step and thus activation of the [18F]TBAF complex, DMTr-Boc-nosyl precursor (4.5 mg) was dissolved in a mixture of DMSO (20 μL) and 2,3-dimethyl-2-butanol (40 μL) to achieve a final concentration of 90.4 mM. One droplet of the DMTr-Boc-nosyl precursor solution (2 μL) was pipetted to loading site 2 and transferred to the dried [18F]TBAF complex on the reaction site at room temperature, followed by the addition of a 3 uL droplet of MeCN. The reaction mixture was gradually heated to 120 °C and held at 120 °C for 3 min to perform the fluorination reaction. After the fluorination reaction, two 3.5 μL droplets of a 4:1 mixture of 1N HCl/MeCN were pipetted to loading site 3, and transported to and mixed with the crude intermediate product at the reaction site. The reaction mixture was slowly heated to 95 °C and held for 1.5 min to complete the hydrolysis reaction. The cover plate was removed and the crude product was extracted using 200 μL H2O, 50 μL MeCN and 18 μL DMSO, sequentially. A small amount of the crude product was used for radio-thin layer chromatography (radio-TLC) and radio-HPLC analyses, while the remainder of the crude product was purified using a miniaturized cartridge as described in the following sections. The total synthesis time and cartridge purification was about 63 minutes.
Cartridge Preparation
A miniaturized purification cartridge was designed based on our previous report (12) to achieve a high purification efficiency (Fig. 3A). The custom-made cartridges consisted of 5 mg of ion retardation resin, 5 mg of cation exchange, 30 mg of neutral alumina (50–100 mesh size), and 150 mg of Oasis HLB resins packed within a 1-mL syringe barrel (Becton, Dickinson and Company, New Jersey). To prevent the formation of air bubbles, two polyethylene frits (20 μm pore size) were used to sandwich the resins. The cartridge was conditioned with methanol (1 mL) and water (2 mL) before use.
Figure 3.
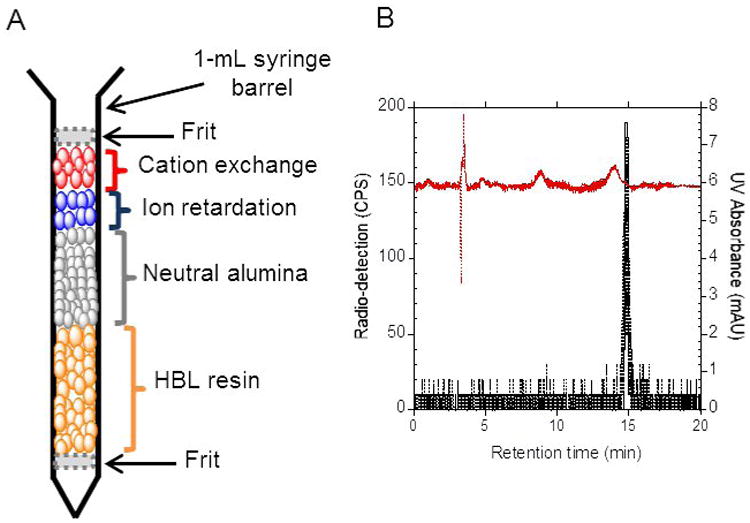
(A) Custom-made purification cartridge used to purify [18F]FLT that was synthesized on EWOD chip. (B) HPLC chromatogram of the cartridge purified [18F]FLT. The red chromatogram represented the absorbance in the UV and the black chromatogram represented the radio detection.
Cartridge Purification of [18F]FLT
Using a 1-mL syringe, the crude reaction mixture collected from the chip was passed through the conditioned miniature cartridge to trap the desired product, followed by sequential elution with 1% ethanol in water (9 mL) and 5% ethanol in water (6 mL) to release the side products to waste. A final washing step was performed using 100% ethanol (0.2 mL, 0.1 mL at a time). After removal of all the side products (analyzed by HPLC), the final [18F]FLT product was eluted from the cartridge using 0.5 mL of ethanol and was collected into a sterile empty vial. The ethanol was evaporated by heating at 75 °C and blowing nitrogen for 5 minutes into the vial, and the resulting dried [18F]FLT residue was redissolved in 0.2 mL of saline.
The residual solvents (ethanol, acetonitrile, DMSO, thexyl alcohol) in the formulated [18F]FLT were analyzed via gas chromatography following the method reported in our previous work (12) (details in Supporting Information). The chemical and radiochemical purity of the formulated [18F[FLT was analyzed using an analytical HPLC using a UV detection at 265 nm and a radio-detector. The product and impurities in the final sample were identified by comparing their retention times to known standards. A calibration curve for FLT and several chemical impurities, such as 2′,3′-didehydro-3′-deoxy-thymidine (stavudine) and thymine, was performed to quantitate the amount of impurities present in the final product.
