Abstract
Abstract
A series of diethyl 2-(4,5-dimethoxycarbonyl-1H-1,2,3-triazol-1-yl)alkylphosphonates was synthesised from ω-azidoalkylphosphonates and dimethyl acetylenedicarboxylate and was further transformed into the respective diamides, dihydrazides, and 5,6-dihydro-1H-[1,2,3]triazolo[4,5-d]pyridazine-4,7-diones as phosphonate analogues of acyclic nucleosides having nucleobases replaced with substituted 1,2,3-triazoles. All compounds containing P–C–C–triazole or P–C–C–CH2–triazole moieties exist in single conformations in which the diethoxyphosphoryl and substituted 1,2,3-triazolyl or substituted (1,2,3-triazolyl)methyl groups are oriented anti. All phosphonates were evaluated in vitro for activity against a variety of DNA and RNA viruses. None of the compounds were endowed with antiviral activity. They were not cytostatic at 100 μM.
Graphical abstract

Electronic supplementary material
The online version of this article (doi:10.1007/s00706-013-1137-x) contains supplementary material, which is available to authorized users.
Keywords: Cycloadditions, Cyclizations, Heterocycles, NMR spectroscopy, Conformation
Introduction
In the past two decades acyclic nucleoside phosphonates (ANPs) have become one of the most important classes of antiviral drugs [1]. Three of them (adefovir, cidofovir, tenofovir; Fig. 1) have been marketed for treatment of viral infection caused by HIV, HBV, HSV and other DNA viruses [2–5]. The concept of acyclic nucleosides is based on the assumption that an acyclic moiety most often bearing an oxygen atom mimics the furanose ring at least partially. Acyclic nucleoside phosphonates require conversion in vivo to their triphosphate metabolites to become active [2, 6]. The replacement of the natural phosphate moiety by a phosphonate group makes analogues less susceptible to enzymatic hydrolysis [7, 8].
Fig. 1.
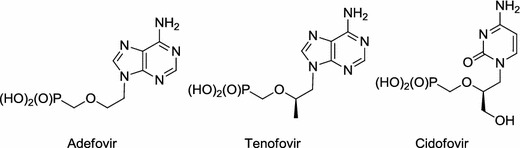
ANPs marketed for treatment of viral infections
Further studies in this field brought a new generation of nucleotide/nucleoside analogues in which natural nucleobases were modified as exemplified by the 2,4-diaminopyrimidine framework present in antiviral HPMPO-DAPy and PMPO-DAPy [9–11] and the 1,2,4-triazole ring in ribavirin (Fig. 2) [12].
Fig. 2.
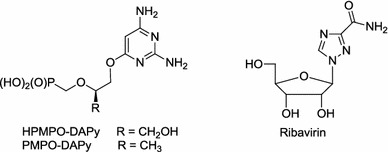
Structures of HPMPO-DAPy, PMPO-DAPy and ribavirin
The antiviral activity of ribavirin stimulated interest in replacing the 1,2,4-triazole system with an isomeric 1,2,3-triazole ring because several 1,2,3-triazoles exhibit antibacterial [13–15], antifungal [15–17], anticancer [18–20], anti-inflammatory [21, 22] and antiviral [23–26] properties. It was found that carbocyclic analogues 1 and phosphonocarbocyclic analogues 2 of ribavirin displayed antiviral activity against HIV-1 (Fig. 3) [27].
Fig. 3.
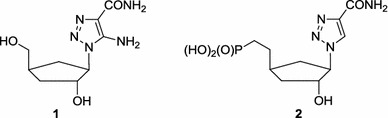
Antiviral analogues of ribavirin having the 1,2,3-triazole ring
Furthermore, preliminary structure–activity relationship evaluation of 1,2,3-triazole nucleoside phosphonates 3 and 4 suggested that this scaffold could be further optimised to afford selective inhibitors of HCV replication (Fig. 4) [28].
Fig. 4.
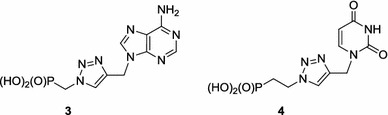
1,2,3-Triazole nucleoside phosphonates as potential inhibitors of HCV replication
On the other hand, it was reported that nucleoside analogues 5 and 6 containing the 5,6-dihydro-1H-imidazo[4,5-d]pyridazine-4,7-dione ring instead of the natural purine framework exhibited antiviral activity by inhibition of a viral helicase from West Nile Virus (WNV) and HCV (Fig. 5). These observations may be useful in designing a lead structure for the development of new classes of antiviral agents [29].
Fig. 5.

Cyclic nucleoside analogues based on the 5,6-dihydro-1H-imidazo[4,5-d]pyridazine-4,7-dione framework
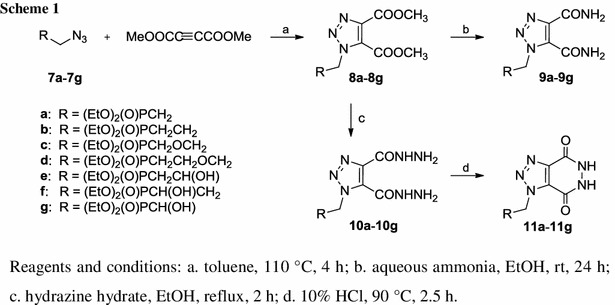
Based on the active compounds already discussed, a novel series of phosphonate analogues 11 having the 5,6-dihydro-1H-[1,2,3]triazolo[4,5-d]pyridazine-4,7-dione system was designed as potential antiviral agents (Scheme 1). Furthermore, because their immediate precursor dihydrazides 10 as well as diamides 9 share several common structural features with ribavirin, they also may show antiviral activity. The key step of our synthetic plan involves the 1,3-dipolar cycloaddition of dimethyl acetylenedicarboxylate and ω-azidoalkylphosphonates 7 which contain structurally diversified alkyl chains to provide the intermediate diesters 8.
Results and discussion
Chemistry
The 1,2,3-triazoles 8a–8d and 8f were synthesised in 64–98 % yield employing the Huisgen 1,3-dipolar cycloaddition of the corresponding azidophosphonates 7 and dimethyl acetylenedicarboxylate at 110 °C in the same way as the known compounds 8e and 8g [30, 31]. They were finally purified either by chromatography on a silica gel column or by crystallisation (Scheme 1).
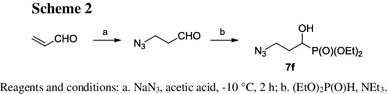
The required azidoalkylphosphonates 7a–7e and 7g have already been described in the literature [30, 32–36]. Azidophosphonate 7f was obtained in the Abramov reaction [37, 38] from 3-azidopropanal [39] and diethyl phosphite in 34 % yield (Scheme 2). It was found that 3-azidopropanal is unstable in the presence of triethylamine used as a catalyst in this reaction. For this reason only 0.4 equiv. of diethyl phosphite was applied to avoid tedious separation of 7f from the reaction mixture.
The diamides 9a–9g were obtained from diesters 8a–8g by ammonolysis according to a standard protocol (Scheme 1) [6, 40]. The crude products were subjected to column chromatography on silica gel and finally purified by crystallisation to give 9a–9g in 37–61 % yields. The 1H NMR spectra of diamides 9a–9g in chloroform-d confirmed the nonequivalence of protons from the carbamoyl groups because in the 6.0–10.8 ppm region four broad singlets were always observed.
The diesters 8a–8g were also converted to the corresponding dihydrazides 10a–10g with hydrazine hydrate (Scheme 1). Preliminary attempts at synthesising dihydrazide 10a followed the literature procedure [41] and showed that refluxing phosphonate 8a and hydrazine hydrate in ethanolic solution for 5 h led to the formation of several products. The 31P NMR spectrum of the reaction mixture revealed the presence of the expected phosphonate 10a (76 %), bicyclic 1,2,3-triazolopyridazinedione 11a (11 %) and their monodealkylated counterparts 12a and 13a (10 and 3 %, respectively) (Fig. 6). When hydrazinolysis of the diester 8a was conducted for 2 h only phosphonates 10a (79 %) and 11a (21 %) were produced. For this reason syntheses of dihydrazides 10b–10g were performed under the same conditions. However, it appeared that for compounds 8c and 8g significant dealkylation still occurred. In both cases the respective crude reaction mixtures contained almost 50 % of by-products. Purifications on silica gel columns and crystallisations gave dihydrazides 10a–10g in 28–66 % yields.
Fig. 6.
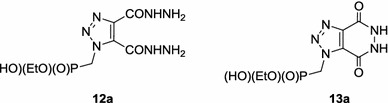
Compounds 12a and 13a
Finally, heating dihydrazides 10a–10g with 10 % hydrochloric acid for 2.5 h gave [1,2,3]triazolo[4,5-d]pyridazine-4,7-diones 11a–11g (Scheme 1) in 30–66 % yields [41].
Conformational analysis
Detailed analyses of 1H and 13C NMR spectral data revealed conformational preferences of phosphonates described in this paper. Compounds 8a–11a contain an 1,2-substituted ethylene fragment which does not freely rotate around a C–C bond because their 1H NMR spectra display AA′XX′P patterns. A similar spectrum was also noticed for 2-azidoethylphosphonate [31]. Antiperiplanar disposition of the diethoxyphosphoryl groups and substituted 1,2,3-triazoles 14a (Fig. 7) was proved by the presence of two identical 3 J(P–HX) = 10.5 Hz couplings which were calculated from the 1H{31P} NMR spectrum of 8a.
