Abstract
The cancer stem cell (CSC) theory is an emerging concept that proposes a hierarchical nature of carcinogenesis, where a small number of tumor cells are capable of driving tumor growth. Despite many unanswered questions surrounding the cancer stem cell model, the hypothesis has rejuvenated hopes for formulating a novel therapeutic strategy for targeting the roots of cancer. This model predicts that cancer stem cells have the capacity to resist conventional radio- and chemotherapy and initiate disease recurrence. We recently investigated the mechanisms of chemoresistance in glioblastoma (GBM), the most common and aggressive adult human brain tumor. Exposure of patient derived glioma xenograft lines to a therapeutic dose of temolozolomide (TMZ), the most commonly used chemotherapy for patients with GBM, consistently increased the glioma stem cell (GSC) frequency over time. Lineage tracing analysis at the single sell level revealed unprecedented cellular plasticity within the glioma cells, allowing them to reprogram from a differentiated state to an undifferentiated CSC-like state. This reprogramming, mediated by cellular plasticity, is driven by TMZ-induced hypoxia inducible factors (HIFs), and provides a novel mechanism for chemoresistance acquisition. We herein discuss the possible role of temozolomide in regulating a cancer stem cell niche that supports GSC resistance, proliferation, and subsequent therapeutic relapse.
Glioblastoma multiforme (GBM) is the most common brain tumor in adults and has a very aggressive phenotype. Of all diagnosed patients, less than 10% survive longer than 5 years and close to 100% will eventually succumb to the disease[1]. Such unfavorable prognoses for GBM patients can be largely attributed to a high rate of recurrence, resulting from the ability of GBM cells to resist conventional radio- and chemotherapy. GBMs are also amongst the first solid tumors in which a stem cell-like tumor initiating cell population has been discovered[2]. The presence of these cells, known as glioma stem cells (GSCs), points to a hierarchical model of gliomagenesis. Such a model suggests that a small subpopulation of glioma cells, in this case GSCs, can resist conventional therapy more effectively than non-GSCs and initiate disease recurrence, thereby sustaining uncontrollable tumor growth.
The therapy resistance property of GSCs has been subject to intense investigation for the past 5 years. While the mechanisms by which GSCs survive radiotherapy are fairly well understood, it remains unclear how GSCs may contribute to GBM chemoresistance[3]. Several reports indicate a marked increase in the resistance of GSC lines against temolozolamide (TMZ), the most commonly used alkylating agent to treat patients with glioma[4–6]. In contrast, recent reports from our laboratory along with others indicate that TMZ can induce a dose and time-dependent depletion of the GSC population[7,8]. The cellular response to alkylating agents, including TMZ is directly correlated to the expression of DNA repair proteins such as O6-methylguanine-methyltransferase (O6-BG/MGMT), which is responsible for removing alkylating adducts and protecting tumor cells from TMZ-induced toxicity[9]. In the clinical setting epigenetic silencing of MGMT is thus far the strongest predictive marker for the therapeutic efficacy of TMZ treatment in GBM patients[10,11]. There is also a consensus in the literature that MGMT expression in GSCs is associated with greater chemo resistance in GBM[7,12,13]. In fact, GSCs with elevated MGMT activity have been reported to be 10-fold less sensitive to TMZ, than those with lower or no expression of MGMT[7]. Such a theory, however, does not address the mechanisms by which GSCs that lack a methylated MGMT promoter can still manage to resist TMZ-based chemotherapy and initiate GBM recurrence. Thus, the interplay between GSC and chemotherapy is more multifaceted than may be previously anticipated, and will require further investigation to elucidate the mechanisms of chemoresistence in the GBM patient.
