Abstract
Drug withdrawal is suggested to play a role in precipitating mood disorders in individuals with familial predisposition. Age-related differences in affective responses to withdrawal might explain the increased risk of mental illnesses when drug use begins during adolescence. Recently we observed that, in contrast to adult male mice, adolescent males exhibited a decrease in immobility in the forced swim test on the third day of withdrawal, as compared with controls. Thus, the present study examined forced swim test behaviors of adolescent female mice during opioid withdrawal. Similar to the male study, adolescent female mice were injected with two morphine regimens which differed in dosage. Three and nine days following discontinuation of morphine administration, forced swim test immobility time and locomotion were evaluated. In contrast to males, which exhibited a decrease in immobility, no significant differences in immobility were observed in female adolescents undergoing withdrawal as compared with saline-injected controls. This sex difference in forced swim test behaviors was not due to changes in overall motor activity, since differences in locomotion were not observed in either male or female adolescent mice. Thus, this study demonstrates sex differences in forced swim test behavior during opioid withdrawal. Forced swim test behaviors are classically used to evaluate mood in rodents, thus this study suggests that opioid withdrawal might affect mood differentially across sexes.
Keywords: morphine, dependence, mood, forced swim test (FST), locomotion, drug addiction
Introduction
Opioids are the most effective analgesics for treating moderate-to-severe pain. However, their use is compromised by their high potential for abuse, development of dependence, and withdrawal. In fact, withdrawal precipitation has long been considered a central component of drug addiction. It has been suggested that the maintenance of opioid abuse, despite the severe consequences, is in part driven by the desire to avoid the onset of withdrawal or to escape the withdrawal state (Aston-Jones and Harris, 2004; Koob et al., 1997; Schulteis and Koob, 1996). Accordingly, sex differences in precipitation of withdrawal might, at least in part, contribute to a differential development of compulsive drug abuse patterns in females and males.
In both humans and rodents, opioid withdrawal is also accompanied by the precipitation of negative affects including aversion and dysphoria (Grasing and Ghosh, 1998; Hand et al., 1988; Schaefer and Michael, 1983; Schulteis et al., 1994). Dysphoria (or depressive-like behavior) during withdrawal was examined in adult rats and mice using, among others, the forced swim test (FST) paradigm (Anraku et al., 2001; Grasing and Ghosh, 1998; Hodgson et al., 2009; Molina et al, 1994; Zurita and Molina, 1999). The FST is a behavioral despair model wherein rodents are placed in a small apparatus filled with enough water so that their tails cannot touch the bottom. Escape from this apparatus is impossible. A period of extreme motor activity is initially observed during which the animal will actively attempt to escape. However, once unsuccessful, they will make fewer and fewer escape attempts – which manifests through longer periods of time in which they present a characteristic immobile posture. Mood is evaluated based on the length of time spent mobile or immobile.
We recently observed age-dependent difference in FST behaviors of male mice undergoing opioid withdrawal (Hodgson et al., 2009). In contrast to adults, on the third day of withdrawal (withdrawal day 3, WD3) adolescent male mice exhibited a decrease in immobility as compared with controls. No significant differences in immobility were observed on withdrawal day 9 (WD9). This effect on FST behaviors was not due to changes in overall motor activity, since no differences in locomotion were observed on either WD3 or WD9 in male adolescent mice.
It has been suggested that the withdrawal from drug use, specifically opioids, might precipitate mood disorders in pre-disposed individuals (Handelsman et al., 1992; Janiri et al., 2005; Schürks et al., 2005; Shobe and Brion, 1971; White, 2004). Accordingly, sex differences in affective responses to withdrawal might contribute to differential development of comorbid mental disorders in females and males. However, there is currently a lack of studies on sex differences in the affective responses to opioid withdrawal, especially during adolescence. This study, therefore, uses the FST paradigm to examine the effects of morphine withdrawal on FST and locomotor behaviors in female adolescents. Similar to the male study (Hodgson et al., 2009), two morphine regimens of differing dosages were used. For both regimens, morphine was administrated repeatedly for six days. Behaviors were examined three and nine days following the discontinuation of morphine administration, i.e. on WD3 and WD9, and compared with the results of our male study.
Materials and methods
Animals
All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and were approved by the Institutional Animal Care and Use Committee. Female C57BL/6 mice, purchased from Harlan Lab (Houston, TX), were housed four to five per cage with food and water ad lib. They were acclimated to the temperature-controlled vivarium with a 12 h/12 h light/dark cycle (light on at 0700) for at least one week prior to treatment. Separate animal groups were used for each behavioral test (FST, locomotion). In a preliminary experiment, we used mice that were bred in our vivarium (breeders were purchased from Harlan Lab, Houston, TX) and their behavioral responses were compared with the responses observed in mice purchased from Harlan Lab (Houston, TX) that were acclimated to the vivarium for at least one week. Our behavioral tests did not indicate any response differences based on breeding history. Thus, in subsequent studies, we used mice that were purchased from Harlan Lab and acclimated to the vivarium for at least one week.