Fig. 7.
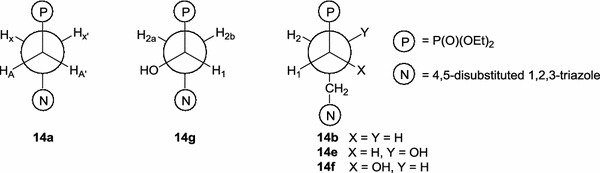
Preferred conformations of the phosphonates described in this study
In the 1H NMR spectra of compounds 8g, 10g, and 11g three hydrogen atoms attached to the two-carbon linker appeared as deceptively simple but very similar ABXP spectral patterns. However, the relevant 3 J(H1–H2a) and 3 J(H1–H2b) coupling constants were precisely calculated (10.2 and 3.6 Hz, respectively) from the 1H NMR spectrum of diamide 9g. These values [42] together with small couplings for 3 J(P–H2a) and 3 J(P–H2b) [43, 44] allowed us to unequivocally establish 14g (Fig. 7) as the preferred conformation of phosphonate 9g although other phosphonates from this series very likely adopt the same anti conformation.
Large values (16.9–19.2 Hz) of 3 J(P–CC–C3) [44–46] observed in the 13C NMR spectra of phosphonates 8–11 containing a three-carbon fragment between the phosphorus atom and the 1,2,3-triazole ring (series b, e, and f) evidenced the preference of antiperiplanar conformations 14b, 14e, and 14f (Fig. 7) for these compounds. This conclusion was further supported by vicinal H1–H2 couplings calculated for 2- and 1-hydroxyphosphonates (series e and f) which clearly indicated gauche (3.0–3.6 Hz) and anti (9.6–10.8 Hz) arrangements of the respective H–C1C2–H protons.
Although values (9.3–12.1 Hz) of 3 J(P–C–O–C) were easily extracted from the 13C NMR spectra of phosphonates 8–11 (series c) they could not be unequivocally applied in the estimation of the extent to which rotation around the PC–OC bond is hindered because the angular dependence of 3 J(P–C–O–C) has not been established so far. However, the rotation around the OC–CN bond is not restricted because vicinal H2C–CH2 coupling constants observed for phosphonates 8c–11c fall in the 4.8–5.2 Hz range. On the other hand, on the basis of the values of 3 J(HC–CH) found for PH2C–CH2O and OH2C–CH2N units (7.2–7.8 and 5.1–5.7 Hz, respectively), full conformational freedom within a five-atom linker in phosphonates 8–11 (series d) is anticipated.
Antiviral activity evaluation
The synthesised compounds 9a–9g, 10a–10g, and 11a–11g were evaluated for their antiviral activities against a wide variety of DNA and RNA viruses using the following cell-based assays: (a) human embryonic lung (HEL) cell: herpes simplex virus-1 (KOS), herpes simplex virus-2 (G), herpes simplex virus-1 (TK− ACVr KOS), vaccinia virus, vesicular stomatitis virus, varicella-zoster virus (TK+ VZV strain OKA and TK¯ VZV strain 07-1) and cytomegalovirus (CMV) (strain AD-169 and Davis); (b) CEM cell cultures: human immunodeficiency virus-1 (HIV-1) and HIV-2; (c) Vero cell cultures: para-influenza-3 virus, reovirus-1, Sindbis virus, Coxsackie virus B4, Punta Toro virus; (d) HeLa cell cultures: vesicular stomatitis virus, Coxackie virus B4 and respiratory syncytial virus; (e) Crandel-Rees feline kidney (CRFK) cell cultures: feline corona virus (FIPV) and feline herpes virus (FHV) and (f) Madin Darby Canine kidney (MDCK) cell culture: influenza A virus H1N1 subtype A/PR/8, influenza H3N2 subtype A/HK/7/87 and influenza B virus (B/HK/5/72). Ganciclovir, cidofovir, acyclovir, brivudin, (S)-9-(2,3-dihydroxypropyl)adenine [(S)-DHPA], ribavirin, oseltamivir carboxylate, amantadine and rimantadine were used as the reference compounds. The antiviral activity was expressed as the EC50: the compound concentration required to reduce virus plaques formation (VZV, CMV) by 50 % or to reduce virus-induced cytopathogenicity by 50 % (other viruses). None of the compounds showed appreciable antiviral activity at subtoxic concentrations.
Evaluation of cytotoxicity
The cytotoxicity of the tested compounds towards the uninfected host cells was defined as the minimum cytotoxic concentration (MMC) that causes a microscopically detectable alteration of normal cell morphology. The 50 % cytotoxic concentration (CC50), i.e. causing a 50 % decrease in cell viability, was determined using a colorimetric 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay system. None of the tested compounds affected the cell morphology of Vero, HEL, HeLa or CRFK cells (MCC or CC50) at compound concentrations up to 100 μM. They were also not cytostatic against murine leukemia and human CEM and HeLa cells at 100 μM.
Conclusions
Phosphonylated 1,2,3-triazoles 8a–8g were obtained in good to excellent yields by 1,3-dipolar cycloaddition between ω-azidophosphonates 7a–7g and dimethyl acetylenedicarboxylate. New series of diamides 9a–9g, dihydrazides 10a–10g and 1,2,3-triazolopyridazinediones 11a–11g were efficiently synthesised as phosphonate analogues of acyclic nucleosides in which nucleobases were replaced by substituted 1,2,3-triazoles.
Compounds 8–11 (series a, b, e, f, g) exist in single conformations in which diethoxyphosphoryl and substituted 1,2,3-triazolyl (series a and b) or (1,2,3-triazolyl)methyl groups (series e, f, g) prefer the anti orientation.
All synthesised compounds were evaluated for their antiviral activity against DNA and RNA viruses and were inactive. None of the compounds were cytotoxic (Vero, HEL, HeLa) or cytostatic (L1210, CEM, HeLa) at a concentration up to 100 μM.
Experimental
The 1H NMR spectra were recorded in CDCl3, CD3OD or D2O on the following spectrometers: Varian Mercury-300 and Bruker Avance III (600 MHz) with TMS as an internal standard; chemical shifts δ in ppm with respect to TMS; coupling constants J in Hz. The 13C NMR spectra were recorded for CDCl3, CD3OD or D2O solutions on Varian Mercury-300 and Bruker Avance III (600 MHz) machines at 75.5 and 150.5 MHz, respectively. The 31P NMR spectra were recorded in CDCl3, CD3OD or D2O on Varian Mercury-300 and Bruker Avance III (600 MHz) spectrometers at 121.5 and 243 MHz, respectively.
IR spectra were measured on an Infinity MI-60 FT-IR spectrometer. Melting points were determined on the Boetius apparatus. Elemental analyses were performed by the microanalytical laboratory of the host institution on Perkin Elmer PE 2400 CHNS analyzer and the results were found to be in good agreement (±0.3 %) with the calculated values.
The following absorbents were used: column chromatography, Merck silica gel 60 (70–230 mesh); analytical TLC, Merck TLC plastic sheets, silica gel 60 F254. TLC plates were developed in chloroform–methanol solvent systems. Visualization of spots was effected with iodine vapours. All solvents were dried according to standard literature methods.
Diethyl 3-azido-1-hydroxypropylphosphonate (7f, C7H16N3O4P)
A mixture of 5.77 g 3-azidopropanal [39] (0.0582 mol), 3.0 cm3 diethyl phosphite (0.023 mol) and 0.81 cm3 triethylamine (0.0058 mol) was stirred at 5 °C for 24 h. The crude product was subjected to chromatography on silica gel with chloroform/methanol (100:1 and 50:1, v/v) to give a yellow oil (4.671 g, 34 %). IR (film): = 3,272, 2,985, 2,934, 2,911, 2,874, 2,102, 1,227, 1,028 cm−1; 1H NMR (CDCl3, 300 MHz): δ = 1.35 (t, J = 7.1 Hz, 6H, 2 × POCH2CH 3), 1.87–2.06 (m, 3H, CH2, OH), 3.55 (t, J = 6.3 Hz, 2H, CH 2N3), 4.01 (dt, J = 9.8, 4.6 Hz, 1H, H-1), 4.12–4.24 (m, 4H, 2 × POCH 2CH3) ppm; 13C NMR (CDCl3, 75.5 MHz): δ = 16.6 and 16.7 (2 d, J = 5.4 Hz, POCC), 30.8 (d, J = 2.6 Hz, C-2), 47.7 (d, J = 15.5 Hz, C-3), 62.9 and 63.2 (2 d, J = 7.2 Hz, POC), 64.6 (d, J = 164.6 Hz, C-1) ppm; 31P NMR (CDCl3, 121.5 MHz): δ = 25.28 ppm.
Synthesis of 1,2,3-triazoles 8a–8g (general procedure)
A solution of azidophosphonate 7a–7g (1.00 mmol) and dimethyl acetylenedicarboxylate 13 (1.00 mmol) in 4 cm3 toluene was refluxed for 4 h. The reaction mixtures were concentrated to dryness to leave yellow oils which were purified on silica gel columns with chloroform/methanol (100:1, v/v) or were crystallised to give 1,2,3-triazoles 8a–8g.