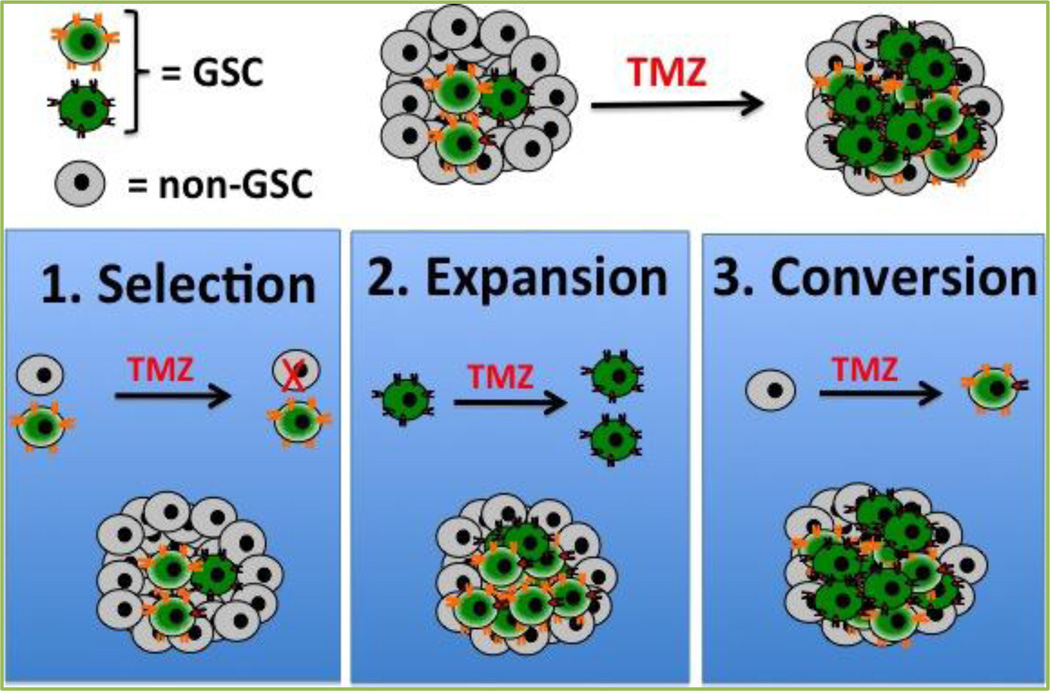
With the goal of filling the knowledge gap, we have investigated the effects of the TMZ-based anti-glioma therapy on the biology of GSCs both in vitro and in vivo by using different patient derived glioma xenograft models. To define the GSC population within the tumor mass we use multiple GSC-specific markers (CD133, CD15, Sox2, Oct4 and Nestin), alone or in combination, and have observed consistent increases in the GSCs pool of glioma patient cell lines when incubated with the therapeutic concentration of TMZ (50 µM)[8]. This increase was time dependent, taking between 6 to 8 days in culture with the TMZ (average increase of GSCs subpopulation 16%). Based on the published reports, as well as our observations, we proposed the following three possible scenarios that may rationalize such expansion of the GSC pool post TMZ therapy: 1) selection, where anti-cancer therapy selectively depletes the non-GSC population, thus increasing the frequency of the GSC pool within the tumor population; 2) expansion, where anti-cancer therapy stimulates only the growth of the GSC populations, thus expanding the pre-therapy pool; 3) conversion, where differentiated glioma cells can dedifferentiate and acquire phenotypic and functional characteristics of GSCs[14,15](Figure 1). In our patient derived orthotopic glioma xenograft models we have observed some degree of spontaneous conversion of non-GSC glioma cells into GSCs over time. However, such conversion of non-GSCs to GSCs was significantly augmented upon long-term exposure to 50 µM of TMZ. This observation was validated by lineage tracing analysis performed at the single cell level using a reporter system based on three different GSC-specific promoters (CD133, Sox2 and Nanog) (Figure 2). The rate of conversion between non-GSCs to GSCs was increased three to four-fold in the presence of TMZ when compared to spontaneous conversion. The BrdU incorporation assay, in turn, demonstrated that TMZ also induced some proliferation and expansion of the pre-therapy GSC pool. Lineage-tracing analysis, however, revealed the GSCs arising from the expansion process were more sensitive to TMZ when compared to newly converted GSCs. Taken together, these results allow us to conclude that TMZ-mediated expansion of the GSC pool is primarily the result of the conversion of non-GSCs to GSCs (Figure 3). Newly converted GSCs expressed GSC-specific markers and possessed a high rate of tumor engraftment capacity. Moreover, they displayed a more invasive phenotype when implanted orthotopically in the brain of nude mice, revealing the infiltrative characteristics of GSCs that may be promoting chemoresistance. While such an occurrence has been widely proposed in the literature, this study represents the first experimental evidence demonstrating the influence of anti-cancer chemotherapy on the intra-conversion of GBM GSCs and non-GSCs[14,16].
