SUMMARY
Congenital cytomegalovirus (CMV) infections are a leading cause of sensorineural hearing loss (SNHL) and neurological impairment. Congenital transmission of CMV can occur with maternal primary infection, reactivation, or reinfection during pregnancy. We reviewed studies of CMV shedding in bodily fluids (defined as CMV detected by culture or CMV DNA detected by polymerase chain reaction). Following diagnosis at birth, children with congenital CMV infection exhibited the highest prevalences of CMV shedding (median = 80%, number of sample population prevalences [N] = 6) and duration of shedding, with a steep decline by age five. Healthy children attending day care shed more frequently (median = 23%, N = 24) than healthy children not attending day care (median = 12%, N = 11). Peak shedding prevalences in children occurred at 1–2 years of age, confirming that young children are the key transmission risk for pregnant women. CMV shedding among children was more prevalent in urine specimens than in oral secretions (median prevalence difference = 11.5%, N = 12). Adults with risk factors such as STD clinic attendance had higher shedding prevalences (median = 22%, N = 20) than adults without risk factors (median = 7%, N = 44). In adults with risk factors, CMV was shed more frequently in urine; in adults without risk factors genital shedding was most common. The prevalence of CMV shedding in nine sample populations of pregnant women increased with advancing gestation. In seven sample populations of children with congenital CMV infection, higher viral load at birth was consistently associated with an elevated risk of SNHL. Higher CMV viral load at birth also consistently correlated with the presence of symptoms of congenital CMV at birth. Published 2011. This article is a US Government work and is in the public domain in the USA.
INTRODUCTION
Adults can become infected with cytomegalovirus (CMV) through a variety of transmission routes, including contact with bodily fluids of children or adults who are shedding the virus, sexual activity, blood transfusion, and organ transplantation [1]. In most cases, CMV infection in immunocompetent adults does not lead to symptomatic disease [1]; however, active CMV infection during pregnancy can result in transmission to the fetus [2] that can in turn result in permanent damage, including SNHL, intellectual disability and, rarely, death [3,4]. The risk of permanent damage appears to be highest among children whose mother experienced primary CMV infection during gestation [5,6]. It has been estimated that more than half a million women of child-bearing age in the United States of America will experience primary CMV infection each year [7].
As with all human herpesviruses, CMV establishes a life-long latent infection during primary infection, and the virus may reactivate at any time with shedding of infectious CMV in saliva, urine and other bodily secretions [1]. Reinfection with a different CMV strain is also associated with shedding [5]. CMV infection and shedding are generally asymptomatic [8], so that individuals are unlikely to know that they have been infected or are shedding the virus. Infants and young children often acquire CMV infection through breastfeeding [9,10] or through contact with other children who are actively shedding virus [11]. Infants and young children may continue shedding virus for a year or more, and probably serve as the leading source for primary infection in women of reproductive age [12–16].
In order to better understand exposures that place women at risk for CMV primary infection or reinfection during pregnancy, we reviewed the available literature on the frequency, magnitude, and risk factors for CMV shedding in various populations.
METHODS
Definition of terms
In this review, we define CMV ‘shedding’ as the presence of CMV detected by culture techniques (e.g. traditional or rapid shell vial) or CMV DNA detected by polymerase chain reaction (PCR; e.g. nested or non-nested)[17]. The testing method is specified in the Tables.
For simplicity and to facilitate comparisons, specimens from the oral cavity, including throat swabs and mouth rinses, were categorized as ‘oral secretions’; cervical and vaginal swabs and lavages were categorized as ‘genital secretions’; and white blood cells, peripheral blood mononuclear cells, and peripheral mononuclear lymphocytes were categorized as ‘blood’.
We grouped adults according to whether or not they had factors that may be associated with CMV infection or shedding. These CMV risk factors included being sexually transmitted disease (STD) clinic attendees, men who have sex with men (MSM), and women with congenitally infected children.
Because of its relatively frequent occurrence in the literature, we created one category for analysis called ‘children with medical conditions’. These children came from studies of children hospitalized for various reasons (other than congenital CMV infection) or children who were institutionalized because of disabilities.
Many studies measured more than one CMV shedding prevalence. This could be because they studied more than one population (e.g. adults and children), more than one specimen type (e.g. urine and oral secretions), or more than one time point (e.g. all three pregnancy trimesters). We used the term ‘sample population prevalence’ to specify each of these shedding prevalences. Thus, some of the figures have more than one data point from a single study. These multiple sample population prevalences are each listed in the Tables.
Study selection and presentation
We identified studies of CMV shedding published from 1965 through 2009 by carrying out Medline searches using key words such as ‘cytomegalovirus’ or ‘CMV’ and ‘shedding’, ‘culture’, ‘PCR’, ‘epidemiology’, or ‘prevalence’. Additional studies were identified through the reference lists in the articles we retrieved. Because there are numerous studies on CMV shedding, we limited the scope of the review by excluding studies that primarily focused on congenital CMV birth prevalence, breast feeding, shedding in amniotic fluid, HIV-infected individuals, organ transplant recipients, or assay validation. In general, we also excluded studies of fewer than 25 participants. However, we included all studies regardless of sample size that examined CMV viral load, duration of shedding, or frequency of sequelae in children with congenital CMV, because few studies addressed these topics.
Because of space constraints, tables (numbered A1–A5) whose data are displayed in figures are placed in the online appendix in order of appearance. Tables whose data are not displayed in figures are shown in the main text and numbered 1–3 in order of appearance. Study categories used in the various tables included congenitally infected children, healthy children enrolled in day care, healthy children not enrolled in day care, children with medical conditions, seroconverters, adolescents who were shedding CMV, adults with CMV risk factors, and adults without CMV risk factors. For congenitally infected children, diagnostic results were excluded because, by definition, the shedding prevalence at diagnosis is 100%.
RESULTS
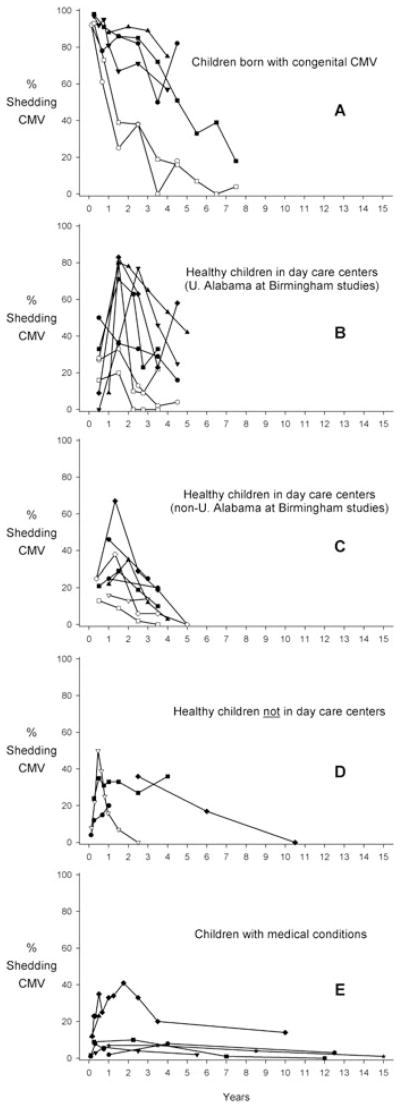
From 56 studies, we identified 112 sample population prevalences measured in a single specimen type (e.g. urine, oral secretions) at a single point in time (Figure 1, Tables A1 and A2). From these prevalences, we found that following their diagnosis at birth, congenitally infected children had the highest prevalences of shedding (median = 80%, N = 6). Among other children, shedding was more common among those in day care centers (median = 23%, N = 24) than among those not in day care centers (median = 12%, N = 11) or those with medical conditions (median = 9%, N = 7). Adults with CMV risk factors had prevalences of shedding similar to children in day care centers (median = 22%, N = 20), whereas adults without risk factors had the lowest prevalences of shedding (median = 7%, N = 44).
Figure 1.
Prevalences of cytomegalovirus (CMV) shedding in various studies that measured a single specimen type (e.g. urine, oral secretions) at a single point in time. Each circle represents the result from an individual study (listed in Tables A2 & A3 [11,14,18–22,27,28,30–32,39,54–78] [79–95]). Studies that measured more than one specimen type have more than one circle plotted above. Prevalences were not stratified by age
Table A1.
CMV shedding prevalence according to specimen typea
| Country | Demographics/Sampling Method | Testing Method | Specimen Type(s) | Sample size | Shedding % |
|---|---|---|---|---|---|
| Category I. Congenitally infected children | |||||
| England [78] | Random selection of specimens from 80 congenitally infected children followed for up to 5 years | Culture | Urine | 109 | 83 |
| Oral secretions | 101 | 63 | |||
| US-Alabama [28] | 104 congenitally infected children followed for up to 7 years | Culture | Urine | 772 | 77 |
| Oral secretions | 484 | 48 | |||
| Category II. Healthy children enrolled in day care centers | |||||
| US-Alabama [72] | Children in day care centers, maximum of one urine and Oral secretions sample per child | Culture | Urine | 231 | 29 |
| Oral secretions | 241 | 13 | |||
| US-Alabama [55] | 58 children in day care centers | Culture | Urine | 54 | 41 |
| Oral secretions | 47 | 30 | |||
| Hands | 44 | 7 | |||
| US-Alabama [14] | 70 children in day care centers | Culture | Urine | 68 | 53 |
| Oral secretions | 29 | 45 | |||
| US-Alabama [56] | 103 children in day care studied at least once (shedding estimated from Figure 2, ref. #117) | Culture | Urine | 198 | 53 |
| Oral secretions | 160 | 34 | |||
| US-California [63] | 100 children in day care centers | Culture | Urine | 90 | 22 |
| Oral secretions | 100 | 11 | |||
| 63 children in infant development centers | Urine | 50 | 22 | ||
| Oral secretions | 62 | 2 | |||
| US-Iowa [66] | 48 children in day care centers | Culture | Urine | 73 | 23 |
| Oral secretions | 80 | 11 | |||
| US-Iowa [67] | 79 children in day care centers followed for 2.5 years | Culture | Urine | 79 | 27 |
| Oral secretions | 79 | 25 | |||
| Category III. Healthy children not enrolled in day care centers | |||||
| The Gambia [61] | 178 infants at 6 months | Culture | Urine | 178 | 40 |
| Oral secretions | 178 | 37 | |||
| Japan [88] | 38 healthy infants between 4–9 months of age | Culture | Urine | 38 | 66 |
| Oral secretions | 33 | 64 | |||
| Category IV. Adults without CMV risk factors | |||||
| Brazil [74] | 102 pregnant and non-pregnant women, 98% CMV seropositive | Nested PCR | Genital secretions | 102 | 13 |
| White blood cells | 98 | 7 | |||
| England [19] | 402 hospitalized patients, ages 15–60+ years old | Culture | Urine | 402 | 0 |
| Oral secretions | 402 | 0 | |||
| The Gambia [61] | 178 post-partum women, 88% CMV seropositive | Culture | Urine | 840 | 4 |
| Oral secretions | 838 | 4 | |||
| Breast milk | 741 | 3 | |||
| Genital secretions | 178 | 7 | |||
| The Gambia [84] | 81 post-partum women, all CMV seropositive | PCR | Urine | 73 | 1 |
| Oral secretions | 74 | 18 | |||
| Genital secretions | 75 | 32 | |||
| Plasma | 79 | 3 | |||
| Colostrum | 77 | 38 | |||
| Japan [88] | 107 pregnant women | Culture | Urine | 76 | 0 |
| Oral secretions | 107 | 0 | |||
| Genital secretions | 153 | 15 | |||
| Taiwan [71] | 350 pregnant women in the 2nd trimester, all CMV seropositive | PCR | Urine | 700 | 6 |
| Genital secretions | 440 | 21 | |||
| Taiwan [22] | 2219 CMV seropositive pregnant women, including multiple specimens from 207 women | PCR | Urine | 2195 | 7 |
| Genital secretions | 600 | 27 | |||
| US-Alabama [86] | 113 healthy, post-partum women, all CMV seropositive | PCR | Urine | 306 | 3 |
| Blood | 248 | 3 | |||
| US-Alabama [73] | 81 post-partum women, all CMV seropositive, over an average of 4 visits, with uninfected infants | Culture | Urine | 76 | 11 |
| Oral secretions | 78 | 9 | |||
| Genital secretions | 78 | 13 | |||
| US-Alabama [18] | 659 pregnant women | Culture | Urine | 600 | 5 |
| Genital secretions | 659 | 10 | |||
| 230 non-pregnant women | Urine | 230 | 3 | ||
| Genital secretions | 202 | 9 | |||
| Category V. Adults with CMV risk factors | |||||
| Italy [27] | 29 seroconverting pregnant women, 27 husbands, 22 children | Culture | Urine | 95 | 42 |
| Oral secretions | 36 | 14 | |||
| US-Alabama [73] | 142 post-partum women, all CMV seropositive, over an average of 4 visits, with infected infants | Culture | Urine | 141 | 45 |
| Oral secretions | 141 | 27 | |||
| Genital secretions | 142 | 23 | |||
| US-Alabama [54] | 6 adolescent seroconverters | Culture | Urine | 54 | 59 |
| Oral secretions | 58 | 14 | |||
| Genital secretions | 45 | 9 | |||
| Blood | 54 | 2 | |||
| PCR | Blood | 50 | 32 | ||
| Plasma | 50 | 14 | |||
| US-California [21] | 52 homosexual men in 1980–81, all CMV seropositive, HIV status unknown | Culture | Urine | 52 | 8 |
| Semen | 52 | 35 | |||
| US-New York [100] | 30 healthy homosexual men | Culture | Urine | 30 | 20 |
| Semen | 30 | 20 | |||
| US-Washington [20] | 951 CMV seropositive women presenting with problems at an STD clinic | Culture | Urine | 890 | 8 |
| Oral secretions | 169 | 2 | |||
| Genital secretions | 890 | 14 | |||
| Rectal secretions | 432 | 1 | |||
Table A2.