Specific Activity Analysis
For specific activity analysis, two batches of [18F]FLT were prepared on the EWOD chip with all volumes doubled to obtain sufficient mass for UV detection on the HPLC. For these experiments, the crude [18F]FLT was first purified via analytical HPLC and the fraction containing the [18F]FLT product was collected. The collected product was diluted in water and passed through the custom-made purification cartridge. The cartridge purification procedure was identical to the single batch experiment. Upon partial evaporation of the ethanol, the concentrated [18F]FLT (∼0.15 mL) was injected into the analytical HPLC for quantification of the cold mass of FLT (UV detection at 254 nm) using the previously obtained calibration curve for FLT. The decay corrected radioactivity of the purified [18F]FLT was used for specific activity analysis.
Pyrogeneticity Test
An aliquot (500 μL) of the final [18F]FLT product in saline was tested for the presence of bacterial endotoxin utilizing a Limulus Amebocyte Lysate (LAL) Test. The sample was further diluted with saline by 20 times before the LAL analysis. The test was performed using a Charles River Endosafe-PTS Portable Testing System.
MicroPET and CT imaging
Details of the small animal imaging can be found in the Supporting Information.
Results
The multistep on-chip reaction begins with the [18F]fluoride ion activation step, followed by the radiofluorination of DMTr-Boc-nosylate FLT precursor, and finally the deprotection of the tert-butyloxycarbonyl (Boc) and the 4,4′-dimethoxytriphenylmethyl (DMTr) groups via acid hydrolysis. A systematic optimization of various reaction parameters including reagent concentration, precursor, precursor to base ratio, reaction temperature, reaction time, and phase transfer catalyst was performed on Teflon-coated glass substrates, which mimic the microdroplet reaction on the EWOD chip. In the first phase of the method development, we found that the optimal fluorination condition used the DMTr-Boc-nosylate FLT precursor and the TBAHCO3 as the phase transfer catalyst in 2:1 molar ratio to achieve 80±7% (n=10) fluorination efficiency. (Efficiencies and yields are reported with standard deviations based on the number of experiments, n.)
Upon further investigation, we found that a significant percentage of radioactivity was lost during the fluorination and hydrolysis step. In our attempt to minimize the loss by reducing the reaction time to 3 min, the fluorination efficiency decreased to 51±7% (n=3). To counteract this effect, the overall reagent concentration was doubled and the reaction temperature was increased from 110 °C to 120 °C. This resulted in an increase in fluorination efficiency to 79±6% (n=2). The fluorination efficiency was further increased to 94±3% (n=9) when the reaction droplet volume was reduced from 4 μL to 2 μL, while maintaining the 2-fold increase in the reagent concentrations. Following the optimized fluorination protocol, the protected [18F]FLT intermediate was hydrolyzed in a mixture of HCl and MeCN at 95 °C for 1.5 min to obtain [18F]FLT in 82±10% (n=10) crude radiochemical yield. The crude radiochemical yield is defined as (radioactivity remaining on chip after synthesis * conversion to [18F]FLT as determined by radio-TLC)/estimated radioactivity loaded onto chip). In comparison to the initial method, the crude radiochemical yield obtained from the optimized method improved from 49±11% (n=10) to 82±10% (n=10).
After the radiosynthesis of [18F]FLT on the EWOD chip, the crude mixture was first collected using a mixture of DMSO, MeCN and water with 84±2% (n=5) collection efficiency. The collected crude reaction mixture (∼270 μL) was then purified on a custom-made miniaturized cartridge to obtain [18F]FLT in >99% radiochemical purity and an overall decay-corrected radiochemical yield of 63±5% (n=5). The cartridge purification efficiency was 89±2% (n=5). This efficiency is defined by the radioactivity of purified [18F]FLT recovered after cartridge purification divided by the total [18F]FLT collected from the EWOD chip (i.e., radioactivity of collected crude product * conversion to [18F]FLT as determined by radio-TLC). Starting with ∼ 333 MBq of radioactivity ([18F]fluoride ion) on the EWOD chip, we have successfully prepared 52 MBq (non-decay corrected) of [18F]FLT upon cartridge purification and formulation.
The purified [18F]FLT sample was subjected to a set of quality control procedures required for testing purity and safety before administering into humans. The final product solution was observed to be clear and free of particulates. The pH was measured to be between 6.5 and 7 using pH paper. The GC analysis showed that the final product contained <20 ppm of MeCN, DMSO, ethanol and thexyl alcohol. The allowable limit for the residual organic solvents are as the following: MeCN (400 ppm), DMSO (5000 ppm), ethanol (5000 ppm), and thexyl alcohol (5000 ppm). The chemical purity of the formulated product was analyzed using an analytical HPLC. Based on the standard UV calibration curves for FLT and the other known impurities, we found that the final sample contained 0.3 ppm of stavudine, 0.05 ppm thymine and 0.07 ppm of FLT. Other UV active peaks that eluted around 3.5 minutes were unidentified and do not match the retention times of any of the known impurities reported in the literature. The LAL test detected less than 1 EU/mL in concentration of the final sample, which is lower than the established USP endotoxin limit of 175 EU/mL per dose for radiopharmaceuticals. The specific activities of [18F]FLT synthesized on the EWOD chips were measured to be 1800 and 2400 GBq/μmol.
