Abstract
Abstract
Two series of novel 4-chloro-2-(benzylthio)-5-(1,3,4-oxadiazol-2-yl)benzenesulfonamides and their N-aroyl derivatives have been synthesized and evaluated for in vitro anticancer activity against the full NCI-60 cell line panel. Most of the compounds exhibited antiproliferative activity. Among them a compound bearing an N-(thien-2-ylcarbonyl) moiety showed broad-spectrum activity with 50% growth inhibition (GI50) values in the range of 2.02–7.82 μM over 50 cell lines.
Graphical abstract
 .
.
Keywords: Acylsulfonamides, 2-Mercaptobenzenesulfonamides, Antitumor agents, Phase-transfer catalysis, Heterocycles
Introduction
Aryl- and heteroarylsulfonamides are an important class of therapeutic agents in current medicinal science [1]. Various arylsulfonamides have been reported to possess anticancer [2–6] and/or anti-human immunodeficiency virus (HIV) properties [6, 7]. Our systematic studies on the synthesis of 1,4,2-benzodithiazine 1,1-dioxides and their subsequent transformations into 2-mercaptobenzenesulfonamide (MBSA) derivatives (Fig. 1) having a variety of heterocyclic ring systems or acyclic polynitrogen moieties at the sulfonamide functionality resulted in promising anticancer [8–13], HIV antiviral [14–16], or antibacterial agents [17] as well as potent inhibitors of transmembrane cancer-associated carbonic anhydrase isozymes hCAIX and hCAXII [18, 19].
Fig. 1.

A number of structurally novel N-acylbenzenesulfonamides have recently been reported either as potent antitumor agents against a broad spectrum of human tumor xenografts (colon, lung, breast, ovary, and prostate) in nude mice [22] (Fig. 2) or clinically investigated drug candidates with cytostatic activity against malignant tumors such as Eli Lilly’s tasisulam sodium [23] or Abbott’s WO-2002024636, ABT-737 [24], and ABT-263 [25] (Fig. 3).
Fig. 2.
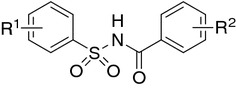
Acyl sulfonamide antiproliferative (ASAP) scaffold [26]
Fig. 3.

Tasisulam sodium (LY573636-sodium): clinically evaluated (phase II/III in metastatic melanoma) antitumor N-acylsulfonamide; pan-Bcl family inhibitors targeting Bcl-2, Bcl-w, and Bcl-xL: WO-2002024636, ABT-737, and ABT-263 [23–25]
This led us to an assumption that expansion of the series of 2-mercapto-N-acylbenzenesulfonamide potential anticancer agents, in which groups of varying size and electronic properties are placed at positions 2, 5, and N- of the benzenesulfonamide ring, may shed light on the structural features contributing to the biological activities.
Results and discussion
Chemistry
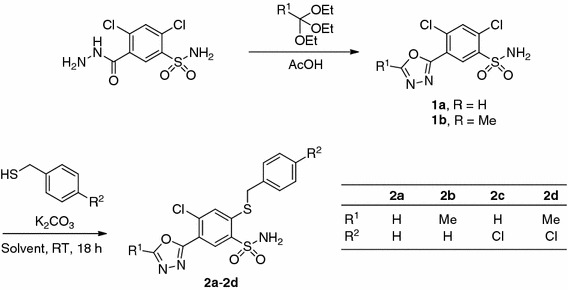
Several methods for synthesis of 2-mercaptobenzenesulfonamides are known. The simplest and most efficient method employs the ring-opening reaction of preformed 3-mercapto-1,1-dioxo-1,4,2-benzodithiazine derivatives under alkaline conditions [27]. Alternatively, access to 2-mercaptobenzenesulfonamides is provided by direct reaction of 2-halogenobenzenesulfonamides with sodium polysulfide (Na2Sx) [28] or conversion of 2-aminobenzenesulfonamides via diazonium salt decomposition utilizing disodium sulfide (Na2S) or potassium ethyl xanthate [28–30]. Herein, we report a direct synthetic route to novel 4-chloro-2-benzylthiobenzenesulfonamides and their N-acylated derivatives. Due to our ongoing research in the field of biologically active 2-mercaptobenzenesulfonamides with five-membered rings incorporated in 5-position of the MBSA scaffold [9], we choose 1,3,4-oxadiazole as our model heterocyclic residue.
The expected 1,3,4-oxadiazoles 1a, 1b were conveniently prepared in good yields by the reaction of 2,4-dichloro-5-sulfamoylbenzhydrazide [31] with orthoesters in refluxing glacial acetic acid (Scheme 1).
Scheme 1.
We found that 2,4-dichloro-5-(1,3,4-oxadiazol-2-yl)benzenesulfonamide (1a) under standard conditions (BnSH/K2CO3/DMF (N,N-dimethylformamide)/RT) undergoes a selective SNAr addition–elimination reaction in 2-position. Moderate yields (14–58%, Table 1, entries 1–4, 6, and 8) of this reaction led us to optimize the conditions. Higher yields were observed when tetrabutylammonium bromide (TBAB) was used as a phase-transfer catalyst, especially in acetonitrile/water (300:1, v/v) reaction environment (Table 1, entry 9). Slight decrease of substrate conversion was observed in the absence of argon atmosphere (Scheme 1).
Table 1.
Reaction of 2,4-dichloro-5-(1,3,4-oxadiazol-2-yl)benzenesulfonamide (1a) with benzyl mercaptan and optimization of the reaction conditions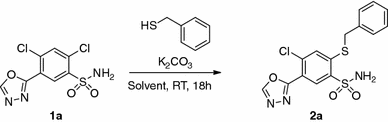
| Entry | Solvent | BnSH/mmol | K2CO3/mmol | Yielda/% |
|---|---|---|---|---|
| 1 | EtOH | 1.0 | 1.2 | Trace |
| 2 | DMF | 1.0 | 1.2 | 32 |
| 3 | DMF | 2.0 | 2.2 | 27 |
| 4 | DMF | 1.0 | 2.2 | 41 |
| 5 | DMF/H2O | 1.0 | 2.2 (cat.)b | 55 |
| 6 | DMSO | 1.0 | 2.2 | 14 |
| 7 | DMSO/H2O | 1.0 | 2.2 (cat.)b | 33 |
| 8 | MeCN | 1.0 | 2.2 | 58 |
| 9 | MeCN/H2O | 1.0 | 2.2 (cat.)a | 81 |
Reaction conditions: 5 cm3 solvent at room temperature (ca. 25 °C) under argon atmosphere
DMSO dimethylsulfoxide
aIsolated yield of 2a
b(n-Bu4 N)+ Br− (0.01 mmol)
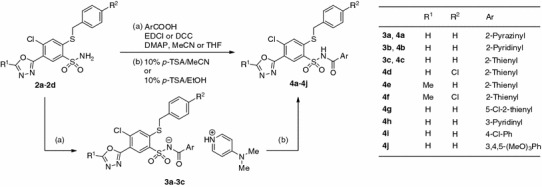
The desired N-acylsulfonamides 4a–4j (Scheme 2) were prepared by carbodiimide-mediated coupling of aromatic carboxylic acids with sulfonamides [32–34] promoted by 4-(N,N-dimethylamino)pyridine (DMAP) in the appropriate solvent. In some cases crystalline 4-(N,N-dimethylamino)pyridinium N-heteroaroylsulfonamidates (3a–3c) were isolated and characterized, which by treatment with 10% (w/v) ethanolic p-toluenesulfonic acid (p-TSA) solution were converted to the desired N-acylsulfonamides 4a–4c.
Scheme 2.
In vitro biological activity
Compounds 2a–2d and 4a–4j submitted to National Cancer Institute (NCI) were evaluated for their in vitro anticancer activity. Sulfonamides 2a and 2c showed significant selectivity toward leukemia cell line CCRF-CEM (Fig. 4), whereas 2d appears to be substantially inactive.
Fig. 4.

Differential cytotoxicity graph for 2a and 2c revealing NCI-60 panel selectivity/resistance pattern expressed in % growth. Sulfonamides 2a and 2c show significant selectivity toward CCRF-CEM human T cell lymphoblast-like cell line. For each agent the difference between mean % growth and % growth of each cell line for that agent is determined, to yield positive values for cell lines more sensitive than average (bars projecting above the horizontal axis) and negative values for cell lines less sensitive than average (bars projecting below the horizontal axis). Mean graph midpoint (the origin of the abscissa) for 2a is 98.22% and for 2c is 92.12%
HOP-92, non-small cell lung cancer, and renal cancer A498 cell lines reveal some insight into structure–activity relationship (SAR). Cytostatic activity of 2a–2c toward those cell lines increases when CLogP and calculated molar refractivity (CMR) of the compound increase (Table 2).
Table 2.
CLogP and CMR molecular descriptors of 2a–2d
| Compd. | Growth (%) | CLogP a | CMRa | |
|---|---|---|---|---|
| HOP-92 | A498 | |||
| 2a | 62.58 | 94.43 | 1.86852 | 9.5054 |
| 2b | 57.35 | 59.49 | 2.13752 | 9.9692 |
| 2c | 48.61 | 56.18 | 2.58152 | 9.9968 |
| 2d | 84.54 | 91.46 | 2.85052 | 10.4606 |
SAR based on HOP-92 and A498 cell line screen at 10 μM concentration of the test agent
aMolecular descriptors calculated using BioByte software package [35]
Over a series of N-(thien-2-ylcarbonyl)benzenesulfonamide derivatives (4c–4g), substitution on the heterocyclic (4e, 4f: R1 = Me) or benzylthio (4d, 4f: R2 = Cl) moiety decreases activity significantly. It seems interesting that closely related six-membered N-heteroaroyl derivatives (4a, 4b, and 4h) showed no activity, which renders 4c as a lead for further optimization.
