Abstract
Nociceptin/orphanin-FQ (N/OFQ) peptide and its receptor (NOP: N/OFQ opioid peptide receptor) are highly expressed in the hippocampus, but their functional role remains poorly understood. We recently showed that hippocampal N/OFQ inhibits learning and memory abilities in mice. Here, we investigated whether the endogenous peptide also regulated emotional responses at the level of the hippocampus. Bilateral infusions of the selective NOP receptor antagonist, UFP-101 (1–3 nmol/side), into the dorsal hippocampus produced antidepressant-like effects in the mouse forced swim and tail suspension tests comparable with those obtained with the prototypical antidepressant, fluoxetine (10–30 mg/kg, intraperitoneal). In the light-dark test, neither UFP-101 (1–3 nmol/side) nor N/OFQ peptide (1–3 nmol/side) modified anxiety measures when injected at behaviorally active doses in the dorsal hippocampus. These findings show a clear dissociation in the involvement of hippocampal N/OFQ system in anxiety- and despair-related behaviors. We conclude that the dorsal hippocampus is a brain region in which there is an important N/OFQ modulation of mnemonic processes and adaptive emotional responses associated to despair states.
Keywords: nociceptin/orphanin-FQ peptide, NOP receptor, anxiety, despair behavior, mice
INTRODUCTION
Nociceptin/orphanin-FQ (N/OFQ) and its receptor (NOP receptor: N/OFQ opioid peptide receptor) show structural similarities to the peptides and receptors of the opioid family but constitute a novel, pharmacologically distinct neurotransmitter system (Darland et al., 1998; Lambert, 2008). N/OFQ and NOP receptors are highly expressed in cortical and limbic structures (septum, amygdaloid complex, and hippocampal formation) suggesting that this neuropeptide system may play an important role in higher order brain functions such as emotion (Darland et al., 1998; Lambert, 2008). Accordingly, studies in rats and mice showed that systemic or intracerebroventricular administration of NOP receptor agonists produce anxiolytic-like effects in a variety of fear and anxiety-provoking situations (Jenck et al., 2000; Gavioli and Calo’, 2006; Hirao et al., 2008; Varty et al., 2008). Conversely, mice with a targeted disruption of the coding region for N/OFQ within the peptide precursor gene display enhanced anxiety-like behavior (Köster et al., 1999; Ouagazzal et al., 2003). However, the deletion of the NOP receptor gene in mice produces bidirectional changes in emotional responses indicating that endogenous N/OFQ may regulate anxiety-related behavior in a complex manner (Gavioli et al., 2007). Evidence also implicate N/OFQ peptide in the control of mood and despair behavior. Clinical studies reported an association of bipolar disorder and postpartum depression with elevated N/OFQ plasma levels (Gu et al., 2003; Wang et al., 2009). Furthermore, pharmacological blockade of NOP receptor signaling produces antidepressant-like effects in rats and mice, independent from any locomotor activity changes, and could be prevented by coinfusion of N/OFQ peptide, indicating that they are mediated by central NOP receptors (Redrobe et al., 2002; Gavioli and Calo’, 2006; Rizzi et al., 2007). More recently, Vitale et al. (2009) showed that chronic treatment with the NOP receptor antagonist, UFP-101, also reverses anhedonia induced by mild chronic stress in rats.
Although there is compelling evidence for the role of N/OFQ system in the control of emotional behavior, little is known about the underlying neuroanatomical substrates. Recent findings showed that the central amygdala and the bed nucleus of the stria terminalis are critical sites for N/OFQ modulation of anxiety states (Rodi et al., 2008; Uchiyama et al., 2008). To the best of our knowledge the potential neuronal sites mediating the antidepressant-like actions of NOP receptor antagonists have not yet been investigated. The hippocampal formation plays an important role in the integration of emotional components of fear and despair behaviors and has been implicated in the action of both anxiolytic and antidepressant drugs (Campbell and Macqueen, 2004; Engin and Treit, 2008). The potent inhibition of hippocampal synaptic activity caused by N/OFQ (Yu et al., 1998; Wei and Xie, 1999; Bongsebandhu-phubhakdi and Manabe, 2007) suggests that the peptide may modulate several processes including mnemonic functions and emotional responses associated to anxiety and despair states. We recently showed that N/OFQ infused in the dorsal hippocampus inhibits learning and memory abilities in mice (Goeldner et al., 2008; see also Sandin et al., 2004). To further clarify the functional role of hippocampal N/OFQ peptide we investigated the effects of pharmacological manipulations of NOP receptor activity in standard paradigms of despair (the forced swim and tail suspension tests) and anxiety (the light-dark test). The former procedures were selected because they are ideal for assessing acute effects of drug treatments on despair behavior. Furthermore, fluoxetine (prototypical antidepressant) was included in each experiment for comparison.