Diethyl 2-(4,5-dimethoxycarbonyl-1H-1,2,3-triazol-1-yl)ethylphosphonate (8a, C12H20N3O7P)
From 0.450 g azidophosphonate 7a (2.17 mmol) and 0.309 g dimethyl acetylenedicarboxylate 13 (2.17 mmol) phosphonate 8a was obtained as a yellowish oil (0.674 g, 89 %) after chromatography on a silica gel column with chloroform/methanol (100:1, v/v). IR (film): = 3,462, 2,985, 2,958, 1,736, 1,468, 1,447, 1,225, 1,140, 1,060, 1,025, 957 cm−1; 1H NMR (CDCl3, 300 MHz): δ = 1.33 (t, J = 7.1 Hz, 6H, CH 3CH2OP), 2.40–2.52 (m, 2H, PCH2), 3.98 (s, 3H, OCH3), 4.02 (s, 3H, OCH3), 4.08–4.18 (m, 4H, CH3CH 2OP), 4.82–4.91 (m, 2H, CH2N) ppm; 13C NMR (CDCl3, 75 MHz): δ = 16.6 (d, J = 5.7 Hz, CCOP), 27.2 (d, J = 140.6 Hz, PC), 45.4 (s, PCCN), 52.9 (s, OCH3), 53.7 (s, OCH3), 62.4 (d, J = 6.3 Hz, CCOP), 129.8 and 140.2 (2 s, C=C), 158.6 and 160.4 (2 s, C=O) ppm; 31P NMR (CDCl3, 121 MHz): δ = 25.9 ppm.
Diethyl 3-(4,5-dimethoxycarbonyl-1H-1,2,3-triazol-1-yl)propylphosphonate (8b, C13H22N3O7P)
From 0.785 g azidophosphonate 7b (3.565 mmol) and 0.507 g dimethyl acetylenedicarboxylate 13 (3.565 mmol) phosphonate 8b was obtained as a yellowish oil (1.067 g, 83 %) after chromatography on a silica gel column with chloroform/methanol (100:1, v/v). IR (film): = 2,953, 2,836, 1,736, 1,463, 1,226, 1,030 cm−1; 1H NMR (CDCl3, 300 MHz): δ = 1.34 (t, J = 7.1 Hz, 6H, CH 3CH2OP), 1.72–1.84 (m, 2H, PCH2CH 2), 2.16–2.31 (m, 2H, PCH 2), 4.00 (s, 3H, OCH3), 4.03 (s, 3H, OCH3), 4.05–4.18 (m, 4H, CH3CH 2OP), 4.71 (t, J = 7.1 Hz, 2H, CH2N) ppm; 13C NMR (CDCl3, 75 MHz): δ = 16.5 (d, J = 6.0 Hz, POCC), 22.6 (d, J = 143.1 Hz, PC), 23.5 (d, J = 4.3 Hz, PCC), 50.4 (d, J = 16.9 Hz, PCCC), 52.7 (s, OCH3), 53.2 (s, OCH3), 61.8 (d, J = 6.3 Hz, POC), 129.7 and 139.8 (2 s, C=C), 158.6 and 160.3 (2 s, C=O) ppm; 31P NMR (CDCl3, 121 MHz): δ = 30.7 ppm.
Diethyl 2-(4,5-dimethoxycarbonyl-1H-1,2,3-triazol-1-yl)ethoxymethylphosphonate (8c, C13H22N3O8P)
From 1.027 g azidophosphonate 7c (4.330 mmol) and 0.615 g dimethyl acetylenedicarboxylate 13 (4.330 mmol) phosphonate 8c was obtained as a yellowish oil (1.538 g, 93 %) after chromatography on a silica gel column with chloroform/methanol (100:1, v/v). IR (film): = 3,459, 2,983, 2,957, 2,908, 1,732, 1,462, 1,225, 1,117, 1,060, 1,027, 960 cm−1; 1H NMR (CDCl3, 600 MHz): δ = 1.31 (t, J = 7.0 Hz, 6H, CH 3CH2OP), 3.74 (d, J = 7.9 Hz, 2H, PCH2), 3.98 (s, 3H, OCH3), 4.02 (s, 3H, OCH3), 4.02 (t, J = 5.2 Hz, 2H, OCH 2CH2N), 4.08–4.13 (m, 4H, CH3CH 2OP), 4.86 (t, J = 5.2 Hz, 2H, OCH2CH 2N) ppm; 13C NMR (CDCl3, 75 MHz): δ = 16.5 (d, J = 5.7 Hz, POCC), 49.8 (s, CN), 52.8 (s, OCH3), 53.5 (s, OCH3), 62.6 (d, J = 6.5 Hz, POCC), 65.2 (d, J = 164.7 Hz, PC), 71.0 (d, J = 9.3 Hz, PCOC), 131.0 and 139.6 (2 s, C=C), 158.8 and 160.3 (2 s, C=O) ppm; 31P NMR (CDCl3, 243 MHz): δ = 20.04 ppm.
Diethyl 2-[2-(4,5-dimethoxycarbonyl-1H-1,2,3-triazol-1-yl)ethoxy]ethylphosphonate (8d, C14H24N3O8P)
From 0.647 g azidophosphonate 7d (2.68 mmol) and 0.382 g dimethyl acetylenedicarboxylate 13 (2.68 mmol) phosphonate 8d (1.030 g, 98 %) was obtained as a yellowish oil. The crude product was sufficiently pure and was used in the next step without further purification. IR (film): = 3,459, 2,983, 2,957, 2,909, 1,736, 1,466, 1,229, 1,118, 1,063, 1,027, 963 cm−1; 1H NMR (CDCl3, 600 MHz): δ = 1.32 (t, J = 7.1 Hz, 6H, CH 3CH2OP), 2.00 (dt, J = 15.3, 7.7 Hz, 2H, PCH2), 3.63 (dt, J = 10.4, 7.7 Hz, 2H, PCH2CH 2O), 3.82 (t, J = 5.2 Hz, 2H, OCH 2CH2N), 4.00 (s, 3H, OCH3), 4.01 (s, 3H, OCH3), 4.05–4.12 (m, 4H, CH3CH 2OP), 4.82 (t, J = 5.2 Hz, 2H, OCH2CH 2N) ppm; 13C NMR (CDCl3, 150 MHz): δ = 16.3 (d, J = 5.7 Hz, POCC), 26.7 (d, J = 139.3 Hz, PC), 49.8 (s, OCCN), 52.6 (s, OCH3), 53.3 (s, OCH3), 61.6 (d, J = 6.4 Hz, CCOP), 65.3 (s, PCC), 68.7 (s, OCCN), 131.4 and 139.5 (2 s, C=C), 159.1 and 160.3 (2 s, C=O) ppm; 31P NMR (CDCl3, 243 MHz): δ = 27.58 ppm.
Diethyl 1-hydroxy-3-(4,5-dimethoxycarbonyl-1H-1,2,3-triazol-1-yl)propylphosphonate (8f, C13H22N3O8P)
From 0.560 g azidophosphonate 7f (2.36 mmol) and 0.335 g dimethyl acetylenedicarboxylate 13 (2.36 mmol) phosphonate 8f (0.850 g, 95 %) was obtained as a white amorphous solid after chromatography on a silica gel column with chloroform/methanol (100:1, v/v). M.p.: 107–109 °C; IR (film): = 3,420, 3,218, 3,081, 2,985, 2,878, 1,675, 1,451, 1,280, 1,223, 1,030, 970 cm−1; 1H NMR (CDCl3, 300 MHz): δ = 1.33 and 1.34 (2 t, J = 7.2 Hz, 6H, CH 3CH2OP), 2.28–2.47 (m, 2H, PCCH 2), 3.70 (dd, J = 6.0, 5.1 Hz, 1H, OH), 3.84 (dddd, J = 10.5, 6.3, 6.0, 3.3 Hz, 1H, PCH), 3.98 (s, OCH3), 4.00 (s, OCH3), 4.10–4.22 (m, 4H, CH3CH 2OP), 4.75–4.90 (m, 2H, CH2N) ppm; 13C NMR (CDCl3, 75 MHz): δ = 16.6 and 16.7 (2 d, J = 5.4 Hz, CCOP), 31.9 (d, J = 3.2 Hz, PCC), 47.2 (d, J = 16.3 Hz, PCCC), 52.8 (s, OCH3), 53.6 (s, OCH3), 63.0 and 63.3 (2 d, J = 7.3 Hz, CCOP), 64.6 (d, J = 164.2 Hz, PC), 130.3 and 139.7 (2 s, C=C), 158.8 and 160.4 (2 s, C=O) ppm; 31P NMR (CDCl3, 121 MHz): δ = 23.5 ppm.
Synthesis of diamides 9a–9g (general procedure)
To a solution of the diester 8a–8g (1.00 mmol) in 14 cm3 ethanol 16 cm3 was added aqueous ammonia. The reaction mixtures were stirred at room temperature for 24 h. Ethanol and excess ammonia were evaporated in vacuo. Diamides 9a–9g were purified on silica gel columns with chloroform/methanol or by crystallisation.