The choice for the age of the adolescent mice was based on studies by Spear and colleagues (reviewed by Spear, 2000) which demonstrated three developmental stages for rodents from weaning to adulthood. Since the adolescent period in rodents is quite short, in this study we chose to conduct behavioral testing during what is considered to be their mid-adolescence/periadolescent period. Accordingly, mice were purchased at postnatal day 22 (PND 22). They were acclimated to the vivarium until PND 28, when morphine injections began, and behavioral testing was performed on PND 38 (WD3). Thus, in this study, mice were injected during what is considered the late phase of their prepubescent period, and they were tested during mid-adolescence/periadolescent period. We refer to this group as adolescents.
Morphine regimen
Mice were injected twice daily (9 a.m. and 5 p.m.) for six consecutive days for a total of 12 injections. We used two morphine regimens, termed a high and low regimen, identical to that used in the male study (Hodgson et al., 2009). Briefly, for the high morphine regimen, mice were injected with increasing doses of morphine (10–40 mg/kg, subcutaneous (s.c.)). On days 1 and 2, the mice were injected with 10 mg/kg morphine. On days 3 and 4, they were injected with 20 mg/kg morphine. On days 5 and 6, they were injected with 40 mg/kg morphine. For the low morphine regimen, mice were injected with a constant 10 mg/kg morphine dose (s.c.) for all 12 injections. Control mice received 12 injections of saline (s.c.). A volume of 10 ml/kg was used for the saline and morphine injections. Morphine sulfate was purchased from Sigma (St. Louis, MO, USA).
Forced swim test
Female adolescent mice (n=13–15 per group) were subjected to the FST three and nine days after the final injection. Since prior FST exposure is known to alter the outcome in subsequent tests, different mice were used for each time period (Porsolt et al., 2001). The FST was performed in an identical manner to the method used in the male study (Hodgson et al., 2009). Briefly, we used Porsolt’s modified version for mice which consists of one exposure to the apparatus (Porsolt et al., 1977). The FST was performed in the second half of the light phase, which was between 3 p.m. and 6 p.m. Mice were habituated to the room for at least 30 minutes prior to testing. One mouse at a time was examined in the testing room. We compared the results obtained for the females with the results obtained in the males, as reported in Hodgson et al. (2009).
We used an apparatus of 10 cm in diameter, 18.3 cm deep, and a 10 cm water level (Jacobson and Cryan, 2007). Water level was sufficient to ensure that the mice were unable to touch the bottom of the test chamber. The water temperature was set to 24.5–25°C, and the water was replaced after each mouse. Mice were placed in the apparatus and videotaped for 10 minutes. Immobility was scored as described by Crowley et al., (2004), as carried out in our earlier study on males (Hodgson et al., 2009). Immobility during each 1-minute interval of the test and for the entire 10-minute period was calculated.
Locomotion
Female adolescent mice (n=6–12) were injected twice daily for six consecutive days with saline, the low morphine regimen, or the high morphine regimen. Their locomotion was recorded on WD3 and WD9. Like the FST, different groups of mice were examined for each time period. Locomotion was recorded in the second part of the light phase, which was between 3 p.m. and 6 p.m. Mice were habituated to the room for at least 30 minutes prior to testing. Mice were placed in an upright cylindrical container (261mm in diameter and 355mm high) and recorded for 60minutes by an overhead camera. The apparatus was cleaned thoroughly with water and completely dried between tests. Total distance traveled (locomotion) was scored using EthoVision 3.1 (Noldus Information Technology).
Data analyses
Separate analyses of variance (ANOVAs) were computed for each of the dependent measures of these studies (immobility time in sec; distance traveled in cm). Analyses were computed using total immobility scores (summated over 10 minutes) or total distance traveled scores (summated over either minutes 1–10 or 1–60) or a within-group factor of time (FST: 1–10 minutes; locomotion: 1–10 min or 1–60 minutes). Additionally, for each sex we calculated the differences in immobility score from controls using the formula: immobility score of X treatment group – immobility score of the sex-matched controls. A factorial analyses consisting of between-group factors of sex (females versus males) and drug treatment (low and high morphine) was performed followed by Bonferroni’s post hoc comparisons. The data for the male study are presented in Hodgson et al. (2009). Differences less than 0.05 were deemed statistically significant.