Compound 4c (NSC 754633) which satisfied predetermined threshold inhibition criteria was selected for the NCI five-dose (0.01–100 μM) assay and exhibited remarkable anticancer activity against most of the tested cell lines representing nine different subpanels (Table 3). Only NCI/ADR-RES (adriamycin-resistant cell line) expressing high levels of MDR1 and Pgp-170 glycoprotein [36, 37] was found to be insensitive at the highest tested concentration (100 μM). The obtained data revealed some subpanel sensitivity toward renal, central nervous system (CNS), and breast cancer cell lines (subpanel selectivity ratio: 1.04–1.46). The CNS cancer subpanel showed highest sensitivity with mean GI50 value of 3.24 μM and mean concentration causing total growth inhibition at 12.68 μM level. It is worth mentioning that the cytotoxic effect of 4c was less pronounced in the leukemia subpanel [50% lethal concentration (LC50) for all tested leukemia cell lines >100 μM]. A relatively large difference in mean cytostatic (mean-graph GI50 = 4.27 μM) and cytotoxic (mean-graph LC50 = 58.88 μM) indicators could be projected to potential low toxicity against normal cells resulting in a broad therapeutic index.
Table 3.
In vitro antiproliferative data (μM) for 4c (NSC 754633) against the full NCI cell lines panel derived from nine clinically isolated human cancer types described by three parameters: molar concentration of the compound causing 50% net cell growth inhibition (GI50), total growth inhibition (TGI), and 50% net cell death (LC50)
| Subpanel | Cell line | GI50/μM | TGI/μM | LC50/μM | ||
|---|---|---|---|---|---|---|
| Conc. per cell line | Subpanel MIDb | SSRd | TGI-MIDe | LC50-MIDf | ||
| Leukemia | 8.27 | 0.52 | 83.57 | –a | ||
| CCRF-CEM | 3.08 | –a | –a | |||
| HL-60(TB) | 12.9 | –a | –a | |||
| K-562 | 3.19 | 29.3 | –a | |||
| MOLT-4 | 3.69 | 72.1 | –a | |||
| RPMI-8226 | 23.4 | –a | –a | |||
| SR | 3.33 | –a | –a | |||
| Non-small cell lung cancer | 4.52 | 0.94 | 31.10 | 71.33 | ||
| A549/ATCC | 2.04 | 4.82 | 13.7 | |||
| EKVX | 6.01 | 36.2 | –a | |||
| HOP-62 | 3.19 | 9.48 | 36.2 | |||
| HOP-92 | 3.14 | –a | –a | |||
| NCI-H226 | 5.07 | 25.0 | –a | |||
| NCI-H23 | 7.25 | –a | –a | |||
| NCI-H322M | 7.82 | 90.8 | –a | |||
| NCI-H460 | 2.29 | 5.42 | 20.7 | |||
| NCI-H522 | 3.90 | 17.1 | –a | |||
| Colon | 6.70 | 0.64 | 36.74 | 64.73 | ||
| COLO 205 | 2.61 | 6.84 | 38.3 | |||
| HCC-2998 | 21.1 | –a | –a | |||
| HCT-116 | 4.01 | 16.1 | 71.1 | |||
| HCT-15 | 10.5 | 69.8 | –a | |||
| HT29 | 3.11 | 8.97 | 35.7 | |||
| KM12 | 3.69 | 51.6 | –a | |||
| SW-620 | 1.88 | 3.88 | 7.99 | |||
| CNS cancer | 3.24 | 1.32 | 12.68 | 36.39 | ||
| SF-268 | 2.07 | 5.32 | 23.4 | |||
| SF-295 | 3.40 | 14.6 | 51.4 | |||
| SF-539 | 3.40 | 3.95 | 27.1 | |||
| SNB-19 | 6.75 | 44.3 | –a | |||
| SNB-75 | 1.96 | 4.25 | 9.18 | |||
| U251 | 1.85 | 3.67 | 7.27 | |||
| Melanoma | 5.87 | 0.73 | 51.09 | 88.23 | ||
| LOX IMVI | 3.09 | –a | –a | |||
| MALME-3 M | 6.79 | 25.1 | –a | |||
| M14 | 4.51 | –a | –a | |||
| MDA-MB-435 | 2.90 | 10.8 | 75.0 | |||
| SK-MEL-2 | 2.64 | 8.73 | 49.2 | |||
| SK-MEL-28 | 5.50 | 21.4 | 69.9 | |||
| SK-MEL-5 | 2.91 | 10.3 | –a | |||
| UACC-257 | 13.6 | 83.5 | –a | |||
| UACC-62 | 10.9 | –a | –a | |||
| Ovarian cancer | 19.60 | 0.22 | 31.02 | 60.09 | ||
| IGROV1 | 10.2 | 39.0 | –a | |||
| OVCAR-3 | 2.29 | 4.37 | 8.34 | |||
| OVCAR-4 | 2.10 | 3.86 | 7.08 | |||
| OVCAR-5 | 16.4 | 46.6 | –a | |||
| OVCAR-8 | 2.55 | 7.74 | 36.6 | |||
| NCI/ADR-RES | –a | –a | –a | |||
| SK-OV-3 | 3.65 | 15.6 | 68.6 | |||
| Renal cancer | 4.09 | 1.04 | 33.44 | 72.36 | ||
| 786-0 | 2.64 | 6.47 | –a | |||
| A498 | 3.18 | –a | –a | |||
| ACHN | 10.9 | –a | –a | |||
| CAKI-1 | 3.65 | 18.3 | 80.4 | |||
| RXF 393 | 2.39 | 5.14 | 14.6 | |||
| SN12C | 3.57 | 15.5 | 75.3 | |||
| TK-10 | 2.68 | 5.77 | 34.6 | |||
| UO-31 | 3.68 | 16.3 | 74.0 | |||
| Prostate cancer | 5.44 | 0.78 | 56.15 | 84.05 | ||
| PC-3 | 7.54 | –a | –a | |||
| DU-145 | 3.33 | 12.3 | 68.1 | |||
| Breast cancer | 2.93 | 1.46 | 25.04 | 83.77 | ||
| MCF7 | 3.22 | 17.7 | –a | |||
| MDA-MB-231/ATCC | 2.02 | 6.10 | 54.0 | |||
| HS 578T | 2.26 | 7.04 | –a | |||
| BT-549 | 4.02 | –a | –a | |||
| T-47D | 2.80 | 7.02 | –a | |||
| MDA-MB-468 | 3.30 | 12.4 | 48.6 | |||
| MG-MIDc | 4.27 | 21.38 | 58.88 | |||
aParameter not determined in five-dose assay, thus assumed 100 μM for the purpose of midpoint calculations
bSubpanel GI50 midpoint = average sensitivity of subpanel cell lines toward the test agent
cMean-graph GI50, TGI, and LC50 midpoints = average sensitivity of all cell lines toward the test agent
dSubpanel selectivity ratio = subpanel MID:MG-MID
eSubpanel TGI midpoint
fSubpanel LC50 midpoint
COMPARE [38, 39] analysis at the NCI of compound 4c showed moderate Pearson correlation coefficient (PCC = 0.446–0.549) with DNA interfering agents such as actinomycin D, echinomycin, bruceantin, chromomycin A3, or didemnin B (Table 4).
Table 4.
COMPARE correlation coefficients (PCC) calculated using compound 4c (NSC 754633) as seed, tested in US NCI-60 cell lines in vitro screen
| Rank | NSC | Number of cell lines | PCC | Compd |
|---|---|---|---|---|
| 1 | 3053 | 59 | 0.549 | Actinomycin D |
| 2 | 325014 | 58 | 0.547 | Bactobolin |
| 3 | 526417 | 56 | 0.520 | Echinomycin |
| 4 | 305884 | 58 | 0.517 | Acodazole HCl |
| 5 | 165563 | 56 | 0.511 | Bruceantin |
| 6 | 267469 | 58 | 0.493 | Deoxydoxorubicin |
| 7 | 58514 | 55 | 0.455 | Chromomycin A3 |
| 8 | 325319 | 57 | 0.446 | Didemnin B |
For definitions and methods of calculation of the correlation coefficient from the COMPARE analysis, see Ref. [39]
Conclusions
We designed a new and efficient method of obtaining substituted 2-mercaptobenzenesulfonamides from readily available 2,4-dichlorobenzenesulfonamides under optimized mild phase-transfer catalysis conditions. This approach offers easy and quick isolation of the products and preparative-scale synthesis. Novel 2-mercaptobenzenesulfonamides and their structurally diverse N-(hetero)aroyl derivatives were evaluated for in vitro antiproliferative activity. The discovered N-acylbenzenesulfonamide 4c shows promising anticancer activity toward 50 human cancer cell lines and could be considered as a lead for further optimization.
Experimental
Melting points were determined with a Boëtius apparatus. Infrared (IR) spectra were taken using a Thermo Mattson Satellite FTIR spectrophotometer, 1H and 13C nuclear magnetic resonance (NMR) were taken with a Varian Gemini 200 MHz or Varian Unity Plus 500 MHz spectrometer. Chemical shifts are reported in ppm ( ). The results of elemental analyses for C, H, and N were in agreement with the calculated values within ±0.4% range. Column chromatography was carried out on silica gel Fluka Silica gel 60 (0.035–0.070 mm). The starting 2,4-dichloro-5-sulfamoylbenzhydrazide was obtained from commercially available 2,4-dichloro-5-sulfamoylbenzoic acid according to methods described previously [31].
