Abstract
Purpose of review
The proximal tubule (PT) plays a critical role in the reabsorption of ions, solutes and low molecular weight proteins from the glomerular filtrate. Although the PT has long been known to acutely modulate ion reabsorption in response to changes in flow rates of the glomerular filtrate, it has only recently been discovered that PT cells can similarly adjust endocytic capacity in response to flow. This review synthesizes our current understanding of mechanosensitive regulation of endocytic capacity in PT epithelia and highlights areas of opportunity for future investigations.
Recent findings
Recent studies have reported that the endocytic capacity of PT cells is dramatically increased upon exposure to flow and the accompanying fluid shear stress (FSS). Modulation of this pathway is dependent on increases in intracellular calcium [Ca2+]i initiated by bending of the primary cilium, and also requires purinergic receptor activation that is mediated by release of extracellular ATP. This article summarizes what is currently known about the signaling cascade that transduces changes in flow into alterations in endocytosis. We discuss the implications of this newly described regulatory pathway with respect to our understanding of protein retrieval by the kidney under normal conditions, and in diseases that present with low molecular weight proteinuria.
Summary
Primary cilia act as mechanotransducers that modulate apical endocytic capacity in PT cells in response to changes in fluid shear stress.
Keywords: Fluid shear stress, primary cilium, calcium, mechanosensation, purinergic receptor
INTRODUCTION
The proximal tubules (PTs) of the kidney are responsible for recovering over two thirds of the water from the glomerular filtrate, as well as a significant fraction of the filtered Na+, K+, Cl−, HCO3−, phosphate, and glucose. Despite sizable daily variations in glomerular filtration rate (GFR), the kidneys maintain consistent fractional fluid and solute reabsorption efficiency. Studies conducted over the past decade have shown that ion reabsorption in the PT is modulated in response to changes in GFR to preserve glomerulotubular balance. The flow of the glomerular filtrate through the PT manifests as fluid shear stress (FSS) on the apical surface of the cells lining the renal tubule and as radial stretch on the wall of the renal tubule. Apical microvilli on PT cells have been suggested to function as mechanosensors that transduce changes in FSS to regulate ion transporter trafficking and activity (1–4). For example, changes in GFR and the accompanying FSS and stretch lead to increases in Na+ reabsorption mediated primarily by the insertion of active transporters into the luminal membrane (3, 5–7). Changes in actin dynamics are thought to play a role in this process; however the exact mechanism for how this leads to altered transporter distribution has not been determined. In addition to mediating mechanosensation upon bending of the microvilli, changes in the actin cytoskeleton resulting from radial stretch may also contribute to FSS-dependent signaling (1, 8).
In the distal tubule, primary cilia have emerged as the organelles that transduce changes in FSS into signals that modulate ion transport and other cellular functions (9–11). Defects in ciliary biogenesis and function lead to diseases (collectively termed ciliopathies) that manifest with a subset of common phenotypes, including cystic kidneys, retinal dystrophies, cognitive impairment, and polydactyly [reviewed in (12)]. Like distal tubule cells, PT cells also express primary cilia, but no role for cilia in modulating PT ion transport has been described (12–14).
Another major function of the PT is to retrieve low molecular weight (LMW) proteins, vitamins, hormones, and other small metabolites from the glomerular filtrate. In the kidney, the multiligand receptors megalin and cubilin are expressed exclusively in the PT, and mediate the apical binding and internalization of these LMW proteins and other ligands into PT cells [reviewed in (15)]. Defects in the receptors that mediate the uptake of these filtered ligands or saturation of the clearance pathway [e.g., in diabetes (16)], lead to LMW proteinuria. Prolonged LMW proteinuria (also referred to as tubular proteinuria because it does not involve glomerular dysfunction) causes further deterioration in kidney function and leads to renal failure (17).
Elevations in GFR increase the amount of filtered megalin and cubilin ligands that pass through the PT. A long-held assumption is that PT cells maintain a sufficiently high constitutive endocytic capacity to efficiently retrieve these proteins even at high GFR. However, until recently, whether PT cells modulate endocytosis in response to changes in flow had not been directly tested. This review summarizes recent findings that implicate cilia mediated mechanotransduction in regulating apical endocytosis in proximal tubule epithelia and discusses new challenges in the field that emerge from this finding.