CMV shedding prevalence according to risk group, categorized by whether single specimens (SS) or multiple specimens (MS) were used and whether they were collected at a single time point (ST) or multiple time points (MT)a
| Country | Demographics/Sampling Method | Testing Method | Specimen Type(s) | Category | Sample size | Shedding % |
|---|---|---|---|---|---|---|
| Category I. Congenitally infected children | ||||||
| Sweden [59] | 35 congenitally infected infants, ages 3 months-4 years | Culture | Urine | SS, ST | 149 | 84 |
| US-Alabama [75] | 38 congenitally infected infants, ages 1–4 years | Culture | Urine | SS, ST | 69 | 87 |
| Category II. Healthy children enrolled in day care centers | ||||||
| England [98] | 117 children in day nurseries over a 1-year period, 2–4 specimens per year | Culture | Urine | SS, MT | 117 | 27 |
| France [65] | 93 children < 1 year old in day care centers | Culture | Urine | SS, ST | 379 | 24 |
| SS, MT | 93 | 36 | ||||
| Italy [69] | 253 children in day care centers | Culture | Oral secretions | SS, ST | 253 | 13 |
| Japan [64] | 54 children in day care centers | PCR | Oral secretions | SS, ST | 54 | 22 |
| Mexico [77] | 152 children at day care centers, sampled at 3 visits | Culture | Oral secretions | SS, ST | 127 | 7 |
| SS, ST | NS | 5 | ||||
| SS, ST | NS | 7 | ||||
| SS, MT | 152 | 11 | ||||
| Sweden [122] | 60 children in day care centers followed 12 weeks | Culture | Urine | SS, MT | 60 | 27 |
| Sweden [68] | 18 children in day care centers, ages 2–3.5 years, mean follow-up of 35 weeks | Culture | Urine | SS, ST | 221 | 55 |
| US-Alabama [72] | Children in day care centers | Culture | Urine, oral secretions | MS, ST | 243 | 32 |
| US-Alabama [97] | 188 children in day care centers | Culture | Urine, oral secretions | MS, ST | 188 | 41 |
| US-Alabama [14] | Children in day care centers | Culture | Urine, oral secretions | MS, ST | 71 | 51 |
| US-Alabama [56] | 103 children in day care centers, 1–3 visits each | Culture | Urine, oral secretions | MS, MT | 103 | 57 |
| US-California [63] | 100 children in day care centers | Culture | Urine, oral secretions | MS, ST | 90 | 22 |
| US-Iowa [66] | 48 children in day care centers | Culture | Urine, oral secretions | MS, ST | 80 | 21 |
| US-Iowa [67] | 79 children in day care centers followed for 2.5 years | Culture | Urine, oral secretions | MS, MT | 79 | 35 |
| US-Iowa [13] | 219 children in day care centers at entry | Culture | Urine, oral secretions | MS, ST | 219 | 15 |
| 219 children in day care centers followed for 2.5–4.5 years | Urine, oral secretions | MS, MT | 219 | 28 | ||
| US-Virginia [11] | 66 children in day care centers cultured 3 times | Culture | Urine | SS, MT | 66 | 24 |
| US-Virginia [123] | Children in day care centers at 2 time points | Culture | Urine, oral secretions | MS, ST | 118 | 44 |
| Category III. Healthy children not enrolled in day care centers | ||||||
| England [62] | Infants without congenital CMV | Culture | Urine | SS, ST | 983 | 12 |
| The Gambia [61] | 178 infants | Culture | Urine, oral secretions | MS, ST | 178 | 53 |
| MS, MT | 150 | 62 | ||||
| Japan [64] | 61 children not in day care centers | PCR | Oral secretions | SS, ST | 61 | 7 |
| Japan [88] | Infants and children ages 1 month-2 years | Culture | Oral secretions | SS, ST | 290 | 25 |
| Mauritius [58] | 121 healthy children | Culture* | Urine | SS, ST | 121 | 2 |
| Sweden [59] | 50 children without congenital CMV, ages 3 months-4 years | Culture | Urine | SS, ST | 225 | 31 |
| US-Alabama [57] | 87 healthy infants ages 9–15 months | Culture | Oral secretions | SS, ST | 87 | 7 |
| US-Alabama [56] | 25 children in home care | Culture | Urine, oral secretions | MS, ST | 25 | 8 |
| US-Iowa [60] | 106 children in child care homes | Culture | Urine | SS, ST | 106 | 8 |
| US-New York [32] | 200 healthy children | Culture | Urine | SS, ST | 200 | 1 |
| US-New York [30] | 38 healthy migrant children | Culture | Urine | SS, ST | 38 | 18 |
| Category IV. Children with medical conditions | ||||||
| Denmark [89] | 262 hospitalized infants, not congenitally infected | Culture | Urine | SS, ST | 262 | 4 |
| England [99] | Hospitalized children | Culture | Urine, oral secretions | MS, ST | 1395 | 3 |
| England [19] | 309 hospitalized patients, ages 2 months-14 years | Culture | Urine, oral secretions | MS, ST | 309 | 5 |
| Finland [31] | 356 hospitalized and outpatient children > 2 months of age | Culture | Urine | SS, ST | 356 | 26 |
| Mauritius [58] | 30 deaf children | Culture | Urine | SS, ST | 30 | 50 |
| 91 mentally retarded children | 91 | 18 | ||||
| Sweden [90] | 661 hospitalized infants | Culture | Urine | SS, ST | 661 | 9 |
| US-California [63] | 63 children in infant development centers | Culture | Urine, oral secretions | MS, ST | 50 | 22 |
| US-California [114] | 93 infants in the intensive care unit, cultured weekly until discharge | Culture | Urine | SS, MT | 93 | 14 |
| US-New York [32] | 100 hospitalized children | Culture | Urine | SS, ST | 100 | 1 |
| US-Texas [124] | 314 hospitalized children in chronic care | Culture | Oral secretions, urine, genital secretions | MS, ST | 314 | 9 |
| US-Virginia [11] | 2729 hospitalized children from newborns up to 18 years old | Culture | Urine | SS, ST | 2729 | 5 |
| V. Adults without CMV risk factors | ||||||
| Egypt [87] | 50 women with recurrent abortions | PCR | Serum | SS, ST | 50 | 12 |
| England [19] | 402 hospitalized patients, ages 15–60+ years old | Culture | Urine, oral secretions | MS, ST | 402 | 0 |
| France [91] | 231 semen donors | Culture | Semen | SS, ST | 635 | 1 |
| SS, MT | 231 | 1 | ||||
| PCR | SS, ST | 551 | 4 | |||
| SS, MT | 197 | 3 | ||||
| France [93] | 97 semen donors | Culture | Semen | SS, ST | 178 | 3 |
| SS, MT | 97 | 2 | ||||
| PCR | SS, ST | 178 | 6 | |||
| SS, MT | 97 | 5 | ||||
| The Gambia [61] | 178 post-partum women, average prevalence in ≥1 of 3 specimen types over 6 cross-sectional visits | Urine, oral secretions, breast milk | MS, ST | 178 | 12 | |
| Greece [92] | 113 men at an infertility clinic | Nested PCR | Semen | SS, ST | 113 | 7 |
| Italy [82] | 66 women receiving routine gynecological care | PCR | Genital secretions | SS, ST | 66 | 73 |
| Italy [85] | 123 patients with fever of unknown origin | PCR | Serum | SS, ST | 123 | 9 |
| Japan [80] | 953 blood donors | PCR | Blood | SS, ST | 953 | 3 |
| Japan [81] | 993 healthy pregnant women, CMV serostatus undetermined, first trimester | PCR | Genital secretions | SS, ST | 993 | 8 |
| Taiwan [83] | 29 post-partum women, CMV serostatus undetermined | PCR | Serum | SS, ST | 29 | 10 |
| Taiwan [71] | 350 pregnant women at 2 time points in the 2nd trimester, all CMV seropositive | PCR | Urine | SS, MT | 350 | 11 |
| Genital secretions | SS, MT | 220 | 30 | |||
| Taiwan [22] | 105 non-pregnant women from an infertility clinic | PCR | Genital secretions | SS, ST | 105 | 8 |
| Taiwan [95] | Men and women attending infertility clinic | DNA hybridization | Genital secretions | SS, ST | 246 | 34 |
| Semen | SS, ST | 248 | 34 | |||
| Turkey [76] | 135 pregnant women, CMV serostatus undetermined, first trimester | PCR | Genital secretions | SS, ST | 135 | 2 |
| US-Alabama [86] | 113 healthy, post-partum women, all CMV seropositive, mean of 4.9 specimens per woman | PCR | Urine, blood | MS, MT | 113 | 14 |
| Urine | SS, MT | 113 | 8 | |||
| Blood | SS, MT | 113 | 6 | |||
| US-Alabama [73] | 81 post-partum women, all CMV seropositive, over an average of 4 visits, with uninfected infants | Culture | Urine, Oral secretions, genital secretions | MS, MT | 81 | 23 |
| US-Massachusetts [94] | 241 male infertility clinic attendees | PCR | Semen | SS, ST | 241 | 9 |
| US-New York [70] | 181 HIV-negative, CMV-seropositive urban, minority women | Culture | Genital secretions | SS, ST | 181 | 4 |
| US-Ohio [121] | 710 CMV seropositive adolescent pregnant women cultured at each antenatal visit | Culture | Urine | SS, MT | 710 | 17 |
| US-Texas [124] | 43 nurses in chronic care | Culture | Oral secretions, urine, genital secretions | MS, ST | 43 | 2 |
| 76 therapists in chronic care | 76 | 3 | ||||
| 69 neonatal nurses | 69 | 3 | ||||
| US-Virginia [12] | 239 CMV seropositive day care workers, average of 3.4 culture sets per woman over two years | Culture | Urine, oral secretions | MS, ST | 239 | 7 |
| MS, MT | 239 | 11 | ||||
| US-Washington [125] | 120 critically ill immunocompetent patients, all CMV seropositive, 1954 samples, 3 samples collected per week, median of 11 samples per patient | PCR | Plasma | SS, MT | 120 | 33 |
| Category VI. Adults with CMV risk factors | ||||||
| Italy [82] | 142 women with abnormal cytology findings | PCR | Genital secretions | SS, ST | 142 | 63 |
| Malawi [126] | 19 KS patients and 58 first-degree family members | PCR | Urine, oral secretions | MS, ST | 77 | 53 |
| US-Alabama [73] | 142 post-partum women, all CMV seropositive, over an average of 4 visits, with infected infants | Culture | Urine, oral secretions, genital secretions | MS, MT | 142 | 56 |
| US-Alabama [79] | 52 women who were attending an STD clinic | PCR | Genital secretions | SS, ST | 52 | 33 |
| US-California [21] | 206 CMV seropositive homosexual men, HIV status unknown, followed for a mean of 14.3 months | Culture | Urine | SS, MT | 206 | 32 |
| US-New York [100] | 30 healthy homosexual men | Culture | Urine, semen | MS, ST | 30 | 37 |
| US-Washington [39] | 191 CMV seropositive women attending an STD clinic, most were symptomatic, mean of 2.7 follow-up visits | Culture | Genital secretions | SS, ST | 163 | 38 |
| SS, MT | 191 | 49 | ||||
| US-Washington [20] | 951 CMV seropositive women presenting with problems at an STD clinic | Culture | Urine, genital secretions | MS, ST | 951 | 17 |
From 26 studies, we identified 34 sample population prevalences measured in a single specimen type across multiple ages (Figure 2, Table A3). Among children born with congenital CMV infection (by definition 100% were shedding at birth), shedding prevalences steadily declined during the first 5 years of life (Figure 2, panel A), although it remained quite high in a few of the studies. Among children who were not congenitally infected, shedding prevalences generally peaked at 1–2 years of age and subsequently declined by the age of five (Figure 2, panels B–E), although this age pattern was less pronounced in healthy children not in day care centers (Figure 2, panel D). Results from the two studies reporting age and shedding among adolescents and adults (Table A3) [18,19] suggest that prevalence may decrease with age [18].