). The results of elemental analyses for C, H, and N were in agreement with the calculated values within ±0.4% range. Column chromatography was carried out on silica gel Fluka Silica gel 60 (0.035–0.070 mm). The starting 2,4-dichloro-5-sulfamoylbenzhydrazide was obtained from commercially available 2,4-dichloro-5-sulfamoylbenzoic acid according to methods described previously [31].
General procedure for the synthesis of 1a, 1b
A mixture of 2.84 g 2,4-dichloro-5-sulfamoylbenzhydrazide (10 mmol) and the appropriate orthoester (60 mmol) in 30 cm3 glacial AcOH was refluxed for 7–12 h. After cooling to room temperature, stirring was continued overnight. The precipitate was filtered off, washed with cold EtOH and petroleum ether, and purified by crystallization from EtOH.
2,4-Dichloro-5-(1,3,4-oxadiazol-2-yl)benzenesulfonamide (1a, C8H5Cl2N3O3S)
Starting from 8.89 g triethyl orthoformate. Yield: 2.42 g (82%); m.p.: 195–197 °C; R
f = 0.59 (benzene/EtOH = 4:1); IR (KBr): 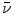 = 3,323, 3,229, 3,165, 3,100, 1,359, 1,340, 1,168 cm−1; 1H NMR (200 MHz, DMSO-d
6):
= 3,323, 3,229, 3,165, 3,100, 1,359, 1,340, 1,168 cm−1; 1H NMR (200 MHz, DMSO-d
6):  = 7.97 (s, 2H, SO2NH2), 8.20 (s, 1H, H-3), 8.56 (s, 1H, H-6), 9.54 (s, 1H, Ar–H) ppm; 13C NMR (50 MHz, DMSO-d
6):
= 7.97 (s, 2H, SO2NH2), 8.20 (s, 1H, H-3), 8.56 (s, 1H, H-6), 9.54 (s, 1H, Ar–H) ppm; 13C NMR (50 MHz, DMSO-d
6):  = 121.84, 131.12, 134.09, 134.56, 136.02, 140.83, 155.47, 160.89 ppm.
= 121.84, 131.12, 134.09, 134.56, 136.02, 140.83, 155.47, 160.89 ppm.
2,4-Dichloro-5-(5-methyl-1,3,4-oxadiazol-2-yl)benzenesulfonamide (1b, C9H7Cl2N3O3S)
Starting from 9.73 g triethyl orthoacetate. Yield: 2.13 g (69%); m.p.: 217–219 °C; R
f = 0.61 (benzene/EtOH = 4:1); IR (KBr): 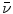 = 3,305, 3,205, 3,094, 1,579, 1,542, 1,460, 1,354, 1,174 cm−1; 1H NMR (200 MHz, DMSO-d
6):
= 3,305, 3,205, 3,094, 1,579, 1,542, 1,460, 1,354, 1,174 cm−1; 1H NMR (200 MHz, DMSO-d
6):  = 2.64 (s, 3H, CH3), 7.95 (s, 2H, SO2NH2), 8.18 (s, 1H, H-3), 8.51 (s, 1H, H-6) ppm; 13C NMR (50 MHz, DMSO-d
6):
= 2.64 (s, 3H, CH3), 7.95 (s, 2H, SO2NH2), 8.18 (s, 1H, H-3), 8.51 (s, 1H, H-6) ppm; 13C NMR (50 MHz, DMSO-d
6):  = 10.93, 122.02, 130.75, 134.08, 134.21, 135.74, 140.77, 161.04, 165.08 ppm.
= 10.93, 122.02, 130.75, 134.08, 134.21, 135.74, 140.77, 161.04, 165.08 ppm.
General procedure for the synthesis of 2a–2d
To a suspension of the appropriate 2,4-dichlorobenzenesulfonamide 1a, 1b (5 mmol) in 30 cm3 MeCN and 0.1 cm3 water, 1.52 g K2CO3 (11 mmol) and 0.016 g TBAB (0.05 mmol) were added. The obtained reaction mixture was vigorously stirred under an argon atmosphere, and slowly the appropriate mercaptan (5 mmol) was added dropwise. After 24 h of stirring at room temperature, the reaction mixture was concentrated under reduced pressure to dryness, and 15 cm3 EtOH was added. The precipitate was filtered off and suspended in 30 cm3 water, stirred for 30 min, and filtered off. The crude product was purified by crystallization from EtOH.
2-Benzylthio-4-chloro-5-(1,3,4-oxadiazol-2-yl)benzenesulfonamide (2a, C15H12ClN3O3S2)
Starting from 1.47 g 1a and 0.62 g benzyl mercaptan. Yield: 1.55 g (81%); m.p.: 153–154 °C; R
f = 0.64 (benzene/EtOH = 4:1); IR (KBr):  = 3,435, 3,332, 3,142, 2,926, 1,590, 1,532, 1,495, 1,450, 1,350, 1,161 cm−1; 1H NMR (500 MHz, DMSO-d
6):
= 3,435, 3,332, 3,142, 2,926, 1,590, 1,532, 1,495, 1,450, 1,350, 1,161 cm−1; 1H NMR (500 MHz, DMSO-d
6):  = 4.54 (s, 2H, SCH2), 7.29–7.32 (m, 1H, Ar–H), 7.36–7.39 (m, 2H, Ar–H), 7.52–7.54 (m, 2H, Ar–H), 7.73 (s, 2H, SO2NH2), 7.84 (s, 1H, H-3), 8.42 (s, 1H, H-6), 9.48 (s, 1H, Ar–H) ppm; 13C NMR (50 MHz, DMSO-d
6):
= 4.54 (s, 2H, SCH2), 7.29–7.32 (m, 1H, Ar–H), 7.36–7.39 (m, 2H, Ar–H), 7.52–7.54 (m, 2H, Ar–H), 7.73 (s, 2H, SO2NH2), 7.84 (s, 1H, H-3), 8.42 (s, 1H, H-6), 9.48 (s, 1H, Ar–H) ppm; 13C NMR (50 MHz, DMSO-d
6):  = 36.16, 118.23, 127.88, 128.89, 129.12, 129.61, 130.29, 135.19, 135.57, 139.55, 143.08, 155.18, 161.33 ppm.
= 36.16, 118.23, 127.88, 128.89, 129.12, 129.61, 130.29, 135.19, 135.57, 139.55, 143.08, 155.18, 161.33 ppm.
2-Benzylthio-4-chloro-5-(5-methyl-1,3,4-oxadiazol-2-yl)benzenesulfonamide (2b, C16H14ClN3O3S2)
Starting from 1.54 g 1b and 0.62 g benzyl mercaptan. Yield: 1.54 g (78%); m.p.: 208–210 °C; R
f = 0.67 (benzene/EtOH = 4:1); IR (KBr): 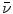 = 3,429, 3,246, 2,924, 2,854, 1,624, 1,591, 1,577, 1,558, 1,525, 1,495, 1,347, 1,165 cm−1; 1H NMR (500 MHz, DMSO-d
6):
= 3,429, 3,246, 2,924, 2,854, 1,624, 1,591, 1,577, 1,558, 1,525, 1,495, 1,347, 1,165 cm−1; 1H NMR (500 MHz, DMSO-d
6):  = 2.58 (s, 3H, CH3), 4.50 (s, 2H, SCH2), 7.27–7.30 (m, 1H, Ar–H), 7.34–7.37 (m, 2H, Ar–H), 7.44–7.46 (m, 2H, Ar–H), 7.74 (s, 2H, SO2NH2), 7.78 (s, 1H, H-3), 8.35 (s, 1H, H-6) ppm; 13C NMR (50 MHz, DMSO-d
6):
= 2.58 (s, 3H, CH3), 4.50 (s, 2H, SCH2), 7.27–7.30 (m, 1H, Ar–H), 7.34–7.37 (m, 2H, Ar–H), 7.44–7.46 (m, 2H, Ar–H), 7.74 (s, 2H, SO2NH2), 7.78 (s, 1H, H-3), 8.35 (s, 1H, H-6) ppm; 13C NMR (50 MHz, DMSO-d
6):  = 10.87, 36.23, 118.89, 127.87, 128.70, 128.96, 129.14, 129.26, 129.33, 129.47, 129.60, 133.49, 135.70, 137.31, 145.04, 161.70, 164.32 ppm.
= 10.87, 36.23, 118.89, 127.87, 128.70, 128.96, 129.14, 129.26, 129.33, 129.47, 129.60, 133.49, 135.70, 137.31, 145.04, 161.70, 164.32 ppm.
4-Chloro-2-(4-chlorobenzylthio)-5-(1,3,4-oxadiazol-2-yl)benzenesulfonamide (2c, C15H11Cl2N3O3S2)
Starting from 1.47 g 1a and 0.79 g 4-chlorobenzyl mercaptan. Yield: 1.58 g (76%); m.p.: 185–187 °C; R
f = 0.63 (benzene/EtOH = 4:1); IR (KBr):  = 3,248, 3,156, 3,087, 2,918, 2,858, 1,589, 1,530, 1,490, 1,440, 1,350, 1,333, 1,162 cm−1; 1H NMR (500 MHz, DMSO-d
6):
= 3,248, 3,156, 3,087, 2,918, 2,858, 1,589, 1,530, 1,490, 1,440, 1,350, 1,333, 1,162 cm−1; 1H NMR (500 MHz, DMSO-d
6):  = 4.55 (s, 2H, SCH2), 7.42–7.44 (m, 2H, Ar–H), 7.55–7.57 (m, 2H, Ar–H), 7.73 (s, 2H, SO2NH2), 7.84 (s, 1H, H-3), 8.42 (s, 1H, H-6), 9.48 (s, 1H, Ar–H) ppm; 13C NMR (50 MHz, DMSO-d
6):
= 4.55 (s, 2H, SCH2), 7.42–7.44 (m, 2H, Ar–H), 7.55–7.57 (m, 2H, Ar–H), 7.73 (s, 2H, SO2NH2), 7.84 (s, 1H, H-3), 8.42 (s, 1H, H-6), 9.48 (s, 1H, Ar–H) ppm; 13C NMR (50 MHz, DMSO-d
6):  = 35.29, 118.41, 128.84, 129.32, 130.29, 131.42, 132.49, 134.86, 135.20, 139.74, 142.55, 155.19, 161.29 ppm.