Figure 1. Possible mechanisms of GSC pool expansion post TMZ therapy.
In our experimental models we observed expansion of GSC frequency after long-term treatment with the therapeutic dose of TMZ (50 µM). We hypothesize three scenarios that can explain such expansion: 1) Selection, where TMZ can selectively deplete less resistant non-GSC GBM cells, thus expanding the GSC pool in a given tumor population; 2) Expansion, where TMZ therapy can promote proliferation in the GSC pool; 3) Conversion, where TMZ can reprogram the non-GSCs into GSC-like cells.
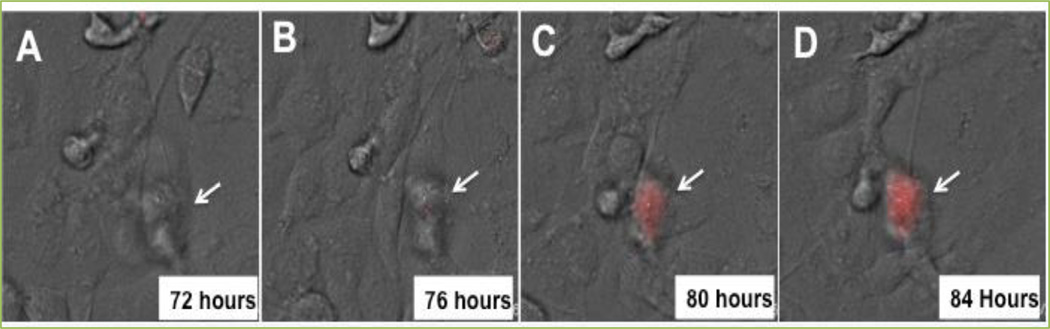
Figure 2. Lineage tracing analysis of conversion of non-GSC to GSC post TMZ therapy.
The U87 glioma cell line was stably transfected with the cancer stem cell specific gene Oct4 promoter-based reporter system expressing red florescent protein. This cell line was cultured with a therapeutic dose of TMZ (50 µM). 72 h post culture time-lapse photographs were collected to examine the conversion of non-GSC (white arrow, A and B) to GSC (white arrow, C and D).
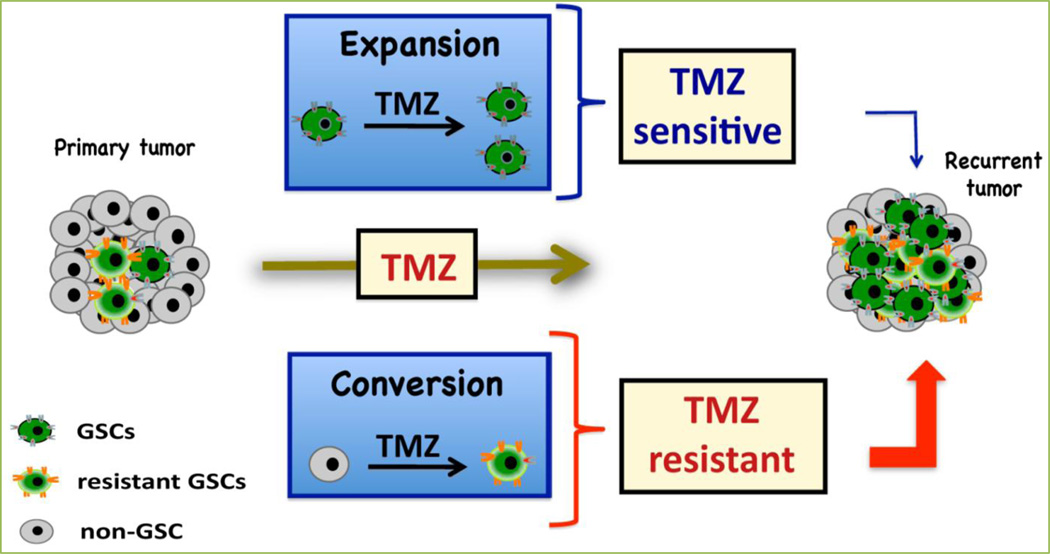
Figure 3. Organogram depicting an overview of our main findings.