Adult C57BL/6N (B6N) male mice (Charles River Laboratory, St-Germain-sur-l’arbresle, France) were used in this study. Hippocampal stereotaxic implantation, intracerebral injections and histology were performed according to the procedures described previously in detail (Goeldner et al., 2008). Mice were implanted under ketamine/xylazine anesthesia with bilateral stainless guide canulae (0.40 mm external diameter) positioned 1 mm above the dorsal hippocampus (AP – 2 mm, L ± 1.6 mm from the bregma and DV – 1.2 mm from the skull surface according to the atlas of Paxinos and Franklin, 2001). Local injections were carried out with stainless-steel injector needles (0.28 mm external diameter) that protruded the guide cannula by 1 mm, into the dorsal hippocampus. Drugs were infused in a volume of 0.25 μl/side at rate of 0.125 μl/min. Following behavioral testing, mice were sacrificed, whole brains were removed and the injection sites determined histologically using 20 μm frozen coronal brain sections stained with cresyl violet acetate. Injection needle tips location was determined according to the standardized atlas plates of Paxinos and Franklin (2001). N/OFQ peptide and the peptidic NOP receptor antagonist, UFP-101 (Calo et al., 2005), (IGBMC peptide synthesis platform) were dissolved in artificial cerebrospinal fluid (acsf (mM): NaCl 126.6, NaHCO3 27.4, KCl 2.4, KH2PO4 0.5, CaCl2 0.89, MgCl2 0.8, Na2HPO4 0.48, and glucose 7.1, pH = 7.4) and infused into the dorsal hippocampus 2 min before testing. Ro64-6168 (Jenck et al., 2000, Hoffmann La Roche, Basel, Switzerland) and fluoxetine (LKT Laboratories, St Paul, USA) were dissolved in saline (0.9% NaCl) and injected intraperitoneally (i.p) at a volume of 10 ml/kg, with 30 min pretreatment time.
The forced swim test was carried out according to Ouagazzal et al. (2003). Naïve mice were placed for 6 min into a glass cylinder (height, 27 cm; diameter, 18 cm) partially filled with water (23 ± 1°C), and floating time was recorded during the last 4 min by an experimenter who was blind to drug treatments. The tail suspension test was carried out in an automated device (MED associates Inc, St Albans, VT) as previously described (Meziane et al., 2007). Mice were suspended to the tail hanger with adhesive tape and the total duration of immobility was recorded during a 6-min test session. The latency to the first episode of immobility was also determined. The light-dark test was carried out according to Ouagazzal et al. (2003). The apparatus consisted of a cupboard containing four identical light-dark boxes (20 × 20 × 14 cm3 high) interconnected with a dark tunnel (5 × 7 × 10 cm3) (Imetronic, Pessac, France). The light box was illuminated with Light Emitting Diodes (LEDs) placed in the ceiling. Mice were placed in the dark compartment, and the total time spent in the lit compartment and number of transitions from the dark to the lit compartment were recorded for 5 min. Further details regarding descriptions and validation of the behavioral procedures can be found at: http://www-mci.u-strasbg.fr/. All data are expressed as mean group-value ± standard error of the mean (s.e.m.) and analyzed using Student’s t-test or one-way analysis of variance (ANOVA) whenever it was appropriate. When relevant, data was submitted to Dunnett’s post hoc test. The criterion for statistical significance was P < 0.05.