THE APICAL ENDOCYTIC PATHWAY
The epithelial cells that line the PT are specialized to internalize and recycle large amounts of apical membrane in order to effectively reabsorb megalin/cubilin ligands that enter the tubule lumen. Despite the critical role of apical endocytosis in PT function, surprisingly little is known about how this pathway is organized or regulated. Our current understanding of the apical endocytic pathway in PT cells comes primarily from electron microscopy studies examining the uptake of fluid phase and membrane markers (18–22). PT cells have highly irregular invaginations at the base of microvilli at the apical surface in PT cells compared with other polarized epithelia (18, 20, 21, 23). Fluid and membrane tracers are internalized into unevenly shaped clathrin-coated pits that form at the base of brush border microvilli. After budding and uncoating, these endocytic vesicles fuse with a dense network of subapical tubules (18). “Fast recycling” (i.e., rapid return of internalized receptors to the apical cell surface without transit through recycling endosomes) may occur from these sites (22). Within 1–15 min after internalization, both membrane and fluid phase markers can be visualized in large spherical compartments termed apical vacuoles (18–20). Tubules that arise from these apical vacuoles are thought to carry recycling proteins back to the apical surface (19, 20), whereas dissociated ligands are delivered to lysosomes for degradation or transcytosed to the basolateral membrane (24).
This model for apical endocytosis in PT cells differs significantly from the pathway described in kidney cells derived from other tubule segments and best characterized in Madin-Darby canine kidney (MDCK) cells. In MDCK cells, apically internalized proteins enter Rab5-positive apical early endosomes, and membrane proteins recycle to the surface via Rab8 positive common endosomes and/or Rab11 positive apical recycling endosomes (25). How the apical tubules and vacuolar compartments in PT cells correspond to the compartments described in MDCK and other polarized kidney cells is not known, although we recently found that the vacuolar structures in primary cultures of PT cells are Rab11a positive (26). Presumably, apical endocytic compartments in PT cells have specialized adaptations that enable them to uniquely handle the large flux of internalized membrane through this pathway. Moreover, as described below, it appears that this pathway can be acutely upregulated in response to increased endocytic demand.
FSS MODULATES APICAL ENDOCYTOSIS IN PT CELLS
Two recent studies have observed that endocytosis in PT cells is modulated by FSS. Opossum kidney (OK) cells incubated under FSS of 1 dyne/cm2 internalized 2–3-fold more fluorescent albumin compared with cells incubated under static conditions (27, 28). This shear stress in the human proximal tubule corresponds to flow rate of roughly 63 nl/min (and a GFR of roughly 115 ml/min/1.73m2) (29, 30). Similar results were obtained with the porcine PT cell model LLC-PK1 (28), and more recently with immortalized human and mouse PT cell lines (Raghavan and Weisz, unpublished). Additionally, exposure to FSS also increased uptake of the fluid phase marker rhodamine dextran, suggesting that FSS stimulates a generic increase in apical endocytic capacity rather than a selective increase in the uptake of megalin/cubilin ligands (28). Consistent with the idea that this response is specific for PT cells, no effect of FSS on apical endocytosis was observed in MDCK Type II cells, which have hybrid characteristics of proximal and distal tubule cells (28, 31).
Endocytosis from the apical surface of differentiated kidney cells occurs exclusively at the base of microvilli via a clathrin-, dynamin-, and actin-dependent pathway (32–37). Addition of chlorpromazine (which disrupts clathrin assembly) or the dynamin inhibitor Dyngo-4a (38) to OK cells inhibited both basal and FSS-stimulated endocytosis, suggesting that the same internalization mechanism operates under both conditions (28). Moreover, the onset of the FSS-stimulated response was rapid and statistically different from control by 15–30 min of exposure to FSS (28). Similarly, within 15 min of removing FSS, the endocytic rate returned to the constitutive level observed in cells maintained under static conditions (28). The delay in onset and rapid reversibility of the FSS-stimulated endocytic response suggest that this is a downstream event in a tightly regulated signaling cascade. The precise mechanism underlying this reversible cascade remains to be determined (see below).
