Abstract
Purpose
Despite significant improvements in treatment for ovarian cancer, survival is poorer for non-Hispanic black (NHB) women compared to non-Hispanic white (NHW) women. Neighborhood socioeconomic status (SES) has been implicated in racial disparities across a variety of health outcomes and may similarly contribute to racial disparities in ovarian cancer survival. The purpose of this analysis is to assess the influence of neighborhood SES on NHB-NHW survival differences after accounting for differences in tumor characteristics and in treatment.
Methods
Data were obtained from 2432 women (443 NHB and 1989 NHW) diagnosed with epithelial ovarian cancer in Cook County, Illinois between 1998 and 2007. Neighborhood (i.e., census tract) SES at the time of diagnosis was calculated for each woman using two well-established composite measures of affluence and disadvantage. Cox proportional hazard models measured the association between NHB race and survival after adjusting for age, tumor characteristics, treatment, year of diagnosis, and neighborhood SES.
Results
There was a strong association between ovarian cancer survival and both measures of neighborhood SES (P < .0001 for both affluence and disadvantage). After adjusting for age, tumor characteristics, treatment, and year of diagnosis, NHB were more likely than NHW to die of ovarian cancer (hazard ratio [HR] = 1.47, 95% confidence interval [CI]: 1.28–1.68). The inclusion of neighborhood affluence and disadvantage into models separately and together attenuated this risk (HRaffluence = 1.37, 95% CI: 1.18 –1.58; HRdisadvantage = 1.28, 95% CI: 1.08–1.52; and HRaffluence + disadvantage = 1.28, 95% CI: 1.08–1.52.
Conclusions
Neighborhood SES, as measured by composite measures of affluence and disadvantage, is a predictor of survival in women diagnosed with ovarian cancer in Cook County, Illinois and may contribute to the racial disparity in survival.
Keywords: Ovarian cancer, Racial disparities, Neighborhood-level factors, SES, Cancer survival
Introduction
Ovarian cancer is the most lethal gynecologic cancer and the fifth-leading cause of cancer deaths among women in the United States. [1]. In 2014, an estimated 21,980 incident cases and 14,270 deaths occurred in the United States. [2]. Whereas non-Hispanic white (NHW) women are at a 30% greater risk of developing ovarian cancer compared to non-Hispanic blacks (NHBs) [3], NHB have significantly poorer survival [3–5], and evidence suggests this disparity is increasing [2,6–8]. Racial disparities in survival from breast [9], prostate [10], and colorectal [11] cancer have been attributed to racial differences in screening rates, which lead to later-stage diagnoses in NHBs. However, ovarian cancer lacks a population-based screening mechanism [12], and findings on racial differences in stage at diagnosis are inconsistent [5,8,13,14]. Even when diagnosed at similar stages, NHB women have consistently poorer survival than NHWs [4,6].
This survival disparity is partly due to racial differences in patient characteristics, in which NHBs have more aggressive tumors [15], a poorer response to treatment [16,17], more limited access to high-quality medical facilities and providers [18], and a lower availability and uptake of treatments than NHWs [6,19,20]. Although these individual-level factors play a role in ovarian cancer survival, they may not fully account for the differential survival observed between NHBs and NHWs, as contextual factors may also contribute to this disparity [21–23]. One important contextual factor is neighborhood socioeconomic status (SES). Area-level measures of SES, including neighborhood SES, have been associated with a variety of health outcomes [24–28]. Significant associations have been observed between components of neighborhood SES and breast cancer screening [29], ovarian cancer tumor characteristics [15], and breast [30–33], ovarian [34], and prostate [35] cancer outcomes. Moreover, the effects of area-level SES appear to differ by race, with some studies reporting stronger associations for NHB than for other racial or ethnic groups [29,36,37]. To our knowledge, comprehensive measures of neighborhood SES have not been evaluated in association with racial disparities in survival from ovarian cancer.
Neighborhood SES provides a contextual basis that may positively or negatively influence ovarian cancer survival independent of individual-level factors [38]. For example, affluent neighborhoods may have strong social networks, whose members have greater knowledge of and access to advances in health care [39,40]. Conversely, disadvantaged neighborhoods may present barriers to seeking and receiving health care [41,42], which may result in poorer overall health and increased psychosocial vulnerability for their residents [43]. In addition, neighborhood SES may influence survival through its effect on the type and availability of treatment. Timely and aggressive treatment is essential to prolonging survival in women with ovarian cancer [44–46], and the presence or absence of factors impacting treatment, such as access to care [47], ability to pay for care [48], or quality of care [18], may cluster geographically.
Cook County, the location of the city of Chicago, is the second most populous county in the United States. It is a demographically diverse geographic area containing 1344 census tracts, of which 866 are in the city of Chicago and 478 are in suburban Cook County [49]. Cook County is highly racially and economically segregated. In 2000, the county’s black Dissimilarity Index [50] was 75.9% in suburban Cook County and 85.6% in Chicago [51], which exceeded other U.S. metropolitan areas with the exception of Milwaukee and Detroit [52]. More than one-third of Cook County’s census tracts (n = 495) are designated by the U.S. Department of Health and Human Services [53] as Medically Underserved Areas or Populations.
Concentrated affluence [54] and concentrated disadvantage [55] are comprehensive and complementary measures of neighborhood SES based on the concept of “ecological differentiation.” One aspect of ecological differentiation is the “economic stratification” that characterizes neighborhood concentrations of disadvantage or affluence [54,56]. Neighborhoods that lack economic resources also tend to be deficient in other areas such as education, employment, and health care [57]. By contrast, neighborhoods with greater physical capital, such as good housing and schools, tend to have residents with greater economic means, higher educational attainment, and greater overall access to resources [58].
The purpose of this analysis is to assess whether racial disparities in survival existed in NHB and NHW women diagnosed with ovarian cancer in Cook County, Illinois from 1998 to 2007 using two measures of neighborhood SES, concentrated affluence and disadvantage. We also examined whether neighborhood SES contributed to any observed survival disparity after accounting for individual-level treatment and clinical characteristics.
Materials and methods
Study population
This study was reviewed and approved by the Institutional Review Board of the University of Illinois at Chicago, and the Illinois Public Health Department, Illinois State Cancer Registry (ISCR). Cancer registry data for eligible ovarian cancer cases diagnosed between January 1, 1998 and July 11, 2007 in Cook County, Illinois were obtained from the ISCR. ISCR is the only source of population-based cancer incidence data for the state of Illinois [59], and data for diagnosis years 1998 through 2007 are estimated to be 98%–100% [60] complete based on North American Association of Central Cancer Registry certification. Cases of invasive epithelial ovarian cancer (ovarian cancer) were eligible for inclusion if they were 20 years or older at diagnosis and either NHB or NHW. Eligible histology codes included International Classification of Diseases for Oncology, Third Edition (ICD-O-3) histology codes (ICD-O-2 for diagnoses before 2001) for epithelial ovarian cancer (specifically, ICD-O-3 codes 8010–8046, 8441–8442, 8460–8462, 8470–8472, 8480–8482, 8380–8382, 8140–8260, 8050–8074, 8562, 8120, 8130, 9014, 8313, 9015, 8800, 8801, 9000, 8310, 8323, 8440, 8450, 8490, 8570, and 8574) [3].