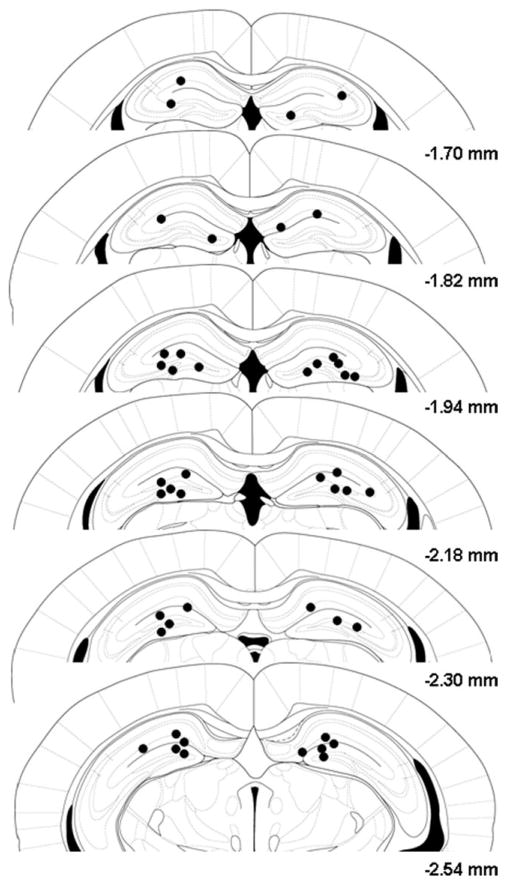
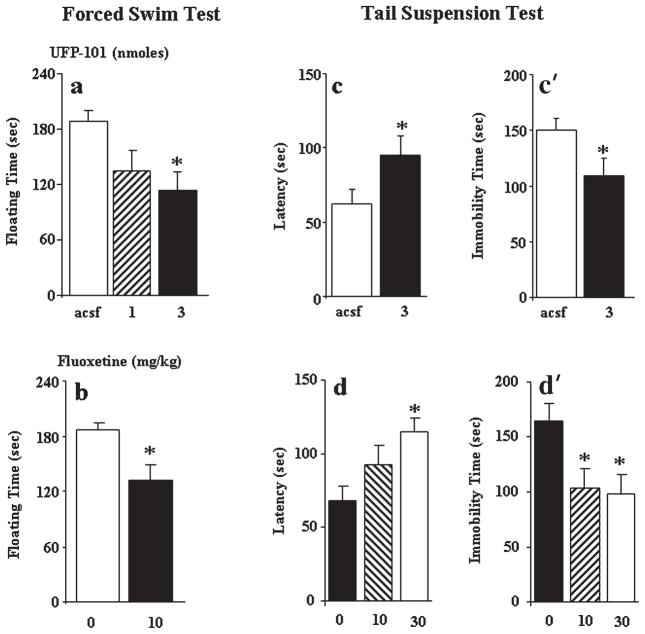
Examples of cannulae track placements located in the dorsal hippocampus are shown in Figure 1 for the subjects tested in the forced swim test experiment. Overall, the injection needle tips of the various groups fell within the dorsal hippocampus in the anterior planes −1.70 to −2.54 mm from bregma according to the atlas of Paxinos and Franklin (2001). Mice with inaccurate cannula placements were excluded from behavioral analysis. As can be seen from Figure 2a, direct infusion of UFP-101 (1 and 3 nmol) into the dorsal hippocampus markedly reduced total floating duration in the forced swim test [F(2,20) = 4.30, P < 0.05] and a significant effect was detected at the highest dose (P < 0.05, Dunnett’s test). Systemic administration of fluoxetine (10 mg/kg) also reduced floating behavior (P < 0.05, Student’s t-test, Fig. 2b). To confirm the antidepressant-like effects of UFP-101, a second group of mice received the active dose (3 nmol) into the dorsal hippocampus and submitted to the tail suspension test. UFP-101 produced a clear antidepressant-like action as reflected by a significant increase in the latency to the first immobility and a decrease in total immobility time (P < 0.05, Student’s t-test, Figs. 2c and c′, respectively). Similar behavioral effects were obtained following systemic administration of fluoxetine ([F(2,19)> 4.0, P < 0.05 for all parameters, Figs. 2d and d′). Post hoc analysis revealed a significant increase in the latency to the first immobility for 30 mg/kg and a significant decrease in immobility time for both 10 and 30 mg/kg doses (P < 0.05, Dunnett’s test).
FIGURE 1.
Coronal mouse brain sections showing histological reconstruction of injection sites in the dorsal hippocampus of the subjects tested in the forced swim test. Values give the distance in mm posterior to the bregma, according to the atlas of Paxinos and Franklin (2001).
FIGURE 2.