Figure 2.
Cytomegalovirus (CMV) shedding as a function of age for different populations of children. Each line represents results from a single study (listed in Table A1 [11,13,14,19,28–32, 55,56,59,62,63,66,69,72,75,78,88–90,96–99]). Black symbols show shedding in urine, white symbols show shedding in oral secretions, and gray symbols show shedding in other specimens. Midpoints were used for ages when an age interval was reported. When age was some value and greater (e.g. ≥7 years) the previous age interval was assumed and the midpoint was taken (e.g. if the previous interval was 1 year [6–7 years], then the interval 7–8 years would be assumed, with a midpoint of 7.5 years). Because of the small age-specific sample sizes and very fine time points (i.e. 1-month), Numazaki et al.[88] was averaged using 2-month intervals
Table A3.
CMV shedding prevalence according to agea
| Country | Demographics/Sampling Method | Testing Method | Specimen Type (s) | Ages | Samples | Shedding % |
|---|---|---|---|---|---|---|
| Category I. Congenitally infected children | ||||||
| England [78] | Congenitally infected children | Culture | Urine | 0–3 months | 48 | 92 |
| 4–12 months | 18 | 78 | ||||
| 1–2 years | 7 | 86 | ||||
| 2–3 years | 17 | 82 | ||||
| 3–4 years | 8 | 50 | ||||
| 4–5 years | 11 | 82 | ||||
| Oral secretions | 0–3 months | 48 | 92 | |||
| 4–12 months | 18 | 61 | ||||
| 1–2 years | 4 | 25 | ||||
| 2–3 years | 13 | 38 | ||||
| 3–4 years | 7 | 0 | ||||
| 4–5 years | 11 | 18 | ||||
| Sweden [59] | Congenitally infected children | Culture | Urine | < 1 week | 35 | 100 |
| 3 months | 29 | 97 | ||||
| 6 months | 26 | 92 | ||||
| 9 months | 22 | 95 | ||||
| 12 months | 27 | 81 | ||||
| 18 months | 21 | 67 | ||||
| 30 months | 17 | 71 | ||||
| 4 years | 7 | 57 | ||||
| US-Alabama [28] | Congenitally infected children | Culture | Urine | 0–6 months | 243 | 98 |
| 7–12 months | 103 | 91 | ||||
| 13–24 months | 101 | 86 | ||||
| 25–36 months | 71 | 85 | ||||
| 37–48 months | 69 | 72 | ||||
| 49–60 months | 53 | 51 | ||||
| 61–72 months | 42 | 33 | ||||
| 73–84 months | 28 | 39 | ||||
| ≥ 85 months | 62 | 18 | ||||
| Oral secretions | 0–6 months | 131 | 93 | |||
| 7–12 months | 59 | 73 | ||||
| 13–24 months | 71 | 39 | ||||
| 25–36 months | 50 | 38 | ||||
| 37–48 months | 42 | 19 | ||||
| 49–60 months | 37 | 16 | ||||
| 61–72 months | 27 | 7 | ||||
| 73–84 months | 22 | 0 | ||||
| ≥ 85 months | 45 | 4 | ||||
| US-Alabama [96] | Asymptomatic congenitally infected children | Culture | Urine and/or oral secretions | 2–3 years | 10 | 90 |
| 4–6 years | 8 | 13 | ||||
| US-Alabama [75] | Asymptomatic congenitally infected children | Culture | Urine | 1 year | 17 | 88 |
| 2 years | 22 | 91 | ||||
| 3 years | 18 | 89 | ||||
| 4 years | 12 | 75 | ||||
| Category II. Healthy children enrolled in day care centers | ||||||
| England [98] | Children in day care centers, shedding at some time during the study | Culture | Urine | < 2 years | 26 | 46 |
| ≥ 2 years | 79 | 25 | ||||
| Italy [69] | Children in day care centers | Culture | Oral secretions | 1 year | 59 | 16 |
| 2 years | 126 | 13 | ||||
| ≥ 3 years | 65 | 14 | ||||
| US-Alabama [72] | Children in day care centers | Culture | Urine | 0–12 months | 10 | 50 |
| 13–24 months | 53 | 36 | ||||
| 25–36 months | 52 | 33 | ||||
| 37–48 months | 52 | 29 | ||||
| > 48 months | 64 | 16 | ||||
| Oral secretions | 0–12 months | 11 | 27 | |||
| 13–24 months | 54 | 33 | ||||
| 25–36 months | 56 | 13 | ||||
| 37–48 months | 53 | 2 | ||||
| > 48 months | 67 | 4 | ||||
| US-Alabama [97] | Children in day care centers | Culture | Urine and/or oral secretions | 0–12 months | 10 | 0 |
| 13–24 months | 38 | 37 | ||||
| 25–36 months | 35 | 77 | ||||
| 37–48 months | 48 | 46 | ||||
| > 48 months | 57 | 25 | ||||
| US-Alabama [55] | Children in day care centers | Culture | Urine | < 12 months | 9 | 33 |
| 13–24 months | 7 | 71 | ||||
| 25–30 months | 8 | 63 | ||||
| 31–36 months | 13 | 23 | ||||
| 37–48 months | 18 | 33 | ||||
| Oral secretions | < 12 months | 7 | 28 | |||
| 13–24 months | 10 | 80 | ||||
| 25–30 months | 10 | 10 | ||||
| 31–36 months | 11 | 9 | ||||
| 37–48 months | 9 | 22 | ||||
| Hands | < 12 months | 6 | 16 | |||
| 13–24 months | 10 | 20 | ||||
| 25–30 months | 9 | 0 | ||||
| 31–36 months | 11 | 0 | ||||
| 37–48 months | 8 | 0 | ||||
| US-Alabama [14] | Children in day care centers | Culture | Urine and/or oral secretions | 0–12 months | 11 | 9 |
| 13–24 months | 18 | 83 | ||||
| 25–36 months | 16 | 63 | ||||
| 37–48 months | 13 | 23 | ||||
| 49–60 months | 12 | 58 | ||||
| US-Alabama [56] | Children in day care centers | Culture | Urine and/or oral secretions | 1 year | 29 | 9 |
| 1.5 years | 25 | 80 | ||||
| 2 years | 21 | 78 | ||||
| 3 years | 46 | 65 | ||||
| 4 years | 37 | 53 | ||||
| 5 years | 40 | 42 | ||||
| US-California [63] | Children in day care centers and infant developmental centers | Culture | Urine | 0–12 months | 33 | 21 |
| 13–24 months | 51 | 29 | ||||
| 25–36 months | 42 | 19 | ||||
| ≥ 37 months | 10 | 10 | ||||
| Oral secretions | 0–12 months | 36 | 13 | |||
| 13–24 months | 64 | 9 | ||||
| 25–36 months | 50 | 2 | ||||
| ≥ 37 months | 12 | 0 | ||||
| US-Iowa [66] | Children in day care centers | Culture | Urine and/or oral secretions | 0–9 months | 8 | 25 |
| 10–24 months | 13 | 62 | ||||
| 25–36 months | 17 | 24 | ||||
| 37–48 months | 17 | 18 | ||||
| 49–72 months | 25 | 0 | ||||
| Urine | 0–9 months | 8 | 25 | |||
| 10–24 months | 12 | 67 | ||||
| 25–36 months | 14 | 29 | ||||
| 37–48 months | 16 | 19 | ||||
| 49–72 months | 23 | 0 | ||||
| Oral secretions | 0–9 months | 8 | 25 | |||
| 10–24 months | 13 | 38 | ||||
| 25–36 months | 17 | 6 | ||||
| 37–48 months | 17 | 6 | ||||
| 49–72 months | 25 | 0 | ||||
| US-Iowa [13] | Children in day care centers | Culture | Urine and/or oral secretions | < 2 years | 74 | 22 |
| 2 years | 23 | 35 | ||||
| 3 years | 50 | 12 | ||||
| ≥ 4 years | 72 | 3 | ||||
| US-Virginia [11] | Children in day care centers | Culture | Urine | 0–2 years | 31 | 25 |
| 2–5 years | 34 | 20 | ||||
| Category III. Healthy children not enrolled in day care centers | ||||||
| England [62] | Infants not congenitally infected | Culture | Urine and/or oral secretions | 6 weeks | 253 | 4 |
| 3 months | 249 | 12 | ||||
| 8 months | 247 | 15 | ||||
| 1 year | 234 | 20 | ||||
| Japan [88] | Healthy children | Culture | Oral secretions | 1 month | 34 | 6 |
| 2 months | 29 | 10 | ||||
| 3 months | 20 | 20 | ||||
| 4 months | 21 | 24 | ||||
| 5 months | 18 | 56 | ||||
| 6 months | 16 | 44 | ||||
| 7 months | 19 | 42 | ||||
| 8 months | 14 | 36 | ||||
| 9 months | 9 | 44 | ||||
| 10 months | 7 | 0 | ||||
| 11 months | 10 | 10 | ||||
| 12 months | 9 | 22 | ||||
| 1 year | 15 | 7 | ||||
| 2 years | 6 | 0 | ||||
| Sweden [59] | Children not congenitally infected | Culture | Urine | < 1 week | 50 | 0 |
| 3 months | 42 | 24 | ||||
| 6 months | 43 | 35 | ||||
| 9 months | 35 | 31 | ||||
| 12 months | 36 | 33 | ||||
| 18 months | 36 | 33 | ||||
| 30 months | 22 | 27 | ||||
| 4 years | 11 | 36 | ||||
| US-New York [30] | Migrant worker children | Culture | Urine | 1–4 years | 14 | 36 |
| 4–8 years | 12 | 17 | ||||
| 8–13 years | 12 | 0 | ||||
| Category IV. Children with medical conditions | ||||||
| Denmark [89] | Hospitalized infants, not congenitally infected | Culture | Urine | < 2 months | 105 | 1 |
| 2–5 months | 76 | 8 | ||||
| 6–12 months | 81 | 5 | ||||
| England [99] | Hospitalized children | Culture | Urine and/or oral secretions | < 3 months | 508 | 2 |
| 3–5 months | 220 | 3 | ||||
| 6–11 months | 207 | 6 | ||||
| 1–4 years | 398 | 4 | ||||
| > 4 years | 62 | 2 | ||||
| England [19] | Hospitalized patients | Culture | Urine and/or oral secretions | 2–5 months | 32 | 9 |
| 6 months - 4 years | 104 | 10 | ||||
| 5–9 years | 101 | 1 | ||||
| 10–14 years | 72 | 0 | ||||
| Finland [31] | Hospitalized and outpatient children | Culture | Urine | 2 months | 43 | 12 |
| 3 months | 30 | 23 | ||||
| 4 months | 26 | 23 | ||||
| 5–6 months | 34 | 35 | ||||
| 7–9 months | 32 | 25 | ||||
| 10–12 months | 18 | 33 | ||||
| 13–18 months | 35 | 34 | ||||
| 19–24 months | 39 | 41 | ||||
| 25–36 months | 30 | 33 | ||||
| 37–48 months | 20 | 20 | ||||
| 5–15 years | 49 | 14 | ||||
| Sweden [90] | Hospitalized infants | Culture | Urine | ≤ 4 weeks | 394 | 1 |
| 5–9 weeks | 52 | 12 | ||||
| 2–11 months | 215 | 23 | ||||
| US-New York [32] | Healthy children and children with chronic conditions | Culture | Urine | 0–2 years | 52 | 2 |
| 2–6 years | 148 | 8 | ||||
| 6–19 years | 145 | 3 | ||||
| US-Virginia [11, 29] | Hospitalized children | Culture | Urine | Newborns | 551 | 3 |
| 0–2 years | 544 | 7 | ||||
| 2–5 years | 913 | 7 | ||||
| 5–12 years | 476 | 4 | ||||
| 12–18 years | 245 | 1 | ||||
| Category V. Adults without CMV risk factors | ||||||
| England [19] | Hospitalized patients | Culture | Urine and/or oral secretions | 15–24 years | 102 | 0 |
| 25–34 years | 100 | 0 | ||||
| 35–59 years | 100 | 0 | ||||
| ≥ 60 years | 100 | 0 | ||||
| US-Alabama [18] | Pregnant women | Culture | Urine | ≤ 14 years | 38 | 8 |
| 15–20 years | 306 | 6 | ||||
| 21–25 years | 123 | 4 | ||||
| 26–30 years | 44 | 0 | ||||
| ≥ 31 years | 29 | 0 | ||||
| Genital secretions | ≤ 14 years | 30 | 17 | |||
| 15–20 years | 372 | 11 | ||||
| 21–25 years | 111 | 11 | ||||
| 26–30 years | 41 | 10 | ||||
| ≥ 31 years | 30 | 0 | ||||
Some of the results from this table are listed in previous tables without stratifying by age.