Data were available for all cases on age, race, Hispanic ethnicity, and Surveillance Epidemiology and End Results (SEER) general summary stage at diagnosis [61]. Vital status was available through July 11, 2012. First course treatment data, based on Facility Oncology Registry Data Standards, were available for all cases diagnosed in 1998–2002 and 2004–2009. Because treatment information was not available for cases diagnosed in 2003, these cases were not included in the analytical sample. Among the 2795 eligible cases available for analysis, 359 were excluded because they lacked information on treatment (262 were diagnosed in 2003, when no treatment data were collected, and 97 were missing treatment data despite availability) or did not have valid FIPS (Federal Information Processing Standards) codes (n = 4). The final sample included 2432 women (1989 NHWs and 443 NHBs). Data were available for the following clinical characteristics: age at diagnosis, stage at diagnosis, tumor grade, year of diagnosis, type of surgical treatment, type of chemotherapy treatment, and patient vital status.
Vital status
Cancer-specific mortality was not available for analysis; therefore, all-cause mortality was used. Because most women with ovarian cancer die of the disease or disease-related complications, all-cause mortality is considered a reasonable estimate of cancer-specific survival for ovarian cancer [3,62]. Five-year survival time was calculated by subtracting the date of ovarian cancer diagnosis from the date of death or censoring (≤5 or >5 years). Vital status was assigned as alive or dead based on vital status reported through July 11, 2012.
Variables
Both neighborhood SES index variables were constructed using U.S. Census data. Patient addresses were geocoded using ArcGIS U.S. Street Locator (ESRI, 12.0; Redlands, CA) and matched to 2000 U.S. Census data to obtain the nine census variables used in the construction of these indices. Final affluence and disadvantage variables were created using methods identical to those used in Peterson et al. [15] that were developed previously [54,55]. Briefly, concentrated affluence (affluence) was constructed from the following three census variables: percent college educated, percent of families with incomes above $75,000, percent in managerial or professional occupations. Each factor was standardized to have a mean of 0 and SD of 1, then summed to create the final affluence variable. Concentrated disadvantage (disadvantage) was created similarly using the following six census variables: percent below poverty, percent unemployed, percent receiving public assistance, percent in female-headed households with children under 18 years, percent under 18 years, and percent NHB. Higher scores for each index variable represented greater concentrated affluence or greater concentrated disadvantage. Both variables were divided into quartiles, with the lowest quartile (Q1) serving as the reference level in multivariate models. These composite measures were used in place of separate SES measures, creating uncorrelated measures accounting for most of the variance with the included census variables [63].
Stage at diagnosis was categorized as early stage (SEER summary stage I/II), late stage (SEER summary stage III/IV) and unknown or unstaged. Pathologic grade was categorized as low grade (well or moderately differentiated), high grade (poor or undifferentiated), or unknown or ungraded [4]. Surgical treatment was categorized as any surgery (debulking cytoreductive or other) or none. Chemotherapy was categorized as administered (multiagent, single agent, or other), recommended but not administered, or contraindicated. Year of diagnosis was included in multivariate models to account for changes in diagnostic procedures or effectiveness of treatments that may have affected survival over time [64] and divided into two periods roughly at the midpoint of the data (1998–2002 and 2004–2007) to account for the lack of treatment data for the year 2003. Age at diagnosis was included in all models (<50, 50–64, 65–74, and ≥75 years) [65].
Statistical analysis
The distribution of individual-level factors (i.e., age, stage, grade, year of diagnosis, receipt of surgical treatment, and chemotherapy) was examined by race, quartile of affluence, quartile of disadvantage, and treatment status. Differences in these distributions were tested using χ2 and t test statistics for categorical and continuous variables, respectively. The Cochran-Armitage test was used to evaluate the linear trend of these variables by quartile of affluence and quartile of disadvantage.
Six multivariate Cox proportional hazards models were constructed to measure the association between NHB race and survival, including (1) age-adjusted; (2) previous covariates plus tumor characteristics and diagnosis year; (3) previous covariates plus treatment; (4) previous covariates plus concentrated affluence; (5) previous covariates plus concentrated disadvantage (excluding concentrated affluence); and (6) previous covariates plus concentrated disadvantage and concentrated affluence. Interaction terms for treatment variables and indices of neighborhood SES, as well as race and both indices were evaluated. No violations of the proportional hazards assumption were observed (P-values for interaction with time ranged from .26 to .28). A sensitivity analysis exploring the potential impact of insurance status on survival was conducted by limiting the data to Medicare-eligible women aged 65 years and older.
Statistical comparisons of all models were performed using log likelihood ratio methods. Statistical significance was set to P less than .05, and all analyses were conducted using SAS 9.3 (SAS Institute Inc., Cary, NC). All statistical tests were two sided.
Results
A total of 300 of 443 (67.7%) NHB and 1055 of 1989 (53%) NHW women died (P <.0001) during the analysis period. Table 1 lists the distribution and association of patient characteristics by race and quartile of concentrated affluence and disadvantage. Mean survival was 0.7 years shorter for NHBs (P < .0001) than for NHWs, and median survival was 2.0 years for NHBs versus 4.2 years for NHWs (P < .0001). NHB were diagnosed at younger ages (60.1 vs. 62.8 years, P = 0.0004) and later stages of disease (69% vs. 60.8% at stage IV, P < .0001). Although fewer tumors in NHBs were high grade (38.4% vs. 43.8%, respectively), a larger proportion were unknown or ungraded (35% vs. 30.2% in NHWs). NHB were less likely to receive surgery (P < .0001) and chemotherapy (P = .003); in addition, NHB lived in neighborhoods with significantly lower mean affluence scores (−2.0 vs. 0.5, P < .0001) and higher mean disadvantage scores (7.0 vs. −1.6, respectively, P < .0001). No difference was observed in year of diagnosis (P = .15).
Table 1.