Effects of UFP-101 and fluoxetine in the forced swim and tail suspension procedures. (a) Mean (± s.e.m.) floating time spent by mice in the forced swim procedure after intrahippocampal infusions of artificial cerebrospinal fluid (acsf, n = 7) or UFP-101 (1 and 3 nmol, n = 8 per dose). (b) Mean (± s.e.m.) floating time after intraperitoneal administration of vehicle (Veh, n = 8) or fluoxetine (10 mg/kg, n = 8). Mean (± s.e.m.) latency to the first immobility (c) and the total immobility time (c′) in the tail suspension test following intrahippocampal infusions of acsf (n = 6) or UFP-101 (3 nmol, n = 8). Mean (± s.e.m.) latency to the first immobility (d) and the total immobility time (d′) following intraperitoneal administration of vehicle (Veh, n = 7) or fluoxetine (10 and 30 mg/kg, n = 8 per dose). *significantly different form corresponding control group, P < 0.05 Student t-test or ANOVA followed by Dunnett’s test.
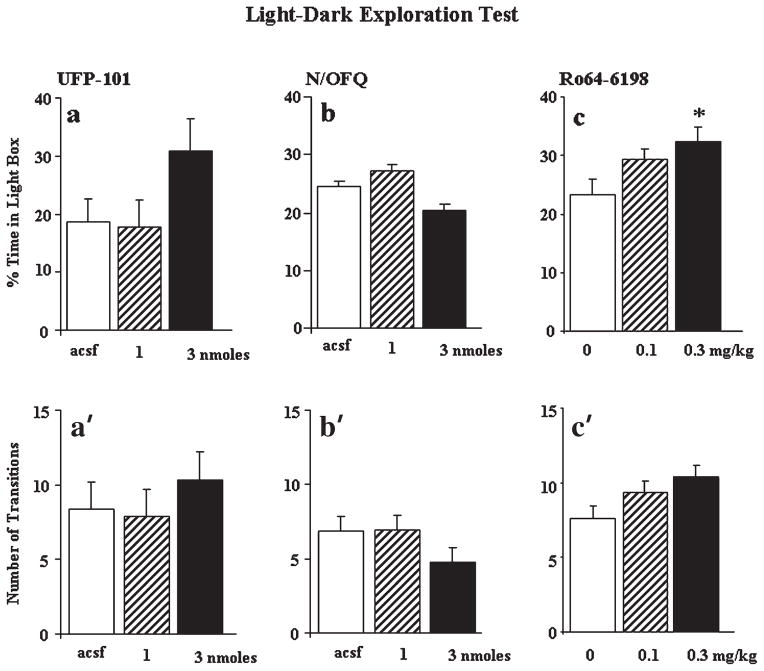
Intrahippocampal infusion of UFP-101 (1 and 3 nmol) did not affect the emotional reactivity of mice in the light-dark test ([F(2,21)<2.42, P > 0.05] for % of time spent in the lit compartment and number of transitions, Figs. 3a and a′, respectively). We next verified whether pharmacological activation of NOP receptor could modify the emotional responses of mice. Intrahippocampal infusion of N/OFQ (1 and 3 nmol) was also ineffective ([F(2,24)<0.75, P > 0.05] for all parameters, Figs. 3b and b′). In contrast, systemic administration of the selective NOP receptor agonist, Ro64–6198 (0.1 and 0.3 mg/ kg), increased the % time spent in the lit compartment [F(2,36) = 4.38, P < 0.05] and a significant effect was detected at the highest dose (P < 0.05 Dunnett’s test, Fig. 3c). Ro64–6198 also enhanced the number of transitions but this effect just failed to reach statistical significance [F(2,36) = 2.57, P = 0.08, Fig. 3c′].
FIGURE 3.
Effects of UFP-101, N/OFQ, and Ro64–6198 in the light-dark test. (a) and (a′) Effects of intrahippocampal infusions of acsf (n = 8) or UFP-101 (1 and 3 nmol, n = 8 per dose) on the percentage of time spent in the lit compartment and the number of transitions from the dark to light compartment, respectively. (b) and (b′) Effects of intrahippocampal infusions of acsf (n = 10) or N/OFQ (1 and 3 nmol, n = 8 and 9, respectively) on the percentage of time spent in the lit compartment and the number of transitions from the dark to the light compartment, respectively. (c) and (c′) Effects of intraperitoneal administration of vehicle (n = 16) or Ro64–6198 (0.1 and 0.3 mg/kg, n = 14 and 9, respectively) on the percentage of time spent in the lit compartment and the number of transitions, respectively. Data are presented as mean ± s.e.m. *Significantly different form corresponding control group, P < 0.05 ANOVA followed by Dunnett’s test.