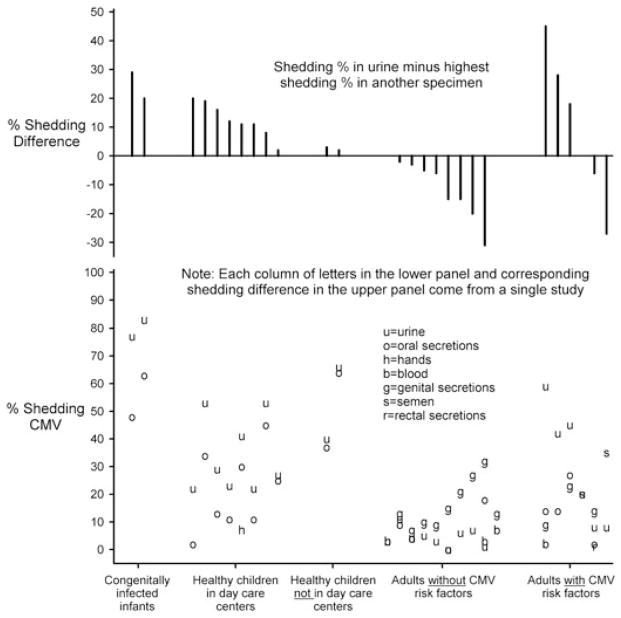
We identified 23 studies with 28 sets of sample population prevalences (Table A1) where CMV shedding was compared in more than one specimen type. Among children, CMV shedding was most commonly detected in urine specimens (Figure 3) but was also prevalent in oral secretions (median prevalence difference = 11.5%, N = 12). In adults without risk factors for CMV shedding (N = 10), genital shedding was most common (although oral secretions were often not tested) (Figure 3). Adults with risk factors for CMV infection (N = 6) most often shed virus in urine, although in two studies [20,21] shedding in other specimen types was more frequent (Figure 3). Studies comparing shedding in multiple specimen types did not include certain specimens, such as semen, blood, and rectal secretions, frequently enough to provide insights into their relative shedding frequencies. In general, however, shedding prevalences in blood were lower than prevalences in other specimen types.
Figure 3.
Comparison of cytomegalovirus (CMV) shedding from different bodily fluids and locations according to risk group. Studies were only included if they measured CMV shedding in multiple specimen types. The lower panel compares shedding in different specimens (e.g. urine vs. oral secretions). Each column of letters comes from a single study (listed in Table A2 [14,18,20–22,27,28,54–56,61,63,66,67,71–74,78,84,86,88,100]). Directly above each column of letters, in the top panel, the corresponding difference is shown between shedding percentage in urine and the highest shedding percentage in another specimen
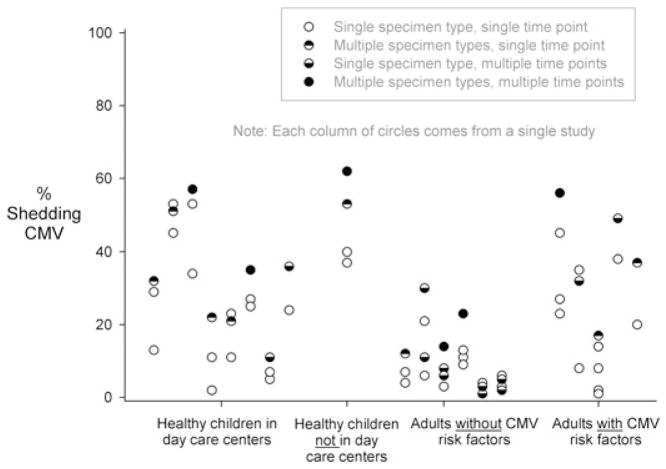
Figure 4 displays the shedding data from 18 studies and 20 sets of sample population prevalences that measured shedding in multiple specimen types and/or multiple study visits. These data demonstrate, unsurprisingly, that if shedding is assessed in multiple specimen types at multiple times it is usually more likely to be found.
Figure 4.
Prevalences of cytomegalovirus (CMV) shedding in various studies that measured not only a single specimen type at a single time point but also multiple specimen types and/or multiple time points. Each column represents results from a single study (listed in Table A3 [14,20,21,39,56,61,63,65–67,71–73,77,86,91,93,100]). Multiple empty circles from a single study occur when CMV shedding was measured separately in more than one specimen type (e.g. one empty circle for shedding in urine, one empty circle for shedding in oral specimens). This figure demonstrates that CMV shedding in individuals is more prevalent if multiple specimen types and/or multiple time points are assessed
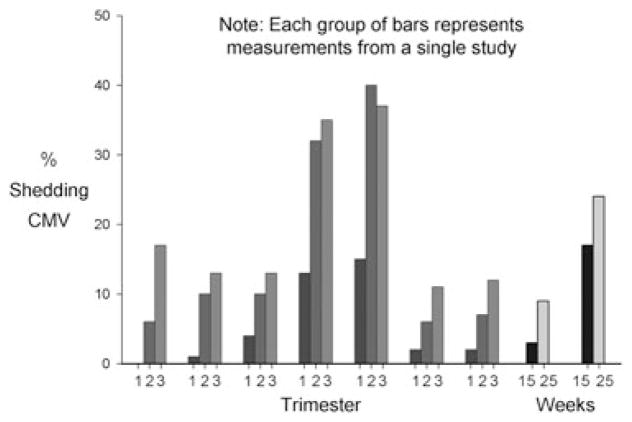
From five studies, we identified nine sets of sample population prevalences measured in a single specimen type at different times during pregnancy (Table A4). Two of these sets came from the same women followed longitudinally throughout pregnancy [22]. The other sets came from cross-sectional samples of women at different times during pregnancy. The prevalence of CMV shedding in pregnant women increased generally with advancing gestation, reaching peak prevalences in the second and third trimesters (Figure 5). Two of the studies had non-pregnant control groups. In one of them [18], shedding was less frequent in the first and second trimesters but equally frequent in the third trimester for the pregnant women compared with the non-pregnant controls. In the other study [22], shedding was more frequent in every trimester for the pregnant women compared with the non-pregnant controls.
Table A4.
CMV shedding among pregnant women according to time of gestation.
| Country | Testing Method | Specimen Type | Time of Gestation | Sample Size | Shedding % |
|---|---|---|---|---|---|
| Japan [88] | Culture | Genital secretions | 1st trimester | 30 | 0 |
| 2nd trimester | 62 | 6 | |||
| 3rd trimester | 61 | 17 | |||
| Taiwan [71] | PCR | Urine | 15 weeks | 350 | 3 |
| 25 weeks | 350 | 9 | |||
| Genital secretions | 15 weeks | 220 | 17 | ||
| 25 weeks | 220 | 24 | |||
| Taiwan [22] | PCR | Urine (longitudinal collection) | 1st trimester | 207 | 1 |
| 2nd trimester | 207 | 10 | |||
| 3rd trimester | 207 | 13 | |||
| Urine (cross-sectional collection) | 1st trimester | 906 | 4 | ||
| 2nd trimester | 395 | 10 | |||
| 3rd trimester | 273 | 13 | |||
| Genital secretions (longitudinal collection) | 1st trimester | 54 | 13 | ||
| 2nd trimester | 54 | 32 | |||
| 3rd trimester | 54 | 35 | |||
| Genital secretions (cross-sectional collection) | 1st trimester | 217 | 15 | ||
| 2nd trimester | 140 | 40 | |||
| 3rd trimester | 81 | 37 | |||
| US-Alabama [18] | Culture | Genital secretions | 1st trimester | 183 | 2 |
| 2nd trimester | 359 | 6 | |||
| 3rd trimester | 317 | 11 | |||
| US-Pennsylvania [101] | Culture | Genital secretions | 1st trimester | 43 | 2 |
| 2nd trimester | 83 | 7 | |||
| 3rd trimester | 49 | 12 |
Figure 5.
Prevalence of cytomegalovirus (CMV) shedding among pregnant women according to time of gestation. Each group of bars represents results from a single study (listed in Table A4 [18,22,49,71,88,101]). Two studies involved longitudinal specimen collection from the same women, the rest of the studies used cross-sectional specimen collection from women having various times of gestation. Where no bar is apparent, the prevalence is zero
We identified four studies (Table 1) outside of the newborn period that assessed differences in CMV viral load by specimen type. No clear differences in viral load were present for the specimen types examined. Because of differences in how viral loads were measured, it was impossible to assess whether viral loads differed according to specimen type. Studies which compared different specimen types in the newborn period (excluded from this review) have found higher viral loads in urine than in blood [23,24].
Table 1.
CMV viral load according to risk group and specimen type
| Country | Demographics/sampling method | Testing method | Specimen type(s) | Sample size | Viral load |
|---|---|---|---|---|---|
| The Gambia [61] | Post-partum women | Culture | Urine | 32 | 3.8 log10 TCID50/mL |
| Oral secretions | 34 | 3.2 log10 TCID50/mL | |||
| Breast milk | 21 | 1.4 log10 TCID50/mL | |||
| Infants at 6 months | Urine | 71 | 3.4 log10 TCID50/mL | ||
| Oral secretions | 66 | 3.7 log10 TCID50/mL | |||
| Japan [108] | Congenitally and postnatally infected newborns | PCR | Urine | 14 | Median = ~107 ge/mL |
| Dried umbilical cord | 30 | Median = ~103 ge/μg cellular DNA | |||
| US-Alabama [104] | Congenitally infected newborns | ||||
| Asymptomatic with hearing loss | Culture | Urine | 4 | Mean = 1.6 × 105 pfu/mL | |
| PCR | Blood | 4 | Mean = 8.7 × 105 ge/mL | ||
| Asymptomatic with normal hearing | Culture | Urine | 54 | Mean = 2.9 × 104 pfu/mL | |
| PCR | Blood | 54 | Mean = 1.1 × 104 ge/mL | ||
| Symptomatic with hearing loss | Culture | Urine | 8 | Mean = 4.9 × 105 pfu/mL | |
| PCR | Blood | 8 | Mean = 1.1 × 105 ge/mL | ||
| Symptomatic with normal hearing | Culture | Urine | 10 | Mean = 3.8 × 104 pfu/mL | |
| PCR | Blood | 10 | Mean = 6.2 × 105 ge/mL | ||
| US-Iowa [66] | Children in day care centers | Culture | Urine | 4 | Mean = 1.7 × 103 pfu/mL |
| Oral secretions | 2 | Mean = 2.3 × 104 pfu/mL |
CMV, cytomegalovirus; PCR, polymerase chain reaction; TCID50, median tissue culture infectious dose; ge, genome equivalents; pfu, plaque forming units; mL, milliliter; μg, micrograms.
In general, the risk for developing SNHL among congenitally infected newborns was strongly and consistently associated with higher viral load measured at birth (Figure 6, Table A5). The six studies used different methods to determine viral load and set variable cut-offs for categorizing viral load. As such, direct comparisons of these studies may be difficult. Nevertheless, the risk for SNHL was not zero among newborns with low viral loads in most of the studies. Higher CMV viral load at birth also correlated with symptoms of congenital CMV at birth (Table 2). Although differences in mean viral load tended to be no more than 1–2 logs, they were consistent across all five studies.
Figure 6.
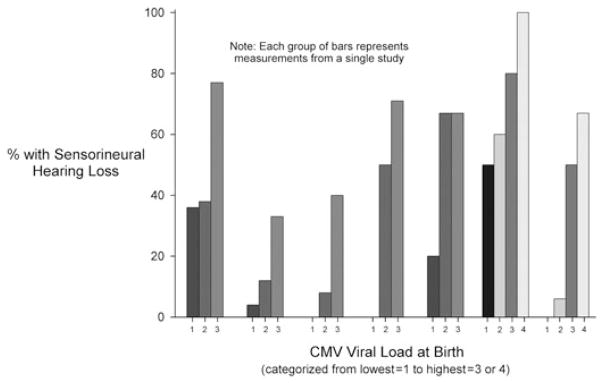
Frequency of sensorineural hearing loss among congenitally infected children according to cytomegalovirus (CMV) viral load at birth. Each group of bars represents measurements from a single study (listed in Table A5 [102–107]). In one study, [104] viral load was measured in urine and blood, so two sets of bars are presented. Viral load categories (i.e. cut-points) varied across different studies and are not necessarily comparable (see Table A5). Those used here correspond to the categorizations chosen by the authors of the different studies. Where no bar is apparent, the prevalence is zero
Table A5.