Our theory is that a combination of factors, between the expansion of previously existent GSCs and the conversion of non-GSCs into newly formed stem-like cells, leads to the observed increases in the GSC population post-long term treatment with clinically relevant doses of TMZ. These newly formed populations play an important role in the generation of a more invasive and infiltrative tumor. They may also lead to increased therapeutic resistance and tumor recurrence. Our results suggest that these newly formed stem-like cells are more resistant to TMZ therapy than the amplified GSC population. The combination of these two processes offers a new explanation for the decreased efficacy of the currently available conventional therapies.
The cancer stem cell (CSC) theory is an emerging concept that proposes a hierarchical nature of carcinogenesis, where a small number of tumor cells are capable of driving tumor growth. A corollary of this theory is that GSCs are the main driving force behind gliomagenesis. Thus, it might be expected that the elimination of GSCs would halt continuous tumor growth. Recent publications, however, argue against such a static hierarchical organization of carcinogenesis and offer the alternate theory that microenvironmental factors such as acidic stress and/or hypoxia within the individual tumor can promote a CSC niche as well as “stemness” in tumor cells resulting in a more plastic hierarchical structure[14,17,18]. It has long been known that anti-cancer therapy can alter the tumor microenvironment and eventually negatively influence therapeutic efficacy. Exposure of tumor-derived fibroblasts to high dose radiation, for example, induces irreversible cellular senescence, which in turn alters the microenvironment through the release of cytokines, chemokines, and growth factors, thereby limiting the therapeutic efficacy[19]. In the case of GBM, the low oxygen tension of the tumor microenvironment, resulting from poor vascularization, promotes a hypoxic condition that initiates the accumulation of hypoxia inducible factors (HIFs), reported to be critical for the generation of a hypoxic niche for GSCs[14,18]. Investigating the role of HIFs in GSC maintenance, Heddleston et al. show that tumor regions with increased HIF expression possess an elevated self-renewing capacity and are enriched for GSC-specific markers CD133 and Nanog. GBM tissue samples collected by image-guided surgery show that the CD133 and Nestin enriched populations are located predominantly within the necrotic center of the tumor, where HIF expression is highest[18]. During TMZ therapy, we observed that HIF expression was significantly up regulated in glioma cells both in vitro and in vivo. In parallel, TMZ treatment in the glioma patient derived orthotopic xenograft models induced elevated hypoxic regions, providing the ideal microenvironment for promoting a HIF induced GSC niche[20]. Moreover, gene expression in TMZ treated GBM samples revealed that the downstream targets of HIFs, including cell cycle related gene CDKN1A, LOX and apoptosis inhibitor gene BIRC3, are also up-regulated upon TMZ therapy. All this data points to the notion that TMZ therapy can induce hypoxia-like responses and the expression of HIFs, which may play an important role in promoting TMZ-induced conversion of non-GSCs to GSCs.
Hypoxic conditions can provide a favorable environment for the maintenance of pluripotency in normal neural stem cells[21]. It has been demonstrated that like normal neural stem cells, GSCs can reside within the hypoxic microenvironment[14,18]. Tumor hypoxia has already been shown to negatively affect the efficacy of many anti-cancer therapeutics including radio- and chemotherapy[22]. One explanation for this is that hypoxia inhibits tumor cell proliferation and induces cell cycle arrest, thus conferring chemo resistance, as the majority of anti-cancer drugs preferentially target rapidly dividing cells. For the same reason, the quiescent nature of cancer stem cells was thought to be a mechanism that partly explained the chemoresistance properties of this subpopulation[23]. Slow cycling CSCs in the colon, breast, and pancreas have been shown to demonstrate the in vivo ability to survive therapies that kill the majority of tumor cells[24,25]. Thus, the slow cycling characteristics of CSCs, in combination with their hypoxic niche behavior, may explain the chemoresistance properties of GSCs. In contrast to this theory, we observed that TMZ-induced GSCs show elevated expression of the proliferation markers Ki67, indicating that the newly converted GSC populations are not quiescent at all and may use other mechanisms for attaining chemoresistance. Several reports have indicated that HIFs may regulate the expression of DNA repair enzyme MGMT[18,26]. Moreover, an analysis of the 10 kb upstream region of the MGMT coding sequence revealed the presence of two separate hypoxia response elements (HREs), and it was demonstrated that HIFs could directly bind to these sequences andto regulate MGMT expression[27].