Results
Withdrawal day 3 (WD3)
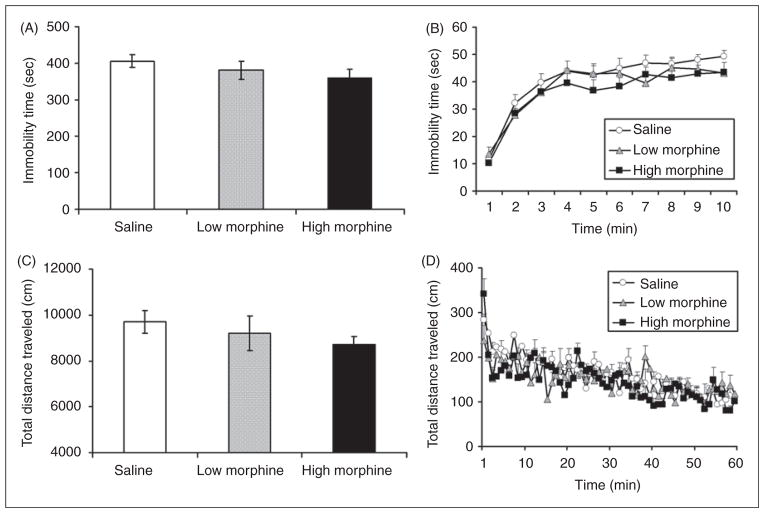
Female adolescent mice did not exhibit a decrease in immobility time on the third day following discontinuation of morphine, as compared with saline-treated controls. One-way ANOVA of the total immobility scores (summed across the entire 10 minute test; Figure 1A) revealed no effect of treatment (F2, 39=1.236, p>0.05). An additional analysis was computed using immobility time during each minute of the 10 minute test period. As expected, in all treatment groups, immobility levels increased with time (Figure 1B). However, no effect of treatment was found (Figure 1B).
Figure 1.
Forced swim test and locomotor behaviors of female adolescent mice on WD3. (A) Total immobility scores during the entire 10-minute test, (B) Immobility scores for each 1-minute interval. (C) Total distance traveled (cm) during the entire 60-minute test. (D) Total distance traveled (cm) in each 1-minute interval. Results are presented as mean±SEM. White bars and ○ – saline-treated control mice; gray bars and ▲ – mice withdrawing from low morphine regimen; black bars and ■ – mice withdrawing from high morphine regimen.
In a separate experiment, we measured locomotor behavior on WD3. One-way ANOVA of the total distance traveled during the 60 minute test (Figure 1C) revealed no significant effect of treatment (F2, 19=1.014, p>0.05). Similarly, one-way ANOVA of the total distance traveled during the first 10 minutes of the test revealed no significant effect of treatment (F2, 19=2.508, p>0.05). Likewise, split-plot analysis of total distance traveled for the one-minute intervals (Figure 1D) revealed no significant effect of treatment at any time interval.
Withdrawal day 9 (WD9)
Similar to WD3, female adolescent mice also did not exhibit a decrease in immobility time on the ninth day following discontinuation of morphine, as compared to saline-treated controls. One-way ANOVA of the total immobility scores (summed across the entire 10 minute test; Figure 2A) revealed no effect of treatment (F2, 41=1.483, p>0.05). An additional analysis was computed using immobility time during each minute of the 10-minute test period. As expected, in all treatment groups, immobility levels increased with time (Figure 2B). However, no effect of treatment was found (Figure 2B).
Figure 2.
Forced swim test and locomotor behaviors of female adolescent mice on WD9. (A) Total immobility scores during the entire 10-minute test, (B) Immobility scores for each 1-minute interval. (C) Total distance traveled (cm) during the entire 60-minute test. (D) Total distance traveled (cm) in each 1-minute interval. Results are presented as mean±SEM. White bars and ○ – saline-treated control mice; gray bars and ▲– mice withdrawing from low morphine regimen; black bars and ■ – mice withdrawing from high morphine regimen.
In a separate experiment, we measured locomotor behavior on WD9. One-way ANOVA of the total distance traveled during the 60-minute test (Figure 2C) revealed no significant effect of treatment (F2, 28=1.078, p>0.05). Similarly, one-way ANOVA of the total distance traveled during the first 10 minutes of the test revealed no significant effect of treatment (F2, 28=2.398, p>0.05). Likewise, split-plot analysis of total distance traveled for the one-minute intervals (Figure 2D) revealed no significant effect of treatment at any time interval.