Percent distribution and association of patient characteristics, by race and quartile* of affluence and disadvantage (N = 2432)
| Variables | Race
|
By quartile of affluence score
|
By quartile of disadvantage score
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NHW
|
NHB
|
P | 1st
|
2nd
|
3rd
|
4th (highest)
|
PTrend | 1st
|
2nd
|
3rd
|
4th (highest)
|
PTrend | |
| n = 1989 (%) | n = 443 (%) | n = 604 (%) | n = 605 (%) | n = 609 (%) | n = 614 (%) | n = 612 (%) | n = 610 (%) | n = 615 (%) | n = 595 (%) | ||||
| Mean survival, y | 3.2 | 2.5 | <.0001 | 4.5 | 4.6 | 5.5 | 5.8 | <.0001 | 5.8 | 5.9 | 4.8 | 3.9 | <.0001 |
| SD | 1.9 | 2.0 | 4.3 | 4.4 | 4.5 | 4.6 | 4.6 | 4.6 | 4.3 | 4.2 | |||
| Median | 4.2 | 2.0 | 2.8 | 2.9 | 4.6 | 5.3 | 5.2 | 5.4 | 3.2 | 2.2 | |||
| Mean age, y | 62.8 | 60.1 | .0004 | 61.5 | 63.8 | 61.5 | 62.3 | .94 | 62.9 | 61.1 | 62.8 | 62.2 | .87 |
| SD | 14.5 | 14.3 | 14.8 | 14.4 | 14.6 | 14.0 | 14.6 | 14.6 | 14.3 | 14.4 | |||
| SEER stage at diagnosis | <.0001 | .002 | .0003 | ||||||||||
| Early (SEER stage I or II) | 627 (31.5) | 92 (20.7) | 170 (28.2) | 164 (27.1) | 199 (32.7) | 186 (30.3) | 196 (32.0) | 208 (34.1) | 174 (28.3) | 141 (23.7) | |||
| Late (SEER stage III or IV) | 1209 (60.8) | 301 (69.0) | 363 (60.1) | 394 (65.1) | 359 (59.0) | 394 (64.2) | 370 (60.5) | 365 (59.8) | 389 (63.3) | 386 (64.9) | |||
| Unknown or unstaged | 153 (7.7) | 50 (11.3) | 71 (11.7) | 47 (7.8) | 51 (8.3) | 34 (5.5) | 46 (7.5) | 37 (6.1) | 52 (8.4) | 68 (11.4) | |||
| Pathologic grade | .0745 | .12 | .02 | ||||||||||
| Low grade | 517 (26.0) | 118 (26.6) | 159 (26.3) | 160 (26.5) | 163 (26.8) | 153 (24.9) | 151 (24.7) | 175 (28.7) | 165 (26.8) | 144 (24.2) | |||
| High grade | 871 (43.8) | 170 (38.4) | 233 (38.6) | 257 (42.5) | 264 (43.3) | 287 (46.7) | 275 (44.9) | 274 (44.9) | 255 (41.5) | 237 (39.8) | |||
| Unknown | 601 (30.2) | 155 (35.0) | 212 (35.1) | 188 (31.0) | 182 (29.9) | 174 (28.3) | 186 (30.4) | 161 (26.4) | 195 (31.7) | 214 (36.0) | |||
| Year of diagnosis | .1471 | .40 | .01 | ||||||||||
| Early (1998–2002) | 1285 (64.6) | 270 (60.9) | 400 (66.2) | 377 (62.3) | 395 (64.9) | 383 (62.4) | 363 (59.3) | 388 (63.6) | 419 (68.1) | 385 (64.7) | |||
| Late (2004–2007) | 704 (35.4) | 173 (39.1) | 204 (33.8) | 228 (37.7) | 214 (35.1) | 231 (37.6) | 249 (40.7) | 222 (36.4) | 196 (31.9) | 210 (35.3) | |||
| Surgery performed | <.0001 | <.0001 | <.0001 | ||||||||||
| Any | 1646 (82.8) | 292 (65.9) | 451 (74.1) | 467 (77.1) | 502 (83.2) | 518 (84.4) | 525 (85.7) | 529 (86.8) | 484 (78.7) | 400 (67.2) | |||
| None | 343 (17.2) | 151 (34.1) | 158 (25.9) | 139 (22.9) | 101 (16.8) | 96 (15.6) | 87 (14.3) | 81 (13.3) | 131 (21.3) | 195 (32.8) | |||
| Chemotherapy administered | .003 | <.0001 | <.0001 | ||||||||||
| Administered | 1344 (67.6) | 263 (59.4) | 348 (57.1) | 403 (66.5) | 411 (68.2) | 445 (72.5) | 436 (71.2) | 415 (68.0) | 413 (67.2) | 343 (57.7) | |||
| Recommended but not administered | 36 (1.8) | 7 (1.6) | 11 (1.8) | 14 (2.3) | 11 (1.8) | 7 (1.1) | 11 (1.8) | 11 (1.8) | 10 (1.6) | 11 (1.8) | |||
| Contraindicated | 609 (30.6) | 173 (39.1) | 250 (41.1) | 189 (31.2) | 181 (30.0) | 162 (26.4) | 165 (27.0) | 184 (30.2) | 192 (31.2) | 241 (40.5) | |||
| Mean affluence score† | 0.5 | −2.0 | <.0001 | −3.1 | −1.4 | 0.6 | 4.0 | <.0001 | 1.6 | 0.9 | −0.1 | −2.4 | <.0001 |
| SD | 2.7 | 2.1 | 0.7 | 0.5 | 0.7 | 1.6 | 2.5 | 2.3 | 2.9 | 1.5 | |||
| Mean disadvantage score‡ | −1.6 | 7.0 | <.0001 | 4.8 | −0.4 | −1.8 | −2.7 | <.0001 | −3.6 | −2.2 | −0.9 | 6.7 | <.0001 |
| SD | 2.4 | 6.5 | 6.6 | 3.2 | 1.9 | 1.5 | 0.9 | 0.3 | 0.6 | 5.5 | |||
| Quartile of affluence | <.0001 | — | — | ||||||||||
| Highest (4th Q) | 587 (29.5) | 27 (6.1) | — | — | — | — | — | — | — | — | |||
| 3rd | 558 (28.0) | 51 (11.5) | — | — | — | — | — | — | — | — | |||
| 2nd | 487 (24.5) | 118 (26.6) | — | — | — | — | — | — | — | — | |||
| 1st | 357 (18.0) | 247 (55.8) | — | — | — | — | — | — | — | — | |||
| Quartile of disadvantage | <.0001 | — | — | ||||||||||
| Highest (4th Q) | 220 (11.1) | 375 (84.7) | — | — | — | — | — | — | — | — | |||
| 3rd | 581 (29.2) | 34 (7.7) | — | — | — | — | — | — | — | — | |||
| 2nd | 597 (30.0) | 13 (2.9) | — | — | — | — | — | — | — | — | |||
| 1st | 591 (29.7) | 21 (4.7) | — | — | — | — | — | — | — | — | |||
Affluence and disadvantage divided into fourths at the quartiles of the sample distribution.
Higher scores reflect greater concentrated affluence.
Higher scores reflect greater concentrated disadvantage.
The highest quartile of affluence was associated with a longer survival time (P <.0001), less advanced tumor stage (P =.002), and receipt of surgery and chemotherapy (P < .0001 for both). The opposite pattern was observed with respect to disadvantage: The highest quartile was associated with a shorter survival time (P <.0001), more advanced tumor stage (P =.0003), and no surgery or chemotherapy (P < .0001 for both). Disadvantage was also associated with tumor grade, with an increasing proportion of ungraded tumors with increasing quartile of disadvantage (P =.02). Because the strongest effects of both affluence and disadvantage were observed in the highest quartile, these variables were evaluated as binary in subsequent Cox models, with the highest quartile compared with the lower three quartiles as the reference category.
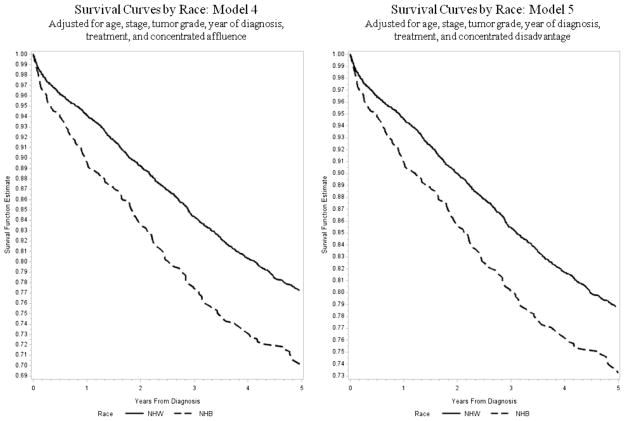
Age-adjusted Cox proportional hazards models estimating the association between race (NHB vs. NHW) and ovarian cancer survival are listed in Table 2. NHB were more likely to die after diagnosis with ovarian cancer (model 1: hazard ratio [HR] = 1.84, 95% confidence interval [CI]: 1.62–2.09), which was similar after adjusting for tumor characteristics (model 2: HR = 1.69, 95% CI: 1.48–1.92). The inclusion of treatment information reduced this risk slightly (model 3: HR = 1.47, 95% CI: 1.28–1.68), as did inclusion of neighborhood SES affluence and disadvantage (model 4: HR = 1.37, 95% CI: 1.18–1.58 and model 5: HR = 1.28, 95% CI: 1.08–1.52). The HR for (model 6, which included both indices, remained unchanged from the previous model: HR = 1.28, 95% CI: 1.08–1.52). None of the interaction terms examined (i.e., treatment and affluence, treatment and disadvantage, race and affluence, and race and disadvantage) were significant. Survival differences between NHB and NHW begin immediately after diagnosis and persisted through the entire 5-year period examined (Fig. 1). Sensitivity analyses restricted to Medicare-eligible women to explore the potential effects of insurance demonstrated similar associations between NHB race and survival in all models (model 4: HR = 1.34, 95% CI: 1.09–1.64; model 5: HR = 1.33, 95% CI: 1.06–1.69; and model 6: HR = 1.33, 95% CI: 1.05–1.68).