Frequency of sensorineural hearing loss among congenitally infected children according to CMV viral load at birth
| Country | Testing Method | Specimen Type(s) | Viral Load | Sample Size | % with Sensorineural Hearing Loss |
|---|---|---|---|---|---|
| Belgium [107] | PCR | Urine | < 3 log ge/mL | 11 | 36 |
| 3.0–4.5 log ge/mL | 8 | 38 | |||
| >4.5 log ge/mL | 13 | 77 | |||
| England [106] | PCR | Dried blood spots | Negative | 10 | 50 |
| 2–2.5 log ge/mL | 5 | 60 | |||
| 2.5–3 log ge/mL | 10 | 80 | |||
| >3 log ge/mL | 9 | 100 | |||
| Italy [105]a | PCR | Blood | < 102 ge/105 PBMCs | 4 | 0 |
| 102–103 ge/105 PBMCs | 16 | 6 | |||
| 103–104 ge/105 PBMCs | 14 | 50 | |||
| > 104 ge/105 PBMCs | 3 | 67 | |||
| US-Alabama [104] | Culture | Urine | < 3500 pfu/mL | 26 | 4 |
| 3500–25000 pfu/mL | 26 | 12 | |||
| >25000 pfu/mL | 24 | 33 | |||
| PCR | Blood | < 3500 ge/mL | 25 | 0 | |
| 3500–25000 ge/mL | 25 | 8 | |||
| >25000 ge/mL | 25 | 40 | |||
| US-Alabama [102] | Culture | Urine | < 5000 pfu/mL | 6 | 0 |
| 5000–50000 pfu/mL | 8 | 50 | |||
| >50000 pfu/mL | 7 | 71 | |||
| US-Alabama, Arkansas, Texas [103] | PCR | Serum | < 200 ge/mL | 5 | 20b |
| 200–5400 ge/mL | 15 | 67b | |||
| >5400 ge/mL | 9 | 67b |
All sequelae rather than sensorineural hearing loss only.
At 12 months follow-up.
ge, genome equivalents; pfu, plaque forming units; mL, milliliter; PBMC, peripheral blood mononuclear cells.
Table 2.
CMV viral load among congenitally infected newborns according to presence or absence of symptoms
| Country | Testing method | Specimen type(s) | Symptoms | Sample size | Viral load |
|---|---|---|---|---|---|
| China [109] | PCR | Urine | Asymptomatic | 25 | Median = 4.50 × 103 ge/mL |
| Symptomatic | 54 | Median = 2.95 × 105 ge/mL | |||
| Italy [105] | PCR | Blood | Asymptomatic | 22 | Log mean = 2.79 ge/105 PBMC |
| Symptomatic | 17 | Log mean = 3.24 ge/105 PBMC | |||
| Italy [110] | Culture | Blood | Asymptomatic | 32 | Median = 0 p72 + fibroblasts/2 × 105 PBMC |
| Symptomatic | 9 | Median = 1.5 p72 + fibroblasts/2 × 105 PBMC | |||
| PCR | Asymptomatic | 32 | Median = 30 ge/105 PBMC | ||
| Symptomatic | 9 | Median = 3000 ge/105 PBMC | |||
| US-Alabama [104] | Culture | Urine | Asymptomatic | 58 | Mean = 3.9 × 104 pfu/mL |
| Symptomatic | 18 | Mean = 2.4 × 105 pfu/mL | |||
| PCR | Blood | Asymptomatic | 58 | Mean = 8.2 × 104 ge/mL | |
| Symptomatic | 18 | Mean = 4.0 × 105 ge/mL | |||
| US-Alabama [28] | Culture | Urine | Asymptomatic | 71 | Mean log TCID50 = 3.82 |
| Symptomatic | 33 | Mean log TCID50 = 4.61 |
CMV, cytomegalovirus; PCR, polymerase chain reaction; TCID50, median tissue culture infectious dose; ge, genome equivalents; pfu, plaque forming units; mL, milliliter; PBMC, peripheral blood mononuclear cells.
Studies of the duration of CMV shedding were carried out in limited situations with highly variable follow-up frequency and duration (Table 3). Consequently, it is not surprising that results varied widely. In general, congenitally infected infants and healthy children often shed for months or years. Among adult seroconverters, shedding typically continued for several months but had usually ceased within half a year. In several studies, shedding was intermittent among children and adults.
Table 3.
Duration of CMV shedding
| Country | Demographics/sampling method | Testing method | Specimen type(s) | Shedding duration | ||||
|---|---|---|---|---|---|---|---|---|
| Category I. Congenitally infected children | ||||||||
| England [62] | 25 congenitally infected infants | Culture | Urine, oral secretions | 96% were shedding at 30 months of age | ||||
| Italy [111] | 14 congenitally infected infants | PCR | Blood | 1–7 days | 8–90 days | 91–180 days | > 180 days | |
| 100% | 93% | 63% | 40% | |||||
| US-New York [112] | 20 congenitally infected children | Culture | Urine | 100% were still shedding through at least 1 year of age | ||||
| US-Ohio [113] | 15 congenitally infected children | Culture | Urine | 73% were still shedding at 4 years of age | ||||
| Category II. Healthy children enrolled in day care centers | ||||||||
| Sweden [68] | 13 children in day care centers who were shedding | Culture | Urine | Virus shedding continued throughout follow-up (6 months–1 year) | ||||
| US-Iowa [67] | 79 children in day care centers | Culture | Urine | Mean duration of shedding = 13 months | ||||
| Oral secretions | Mean duration of shedding = 7 months | |||||||
| Category III. Healthy children not enrolled in day care centers | ||||||||
| Finland [31] | 39 hospitalized and outpatient children who were shedding | Culture | Urine | 29 children always shed during a mean of 10 months follow-up, 10 children shed intermittently | ||||
| Japan [88] | 17 healthy children who were shedding | Culture | Oral secretions | 15 stopped shedding within 12 months; most shed for 3–9 months. | ||||
| Sweden [90] | 27 children who were shedding but who were not infected congenitally | Culture | Urine | Up to 2 years of age, 98% of samples were positive | ||||
| Oral secretions | Up to 2 years of age, 84% of samples were positive | |||||||
| US-California [114] | 13 infants in ICU who were shedding | Culture | Urine | All infants shed weekly for the duration of their hospital stay or the duration of the study (duration of follow-up not shown) | ||||
| Category IV. Seroconverters | ||||||||
| Austria [115] | 48 immunocompetent adult seroconverters | Culture | Serum | Duration of shedding less than approximately 90 days for all (estimated from Figure 1C of [115]) | ||||
| Italy [116] | 35 IgM + or indeterminate adults, presumed seroconverters | Culture | Blood | Up to 120 days, shedding prevalence > 50%; after 150 days, shedding prevalence = approximately 33% (estimated from Figure 5 of Ref. [116]) | ||||
| Italy [117] | 52 immunocompetent seroconverters (including 40 pregnant women) | Blood | 1–30 days | 31–60 days | 61–90 days | 91– 180 days | >180 days | |
| Culture | (by specimen) | 21% | 0 | 0 | 0 | 0 | ||
| (by patient) | 26% | 0 | 0 | 0 | 0 | |||
| PCR | (by specimen) | 100% | 81% | 39% | 17% | 0 | ||
| (by patient) | 100% | 89% | 47% | 27% | 0 | |||
| Italy [111] | 32 seroconverting pregnant women | PCR | Blood | 4–30 days | 31–60 days | 61–90 days | 91– 180 days | >180 days |
| 100% | 71% | 46% | 30% | 8% | ||||
| Italy [118] | Culture | Blood | Mean ge/10 μL (estimated from Figure 1 of [118]) for different numbers of days after seroconversion | |||||
| 20 days | 70 days | 150 days | ||||||
| 74 seroconverting pregnant women | 45 ge | 3 ge | 0 ge | |||||
| 16 seroconverting men and 13 seroconverting non-pregnant women | 42 ge | 2 ge | 0 ge | |||||
| US-Alabama [119] | 23 seroconverting post-partum women | Culture | Urine, oral secretions, genital secretions | Median time from seroconversion to shedding = 2 weeks, range = 0–12 weeks. All shed CMV from at least one site at their subsequent visits, with follow-up as long as 3.5 years | ||||
| US-California [21] | 22 seroconverting homosexual men | Culture | Urine | 27% shed at some time during follow-up, mean follow-up of 9.3 months | ||||
| US-Washington [40] | 36 seroconverting women seen at an STD clinic | Culture | Urine, genital secretions | Median of shedding = 240 days for 14 women shedding from the cervix only | ||||
| Median of shedding = 70 days for four women shedding in urine and cervix | ||||||||
| Category V. Adolescents who were shedding CMV | ||||||||
| US-Alabama [120] | 18 adolescent women who were shedding | Culture | Primarily genital secretions | Over 4 years of follow-up, 17% shed at every visit, 67% shed intermittently, and 17% never shed again | ||||
| US-Ohio [121] | 121 adolescent pregnant women who were shedding | Culture | Urine | In the majority, shedding was intermittent over a period of several months. | ||||
CMV, cytomegalovirus; PCR, polymerase chain reaction; ge, genome equivalents.
DISCUSSION
Studies of CMV shedding in various populations have provided valuable insights into the risk of acquiring CMV infection among women of reproductive age. This comprehensive analysis of CMV shedding among a variety of age groups, demographics and social settings, supports other findings that suggest that exposure to young children, especially those ages 1–2 years, poses a great risk for CMV infection [25–27]. Among young children shedding prevalences were lowest among infants less than 1-year-old. Prevalence of CMV shedding was uniformly high among 1-and 2-year-old children, whether they were enrolled in day care centers with large numbers of children or in home care. Thereafter, the prevalence of shedding declined markedly, although still continuing at relatively high levels among 3–4-year-olds. In the several studies that examined children older than five [11,19,28–32], shedding prevalences were similar to those observed in adults (5–10%). Children in day care studied at the University of Alabama at Birmingham had higher prevalences of shedding overall than children in day care studied elsewhere (Figure 1, panels B and C), which may reflect differences in the underlying study populations such as race, socioeconomic status, or mother’s serostatus. One notable difference in shedding patterns occurred among children with serious medical conditions, where in several studies the prevalence of shedding stayed at low levels throughout childhood (Figure 2, panel E), perhaps reflecting fewer opportunities for exposure.
Children ages 1–2 years may be a key transmission risk not only because of their high shedding prevalences, but because they are more mobile than younger children and are more likely than older children to spread urine or oral secretions to others because they are not yet toilet trained (e.g. still in diapers) and are more likely to drool (e.g. because of teething). Thus, caregivers who are pregnant or planning a pregnancy should make extra efforts to follow hygienic precautions with 1–2-year-olds [33,34]. Furthermore, providers in day care centers may have lower risk when caring for infants or for older children.
Among children, CMV shedding occurs most frequently in urine but is also commonly found in oral secretions. These studies support the existing consensus that pregnant women should avoid getting urine or saliva in their eyes, nose, or mouth [35–38]. Based on these data, sensible prevention activities for pregnant women would include hand washing after diaper changes or wiping a child’s nose or face and avoiding saliva when kissing young children and not sharing food, drink, utensils, or towels.
Adults typically shed CMV less frequently than children, but their prevalence of shedding is rarely zero. Furthermore, contrary to what is sometimes stated in the literature, seroconverting adults shed for many months rather than weeks. Among adults, genital secretions are a common fluid for CMV shedding, consistent with other studies that identified sexual risk factors for CMV seropositivity or seroconversion [25,39–43]. However, adults also shed CMV in oral secretions, suggesting that CMV transmission among adults can occur through kissing or oral sex. Unfortunately, it may be difficult if not impossible to assess the relative risk of these frequently co-occurring intimate behaviors. This presents a challenge for advising women on precautions to take with intimate partners during pregnancy. At a minimum, it would be prudent to limit new sex partners during pregnancy and to use a condom with new partners, even though these practices would not eliminate the risk for oral transmission. In many cases, the risk from regular partners may be less because they typically share the same CMV serostatus—for example, in one very large study in Belgium only 20% of seronegative women had a seropositive partner [26].
Among the different risk groups, children born with congenital CMV infection displayed the highest prevalences of subsequent CMV shedding (Figures 1 and 2). However, a substantial proportion of healthy children also shed CMV, reinforcing the notion that children with congenital CMV do not pose a special risk in day care or classroom settings and should not be excluded or treated differently.
Although the risk of CMV exposure for women of reproductive age may be elevated when their child attends day care, the risk is by no means eliminated for women who care for their children at home. About 20% of children in day care centers are shedding CMV, but about 10% of children not in day care centers are also shedding (Figure 2). As such, pregnant women should follow hygienic precautions whether or not their young children attend day care centers.
Studies that only measure CMV shedding in one specimen type at one point in time invariably underestimate the number of individuals who are shedders. This is because CMV shedding is frequently intermittent [44] and because CMV is shed in some specimen types more frequently than others. Thus, the shedding prevalences in the different risk groups (Figure 1) should be treated as minimum estimates of the prevalence of individuals shedding in bodily fluids. Furthermore, care must be taken when comparing shedding prevalences across studies, because some may present results from a single specimen and time point whereas others combine results from multiple specimens or time points.
Viral shedding was more common among pregnant women as gestation progressed for all five studies that have examined this phenomenon (Table A4). It is not clear why this phenomenon occurs, but because there is no apparent reason why exposure risk should increase with advancing gestation, it may be that this trend is related to a possible altered state of immunity during pregnancy [45,46], which could increase the likelihood of CMV primary infection, reactivation, or reinfection, as well as the duration of viral shedding [47–49].