Sex differences in FST behaviors during morphine withdrawal
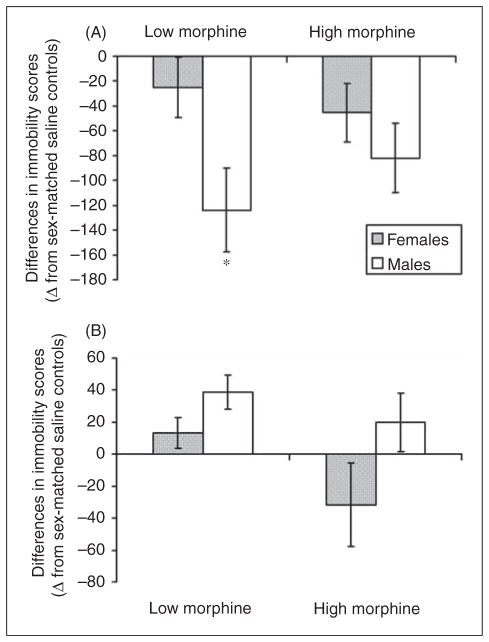
We compared the results obtained for females with the results previously obtained for male adolescent mice (Hodgson et al., 2009). We first examined whether there were baseline differences in FST immobility between female and male adolescent mice. Analysis revealed no significant differences in baseline immobility time, i.e. female and male control (saline-treated) mice spent similar amounts of time immobile (405.5±65.2 vs. 400.7±73.2; t=0.197, df=31, p>0.05). Thus, we next calculated the differences in immobility between the two treatment groups (i.e. low and high morphine) and their sex-matched controls (i.e. saline-injected mice). Sex differences in FST behaviors in mice during opioid withdrawal were observed on WD3. Two-way ANOVA (sex × treatment) revealed a significant effect of sex (F1, 54=6.138, p=0.016; Figure 3A). Bonferroni post-hoc contrasts revealed a significant difference between female and male mice withdrawing from the low morphine regimen (p<0.05). Sex differences in FST behaviors were also observed on WD9 (F1, 55=7.42, p=0.034; Figure 3B); however, no significant differences were revealed in the Bonferroni post-hoc contrasts.
Figure 3.
Sex differences in forced swim test behaviors of adolescent mice during morphine withdrawal. Results of the differences in immobility scores during withdrawal from low and high morphine on (A) WD3 and (B) WD9 as compared with sex-matched controls are presented as mean±SEM. Male data is from Hodgson et al. (2009). Gray bars - females; White bars - males. (*) indicates a significant difference from females (p<0.05).
Note that we did not observe significant differences between the two morphine regimens. In other words, as compared with controls, treatment with both morphine regimens yielded similar trends (for example, on WD3 adolescent males exhibited less immobility during morphine withdrawal from both low and high morphine treatment, while no significant effect on FST behaviors was observed in the females for either regimen). Thus, there was no main effect of treatment, i.e. low vs. high morphine (WD3: F1, 54=0.25, p>0.05; WD9: F1, 55=5.08, p>0.05) and no significant interaction between sex and treatment (WD3: F1, 54=2.13, p>0.05; WD9: F1, 55=0.84, p>0.05).
Discussion
This study demonstrates sex differences in FST behaviors in mice during opioid withdrawal. In this study, no change in FST behaviors was observed in female adolescent mice undergoing withdrawal on WD3 or WD9 as compared with controls. This was not due to changes in motor activity since no differences in locomotion were observed on either WD3 or WD9. In contrast, male adolescent mice exhibited a decrease in immobility on WD3 and no change in FST behaviors on WD9 (Hodgson et al., 2009). In the males, the change in FST behavior was also not due to changes in motor activity during withdrawal (Hodgson et al., 2009). The sex differences in FST behaviors during withdrawal are probably not due to sex differences in morphine pharmacokinetics, given that no significant pharmacokinetical differences were found between prepubertal female and male mice (Diaz et al., 2007). Furthermore, this sex-related difference in FST behaviors most likely does not represent general sex differences in the severity of withdrawal precipitation. In mice, there was no sex-related difference in the severity of somatic withdrawal symptoms during naloxone-precipitated withdrawal (Ali et al., 1995; El-Kadi and Sharif, 1994; Kest et al., 2001). Additionally, during spontaneous withdrawal, females’ overall somatic symptoms scores were actually higher in magnitude and longer in duration for 10–50 mg/kg increasing morphine doses (similar to the high morphine regimen in this study) as compared with males (Papaleo and Contarino, 2006).
Given that no significant differences in morphine pharmacokinetics were found between prepubertal female and male mice (Diaz et al., 2007), we examined the female mice in this study on the same days in which the males were examined (Hodgson et al., 2009). The rationale for using these days in the male study was because prior studies in adult rats undergoing opioid withdrawal demonstrated that the dysphoric effect (i.e. increased FST immobility) started around WD3 (Anraku et al., 2001; Molina et al, 1994) and lasted well into the second and third week of withdrawal (Grasing and Ghosh, 1998). Therefore, when examining adolescents (males and females) we chose to examine the effects of withdrawal on WD3 and WD9. We specifically did not examine the effects of withdrawal on day 1 and 2 when, even in the adults, depression-like behaviors are not yet observed, thus the lack of them in the adolescents would not have been surprising. Also, we did not examine the response in the third week of withdrawal, given that adolescence in mice only lasts a short period of time, thus by the third week of withdrawal the mice are already considered young adults (Spear, 2000).