Discussion
Since the first radiosynthesis of [18F]FLT reported by Grierson and coworkers (18), there have been a multitude of methods developed to achieve higher and more reliable yield (19). Notably, Eisenhut and co-workers developed a new FLT precursor, DMTr-Boc-nosyl to facilitate synthesis automation and it has become the precursor of choice to achieve high radiosynthesis yield (20). Since then, multiple research groups have reported the radiosynthesis of [18F]FLT using this DMTr-Boc-nosyl FLT precursor based on the conventional no-carrier-added radiofluorination method with radiochemical yields ranging from 23-50% (19, 21-24). Particularly, the use of a bulky alcohol as co-solvent in assisting the no-carrier-added radiofluorination is attractive and has been reported to attain [18F]FLT in a high radiochemical yield (60-65%) (25-28). We adapted this high yielding, high selectivity protic solvent chemistry to the EWOD microdevice, and developed a miniature cartridge purification method to eliminate the expensive and complicated HPLC purification.
During this developmental process, the majority of the processes were performed manually, such as reagent loading, product collection and cartridge purification, which limits the amount of radioactivity that was used in this work. While this report focused on the development of a reliable microscale radiochemistry on the EWOD chip, we are also currently developing an automated reagent delivery, product collection and purification system (29-32). We anticipate that high activity production within microliter volume can easily be achieved based on the recent demonstration of the production of clinical dose amounts of [18F]FDG and [18F]fallypride in batch microfluidic devices (9, 33). For example, a miniaturized anion exchange cartridge was able to concentrate ∼32 GBq of [18F]fluoride/[18O]H2O into a 5 μL volume of eluent. Such volume is commensurate with the design of EWOD chip reaction site, which can accommodate up to ∼17 μL droplet size.
Starting from the original report of the radiosynthesis of [18F]FLT in protic solvent (26), we first performed systematic screening of various reaction parameters including reagent concentrations, precursors, precursor to base ratios, and phase transfer catalysts on the microscale using Teflon-coated glass substrates. In contrast to the macroscale method, we chose to use a mixture of DMSO and thexyl alcohol to improve the solubilization of the precursor throughout the entire fluorination reaction, which is critical for synthesis automation and to increase the reaction reliability. While macroscale syntheses generally avoid the use of DMSO (bp: 182 °C) and thexyl alcohol (bp: 120 °C) due to the difficulty in removing these solvents after the synthesis, the much smaller volume used in the microscale synthesis (2 μL versus 500 μL – 1000 μL) facilitates the rapid removal of these solvents. At the end of the synthesis, the droplet volume has already shrunk to ∼0.2 μL due to solvent evaporation during the fluorination reaction (Fig. S-1). Without further evaporation, we have confirmed that the level of residual solvents of the final purified product is below the recommended limits for injection. A summary of results from the optimization studies performed on Teflon-coated glass is presented in Table 1. We found that the precursor to base concentration ratio is one of the critical parameters affecting the fluorination efficiency, which is consistent with the work by Suehiro et al (34). In addition to the precursor to base ratio, we have also investigated the use of the cryptand complex as the phase transfer catalyst. As shown in Table 1, the fluorination yield using the cryptand complex is lower (58%; Table 1, condition 2) in comparison to the TBAHCO3 (80%; Table 1, condition 4) when performed under similar condition (i.e.: precursor to base ratio ∼ 2), which is also consistent with the report by Kim et al (35). To confirm the catalytic effect of the alcohol in the radiosynthesis, we have also performed a control experiment by replacing the DMSO/thexyl alcohol with an aprotic solvent mixture of DMSO and MeCN. The DMSO/MeCN solvent ratio was determined empirically such that the droplet size at the end of fluorination reaction was a similar size for the two cases. We observed significantly lower fluorination efficiency of 38±4% (n=2) when DMSO/MeCN was used versus 80±7% (n=10) when thexyl alcohol was used in the fluorination reaction (Table 1, Conditions 4 and 5), which confirmed the advantage of thexyl alcohol in assisting the nucleophilic substitution reaction. In our attempt of using the DMTr-nosyl FLT precursor, we found that the fluorination yield was lower and less reliable in comparison to the DMTr-Boc-nosylate FLT precursor under similar condition (Table 1, condition 1 versus 3). The final hydrolysis step was performed at 95 °C for 5 minutes. The completion of the hydrolysis reaction was tentatively confirmed by the emergence of a single radio peak at around 15 minutes, which corresponds to [18F]FLT in the radio-HPLC (in supporting information; Fig. S-3). This first phase of optimized method is referred to as Method 1 in Table 2.