= 35.29, 118.41, 128.84, 129.32, 130.29, 131.42, 132.49, 134.86, 135.20, 139.74, 142.55, 155.19, 161.29 ppm.
4-Chloro-2-(4-chlorobenzylthio)-5-(5-methyl-1,3,4-oxadiazol-2-yl)benzenesulfonamide (2d, C16H13Cl2N3O3S2)
Starting from 1.54 g 1b and 0.79 g 4-chlorobenzyl mercaptan. Yield: 1.79 g (83%); m.p.: 250–252 °C; R
f = 0.68 (benzene/EtOH = 4:1); IR (KBr):  = 3,363, 3,239, 2,925, 2,853, 1,636, 1,587, 1,574, 1,559, 1,520, 1,493, 1,456, 1,349, 1,167 cm−1; 1H NMR (500 MHz, DMSO-d
6):
= 3,363, 3,239, 2,925, 2,853, 1,636, 1,587, 1,574, 1,559, 1,520, 1,493, 1,456, 1,349, 1,167 cm−1; 1H NMR (500 MHz, DMSO-d
6):  = 2.59 (s, 3H, CH3), 4.51 (s, 2H, SCH2), 7.41–7.43 (m, 2H, Ar–H), 7.47–7.49 (m, 2H, Ar–H), 7.76–7.77 (m, 3H, H-3 and SO2NH2), 8.35 (s, 1H, H-6) ppm; 13C NMR (50 MHz, DMSO-d
6):
= 2.59 (s, 3H, CH3), 4.51 (s, 2H, SCH2), 7.41–7.43 (m, 2H, Ar–H), 7.47–7.49 (m, 2H, Ar–H), 7.76–7.77 (m, 3H, H-3 and SO2NH2), 8.35 (s, 1H, H-6) ppm; 13C NMR (50 MHz, DMSO-d
6):  = 10.87, 35.36, 119.03, 128.78, 128.94, 129.48, 131.27, 132.50, 133.51, 134.94, 137.44, 144.62, 161.67, 164.34 ppm.
= 10.87, 35.36, 119.03, 128.78, 128.94, 129.48, 131.27, 132.50, 133.51, 134.94, 137.44, 144.62, 161.67, 164.34 ppm.
General procedure for the synthesis of 2-benzylthio-4-chloro-5-(1,3,4-oxadiazol-2-yl)-4-(N,N-dimethylamino)pyridinium N-acylbenzenesulfonamidates 3a–3c
To the appropriate carboxylic acid (1.1 mmol) in 5 cm3 dry MeCN, 0.212 g 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDCI, 1.1 mmol) was added and stirred for 5 min. 2a (0.382 g, 1 mmol) and 0.256 g DMAP (2.1 mmol) were added, and the reaction mixture was stirred at room temperature overnight. The precipitate was filtered off and washed with cold MeCN and MeOH. The crude salt was purified by crystallization from MeOH.
4-(N,N-Dimethylamino)pyridinium 2-benzylthio-4-chloro-5-(1,3,4-oxadiazol-2-yl)-N-(pyrazine-2-carbonyl)benzenesulfonamidate (3a, C27H24ClN7O4S2)
Starting from 0.137 g pyrazine-2-carboxylic acid. Yield: 0.338 g (55%); m.p.: 209–210 °C; R
f = 0.14 (benzene/EtOH = 4:1); IR (KBr): 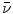 = 3,198, 3,109, 3,056, 2,924, 1,646, 1,612, 1,589, 1,562, 1,498, 1,323, 1,142 cm−1; 1H NMR (200 MHz, DMSO-d
6):
= 3,198, 3,109, 3,056, 2,924, 1,646, 1,612, 1,589, 1,562, 1,498, 1,323, 1,142 cm−1; 1H NMR (200 MHz, DMSO-d
6):  = 3.17 (s, 6H, N(CH3)2), 4.35 (s, 2H, SCH2), 6.94–6.98 (m, 2H, Ar–H), 7.19–7.22 (m, 3H, Ar–H), 7.32–7.37 (m, 2H, Ar–H), 7.58 (s, 1H, H-3), 8.20–8.23 (m, 2H, Ar–H), 8.49 (s, 1H, H-6), 8.62–8.63 (m, 2H, Ar–H), 9.09 (s, 1H, Ar–H), 9.44 (s, 1H, Ar–H), 13.22 (br s, 1H, NH+) ppm; 13C NMR (50 MHz, DMSO-d
6):
= 3.17 (s, 6H, N(CH3)2), 4.35 (s, 2H, SCH2), 6.94–6.98 (m, 2H, Ar–H), 7.19–7.22 (m, 3H, Ar–H), 7.32–7.37 (m, 2H, Ar–H), 7.58 (s, 1H, H-3), 8.20–8.23 (m, 2H, Ar–H), 8.49 (s, 1H, H-6), 8.62–8.63 (m, 2H, Ar–H), 9.09 (s, 1H, Ar–H), 9.44 (s, 1H, Ar–H), 13.22 (br s, 1H, NH+) ppm; 13C NMR (50 MHz, DMSO-d
6):  = 35.76, 107.14, 117.12, 127.52, 127.67, 128.64, 129.25, 132.10, 133.50, 136.12, 139.51, 141.52, 143.23, 144.00, 145.22, 145.57, 150.80, 154.98, 157.13, 161.75, 167.83 ppm.
= 35.76, 107.14, 117.12, 127.52, 127.67, 128.64, 129.25, 132.10, 133.50, 136.12, 139.51, 141.52, 143.23, 144.00, 145.22, 145.57, 150.80, 154.98, 157.13, 161.75, 167.83 ppm.
4-(N,N-Dimethylamino)pyridinium 2-benzylthio-4-chloro-5-(1,3,4-oxadiazol-2-yl)-N-(pyridine-2-carbonyl)benzenesulfonamidate (3b, C28H25ClN6O4S2)
Starting from 0.135 g pyridine-2-carboxylic acid. Yield: 0.219 g (36%); m.p.: 217–219 °C; R
f = 0.22 (benzene/EtOH = 4:1); IR (KBr): 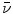 = 3,195, 3,107, 2,924, 1,646, 1,607, 1,588, 1,562, 1,496, 1,324, 1,141 cm−1; 1H NMR (200 MHz, DMSO-d
6):
= 3,195, 3,107, 2,924, 1,646, 1,607, 1,588, 1,562, 1,496, 1,324, 1,141 cm−1; 1H NMR (200 MHz, DMSO-d
6):  = 3.16 (s, 6H, N(CH3)2), 4.32 (s, 2H, SCH2), 6.91–6.95 (m, 2H, Ar–H), 7.18–7.21 (m, 3H, Ar–H), 7.31–7.32 (m, 2H, Ar–H), 7.41–7.45 (m, 1H, Ar–H), 7.57 (s, 1H, H-3), 7.78–7.86 (m, 1H, Ar–H), 7.94–7.98 (m, 1H, Ar–H), 8.23–8.26 (m, 2H, Ar–H), 8.50 (s, 1H, H-6), 8.55–8.57 (m, 2H, Ar–H), 9.45 (s, 1H, Ar–H), 13.20 (br s, 1H, NH+) ppm; 13C NMR (50 MHz, DMSO-d
6):
= 3.16 (s, 6H, N(CH3)2), 4.32 (s, 2H, SCH2), 6.91–6.95 (m, 2H, Ar–H), 7.18–7.21 (m, 3H, Ar–H), 7.31–7.32 (m, 2H, Ar–H), 7.41–7.45 (m, 1H, Ar–H), 7.57 (s, 1H, H-3), 7.78–7.86 (m, 1H, Ar–H), 7.94–7.98 (m, 1H, Ar–H), 8.23–8.26 (m, 2H, Ar–H), 8.50 (s, 1H, H-6), 8.55–8.57 (m, 2H, Ar–H), 9.45 (s, 1H, Ar–H), 13.20 (br s, 1H, NH+) ppm; 13C NMR (50 MHz, DMSO-d
6):  = 35.80, 107.05, 117.09, 123.74, 125.21, 127.49, 127.65, 128.63, 129.26, 132.30, 133.45, 136.04, 136.91, 139.89, 141.62, 143.21, 148.68, 154.99, 155.48, 157.00, 161.77, 169.37 ppm.
= 35.80, 107.05, 117.09, 123.74, 125.21, 127.49, 127.65, 128.63, 129.26, 132.30, 133.45, 136.04, 136.91, 139.89, 141.62, 143.21, 148.68, 154.99, 155.48, 157.00, 161.77, 169.37 ppm.