Table 2.
HR for NHB versus NHW survival among women with ovarian cancer, all models adjusted for age at diagnosis (N = 2432)
| Variables | Model 1
|
Model 2
|
Model 3
|
Model 4
|
Model 5
|
Model 6
|
|---|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| Race | ||||||
| NHW | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| NHB | 1.84 (1.62– 2.09) | 1.69 (1.48–1.92) | 1.47 (1.28–1.68) | 1.37 (1.18–1.58) | 1.28 (1.08–1.52) | 1.28 (1.08–1.52) |
| Stage at diagnosis | ||||||
| Early (SEER Stage I or II) | — | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Late (SEER Stage III or IV) | — | 5.13 (4.27–6.17) | 4.76 (3.91–5.78) | 4.77 (3.92–5.80) | 4.71 (3.88–5.73) | 4.75 (3.91–5.78) |
| Other or unstaged | — | 3.40 (2.65–4.35) | 2.92 (2.27–3.75) | 2.92 (2.27–3.75) | 2.94 (2.28–3.77) | 2.95 (2.30–3.80) |
| Pathologic grade | ||||||
| Low grade | — | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| High grade | — | 1.26 (1.09–1.47) | 1.36 (1.17–1.59) | 1.37 (1.18–1.60) | 1.37 (1.17–1.60) | 1.38 (1.18–1.61) |
| Other or unknown | — | 1.66 (1.42–1.95) | 1.18 (1.00–1.40) | 1.19 (1.01–1.41) | 1.19 (1.01–1.40) | 1.20 (1.01–1.41) |
| Year of diagnosis* | ||||||
| Early (1998–2002) | — | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Late (2004–2007) | — | 1.01 (0.91–1.13) | 0.99 (0.88–1.11) | 1.01 (0.90–1.13) | 1.02 (0.91–1.14) | 1.02 (0.91–1.15) |
| Surgery performed | ||||||
| Any | — | — | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| None | — | — | 3.14 (2.75–3.59) | 3.09 (2.71–3.53) | 3.04 (2.65–3.47) | 3.02 (2.64–3.45) |
| Chemotherapy administered | ||||||
| Administered | — | — | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Recommended but not administered | — | — | 2.67 (1.90–3.75) | 2.62 (1.87–3.68) | 2.71 (1.93–3.81) | 2.67 (1.90–3.75) |
| Contraindicated | — | — | 1.44 (1.26–1.65) | 1.43 (1.25–1.64) | 1.41 (1.24–1.61) | 1.43 (1.25–1.63) |
| Affluence | ||||||
| 1st quartile | — | — | — | 1.00 (reference) | — | 1.00 (reference) |
| 2nd quartile | — | — | — | 1.08 (0.93–1.26) | — | 1.15 (0.98–1.35) |
| 3rd quartile | — | — | — | 0.91 (0.78–1.07) | — | 1.02 (0.85–1.21) |
| 4th quartile (highest) | — | — | — | 0.77 (0.65–0.91) | — | 0.86 (0.72–1.04) |
| Disadvantage | ||||||
| 1st quartile | — | — | — | — | 1.00 (reference) | 1.00 (reference) |
| 2nd quartile | — | — | — | — | 0.97 (0.82–1.14) | 0.96 (0.82–1.13) |
| 3rd quartile | — | — | — | — | 1.25 (1.07–1.46) | 1.21 (1.03–1.43) |
| 4th quartile (highest) | — | — | — | — | 1.30 (1.08–1.56) | 1.23 (1.00–1.51) |
All models adjusted for age in four categories: less than 50, 50–64, 65–74, and 75 years or more.
Patients diagnosed in 2003 excluded due to missing treatment data.
Fig. 1.
Probability of survival by race after diagnosis with ovarian cancer, Cook County, Illinois, 1998–2007.
Discussion
These results demonstrate a significant disparity in survival between NHW and NHB diagnosed with ovarian cancer in highly segregated Cook County, Illinois, which is consistent with analyses of nationally representative data [4,6,66]. Survival disparities persisted after accounting for individual-level prognostic factors and treatment. Importantly, racial differences in treatment, as observed in this study and described in detail in our earlier work [20], contribute to the racial disparity in survival. Both neighborhood affluence and neighborhood disadvantage were significantly associated with survival. After accounting for these variables in separate models, the risk of death for NHB women was attenuated, although the HRs remained statistically significant. These results suggest that neighborhood SES may contribute to the racial disparity in survival among women diagnosed with ovarian cancer, which is consistent with a previous analysis examining the effects of neighborhood disadvantage on ovarian cancer-specific survival in a hospital-based study of cases diagnosed in Cook County, Illinois during an earlier time period [34]. The mechanisms through which neighborhood SES affects cancer survival are difficult to measure; however, we hypothesize several ways in which indicators of neighborhood affluence and disadvantage might influence ovarian cancer survival.
Neighborhood affluence is associated with healthier environments [67], better self-rated health [68], and higher levels of social support for residents [69], each of which improves cancer survival [70–72]. Neighborhood-level affluence and individual-level affluence are strongly correlated [69]. Affluent individuals possess greater economic resources and social capital, which enable them to avail themselves of the treatment advances associated with better cancer survival [39,40]. In addition, individual-level affluence is associated with private health insurance [73], which is associated in turn with greater access to gynecologic oncologists [74] and participation in clinical trials [75], factors that also result in improved ovarian cancer survival [76,77].
Neighborhood disadvantage has a negative effect on the overall environment and also on individual residents. Disadvantage at the neighborhood level creates an environment of physical and social disorder [78,79], producing conditions that challenge health-seeking behaviors [41,80–83], including delays in seeking timely care or treatment which negatively impacts cancer survival [84,85]. Residents of disadvantaged neighborhoods suffer from greater social isolation [86], which also adversely impacts health-seeking behaviors, in turn leading to later-stage diagnoses, suboptimal treatment [87], and increased cancer mortality [88]. Moreover, residents of disadvantaged neighborhoods are more likely to be disadvantaged themselves [67]. Individual-level disadvantage is associated with poor health care [83] and a greater number of comorbidities [89–92], both of which have been associated with suboptimal cancer treatment [93–95], failure to complete treatment [96], and treatment complications [95].
Finally, the decrease in the survival disparity after adjustment for the two measures of neighborhood SES may reflect underlying differences in a variety of factors that tend to cluster geographically, such as access and quality of care [18,47], cultural or religious beliefs [97,98], health-seeking behaviors, trust in health care providers [99], and environmental stress [100,101].
