Abstract
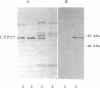
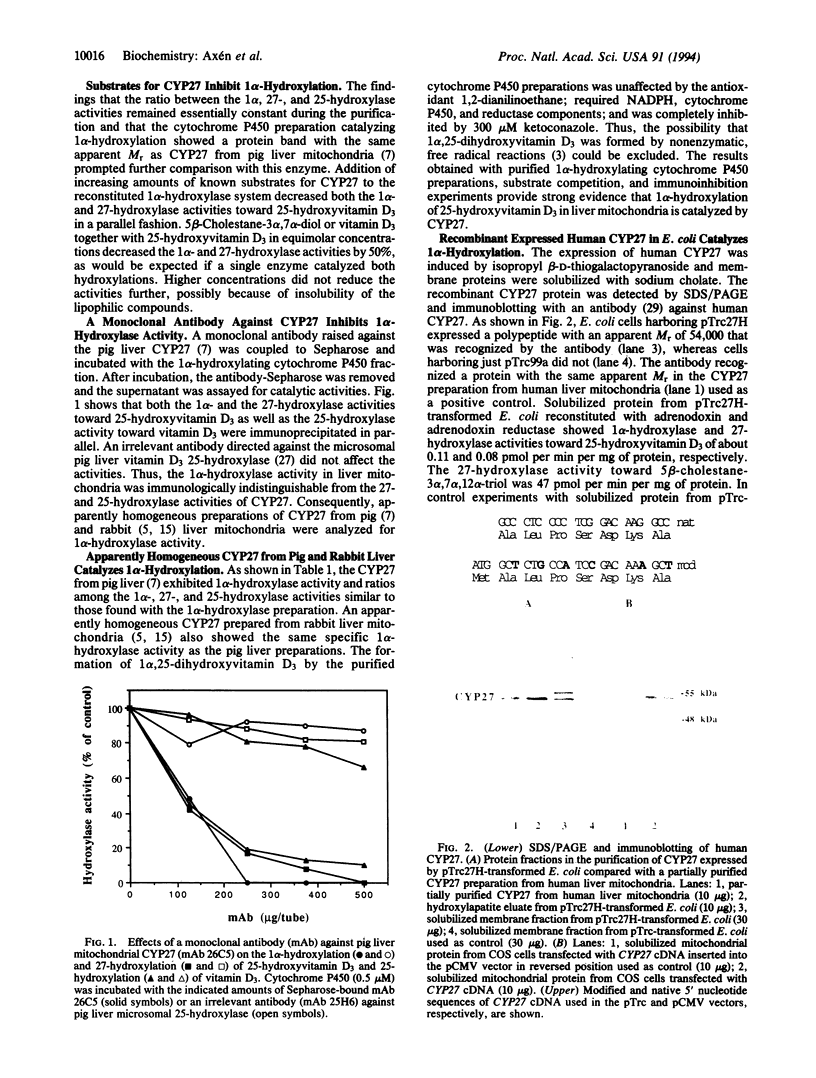
A cytochrome P450 catalyzing 1 alpha-hydroxylation of 25-hydroxyvitamin D3 was purified from pig liver mitochondria. It also catalyzed 27-hydroxylation of 25-hydroxyvitamin D3 and 25-hydroxylation of vitamin D3. The ratio between the 1 alpha-, 27-, and 25-hydroxylase activities remained essentially constant during the purification. Substrates for sterol 27-hydroxylase CYP27 inhibited and a monoclonal antibody raised against CYP27 immunoprecipitated the 1 alpha-, 27-, and 25-hydroxylase activities. Apparently homogeneous preparations of CYP27 from pig and rabbit liver mitochondria catalyzed 1 alpha-hydroxylation. Human liver mitochondrial CYP27 was expressed from its cDNA in Escherichia coli. The nucleotide sequence encoding the N terminus of CYP27 was modified in the first eight codons to achieve expression in E. coli. The purified recombinant-expressed CYP27 reconstituted with the electron-transferring system of adrenal mitochondria catalyzed 1 alpha-hydroxylation of 25-hydroxyvitamin D3. Expression of unmodified CYP27 cDNA in simian COS cells confirmed the 1 alpha-hydroxylase activity toward 25-hydroxyvitamin D3.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyoshi-Shibata M., Usui E., Sakaki T., Yabusaki Y., Noshiro M., Okuda K., Ohkawa H. Expression of rat liver vitamin D3 25-hydroxylase cDNA in Saccharomyces cerevisiae. FEBS Lett. 1991 Mar 25;280(2):367–370. doi: 10.1016/0014-5793(91)80333-x. [DOI] [PubMed] [Google Scholar]
- Andersson S., Davis D. L., Dahlbäck H., Jörnvall H., Russell D. W. Cloning, structure, and expression of the mitochondrial cytochrome P-450 sterol 26-hydroxylase, a bile acid biosynthetic enzyme. J Biol Chem. 1989 May 15;264(14):8222–8229. [PubMed] [Google Scholar]
- Andersson S., Jörnvall H. Sex differences in cytochrome P-450-dependent 25-hydroxylation of C27-steroids and vitamin D3 in rat liver microsomes. J Biol Chem. 1986 Dec 25;261(36):16932–16936. [PubMed] [Google Scholar]
- Axén E., Bergman T., Wikvall K. Purification and characterization of a vitamin D3 25-hydroxylase from pig liver microsomes. Biochem J. 1992 Nov 1;287(Pt 3):725–731. doi: 10.1042/bj2870725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes H. J., Arlotto M. P., Waterman M. R. Expression and enzymatic activity of recombinant cytochrome P450 17 alpha-hydroxylase in Escherichia coli. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5597–5601. doi: 10.1073/pnas.88.13.5597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berginer V. M., Shany S., Alkalay D., Berginer J., Dekel S., Salen G., Tint G. S., Gazit D. Osteoporosis and increased bone fractures in cerebrotendinous xanthomatosis. Metabolism. 1993 Jan;42(1):69–74. doi: 10.1016/0026-0495(93)90174-m. [DOI] [PubMed] [Google Scholar]