Importantly, the endocytic response to FSS is tuned within the normal physiological range of flow across the PT. GFR ranges between 60 and 120 mL/min/1.73m2 in healthy individuals, but can reach >140 mL/min/1.73m2 in diabetic patients (39). Our study showed that the endocytic response trended upwards starting at low FSS (>0.2 dyne/cm2) and reaches statistical significance compared with control cells (maintained under static conditions) between 0.7 dyne/cm2 (equivalent to a GFR of ~60 mL/min/1.73m2) and 1 dyne/cm2 (28). Interestingly, exposure to higher FSS (1.5 dyne/cm2, equivalent to a GFR of ~150 mL/min/1.73m2) did not further enhance uptake of albumin (28). This suggests that PT cells tune their endocytic capacity within the normal range, and that saturation of this response at the higher FSS encountered in hyperfiltering disease states may contribute to tubular proteinuria.
PRIMARY CILIA, PURINERGIC SIGNALING AND Ca2+ RELEASE FROM ER STORES MEDIATE THE ENDOCYTIC RESPONSE TO FSS
In distal tubule cells, bending of primary cilia in response to FSS leads to an acute and transient increase in [Ca2+]i (11, 40). Similarly, FSS triggered a rapid increase in [Ca2+]i in polarized OK cells that was not observed when Ca2+ was omitted from the perfusion buffer, or when cells were deciliated by treatment with ammonium sulfate (28).
The FSS-stimulated, cilium-dependent increase in [Ca2+]i in collecting duct cells is mediated by Ca2+-stimulated Ca2+ release from endoplasmic reticulum (ER) reserves (11). Consistent with this, FSS had no effect on [Ca2+]i in OK cells treated with the SERCA inhibitor tBuBHQ (to deplete ER reserves of Ca2+) or ryanodine (to deplete ryanodine-sensitive stores of ER Ca2+) (28). Ciliary bending in distal tubule cells is also known to cause extracellular release of ATP that can trigger P2XR and P2YR purinergic receptors and cause a further increase in [Ca2+]i (9, 41). Consistent with this possibility, inclusion of the ATP hydrolyzing enzyme apyrase in the perfusion medium attenuated the FSS-stimulated increase in [Ca2+]i. Similarly, treatment with suramin, a pan-inhibitor of purinergic receptors, blocked this response. Conversely, addition of ATP or ryanodine to cells incubated under static conditions was sufficient to trigger enhanced endocytosis of albumin (28). Together, these results suggest an important role for ciliary mechanosensitive Ca2+ channels, extracellular ATP, purinergic signaling, and ER stores of Ca2+ in regulating the endocytic response to FSS.