The role of TMZ-induced HIFs in regulating MGMT in the converted GSC compartment requires further investigation, however, our preliminary data point towards the notion that TMZ-induced hypoxic responses may not only promote conversion of the non-GSCs to GSCs, but also may regulate the expression of the chemoresistance gene in the newly converted GSC compartment. In light of our observations one can postulate that even if anti-cancer therapy can target preexisting GSCs, more may arise from the stress-induced conversion of non-GSCs to GSCs and initiate therapeutic resistance. This has important clinical implications regarding the development of an effective anti-glioma therapy because formulating such a therapy may not only be dependent on its ability to target preexisting GSCs but also on the sensitivity of the newly converted GSCs and the rate at which they are generated.
Until recently, the cellular hierarchy was considered to be unidirectional, where undifferentiated tissue stem cells exit from their self-renewing state and enter into a committed phase to become differentiated progeny. Such a mature fate is thought to be permanent, as their phenotypes are considered to be inelastic. However, a growing body of evidence, ranging from developmental biology to disease pathology, argues against such a unidirectional flow of the cellular hierarchy. What is proposed instead is the possibility that cell fate is a dynamic process that can be bidirectional. In this case, differentiated cells in the presence of appropriate cue(s) can reverse their mature fate and acquire stem-like states. Recently, phenomena of dedifferentiation have been demonstrated during the generation of the induced pluripotent stem cells (iPSCs), where targeted expression of c-Myc, Sox-2, Oct-4 and Klf-4 converted adult mouse fibroblasts into pluripotent ESC-like cells[28]. This example of reprogramming is not only demonstrated in the experimental condition after artificial manipulations/stimulations, but also observed in physiological conditions in vivo. In Drosophila, differentiated cells under specific conditions can be dedifferentiated into gonadal stem cells[29]. The mature luminal secretory cells, furthermore, can acquire stemness and convert into basal stem-like cells with indistinguishable stem cell morphology and functional characteristics[30]. In breast cancer, the basal-like human mammary epithelial cells spontaneously acquire a cancer stem cell phenotype and, most importantly, oncogenic transformations that accelerate such dedifferentiation processes[15]. Recently, radiation induced stress was shown to reprogram the polyploidy subpopulation of breast cancer cells by inducing Oct4, Nanog and Klf4 expression, thereby generating breast cancer stem-like cells[31]. These reports along with our observations emphasize the phenotypic plasticity of cancer cells in support of a clonal evolution model that suggests such reprograming may be less random than it is believed to be.
Carcinogenesis is an evolutionary process that is governed by the natural selection of cell clones that have obtained advantageous heritable phenotypes. Such Darwinian nature of cancer lies at the heart of therapeutic resistance. The role of cellular hierarchy in this evolutionary selection process is yet to be determined. However, our data raises the possibility that the intrinsic therapy-resistance properties of cancer may not be only associated with a static hierarchical state but also be influenced by the cellular plasticity of cancer cells as well. Such cellular plasticity may enhance the ability of cancers to adapt and empower certain cells or subpopulation of cells, over others, to thrive during therapy. Thus, a more detailed understanding of the molecular mechanisms of tumor cellular plasticity, and its role in promoting therapeutic resistance, will be critical for developing effective therapeutic strategies to improve the prognosis of patients diagnosed with GBM.
Acknowledgements
This work was supported by the National Institute of Health funding R00CA160775 (AUA) and R01CA122930 (MSL).