Diethyl 2-(4,5-dicarbamoyl-1H-1,2,3-triazol-1-yl)ethylphosphonate (9a, C10H18N5O5P)
From 0.165 g diester 8a (0.472 mmol) diamide 9a was obtained as a white amorphous solid (0.065 g, 42 %) after chromatography on a silica gel column with chloroform/methanol (50:1, 20:1, v/v) followed by crystallisation from ethanol/diethyl ether. M.p.: 172–173 °C; IR (KBr): = 3,428, 3,228, 3,113, 2,986, 2,932, 1,687, 1,451, 1,221, 1,023 cm−1; 1H NMR (CDCl3, 300 MHz): δ = 1.34 (t, J = 6.9 Hz, 6H, CH 3CH2OP), 2.41–2.53 (m, 2H, PCH2), 4.09–4.21 (m, 4H, CH3CH 2OP), 5.08–5.17 (m, 2H, CH2N), 6.01 (s, 2H, NH2), 7.58 (s, 1H, NH), 10.77 (s, 1H, NH) ppm; 13C NMR (CD3OD, 75 MHz): δ = 16.8 (d, J = 6.0 Hz, CCOP), 27.3 (d, J = 140.3 Hz, PC), 47.2 (s, PCCN), 63.8 (d, J = 6.6 Hz, CCOP), 132.1 and 140.4 (2 s, C=C), 160.3 and 165.2 (2 s, C=O) ppm; 31P NMR (CDCl3, 121 MHz): δ = 26.5 ppm.
Diethyl 3-(4,5-dicarbamoyl-1H-1,2,3-triazol-1-yl)propylphosphonate (9b, C11H20N5O5P)
From 0.114 g diester 8b (0.314 mmol) diamide 9b was obtained as a white amorphous solid (0.070 g, 61 %) after chromatography on a silica gel column with chloroform/methanol (50:1, v/v) and crystallisation from ethanol. M.p.: 143–144 °C; IR (KBr): = 3,455, 3,270, 2,984, 2,909, 1,694, 1,629, 1,592, 1,454, 1,205, 1,018, 961 cm−1; 1H NMR (CDCl3, 300 MHz): δ = 1.31 (t, J = 6.9 Hz, 6H, CH 3CH2OP), 1.74–1.86 (m, 2H, PCH2CH 2), 2.18–2.32 (m, 2H, PCH2), 4.04–4.16 (m, 4H, CH3CH 2OP), 4.98 (t, J = 6.9 Hz, 2H, CH2N), 6.13 (s, 1H, NH), 6.25 (s, 1H, NH), 7.62 (s, 1H, NH), 10.79 (s, 1H, NH) ppm; 13C NMR (CDCl3, 75 MHz): δ = 16.7 (d, J = 6.6 Hz, CCOP), 23.0 (d, J = 141.9 Hz, PC), 23.8 (d, J = 4.3 Hz, PCC), 51.8 (d, J = 19.2 Hz, PCCC), 62.0 (d, J = 6.6 Hz, CCOP), 130.7 and 138.7 (2 s, C=C), 158.6 and 163.7 (2 s, C=O) ppm; 31P NMR (CDCl3, 121 MHz): δ = 31.4 ppm.
Diethyl [2-(4,5-dicarbamoyl-1H-1,2,3-triazol-1-yl)ethoxy]methylphosphonate (9c, C11H20N5O6P)
From 0.280 g diester 8c (0.738 mmol) diamide 9c was obtained as a white amorphous solid (0.139 g, 54 %) after chromatography on a silica gel column with chloroform/methanol (50:1, v/v). M.p.: 100–101 °C; IR (KBr): = 3,361, 3,259, 3,110, 2,990, 2,959, 2,901, 1,689, 1,613, 1,454, 1,303, 1,219, 1,118, 1,048, 1,019, 967, 943 cm−1; 1H NMR (CD3OD, 600 MHz): δ = 1.29 (t, J = 7.1 Hz, 6H, CH 3CH2OP), 3.89 (d, J = 8.5 Hz, 2H, PCH2), 4.06 (t, J = 5.2 Hz, 2H, OCH 2CH2N), 4.06–4.10 (m, 4H, CH3CH 2OP), 5.14 (t, J = 5.2 Hz, 2H, CH2N) ppm; 13C NMR (CD3OD, 150 MHz): δ = 15.3 (d, J = 5.9 Hz, CCOP), 50.3 (s, CN), 62.8 (d, J = 6.5 Hz, CCOP), 64.0 (d, J = 164.7 Hz, PC), 71.1 (d, J = 11.9 Hz, PCOC), 131.0 and 139.0 (2 s, C=C), 159.1 and 163.9 (2 s, C=O) ppm; 31P NMR (CD3OD, 243 MHz): δ = 21.3 ppm.
Diethyl 2-[2-(4,5-dicarbamoyl-1H-1,2,3-triazol-1-yl)ethoxy]ethylphosphonate (9d, C12H22N5O6P)
From 0.264 g diester 8d (0.671 mmol) diamide 9d was obtained as a white amorphous solid (0.090 g, 37 %) after chromatography on a silica gel column with chloroform/methanol (50:1, v/v) and crystallisation from ethyl acetate. M.p.: 102–103 °C; IR (KBr): = 3,400, 3,306, 3,214, 2,986, 2,906, 1,672, 1,608, 1,453, 1,245, 1,109, 1,024, 961 cm−1; 1H NMR (CDCl3, 300 MHz): δ = 1.31 (t, J = 6.9 Hz, 6H, CH 3CH2OP), 2.05 (dt, J = 15.3, 7.8 Hz, 2H, PCH2), 3.69 (dt, J = 11.1, 7.8 Hz, 2H, PCH2CH 2O), 3.91 (t, J = 5.4 Hz, 2H, OCH 2CH2N), 4.01–4.12 (m, 4H, CH3CH 2OP), 5.11 (t, J = 5.4 Hz, 2H, OCH2CH 2N), 6.00 (s, 1H, NH), 6.07 (s, 1H, NH), 7.60 (s, 1H, NH), 10.77 (s, 1H, NH) ppm; 13C NMR (CDCl3, 75 MHz): δ = 16.6 (d, J = 6.0 Hz, CCOP), 26.9 (d, J = 139.1 Hz, PC), 51.0 (s, OCCN), 61.9 (d, J = 6.3 Hz, CCOP), 65.0 (s, PCCO), 69.1 (s, OCCN), 130.9 and 139.7 (2 s, C=C), 158.7 and 163.7 (2 s, C=O) ppm; 31P NMR (CDCl3, 121 MHz): δ = 29.2 ppm.
Diethyl 3-(4,5-dicarbamoyl-1H-1,2,3-triazol-1-yl)-2-hydroxypropylphosphonate (9e, C11H20N5O6P)
From 0.145 g diester 8e (0.382 mmol) diamide 9e was obtained as a white amorphous solid (0.060 g, 45 %) after chromatography on a silica gel column with chloroform/methanol (50:1, v/v) and crystallisation from methanol. M.p.: 142–143 °C; IR (KBr): = 3,428, 3,308, 3,034, 2,988, 1,686, 1,667, 1,599, 1,443, 1,375, 1,293, 1,113, 1,083, 1,055, 1,029, 960 cm−1; 1H NMR (CD3OD, 300 MHz): δ = 1.32 (t, J = 7.2 Hz, 6H, CH 3CH2OP), 2.06 (ddd, J = 22.8, 15.3, 7.8 Hz, 1H, PCH aHb), 2.18 (ddd, J = 20.4, 15.3, 5.1 Hz, 1H, PCHa H b), 4.07–4.19 (m, 4H, CH3CH 2OP), 4.37–4.50 (m, 1H, PCCH), 4.99 (dd, J = 13.5, 8.1 Hz, 1H, PCCCH aHb), 5.02 (dd, J = 13.5, 4.2 Hz, 1H, PCCCHa H b) ppm; 13C NMR (CD3OD, 75 MHz): δ = 16.9 (d, J = 6.3 Hz, CCOP), 32.3 (d, J = 141.7 Hz, PC), 58.1 (d, J = 18.0 Hz, PCCC), 63.4 and 63.7 (2 d, J = 6.6 Hz, CCOP), 67.0 (d, J = 4.0 Hz, PCC), 132.6 and 140.4 (2 s, C=C), 160.6 and 165.3 (2 s, C=O) ppm; 31P NMR (CD3OD, 121 MHz): δ = 30.1 ppm.
Diethyl 3-(4,5-dicarbamoyl-1H-1,2,3-triazol-1-yl)-1-hydroxypropylphosphonate (9f, C11H20N5O6P)
From 0.180 g diester 8f (0.475 mmol) diamide 9f was obtained as a white amorphous solid (0.093 g, 56 %) after crystallisation from methanol. M.p.: 179–180 °C; IR (KBr): = 3,433, 3,319, 3,212, 2,974, 2,930, 2,874, 1,668, 1,605, 1,455, 1,399, 1,221, 1,167, 1,066, 1,014, 953 cm−1; 1H NMR (CD3OD, 300 MHz): δ = 1.32 (t, J = 7.2 Hz, 6H, CH 3CH2OP), 2.13–2.28 (m, 1H, PCCH aHb), 2.31–2.44 (m, 1H, PCCHa H b), 3.89 (ddd, J = 10.5, 7.2, 3.0 Hz, 1H, PCH), 4.10–4.21 (m, 4H, CH3CH 2OP), 4.95–5.14 (m, 2H, CH2N) ppm; solubility of 9f in D2O or CD3OD was not sufficient to measure the 13C NMR spectrum; 31P NMR (CD3OD, 121 MHz): δ = 25.4 ppm.