Table 1.
Optimization of the radiofluorination with varying parameters and precursors on Teflon-glass substrates.
| Condition | Precursor* (mM) | PTC/Base* (mM) | Solvents (v/v) μL | Temp. (°C) | Time (min) | Average Fluorination efficiency (%)† |
|---|---|---|---|---|---|---|
| 1 | Non-Boc (40) | TBAHCO3 (39) | DMSO/TA (0.8/3.2) | 100 | 5 | 47±18 (n=8) |
| 2 | Boc (40) | K222/K2CO3 (36/18) | DMSO/TA (0.8/3.2) | 100 | 5 | 58±6 (n=2) |
| 3 | Boc (40) | TBAHCO3 (39) | DMSO/TA (0.8/3.2) | 100 | 5 | 55±19 (n=3) |
| 4 | Boc (45) | TBAHCO3 (22.5) | DMSO/TA (0.8/3.2) | 110 | 5 | 80±7 (n=10) |
| 5 | Boc (45) | TBAHCO3 (22.5) | DMSO/MeCN (1/3) | 110 | 5 | 38±4 (n=2) |
Abbreviations: Boc: 3-N-Boc-5′-O-dimethoxytrityl-3′-O-nosyl-thymidine; non-Boc: 5′-O-Dimethoxytrityl-3′-O-nosyl-thymidine; PTC: Phase transfer catalyst; TBAHCO3: tetrabutylammonium bicarbonate; K222: Kryptofix 2.2.2; K2CO3: potassium carbonate; DMSO: dimethylsulfoxide; TA: thexyl alcohol; MeCN: acetonitrile.
The fluorination efficiencies are reported with standard deviations based on the number of experiments, n.
Table 2. Summary of the reaction conditions for the radiosynthesis of [18F]FLT on EWOD chip based on Method 1.
| Process | Temp. (°C) | Reaction time (min) | Reagent | Conc. (mM) | Droplet volume (μL) | Fluorination efficiency (%)† | Radioactivity remaining on chip (%)* | Crude Radiochemical yield (%)† |
|---|---|---|---|---|---|---|---|---|
| (1) Load [18F]TBAF | RT | TBAHCO3 | 22.5 | 6 | n/a | n/a | ||
| (2) MeCN azeotropic distillation | 105 | 2 | MeCN | n/a | 9 | n/a | n/a | |
| (3) Fluorination | 120 | 5 | FLT precursor | 45 | 4 | 74±6 (n=8) | 80 (n=4) | n/a |
| (4) Hydrolysis | 95 | 5 | HCl | 0.75 | 7 | n/a | 67 (n=4) | 49±11 (n=10) |
To determine radioactivity remaining on chip, the chip was removed after each step and the radioactivity on the entire chip was measured using a dose calibrator for the radioactivity measurement.
Fluorination efficiencies and yields are reported with standard deviations based on the number of experiments, n.
Though Method 1 exhibited high fluorination and hydrolysis efficiencies, we found that the overall crude radiochemical yield of [18F]FLT at the end of the synthesis was relatively low (47±19%; n=7). To understand the discrepancy between overall efficiency and yields of individual steps, we measured the radioactivity losses after each step of the synthesis on a Teflon-coated glass substrate. The sandwiched Teflon-glass substrate was removed from the Peltier heater and the radioactivity on the substrate was measured using the dose calibrator after each step of the Method I synthesis (Table 2). Based on this study, we found that the major loss occurred after both the fluorination and hydrolysis steps. While the exact mechanism of the radioactivity loss is yet to be determined, our initial approach was to reduce the reaction times for both the fluorination and hydrolysis reaction to reduce the overall radioactivity losses, while ideally not diminishing the yields.
In order to do so, we first investigated the reaction kinetics on Teflon-glass substrate and found that a reduction of the fluorination reaction time from 5 min to 3 min resulted in a significant decrease in the fluorination efficiency. Through a series of experiments, we found that the fluorination reaction kinetics can be improved by increasing the reagent concentration and the reaction temperature. Interestingly, we also found that the fluorination yield was further increased (from 79% to 94%) by simply reducing the droplet size from 4 μL to 2 μL, while maintaining the two-fold increase in the concentration of the reagent. The enhanced fluorination yield could be speculated to be attributable to the increase in the [18F]fluoride ion concentration as the reaction volume was decreased. While consistent with speculations that this is an advantage of microfluidic synthesis platforms (15), further investigation of this effect is necessary.