TOWARDS A COMPREHENSIVE MODEL FOR FLOW-STIMULATED ENDOCYTOSIS
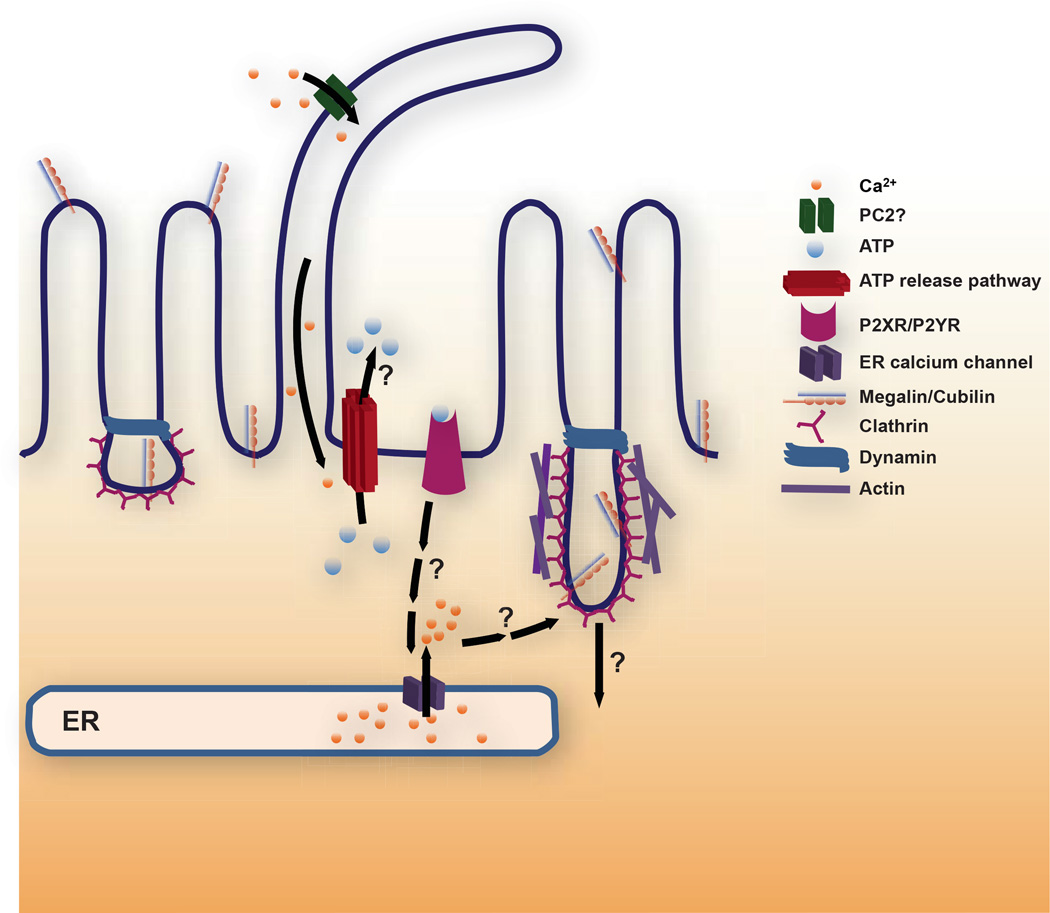
The observations above suggest a model whereby ciliary bending activates cation channels (possibly polycystin-2) that lead to a small increase in [Ca2+]i which triggers extracellular ATP release to activate P2Rs and stimulates further Ca2+ release from ER stores, ultimately leading to enhanced endocytosis (Fig. 1). Needless to say, many gaps in our understanding of the steps and implications of this model remain, and are discussed further below.
Figure 1. Working model for FSS-stimulated endocytosis in the proximal tubule.
We hypothesize that ciliary bending activates a cation channel [possibly polycystin-2 (PC2)] that elevates ciliary and periciliary [Ca2+]i to trigger luminal release of ATP via any of several pathways (see text for details). ATP activates purinergic P2X or P2Y receptors that ultimately leads to Ca2+ release from ER stores via ryanodine and/or IP3 receptor mediated pathways. Increased [Ca2+]i in turn ultimately leads to enhanced apical endocytosis. The downstream signaling cascade leading to the endocytic response remains to be determined. Endocytosis from the apical surface of polarized cells occurs at the base of microvilli via a pathway that requires clathrin and dynamin. Coated pit morphology at the apical surface of PT cells is highly irregular compared with other polarized epithelial cells consistent with the possibility that endocytosis is stimulated by an increase in individual endocytic pit size. Alternatively or in addition, the increase in endocytic capacity in response to FSS may be caused by an increase in the number of apical clathrin coated pits that form.
Although primary cilia are required for the endocytic response to FSS, the mechanosensitive channel that initiates the response remains to be identified. An obvious candidate is the transient receptor potential (TRP) channel polycystin-2 (PC-2), which is important for flow-dependent responses in the distal tubule (11). The related TRPV4 channel has also been implicated in mechanosensation in the distal tubule, possibly by forming a complex with PC-2 (42–45); however, TRPV4 is not expressed in the PT (46).