Diethyl 2-(4,5-dicarbamoyl-1H-1,2,3-triazol-1-yl)-1-hydroxyethylphosphonate (9g, C10H18N5O6P)
From 0.230 g diester 8g (0.630 mmol) diamide 9g was obtained as a white amorphous solid (0.112 g, 53 %) after chromatography on a silica gel column with chloroform/methanol (20:1, v/v) and crystallisation from methanol. M.p.: 200–202 °C; IR (KBr): = 3,431, 3,223, 3,081, 2,990, 2,847, 1,671, 1,599, 1,452, 1,393, 1,289, 1,225, 1,031, 979 cm−1; 1H NMR (CD3OD, 600 MHz): δ = 1.39 and 1.40 (2 t, J = 7.2 Hz, 6H, CH 3CH2OP), 4.22-4.29 (m, 4H, CH3CH 2OP), 4.55 (ddd, J = 10.2, 9.9 Hz, 3.6 Hz, 1H, PCH), 5.10 (ddd, J = 13.5, 10.2, 8.7 Hz, 1H, PCCH aHb), 5.22 (ddd, J = 13.5, 3.6, 3.1 Hz, 1H, PCCHa H b) ppm; solubility of 9g in D2O and CD3OD was not sufficient to measure the 13C NMR spectrum; 31P NMR (CD3OD, 243 MHz): δ = 21.2 ppm.
Synthesis of dihydrazides 10a–10g (general procedure)
A solution of the diester 8a–8g (0.73 mmol) and 0.530 cm3 hydrazine hydrate (10.8 mmol) in 6 cm3 ethanol was refluxed for 2 h. The reaction mixtures were concentrated to give yellow oils or solids which were subjected to chromatography on a silica gel column with chloroform/methanol or crystallisation to obtain dihydrazides 10a–10g.
Diethyl 2-[4,5-bis(hydrazinocarbonyl)-1H-1,2,3-triazol-1-yl]ethylphosphonate (10a, C10H20N7O5P)
From 0.115 g diester 8a (0.330 mmol) dihydrazide 10a was obtained (0.070 g, 61 %) after chromatography on a silica gel column with chloroform/methanol (50:1, 20:1, 10:1, v/v) as a white amorphous solid. M.p.: 84–86 °C; IR (KBr): = 3,335, 3,282, 2,984, 2,932, 1,661, 1,606, 1,551, 1,491, 1,262, 1,223, 1,056, 1,020, 963 cm−1; 1H NMR (CD3OD, 300 MHz): δ = 1.32 (t, J = 6.6 Hz, 6H, CH 3CH2O), 2.48–2.59 (m, 2H, PCH2), 4.07–4.18 (m, 4H, CH3CH 2O), 5.03–5.14 (m, 2H, CH2N) ppm; 13C NMR (CD3OD, 75 MHz): δ = 16.9 (d, J = 6.0 Hz, CCOP), 27.4 (d, J = 140.0 Hz, PC), 47.1 (s, PCCN), 63.8 (d, J = 6.3 Hz, CCOP × 2), 130.8 and 139.3 (2 s, C=C), 157.6 and 161.8 (2 s, C=O) ppm; 31P NMR (CD3OD, 121 MHz): δ = 28.2 ppm.
Diethyl 3-[4,5-bis(hydrazinocarbonyl)-1H-1,2,3-triazol-1-yl]propylphosphonate (10b, C11H22N7O5P)
From 0.170 g diester 8b (0.468 mmol) dihydrazide 10b was obtained as a white amorphous solid (0.113 g, 66 %) after chromatography on a silica gel column with chloroform/methanol (50:1, 20:1, 10:1, v/v). M.p.: 73–75 °C; IR (KBr): = 3,300, 3,192, 2,983, 2,931, 1,656, 1,551, 1,209, 1,024, 969 cm−1; 1H NMR (CDCl3, 300 MHz): δ = 1.28 (t, J = 6.9 Hz, 6H, CH 3CH2OP), 1.71–1.82 (m, 2H, PCH2CH 2), 2.13–2.27 (m, 2H, PCH2), 4.00–4.13 (m, 4H, CH3CH 2OP), 4.94 (t, J = 7.2 Hz, 2H, CH2N), 5.15–6.03 (brs, 6H, NHNH2) ppm; 13C NMR (CDCl3, 75 MHz): δ = 16.7 (d, J = 6.0 Hz, CCOP), 22.9 (d, J = 139.9 Hz, PC), 23.9 (d, J = 7.4 Hz, PCC), 51.8 (d, J = 18.9 Hz, PCCC), 62.0 (d, J = 6.6 Hz, CCOP), 129.5 and 137.6 (2 s, C=C), 156.3 and 161.3 (2 s, C=O) ppm; 31P NMR (CDCl3, 121 MHz): δ = 31.5 ppm.
Diethyl [2-[4,5-bis(hydrazinocarbonyl)-1H-1,2,3-triazol-1-yl]ethoxy]methylphosphonate (10c, C11H22N7O6P)
From 0.430 g diester 8c (1.13 mmol) dihydrazide 10c was obtained as a colourless oil (0.139 g, 32 %) after chromatography on a silica gel column with chloroform/methanol (50:1, 20:1, 10:1, v/v). IR (film): = 3,326, 3,248, 2,985, 2,918, 2,890, 1,666, 1,544, 1,440, 1,300, 1,238, 1,117, 1,024, 968 cm−1; 1H NMR (CD3OD, 600 MHz): δ = 1.28 (t, J = 7.0 Hz, 6H, CH 3CH2OP), 3.89 (d, J = 8.5 Hz, 2H, PCH2), 4.05–4.10 (m, 6H, CH3CH 2OP and OCH 2CH2N), 5.14 (t, J = 4.8 Hz, 2H, OCH2CH 2N) ppm; 13C NMR (CD3OD, 150 MHz): δ = 15.3 (d, J = 5.5 Hz, CCOP), 50.2 (s, CN), 62.8 (d, J = 6.5 Hz, CCOP), 64.0 (d, J = 164.9 Hz, PC), 71.1 (d, J = 11.9 Hz, PCOC), 129.9 and 137.9 (2 s, C=C), 156.5 and 160.5 (2 s, C=O) ppm; 31P NMR (CD3OD, 243 MHz): δ = 21.4 ppm.
Diethyl 2-[2-[4,5-bis(hydrazinocarbonyl)-1H-1,2,3-triazol-1-yl]ethoxy]ethylphosphonate (10d, C12H24N7O6P)
From 0.216 g diester 8d (0.549 mmol) dihydrazide 10d was obtained as a white amorphous solid (0.128 g, 59 %) after chromatography on a silica gel column with chloroform/methanol (50:1, 20:1, v/v) and crystallisation from ethyl acetate. M.p: 95–96 °C; IR (KBr): = 3,300, 3,232, 2,983, 2,913, 2,827, 1,667, 1,545, 1,550, 1,252, 1,023, 966, 918 cm−1; 1H NMR (CDCl3, 300 MHz): δ = 1.31 (t, J = 7.2 Hz, 6H, CH 3CH2OP), 2.03 (dt, J = 15.0, 7.5 Hz, 2H, PCH2), 3.69 (dt, J = 11.1, 7.5 Hz, 2H, PCH2CH 2O), 3.91 (t, J = 5.7 Hz, 2H, OCH 2CH2N), 4.01–4.13 (m, 4H, CH3CH 2OP), 4.20 (brs, 4H, NH2), 5.13 (t, J = 5.7 Hz, 2H, OCH2CH 2N), 8.81 (s, 1H, NH), 12.0 (s, 1H, NH) ppm; 13C NMR (CDCl3, 75 MHz): δ = 16.6 (d, J = 6.0 Hz, CCOP), 27.0 (d, J = 138.5 Hz, PC), 51.0 (s, OCCN), 61.9 (d, J = 6.3 Hz, CCOP), 65.0 (s, PCCO), 69.1 (s, OCCN), 129.9 and 137.5 (2 s, C=C), 156.5 and 161.3 (2 s, C=O) ppm; 31P NMR (CDCl3, 121 MHz): δ = 28.5 ppm.
Diethyl 3-[4,5-bis(hydrazinocarbonyl)-1H-1,2,3-triazol-1-yl]-2-hydroxypropylphosphonate (10e, C11H22N7O6P)
From 0.290 g diester 8e (0.764 mmol) dihydrazide 10e was obtained as a white amorphous solid (0.140 g, 48 %) after chromatography on a silica gel column with chloroform/methanol (50:1, 20:1, v/v) and crystallisation from ethanol. M.p.: 140–142 °C; IR (KBr): = 3,336, 3,275, 2,980, 2,930, 1,668, 1,578, 1,549, 1,443,1,260, 1,217, 1,067, 1,027, 957 cm−1; 1H NMR (CD3OD, 600 MHz): δ = 1.34 and 1.35 (2 t, J = 7.1 Hz, 6H, CH 3CH2OP), 2.12 (ddd, J = 23.6, 15.4, 8.2 Hz, 1H, PCH aHb), 2.18 (ddd, J = 20.1, 15.4, 4.7 Hz, 1H, PCHa H b), 4.11–4.19 (m, 4H, CH3CH 2OP), 4.44–4.47 (m, 1H, PCCH), 4.96 (dd, J = 13.4, 8.4 Hz, 1H, PCCCH aHb), 5.05 (dd, J = 13.4, 4.1 Hz, 1H, PCCCHa H b) ppm; 13C NMR (CD3OD, 150 MHz): δ = 15.3 (d, J = 5.8 Hz, CCOP), 30.8 (d, J = 141.6 Hz, PC), 56.4 (d, J = 17.3 Hz, PCCC), 61.9 and 62.2 (2 d, J = 6.5 Hz, CCOP), 65.6 (d, J = 3.6 Hz, PCC), 130.1 and 137.9 (2 s, C=C), 156.7 and 160.6 (2 s, C=O) ppm; 31P NMR (CD3OD, 243 MHz): δ = 28.9 ppm.