Based on the new optimized synthesis (Method 2, Table 3), we indeed observed a reduction in radioactivity loss from 33% loss to only about 15% loss (Table 2). The new microscale [18]FLT conditions also yielded higher fluorination efficiency (91±4% (n=10)). Overall, the crude radiochemical yield of [18F]FLT increased from 49±11% (Table 2) to 82±10% (Table 3).
Table 3.
Summary of the reaction conditions for the radiosynthesis of [18F]FLT on EWOD chip based on Method 2.
| Process | Temp. (°C) | Reaction time (min) | Reagent | Conc.(mM) | Droplet volume (μL) | Fluorination efficiency (%)† | Radioactivity remained on chip (%)* | Crude Radiochemical yield (%)† |
|---|---|---|---|---|---|---|---|---|
| (1) Load [18F]TBAF | RT | TBAHCO3 | 45 | 6 | n/a | n/a | ||
| (2) MeCN azeotropic distillation | 105 | 2 | MeCN | n/a | 9 | n/a | n/a | |
| (3) Fluorination | 120 | 3 | FLT precursor | 90 | 2 | 91±4 (n=10) | 94 (n=4) | n/a |
| (4) Hydrolysis | 95 | 1.5 | HCl | 75 | 7 | n/a | 85 (n=4) | 82±10 (n=11) |
To determine radioactivity remaining on chip, the chip was removed after each step and the radioactivity on the entire chip was measured using a dose calibrator for the radioactivity measurement.
Fluorination efficiencies and yields are reported with standard deviations based on the number of experiments, n.
With the exception of [18F]FDG and [18F]NaF, other PET probes generally require final purification via HPLC to remove excess precursor or other radiolabelled or toxic side products that cannot be easily removed via solid phase extraction (SPE). However, this technique is not easily miniaturized as would be needed for a benchtop radiosynthesis platform. These shortcomings of HPLC have led to the emergence of several HPLC-free radiochemistry methods (36-38).
From the point of view of the cartridge purification, the most desirable solvent to collect the product from the chip is water. However, in the microscale, where the surface to volume ratio is large, we found that the collection efficiency (ratio of radioactivity recovered from the chip versus the total radioactivity that was measured on-chip after the synthesis) of the final crude [18F]FLT from the EWOD chip was below 15% when only water was used as the extraction solvent. Using 50:50 v/v MeCN/H2O as the extraction solvent, the collection efficiency improved to 47±10% (n=5). Finally, we attempted a mixture of DMSO, MeCN and water (18 μL, 50 μL and 200 μL, respectively) and obtained 84±2% (n=5) collection efficiency. However, in this solvent mixture, we found that the [18F]FLT was not able to be separated from the other side products when the conventional reversed phase C18 resins were used in the cartridge purification. This observation can be explained by the increasing composition of nonpolar solvent during the cartridge purification, which is sufficient to disrupt the van der Waals forces between the [18F]FLT analyte and the reversed phase resin (39). To address this issue, we investigated a new type of sorbent known as the Oasis Hydrophilic-Lipophilic-Balanced resins (HLB). Based on systematic cartridge purification studies, we determined that the optimal HLB resin to be 150 mg to efficiently retain [18F]FLT from a solvent mixture of MeCN, water and DMSO, while enabling elution of the other side products from the cartridge. This resin was combined in a miniaturized cartridge with additional resins for removal of other impurities. Upon the cartridge purification of [18F]FLT, the final purified [18F]FLT product was collected in 500 μL of 100% ethanol. Upon evaporation and reformulation in saline, 52 MBq of [18F]FLT was obtained for micro-PET imaging studies of several A431 tumor bearing mice. As expected, the micro-PET images showed a high accumulation of [18F]FLT in the tumor due to the high level of expression of the thymidine kinase-1 (TK-1) enzyme in rapidly proliferating cells (Fig. S-7). No adverse effect on the physiology of the mice was observed after injection.
Based on several [18F]FLT samples that were subjected to a standard quality control procedure, the final [18F]FLT solutions were found to have negligible amount of impurities upon formulation in ∼ 0.2 mL of saline. The only radioactive component present in the final compound was [18F]FLT upon successful removal of the unreacted [18F]fluoride ion using the custom-made cartridge (Fig. 3B and radio-TLC in Fig. S-6). Due to the minute amount of reagent used on the EWOD microfluidic device for radiosynthesis, the absolute amounts of impurities and residual solvent reported here and in our previous report (12) were extremely small. Additionally, the level of impurities reported here would be ∼ 50 times lower upon diluting the single dose of [18F]FLT from the EWOD chip in 10 mL of saline for clinical PET imaging. For clinical production, we anticipate to only increase the amount of radioactivity loaded onto the EWOD chip while keeping all other aspects of the synthesis process the same. Therefore there will be a significant reduction in the level of impurities, bacterial endotoxins, and residual solvents that are present in a single dose of PET radiopharmaceuticals for clinical studies.