An unresolved general question of broader significance is the relationship between cilia length, Ca2+ signaling, and the endocytic response. Mutations in many proteins involved in ciliary biogenesis lead to cystic kidney disease, and both shorter and longer cilia can be observed under these conditions. However, the relationship between FSS, ciliary length, and Ca2+ release from ER stores is unknown: is Ca2+ release graduated or binary when the other variables are altered? Surprisingly, there have been no studies where the effect of different FSS on [Ca2+]i in cells with varying cilia length has been systematically measured. A modeling study predicts that longer cilia are more sensitive to flow, and consistent with this idea, two reports suggest that FSS-stimulated Ca2+ signaling is blunted in cells with shortened cilia (47–49). However, one of these groups also observed blunted Ca2+ responses in young mice, which have longer cilia than their older counterparts (48). Thus, there may be an optimal cilia length that enables maximal signaling.
Despite the sizable body of evidence demonstrating a role for primary cilia in flow sensing, the availability of new methods to directly record and quantify the concentration of Ca2+ in the cilioplasm has sparked controversy surrounding the role of this organelle in mechanosensation Studies by the Clapham group clearly demonstrate that the cilioplasm is segregated from the cytosol, and can function as a discrete Ca2+ signaling domain (50). In these experiments, increases in ciliary Ca2+ did not lead to substantial alterations in intracellular calcium levels. Moreover, the authors found that polycystins were not especially sensitive to changes in pressure (although they did not apparently measure activity in response to changes in fluid shear stress (51). Taken to the extreme, these observations could argue that ciliary bending does not trigger mechanosensation. However, more recent work by Nauli and colleagues clearly demonstrates that exposure to flow causes a PC-2 mediated increase in [Ca2+]I (52). These studies also found differences in [Ca2+]i responses to chemical vs mechanical stimuli, as the increase in Ca2+ in response to chemical stimulation of the ciliary CaV1.2 channel was restricted to the cilioplasm (52).
Another essential question to address is what maintains the endocytic response to flow after the initial and transient spike in [Ca2+]i? Endocytosis rates increase in PT cells within 15–30 min after exposure to FSS, and this increased rate of uptake persists long after [Ca2+]i has returned to baseline levels, so long as the FSS is maintained [at least up to 3 h (28)]. Moreover, once the flow is removed, endocytic rates return to normal very rapidly. This suggests that an as yet undetermined and sustained signal/secondary messenger is initiated by the increase in [Ca2+]i that continues to sense the level of FSS and maintain enhanced endocytic capacity.
Our model hypothesizes that subsequent to ciliary bending, the release of ATP into the extracellular milieu is critically important for the FSS-stimulated response. Based on recent studies from other labs, ATP could also initiate signaling upstream of ciliary bending (50, 53) Moving forward, it is important to define the mechanism(s) underlying flow-mediated ATP release from PT cells. Several pathways are known to enable ATP release in the kidney and other organs (54). First, ATP can be released by the connexin (Cx) family of gap-junction channels. Cx30 hemichannels have been demonstrated to play a role in luminal ATP release in the distal tubule (55), and several members of the connexin family are reported to be expressed in the PT, including Cx26, Cx32, Cx37, Cx40, and Cx43 (56–60). Second, the channel-forming pannexin1 (Panx1) protein is widely expressed in the kidney, including the PT, and has been found to play a role in luminal ATP release (61). Several chloride channels, including maxi anion channels, voltage-regulated ion channels, and tweety anion channels have also been implicated in ATP release from cells (54). Finally, ATP may be delivered to the extracellular milieu through constitutive vesicular release or through Ca2+-triggered exocytosis, as has been demonstrated in MDCK cells (62).
We consistently observed that the pan-purinergic receptor blocker suramin inhibits both the increase in [Ca2+]i and endocytosis in response to FSS, consistent with a critical role for purinergic signaling in mediating these FSS dependent responses. Purinergic receptors that have been variously reported to be expressed in the PT include P2X1, P2X4, P2X5, P2X6, and P2X7, and P2Y1, P2Y2, P2Y4, P2Y6, and P2Y11 (63–68). Some discrepancies in expression of these receptors have been reported in different studies, and there may also be species-dependent differences. The purinergic receptor involved in this cascade can be identified by knocking down candidates and/or through pharmacological interventions.