4-(N,N-Dimethylamino)pyridinium 2-benzylthio-4-chloro-5-(1,3,4-oxadiazol-2-yl)-N-(thien-2-ylcarbonyl)benzenesulfonamidate (3c, C27H24ClN5O4S3)
Starting from 0.141 g thiophene-2-carboxylic acid. Yield: 0.295 g (48%); m.p.: 201–202 °C; R
f = 0.16 (benzene/EtOH = 4:1); IR (KBr):  = 3,214, 3,090, 2,924, 1,649, 1,591, 1,565, 1,315, 1,138 cm−1; 1H NMR (200 MHz, DMSO-d
6):
= 3,214, 3,090, 2,924, 1,649, 1,591, 1,565, 1,315, 1,138 cm−1; 1H NMR (200 MHz, DMSO-d
6):  = 3.17 (s, 6H, N(CH3)2), 4.33 (s, 2H, SCH2), 6.94–6.98 (m, 3H, Ar–H), 7.18–7.21 (m, 3H, Ar–H), 7.36–7.38 (m, 3H, Ar–H), 7.49–7.50 (m, 2H, H-3 and Ar–H), 8.18–8.22 (m, 2H, Ar–H), 8.44 (s, 1H, H-6), 9.43 (s, 1H, Ar–H), 13.18 (br s, 1H, NH+) ppm; 13C NMR (50 MHz, DMSO-d
6):
= 3.17 (s, 6H, N(CH3)2), 4.33 (s, 2H, SCH2), 6.94–6.98 (m, 3H, Ar–H), 7.18–7.21 (m, 3H, Ar–H), 7.36–7.38 (m, 3H, Ar–H), 7.49–7.50 (m, 2H, H-3 and Ar–H), 8.18–8.22 (m, 2H, Ar–H), 8.44 (s, 1H, H-6), 9.43 (s, 1H, Ar–H), 13.18 (br s, 1H, NH+) ppm; 13C NMR (50 MHz, DMSO-d
6):  = 35.75, 107.20, 116.90, 127.31, 127.49, 128.66, 128.90, 129.04, 129.31, 129.56, 132.09, 133.14, 136.22, 139.51, 142.21, 143.27, 145.46, 154.93, 157.14, 161.81, 165.81 ppm.
= 35.75, 107.20, 116.90, 127.31, 127.49, 128.66, 128.90, 129.04, 129.31, 129.56, 132.09, 133.14, 136.22, 139.51, 142.21, 143.27, 145.46, 154.93, 157.14, 161.81, 165.81 ppm.
General procedure for the synthesis of N-acylbenzenesulfonamides 4a–4c
To a suspension of the appropriate pyridinium salt 3a–3c (0.5 mmol) in 5 cm3 EtOH, 2 cm3 10% p-TSA solution in EtOH was added and stirred at room temperature for 1 h. The precipitate was filtered off and washed with EtOH and water.
2-Benzylthio-4-chloro-5-(1,3,4-oxadiazol-2-yl)-N-(pyrazine-2-carbonyl)benzenesulfonamide (4a, C20H14ClN5O4S2)
Yield: 0.242 g (99%); m.p.: 294–296 °C; R
f = 0.10 (benzene/EtOH = 4:1); IR (KBr): 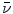 = 3,485, 3,364, 3,298, 3,203, 2,871, 1,612, 1,585, 1,549, 1,492, 1,450, 1,362, 1,159 cm−1; 1H NMR (500 MHz, DMSO-d
6):
= 3,485, 3,364, 3,298, 3,203, 2,871, 1,612, 1,585, 1,549, 1,492, 1,450, 1,362, 1,159 cm−1; 1H NMR (500 MHz, DMSO-d
6):  = 4.51 (s, 2H, SCH2), 7.08–7.17 (m, 3H, Ar–H), 7.29–7.31 (m, 2H, Ar–H), 7.89 (s, 1H, H-3), 8.58 (s, 1H, H-6), 8.81 (s, 1H, Ar–H), 8.94 (s, 1H, Ar–H), 9.08 (s, 1H, Ar–H), 9.47 (s, 1H, Ar–H) ppm; 13C NMR (50 MHz, DMSO-d
6):
= 4.51 (s, 2H, SCH2), 7.08–7.17 (m, 3H, Ar–H), 7.29–7.31 (m, 2H, Ar–H), 7.89 (s, 1H, H-3), 8.58 (s, 1H, H-6), 8.81 (s, 1H, Ar–H), 8.94 (s, 1H, Ar–H), 9.08 (s, 1H, Ar–H), 9.47 (s, 1H, Ar–H) ppm; 13C NMR (50 MHz, DMSO-d
6):  = 35.98, 118.54, 127.81, 128.61, 129.27, 129.74, 133.91, 135.58, 136.99, 143.88, 144.09, 144.87, 148.89, 155.25, 161.03, 163.39, 163.44 ppm.
= 35.98, 118.54, 127.81, 128.61, 129.27, 129.74, 133.91, 135.58, 136.99, 143.88, 144.09, 144.87, 148.89, 155.25, 161.03, 163.39, 163.44 ppm.
2-Benzylthio-4-chloro-5-(1,3,4-oxadiazol-2-yl)-N-(pyridine-2-carbonyl)benzenesulfonamide (4b, C20H14ClN5O4S2)
Yield: 0.241 g (99%); m.p.: 173–175 °C; R
f = 0.40 (benzene/EtOH = 4:1); IR (KBr): 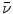 = 3,138, 2,924, 2,854, 1,730, 1,647, 1,590, 1,530, 1,496, 1,450, 1,347, 1,174 cm−1; 1H NMR (200 MHz, DMSO-d
6):
= 3,138, 2,924, 2,854, 1,730, 1,647, 1,590, 1,530, 1,496, 1,450, 1,347, 1,174 cm−1; 1H NMR (200 MHz, DMSO-d
6): 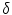 = 4.47 (s, 2H, SCH2), 7.02–7.19 (m, 3H, Ar–H), 7.26–7.30 (m, 2H, Ar–H), 7.82 (s, 1H, H-3), 7.88–7.95 (m, 1H, Ar–H), 8.12–8.16 (m, 1H, Ar–H), 8.26–8.35 (m, 1H, Ar–H), 8.57 (s, 1H, H-6), 8.76–8.78 (m, 1H, Ar–H), 9.47 (s, 1H, Ar–H) ppm; 13C NMR (50 MHz, DMSO-d
6):
= 4.47 (s, 2H, SCH2), 7.02–7.19 (m, 3H, Ar–H), 7.26–7.30 (m, 2H, Ar–H), 7.82 (s, 1H, H-3), 7.88–7.95 (m, 1H, Ar–H), 8.12–8.16 (m, 1H, Ar–H), 8.26–8.35 (m, 1H, Ar–H), 8.57 (s, 1H, H-6), 8.76–8.78 (m, 1H, Ar–H), 9.47 (s, 1H, Ar–H) ppm; 13C NMR (50 MHz, DMSO-d
6):  = 35.82, 118.20, 124.54, 127.67, 128.58, 128.79, 129.19, 129.30, 133.37, 135.73, 136.07, 136.66, 141.76, 143.52, 146.77, 147.41, 155.18, 161.20, 162.66 ppm.
= 35.82, 118.20, 124.54, 127.67, 128.58, 128.79, 129.19, 129.30, 133.37, 135.73, 136.07, 136.66, 141.76, 143.52, 146.77, 147.41, 155.18, 161.20, 162.66 ppm.
2-Benzylthio-4-chloro-5-(1,3,4-oxadiazol-2-yl)-N-(thien-2-ylcarbonyl)benzenesulfonamide (4c, C20H14ClN3O4S3)
Yield: 0.244 g (99%); m.p.: 282–284 °C; R
f = 0.12 (benzene/EtOH = 4:1); IR (KBr):  = 3,382, 3,354, 3,253, 3,106, 1,614, 1,601, 1,579, 1,565, 1,549, 1,332, 1,318, 1,176 cm−1; 1H NMR (200 MHz, DMSO-d
6):
= 3,382, 3,354, 3,253, 3,106, 1,614, 1,601, 1,579, 1,565, 1,549, 1,332, 1,318, 1,176 cm−1; 1H NMR (200 MHz, DMSO-d
6):  = 4.51 (s, 2H, SCH2), 7.30–7.76 (m, 12H, H-3 and Ar–H), 8.35 (s, 1H, H-6) ppm; 13C NMR (50 MHz, DMSO-d
6):
= 4.51 (s, 2H, SCH2), 7.30–7.76 (m, 12H, H-3 and Ar–H), 8.35 (s, 1H, H-6) ppm; 13C NMR (50 MHz, DMSO-d
6):  = 36.26, 118.90, 119.01, 126.03, 127.87, 128.46, 128.72, 128.96, 129.25, 129.50, 132.64, 135.75, 137.16, 137.65, 144.38, 155.74, 159.68 ppm.
= 36.26, 118.90, 119.01, 126.03, 127.87, 128.46, 128.72, 128.96, 129.25, 129.50, 132.64, 135.75, 137.16, 137.65, 144.38, 155.74, 159.68 ppm.