The interpretation of the FST behavioral results, namely the extrapolation from motor activity to mood, should be performed with caution, especially when extrapolating from a measure of rodent response to the possible mental state of humans during opioid withdrawal. Furthermore, additional tests such as tail suspension test, sucrose consumption/preference, and intracranial self-stimulation should be conducted to further support any conclusion. Nonetheless, when extrapolating using the traditional understanding of FST results, we conclude that opioid withdrawal might precipitate different mood responses in female and male adolescent mice. In this regard, the decreased immobility exhibited by male adolescents (Hodgson et al., 2009) is interpreted to represent mood elation, while females did not exhibit a significant change in mood during opioid withdrawal.
In adult rodents, the opioid system is known to be involved in modulating emotional states. More specifically, the opioid system was demonstrated to modulate FST behaviors. The dynorphin/kappa opioid receptor system mediates depressive-like behaviors (Carlezon et al., 2006; Mague et al., 2003; Shirayama et al., 2004; Todtenkopf et al., 2004), while activation of the enkephalin/delta receptors system has an antidepressant-like effect (Baamonde et al., 1992; Nieto et al., 2005). Thus, sex differences in the expression and functionality of various components of the opioid system might explain the differences observed in FST behavior. Indeed, sex-linked differences have been observed in the distribution of kappa opioid receptors in the spinal cord (Harris et al., 2004). Additionally, females were suggested to express lower levels of the mu opioid receptors in the ventral periaqueductal gray (Bernal et al., 2007). Sex differences were also observed in expression levels of preproenkephalins, where female mice had higher expression levels in the striatum as compared with males (Gerald et al., 2008). Unfortunately, there is limited literature examining sex differences in the expression of different components of the opioid system, especially in those key brain areas suggested to be involved in the modulation of FST behaviors.
Alternatively, the observed sex differences during adolescence might be explained by differences in the expression and functionality of the opioid system at different stages during development (i.e. during adolescence). Indeed, some literature suggests that the opioid system is still immature during adolescence. For instance, in male rodents, different expression levels of opioid receptors are observed across lifespan (Carretero et al., 2004; Gazyakan et al., 2000; Kivell et al., 2004; Ueno et al., 1988). Also, there are changes in the cellular localization and functionality of the opioid system throughout adolescence (Wang et al., 2003a, b). However, there is limited information on the ontogenesis of the opioid system in males and even less data on the ontogenesis in females. One study demonstrated no significant baseline sex differences in the expression of the mu opioid receptor in different brain areas between prepubertal male and female mice (Diaz et al., 2006). However, there is a lack of literature on sex-dependent developmental differences of the kappa and delta opioid receptors, which are known to modulate FST behaviors in adults. Thus, it is still possible that sex differences in the ontogenesis of the kappa and delta opioid receptors underlie the difference in FST behaviors between male and female adolescent mice withdrawing from morphine.
A third possible explanation for these sex differences during withdrawal is that morphine exposure or withdrawal might modulate the expression of opioid receptors differently in female and male adolescent mice. Indeed, sex differences in the expression of the mu opioid receptor in different brain areas were observed between prepubertal male and female mice undergoing morphine withdrawal (Diaz et al., 2006). However, as mentioned earlier, there is limited information on the effect of morphine exposure or withdrawal on the expression levels of different components of the opioid system in both males and females. Thus, further studies are needed to examine both the ontogenesis of the opioid system in both males and females, and the effect of morphine exposure on the expression levels of different components of the opioid system during different developmental stages.
This study demonstrates the existence of sex differences in the affective responses to opioid withdrawal. Studying the consequences of opioid withdrawal in adolescents is clearly important, given the recent surge in prescription medication abuse among adolescents, especially pain killers such as oxycodone (National Survey, 2006). We focused on FST and locomotor behaviors, given the high comorbidity of mood disorders in drug addicts, even after years of abstinence (reviewed in Maremmani et al., 2006). Moreover, this comorbidity was demonstrated to increase when drug use began at a young age (Caspi et al., 2005; Degenhardt et al., 2007; Gfroerer et al., 2002; Mathers et al., 2006; Tucker et al., 2006). However, the underlying biological mechanisms for this comorbidity are still elusive. We demonstrated that opioid withdrawal induces different FST behaviors in female and male adolescents. Thus, this study raises the question: is sex also a contributing factor to the precipitation of differential mood disorders in addicts? Specifically, is sex an important factor for the observed comorbidity of hypomania in heroin addicts (reviewed by Maremmani et al., 2006)? This study may provide some insights into the role of withdrawal from drugs of abuse in precipitating future mental illnesses, and will potentially have clinical implications on treatment approaches for drug addiction during adolescence. Future studies will examine the receptor mechanisms involved, as well as other brain pathways and signaling cascades that are important in the manifestation of sex differences during drug withdrawal.