Diethyl 3-[4,5-bis(hydrazinocarbonyl)-1H-1,2,3-triazol-1-yl]-1-hydroxypropylphosphonate (10f, C11H22N7O6P)
From 0.262 g diester 8f (0.691 mmol) dihydrazide 10f was obtained as a white amorphous solid (0.155 g, 60 %) after chromatography on a silica gel column with chloroform/methanol (50:1, 30:1, v/v). M.p.: 119–120 °C; IR (KBr): = 3,316, 3,266, 3,160, 2,976, 2,930, 1,659, 1,572, 1,532, 1,249, 1,210, 1,072, 1,022, 955 cm−1; 1H NMR (CD3OD, 300 MHz): δ = 1.31 (t, J = 7.5 Hz, 6H, CH 3CH2OP), 2.13-2.29 (m, 1H, PCCH aHb), 2.31–2.44 (m, 1H, PCCHa H b), 3.91 (ddd, J = 10.8, 7.8, 3.3 Hz, 1H, PCH), 4.09–4.21 (m, 4H, CH3CH 2OP), 4.96–5.15 (m, 2H, CH2N) ppm; 13C NMR (CD3OD, 75 MHz): δ = 17.0 (d, J = 5.4 Hz, CCOP), 33.4 (s, PCC), 48.4 (d, J = 18.9 Hz, PCCC), 64.2 and 64.5 (d, J = 7.1 Hz, CCOP), 65.6 (d, J = 166.7 Hz, PC), 130.9 and 139.2 (2 s, C=C), 157.7 and 161.8 (2 s, C=O × 2) ppm; 31P NMR (CD3OD, 121 MHz): δ = 25.5 ppm.
Diethyl 2-[4,5-bis(hydrazinocarbonyl)-1H-1,2,3-triazol-1-yl]-1-hydroxyethylphosphonate (10g, C10H20N7O6P)
From 0.450 g diester 8g (1.23 mmol) dihydrazide 10g was obtained as a white amorphous solid (0.128 g, 28 %) after chromatography on a silica gel column with chloroform/methanol (50:1, 30:1, v/v). M.p.: 149–152 °C; IR (KBr): = 3,334, 3,207, 2,980, 2,930, 2,870, 1,668, 1,578, 1,449, 1,217, 1,067, 1,028, 953 cm−1; 1H NMR (D2O, 600 MHz): δ = 1.31 (t, J = 7.0 Hz, 6H, CH 3CH2OP), 4.18–4.24 (m, 4H, CH3CH 2OP), 4.53–4.58 (m, 1H, PCH), 4.94–5.01 (m, 1H, PCCH aHb), 5.11–5.16 (m, 1H, PCCHa H b) ppm; 13C NMR (D2O, 150 MHz): δ = 15.7 (d, J = 4.6 Hz, CCOP), 51.9 (d, J = 11.2 Hz, PCCN), 64.7 and 64.8 (2 d, J = 7.4 Hz, CCOP), 65.5 (d, J = 165.8 Hz, PC), 130.4 and 137.5 (2 s, C=C), 156.5 and 160.2 (2 s, C=O) ppm; 31P NMR (D2O, 243 MHz): δ = 21.8 ppm.
Synthesis of 1,2,3-triazolopyridazinediones 11a–11g (general procedure)
A mixture of the dihydrazide 10a–10g (0.50 mmol) and 5 cm3 10 % hydrochloric acid was heated at 90 °C for 2.5 h. After concentration in vacuo, crude products were purified on a silica gel column with chloroform/methanol or crystallised from the appropriate solvent.
Diethyl 2-(4,7-dioxo-5,6-dihydro-1H-1,2,3-triazolo[4,5-d]pyridazin-1-yl)ethylphosphonate (11a, C10H16N5O5P)
From 0.167 g dihydrazide 10a (0.478 mmol) compound 11a was obtained as a white amorphous solid (0.045 g, 30 %) after chromatography on a silica gel column with chloroform/methanol (50:1, 20:1, 10:1, v/v) followed by crystallisation from ethanol. M.p.: 198–200 °C; IR (KBr): = 3,385, 2,922, 2,672, 1,687, 1,582, 1,460, 1,262, 1,214, 1,014, 1,019, 980 cm−1; 1H NMR (CD3OD, 300 MHz): δ = 1.28 (t, J = 6.6 Hz, 6H, CH 3CH2OP), 2.58 (dt, J = 14.7 Hz, J = 7.5 Hz, PCH2), 4.03–4.13 (m, 4H, CH3CH 2OP), 5.12 (dt, J = 12.6 Hz, J = 7.5 Hz, CH2N) ppm; 13C NMR (CD3OD, 150 MHz): δ = 17.5 (d, J = 5.7 Hz, CCOP), 28.2 (d, J = 141.0 Hz, PC), 46.5 (d, J = 3.0 Hz, PCCN), 64.7 (d, J = 6.6 Hz, CCOP), 131.3 and 141.8 (2 s, C=C), 152.0 and 154.6 (2 s, C=O) ppm; 31P NMR (CD3OD, 121 MHz): δ = 27.7 ppm.
Diethyl 3-(4,7-dioxo-5,6-dihydro-1H-1,2,3-triazolo[4,5-d]pyridazin-1-yl)propylphosphonate (11b, C11H18N5O5P)
From 0.055 g dihydrazide 10b (0.151 mmol) compound 11b was obtained as a white amorphous solid (0.033 g, 66 %) after chromatography on a silica gel column with chloroform/methanol (50:1, 20:1, 10:1, v/v). M.p.: 148–150 °C; IR (KBr): = 3,446, 3,074, 2,991, 2,960, 1,662, 1,555, 1,462, 1,294, 1,218, 1,032, 972, 805 cm−1; 1H NMR (CD3OD, 300 MHz): δ = 1.31 (t, J = 6.9 Hz, 6H, CH 3CH2OP), 1.84–1.95 (m, 2H, PCH2CH 2), 2.24–2.38 (m, 2H, PCH2), 4.02–4.15 (m, 4H, CH3CH 2OP), 4.93 (t, J = 6.9 Hz, 2H, CH2N) ppm; 13C NMR (CD3OD, 75 MHz): δ = 16.7 (d, J = 5.7 Hz, CCOP), 22.7 (d, J = 141.9 Hz, PC), 23.9 (s, PCC), 50.5 (d, J = 18.5 Hz, PCCC), 62.6 (d, J = 6.5 Hz, CCOP), 129.1 and 139.7 (2 s, C=C), 150.5 and 152.7 (2 s, C=O) ppm; 31P NMR (CD3OD, 121 MHz): δ = 32.6 ppm.
Diethyl [2-(4,7-dioxo-5,6-dihydro-1H-1,2,3-triazolo[4,5-d]pyridazin-1-yl)ethoxy]methylphosphonate (11c, C11H18N5O6P)
From 0.090 g dihydrazide 10c (0.237 mmol) compound 11c was obtained as a white amorphous solid (0.043 g, 52 %) after chromatography on a silica gel column with chloroform/methanol (50:1, 20:1, v/v). M.p.: 170–172 °C; IR (KBr): = 3,348, 2,985, 2,930, 2,918, 1,658, 1,552, 1,420, 1,260, 1,070, 1,028, 975 cm−1; 1H NMR (CD3OD, 600 MHz): δ = 1.25 (t, J = 7.0 Hz, 6H, CH 3CH2OP), 3.89 (d, J = 8.5 Hz, 2H, PCH2), 4.01–4.04 (m, 4H, CH3CH 2OP), 4.16 (t, J = 5.1 Hz, 2H, OCH 2CH2N), 5.09 (t, J = 5.1 Hz, 2H, OCH2CH 2N) ppm; 13C NMR (CD3OD, 150 MHz): δ = 15.2 (d, J = 5.7 Hz, CCOP), 49.2 (s, CN), 62.7 (d, J = 6.6 Hz, CCOP), 63.9 (d, J = 164.8 Hz, PC), 70.9 (d, J = 12.1 Hz, PCOC), 129.2 and 139.3 (2 s, C=C), 149.6 and 152.3 (2 s, C=O) ppm; 31P NMR (CD3OD, 243 MHz): δ = 21.2 ppm.
Diethyl 2-[2-(4,7-dioxo-5,6-dihydro-1H-1,2,3-triazolo[4,5-d]pyridazin-1-yl)ethoxy]ethylphosphonate (11d, C12H20N5O6P)
From 0.165 g dihydrazide 10d (0.419 mmol) compound 11d was obtained as a white amorphous solid (0.087 g, 58 %) after chromatography on a silica gel column with chloroform/methanol (50:1, 20:1, v/v) and crystallisation from ethyl acetate/chloroform. M.p.: 134–135 °C; IR (KBr): = 3,433, 2,974, 2,928, 2,872, 1,715, 1,645, 1,206, 1,113, 1,065, 1,026, 982, 952 cm−1; 1H NMR (CDCl3, 300 MHz): δ = 1.26 (t, J = 7.2 Hz, 6H, CH 3CH2OP), 2.07 (dt, J = 15.0, 7.2 Hz, 2H, PCH2), 3.73 (dt, J = 13.5, 7.5 Hz, 2H, PCH2CH 2O), 3.96–4.10 (m, 6H, CH3CH 2OP and OCH 2CH2N), 5.01 (t, J = 5.1 Hz, 2H, OCH2CH 2N), 10.49 (brs, 2H, NH) ppm; 13C NMR (CDCl3, 75 MHz): δ = 16.6 (d, J = 6.3 Hz, CCOP), 26.8 (d, J = 138.9 Hz, PC), 49.9 (s, OCCN), 62.2 (d, J = 6.5 Hz, CCOP), 65.0 (s, PCCO), 68.8 (s, OCCN), 129.4 and 139.4 (2 s, C=C), 151.1 and 152.8 (2 s, C=O) ppm; 31P NMR (CDCl3, 121 MHz): δ = 29.3 ppm.