Approximately from 10% to 15% of congenitally infected children who have asymptomatic infections at birth will develop disabilities within 2 years, most often SNHL [4,50]. It does not appear that CMV shedding or viral load measured after the newborn period is a strong predictor of SNHL [44,51]. In contrast, the observation that the highest viral loads at birth are consistently associated with the greatest risk for developing hearing impairments should be useful for clinical prognoses. However, the positive predictive value of high viral load for SNHL is probably only 30%–80% whereas the negative predictive value of low viral load probably ranges from 50% to 100% depending on the chosen assay cut-off (Figure 6). Nevertheless, all congenitally infected children would probably benefit from regular follow-up audiological testing, but such follow up would be especially important for children with high viral loads at birth.
The findings from CMV shedding studies tended to be consistent with serologic studies that addressed risk factors for CMV transmission [25,52]. Nonetheless, the studies we reviewed had significant differences in study design, population demographics, number of participants studied, and other factors that limited the conclusions we could draw. The majority of studies used viral culture to isolate infectious virus. However, for those studies that used PCR, there was no way to know how well the presence of viral DNA correlated with the presence of infectious virus in any given bodily fluid. Additional studies are needed in a household setting where much child- to-mother transmission is likely to occur [15,53]. Finally, studies comparing a broad selection of clinical specimens (urine, saliva, blood in children; urine, saliva, blood, genital secretions, semen in adults) at multiple time points are needed to better understand and prevent CMV transmission to pregnant women.
Acknowledgments
We thank the European Congenital Cytomegalovirus Initiative (ECCI) for proposing and encouraging this review.
Abbreviations used
- MSM
men who have sex with men
- SES
socioeconomic status
- SNHL
sensory neural hearing loss
- STD
sexually transmitted disease
Footnotes
CONFLICT OF INTEREST
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Supporting information may be found in the online version of this article.
References
- 1.Mocarski ES, Jr, Shenk T, Pass RF. Cytomegaloviruses. In: Knipe DM, Howley PM, editors. Fields’ Virology. 5. Lippincott Williams & Wilkins; Philadelphia: 2007. pp. 2702–2772. [Google Scholar]
- 2.Kenneson A, Cannon MJ. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Reviews in Medical Virology. 2007;17:253–276. doi: 10.1002/rmv.535. [DOI] [PubMed] [Google Scholar]
- 3.Demmler GJ Infectious Diseases Society of America and Centers for Disease Control. Summary of a workshop on surveillance for congenital cytomegalovirus disease. Reviews of Infectious Diseases. 1991;13:315–329. doi: 10.1093/clinids/13.2.315. [DOI] [PubMed] [Google Scholar]
- 4.Dollard SC, Grosse SD, Ross DS. New estimates of the prevalence of neurological and sensory sequelae and mortality associated with congenital cytomegalovirus infection. Reviews in Medical Virology. 2007;17:355–363. doi: 10.1002/rmv.544. [DOI] [PubMed] [Google Scholar]
- 5.Boppana SB, Rivera LB, Fowler KB, Mach M, Britt WJ. Intrauterine transmission of cytomegalovirus to infants of women with preconceptional immunity. The New England Journal of Medicine. 2001;344:1366–1371. doi: 10.1056/NEJM200105033441804. [DOI] [PubMed] [Google Scholar]
- 6.Fowler KB, Stagno S, Pass RF, Britt WJ, Boll TJ, Alford CA. The outcome of congenital cytomegalovirus infection in relation to maternal antibody status. The New England Journal of Medicine. 1992;326:663–667. doi: 10.1056/NEJM199203053261003. [DOI] [PubMed] [Google Scholar]
- 7.Staras SAS, Dollard SC, Radford KW, Flanders WD, Pass RF, Cannon MJ. Seroprevalence of cytomegalovirus infection in the United States, 1988–1994. Clinical Infectious Diseases. 2006;43:1143–1151. doi: 10.1086/508173. [DOI] [PubMed] [Google Scholar]
- 8.Pass RF, Zhang C, Evans A, et al. Vaccine prevention of maternal cytomegalovirus infection. The New England Journal of Medicine. 2009;360:1191–1199. doi: 10.1056/NEJMoa0804749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schleiss MR. Role of breast milk in acquisition of cytomegalovirus infection: recent advances. Current Opinion in Pediatrics. 2006;18:48–52. doi: 10.1097/01.mop.0000192520.48411.fa. [DOI] [PubMed] [Google Scholar]
- 10.Hamprecht K, Maschmann J, Jahn G, Poets CF, Goelz R. Cytomegalovirus transmission to preterm infants during lactation. Journal of Clinical Virology. 2008;41:198–205. doi: 10.1016/j.jcv.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 11.Adler SP. The molecular epidemiology of cytomegalovirus transmission among children attending a day care center. The Journal of Infectious Diseases. 1985;152:760–768. doi: 10.1093/infdis/152.4.760. [DOI] [PubMed] [Google Scholar]
- 12.Adler SP. Cytomegalovirus and child day care. Evidence for an increased infection rate among day-care workers. The New England Journal of Medicine. 1989;321:1290–1296. doi: 10.1056/NEJM198911093211903. [DOI] [PubMed] [Google Scholar]
- 13.Murph JR, Baron JC, Brown CK, Ebelback CL, Bale JF. The occupational risk of cytomegalovirus infection among day-care providers. The Journal of the American Medical Association. 1991;265:603–608. [PubMed] [Google Scholar]
- 14.Pass RF, August AM, Dworsky M, Reynolds DW. Cytomegalovirus infection in a day-care center. The New England Journal of Medicine. 1982;307:477–479. doi: 10.1056/NEJM198208193070804. [DOI] [PubMed] [Google Scholar]
- 15.Pass RF, Hutto C, Ricks R, Cloud GA. Increased rate of cytomegalovirus infection among parents of children attending day-care centers. The New England Journal of Medicine. 1986;314:1414–1418. doi: 10.1056/NEJM198605293142204. [DOI] [PubMed] [Google Scholar]
- 16.Pass RF, Little EA, Stagno S, Britt WJ, Alford CA. Young children as a probable source of maternal and congenital cytomegalovirus infection. The New England Journal of Medicine. 1987;316:1366–1370. doi: 10.1056/NEJM198705283162203. [DOI] [PubMed] [Google Scholar]
- 17.Revello MG, Gerna G. Diagnosis and management of human cytomegalovirus infection in the mother, fetus, and new-born infant. Clinical Microbiology Reviews. 2002;15:680–715. doi: 10.1128/CMR.15.4.680-715.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stagno S, Reynolds D, Tsiantos A, et al. Cervical cytomegalovirus excretion in pregnant and nonpregnant women: suppression in early gestation. The Journal of Infectious Diseases. 1975;131:522–527. doi: 10.1093/infdis/131.5.522. [DOI] [PubMed] [Google Scholar]
- 19.Stern H. Isolation of cytomegalovirus and clinical manifestations of infection at different ages. British Medical Journal. 1968;1:665–669. doi: 10.1136/bmj.1.5593.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collier AC, Handsfield HH, Ashley R, et al. Cervical but not urinary excretion of cytomegalovirus is related to sexual activity and contraceptive practices in sexually active women. The Journal of Infectious Diseases. 1995;171:33–38. doi: 10.1093/infdis/171.1.33. [DOI] [PubMed] [Google Scholar]
- 21.Mintz L, Drew WL, Miner RC, Braff EH. Cytomegalovirus infections in homosexual men. An epidemiological study. Annals of Internal Medicine. 1983;99:326–329. doi: 10.7326/0003-4819-99-3-326. [DOI] [PubMed] [Google Scholar]
- 22.Shen CY, Chang SF, Yen MS, Ng HT, Huang ES, Wu CW. Cytomegalovirus excretion in pregnant and nonpregnant women. Journal of Clinical Microbiology. 1993;31:1635–1636. doi: 10.1128/jcm.31.6.1635-1636.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Halwachs-Baumann G, Genser B, Pailer S, et al. Human cytomegalovirus load in various body fluids of congenitally infected newborns. Journal of Clinical Virology. 2002;25:81–87. doi: 10.1016/s1386-6532(02)00188-9. [DOI] [PubMed] [Google Scholar]
- 24.Inoue N, Koyano S. Evaluation of screening tests for congenital cytomegalovirus infection. The Pediatric Infectious Disease Journal. 2008;27:182–184. doi: 10.1097/INF.0b013e318161a2d5. [DOI] [PubMed] [Google Scholar]
- 25.Hyde TB, Schmid DS, Cannon MJ. Cytomegalovirus seroconversion rates and risk factors: implications for congenital CMV. Reviews in Medical Virology. 2010;20:311–326. doi: 10.1002/rmv.659. [DOI] [PubMed] [Google Scholar]
- 26.Francisse S, Revelard P, De Maertelaer V, Strebelle E, Englert Y, Liesnard C. Human cytomegalovirus seroprevalence and risk of seroconversion in a fertility clinic population. Obstetrics & Gynecology. 2009;114:285–291. doi: 10.1097/AOG.0b013e3181af3d6f. [DOI] [PubMed] [Google Scholar]
- 27.Revello MG, Campanini G, Piralla A, et al. Molecular epidemiology of primary human cytomegalovirus infection in pregnant women and their families. Journal of Medical Virology. 2008;80:1415–1425. doi: 10.1002/jmv.21243. [DOI] [PubMed] [Google Scholar]
- 28.Pass RF, Stagno S, Britt WJ, Alford CA. Specific cell-mediated immunity and the natural history of congenital infection with cytomegalovirus. The Journal of Infectious Diseases. 1983;148:953–961. doi: 10.1093/infdis/148.6.953. [DOI] [PubMed] [Google Scholar]
- 29.Adler SP. The prevalence of cytomegalovirus viruria among hospitalized children and the risk of cytomegalovirus acquisition by nurses. The New England Journal of Medicine. 1984;310:1388. doi: 10.1056/NEJM198405243102115. [DOI] [PubMed] [Google Scholar]
- 30.Li F, Hanshaw JB. Cytomegalovirus infection among migrant children. American Journal of Epidemiology. 1967;86:137–141. doi: 10.1093/oxfordjournals.aje.a120718. [DOI] [PubMed] [Google Scholar]
- 31.Leinikki P, Heinonen K, Pettay O. Incidence of cytomegalovirus infections in early childhood. Scandinavian Journal of Infectious Diseases. 1972;4:1–5. doi: 10.3109/inf.1972.4.issue-1.01. [DOI] [PubMed] [Google Scholar]
- 32.Hanshaw JB, Betts RF, Simon G, Boynton RC. Acquired cytomegalovirus infection: association with hepatomegaly and abnormal liver-function tests. The New England Journal of Medicine. 1965;272:602–609. doi: 10.1056/NEJM196503252721202. [DOI] [PubMed] [Google Scholar]
- 33.Knowledge and practices of obstetricians and gynecologists regarding cytomegalovirus infection during pregnancy--United States, 2007. MMWR. Morbidity and Mortality Weekly Report. 2008;57:65–68. [PubMed] [Google Scholar]
- 34.Cannon MJ, Davis KF. Washing our hands of the congenital cytomegalovirus disease epidemic. BMC Public Health. 2005;5:70. doi: 10.1186/1471-2458-5-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adler SP, Finney JW, Manganello AM, Best AM. Prevention of child-to-mother transmission of cytomegalovirus by changing behaviors: a randomized controlled trial. The Pediatric Infectious Disease Journal. 1996;15:240–246. doi: 10.1097/00006454-199603000-00013. [DOI] [PubMed] [Google Scholar]
- 36.Demmler-Harrison GJ. Congenital cytomegalovirus: public health action towards awareness, prevention, and treatment. Journal of Clinical Virology. 2009;46(Suppl 4):S1–S5. doi: 10.1016/j.jcv.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 37.Picone O, Vauloup-Fellous C, Cordier AG, et al. A 2-year study on cytomegalovirus infection during pregnancy in a French hospital. BJOG: An International Journal of Obstetrics & Gynaecology. 2009;116:818–823. doi: 10.1111/j.1471-0528.2009.02139.x. [DOI] [PubMed] [Google Scholar]