References
- 1.Deen DF, Chiarodo A, Grimm EA, Fike JR, Israel MA, Kun LE, et al. Brain Tumor Working Group Report on the 9th International Conference on Brain Tumor Research and Therapy. Organ System Program, National Cancer Institute. J Neurooncol. 1993;16:243–272. doi: 10.1007/BF01057041. [DOI] [PubMed] [Google Scholar]
- 2.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 3.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 4.Eramo A, Ricci-Vitiani L, Zeuner A, Pallini R, Lotti F, Sette G, et al. Chemotherapy resistance of glioblastoma stem cells. Cell Death Differ. 2006;13:1238–1241. doi: 10.1038/sj.cdd.4401872. [DOI] [PubMed] [Google Scholar]
- 5.Bleau AM, Hambardzumyan D, Ozawa T, Fomchenko EI, Huse JT, Brennan CW, et al. PTEN/PI3K/Akt pathway regulates the side population phenotype and ABCG2 activity in glioma tumor stem-like cells. Cell Stem Cell. 2009;4:226–235. doi: 10.1016/j.stem.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pistollato F, Rampazzo E, Persano L, Abbadi S, Frasson C, Denaro L, et al. Interaction of hypoxia-inducible factor-1alpha and Notch signaling regulates medulloblastoma precursor proliferation and fate. Stem Cells. 2010;28:1918–1929. doi: 10.1002/stem.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beier D, Rohrl S, Pillai DR, Schwarz S, Kunz-Schughart LA, Leukel P, et al. Temozolomide preferentially depletes cancer stem cells in glioblastoma. Cancer Res. 2008;68:5706–5715. doi: 10.1158/0008-5472.CAN-07-6878. [DOI] [PubMed] [Google Scholar]
- 8.Auffinger B, Tobias AL, Han Y, Lee G, Guo D, Dey M, et al. Conversion of differentiated cancer cells into cancer stem-like cells in a glioblastoma model after primary chemotherapy. Cell Death Differ. 2014;21:1119–1131. doi: 10.1038/cdd.2014.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaina B, Christmann M, Naumann S, Roos WP. MGMT: key node in the battle against genotoxicity, carcinogenicity and apoptosis induced by alkylating agents. DNA Repair (Amst) 2007;6:1079–1099. doi: 10.1016/j.dnarep.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 10.Hegi ME, Liu L, Herman JG, Stupp R, Wick W, Weller M, et al. Correlation of O6-methylguanine methyltransferase (MGMT) promoter methylation with clinical outcomes in glioblastoma and clinical strategies to modulate MGMT activity. J Clin Oncol. 2008;26:4189–4199. doi: 10.1200/JCO.2007.11.5964. [DOI] [PubMed] [Google Scholar]
- 11.Gorlia T, van den Bent MJ, Hegi ME, Mirimanoff RO, Weller M, Cairncross JG, et al. Nomograms for predicting survival of patients with newly diagnosed glioblastoma: prognostic factor analysis of EORTC and NCIC trial 26981–22981/CE.3. Lancet Oncol. 2008;9:29–38. doi: 10.1016/S1470-2045(07)70384-4. [DOI] [PubMed] [Google Scholar]
- 12.Liu G, Yuan X, Zeng Z, Tunici P, Ng H, Abdulkadir IR, et al. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol Cancer. 2006;5:67. doi: 10.1186/1476-4598-5-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blough MD, Beauchamp DC, Westgate MR, Kelly JJ, Cairncross JG. Effect of aberrant p53 function on temozolomide sensitivity of glioma cell lines and brain tumor initiating cells from glioblastoma. J Neurooncol. 2011;102:1–7. doi: 10.1007/s11060-010-0283-9. [DOI] [PubMed] [Google Scholar]
- 14.Heddleston JM, Li Z, McLendon RE, Hjelmeland AB, Rich JN. The hypoxic microenvironment maintains glioblastoma stem cells and promotes reprogramming towards a cancer stem cell phenotype. Cell Cycle. 2009;8:3274–3284. doi: 10.4161/cc.8.20.9701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chaffer CL, Brueckmann I, Scheel C, Kaestli AJ, Wiggins PA, Rodrigues LO, et al. Normal and neoplastic nonstem cells can spontaneously convert to a stem-like state. Proc Natl Acad Sci U S A. 2011;108:7950–7955. doi: 10.1073/pnas.1102454108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta PB, Chaffer CL, Weinberg RA. Cancer stem cells: mirage or reality? Nat Med. 2009;15:1010–1012. doi: 10.1038/nm0909-1010. [DOI] [PubMed] [Google Scholar]