4-Chloro-2-(4-chlorobenzylthio)-5-(1,3,4-oxadiazol-2-yl)-N-(thien-2-ylcarbonyl)benzenesulfonamide (4d, C20H13Cl2N3O4S3)
To a solution of 0.128 g thiophene-2-carboxylic acid (1 mmol) in 3 cm3 dry MeCN, 0.192 g EDCI (1 mmol) was added and stirred at room temperature for 5 min. 2c (0.416 g, 1 mmol) and 0.184 g DMAP (1.5 mmol) were added and stirred at room temperature for 18 h. The reaction mixture was acidified with 2 cm3 10% p-TSA/MeCN and concentrated under reduced pressure, and the residue was chromatographed with CH2Cl2/MeOH/AcOH (97:1:2) on silica gel column giving pure 4d. Yield: 0.248 g (47%); R
f = 0.16 (benzene/EtOH = 4:1); m.p.: 205–207 °C; IR (KBr): 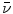 = 3,164, 3,094, 2,841, 1,678, 1,591, 1,526, 1,491, 1,450, 1,352, 1,262, 1,170 cm−1; 1H NMR (200 MHz, DMSO-d
6):
= 3,164, 3,094, 2,841, 1,678, 1,591, 1,526, 1,491, 1,450, 1,352, 1,262, 1,170 cm−1; 1H NMR (200 MHz, DMSO-d
6):  = 4.55 (s, 2H, SCH2), 7.08–7.12 (m, 2H, Ar–H), 7.23–7.27 (m, 1H, Ar–H), 7.32–7.37 (m, 2H, Ar–H), 7.44–7.46 (m, 1H, Ar–H), 7.90 (s, 1H, H-3), 7.97–8.00 (m, 1H, Ar–H), 8.53 (s, 1H, H-6), 9.48 (s, 1H, Ar–H) ppm; 13C NMR (50 MHz, DMSO-d
6):
= 4.55 (s, 2H, SCH2), 7.08–7.12 (m, 2H, Ar–H), 7.23–7.27 (m, 1H, Ar–H), 7.32–7.37 (m, 2H, Ar–H), 7.44–7.46 (m, 1H, Ar–H), 7.90 (s, 1H, H-3), 7.97–8.00 (m, 1H, Ar–H), 8.53 (s, 1H, H-6), 9.48 (s, 1H, Ar–H) ppm; 13C NMR (50 MHz, DMSO-d
6):  = 35.02, 118.77, 128.57, 129.02, 129.97, 131.00, 131.35, 132.37, 133.00, 134.08, 134.90, 135.32, 135.43, 136.59, 136.95, 142.89, 155.23, 159.97, 160.99 ppm.
= 35.02, 118.77, 128.57, 129.02, 129.97, 131.00, 131.35, 132.37, 133.00, 134.08, 134.90, 135.32, 135.43, 136.59, 136.95, 142.89, 155.23, 159.97, 160.99 ppm.
2-Benzylthio-4-chloro-5-(5-methyl-1,3,4-oxadiazol-2-yl)-N-(thien-2-ylcarbonyl)benzenesulfonamide (4e, C21H16ClN3O4S3)
To a solution of 0.128 g thiophene-2-carboxylic acid (1 mmol) in 3 cm3 dry MeCN, 0.192 g EDCI (1 mmol) was added and stirred for 5 min. 2b (0.396 g, 1 mmol) and 0.184 g DMAP (1.5 mmol) were added and stirred at room temperature for 18 h. The obtained solution was concentrated under reduced pressure, and 2 cm3 10% p-TSA/EtOH was added with vigorous stirring. The obtained suspension was left in the refrigerator overnight. The formed crystalline solid was filtered off and washed with cold EtOH. Yield: 0.213 g (42%); m.p.: 230–231 °C; R
f = 0.17 (benzene/EtOH = 4:1); IR (KBr):  = 3,098, 2,925, 2,854, 1,658, 1,591, 1,577, 1,525, 1,495, 1,453, 1,361, 1,176 cm−1; 1H NMR (500 MHz, DMSO-d
6):
= 3,098, 2,925, 2,854, 1,658, 1,591, 1,577, 1,525, 1,495, 1,453, 1,361, 1,176 cm−1; 1H NMR (500 MHz, DMSO-d
6):  = 2.62 (s, 3H, CH3), 4.50 (s, 2H, SCH2), 7.21–7.23 (m, 1H, Ar–H), 7.28–7.31 (m, 1H, Ar–H), 7.34–7.37 (m, 2H, Ar–H), 7.44–7.46 (m, 2H, Ar–H), 7.79 (s, 1H, H-3), 7.97–7.98 (m, 1H, Ar–H), 8.15–8.16 (m, 1H, Ar–H), 8.50 (s, 1H, H-6) ppm; 13C NMR (50 MHz, DMSO-d
6):
= 2.62 (s, 3H, CH3), 4.50 (s, 2H, SCH2), 7.21–7.23 (m, 1H, Ar–H), 7.28–7.31 (m, 1H, Ar–H), 7.34–7.37 (m, 2H, Ar–H), 7.44–7.46 (m, 2H, Ar–H), 7.79 (s, 1H, H-3), 7.97–7.98 (m, 1H, Ar–H), 8.15–8.16 (m, 1H, Ar–H), 8.50 (s, 1H, H-6) ppm; 13C NMR (50 MHz, DMSO-d
6):  = 10.87, 36.39, 118.83, 127.98, 128.56, 129.02, 129.56, 132.56, 132.64, 133.06, 133.48, 135.35, 135.48, 136.27, 147.66, 160.11, 161.39, 164.43 ppm.
= 10.87, 36.39, 118.83, 127.98, 128.56, 129.02, 129.56, 132.56, 132.64, 133.06, 133.48, 135.35, 135.48, 136.27, 147.66, 160.11, 161.39, 164.43 ppm.
4-Chloro-2-(4-chlorobenzylthio)-5-(5-methyl-1,3,4-oxadiazol-2-yl)-N-(thien-2-ylcarbonyl)benzenesulfonamide (4f, C21H15Cl2N3O4S3)
To a solution of 0.128 g thiophene-2-carboxylic acid (1 mmol) in 5 cm3 dry tetrahydrofuran (THF), 0.206 g 1,3-dicyclohexylcarbodiimide (DCC, 1 mmol) was added and stirred for 5 min at room temperature. 2d (0.430 g, 1 mmol) and 0.184 g DMAP (1.5 mmol) were added and stirred at room temperature for 48 h. By-products were filtered out and washed thoroughly with THF. The filtrate was acidified with 2 cm3 10% p-TSA/EtOH and concentrated under reduced pressure, and the resulting oily residue was chromatographed with AcOEt/petroleum ether (1:1) on silica gel column giving pure 4f. Yield: 0.135 g (25%); m.p.: 134–136 °C; R
f = 0.22 (benzene/EtOH = 4:1); IR (KBr): 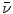 = 3,422, 2,925, 2,855, 1,654, 1,575, 1,523, 1,490, 1,360, 1,261, 1,169 cm−1; 1H NMR (200 MHz, DMSO-d
6):
= 3,422, 2,925, 2,855, 1,654, 1,575, 1,523, 1,490, 1,360, 1,261, 1,169 cm−1; 1H NMR (200 MHz, DMSO-d
6):  = 2.63 (s, 3H, CH3), 4.52 (s, 2H, SCH2), 7.20–7.24 (m, 1H, Ar–H), 7.39–7.51 (m, 4H, Ar–H), 7.77 (s, 1H, H-3), 7.97–7.99 (m, 2H, Ar–H), 8.15–8.17 (m, 1H, Ar–H), 8.51 (s, 1H, H-6) ppm; 13C NMR (50 MHz, DMSO-d
6):
= 2.63 (s, 3H, CH3), 4.52 (s, 2H, SCH2), 7.20–7.24 (m, 1H, Ar–H), 7.39–7.51 (m, 4H, Ar–H), 7.77 (s, 1H, H-3), 7.97–7.99 (m, 2H, Ar–H), 8.15–8.17 (m, 1H, Ar–H), 8.51 (s, 1H, H-6) ppm; 13C NMR (50 MHz, DMSO-d
6):  = 10.58, 35.22, 118.62, 128.30, 128.48, 128.71, 131.06, 132.34, 132.76, 133.20, 134.27, 135.18, 135.97, 146.95, 159.82, 161.06, 164.15 ppm.
= 10.58, 35.22, 118.62, 128.30, 128.48, 128.71, 131.06, 132.34, 132.76, 133.20, 134.27, 135.18, 135.97, 146.95, 159.82, 161.06, 164.15 ppm.
2-Benzylthio-4-chloro-N-(5-chlorothien-2-ylcarbonyl)-5-(1,3,4-oxadiazol-2-yl)benzenesulfonamide (4g, C20H13Cl2N3O4S3)
To a solution of 0.164 g 5-chlorothiophene-2-carboxylic acid (1 mmol) in 5 cm3 dry MeCN, 0.192 g EDCI (1 mmol) was added and stirred at room temperature for 5 min. 2a (0.382 g, 1 mmol) and 0.184 g DMAP (1.5 mmol) were added and stirred at room temperature for 12 h. The obtained solution was acidified with 2 cm3 10% p-TSA/MeCN and stirred under cooling (ice bath) for 2 h. The precipitated white solid was filtered off and purified by crystallization from MeCN. Yield: 0.268 g (51%); m.p.: 254–255 °C; R
f = 0.12 (benzene/EtOH = 4:1); IR (KBr):  = 3,160, 3,098, 2,924, 2,855, 2,717, 1,683, 1,592, 1,559, 1,531, 1,472, 1,351, 1,328, 1,168 cm−1; 1H NMR (500 MHz, DMSO-d
6):
= 3,160, 3,098, 2,924, 2,855, 2,717, 1,683, 1,592, 1,559, 1,531, 1,472, 1,351, 1,328, 1,168 cm−1; 1H NMR (500 MHz, DMSO-d
6):  = 4.51 (s, 2H, SCH2), 7.15–7.22 (m, 3H, Ar–H), 7.24–7.25 (s, 1H, Ar–H), 7.33–7.35 (m, 2H, Ar–H), 7.83 (s, 1H, H-3), 7.87 (s, 1H, Ar–H), 8.49 (s, 1H, H-6), 9.46 (s, 1H, Ar–H) ppm; 13C NMR (50 MHz, DMSO-d
6):
= 4.51 (s, 2H, SCH2), 7.15–7.22 (m, 3H, Ar–H), 7.24–7.25 (s, 1H, Ar–H), 7.33–7.35 (m, 2H, Ar–H), 7.83 (s, 1H, H-3), 7.87 (s, 1H, Ar–H), 8.49 (s, 1H, H-6), 9.46 (s, 1H, Ar–H) ppm; 13C NMR (50 MHz, DMSO-d
6):  = 35.64, 118.22, 127.52, 128.39, 128.81, 129.01, 129.37, 132.56, 133.56, 134.98, 135.25, 135.75, 136.51, 136.62, 143.19, 154.91, 159.11, 160.73 ppm.