The specific activity of [18F]FLT synthesized on the EWOD chip was measured to be more than 10 times higher than literature reports using conventional macroscale radiosynthesizers. The high specific activity of [18F]FLT synthesized on the EWOD chip suggests that the Teflon layer on the chip does not lead to significant amount of carrier fluoride contamination through radiolysis or other mechanisms. This result is consistent with the recent report by Rensch et al (40) on the reduction of radiolysis on microfluidic devices due to the geometric confinement. The simulation and experimental studies conducted by Rensch suggested that the majority of the energy of positrons is deposited into the walls of microfluidic chips rather than in the reaction mixture when the dimension of the microfluidic channel or gap is smaller than the positron range (∼ 400 μm). The high specific activity suggests an advantage of microfluidic platforms for the production of high specific activity radiopharmaceuticals for imaging low abundance receptors. We are currently investigating key contributing factors that lead to this high specific activity.
Conclusions
In this report, we have developed an optimal two-step, one-pot procedure to synthesize [18F]FLT on the EWOD radiosynthesizer. The method uses a bulky alcohol (thexyl alcohol) as a co-solvent and exhibits a high and reliable decay-corrected radiochemical yield of 63±5% (n=5). The synthesis time (including cartridge purification) was 63 min. The optimized microscale radiofluorination strategy yielded up to 52 MBq (non-decay corrected) [18F]FLT. The final product passed all quality control tests, including pH, chemical purity, residual solvent analysis and pyrogenecity test that are required before administering into humans.
The small size of the microfluidic platform suggests that the overall need for radiation shielding could be dramatically reduced in comparison to the size of hot cells and mini cells. The reduction in the overall size and shielding enable the synthesizer to be self-shielded and placed on a standard laboratory bench-top, thus eliminating some of the barriers to PET probe production. With increasing degree of automation of the EWOD microfluidic platform, such as radioactivity concentration, reagent delivery, cartridge purification and an integrated quality control module, a compact, robust radiosynthesizer that produced probes on-demand on a benchtop may be possible. Currently, the EWOD chip is designed to be one-time used, similar to the disposable reagent cassettes used in many recent macroscale radiosynthesizers. This report demonstrates that a similar microfluidic chip design as reported in our previous publication can be used to synthesize different PET tracers. To date, we have demonstrated the syntheses of [18F]FDG, [18F]FLT and other PET probes (manuscript in preparation) by simply changing the reagents and reaction conditions.
Supplementary Material
Acknowledgments
This work was supported in part by funds from the UCLA Department of Molecular and Medical Pharmacology and the UCLA Foundation from a donation made by Ralph and Marjorie Crump for the Crump Institute for Molecular imaging. We thank Dr. David Stout at the Crump Radiochemistry Cyclotron facility and Dr. Saman Sadeghi at the UCLA Biomedical Cyclotron facility for providing [18F]fluoride ion for these studies and performing the pyrogenecity test, Dr. Caius Radu for performing the [18F]FLT in vivo studies and Jeffrey Collins for performing residual solvent analysis.
References
- 1.Ametamey SM, Honer M, Schubiger AP. Molecular imaging with PET. Chem Rev. 2008;108:1501–1516. doi: 10.1021/cr0782426. [DOI] [PubMed] [Google Scholar]
- 2.Phelps ME. Positron emission tomography provides molecular imaging of biological processes. Proc Natl Acad Sci. 2000;97(16):9226–9233. doi: 10.1073/pnas.97.16.9226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen K, Chen X. Positron emission tomography imaging of cancer biology: Current status and future prospects. Semin Oncol. 2011;38(1):70–86. doi: 10.1053/j.seminoncol.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keng PY, Esterby M, Van Dam M. Emerging Technologies for Decentralized Production of PET Tracers. In: Hsieh CH, editor. Positron Emission Tomography-Current Clinical and Research Aspects. 1st. New York, USA: InTech; 2012. pp. 163–192. [Google Scholar]