Diethyl 3-(4,7-dioxo-5,6-dihydro-1H-1,2,3-triazolo[4,5-d]pyridazin-1-yl)-2-hydroxypropylphosphonate (11e, C11H18N5O6P)
From 0.270 g dihydrazide 10e (0.719 mmol) compound 11e was obtained as a white amorphous solid (0.087 g, 39 %) after chromatography on a silica gel column with chloroform/methanol (20:1, 10:1, v/v) and crystallisation from water. M.p.: 168–170 °C; IR (KBr): = 3,476, 2,982, 2,932, 2,867, 1,655, 1,550, 1,427, 1,405, 1,256, 1,222, 1,108, 1,054, 957 cm−1; 1H NMR (D2O, 600 MHz): δ = 1.30 and 1.31 (2 t, J = 6.1 Hz, 6H, CH 3CH2OP), 2.25 (td, J = 16.1, 8.8 Hz, 1H, PCH aHb), 2.35 (td, J = 15.8, 3.2 Hz, 1H, PCHa H b), 4.10–4.17 (m, 4H, CH3CH 2OP), 4.52–4.59 (m, 1H, PCCH), 4.91 (dd, J = 12.8, 9.2 Hz, 1H, PCCCH aHb), 4.98 (dd, J = 12.8, 2.6 Hz, 1H, PCCCHa H b) ppm; 13C NMR (D2O, 150 MHz): δ = 15.6 (d, J = 5.7 Hz, CCOP), 30.0 (d, J = 139.9 Hz, PC), 55.7 (d, J = 17.8 Hz, PCCC), 63.5 and 63.6 (2 d, J = 6.4 Hz, CCOP), 65.4 (d, J = 3.8 Hz, PCC), 130.1 and 140.1 (2 s, C=C), 149.8 and 153.4 (2 s, C=O) ppm; 31P NMR (D2O, 243 MHz): δ = 30.3 ppm.
Diethyl 3-(4,7-dioxo-5,6-dihydro-1H-1,2,3-triazolo[4,5-d]pyridazin-1-yl)-1-hydroxypropylphosphonate (11f, C11H18N5O6P)
From 0.163 g dihydrazide 10f (0.430 mmol) compound 11f was obtained as a white amorphous solid (0.086 g, 58 %) after chromatography on a silica gel column with chloroform/methanol (50:1, 20:1, 10:1, v/v). M.p.: 66–68 °C; IR (KBr): = 3,362, 2,987, 2,920, 1,666, 1,580, 1,449, 1,212, 1,050, 1,021, 970 cm−1; 1H NMR (CD3OD, 300 MHz): δ = 1.32 (t, J = 7.2 Hz, 6H, CH 3CH2OP), 2.19–2.28 (m, 1H, PCCH aHb), 2.31–2.44 (m, 1H, PCCHa H b), 3.89 (ddd, J = 10.5, 7.2, 3.0 Hz, 1H, PCH), 4.10–4.21 (m, 4H, CH3CH 2OP), 4.95–5.12 (m, 2H, CH2N) ppm; 13C NMR (CD3OD, 75 MHz): δ = 17.0 (d, J = 5.4 Hz, CCOP), 33.3 (d, J = 4.0 Hz, PCC), 48.1 (d, J = 16.2 Hz, PCCC), 64.2 and 64.5 (d, J = 6.9 Hz, CCOP), 65.4 (d, J = 167.3 Hz, PC), 130.2 and 140.8 (2 s, C=C), 148.9 and 152.2 (2 s, C=O) ppm; 31P NMR (CD3OD, 121 MHz): δ = 25.3 ppm.
Diethyl 2-(4,7-dioxo-5,6-dihydro-1H-1,2,3-triazolo[4,5-d]pyridazin-1-yl)-1-hydroxyethylphosphonate (11g, C10H16N5O6P)
From 0.100 g dihydrazide 10g (0.274 mmol) compound 11g was obtained as a colourless oil (0.036 g, 40 %) after chromatography on a silica gel column with chloroform/methanol (50:1, 10:1, v/v). IR (film): = 3,362, 2,988, 2,971, 1,667, 1,580, 1,212, 1,021 cm−1; 1H NMR (CD3OD, 600 MHz): δ = 1.38 and 1.39 (2 t, J = 7.0 Hz, 6H, CH 3CH2OP), 4.23–4.29 (m, 4H, CH3CH 2OP), 4.64–4.69 (m, 1H, PCH), 5.09–5.15 (m, 2H, CH2N) ppm; 13C NMR (CD3OD, 150 MHz): δ = 15.3 and 15.4 (d, J = 5.2 Hz, CCOP), 51.3 (d, J = 11.6 Hz, PCCN), 63.3 and 63.5 (2 d, J = 6.8 Hz, COP), 66.4 (d, J = 166.2 Hz, PC), 129.2 and 139.4 (2 s, C=C), 150.1 and 152.4 (2 s, C=O) ppm; 31P NMR (CD3OD, 243 MHz): δ = 20.5 ppm.
Assays for antiviral activity other than HIV
The compounds were evaluated against the following viruses: herpes simplex virus type 1 (HSV-1) strain KOS, thymidine kinase-deficient (TK−) HSV-1 KOS strain resistant to ACV (ACVr), herpes simplex virus type 2 (HSV-2) strain G, cytomegalovirus (strains AD-169 and Davis), varicella-zoster virus (VZV) (strains OKA and YS), vaccinia virus Lederle strain, respiratory syncytial virus (RSV) strain Long, vesicular stomatitis virus (VSV), coxsackie b4 virus, parainfluenza 3, influenza virus A (subtypes H1N1, H3N2), influenza virus B, reovirus-1, Sindbis virus and Punta Toro virus. The antiviral assays were based on inhibition of virus-induced cytopathicity or plaque formation in human embryonic lung (HEL) fibroblasts, African green monkey cells (Vero), human epithelial cervix carcinoma cells (HeLa) or MDCK. Confluent cell cultures in microtiter 96-well plates were inoculated with 100 CCID50 of virus (1 CCID50 being the virus dose to infect 50 % of the cell cultures) or 20 or 100 plaque forming units (PFU) (for VZV and CMV, respectively) in the presence of varying concentrations of the test compounds. Viral cytopathicity or plaque formation was recorded as soon as it reached completion in the control virus-infected cell cultures that were not treated with the test compounds. Antiviral activity was expressed as the EC50 or compound concentration required to reduce virus-induced cytopathogenicity or viral plaque formation by 50 %.
Anti-HIV activity assays
Inhibition of HIV-1(IIIB)- and HIV-2(ROD)-induced cytopathicity in CEM cell cultures was measured in microtiter 96-well plates containing 3 × 105 CEM cells/cm3 infected with 100 CCID50 of HIV per cm3 and containing appropriate dilutions of the test compounds. After 4–5 days of incubation at 37 °C in a CO2-controlled humidified atmosphere, CEM giant cell (syncytium) formation was examined microscopically. The EC50 (50 % effective concentration) was defined as the compound concentration required to inhibit HIV-induced giant cell formation by 50 %.
Cytostatic activity assays
All assays were performed in 96-well microtiter plates. To each well were added (5–7.5) × 104 tumour cells and a given amount of the test compound. The cells were allowed to proliferate for 48 h (murine leukemia L1210 cells) or 72 h (human lymphocytic CEM and human cervix carcinoma HeLa cells) at 37 °C in a humidified CO2-controlled atmosphere. At the end of the incubation period, the cells were counted in a Coulter counter. The IC50 (50 % inhibitory concentration) was defined as the concentration of the compound that inhibited cell proliferation by 50 %.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
The authors are grateful to Leen Ingels, Leentje Persoons, Frieda De Meyer, Lizette van Berckelaer, Anita Camps, Steven Carmans and Lies Van den Heurck for excellent technical assistance. The work was supported by the KU Leuven (GOA 10/14) and by the Medical University of Lodz (502-34-009 and 503/3-014-01/503-1).