- 38.Vauloup-Fellous C, Picone O, Cordier AG, et al. Does hygiene counseling have an impact on the rate of CMV primary infection during pregnancy? Results of a 3-year prospective study in a French hospital. Journal of Clinical Virology. 2009;46:S49–S53. doi: 10.1016/j.jcv.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 39.Chandler SH, Holmes KK, Wentworth BB, et al. The epidemiology of cytomegalovirial infection in women attending sexually transmitted disease clinic. The Journal of Infectious Diseases. 1985;152:597–605. doi: 10.1093/infdis/152.3.597. [DOI] [PubMed] [Google Scholar]
- 40.Coonrod D, Collier AC, Ashley R, DeRouen T, Corey L. Association between cytomegalovirus seroconversion and upper genital tract infection among women attending a sexually transmitted disease clinic: a prospective study. The Journal of Infectious Diseases. 1998;177:1188–1193. doi: 10.1086/515292. [DOI] [PubMed] [Google Scholar]
- 41.Fowler KB, Pass RF. Risk factors for congenital cytomegalovirus infection in the offspring of young women: exposure to young children and recent onset of sexual activity. Pediatrics. 2006;118:e286–e292. doi: 10.1542/peds.2005-1142. [DOI] [PubMed] [Google Scholar]
- 42.Staras SA, Flanders WD, Dollard SC, Pass RF, McGowan JE, Jr, Cannon MJ. Influence of sexual activity on cytomegalovirus seroprevalence in the United States, 1988–1994. Sexually Transmitted Diseases. 2008;35:472–479. doi: 10.1097/OLQ.0b013e3181644b70. [DOI] [PubMed] [Google Scholar]
- 43.Stover CT, Smith DK, Schmid DS, et al. Prevalence of and risk factors for viral infections among human immunodeficiency virus (HIV)-infected and high-risk HIV-uninfected women. The Journal of Infectious Diseases. 2003;187:1388–1396. doi: 10.1086/374649. [DOI] [PubMed] [Google Scholar]
- 44.Rosenthal LS, Fowler KB, Boppana SB, et al. Cytomegalovirus shedding and delayed sensorineural hearing loss: results from longitudinal follow-up of children with congenital infection. The Pediatric Infectious Disease Journal. 2009;28:515–520. doi: 10.1097/INF.0b013e318198c724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trowsdale J, Betz AG. Mother’s little helpers: mechanisms of maternal-fetal tolerance. Nature Immunology. 2006;7:241–246. doi: 10.1038/ni1317. [DOI] [PubMed] [Google Scholar]
- 46.Redman CWG, Sargent IL. Microparticles and immunomodulation in pregnancy and pre-eclampsia. Journal of Reproductive Immunology. 2007;76:61–67. doi: 10.1016/j.jri.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 47.Baboonian C, Grundy JE, Lever AM, Griffiths PD. Effect of pregnancy plasma upon in vitro parameters of cell mediated immunity. FEMS Microbiology Immunology. 1989;1:189–197. doi: 10.1111/j.1574-6968.1989.tb02382.x. [DOI] [PubMed] [Google Scholar]
- 48.Kumar A, Madden DL, Nankervis GA. Humoral and cell-mediated immune-responses to herpesvirus antigens during pregnancy - a longitudinal-study. Journal of Clinical Immunology. 1984;4:12–17. doi: 10.1007/BF00915281. [DOI] [PubMed] [Google Scholar]
- 49.Baboonian C, Griffiths P. Is pregnancy immunosuppressive? Humoral immunity against viruses. BJOG: An International Journal of Obstetrics & Gynaecology. 1983;90:1168–1175. doi: 10.1111/j.1471-0528.1983.tb06466.x. [DOI] [PubMed] [Google Scholar]
- 50.Grosse SD, Ross DS, Dollard SC. Congenital cytomegalovirus (CMV) infection as a cause of permanent bilateral hearing loss: a quantitative assessment. Journal of Clinical Virology. 2008;41:57–62. doi: 10.1016/j.jcv.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 51.Ross SA, Novak Z, Fowler KB, Arora N, Britt WJ, Boppana SB. Cytomegalovirus blood viral load and hearing loss in young children with congenital infection. The Pediatric Infectious Disease Journal. 2009;28:588–592. doi: 10.1097/INF.0b013e3181979a27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cannon MJ, Schmid DS, Hyde TB. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Reviews in Medical Virology. 2010;20:202–213. doi: 10.1002/rmv.655. [DOI] [PubMed] [Google Scholar]
- 53.Staras SA, Flanders WD, Dollard SC, Pass RF, McGowan JE, Jr, Cannon MJ. Cytomegalovirus seroprevalence and childhood sources of infection: a population-based study among pre-adolescents in the United States. Journal of Clinical Virology. 2008;43:266–271. doi: 10.1016/j.jcv.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 54.Zanghellini F, Boppana SB, Emery VC, Griffiths PD, Pass RF. Asymptomatic primary cytomegalovirus infection: virologic and immunologic features. The Journal of Infectious Diseases. 1999;180:702–707. doi: 10.1086/314939. [DOI] [PubMed] [Google Scholar]
- 55.Hutto C, Little EA, Ricks R, Lee JD, Pass RF. Isolation of cytomegalovirus from toys and hands in a day care center. The Journal of Infectious Diseases. 1986;154:527–530. doi: 10.1093/infdis/154.3.527. [DOI] [PubMed] [Google Scholar]
- 56.Pass RF, Hutto SC, Reynolds DW, Polhill RB. Increased frequency of cytomegalovirus infection in children in group day care. Pediatrics. 1984;74:121–126. [PubMed] [Google Scholar]
- 57.Dworsky M, Yow M, Stagno S, Pass RF, Alford C. Cytomegalovirus infection of breast milk and transmission in infancy. Pediatrics. 1983;72:295–299. [PubMed] [Google Scholar]
- 58.Pultoo A, Jankee H, Meetoo G, Pyndiah MN, Khittoo G. Detection of cytomegalovirus in urine of hearing-impaired and mentally retarded children by PCR and cell culture. The Journal of Communicable Diseases. 2000;32:101–108. [PubMed] [Google Scholar]
- 59.Ahlfors K, Ivarsson SA, Harris S. Report on a long-term study of maternal and congenital cytomegalovirus infection in Sweden. Review of prospective studies available in the literature. Scandinavian Journal of Infectious Diseases. 1999;31:443–457. doi: 10.1080/00365549950163969. [DOI] [PubMed] [Google Scholar]
- 60.Bale JF, Jr, Zimmerman B, Dawson JD, Souza IE, Petheram SJ, Murph JR. Cytomegalovirus transmission in child care homes. Archives of Pediatrics & Adolescent Medicine. 1999;153:75–79. doi: 10.1001/archpedi.153.1.75. [DOI] [PubMed] [Google Scholar]
- 61.Bello C, Whittle H. Cytomegalovirus infection in Gambian mothers and their babies. Journal of Clinical Pathology. 1991;44:366–369. doi: 10.1136/jcp.44.5.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Peckham CS, Johnson C, Ades A, Pearl K, Chin KS. Early acquisition of cytomegalovirus infection. Archives of Disease in Childhood. 1987;62:780–785. doi: 10.1136/adc.62.8.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jones LA, Dukes-Duncan PM, Yeager AS. Cytomegaloviral infections in infant-toddler centers: centers for the developmentally delayed versus regular day care. The Journal of Infectious Diseases. 1985;151:953–955. doi: 10.1093/infdis/151.5.953. [DOI] [PubMed] [Google Scholar]
- 64.Kashiwagi Y, Nemoto S, Hisashi, et al. Cytomegalovirus DNA among children attending two day-care centers in Tokyo. Pediatrics International. 2001;43:493–495. doi: 10.1046/j.1442-200x.2001.01433.x. [DOI] [PubMed] [Google Scholar]
- 65.Lasry S, Deny P, Asselot C, et al. Inter-strain variations in the cytomegalovirus (CMV) glycoprotein B gene sequence among CMV-infected children attending six day care centers. The Journal of Infectious Diseases. 1996;174:606–609. doi: 10.1093/infdis/174.3.606. [DOI] [PubMed] [Google Scholar]
- 66.Murph JR, Bale JF, Murray JC, Stinski MF, Perlman S. Cytomegalovirus transmission in a Midwest day care center: possible relationship to child care practices. The Journal of Pediatrics. 1986;109:35–39. doi: 10.1016/s0022-3476(86)80568-6. [DOI] [PubMed] [Google Scholar]
- 67.Murph JR, Bale JF. The natural history of acquired cytomegalovirus infection among children in group day care. American Journal of Diseases of Children. 1988;142:843–846. doi: 10.1001/archpedi.1988.02150080049020. [DOI] [PubMed] [Google Scholar]
- 68.Strom J. A study of infections and illnesses in a day nursery based on inclusion-bearing cells in the urine and infectious agent in feces, urine and nasal secretion. Scandinavian Journal of Infectious Disease. 1979;11:265–269. doi: 10.3109/inf.1979.11.issue-4.02. [DOI] [PubMed] [Google Scholar]
- 69.Volpi A, Pica F, Cauletti A, Pana A, Rocchi G. Cytomegalovirus infection in day care centers in Rome, Italy: viral excretion in children and occupational risk among workers. Journal of Medical Virology. 1988;26:119–125. doi: 10.1002/jmv.1890260203. [DOI] [PubMed] [Google Scholar]
- 70.Clarke LM, Duerr A, Feldman J, Sierra MF, Daidone BJ, Landesman SH. Factors associated with cytomegalovirus infection among human immunodeficiency virus type 1-seronegative and -seropositive women from an urban minority community. The Journal of Infectious Diseases. 1996;173:77–82. doi: 10.1093/infdis/173.1.77. [DOI] [PubMed] [Google Scholar]
- 71.Shen CY, Chang SF, Chao MF, et al. Cytomegalovirus recurrence in seropositive pregnant women attending obstetric clinics. Journal of Medical Virology. 1993;41:24–29. doi: 10.1002/jmv.1890410106. [DOI] [PubMed] [Google Scholar]
- 72.Prevalence of cytomegalovirus excretion from children in five day-care centers--Alabama. MMWR. Morbidity and Mortality Weekly Report. 1985;34:49–51. [PubMed] [Google Scholar]
- 73.Pass RF, Stagno S, Dworsky ME, Smith RJ, Alford CA. Excretion of cytomegalovirus in mothers: observations after delivery of congenitally infected and normal infants. The Journal of Infectious Diseases. 1982;146:1–6. doi: 10.1093/infdis/146.1.1. [DOI] [PubMed] [Google Scholar]
- 74.Spano LC, Gatti J, Nascimento JP, Leite JP. Prevalence of human cytomegalovirus infection in pregnant and non-pregnant women. The Journal of Infection. 2004;48:213–220. doi: 10.1016/S0163-4453(03)00128-2. [DOI] [PubMed] [Google Scholar]
- 75.Stagno S, Reynolds DW, Tsiantos A, Fuccillo DA, Long W, Alford CA. Comparative serial virologic and serologic studies of symptomatic and subclinical congenitally and natally acquired cytomegalovirus infections. The Journal of Infectious Diseases. 1975;132:568–577. doi: 10.1093/infdis/132.5.568. [DOI] [PubMed] [Google Scholar]
- 76.Biri A, Bozdayi AG, Ab C, Dinc AB, Yucel AA, Rota AS. The detection of CMV in amniotic fluid and cervicovaginal smear samples by real-time PCR assay in prenatal diagnosis. Archives of Gynecology and Obstetrics. 2006;273:261–266. doi: 10.1007/s00404-005-0020-3. [DOI] [PubMed] [Google Scholar]
- 77.Noyola DE, Valdez-Lopez BH, Hernandez-Salinas AE, et al. Cytomegalovirus excretion in children attending day-care centers. Archives of Medical Research. 2005;36:590–593. doi: 10.1016/j.arcmed.2005.03.045. [DOI] [PubMed] [Google Scholar]
- 78.MacDonald H, Tobin JOH. Congenital cytomegalovirus infection: a collaborative study on epidemiological, clinical and laboratory findings. Developmental Medicine and Child Neurology. 1978;20:471–482. doi: 10.1111/j.1469-8749.1978.tb15248.x. [DOI] [PubMed] [Google Scholar]
- 79.Ross SA, Novak Z, Ashrith G, et al. Association between genital tract cytomegalovirus infection and bacterial vaginosis. The Journal of Infectious Diseases. 2005;192:1727–1730. doi: 10.1086/497150. [DOI] [PubMed] [Google Scholar]
- 80.Nishiwaki M, Fujimuro M, Teishikata Y, et al. Epidemiology of Epstein-Barr virus, cytomegalovirus, and Kaposi’s sarcoma-associated herpesvirus infections in peripheral blood leukocytes revealed by a multiplex PCR assay. Journal of Medical Virology. 2006;78:1635–1642. doi: 10.1002/jmv.20748. [DOI] [PubMed] [Google Scholar]
- 81.Tanaka K, Yamada H, Minami M, et al. Screening for vaginal shedding of cytomegalovirus in healthy pregnant women using real-time PCR: correlation of CMV in the vagina and adverse outcome of pregnancy. Journal of Medical Virology. 2006;78:757–759. doi: 10.1002/jmv.20619. [DOI] [PubMed] [Google Scholar]
- 82.Broccolo F, Cassina G, Chiari S, et al. Frequency and clinical significance of human beta-herpesviruses in cervical samples from Italian women. Journal of Medical Virology. 2008;80:147–153. doi: 10.1002/jmv.21054. [DOI] [PubMed] [Google Scholar]