The use of two composite measures to reflect neighborhood SES is a strength of this analysis. Concentrated affluence is conceptually related to, but distinct from, concentrated disadvantage. While poverty has become more geographically concentrated, so has the concentration of high-level resources (i.e., education, occupation, and income) [54]. Rather than considering the distribution of socioeconomic resources on a continuum from highly disadvantaged to highly affluent, Sampson et al. [56] point out the need to separate affluence and disadvantage analytically. That is, it is not simply the absence of socioeconomic resources can negatively impact health; instead, the presence of socioeconomic resources may additionally exert a positive effect on health. Together, these measures reflect the contextual effect of neighborhood SES on the survival experience of women with ovarian cancer. Importantly, these two composite measures are more appropriate representations of the construct of neighborhood SES than are single measures. Moreover, they address the problem of collinearity that occurs when multiple single-SES measures are used in multivariate models [99].
These findings should be interpreted in light of certain limitations. First, cases were limited women diagnosed with ovarian cancer exclusively in Cook County, Illinois, which may not be representative of other regions of the United States. Cook County is highly segregated geographically [52], so the effects of neighborhood SES in this region may be greater than such effects seen in other geographic areas. A second limitation is the unknown insurance status of patients, which has been shown to impact survival with ovarian and other cancers [4,35,102]. However, the sensitivity analysis restricted to Medicare-eligible women aged 65 years and older [5] produced estimates similar to the original analysis.
In summary, neighborhood SES, as measured by concentrated affluence and concentrated disadvantage, was associated with significantly poorer survival for NHB women compared to NHW women. Our findings suggest a greater proportion of NHB women lived in neighborhoods with higher concentrated disadvantage and lower concentrated affluence, compared to NHWs. These results suggest that the contextual effect of neighborhood SES may partly explain the observed survival differences between NHB and NHW diagnosed with ovarian cancer. Further research should investigate how these contextual differences impact factors more proximate to survival, including patient-related factors (e.g., barriers to care, health-seeking behavior, religiosity, reasons for treatment refusal) as well patient-provider characteristics (e.g., communication, mistrust, perceived racism).
Acknowledgments
This research was funded by the NIH-NCMHD, 1 P60 MD003424 and American Cancer Society, RSG-13-380-01-CPHPS.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 3.Altekruse SF, Kosary CL, Krapcho M, Neyman N, Aminou R, Waldron W, et al. SEER Cancer Statistics Review, 1975–2007, based on November 2009 SEER data submission, posted to the SEER web site. Bethesda, MD: National Cancer Institute; 2010. [Google Scholar]
- 4.Bristow RE, Powell MA, Al-Hammadi N, Chen L, Miller JP, Roland PY, et al. Disparities in ovarian cancer care quality and survival according to race and socioeconomic status. J Natl Cancer Inst. 2013;105(11):823–32. doi: 10.1093/jnci/djt065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Howell EA, Egorova N, Hayes MP, Wisnivesky J, Franco R, Bickell N. Racial disparities in the treatment of advanced epithelial ovarian cancer. Obstet Gynecol. 2013;122(5):1025–32. doi: 10.1097/AOG.0b013e3182a92011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan JK, Zhang M, Hu JM, Shin JY, Osann K, Kapp DS. Racial disparities in surgical treatment and survival of epithelial ovarian cancer in United States. J Surg Oncol. 2008;97(2):103–7. doi: 10.1002/jso.20932. [DOI] [PubMed] [Google Scholar]
- 7.McGuire V, Herrinton L, Whittemore AS. Race, epithelial ovarian cancer survival, and membership in a large health maintenance organization. Epidemiology. 2002;13(2):231–4. doi: 10.1097/00001648-200203000-00021. [DOI] [PubMed] [Google Scholar]
- 8.McGuire V, Jesser CA, Whittemore AS. Survival among U.S. women with invasive epithelial ovarian cancer. Gynecol Oncol. 2002;84(3):399–403. doi: 10.1006/gyno.2001.6536. [DOI] [PubMed] [Google Scholar]
- 9.Sprague BL, Trentham-Dietz A, Gangnon RE, Ramchandani R, Hampton JM, Robert SA, et al. Socioeconomic status and survival after an invasive breast cancer diagnosis. Cancer. 2011;117(7):1542–51. doi: 10.1002/cncr.25589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones BA, Liu WL, Araujo AB, Kasl SV, Silvera SN, Soler-Vila H, et al. Explaining the race difference in prostate cancer stage at diagnosis. Cancer Epidemiol Biomarkers Prev. 2008;17(10):2825–34. doi: 10.1158/1055-9965.EPI-08-0203. [DOI] [PubMed] [Google Scholar]
- 11.Irby K, Anderson WF, Henson DE, Devesa SS. Emerging and widening colorectal carcinoma disparities between blacks and whites in the United States (1975–2002) Cancer Epidemiol Biomarkers Prev. 2006;15(4):792–7. doi: 10.1158/1055-9965.EPI-05-0879. [DOI] [PubMed] [Google Scholar]
- 12.Nolen BM, Lokshin AE. Biomarker testing for ovarian cancer: clinical utility of multiplex assays. Mol Diagn Ther. 2013;17(3):139–46. doi: 10.1007/s40291-013-0027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim S, Dolecek TA, Davis FG. Racial differences in stage at diagnosis and survival from epithelial ovarian cancer: a fundamental cause of disease approach. Soc Sci Med. 2010;71(2):274–81. doi: 10.1016/j.socscimed.2010.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farley JH, Tian C, Rose GS, Brown CL, Birrer M, Maxwell GL. Race does not impact outcome for advanced ovarian cancer patients treated with cisplatin/paclitaxel: an analysis of Gynecologic Oncology Group trials. Cancer. 2009;115(18):4210–7. doi: 10.1002/cncr.24482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peterson C, Rauscher G, Johnson T, Kirschner C, Barrett R, Kim S, et al. The association between neighborhood socioeconomic status and ovarian cancer tumor characteristics. Cancer Causes Control. 2014;25(5):633–7. doi: 10.1007/s10552-014-0357-7. [DOI] [PubMed] [Google Scholar]
- 16.Terplan M, Temkin S, Tergas A, Lengyel E. Does equal treatment yield equal outcomes? The impact of race on survival in epithelial ovarian cancer. Gynecol Oncol. 2008;111(2):173–8. doi: 10.1016/j.ygyno.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.John EM, Whittemore A, Harris R, Itnyre J. Characteristics relating to ovarian cancer risk: collaborative analysis of seven U.S. case-control studies. Epithelial ovarian cancer in black women. Collaborative Ovarian Cancer Group. J Natl Cancer Inst. 1993;85(2):142–7. doi: 10.1093/jnci/85.2.142. [DOI] [PubMed] [Google Scholar]