- 17.Hjelmeland AB, Wu Q, Heddleston JM, Choudhary GS, MacSwords J, Lathia JD, et al. Acidic stress promotes a glioma stem cell phenotype. Cell Death Differ. 2011;18:829–840. doi: 10.1038/cdd.2010.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pistollato F, Abbadi S, Rampazzo E, Persano L, Della Puppa A, Frasson C, et al. Intratumoral hypoxic gradient drives stem cells distribution and MGMT expression in glioblastoma. Stem Cells. 2010;28:851–862. doi: 10.1002/stem.415. [DOI] [PubMed] [Google Scholar]
- 19.Hellevik T, Pettersen I, Berg V, Bruun J, Bartnes K, Busund LT, et al. Changes in the Secretory Profile of NSCLC-Associated Fibroblasts after Ablative Radiotherapy: Potential Impact on Angiogenesis and Tumor Growth. Transl Oncol. 2013;6:66–74. doi: 10.1593/tlo.12349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Z, Bao S, Wu Q, Wang H, Eyler C, Sathornsumetee S, et al. Hypoxia-inducible factors regulate tumorigenic capacity of glioma stem cells. Cancer Cell. 2009;15:501–513. doi: 10.1016/j.ccr.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ezashi T, Das P, Roberts RM. Low O2 tensions and the prevention of differentiation of hES cells. Proc Natl Acad Sci U S A. 2005;102:4783–4788. doi: 10.1073/pnas.0501283102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rohwer N, Cramer T. Hypoxia-mediated drug resistance: novel insights on the functional interaction of HIFs and cell death pathways. Drug Resist Updat. 2011;14:191–201. doi: 10.1016/j.drup.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 23.Moore N, Lyle S. Quiescent, slow-cycling stem cell populations in cancer: a review of the evidence and discussion of significance. J Oncol. 2011;2011 doi: 10.1155/2011/396076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dembinski JL, Krauss S. Characterization and functional analysis of a slow cycling stem cell-like subpopulation in pancreas adenocarcinoma. Clin Exp Metastasis. 2009;26:611–623. doi: 10.1007/s10585-009-9260-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naumov GN, Townson JL, MacDonald IC, Wilson SM, Bramwell VH, Groom AC, et al. Ineffectiveness of doxorubicin treatment on solitary dormant mammary carcinoma cells or late-developing metastases. Breast Cancer Res Treat. 2003;82:199–206. doi: 10.1023/B:BREA.0000004377.12288.3c. [DOI] [PubMed] [Google Scholar]
- 26.Pistollato F, Chen HL, Rood BR, Zhang HZ, D'Avella D, Denaro L, et al. Hypoxia and HIF1alpha repress the differentiative effects of BMPs in high-grade glioma. Stem Cells. 2009;27:7–17. doi: 10.1634/stemcells.2008-0402. [DOI] [PubMed] [Google Scholar]
- 27.Persano L, Pistollato F, Rampazzo E, Della Puppa A, Abbadi S, Frasson C, et al. BMP2 sensitizes glioblastoma stem-like cells to Temozolomide by affecting HIF-1alpha stability and MGMT expression. Cell Death Dis. 2012;3:e412. doi: 10.1038/cddis.2012.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 29.Hay ED, Fischman DA. Origin of the blastema in regenerating limbs of the newt Triturus viridescens. An autoradiographic study using tritiated thymidine to follow cell proliferation and migration. Dev Biol. 1961;3:26–59. doi: 10.1016/0012-1606(61)90009-4. [DOI] [PubMed] [Google Scholar]
- 30.Tata PR, Mou H, Pardo-Saganta A, Zhao R, Prabhu M, Law BM, et al. Dedifferentiation of committed epithelial cells into stem cells in vivo. Nature. 2013;503:218–223. doi: 10.1038/nature12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lagadec C, Vlashi E, Della Donna L, Dekmezian C, Pajonk F. Radiation-induced reprogramming of breast cancer cells. Stem Cells. 2012;30:833–844. doi: 10.1002/stem.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]