- Bergman T., Postlind H. Characterization of pig kidney microsomal cytochrome P-450 catalysing 25-hydroxylation of vitamin D3 and C27 steroids. Biochem J. 1990 Sep 1;270(2):345–350. doi: 10.1042/bj2700345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björkhem I., Holmberg I., Kristiansen T., Pedersen J. I. Assay of 1,25-dihydroxy vitamin D3 by isotope dilution--mass fragmentography. Clin Chem. 1979 Apr;25(4):584–588. [PubMed] [Google Scholar]
- Cali J. J., Hsieh C. L., Francke U., Russell D. W. Mutations in the bile acid biosynthetic enzyme sterol 27-hydroxylase underlie cerebrotendinous xanthomatosis. J Biol Chem. 1991 Apr 25;266(12):7779–7783. [PMC free article] [PubMed] [Google Scholar]
- Cali J. J., Russell D. W. Characterization of human sterol 27-hydroxylase. A mitochondrial cytochrome P-450 that catalyzes multiple oxidation reaction in bile acid biosynthesis. J Biol Chem. 1991 Apr 25;266(12):7774–7778. [PubMed] [Google Scholar]
- Clark B. J., Waterman M. R. Heterologous expression of mammalian P450 in COS cells. Methods Enzymol. 1991;206:100–108. doi: 10.1016/0076-6879(91)06081-d. [DOI] [PubMed] [Google Scholar]
- Dahlbäck H. Characterization of the liver mitochondrial cytochrome P-450 catalyzing the 26-hydroxylation of 5 beta-cholestane-3 alpha, 7 alpha, 12 alpha-triol. Biochem Biophys Res Commun. 1988 Nov 30;157(1):30–36. doi: 10.1016/s0006-291x(88)80006-8. [DOI] [PubMed] [Google Scholar]
- Dahlbäck H., Wikvall K. 25-Hydroxylation of vitamin D3 by a cytochrome P-450 from rabbit liver mitochondria. Biochem J. 1988 May 15;252(1):207–213. doi: 10.1042/bj2520207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esser V., Limbird L. E., Brown M. S., Goldstein J. L., Russell D. W. Mutational analysis of the ligand binding domain of the low density lipoprotein receptor. J Biol Chem. 1988 Sep 15;263(26):13282–13290. [PubMed] [Google Scholar]
- Gray R. W., Ghazarian J. G. Solubilization and reconstitution of kidney 25-hydroxyvitamin D3 1 alpha- and 24-hydroxylases from vitamin D-replete pigs. Biochem J. 1989 Apr 15;259(2):561–568. doi: 10.1042/bj2590561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y. D., Strugnell S., Back D. W., Jones G. Transfected human liver cytochrome P-450 hydroxylates vitamin D analogs at different side-chain positions. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8668–8672. doi: 10.1073/pnas.90.18.8668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry H. L. Vitamin D hydroxylases. J Cell Biochem. 1992 May;49(1):4–9. doi: 10.1002/jcb.240490103. [DOI] [PubMed] [Google Scholar]