P2X receptors typically trigger ER Ca2+ release via activation of ryanodine receptors, whereas P2YRs are commonly thought to signal via IP3 receptors. While we observed that ryanodine added at concentrations where it functions as an agonist of the ryanodine receptor can trigger endocytosis even in cells maintained under static conditions, we do not know whether it is required for the FSS-stimulated endocytic response. Identifying the ER Ca2+ release channel involved in the endocytic response to FSS is challenging, but may also provide clues as to which purinergic signaling mechanism (P2X or P2Y) is relevant.
Finally, we lack an understanding of how increases in [Ca2+]i are transduced into enhanced endocytic capacity. The increase in endocytosis could be due to an increase in the number of coated pits and/or an increase in their average size. There is precedence for actin-dependent modulation of coated vesicle size, as recent studies have shown that actin is required for clathrin-mediated uptake of larger cargoes such as virus particles, whereas endocytosis of most cargoes in nonpolarized cells is largely actin-independent (69). Coated pit morphology at the apical surface of PT cells is highly irregular compared with other polarized epithelial cells consistent with dynamic variation in endocytic pit capacity. Our recent unpublished results suggest that Cdc42 a small GTPase involved in regulating actin dynamics may play a role in coupling the initial Ca2+ response to flow into enhanced apical endocytosis.
CONCLUSION
This review describes our current understanding of how PT cells respond endocytically to changes in FSS. A limitation of the studies to date is that they have been performed only in immortalized cell culture models of PT. Clearly, more work is necessary to assess the implications of these findings with respect to kidney function. For example, how are the apparently distinct FSS-dependent signals that regulate the abundance of surface ion transporters and the modulation of endocytic capacity integrated to maintain both aspects of PT function?
A confounding variable in assessing the physiological relevance of these observations is the current controversy regarding the role and magnitude of PT endocytic uptake in maintaining efficient clearance of proteins that enter the tubule lumen. Several recent studies have concluded that the fraction of albumin that escapes the glomerular filtration barrier and enters the tubule lumen is considerably higher than previously reported using microperfusion and other approaches (70–72). These studies further suggested that albumin is efficiently endocytosed by PT cells, and rather than being degraded, is largely transcytosed to the basolateral surface and released into the extracellular fluid for reclamation (73). An implication of these conclusions is that defects in proximal tubule function rather than in the glomerular barrier account for clinical proteinuria (74). In this model, constitutive PT endocytic capacity is predicted to be considerably higher than previously assumed, and might be expected to be less sensitive to changes in GFR.
A related issue to be addressed is whether defects in the modulation of FSS-mediated endocytosis contribute to the LMW proteinuria observed in acute, chronic, and genetic diseases. Understanding the molecular mechanism that drives the endocytic response to FSS could lead to the development of therapeutic interventions to improve kidney function, as reducing GFR by modulating hemodynamics could be effective in retarding kidney damage in these patients. One disease that may link the response to FSS with LMW proteinuria is Lowe syndrome, an X-linked disease caused by mutations in the phosphatidylinositol 5’-phosphatase OCRL1. The primary cause of mortality of patients with Lowe syndrome is renal failure as a consequence of chronic LMW proteinuria (75). Interestingly, loss of OCRL1 has been demonstrated to alter cilia length in kidney and other cells (76–78), suggesting a possible link between OCRL1 function and the endocytic response to FSS. Whether this is the case for Lowe syndrome and other diseases that present with LMW proteinuria is an important next question to be addressed in the field.
KEY POINTS.
Apical endocytosis in proximal tubule cells is acutely modulated by changes in fluid shear stress.
The endocytic response to fluid shear stress requires the primary cilium, ATP release, purinergic signaling, and release of Ca2+ from endoplasmic reticulum stores.
The endocytic response is tuned to physiologically relevant levels of fluid shear stress, and occurs via a clathrin- and dynamin-mediated internalization mechanism.
Defective regulation of the endocytic response to fluid shear stress may underlie the low molecular weight proteinuria observed in some kidney diseases.
Acknowledgements
We thank Dr. Osama Alshogran for insightful comments on the manuscript.