- 83.Hou GQ, Chen SS, Lee CP. Pathogens in maternal blood and fetal cord blood using Q-PCR assay. Taiwanese Journal of Obstetrics & Gynecology. 2006;45:114–119. doi: 10.1016/S1028-4559(09)60207-2. [DOI] [PubMed] [Google Scholar]
- 84.Kaye S, Miles D, Antoine P, et al. Virological and immunological correlates of mother-to-child transmission of cytomegalovirus in The Gambia. The Journal of Infectious Diseases. 2008;197:1307–1314. doi: 10.1086/586715. [DOI] [PubMed] [Google Scholar]
- 85.Manfredi R, Calza L, Chiodo F. Primary cytomegalovirus infection in otherwise healthy adults with fever of unknown origin: a 3-year prospective survey. Infection. 2006;34:87–90. doi: 10.1007/s15010-006-5012-0. [DOI] [PubMed] [Google Scholar]
- 86.Novak Z, Ross SA, Patro RK, et al. Cytomegalovirus strain diversity in seropositive women. Journal of Clinical Microbiology. 2008;46:882–886. doi: 10.1128/JCM.01079-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zaki MES, Goda H. Relevance of parvovirus B19, herpes simplex virus 2, and cytomegalovirus virologic markers in maternal serum for diagnosis of unexplained recurrent abortions. Archives of Pathology & Laboratory Medicine. 2007;131:956–960. doi: 10.5858/2007-131-956-ROPBHS. [DOI] [PubMed] [Google Scholar]
- 88.Numazaki Y, Yano N, Morizuka T, Takai S, Ishida N. Primary infection with human cytomegalovirus: virus isolation from healthy infants and pregnant women. American Journal of Epidemiology. 1970;91:410–417. doi: 10.1093/oxfordjournals.aje.a121151. [DOI] [PubMed] [Google Scholar]
- 89.Andersen HK, Gravesen JJ, Iversen T. Cytomegalovirus infection among infants admitted to a paediatric department. Acta Paediatrica Scandinavica. 1972;61:445–451. doi: 10.1111/j.1651-2227.1972.tb15862.x. [DOI] [PubMed] [Google Scholar]
- 90.Ahlfors K, Ivarsson SA, Johnsson T, Svensson I. Congenital and acquired cytomegalovirus infections. Virological and clinical studies on a Swedish infant population. Acta Paediatrica Scandinavica. 1978;67:321–328. doi: 10.1111/j.1651-2227.1978.tb16328.x. [DOI] [PubMed] [Google Scholar]
- 91.Bresson JL, Clavequin MC, Mazeron MC, et al. Risk of cytomegalovirus transmission by cryopreserved semen: a study of 635 semen samples from 231 donors. Human Reproduction. 2003;18:1881–1886. doi: 10.1093/humrep/deg362. [DOI] [PubMed] [Google Scholar]
- 92.Kapranos N, Petrakou E, Anastasiadou C, Kotronias D. Detection of herpes simplex virus, cytomegalovirus, and Epstein-Barr virus in the semen of men attending an infertility clinic. Fertility and Sterility. 2003;79:1566–1570. doi: 10.1016/s0015-0282(03)00370-4. [DOI] [PubMed] [Google Scholar]
- 93.Mansat A, Mengelle C, Chalet M, et al. Cytomegalovirus detection in cryopre-served semen samples collected for therapeutic donor insemination. Human Reproduction. 1997;12:1663–1666. doi: 10.1093/humrep/12.8.1663. [DOI] [PubMed] [Google Scholar]
- 94.Bezold G, Politch JA, Kiviat NB, Kuypers JM, Wolff H, Anderson DJ. Prevalence of sexually transmissible pathogens in semen from asymptomatic male infertility patients with and without leukocytospermia. Fertililty and Sterility. 2007;87:1087–1097. doi: 10.1016/j.fertnstert.2006.08.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yang YS, Ho HN, Chen HF, et al. Cytomegalovirus-infection and viral shedding in the genital-tract of infertile couples. Journal of Medical Virology. 1995;45:179–182. doi: 10.1002/jmv.1890450212. [DOI] [PubMed] [Google Scholar]
- 96.Reynolds DW, Stagno S, Stubbs G, et al. Inapparent congenital cytomegalovirus infection with elevated cord IgM levels. The New England Journal of Medicine. 1974;290:291–296. doi: 10.1056/NEJM197402072900601. [DOI] [PubMed] [Google Scholar]
- 97.Hutto C, Ricks R, Garvie M, Pass RF. Epidemiology of cytomegalovirus infections in young children: day care vs. home care. The Pediatric Infectious Disease Journal. 1985;4:149–152. doi: 10.1097/00006454-198503000-00008. [DOI] [PubMed] [Google Scholar]
- 98.Nelson DB, Peckham CS, Pearl KN, Chin KS, Garrett AJ, Warren DE. Cytomegalovirus infection in day nurseries. Archives of Disease in Childhood. 1987;62:329–332. doi: 10.1136/adc.62.4.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Walker GH, Tobin JO. Cytomegalovirus infection in the North West of England. A report on a two-year study. Archives of Disease in Childhood. 1970;45:513–522. doi: 10.1136/adc.45.242.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lange M, Klein EB, Kornfield H, Cooper LZ, Grieco MH. Cytomegalovirus isolation from healthy homosexual men. JAMA: The Journal of the American Medical Association. 1984;252:1908–1910. [PubMed] [Google Scholar]
- 101.Montgomery R, Youngblood L, Medearis DN., Jr Recovery of cytomegalovirus from the cervix in pregnancy. Pediatrics. 1972;49:524–531. [PubMed] [Google Scholar]
- 102.Rivera LB, Boppana SB, Fowler KB, Britt WJ, Stagno S, Pass RF. Predictors of hearing loss in children with symptomatic congenital cytomegalovirus. Pediatrics. 2002;110:762–767. doi: 10.1542/peds.110.4.762. [DOI] [PubMed] [Google Scholar]
- 103.Bradford RD, Cloud G, Lakeman AD, et al. Detection of cytomegalovirus (CMV) DNA by polymerase chain reaction is associated with hearing loss in newborns with symptomatic congenital CMV infection involving the central nervous system. The Journal of Infectious Diseases. 2005;191:227–233. doi: 10.1086/426456. [DOI] [PubMed] [Google Scholar]
- 104.Boppana SB, Fowler KB, Pass RF, et al. Congenital cytomegalovirus infection: association between virus burden in infancy and hearing loss. The Journal of Pediatrics. 2005;146:817–823. doi: 10.1016/j.jpeds.2005.01.059. [DOI] [PubMed] [Google Scholar]
- 105.Lanari M, Lazzarotto T, Venturi V, et al. Neonatal cytomegalovirus blood load and risk of sequelae in symptomatic and asymptomatic congenitally infected newborns. Pediatrics. 2006;117:e76–e83. doi: 10.1542/peds.2005-0629. [DOI] [PubMed] [Google Scholar]
- 106.Walter S, Atkinson C, Sharland M, et al. Congenital cytomegalovirus: association between dried blood spot viral load and hearing loss. Archives of Disease in Childhood Fetal and Neonatal Edition. 2008;93:F280–F285. doi: 10.1136/adc.2007.119230. [DOI] [PubMed] [Google Scholar]
- 107.Verbeeck J, Van Kerschaver E, Wollants E, Beuselinck K, Stappaerts L, Van Ranst M. Detection of perinatal cytomegalovirus infection and sensorineural hearing loss in Belgian infants by measurement of automated auditory brainstem response. Journal of Clinical Microbiology. 2008;46:3564–3568. doi: 10.1128/JCM.00757-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yan H, Koyano S, Inami Y, et al. Genetic variations in the gB, UL144 and UL149 genes of human cytomegalovirus strains collected from congenitally and postnatally infected Japanese children. Archives of Virology. 2008;153:667–674. doi: 10.1007/s00705-008-0044-7. [DOI] [PubMed] [Google Scholar]
- 109.Yu ZS, Zou CC, Zheng JY, Zhao ZY. Cytomegalovirus gB genotype and clinical features in Chinese infants with congenital infections. Intervirology. 2006;49:281–285. doi: 10.1159/000093458. [DOI] [PubMed] [Google Scholar]
- 110.Revello MG, Zavattoni M, Baldanti F, Sarasini A, Paolucci S, Gerna G. Diagnostic and prognostic value of human cytomegalovirus load and IgM antibody in blood of congenitally infected newborns. Journal of Clinical Virology. 1999;14:57–66. doi: 10.1016/s1386-6532(99)00016-5. [DOI] [PubMed] [Google Scholar]
- 111.Revello MG, Lilleri D, Zavattoni M, et al. Human cytomegalovirus immediate-early messenger RNA in blood of pregnant women with primary infection and of congenitally infected newborns. The Journal of Infectious Diseases. 2001;184:1078–1081. doi: 10.1086/323425. [DOI] [PubMed] [Google Scholar]
- 112.Melish ME, Hanshaw JB. Congenital cytomegalovirus infection. Developmental progress of infants detected by routine screening. American Journal of Diseases of Children. 1973;126:190–194. doi: 10.1001/archpedi.1973.02110190168011. [DOI] [PubMed] [Google Scholar]
- 113.Kumar ML, Nankervis GA, Gold E. Inapparent congenital cytomegalovirus infection. The New England Journal of Medicine. 1973;288:1370–1372. doi: 10.1056/NEJM197306282882603. [DOI] [PubMed] [Google Scholar]
- 114.Spector SA, Schmidt K, Ticknor W, Grossman M. Cytomegaloviruria in older infants in intensive care nurseries. The Journal of Pediatrics. 1979;95:444–446. doi: 10.1016/s0022-3476(79)80532-6. [DOI] [PubMed] [Google Scholar]
- 115.Steininger C, Kundi M, Kletzmayr J, Aberle SW, Popow-Kraupp T. Antibody maturation and viremia after primary cytomegalovirus infection, in immunocompetent patients and kidney-transplant patients. The Journal of Infectious Diseases. 2004;190:1908–1912. doi: 10.1086/424677. [DOI] [PubMed] [Google Scholar]
- 116.Natali A, Valcavi P, Medici MC, Dieci E, Montali S, Chezzi C. Cytomegalovirus infection in an Italian population: antibody prevalence, virus excretion and maternal transmission. The New Microbiologica. 1997;20:123–133. [PubMed] [Google Scholar]
- 117.Revello MG, Zavattoni M, Sarasini A, Percivalle E, Simoncini L, Gerna G. Human cytomegalovirus in blood in immunocompetent persons during primary infection: prognostic implications for pregnancy. The Journal of Infectious Diseases. 1998;177:1170–1175. doi: 10.1086/515277. [DOI] [PubMed] [Google Scholar]
- 118.Revello MG, Lilleri D, Zavattoni M, et al. Lymphoproliferative response in primary human cytomegalovirus (HCMV) infection is delayed in HCMV transmitter mothers. The Journal of Infectious Diseases. 2006;193:269–276. doi: 10.1086/498872. [DOI] [PubMed] [Google Scholar]
- 119.Zhang C, Buchanan H, Andrews W, Evans A, Pass RF. Detection of cytomegalovirus infection during a vaccine clinical trial in healthy young women: seroconversion and viral shedding. Journal of Clinical Virology. 2006;35:338–342. doi: 10.1016/j.jcv.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 120.Griffiths PD, Stagno S, Reynolds DW, Alford CA. A longitudinal study of the serological and virological status of 18 women infected with cytomegalovirus. Archives of Virology. 1978;58:111–118. doi: 10.1007/BF01315403. [DOI] [PubMed] [Google Scholar]
- 121.Nankervis GA, Kumar ML, Cox FE, Gold E. A prospective study of maternal cytomegalovirus infection and its effect on the fetus. American Journal of Obstetrics and Gynecology. 1984;149:435–440. doi: 10.1016/0002-9378(84)90159-5. [DOI] [PubMed] [Google Scholar]
- 122.Strangert K, Carlstrom G, Jeanson S, Nord CE. Infections in preschool children in group day care. Acta paediatrica Scandinavica. 1976;65:455–463. doi: 10.1111/j.1651-2227.1976.tb04914.x. [DOI] [PubMed] [Google Scholar]
- 123.Adler SP. Molecular epidemiology of cytomegalovirus: viral transmission among children attending a day care center, their parents, and caretakers. The Journal of Pediatrics. 1988;112:366–372. doi: 10.1016/s0022-3476(88)80314-7. [DOI] [PubMed] [Google Scholar]
- 124.Demmler GJ, Yow MD, Spector SA, et al. Nosocomial cytomegalovirus infections within two hospitals caring for infants and children. The Journal of Infectious Diseases. 1987;156:9–16. doi: 10.1093/infdis/156.1.9. [DOI] [PubMed] [Google Scholar]
- 125.Limaye AP, Kirby KA, Rubenfeld GD, et al. Cytomegalovirus reactivation in critically ill immunocompetent patients. JAMA: The Journal of American Medical Association. 2008;300:413–422. doi: 10.1001/jama.300.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Beyari MM, Hodgson TA, Kondowe W, et al. Inter- and intra-person cytomegalovirus infection in Malawian families. Journal of Medical Virology. 2005;75:575–582. doi: 10.1002/jmv.20312. [DOI] [PubMed] [Google Scholar]