- 5.Vallabhajosula S. 18F-labeled positron emission tomographic radiopharmaceuticals in oncology: An overview of radiochemistry and mechanisms of tumor localization. Semin Nucl Med. 2011;37:400–419. doi: 10.1053/j.semnuclmed.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Chen W, Cloughesy T, Kamdar N, et al. Imaging proliferation in brain tumors with 18F-FLT PET: Comparison with 18F-FDG. J Nucl Med. 2005;46(6):945–952. [PubMed] [Google Scholar]
- 7.Gillies JM, Prenant C, CHimon GN, et al. Microfluidic reactor for the radiosynthesis of pet radiotracers. Appl Radiat Isot. 2006;64:325–332. doi: 10.1016/j.apradiso.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Steel CJ, O'Brien AT, Luthra SK, Brady F. Automated PET radiosyntheses using microfluidic devices. J Label Compd Radiopharm. 2007;50:308–311. [Google Scholar]
- 9.Elizarov AM, van Dam RM, Shin YS, et al. Design and optimization of coin-shaped microreactor chips for pet radiopharmaceutical synthesis. J Nucl Med. 2010;51(2):282–287. doi: 10.2967/jnumed.109.065946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bejot R, Elizarov AM, Ball E, et al. Batch-mode microfluidic radiosynthesis of n-succinimidyl-4-[18F]fluorobenzoate for protein labelling. J Label Compd Radiopharm. 2011;54:117–122. [Google Scholar]
- 11.Arima V, Pascali G, Lade O, et al. Radiochemistry on chip: Towards dose-on-demand synthesis of PET radiopharmaceuticals. Lab Chip. 2013;13:2328. doi: 10.1039/c3lc00055a. [DOI] [PubMed] [Google Scholar]
- 12.Keng PY, Chen S, Sadeghi S, et al. Digital microfluidics for multi-step batch chemical synthesis: Application to synthesis of radiotracers for positron emission tomography (PET) Proc Natl Acad Sci USA. 2012;109(3):690–695. doi: 10.1073/pnas.1117566109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whitesides GM. The origins and the future of microfluidics. Nature. 2006;442(7101):368–373. doi: 10.1038/nature05058. [DOI] [PubMed] [Google Scholar]
- 14.Fletcher PDI, Haswell SJ, Pombo-Villar E, et al. Micro reactors: Principles and applications in organic synthesis. Tetrahedron. 2002;58(24):4735–4757. [Google Scholar]
- 15.Elizarov AM. Microreactors for radiopharmaceutical synthesis. Lab Chip. 2009;9(10):1326–1333. doi: 10.1039/b820299k. [DOI] [PubMed] [Google Scholar]
- 16.Moon M, Cho SK, Garrell RL, Kim CJ. Low voltage electrowetting-on-dielectric. J App Phys. 2002;92:4080–4087. [Google Scholar]
- 17.Kim HK, Chen S, Javed MR, Kim CJ, van Dam RM, Keng Py. Optimization of microdroplet radiofluorination towards on-demand synthesis of n-succinimidyl-4-[18F]fluorobenzoate (SFB) on an ewod microdevice. Society of Nuclear Medicine Annual Meeting Abstracts. 2012;53(Supplement 1) 2012/05/01. [Google Scholar]
- 18.Gierson JR, Shields AF. Radiosynthesis of 3′-deoxy-3′-[18F]fluorothymidine: [18F]FLT for imaging of cecullar proliferation in vivo. Nuc Med Biol. 2000;27:143–156. doi: 10.1016/s0969-8051(99)00104-3. [DOI] [PubMed] [Google Scholar]
- 19.Roeda D, Dollé F. Aliphatic nucleophilic radiofluorination. Current Radiopharmaceuticals. 2010;3(2):81–108. [Google Scholar]
- 20.Martin SJ, Eisenbarth JA, Wagner-Utermann U, et al. A new precursor for the radiosynthesis of [18F]FLT. Nucl Med Biol. 2002;29(2):263–273. doi: 10.1016/s0969-8051(01)00289-x. [DOI] [PubMed] [Google Scholar]
- 21.Yun M, Oh SJ, Ha HJ, Ryu JS, Moon DH. High radiochemical yield synthesis of 3′-deoxy-3′-[18F]fluorothymidine using (5′-o-dimethoxytrityl-2′-deoxy-3′-o-nosyl-β-d-threo pentofuranosyl) thymine and its 3-n-boc-protected analogue as a labeling precursor. Nucl Med Biol. 2003;30(2):151–157. doi: 10.1016/s0969-8051(02)00409-2. [DOI] [PubMed] [Google Scholar]
- 22.Oh SJ, Mosdzianowski C, Chi DY, et al. Fully automated synthesis system of 3′-deoxy-3′-[18F]fluorothymidine. Nucl Med Biol. 2004;31(6):803–809. doi: 10.1016/j.nucmedbio.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 23.Moon BS, Lee KC, An GI, et al. Preparation of 3′‐deoxy‐3′‐[18F]fluorothymidine ([18F]FLT) in ionic liquid, [bmim][otf] J Label Compd Radiopharm. 2006;49(3):287–293. [Google Scholar]