- 18.Bristow RE, Chang J, Ziogas A, Randall LM, Anton-Culver H. High-volume ovarian cancer care: survival impact and disparities in access for advanced-stage disease. Gynecol Oncol. 2014;132(2):403–10. doi: 10.1016/j.ygyno.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 19.Aranda MA, McGory M, Sekeris E, Maggard M, Ko C, Zingmond DS. Do racial/ethnic disparities exist in the utilization of high-volume surgeons for women with ovarian cancer? Gynecol Oncol. 2008;111(2):166–72. doi: 10.1016/j.ygyno.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joslin CE, Brewer KC, Davis FG, Hoskins K, Peterson CE, Pauls HA. The effect of neighborhood-level socioeconomic status on racial differences in ovarian cancer treatment in a population-based analysis in Chicago. Gynecol Oncol. 2014;135(2):285–91. doi: 10.1016/j.ygyno.2014.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Warner ET, Gomez SL. Impact of neighborhood racial composition and metropolitan residential segregation on disparities in breast cancer stage at diagnosis and survival between black and white women in California. J Community Health. 2010;35(4):398–408. doi: 10.1007/s10900-010-9265-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Osypuk TL. Future research directions for understanding neighborhood contributions to health disparities. Rev Epidemiol Sante Publique. 2013;61(Suppl 2):S61–8. doi: 10.1016/j.respe.2013.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson RC, Schoeni RF, Rogowski JA. Health disparities in mid-to-late life: the role of earlier life family and neighborhood socioeconomic conditions. Soc Sci Med. 2012;74(4):625–36. doi: 10.1016/j.socscimed.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reagan PB, Salsberry PJ. Race and ethnic differences in determinants of preterm birth in the USA: broadening the social context. Soc Sci Med. 2005;60(10):2217–28. doi: 10.1016/j.socscimed.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 25.Acevedo-Garcia D. Residential segregation and the epidemiology of infectious diseases. Soc Sci Med. 2000;51(8):1143–61. doi: 10.1016/s0277-9536(00)00016-2. [DOI] [PubMed] [Google Scholar]
- 26.Browning CR, Cagney KA, Wen M. Explaining variation in health status across space and time: implications for racial and ethnic disparities in self-rated health. Soc Sci Med. 2003;57(7):1221–35. doi: 10.1016/s0277-9536(02)00502-6. [DOI] [PubMed] [Google Scholar]
- 27.Doubeni CA, Schootman M, Major JM, Stone RA, Laiyemo AO, Park Y, et al. Health status, neighborhood socioeconomic context, and premature mortality in the United States: the National Institutes of Health-AARP Diet and Health Study. Am J Public Health. 2012;102(4):680–8. doi: 10.2105/AJPH.2011.300158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shariff-Marco S, Yang J, John EM, Sangaramoorthy M, Hertz A, Koo J, et al. Impact of neighborhood and individual socioeconomic status on survival after breast cancer varies by race/ethnicity: the Neighborhood and Breast Cancer Study. Cancer Epidemiol Biomarkers Prev. 2014;23(5):793–811. doi: 10.1158/1055-9965.EPI-13-0924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dailey AB, Kasl SV, Holford TR, Calvocoressi L, Jones BA. Neighborhood-level socioeconomic predictors of nonadherence to mammography screening guidelines. Cancer Epidemiol Biomarkers Prev. 2007;16(11):2293–303. doi: 10.1158/1055-9965.EPI-06-1076. [DOI] [PubMed] [Google Scholar]
- 30.Whitman S, Orsi J, Hurlbert M. The racial disparity in breast cancer mortality in the 25 largest cities in the United States. Cancer Epidemiol. 2012;36(2):e147–51. doi: 10.1016/j.canep.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 31.Robert S, Strombom I, Trentham-Dietz A, Hampton JM, McElroy JA, Newcomb PA, et al. Socioeconomic risk factors for breast cancer: distinguishing individual- and community-level effects. Epidemiology. 2004;5(4):442–50. doi: 10.1097/01.ede.0000129512.61698.03. [DOI] [PubMed] [Google Scholar]
- 32.Flores YN, Davidson P, Nakazono TT, Carreon DC, Mojica CM, Bastani R. Neighborhood socio-economic disadvantage and race/ethnicity as predictors of breast cancer stage at diagnosis. BMC Public Health. 2013;13:1061. doi: 10.1186/1471-2458-13-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bethea TN, Palmer JR, Rosenberg L, Cozier YC. Neighborhood socioeconomic status in relation to cancer mortality in the Black Women’s Health Study, 1995–2011. Cancer Epidemiol Biomarkers Prev. 2014;23:563. [Google Scholar]
- 34.Peterson CE, Rauscher GH, Johnson TP, Kirschner CV, Freels S, Barrett RE, et al. The effect of neighborhood disadvantage on the racial disparity in ovarian cancer-specific survival in a large hospital-based study in Cook County, Illinois. Front Public Health. 2015;3:8. doi: 10.3389/fpubh.2015.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Freeman VL, Ricardo AC, Campbell RT, Barrett RE, Warnecke RB. Association of census tract-level socioeconomic status with disparities in prostate cancer-specific survival. Cancer Epidemiol Biomarkers Prev. 2011;20(10):2150–9. doi: 10.1158/1055-9965.EPI-11-0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lim JW, Ashing-Giwa KT. Examining the effect of minority status and neighborhood characteristics on cervical cancer survival outcomes. Gynecol Oncol. 2011;121(1):87–93. doi: 10.1016/j.ygyno.2010.11.041. [DOI] [PubMed] [Google Scholar]
- 37.Borrell LN, Diez Roux AV, Rose K, Catellier D, Clark BL. Neighbourhood characteristics and mortality in the Atherosclerosis Risk in Communities Study. Int J Epidemiol. 2004;33(2):398–407. doi: 10.1093/ije/dyh063. [DOI] [PubMed] [Google Scholar]
- 38.Ellen IG, Mijanovich T, Dillman K-N. Neighborhood effects on health: exploring the links and assessing the evidence. J Urban Aff. 2001;23(3–4):391–408. [Google Scholar]
- 39.Link BG, Phelan J. Social conditions as fundamental causes of disease. J Health Soc Behav. 1995;35(Spec):80–94. [PubMed] [Google Scholar]
- 40.Link BG, Phelan JC. McKeown and the idea that social conditions are fundamental causes of disease. Am J Public Health. 2002;92(5):730–2. doi: 10.2105/ajph.92.5.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barrett RE, Cho YI, Weaver KE, Ryu K, Campbell RT, Dolecek TA, et al. Neighborhood change and distant metastasis at diagnosis of breast cancer. Ann Epidemiol. 2008;18(1):43–7. doi: 10.1016/j.annepidem.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 42.Brown AF, Liang LJ, Vassar SD, Merkin SS, Longstreth WT, Jr, Ovbiagele B, et al. Neighborhood socioeconomic disadvantage and mortality after stroke. Neurology. 2013;80(6):520–7. doi: 10.1212/WNL.0b013e31828154ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Jaarsveld CH, Miles A, Wardle J. Pathways from deprivation to health differed between individual and neighborhood-based indices. J Clin Epidemiol. 2007;60(7):712–9. doi: 10.1016/j.jclinepi.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 44.Aletti GD, Dowdy SC, Gostout BS, Jones MB, Stanhope CR, Wilson TO, et al. Aggressive surgical effort and improved survival in advanced-stage ovarian cancer. Obstetrics Gynecol. 2006;107(1):77–85. doi: 10.1097/01.AOG.0000192407.04428.bb. [DOI] [PubMed] [Google Scholar]
- 45.Chi DS, Eisenhauer EL, Zivanovic O, Sonoda Y, Abu-Rustum NR, Levine DA, et al. Improved progression-free and overall survival in advanced ovarian cancer as a result of a change in surgical paradigm. Gynecol Oncol. 2009;114(1):26–31. doi: 10.1016/j.ygyno.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 46.Randall TC, Rubin SC. Cytoreductive surgery for ovarian cancer. Surg Clin North Am. 2001;81(4):871–83. doi: 10.1016/s0039-6109(05)70171-7. [DOI] [PubMed] [Google Scholar]
- 47.Polsky D, Armstrong K, Randall TC, Ross RN, Even-Shoshan O, Rosenbaum PR, et al. Variation in chemotherapy utilization in ovarian cancer: the relative contribution of geography. Health Serv Res. 2006;41(6):2201–18. doi: 10.1111/j.1475-6773.2006.00596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harlan LC, Greene AL, Clegg LX, Mooney M, Stevens JL, Brown ML. Insurance status and the use of guideline therapy in the treatment of selected cancers. J Clin Oncol. 2005;23(36):9079–88. doi: 10.1200/JCO.2004.00.1297. [DOI] [PubMed] [Google Scholar]
- 49.U.S. Census Bureau. [Accessed May 14, 2014];County Population Estimates, 100 largest counties. 2008 Available from: http://www.census.gov/popest/counties/CO-EST2008-07.html.