Acknowledgments
These studies were supported by NIDA (DA022402); KWR is supported by NIH (P50DA05010). We also would like to thank Mr. Menachum M Slodowitz for his editorial assistance.
Footnotes
Disclosure/conflict of interest
The authors have no financial interests to disclose.
References
- Ali BH, Sharif SI, Elkadi A. Sex differences and the effect of gonadectomy on morphine-induced antinociception and dependence in rats and mice. Clin Exp Pharmacol P. 1995;22:342–344. doi: 10.1111/j.1440-1681.1995.tb02012.x. [DOI] [PubMed] [Google Scholar]
- Anraku T, Ikegaya Y, Matsuki N, Nishiyama N. Withdrawal from chronic morphine administration causes prolonged enhancement of immobility in rat forced swimming test. Psychopharmacology (Berlin) 2001;157:217–20. doi: 10.1007/s002130100793. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Harris GC. Brain substrates for increased drug seeking during protracted withdrawal. Neuropharmacology. 2004;47:167–179. doi: 10.1016/j.neuropharm.2004.06.020. [DOI] [PubMed] [Google Scholar]
- Baamonde A, Daugé V, Ruiz-Gayo M, et al. Antidepressant-type effects of endogenous enkephalins protected by systemic RB 101 are mediated by opioid delta and dopamine D1 receptor stimulation. Eur J Pharmacol. 1992;216:157–166. doi: 10.1016/0014-2999(92)90356-9. [DOI] [PubMed] [Google Scholar]
- Bernal SA, Morgan MM, Craft RM. PAG mu opioid receptor activation underlies sex differences in morphine antinociception. Behav Brain Res. 2007;177:126–133. doi: 10.1016/j.bbr.2006.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Béguin C, DiNieri JA, et al. Depressive-like effects of the kappa-opioid receptor agonist salvinorin A on behavior and neurochemistry in rats. J Pharmacol Exp Ther. 2006;316:440–447. doi: 10.1124/jpet.105.092304. [DOI] [PubMed] [Google Scholar]
- Carretero J, Bodego P, Rodriguez RE, Rubio M, Blanco E, Burks DJ. Expression of the mu-opioid receptor in the anterior pituitary gland is influenced by age and sex. Neuropeptides. 2004;38:63–68. doi: 10.1016/j.npep.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE, Cannon M, et al. Moderation of the effect of adolescent-onset cannabis use on adult psychosis by a functional polymorphism in the catechol-O-methyltransferase gene: longitudinal evidence of a gene X environment interaction. Biol Psychiat. 2005;57:1117–1127. doi: 10.1016/j.biopsych.2005.01.026. [DOI] [PubMed] [Google Scholar]
- Crowley JJ, Jones MD, O’Leary OF, Lucki I. Automated tests for measuring the effects of antidepressants in mice. Pharmacol Biochem Be. 2004;78:269–274. doi: 10.1016/j.pbb.2004.03.014. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Coffey C, Moran P, Carlin JB, Patton GC. The predictors and consequences of adolescent amphetamine use: findings from the Victoria Adolescent Health Cohort Study. Addiction. 2007;102:1076–1084. doi: 10.1111/j.1360-0443.2007.01839.x. [DOI] [PubMed] [Google Scholar]
- Diaz SL, Barros VG, Antonelli MC, Rubio MC, Balerio GN. Morphine withdrawal syndrome and its prevention with baclofen: Autoradiographic study of mu-opioid receptors in prepubertal male and female mice. Synapse. 2006;60:132–140. doi: 10.1002/syn.20279. [DOI] [PubMed] [Google Scholar]
- Diaz SL, Hermida MP, Joannas LD, et al. Pharmacokinetic aspects of naloxone-precipitated morphine withdrawal in male and female prepubertal mice. Biopharm Drug Dispos. 2007;28:283–289. doi: 10.1002/bdd.554. [DOI] [PubMed] [Google Scholar]
- El-Kadi AO, Sharif SI. The influence of various experimental conditions on the expression of naloxone-induced withdrawal symptoms in mice. Gen Pharmacol. 1994;25:1505–1510. doi: 10.1016/0306-3623(94)90181-3. [DOI] [PubMed] [Google Scholar]
- Gazyakan E, Disko U, Haaf A, Heimrich B, Jackisch R. Postnatal development of opioid receptors modulating acetylcholine release in hippocampus and septum of the rat. Brain Res Dev Brain Res. 2000;123:135–141. doi: 10.1016/s0165-3806(00)00091-2. [DOI] [PubMed] [Google Scholar]