Forced swim and tail suspension tests involve exposure of animals to inescapable aversive situations and the immobility observed during testing is considered to reflect a state of despair on the assumption that animals have given up hope to escape (Cryan et al., 2005a, b). NOP receptor antagonists of diverse chemical structures (e.g., [Nphe1]N/OFQ(1–13)-NH2, UFP-101, J-113397, SB-612111) display antidepressant-like activity in these procedures following systemic or intracerebroventricular administrations (Redrobe et al., 2005; Gavioli and Calo’, 2006; Rizzi et al., 2007). Our results show that the dorsal hippocampus is one of the neuronal sites mediating these effects. In both procedures, intrahippocampal infusions of UFP-101 produced anti-depressant-like effects of comparable magnitude to those obtained with fluoxetine. The observed effects of UFP-101 cannot be attributed to psychomotor activation. We previously showed that intrahippocampal infusion of UFP-101 at a comparable dose range (5 nmol/side) was without any effect on locomotor activity or exploration of a novel object in the open field test, unlike the psychotomimetic MK-801 (Goeldner et al., 2008). Together, these findings suggest that the endogenous N/OFQ tone in the dorsal hippocampus facilitates passive coping responses under behavioral despair stress.
In the light-dark test, neither UFP-101 nor N/OFQ peptide changed anxiety measures when infused at behaviorally active doses (Goeldner et al., 2008) in the dorsal hippocampus. In contrast, clear anxiolytic-like effects were obtained with systemic administration of the NOP receptor agonist, Ro64–6198, thus confirming previous studies (Jenck et al., 2000; Varty et al., 2005). The absence of N/OFQ effects was somewhat unexpected given the role of the hippocampus in modulation of fear/anxiety states (Engin and Treit, 2008) and the high expression of NOP receptor in this brain structure (Darland et al., 1998). Despite its potent suppressive actions on hippocampal synaptic activity, N/OFQ peptide seems to differ from other inhibitory neurotransmitters, such as GABA, in that its action cannot be detected under anxiogenic-provoking situations (Gonzalez et al., 1998). It may be argued that the doses used in this study are not optimal for detecting anxiolytic-like effects of N/OFQ since changes in anxiety and fear learning were found with lower dose-ranges in other structures such as the amygdala (Roozendaal et al., 2007; Uchiyama et al., 2008). As previously shown by Uchiyama et al. (2008), intrahippocampal injections of N/OFQ at a dose-range producing anxiolytic-like effects in the central amygdala had no effects on anxiety, thus ruling out the former possibility. Collectively, the above findings suggest that N/OFQ system in the dorsal hippocampus may not play a major role in the modulation of adaptive fear/anxiety responses, and that other brain structures (e.g., central amygdala and bed nucleus of the stria terminalis) mediate the anxiolytic-like effects observed with systemic administration of the NOP receptor agonists (present study, Jenck et al., 2000; Hirao et al., 2008; Varty et al., 2008).
The pattern of effects we obtained with central drug injections reveals differential contribution of N/OFQ to distinct hippocampal functions. This neuropeptide appears to play a predominant role in the modulation of specific behavioral processes, particularly those involving learning (Goeldner et al., 2008) and emotional responses associated to despair state (the present study). The inhibitory influence of N/OFQ on cognitive processing was previously shown to involve suppression of glutamatergic signaling at the NMDA receptor subtype (Mamiya et al., 2003; Goeldner et al., 2008, 2009). It is unlikely that similar mechanisms underlie the action of N/OFQ on despair behavior since inactivation of NMDA receptor within the dorsal hippocampus was suggested to produce antidepressant-like effects (Przegalinski et al., 1997; Maeng et al., 2008). Hence, the prodepressive-like action of endogenous N/OFQ may be mediated through suppression of the function of glutamatergic AMPA receptor (Maeng et al., 2008) or other neurotransmitter systems that promote active coping responses under inescapable stress. Modulation of serotonin transmission is a likely possibility as brain serotonin depletion attenuates the antidepressant-like effects of intracerebroventricular infusion of UFP-101 (Gavioli and Calo’, 2006). N/OFQ also exerts a tonic inhibitory action on hippocampal noradrena-line release (Schlicker and Morari, 2000) and activation of adrenergic receptors in the dorsal hippocampus produces antidepressant-like effects (Plaznik and Kostowski, 1985; Zhang et al., 2001). Further antagonism studies are therefore needed to determine which neurotransmitters are involved in the behavioral effects of endogenous hippocampal N/OFQ.