= 35.64, 118.22, 127.52, 128.39, 128.81, 129.01, 129.37, 132.56, 133.56, 134.98, 135.25, 135.75, 136.51, 136.62, 143.19, 154.91, 159.11, 160.73 ppm.
2-Benzylthio-4-chloro-5-(1,3,4-oxadiazol-2-yl)-N-(pyridine-3-carbonyl)benzenesulfonamide (4h, C21H15ClN4O4S2)
To a suspension of 0.135 g pyridine-3-carboxylic acid (1.1 mmol) in 5 cm3 dry MeCN, 0.212 g EDCI (1.1 mmol) was added and stirred for 5 min at room temperature. 2a (0.382 g, 1 mmol) and 0.184 g DMAP (1.5 mmol) were added and stirred for 18 h at room temperature. The precipitate was filtered off, washed with MeCN, and then suspended in 1 cm3 EtOH, acidified with 1 cm3 10% p-TSA/EtOH, and stirred for 2 h at room temperature. The precipitate was filtered off, washed with EtOH, and purified by extraction of contaminants with hot MeCN. Yield: 0.122 g (25%); m.p.: 282–284 °C; R
f = 0.30 (benzene/EtOH = 4:1); IR (KBr):  = 3,436, 3,096, 3,060, 2,926, 1,633, 1,589, 1,565, 1,520, 1,495, 1,355, 1,135 cm−1; 1H NMR (200 MHz, DMSO-d
6):
= 3,436, 3,096, 3,060, 2,926, 1,633, 1,589, 1,565, 1,520, 1,495, 1,355, 1,135 cm−1; 1H NMR (200 MHz, DMSO-d
6):  = 4.47 (s, 2H, SCH2), 7.20–7.33 (m, 5H, Ar–H), 7.73–7.78 (m, 2H, H-3 and Ar–H), 8.49–8.55 (m, 2H, H-6 and Ar–H), 8.85–8.87 (m, 1H, Ar–H), 8.09 (s, 1H, Ar–H), 8.47 (s, 1H, Ar–H) ppm; 13C NMR (50 MHz, DMSO-d
6):
= 4.47 (s, 2H, SCH2), 7.20–7.33 (m, 5H, Ar–H), 7.73–7.78 (m, 2H, H-3 and Ar–H), 8.49–8.55 (m, 2H, H-6 and Ar–H), 8.85–8.87 (m, 1H, Ar–H), 8.09 (s, 1H, Ar–H), 8.47 (s, 1H, Ar–H) ppm; 13C NMR (50 MHz, DMSO-d
6):  = 35.88, 117.98, 125.11, 127.74, 127.96, 128.74, 129.29, 131.77, 133.19, 135.59, 135.76, 137.50, 139.95, 143.52, 146.76, 149.56, 155.14, 161.32, 164.82 ppm.
= 35.88, 117.98, 125.11, 127.74, 127.96, 128.74, 129.29, 131.77, 133.19, 135.59, 135.76, 137.50, 139.95, 143.52, 146.76, 149.56, 155.14, 161.32, 164.82 ppm.
2-Benzylthio-4-chloro-N-(4-chlorobenzoyl)-5-(1,3,4-oxadiazol-2-yl)benzenesulfonamide (4i, C22H15Cl2N3O4S2)
To a solution of 0.172 g 4-chlorobenzoic acid (1.1 mmol) in 5 cm3 dry MeCN, 0.227 g DCC (1.1 mmol) was added and stirred at room temperature for 5 min. 2a (0.382 g, 1 mmol) and 0.184 g DMAP (1.5 mmol) were added and stirred at room temperature for 72 h. By-products were filtered out and washed thoroughly with MeCN. The filtrate was concentrated under reduced pressure to dryness. MeOH (2 cm3) was added, and the obtained mixture was slowly acidified with 5 M hydrochloric acid. The formed precipitate was filtered off and washed with EtOH and water. The crude product was purified by crystallization from EtOH. Yield: 0.292 g (56%); m.p.: 275–277 °C; R
f = 0.25 (benzene/EtOH = 4:1); IR (KBr):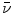 = 3,162, 3,080, 2,929, 2,854, 1,698, 1,592, 1,531, 1,492, 1,462, 1,348, 1,168 cm−1; 1H NMR (500 MHz, DMSO-d
6):
= 3,162, 3,080, 2,929, 2,854, 1,698, 1,592, 1,531, 1,492, 1,462, 1,348, 1,168 cm−1; 1H NMR (500 MHz, DMSO-d
6):  = 4.56 (s, 2H, SCH2), 7.14–7.21 (m, 3H, Ar–H), 7.32–7.36 (m, 2H, Ar–H), 7.57–7.62 (m, 2H, H-3 and Ar–H), 7.88–7.95 (m, 3H, Ar–H), 8.57 (s, 1H, H-6), 9.48 (s, 1H, Ar–H) ppm; 13C NMR (50 MHz, DMSO-d
6):
= 4.56 (s, 2H, SCH2), 7.14–7.21 (m, 3H, Ar–H), 7.32–7.36 (m, 2H, Ar–H), 7.57–7.62 (m, 2H, H-3 and Ar–H), 7.88–7.95 (m, 3H, Ar–H), 8.57 (s, 1H, H-6), 9.48 (s, 1H, Ar–H) ppm; 13C NMR (50 MHz, DMSO-d
6):  = 35.99, 118.53, 127.90, 128.80, 128.99, 129.36, 129.51, 130.41, 130.80, 134.00, 134.79, 135.39, 136.95, 138.61, 143.69, 155.24, 161.03, 164.52 ppm.
= 35.99, 118.53, 127.90, 128.80, 128.99, 129.36, 129.51, 130.41, 130.80, 134.00, 134.79, 135.39, 136.95, 138.61, 143.69, 155.24, 161.03, 164.52 ppm.
2-Benzylthio-4-chloro-5-(1,3,4-oxadiazol-2-yl)-N-(3,4,5-trimethoxybenzoyl)benzenesulfonamide (4j, C25H22ClN3O7S2)
To a suspension of 0.233 g 3,4,5-trimethoxybenzoic acid (1.1 mmol) in 5 cm3 dry MeCN, 0.227 g DCC (1.1 mmol) was added and stirred at room temperature for 5 min. 2a (0.382 g, 1 mmol) and 0.184 g DMAP (1.5 mmol) were added and stirred at room temperature for 20 h. The precipitate was filtered off and suspended in 5 cm3 EtOH, acidified with 2 cm3 10% p-TSA/EtOH, and stirred under cooling (ice bath) for 5 min. The crude product was filtered off and purified by crystallization from EtOH. Yield: 0.366 g (64%); m.p.: 245–247 °C; R
f = 0.29 (benzene/EtOH = 4:1); IR (KBr):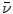 = 3,442, 3,158, 3,092, 2,962, 2,931, 2,841, 1,697, 1,595, 1,526, 1,511, 1,460, 1,331, 1,162 cm−1; 1H NMR (200 MHz, DMSO-d
6):
= 3,442, 3,158, 3,092, 2,962, 2,931, 2,841, 1,697, 1,595, 1,526, 1,511, 1,460, 1,331, 1,162 cm−1; 1H NMR (200 MHz, DMSO-d
6):  = 3.73 (s, 3H, OCH3), 3.78 (s, 6H, 2OCH3), 4.56 (s, 2H, SCH2), 7.16–7.36 (m, 7H, Ar–H), 7.90 (s, 1H, H-3), 8.59 (s, 1H, H-6), 9.49 (s, 1H, Ar–H) ppm; 13C NMR (50 MHz, DMSO-d
6):
= 3.73 (s, 3H, OCH3), 3.78 (s, 6H, 2OCH3), 4.56 (s, 2H, SCH2), 7.16–7.36 (m, 7H, Ar–H), 7.90 (s, 1H, H-3), 8.59 (s, 1H, H-6), 9.49 (s, 1H, Ar–H) ppm; 13C NMR (50 MHz, DMSO-d
6):  = 36.01, 56.38, 60.45, 106.49, 118.49, 125.99, 127.87, 128.74, 129.39, 134.16, 134.79, 135.32, 136.94, 142.05, 143.73, 152.93, 155.25, 161.05, 164.81 ppm.
= 36.01, 56.38, 60.45, 106.49, 118.49, 125.99, 127.87, 128.74, 129.39, 134.16, 134.79, 135.32, 136.94, 142.05, 143.73, 152.93, 155.25, 161.05, 164.81 ppm.
NCI in vitro anticancer screen
As of early 2007 all compounds submitted to the NCI-60 cell screen are tested initially at a single high dose (10 μM) in the full NCI-60 cell panel representing human leukemia, melanoma and lung, colon, brain, breast, ovary, kidney, and prostate cancers. Briefly, the compounds were solubilized in DMSO and added at a single concentration, and the cell culture was incubated for 48 h at 37 °C, 5% CO2, 95% air, and 100% relative humidity. End points were determined by colorimetric sulforhodamine B (SRB) assay [40]. Results for each compound were reported as a mean-graph of the percent growth of the treated cells relative to the no-drug control, and relative to the time-zero number of cells. This allows detection of both growth inhibition (values between 0 and 100) and lethality (values less than 0) [41]. According to Developmental Therapeutics Program (DTP) anticancer screening paradigm, after obtaining the results for one-dose assay, careful analysis of DTP screening data was performed and compound 4c (NSC 754633) which satisfied predetermined threshold inhibition criteria was selected for the NCI five-dose (0.01–100 μM) assay. The results were used to create dose–response curves (log10 of sample concentration versus % growth), and three response parameters (GI50, TGI, and LC50) were calculated for each cell line. GI50 measures the growth inhibitory power of the test agent, TGI signifies a cytostatic effect, and LC50 signifies a cytotoxic effect.