- Hollis B. W. 25-Hydroxyvitamin D3-1 alpha-hydroxylase in porcine hepatic tissue: subcellular localization to both mitochondria and microsomes. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6009–6013. doi: 10.1073/pnas.87.16.6009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmberg-Betsholtz I., Lund E., Björkhem I., Wikvall K. Sterol 27-hydroxylase in bile acid biosynthesis. Mechanism of oxidation of 5 beta-cholestane-3 alpha,7 alpha,12 alpha,27-tetrol into 3 alpha,7 alpha,12 alpha-trihydroxy-5 beta-cholestanoic acid. J Biol Chem. 1993 May 25;268(15):11079–11085. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Littledike E. T., Horst R. L. Metabolism of vitamin D3 in nephrectomized pigs given pharmacological amounts of vitamin D3. Endocrinology. 1982 Dec;111(6):2008–2013. doi: 10.1210/endo-111-6-2008. [DOI] [PubMed] [Google Scholar]
- Lund E., Björkhem I., Furster C., Wikvall K. 24-, 25- and 27-hydroxylation of cholesterol by a purified preparation of 27-hydroxylase from pig liver. Biochim Biophys Acta. 1993 Feb 24;1166(2-3):177–182. doi: 10.1016/0005-2760(93)90094-p. [DOI] [PubMed] [Google Scholar]
- Mandel M. L., Swartz S. J., Ghazarian J. G. Avian kidney mitochondrial hemeprotein P-4501 alpha: isolation, characterization and NADPH-ferredoxin reductase-dependent activity. Biochim Biophys Acta. 1990 Jun 20;1034(3):239–246. doi: 10.1016/0304-4165(90)90044-w. [DOI] [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. II. SOLUBILIZATION, PURIFICATION, AND PROPERTIES. J Biol Chem. 1964 Jul;239:2379–2385. [PubMed] [Google Scholar]
- Ohyama Y., Masumoto O., Usui E., Okuda K. Multi-functional property of rat liver mitochondrial cytochrome P-450. J Biochem. 1991 Mar;109(3):389–393. doi: 10.1093/oxfordjournals.jbchem.a123391. [DOI] [PubMed] [Google Scholar]
- Postlind H. Separation of the cytochromes P-450 in pig kidney mitochondria catalyzing 1 alpha-, 24- and 26-hydroxylations of 25-hydroxyvitamin D3. Biochem Biophys Res Commun. 1990 Apr 16;168(1):261–266. doi: 10.1016/0006-291x(90)91702-t. [DOI] [PubMed] [Google Scholar]
- Richardson T. H., Hsu M. H., Kronbach T., Barnes H. J., Chan G., Waterman M. R., Kemper B., Johnson E. F. Purification and characterization of recombinant-expressed cytochrome P450 2C3 from Escherichia coli: 2C3 encodes the 6 beta-hydroxylase deficient form of P450 3b. Arch Biochem Biophys. 1993 Jan;300(1):510–516. doi: 10.1006/abbi.1993.1069. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Usui E., Noshiro M., Ohyama Y., Okuda K. Unique property of liver mitochondrial P450 to catalyze the two physiologically important reactions involved in both cholesterol catabolism and vitamin D activation. FEBS Lett. 1990 Nov 12;274(1-2):175–177. doi: 10.1016/0014-5793(90)81357-t. [DOI] [PubMed] [Google Scholar]
- Wikvall K. Hydroxylations in biosynthesis of bile acids. Isolation of a cytochrome P-450 from rabbit liver mitochondria catalyzing 26-hydroxylation of C27-steroids. J Biol Chem. 1984 Mar 25;259(6):3800–3804. [PubMed] [Google Scholar]