References
- 1.De Clercq E. Antiviral Res. 2007;75:1. doi: 10.1016/j.antiviral.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 2.De Clercq E, Holý A. Nat Rev Drug Disc. 2005;4:928. doi: 10.1038/nrd1877. [DOI] [PubMed] [Google Scholar]
- 3.Naesens L, Snoeck R, Andrei G, Balzarini J, Neyts J, De Clercq E. Antivir Chem Chemother. 1997;8:1. [Google Scholar]
- 4.Starret JE, Jr, Tortolani DR, Hitchock MJ, Martin JC, Mansuri MM. Antiviral Res. 1992;19:1484. doi: 10.1016/0166-3542(92)90084-I. [DOI] [PubMed] [Google Scholar]
- 5.De Clercq E. Clin Microbiol Rev. 2003;16:569. doi: 10.1128/CMR.16.4.569-596.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen HM, Hosmane RH. Nucleosides Nucleotides Nucleic Acids. 2001;8:1599. doi: 10.1081/NCN-100105250. [DOI] [PubMed] [Google Scholar]
- 7.Baszczyňski O, Jansa P, Dračinsky M, Klepetářová B, Holý A, Votruba I, De Clercq E, Balzarini J, Janeba Z. Bioorg Med Chem. 2011;19:2114. doi: 10.1016/j.bmc.2011.02.050. [DOI] [PubMed] [Google Scholar]
- 8.De Clercq E, Holý A, Rosenberg I, Sakuma T, Balzarini J, Maudgal PC. Nature. 1986;323:464. doi: 10.1038/323464a0. [DOI] [PubMed] [Google Scholar]
- 9.Holý A, Votruba I, Masojidková M, Andrei G, Snoeck R, Naesens L, De Clercq E, Balzarini J. J Med Chem. 2002;45:1918. doi: 10.1021/jm011095y. [DOI] [PubMed] [Google Scholar]
- 10.Hocková D, Holý A, Masojidková M, Andrei G, Snoeck R, De Clercq E, Balzarini J. J Med Chem. 2002;46:5064. doi: 10.1021/jm030932o. [DOI] [PubMed] [Google Scholar]
- 11.Balzarini J, Pannecouque C, Naesens L, Andrei G, Snoeck R, De Clercq E, Hocková D, Holý A. Nucleosides Nucleotides Nucleic Acids. 2004;23:1321. doi: 10.1081/NCN-200027573. [DOI] [PubMed] [Google Scholar]
- 12.Maag D, Castro C, Hong Z, Cameron CE. J Biol Chem. 2001;276:46094. doi: 10.1074/jbc.C100349200. [DOI] [PubMed] [Google Scholar]
- 13.Wang XL, Wan K, Zhou CH. Eur J Med Chem. 2010;45:4631. doi: 10.1016/j.ejmech.2010.07.031. [DOI] [PubMed] [Google Scholar]
- 14.Weide T, Saldanha SA, Minond D, Spicer TP, Fotsing JR, Spaargaren M, Frere JM, Bebrone C, Sharpless KB, Hodder PS, Fokin VV. Med Chem Lett. 2010;1:150. doi: 10.1021/ml900022q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chandrika PM, Yakaiah T, Gayatri G, Pranay Kumar K, Narsaiah B, Murthy USN, Raghu Ram Rao A. Eur J Med Chem. 2010;45:78. doi: 10.1016/j.ejmech.2009.09.027. [DOI] [PubMed] [Google Scholar]
- 16.Aher NG, Pore VS, Mishra NN, Kumar A, Shukla PK, Sharma A, Bhat MK. Bioorg Med Chem Lett. 2009;19:759. doi: 10.1016/j.bmcl.2008.12.026. [DOI] [PubMed] [Google Scholar]
- 17.Chaudhary PM, Chavan SR, Shirazi F, Razdan M, Nimkar P, Maybhate SP, Likhite AP, Gonnade R, Hazara BG, Deshpande MV, Deshpande SR. Bioorg Med Chem. 2009;17:2433. doi: 10.1016/j.bmc.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 18.Stefely JA, Palchaudhuri R, Miller PA, Peterson RJ, Moraski GC, Hergenrother PJ, Miller MJ. J Med Chem. 2010;53:3389. doi: 10.1021/jm1000979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park SM, Yang H, Park SK, Kim HM, Kim BH. Bioorg Med Chem Lett. 2010;20:5831. doi: 10.1016/j.bmcl.2010.07.126. [DOI] [PubMed] [Google Scholar]
- 20.Odlo K, Fournier-Dit-Chabert J, Ducki S, Gani OABSM, Sylte I, Hansen TV. Bioorg Med Chem. 2010;18:6874. doi: 10.1016/j.bmc.2010.07.032. [DOI] [PubMed] [Google Scholar]
- 21.Wu J, Green N, Hotchandani R, Hu Y, Condon J, Huang A, Kaila N, Li HQ, Guler S, Li W, Tam SY, Wang Q, Pelker J, Marusic S, Hsu S, Hall JP, Telliez JP, Cui J, Lin LL. Bioorg Med Chem Lett. 2009;19:3485. doi: 10.1016/j.bmcl.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 22.Yoon J, Cho L, Lee SK, Ryu JS. Bioorg Med Chem Lett. 2011;21:1953. doi: 10.1016/j.bmcl.2011.02.034. [DOI] [PubMed] [Google Scholar]
- 23.Velázquez S, Alvarez R, Pérez C, De Clercq E, Balzarini J, Camarasa MJ. Antivir Chem Chemother. 1998;9:481. doi: 10.1177/095632029800900604. [DOI] [PubMed] [Google Scholar]
- 24.Joradão AK, Ferreira VF, Souza TML, De Souza Faria GG, Machado V, Abrantes JL, De Souza MCBV, Cunha AC. Bioorg Med Chem. 2011;19:1860. doi: 10.1016/j.bmc.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 25.Montagu A, Roy V, Balzarini J, Snoeck R, Andrei G, Agrofoglio LA. Eur J Med Chem. 2011;46:778. doi: 10.1016/j.ejmech.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 26.Da Silva FC, De Souza MCBV, Frugulhetti IIP, Castro HC, De Souza SL, De Souza TML, Rodrigues DQ, Souza AMT, Abreu PA, Passamani F, Rodrigues RC, Ferreira VF. Eur J Med Chem. 2009;44:373. doi: 10.1016/j.ejmech.2008.02.047. [DOI] [PubMed] [Google Scholar]
- 27.Saito Y, Escuret V, Durantel D, Zoulim F, Schinazi RF, Agrofolio LA. Bioorg Med Chem. 2003;11:3633. doi: 10.1016/S0968-0896(03)00349-3. [DOI] [PubMed] [Google Scholar]
- 28.Elayadi H, Smietana M, Pannecouque C, Leyssen P, Neyts J, Vasseur JJ, Lazrek HB. Bioorg Med Chem. 2010;20:7365. doi: 10.1016/j.bmcl.2010.10.046. [DOI] [PubMed] [Google Scholar]
- 29.Borowski P, Lang M, Haag A, Schmitz H, Choe J, Chen HM, Hosmane RS. Antimicrob Agents Chemother. 2002;46:1231. doi: 10.1128/AAC.46.5.1231-1239.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Głowacka IE, Cieślak M, Piotrowska DG. Phosphorus Sulfur Silicon Relat Elem. 2011;186:431. doi: 10.1080/10426507.2010.494646. [DOI] [Google Scholar]
- 31.Palacios F, Ochoa de Retana AM, Pagalday J. Heterocycles. 1994;38:95. doi: 10.3987/COM-93-6516. [DOI] [Google Scholar]
- 32.Głowacka IE, Balzarini J, Wróblewski AE. Nucleosides Nucleotides Nucleic Acids. 2012;31:293. doi: 10.1080/15257770.2012.662611. [DOI] [PubMed] [Google Scholar]
- 33.Eger K, Klünder EM, Schmidt M. J Med Chem. 1994;37:3057. doi: 10.1021/jm00045a010. [DOI] [PubMed] [Google Scholar]
- 34.Koszytkowska-Stawińska M, De Clercq E, Balzarini J. Bioorg Med Chem. 2009;17:3756. doi: 10.1016/j.bmc.2009.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Głowacka IE, Balzarini J, Wróblewski AE. Arch Pharm Chem Life Sci. 2013;346:278. doi: 10.1002/ardp.201200421. [DOI] [PubMed] [Google Scholar]
- 36.Głowacka IE. Tetrahedron Asymmetry. 2009;20:2270. doi: 10.1016/j.tetasy.2009.09.005. [DOI] [Google Scholar]
- 37.Abramov VS. Zh Obshch Khim. 1952;22:647. [Google Scholar]
- 38.Abramov VS. Chem Abstr. 1953;47:5351e. [Google Scholar]
- 39. Davies AJ, Donald ASR, Marks RE (1967) J Chem Soc C 1967:2109
- 40.Wang Y, Inguaggioto G, Jasamai M, Shah M, Hughes D, Slater M, Simons C. Bioorg Med Chem. 1999;7:481. doi: 10.1016/S0968-0896(98)00255-7. [DOI] [PubMed] [Google Scholar]
- 41.Biagi G, Ciambrone F, Livi O, Scartoni V. J Heterocycl Chem. 2002;39:889. doi: 10.1002/jhet.5570390506. [DOI] [Google Scholar]
- 42.Karplus M. J Am Chem Soc. 1963;85:2870. doi: 10.1021/ja00901a059. [DOI] [Google Scholar]
- 43.Benezra C. J Am Chem Soc. 1973;95:6890. doi: 10.1021/ja00802a003. [DOI] [Google Scholar]
- 44.Neeser JR, Tronchet JMJ, Charollais EJ. Can J Chem. 1983;61:2112. doi: 10.1139/v83-367. [DOI] [Google Scholar]
- 45.Adiwidjaja G, Meyer B, Thiem J. Z Naturforsch. 1979;34b:1547. [Google Scholar]
- 46.Buchanan GW, Bourque K, Seeley A. Magn Res Chem. 1986;24:360. doi: 10.1002/mrc.1260240418. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