- Gerald TM, Howlett AC, Ward GR, Ho C, Franklin SO. Gene expression of opioid and dopamine systems in mouse striatum: effects of CB1 receptors, age and sex. Psychopharmacology (Berlin) 2008;198:497–508. doi: 10.1007/s00213-008-1141-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gfroerer JC, Wu LT, Penne MA. Initiation of marijuana use: Trends, patterns, and implications. Rockville, MD: Substance Abuse and Mental Health Services Administration, Office of Applied Studies; 2002. (DHHS Publication No. SMA 02–3711, Analytic Series A–17) [Google Scholar]
- Grasing K, Ghosh S. Selegiline prevents long-term changes in dopamine efflux and stress immobility during the second and third weeks of abstinence following opiate withdrawal. Neuropharmacology. 1998;37:1007–10017. doi: 10.1016/s0028-3908(98)00093-8. [DOI] [PubMed] [Google Scholar]
- Hand TH, Koob GF, Stinus L, Le Moal M. Aversive properties of opiate receptor blockade: evidence for exclusively central mediation in naive and morphine-dependent rats. Brain Res. 1988;474:364–368. doi: 10.1016/0006-8993(88)90452-0. [DOI] [PubMed] [Google Scholar]
- Handelsman L, Aronson MJ, Ness R, Cochrane KJ, Kanof PD. The dysphoria of heroin addiction. Am J Drug Alcohol Ab. 1992;18:275–287. doi: 10.3109/00952999209026067. [DOI] [PubMed] [Google Scholar]
- Harris JA, Chang PC, Drake CT. Kappa opioid receptors in rat spinal cord: sex-linked distribution differences. Neuroscience. 2004;124:879–890. doi: 10.1016/j.neuroscience.2003.12.042. [DOI] [PubMed] [Google Scholar]
- Hodgson SR, Hofford RS, Wellman PJ, Eitan S. Different affective response to opioid withdrawal in adolescent and adult mice. Life Sci. 2009;84:52–60. doi: 10.1016/j.lfs.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson LH, Cryan JF. Feeling strained? Influence of genetic background on depression-related behavior in mice: a review. Behav Genet. 2007;37:171–213. doi: 10.1007/s10519-006-9106-3. [DOI] [PubMed] [Google Scholar]
- Janiri L, Martinotti G, Dario T, et al. Anhedonia and substance- related symptoms in detoxified substance-dependent subjects: a correlation study. Neuropsychobiology. 2005;52:37–44. doi: 10.1159/000086176. [DOI] [PubMed] [Google Scholar]
- Kest B, Palmese CA, Hopkins E, Adler M, Juni A. Assessment of acute and chronic morphine dependence in male and female mice. Pharmacol Biochem Be. 2001;70:149–56. doi: 10.1016/s0091-3057(01)00600-1. [DOI] [PubMed] [Google Scholar]
- Kivell BM, Day DJ, McDonald FJ, Miller JH. Developmental expression of mu and delta opioid receptors in the rat brainstem: evidence for a postnatal switch in mu isoform expression. Brain Res Dev Brain Res. 2004;148:185–196. doi: 10.1016/j.devbrainres.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Koob GF, Caine SB, Parsons L, Markou A, Weiss F. Opponent process model and psychostimulant addiction. Pharmacol Biochem Be. 1997;57:513–521. doi: 10.1016/s0091-3057(96)00438-8. [DOI] [PubMed] [Google Scholar]
- Mague SD, Pliakas AM, Todtenkopf MS, et al. Antidepressant-like effects of kappa-opioid receptor antagonists in the forced swim test in rats. J Pharmacol Exp Ther. 2003;305:323–330. doi: 10.1124/jpet.102.046433. [DOI] [PubMed] [Google Scholar]
- Maremmani I, Perugi G, Pacini M, Akiskal HS. Toward a unitary perspective on the bipolar spectrum and substance abuse: opiate addiction as a paradigm. J Affect Disorders. 2006;93:1–12. doi: 10.1016/j.jad.2006.02.022. [DOI] [PubMed] [Google Scholar]
- Mathers M, Toumbourou JW, Catalano RF, Williams J, Patton GC. Consequences of youth tobacco use: a review of prospective behavioural studies. Addiction. 2006;101:948–958. doi: 10.1111/j.1360-0443.2006.01438.x. [DOI] [PubMed] [Google Scholar]
- Molina VA, Heyser CJ, Spear LP. Chronic variable stress or chronic morphine facilitates immobility in a forced swim test: reversal by naloxone. Psychopharmacology (Berlin) 1994;114:433–440. doi: 10.1007/BF02249333. [DOI] [PubMed] [Google Scholar]
- National Survey. Results from the 2005 National Survey on Drug Use & Health: National Findings. Office of Applied Studies, Substance Abuse & Mental Health Services Administration (SAMHSA), US department of Health and Human Services; Rockville, Maryland, USA: 2006. [Google Scholar]