In summary, the above findings indicate that the dorsal hippocampus is a brain region in which there is an important N/OFQ modulation of mnemonic processes and adaptive emotional responses associated to despair states. They also provide new evidence for the view that anxiety and despair behaviors are subserved by overlapping but not identical neural substrates.
Acknowledgments
Grant sponsor: NIDA, Center for Opioid Receptors and Drugs of Abuse; Grant number: #DA 005010; Grant sponsors: Centre National de la Recherche Scientifique (CNRS), the Institut National de la Santé et de la Recherche Médicale (INSERM), the University of Strasbourg.
The authors thank Dr. Wichmann Jürgen from Hoffmann-La Roche for the generous gift of the synthetic NOP receptor agonist, Ro64–6198.
References
- Bongsebandhu-phubhakdi S, Manabe T. The neuropeptide nociceptin is a synaptically released endogenous inhibitor of hippocampal long-term potentiation. J Neurosci. 2007;27:4850–4858. doi: 10.1523/JNEUROSCI.0876-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calo’ G, Guerrini R, Rizzi A, Salvadori S, Burmeister M, Kapusta DR, Lambert DG, Regoli D. UFP-101, a peptide antagonist selective for the nociceptin/orphanin FQ receptor. CNS Drug Rev. 2005;11:97–112. doi: 10.1111/j.1527-3458.2005.tb00264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell S, Macqueen G. The role of the hippocampus in the pathophysiology of major depression. J Psychiatry Neurosci. 2004;29:417–426. [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Mombereau C, Vassout A. The tail suspension test as a model for assessing antidepressant activity: Review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev. 2005a;29:571–625. doi: 10.1016/j.neubiorev.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Valentino RJ, Lucki I. Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci Biobehav Rev. 2005b;29:547–569. doi: 10.1016/j.neubiorev.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Darland T, Heinricher MM, Grandy DK. Orphanin FQ/nociceptin: A role in pain and analgesia, but so much more. Trends Neurosci. 1998;21:215–221. doi: 10.1016/s0166-2236(97)01204-6. [DOI] [PubMed] [Google Scholar]
- Engin E, Treit D. The effects of intra-cerebral drug infusions on animals’ unconditioned fear reactions: A systematic review. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1399–1419. doi: 10.1016/j.pnpbp.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Gavioli EC, Calo’ G. Antidepressant- and anxiolytic-like effects of nociceptin/orphanin FQ receptor ligands. Naunyn Schmiedebergs Arch Pharmacol. 2006;372:319–330. doi: 10.1007/s00210-006-0035-8. [DOI] [PubMed] [Google Scholar]
- Gavioli EC, Rizzi A, Marzola G, Zucchini S, Regoli D, Calo’ G. Altered anxiety-related behavior in nociceptin/orphanin FQ receptor gene knockout mice. Peptides. 2007;28:1229–1239. doi: 10.1016/j.peptides.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Goeldner C, Reiss D, Wichmann J, Meziane H, Kieffer BL, Ouagazzal AM. Nociceptin receptor impairs recognition memory via interaction with NMDA receptor-dependent mitogen-activated protein kinase/extracellular signal-regulated kinase signaling in the hippocampus. J Neurosci. 2008;28:2190–2198. doi: 10.1523/JNEUROSCI.3711-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeldner C, Reiss D, Wichmann J, Kieffer BL, Ouagazzal AM. Activation of nociceptin opioid peptide (NOP) receptor impairs contextual fear learning in mice through glutamatergic mechanisms. Neurobiol Learn Mem. 2009;91:393–401. doi: 10.1016/j.nlm.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez LE, Ouagazzal AM, File SE. Stimulation of benzodiazepine receptors in the dorsal hippocampus and median raphé reveals differential GABAergic control in two animal tests of anxiety. Eur J Neurosci. 1998;10:3673–3680. doi: 10.1046/j.1460-9568.1998.00375.x. [DOI] [PubMed] [Google Scholar]