- 50.Jahn JSCF, Schrag C. The measurement of ecological segregation. Am Sociol Rev. 1947;12(3):293–303. [Google Scholar]
- 51.Hall M, Iceland J, Sanchez L, Marsh K. Changing American neighborhoods and communities report series. 2010 [Google Scholar]
- 52.Frey WH. [Accessed May 14, 2014];New Racial Segregation Measures for States and Large Metropolitan Areas: Analysis of the 2005–2009 American Community Survey and US Census. 2000 Available from: http://censusscope.org/ACS/Segregation.html.
- 53.Administration HRaS. Find shortage areas: MUA/P by state and county. US Department of Health and Human Services; 2003. [Accessed May 30, 2014]. Available from: http://muafind.hrsa.gov/index.aspx. [Google Scholar]
- 54.Sampson RJ, Morenoff JD, Earls F. Beyond social capital:spatial dynamics of collective efficacy for children. Am Social Rev. 1999;64(5):633–60. [Google Scholar]
- 55.Browning CR, Cagney KA. Neighborhood structural disadvantage, collective efficacy, and self-rated physical health in an urban setting. J Health Soc Behav. 2002;43(4):383–99. [PubMed] [Google Scholar]
- 56.Sampson RJ, Morenoff JD, Gannon-Rowley T. Assessing “neighborhood effects”: social processes and new directions in research. Annu Rev Sociol. 2002;28:443–78. [Google Scholar]
- 57.Rossen LM. Neighbourhood economic deprivation explains racial/ethnic disparities in overweight and obesity among children and adolescents in the U.S. A. J Epidemiol Community Health. 2014;68(2):123–9. doi: 10.1136/jech-2012-202245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Solari CD. Affluent neighborhood persistence and change in U.S. cities. City Community. 2012;11(4):370–88. doi: 10.1111/j.1540-6040.2012.01422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Garner K, Shen T. Illinios County Cancer Statistics Review, 2006–2010. Springfield, IL: Illinois Department of Public Health; 2013. [Google Scholar]
- 60.Illinois Department of Public Health Division of Epidemiologic Studies Illinois State Cancer Registry. [Accessed May 12, 2014];User Manual. 2013 Available from: http://www.idph.state.il.us/cancer/13/READMEv20.txt.
- 61.Young JLJ, Roffers SD, Ries LA, Fritz AG, Hurlbert AA. SEER summary staging manual—2000: codes and coding instructions. Bethesda, MD: National Cancer Institute; 2001. [Google Scholar]
- 62.Dolecek TA, McCarthy BJ, Joslin CE, Peterson CE, Kim S, Freels SA, et al. Prediagnosis food patterns are associated with length of survival from epithelial ovarian cancer. J Am Diet Assoc. 2010;110(3):369–82. doi: 10.1016/j.jada.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 63.Vyas S, Kumaranayake L. Constructing socio-economic status indices: how to use principal components analysis. Health Policy Plan. 2006;21(6):459–68. doi: 10.1093/heapol/czl029. [DOI] [PubMed] [Google Scholar]
- 64.Morgan RJ, Jr, Alvarez RD, Armstrong DK, Burger RA, Castells M, Chen LM, et al. Ovarian cancer, version 3 2012. J Natl Compr Canc Netw. 2012;10(11):1339–49. doi: 10.6004/jnccn.2012.0140. [DOI] [PubMed] [Google Scholar]
- 65.Baldwin LA, Huang B, Miller RW, Tucker T, Goodrich ST, Podzielinski I, et al. Ten-year relative survival for epithelial ovarian cancer. Obstet Gynecol. 2012;120(3):612–8. doi: 10.1097/AOG.0b013e318264f794. [DOI] [PubMed] [Google Scholar]
- 66.Terplan M, Smith E, Temkin S. Race in ovarian cancer treatment and survival: a systematic review with meta-analysis. Cancer Causes Control. 2009;20(7):1139–50. doi: 10.1007/s10552-009-9322-2. [DOI] [PubMed] [Google Scholar]
- 67.Carroll-Scott A, Gilstad-Hayden K, Rosenthal L, Peters SM, McCaslin C, Joyce R, et al. Disentangling neighborhood contextual associations with child body mass index, diet, and physical activity: the role of built, socioeconomic, and social environments. Soc Sci Med. 2013;95:106–14. doi: 10.1016/j.socscimed.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Diez Roux AV, Mair C. Neighborhoods and health. Ann N Y Acad Sci. 2010;1186:125–45. doi: 10.1111/j.1749-6632.2009.05333.x. [DOI] [PubMed] [Google Scholar]
- 69.Wen M, Browning CR, Cagney KA. Poverty, affluence, and income inequality: neighborhood economic structure and its implications for health. Soc Sci Med. 2003;57(5):843–60. doi: 10.1016/s0277-9536(02)00457-4. [DOI] [PubMed] [Google Scholar]
- 70.Pelser C, Arem H, Pfeiffer RM, Elena JW, Alfano CM, Hollenbeck AR, et al. Prediagnostic lifestyle factors and survival after colon and rectal cancer diagnosis in the National Institutes of Health (NIH)-AARP Diet and Health Study. Cancer. 2014;120(10):1540–7. doi: 10.1002/cncr.28573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ikeda A, Kawachi I, Iso H, Iwasaki M, Inoue M, Tsugane S. Social support and cancer incidence and mortality: the JPHC study cohort II. Cancer Causes Control. 2013;24(5):847–60. doi: 10.1007/s10552-013-0147-7. [DOI] [PubMed] [Google Scholar]
- 72.Johnson AM, Hines RB, Johnson JA, 3rd, Bayakly AR. Treatment and survival disparities in lung cancer: the effect of social environment and place of residence. Lung Cancer. 2014;83(3):401–7. doi: 10.1016/j.lungcan.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 73.Adler NE, Newman K. Socioeconomic disparities in health: pathways and policies. Health Aff (Millwood) 2002;21(2):60–76. doi: 10.1377/hlthaff.21.2.60. [DOI] [PubMed] [Google Scholar]
- 74.Sateren WB, Trimble EL, Abrams J, Brawley O, Breen N, Ford L, et al. How sociodemographics, presence of oncology specialists, and hospital cancer programs affect accrual to cancer treatment trials. J Clin Oncol. 2002;20(8):2109–17. doi: 10.1200/JCO.2002.08.056. [DOI] [PubMed] [Google Scholar]
- 75.Klamerus JF, Bruinooge SS, Ye X, Klamerus ML, Damron D, Lansey D, et al. The impact of insurance on access to cancer clinical trials at a comprehensive cancer center. Clin Cancer Res. 2010;16(24):5997–6003. doi: 10.1158/1078-0432.CCR-10-1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Robinson WR, Ritter J, Rogers AS, Tedjarati S, Lieberenz C. Clinical trial participation is associated with improved outcome in women with ovarian cancer. Int J Gynecol Cancer. 2009;19(1):124–8. doi: 10.1111/IGJ.0b013e31819a1ce8. [DOI] [PubMed] [Google Scholar]
- 77.Chan JK, Kapp DS, Shin JY, Husain A, Teng NN, Berek JS, et al. Influence of the gynecologic oncologist on the survival of ovarian cancer patients. Obstet Gynecol. 2007;109(6):1342–50. doi: 10.1097/01.AOG.0000265207.27755.28. [DOI] [PubMed] [Google Scholar]