- 24.Teng B, Wang S, Fu Z, Dang Y, Wu Z, Liu L. Semiautomatic synthesis of 3′-deoxy-3′-[18F]fluorothymidine using three precursors. Appl Radiat Isot. 2006;64(2):187–193. doi: 10.1016/j.apradiso.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 25.Lee S, Oh S, Chi D, et al. Simple and highly efficient synthesis of 3′-deoxy-3′-[18F]fluorothymidine using nucleophilic fluorination catalyzed by protic solvent. Eur J Nucl Med Mol Imaging. 2007;34(9):1406–1409. doi: 10.1007/s00259-007-0391-8. [DOI] [PubMed] [Google Scholar]
- 26.Lee SJ, Oh SJ, Chi DY, Lee BS, Ryu JS, Moon DH. Comparison of synthesis yields of 3′‐deoxy‐3′‐[18F]fluorothymidine by nucleophilic fluorination in various alcohol solvents. J Label Compd Radiopharm. 2008;51(1):80–82. [Google Scholar]
- 27.Kim DW, Jeong, Lim ST, Sohn MH, Katzenellenbogen JA, Chi DY. Facile nucleophilic fluorination reactions using tert-alcohols as a reaction medium: Significantly enhanced reactivity of alkali metal fluorides and improved selectivity. J Org Chem. 2008;73(3):957–962. doi: 10.1021/jo7021229. [DOI] [PubMed] [Google Scholar]
- 28.Kim DW, Jeong HJ, Lim ST, Sohn MH. Recent trends in the nucleophilic [18F]-radiolabeling method with no-carrier-added [18F]fluoride. Nuclear Medicine and Molecular Imaging. 2010;44(1):25–32. doi: 10.1007/s13139-009-0008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shah GJ, Javed MR, Yang A, Keng PY, van Dam RM. Integration of [18F]fluoride concentration into a digital microfluidics-based radiosynthesizer for the benchtop. J NucI Med. 2012;53(Supplement 1):575. [Google Scholar]
- 30.Shah GJ, Lei J, Chen S, Kim CJC, Keng PY, van Dam RM. Proof-of-concept automated synthesis and hplc purification of [18F]fallypride on a prototype digital microfluidic radiosynthesizer; World Molecular Imaging Conference; Dublin, Ireland. 2012. [Google Scholar]
- 31.Shah GJ, Keng PY, Chen S, et al. Integrated digital microchemistry platform: Automation of multi-reagent loading, on-chip high-temperature reactions, and product extraction. International Symposium on MicroChemistry and Microsystem; Hsinchu, Taiwan: 2012. [Google Scholar]
- 32.Shah GJ, Ding HJ, Sadeghi S, Chen S, Kim CJ, Van Dam M. On-demand droplet loading for automated organic chemistry on digital microfluidics. Lab Chip. 2013 doi: 10.1039/c3lc41363b. Advanced article. [DOI] [PubMed] [Google Scholar]
- 33.Lebedev A, Miraghaie R, Kotta K, et al. Batch-reactor microfluidic device: First human use of a microfluidically produced pet radiotracer. Lab Chip. 2012;13:136. doi: 10.1039/c2lc40853h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suehiro M, Vallabhajosula S, Goldsmith SJ, Ballon DJ. Investigation of the role of the base in the synthesis of [18F]FLT. Appl Radiat Isot. 2007;65(12):1350–1358. doi: 10.1016/j.apradiso.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 35.Kim DW, Jeong HJ, Lim ST, Sohn MH. Facile nucleophilic fluorination of primary alkyl halides using tetrabutylammonium fluoride in a tert-alcohol medium. Tetrahedron Lett. 2010;51(2):432–434. [Google Scholar]
- 36.Nandy SK, Rajan MGR. Fully automated and simplified radiosynthesis of [18F]-3-deoxy-3′-fluorothymidine using anhydro precursor and single neutral alumina column purification. J Radioanal Nucl Chem. 2010;283:741–748. [Google Scholar]
- 37.Tang G, Tang X, Wen F, Wang M, Li B. A facile and rapid automated synthesis of 3′-deoxy-3′-[18F]fluorothymidine. Appl Radiat Isot. 2010;68(9):1734–1739. doi: 10.1016/j.apradiso.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 38.Pascali C, Bogni A, Fugazza L, et al. Simple preparation and purification of ethanol-free solutions of 3′-deoxy-3′-[18F]fluorothymidine by means of disposable solid-phase extraction cartridges. Nucl Med Biol. 2012;39(4):540–550. doi: 10.1016/j.nucmedbio.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 39.Zwir-Ferenc A, Bizuik M. Solid phase extraction technique-trends, opportunities and applications. Polish J of Environ Stud. 2006;15:677–690. [Google Scholar]
- 40.Rensch C, Waengler B, Yaroshenko A, et al. Microfluidic reactor geometries for radiolysis reduction in radiopharmaceuticals. Appl Radiat Isot. 2012;70:1691–1697. doi: 10.1016/j.apradiso.2012.03.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