- Gu H, Hu D, Hong XR, Mao J, Cui Y, Hui N, Sha JY. Changes and significance of orphanin and serotonin in patients with postpartum depression. Zhonghua Fu Chan Ke Za Zhi. 2003;38:727–728. [PubMed] [Google Scholar]
- Hirao A, Imai A, Sugie Y, Yamada Y, Hayashi S, Toide K. Pharmacological characterization of the newly synthesized nociceptin/ orphanin FQ-receptor agonist 1-[1-(1-methylcyclooctyl)-4-piperi-dinyl]-2-[(3R)-3-piperidinyl]-1H-benzimidazole as an anxiolytic agent. J Pharmacol Sci. 2008;106:361–368. doi: 10.1254/jphs.fp0071742. [DOI] [PubMed] [Google Scholar]
- Jenck F, Ouagazzal A, Pauly-Evers M, Moreau JL. OrphaninFQ: Role in behavioral fear responses and vulnerability to stress? Mol Psychiatry. 2000;5:572–574. doi: 10.1038/sj.mp.4000793. [DOI] [PubMed] [Google Scholar]
- Köster A, Montkowski A, Schulz S, Stübe EM, Knaudt K, Jenck F, Moreau JL, Nothacker HP, Civelli O, Reinscheid RK. Targeted disruption of orphanin FQ/nociceptin gene increases stress susceptibility and impairs stress adaptation in mice. Proc Natl Acad Sci. 1999;96:10444–10449. doi: 10.1073/pnas.96.18.10444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert DG. The nociceptin/orphanin FQ receptor: A target with broad therapeutic potential. Nat Rev Drug Discov. 2008;7:694–710. doi: 10.1038/nrd2572. [DOI] [PubMed] [Google Scholar]
- Maeng S, Zarate CA, Jr, Du J, Schloesser RJ, McCammon J, Chen G, Manji HK. Cellular mechanisms underlying the antidepressant effects of ketamine: Role of alpha-amino-3-hydroxy-5-methyl-isoxazole-4-propionic acid receptors. Biol Psychiatry. 2008;63:349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Mamiya T, Yamada K, Miyamoto Y, Konig N, Watanabe Y, Noda Y, Nabeshima T. Neuronal mechanism of nociceptin-induced modulation of learning and memory: Involvement of N-methyl-D-aspartate receptors. Mol Psychiatry. 2003;8:752–765. doi: 10.1038/sj.mp.4001313. [DOI] [PubMed] [Google Scholar]
- Meziane H, Ouagazzal AM, Aubert L, Wietrzych M, Krezel W. Estrous cycle effects on behavior of C57BL/6J and BALB/cByJ female mice: Implications for phenotyping strategies. Genes Brain Behav. 2006;6:192–200. doi: 10.1111/j.1601-183X.2006.00249.x. [DOI] [PubMed] [Google Scholar]
- Ouagazzal AM, Moreau JL, Pauly-Evers M, Jenck F. Impact of environmental housing conditions on the emotional responses of mice deficient for nociceptin/orphanin FQ peptide precursor gene. Behav Brain Res. 2003;144:111–117. doi: 10.1016/s0166-4328(03)00066-4. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. 2. San Diego: Academic; 2001. [Google Scholar]
- PłaŸnik A, Kostowski W. Modification of behavioral response to intra-hippocampal injections of noradrenaline and adrenoceptor agonists by chronic treatment with desipramine and citalopram: Functional aspects of adaptive receptor changes. Eur J Pharmacol. 1985;101:305–306. doi: 10.1016/0014-2999(85)90609-0. [DOI] [PubMed] [Google Scholar]
- Przegaliński E, Tatarczyńska E, Dereń-Wesołek A, Chojnacka-Wojcik E. Antidepressant-like effects of a partial agonist at strychnine-insensitive glycine receptors and a competitive NMDA receptor antagonist. Neuropharmacology. 1997;36:31–37. doi: 10.1016/s0028-3908(96)00157-8. [DOI] [PubMed] [Google Scholar]
- Redrobe JP, Calo’ G, Regoli D, Quirion R. Nociceptin receptor antagonists display antidepressant-like properties in the mouse forced swimming test. Naunyn Schmiedeberg’s Arch Pharmacol. 2002;365:164–167. doi: 10.1007/s00210-001-0511-0. [DOI] [PubMed] [Google Scholar]