- Nieto MM, Guen SL, Kieffer BL, Roques BP, Noble F. Physiological control of emotion-related behaviors by endogenous enkephalins involves essentially the delta opioid receptors. Neuroscience. 2005;135:305–313. doi: 10.1016/j.neuroscience.2005.06.025. [DOI] [PubMed] [Google Scholar]
- Papaleo F, Contarino A. Gender- and morphine dose-linked expression of spontaneous somatic opiate withdrawal in mice. Behav Brain Res. 2006;170:110–118. doi: 10.1016/j.bbr.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacod T. 1977;229:327–336. [PubMed] [Google Scholar]
- Porsolt RD, Brossard G, Hautbois C, Roux S. Rodent models of depression: forced swimming and tail suspension behavioral despair tests in rats and mice. In: Crawley JN, et al., editors. Curr Protoc Neurosci. Unit 8.10A. Chapter 8. J. Wiley; United States: 2001. [DOI] [PubMed] [Google Scholar]
- Schaefer GJ, Michael RP. Morphine withdrawal produces differential effects on the rate of lever-pressing for brain self-stimulation in the hypothalamus and midbrain in rats. Pharmacol Biochem Be. 1983;18:571–577. doi: 10.1016/0091-3057(83)90283-6. [DOI] [PubMed] [Google Scholar]
- Schulteis G, Koob GF. Reinforcement processes in opiate addiction: a homeostatic model. Neurochem Res. 1996;21:1437–1454. doi: 10.1007/BF02532385. [DOI] [PubMed] [Google Scholar]
- Schulteis G, Markou A, Gold LH, Stinus L, Koob GF. Relative sensitivity to naloxone of multiple indices of opiate withdrawal: a quantitative dose-response analysis. J Pharmacol Exp Ther. 1994;271:1391–1398. [PubMed] [Google Scholar]
- Schürks M, Overlack M, Bonnet U. Naltrexone treatment of combined alcohol and opioid dependence: deterioration of comorbid major depression. Pharmacopsychiatry. 2005;38:100–102. doi: 10.1055/s-2005-837812. [DOI] [PubMed] [Google Scholar]
- Shirayama Y, Ishida H, Iwata M, Hazama GI, Kawahara R, Duman RS. Stress increases dynorphin immunoreactivity in limbic brain regions and dynorphin antagonism produces antidepressant-like effects. J Neurochem. 2004;90:1258–1268. doi: 10.1111/j.1471-4159.2004.02589.x. [DOI] [PubMed] [Google Scholar]
- Shobe FO, Brion P. Long-term prognosis in manic-depressive illness. Arch Gen Psychiat. 1971;24:334–337. doi: 10.1001/archpsyc.1971.01750100044006. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav R. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Todtenkopf MS, Marcus JF, Portoghese PS, Carlezon WA., Jr Effects of kappa-opioid receptor ligands on intracranial self-stimulation in rats. Psychopharmacology (Berlin) 2004;172:463–470. doi: 10.1007/s00213-003-1680-y. [DOI] [PubMed] [Google Scholar]
- Tucker JS, Ellickson PL, Collins RL, Klein DJ. Does solitary substance use increase adolescents’ risk for poor psychosocial and behavioral outcomes? A 9-year longitudinal study comparing solitary and social users. Psychol Addict Behav. 2006;20:363–372. doi: 10.1037/0893-164X.20.4.363. [DOI] [PubMed] [Google Scholar]
- Ueno E, Liu DD, Ho IK, Hoskins B. Opiate receptor characteristics in brains from young, mature and aged mice. Neurobiol Aging. 1988;9:279–283. doi: 10.1016/s0197-4580(88)80066-6. [DOI] [PubMed] [Google Scholar]
- Wang H, Cuzon VC, Pickel VM. Postnatal development of mu-opioid receptors in the rat caudate-putamen nucleus parallels asymmetric synapse formation. Neuroscience. 2003a;118:695–708. doi: 10.1016/s0306-4522(02)00926-0. [DOI] [PubMed] [Google Scholar]
- Wang H, Cuzon VC, Pickel VM. Ultrastructural localization of delta-opioid receptors in the rat caudate-putamen nucleus during postnatal development: relation to synaptogenesis. J Comp Neurol. 2003b;467:343–353. doi: 10.1002/cne.10920. [DOI] [PubMed] [Google Scholar]
- White JM. Pleasure into pain: the consequences of long-term opioid use. Addict Behav. 2004;29:1311–1324. doi: 10.1016/j.addbeh.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Zurita A, Molina V. Prior morphine facilitates the occurrence of immobility and anhedonia following stress. Physiol Behav. 1999;65:833–837. doi: 10.1016/s0031-9384(98)00247-9. [DOI] [PubMed] [Google Scholar]