Acknowledgments
The authors are very grateful to Dr. Joel Morris, Chief of Drug Synthesis and Chemistry Branch (DSCB), National Cancer Institute (Bethesda, MD) for the in vitro screening.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
References
- 1.Negwer M. Organic-chemical drugs and their synonyms. Berlin: Akademie Verlag; 1994. [Google Scholar]
- 2.Winum JY, Rami M, Scozzafava A, Montero JL, Supuran C. Med Res Rev. 2008;28:445. doi: 10.1002/med.20112. [DOI] [PubMed] [Google Scholar]
- 3.Neri D, Supuran CT. Nat Rev Drug Discov. 2011 doi: 10.1038/nrd3554. [DOI] [PubMed] [Google Scholar]
- 4.Bouchain G, Delorme D. Curr Med Chem. 2003;10:2359. doi: 10.2174/0929867033456585. [DOI] [PubMed] [Google Scholar]
- 5.Owa T, Yoshino H, Okauchi T, Okabe T, Ozawa Y, Sugi NH, Yoshimatsu K, Nagasu T, Koyanagi N, Kitoh K. Bioorg Med Chem Lett. 2002;12:2097. doi: 10.1016/S0960-894X(02)00376-1. [DOI] [PubMed] [Google Scholar]
- 6.Scozzafava A, Owa T, Mastrolorenzo A, Supuran CT. Curr Med Chem. 2003;10:925. doi: 10.2174/0929867033457647. [DOI] [PubMed] [Google Scholar]
- 7.De Clercq E. Int J Biochem Cell Biol. 2004;36:1800. doi: 10.1016/j.biocel.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 8.Sławiński J. Eur J Med Chem. 2003;39:179. doi: 10.1016/j.ejmech.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 9.Sławiński J, Brożewicz K, Fruziński A, Główka ML. Heterocycles. 2011;83:1093. doi: 10.3987/COM-11-12164. [DOI] [Google Scholar]
- 10.Sławiński J, Bednarski P, Reszka P. Polish J Chem. 2004;39:179. [Google Scholar]
- 11.Sławiński J, Gdaniec M. Eur J Med Chem. 2005;40:377. doi: 10.1016/j.ejmech.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 12.Sławiński J, Brzozowski Z. Eur J Med Chem. 2006;41:1180. doi: 10.1016/j.ejmech.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 13.Brzozowski Z, Sączewski F, Sławiński J, Bednarski PJ, Grünert R, Gdaniec M. Bioorg Med Chem. 2007;15:2560. doi: 10.1016/j.bmc.2007.01.052. [DOI] [PubMed] [Google Scholar]
- 14.Kuo CL, Assefa H, Kamath S, Brzozowski Z, Sławiński J, Sączewski F, Buolamwini JK, Neamati N. J Med Chem. 2004;47:385. doi: 10.1021/jm030378i. [DOI] [PubMed] [Google Scholar]
- 15.Brzozowski Z, Sławiński J, Sączewski F, Sanchez T, Neamati N. Eur J Med Chem. 2008;43:1188. doi: 10.1016/j.ejmech.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 16.Brzozowski Z, Sączewski F, Sławiński J, Sanchez T, Neamati N. Eur J Med Chem. 2009;44:190. doi: 10.1016/j.ejmech.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 17.Sławiński J, Żołnowska B, Pirska D, Kędzia A, Kwapisz E. J Enzym Inhib Med Chem. 2011 doi: 10.3109/14756366.2011.625024. [DOI] [PubMed] [Google Scholar]
- 18.Sączewski F, Innocenti A, Brzozowski Z, Sławiński J, Pomarnacka E, Kornicka A, Scozzafava A, Supuran CT. J Enzym Inhib Med Chem. 2006;21:563. doi: 10.1080/14756360600648146. [DOI] [PubMed] [Google Scholar]
- 19.Sączewski F, Sławiński J, Kornicka A, Brzozowski Z, Pomarnacka E, Innocenti A, Scozzafava A, Supuran CT. Bioorg Med Chem Lett. 2006;16:4846. doi: 10.1016/j.bmcl.2006.06.064. [DOI] [PubMed] [Google Scholar]
- 20.Neamati N, Mazumder A, Sunder S, Owen JM, Schultz RJ, Pommier Y. Antivir Chem Chemother. 1997;8:485. [Google Scholar]
- 21.Kuo CL, Assefa H, Kamath S, Brzozowski Z, Sławiński J, Sączewski F, Buolamwini JK, Neamati N. J Med Chem. 2004;47:385. doi: 10.1021/jm030378i. [DOI] [PubMed] [Google Scholar]
- 22.Corbett TH, White K, Polin L, Kushner J, Paluch J, Shih C, Grossman CS. Investig New Drug. 2003;21:33. doi: 10.1023/A:1022912208877. [DOI] [PubMed] [Google Scholar]
- 23.Kirkwood JM, Gonzalez R, Reintgen D, Clingan PR, McWilliams RR, De Alwis DP, Zimmermann A, Brown MP, Ilaria RL, Jr, Millward MJ. Cancer. 2011 doi: 10.1002/cncr.26068. [DOI] [PubMed] [Google Scholar]
- 24.Dömling A, Antuch W, Beck B, Schauer-Vukašinović V. Bioorg Med Chem Lett. 2008;18:4115. doi: 10.1016/j.bmcl.2008.05.096. [DOI] [PubMed] [Google Scholar]
- 25.Ackler S, Mitten MJ, Foster K, Oleksijew A, Refici M, Tahir SK, Xiao Y, Tse C, Frost DJ, Fesik SW, Rosenberg SH, Elmore SW, Shoemaker AR. Cancer Chemother Pharmacol. 2010;66:869. doi: 10.1007/s00280-009-1232-1. [DOI] [PubMed] [Google Scholar]
- 26.Lobb KL, Hipskind PA, Aikins JA, Alvarez E, Cheung YY, Considine EL, De Dios A, Durst GL, Ferritto R, Grossman CS, Giera DD, Hollister BA, Huang Z, Iversen PW, Law KL, Li T, Lin HS, Lopez B, Lopez JE, Cabrejas LMM, McCann DJ, Molero V, Reilly JE, Richett ME, Shih C, Teicher B, Wikel JH, White WT, Mader MM. J Med Chem. 2004;47:5367. doi: 10.1021/jm030594r. [DOI] [PubMed] [Google Scholar]
- 27.Brzozowski Z, Sławiński J. Acta Pol Pharm. 1984;41:5. [PubMed] [Google Scholar]
- 28.Novello FC, Jones JH (1965) Belgium Patent 669,534; (1965) Chem Abstr 65:5467
- 29.Goralski CT, Pews RG, Burk GA (1997) US Patent 4,041,073; (1997) Chem Abstr 87:151851
- 30.Szczepanski H, Meyer W, Weibel F (1991) Eur Patent 420,815; (1991) Chem Abstr 115:71151
- 31.Pomarnacka E, Angielski S, Hoppe A. Acta Pol Pharm. 1984;41:141. [Google Scholar]
- 32.Sturino CF, Labelle M. Tetrahedron Lett. 1998;39:5891. doi: 10.1016/S0040-4039(98)01240-4. [DOI] [Google Scholar]
- 33.Donkor IO, Abdel-Ghany YS, Kador PF, Mizoguchi T, Bartoszko-Malik A, Miller DD. Eur J Med Chem. 1998;33:15. doi: 10.1016/S0223-5234(99)80071-3. [DOI] [Google Scholar]
- 34.Matassa VG, Maduskuie TP, Jr, Shapiro HS, Hesp B, Snyder DW, Aharony D, Krell RD, Keith RA. J Med Chem. 1990;33:1781. doi: 10.1021/jm00168a037. [DOI] [PubMed] [Google Scholar]
- 35.BioByte Corp., Claremont, CA, USA (2005), CLogP v5.01; www.biobyte.com
- 36.Alvarez M, Paull K, Monks A, Hose C, Lee JS, Weinstein J, Grever M, Bates S, Fojo T. J Clin Invest. 1995;95:2205. doi: 10.1172/JCI117910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee JS, Paull K, Alvarez M, Hose C, Monks A, Grever M, Fojo AT, Bates SE. Mol Pharmacol. 1994;46:627. [PubMed] [Google Scholar]
- 38.Weinstein JN, Myers TG, O’Connor PM, Friend SH, Fornace AJ, Jr, Kohn KW, Fojo T, Bates SE, Rubinstein LV, Anderson NL, Buolamwini JK, Van Osdol WW, Monks AP, Scudiero DA, Sausville EA, Zaharevitz DW, Bunow B, Viswanadhan VN, Johnson GS, Wittes RE, Paull KD. Science. 1997;275:343. doi: 10.1126/science.275.5298.343. [DOI] [PubMed] [Google Scholar]
- 39.Paull KD, Hamel E, Malspeis L. Cancer chemotherapeutic agents. Washington DC: Oxford University Press; 1995. [Google Scholar]
- 40.Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd MR. J Natl Cancer Inst. 1990;82:1107. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- 41.Paull KD, Shoemaker RH, Hodes L, Monks A, Scudiero DA, Rubinstein L, Plowman J, Boyd MR. J Natl Cancer Inst. 1989;81:1088. doi: 10.1093/jnci/81.14.1088. [DOI] [PubMed] [Google Scholar]