- Rizzi A, Gavioli EC, Marzola G, Spagnolo B, Zucchini S, Ciccocioppo R, Trapella C, Regoli D, Calò G. Pharmacological characterization of the nociceptin/orphanin FQ receptor antagonist SB-612111 [(−)-cis-1-methyl-7-[[4-(2,6-dichlorophenyl)piperidin-1-yl]methyl]-6,7,8,9-tetrahydro-5H-benzocyclohepten-5-ol]: In vivo studies. J Pharmacol Exp Ther. 2007;321:968–974. doi: 10.1124/jpet.106.116780. [DOI] [PubMed] [Google Scholar]
- Rodi D, Zucchini S, Simonato M, Cifani C, Massi M, Polidori C. Functional antagonism between nociceptin/orphanin FQ (N/ OFQ) and corticotropin-releasing factor (CRF) in the rat brain: Evidence for involvement of the bed nucleus of the stria terminalis. Psychopharmacology. 2008;196:523–531. doi: 10.1007/s00213-007-0985-7. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Lengvilas R, McGaugh JL, Civelli O, Reinscheid RK. Orphanin FQ/nociceptin interacts with the basolateral amygdala noradrenergic system in memory consolidation. Learn Mem. 2007;14:29–35. doi: 10.1101/lm.403607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandin J, Ogren SO, Terenius L. Nociceptin/orphanin FQ modulates spatial learning via ORL-1 receptors in the dorsal hippocampus of the rat. Brain Res. 2004;997:222–233. doi: 10.1016/j.brainres.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Schlicker E, Morari M. Nociceptin/orpahin FQ, neurotransmitter release in the central nervous system. Peptides. 2000;21:1023–1029. doi: 10.1016/s0196-9781(00)00233-3. [DOI] [PubMed] [Google Scholar]
- Uchiyama H, Yamaguchi T, Toda A, Hiranita T, Watanabe S, Eyanagi R. Involvement of the GABA/benzodiazepine receptor in the anxiolytic-like effect of nociceptin/orphanin FQ. Eur J Pharmacol. 2008;590:185–189. doi: 10.1016/j.ejphar.2008.05.031. [DOI] [PubMed] [Google Scholar]
- Varty GB, Lu SX, Morgan CA, Cohen-Williams ME, Hodgson RA, Smith-Torhan A, Zhang H, Fawzi AB, Graziano MP, Ho GD, Matasi J, Tulshian D, Coffin VL, Carey GJ. The anxiolytic-like effects of the novel, orally active nociceptin opioid receptor agonist 8-[bis(2-methylphenyl)methyl]-3-phenyl-8-azabicyclo[3.2.1] octan-3-ol (SCH 221510) J Pharmacol Exp Ther. 2008;326:672–682. doi: 10.1124/jpet.108.136937. [DOI] [PubMed] [Google Scholar]
- Vitale G, Ruggieri V, Filaferro M, Frigeri C, Alboni S, Tascedda F, Brunello N, Guerrini R, Cifani C, Massi M. Chronic treatment with the selective NOP receptor antagonist [Nphe(1),Arg (14),Lys (15)]N/OFQ-NH (2) (UFP-101) reverses the behavioral and biochemical effects of unpredictable chronic mild stress in rats. Psychopharmacology. 2009;207:173–189. doi: 10.1007/s00213-009-1646-9. [DOI] [PubMed] [Google Scholar]
- Wang LN, Liu LF, Zhang JX, Zhao GF. Plasma levels of nociceptin/orphanin FQ in patients with bipolar disorders and health adults. Zhonghua Yi Xue Za Zhi. 2009;89:916–918. [PubMed] [Google Scholar]
- Wei WZ, Xie CW. Orphanin FQ suppresses NMDA receptor-dependent long-term depression and depotentiation in hippocampal dentate gyrus. Learn Mem. 1999;6:467–477. doi: 10.1101/lm.6.5.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu TP, Xie CW. Orphanin FQ/nociceptin inhibits synaptic transmission and long-term potentiation in rat dentate gyrus through postsynaptic mechanisms. J Neurophysiol. 1998;80:1277–1284. doi: 10.1152/jn.1998.80.3.1277. [DOI] [PubMed] [Google Scholar]
- Zhang HT, Frith SA, Wilkins J, O’Donnell JM. Comparison of the effects of isoproterenol administered into the hippocampus, frontal cortex, or amygdala on behavior of rats maintained by differential reinforcement of low response rate. Psychopharmacology. 2001;159:89–97. doi: 10.1007/s002130100889. [DOI] [PubMed] [Google Scholar]