- 78.Echeverria SE, Borrell LN, Brown D, Rhoads G. A local area analysis of racial, ethnic, and neighborhood disparities in breast cancer staging. Cancer Epidemiol Biomarkers Prev. 2009;18(11):3024–9. doi: 10.1158/1055-9965.EPI-09-0390. [DOI] [PubMed] [Google Scholar]
- 79.Sampson RJ. The neighborhood context of well-being. Perspect Biol Med. 2003;46(3 Suppl):S53–64. [PubMed] [Google Scholar]
- 80.Sudano JJ, Baker DW. Explaining US racial/ethnic disparities in health declines and mortality in late middle age: the roles of socioeconomic status, health behaviors, and health insurance. Soc Sci Med. 2006;62(4):909–22. doi: 10.1016/j.socscimed.2005.06.041. [DOI] [PubMed] [Google Scholar]
- 81.King-Shier KM, Mather C, LeBlanc P. Understanding the influence of urban-or rural-living on cardiac patients’ decisions about diet and physical activity: descriptive decision modeling. Int J Nurs Stud. 2013;50(11):1513–23. doi: 10.1016/j.ijnurstu.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 82.Gornick ME. A decade of research on disparities in medicare utilization: lessons for the health and health care of vulnerable men. Am J Public Health. 2008;98(9 Suppl):S162–8. doi: 10.2105/ajph.98.supplement_1.s162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kirby JB, Kaneda T. Neighborhood socioeconomic disadvantage and access to health care. J Health Soc Behav. 2005;46(1):15–31. doi: 10.1177/002214650504600103. [DOI] [PubMed] [Google Scholar]
- 84.Forrest LF, Adams J, Wareham H, Rubin G, White M. Socioeconomic inequalities in lung cancer treatment: systematic review and meta-analysis. PLoS Med. 2013;10(2):e1001376. doi: 10.1371/journal.pmed.1001376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Forrest LF, Sowden S, Rubin G, White M, Adams J. Socio-economic inequalities in patient, primary care, referral, diagnostic, and treatment intervals on the lung cancer care pathway: protocol for a systematic review and meta-analysis. Syst Rev. 2014;3:30. doi: 10.1186/2046-4053-3-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Massey DS, Denton NA. American apartheid: segregation and the making of the underclass. Cambridge, MA: Harvard University Press; 1993. [Google Scholar]
- 87.Gehlert S, Sohmer D, Sacks T, Mininger C, McClintock M, Olopade O. Targeting health disparities: a model linking upstream determinants to downstream interventions. Health Aff (Millwood) 2008;27(2):339–49. doi: 10.1377/hlthaff.27.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kroenke CH, Kubzansky LD, Schernhammer ES, Holmes MD, Kawachi I. Social networks, social support, and survival after breast cancer diagnosis. J Clin Oncol. 2006;24(7):1105–11. doi: 10.1200/JCO.2005.04.2846. [DOI] [PubMed] [Google Scholar]
- 89.Diez-Roux AV, Nieto FJ, Muntaner C, Tyroler HA, Comstock GW, Shahar E, et al. Neighborhood environments and coronary heart disease: a multilevel analysis. Am J Epidemiol. 1997;146(1):48–63. doi: 10.1093/oxfordjournals.aje.a009191. [DOI] [PubMed] [Google Scholar]
- 90.Barr RG, Diez-Roux AV, Knirsch CA, Pablos-Mendez A. Neighborhood poverty and the resurgence of tuberculosis in New York City, 1984–1992. Am J Public Health. 2001;91(9):1487–93. doi: 10.2105/ajph.91.9.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Halonen JI, Vahtera J, Oksanen T, Pentti J, Virtanen M, Jokela M, et al. Socioeconomic characteristics of residential areas and risk of death: is variation in spatial units for analysis a source of heterogeneity in observed associations? BMJ Open. 2013;3(4):1–9. doi: 10.1136/bmjopen-2012-002474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Friel S, Chopra M, Satcher D. Unequal weight: equity oriented policy responses to the global obesity epidemic. BMJ. 2007;335(7632):1241–3. doi: 10.1136/bmj.39377.622882.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Maas HA, Kruitwagen RF, Lemmens VE, Goey SH, Janssen-Heijnen ML. The influence of age and co-morbidity on treatment and prognosis of ovarian cancer: a population-based study. Gynecol Oncol. 2005;97(1):104–9. doi: 10.1016/j.ygyno.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 94.Jorgensen TL, Teiblum S, Paludan M, Poulsen LO, Jorgensen AY, Bruun KH, et al. Significance of age and comorbidity on treatment modality, treatment adherence, and prognosis in elderly ovarian cancer patients. Gynecol Oncol. 2012;127(2):367–74. doi: 10.1016/j.ygyno.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 95.Erickson BK, Martin JY, Shah MM, Straughn JM, Jr, Leath CA., 3rd Reasons for failure to deliver National Comprehensive Cancer Network (NCCN)-adherent care in the treatment of epithelial ovarian cancer at an NCCN cancer center. Gynecol Oncol. 2014;133(2):142–6. doi: 10.1016/j.ygyno.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hu CY, Delclos GL, Chan W, Du XL. Assessing the initiation and completion of adjuvant chemotherapy in a large nationwide and population-based cohort of elderly patients with stage-III colon cancer. Med Oncol. 2011;28(4):1062–74. doi: 10.1007/s12032-010-9644-7. [DOI] [PubMed] [Google Scholar]
- 97.Powell LH, Shahabi L, Thoresen CE. Religion and spirituality: linkages to physical health. Am Psychol. 2003;58(1):36–52. doi: 10.1037/0003-066x.58.1.36. [DOI] [PubMed] [Google Scholar]
- 98.Johnson KS, Elbert-Avila KI, Tulsky JA. The influence of spiritual beliefs and practices on the treatment preferences of African Americans: a review of the literature. J Am Geriatr Soc. 2005;53(4):711–9. doi: 10.1111/j.1532-5415.2005.53224.x. [DOI] [PubMed] [Google Scholar]
- 99.Pickett KE, Pearl M. Multilevel analyses of neighbourhood socioeconomic context and health outcomes: a critical review. J Epidemiol Community Health. 2001;55(2):111–22. doi: 10.1136/jech.55.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Matheson FI, Moineddin R, Dunn JR, Creatore MI, Gozdyra P, Glazier RH. Urban neighborhoods, chronic stress, gender and depression. Soc Sci Med. 2006;63(10):2604–16. doi: 10.1016/j.socscimed.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 101.Sampson RJ, Raudenbush SW, Earls F. Neighborhoods and violent crime: a multilevel study of collective efficacy. Science. 1997;277(5328):918–24. doi: 10.1126/science.277.5328.918. [DOI] [PubMed] [Google Scholar]
- 102.Du XL, Sun CC, Milam MR, Bodurka DC, Fang S. Ethnic differences in socioeconomic status, diagnosis, treatment, and survival among older women with epithelial ovarian cancer. Int J Gynecol Cancer. 2008;18(4):660–9. doi: 10.1111/j.1525-1438.2007.01081.x. [DOI] [PubMed] [Google Scholar]