Financial support and sponsorship: Research in the Weisz lab is supported by NIH grants DK101484 and DK100357 and by the Lowe Syndrome Association.
Footnotes
Conflicts of interest: None
REFERENCES
Papers of particular interest published within the annual period of review have been highlighted as:
* of special interest
** of outstanding interest
- 1.Du Z, Duan Y, Yan Q, Weinstein AM, Weinbaum S, Wang T. Mechanosensory function of microvilli of the kidney proximal tubule. Proc Natl Acad Sci U S A. 2004;101(35):13068–13073. doi: 10.1073/pnas.0405179101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Du Z, Yan Q, Duan Y, Weinbaum S, Weinstein AM, Wang T. Axial flow modulates proximal tubule NHE3 and H-ATPase activities by changing microvillus bending moments. Am J Physiol Renal Physiol. 2006;290(2):F289–F296. doi: 10.1152/ajprenal.00255.2005. [DOI] [PubMed] [Google Scholar]
- 3.Duan Y, Weinstein AM, Weinbaum S, Wang T. Shear stress-induced changes of membrane transporter localization and expression in mouse proximal tubule cells. Proc Natl Acad Sci U S A. 2010;107(50):21860–21865. doi: 10.1073/pnas.1015751107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weinbaum S, Duan Y, Satlin LM, Wang T, Weinstein AM. Mechanotransduction in the renal tubule. Am J Physiol Renal Physiol. 2010;299(6):F1220–F1236. doi: 10.1152/ajprenal.00453.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McDonough AA. Mechanisms of proximal tubule sodium transport regulation that link extracellular fluid volume and blood pressure. American journal of physiology Regulatory, integrative and comparative physiology. 2010;298(4):R851–R861. doi: 10.1152/ajpregu.00002.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weinstein AM, Weinbaum S, Duan Y, Du Z, Yan Q, Wang T. Flow-dependent transport in a mathematical model of rat proximal tubule. American journal of physiology Renal physiology. 2007;292(4):F1164–F1181. doi: 10.1152/ajprenal.00392.2006. [DOI] [PubMed] [Google Scholar]
- 7.Weinstein AM, Sontag ED. Modeling proximal tubule cell homeostasis: tracking changes in luminal flow. Bull Math Biol. 2009;71(6):1285–1322. doi: 10.1007/s11538-009-9402-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duan Y, Gotoh N, Yan Q, Du Z, Weinstein AM, Wang T, et al. Shear-induced reorganization of renal proximal tubule cell actin cytoskeleton and apical junctional complexes. Proc Natl Acad Sci U S A. 2008;105(32):11418–11423. doi: 10.1073/pnas.0804954105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Praetorius HA. The primary cilium as sensor of fluid flow - new building blocks to the model. Am J Physiol Cell Physiol. 2014 doi: 10.1152/ajpcell.00336.2014. ajpcell 00336 2014. A thoughtful and historical review that describes the research trajectory leading to our current understanding of primary cilia as a flow sensor.
- 10.Jones TJ, Nauli SM. Mechanosensory calcium signaling. Advances in experimental medicine and biology. 2012;740:1001–1015. doi: 10.1007/978-94-007-2888-2_46. [DOI] [PubMed] [Google Scholar]
- 11.Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet. 2003;33(2):129–137. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- 12.Ware SM, Aygun MG, Hildebrandt F. Spectrum of clinical diseases caused by disorders of primary cilia. Proceedings of the American Thoracic Society. 2011;8(5):444–450. doi: 10.1513/pats.201103-025SD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoder BK. Role of primary cilia in the pathogenesis of polycystic kidney disease. J Am Soc Nephrol. 2007;18(5):1381–1388. doi: 10.1681/ASN.2006111215. [DOI] [PubMed] [Google Scholar]
- 14.Rodat-Despoix L, Delmas P. Ciliar functions in the nephron. Pflugers Archiv : European journal of physiology. 2009;458(1):179–187. doi: 10.1007/s00424-008-0632-0. [DOI] [PubMed] [Google Scholar]
- 15.Christensen EI, Birn H, Storm T, Weyer K, Nielsen R. Endocytic receptors in the renal proximal tubule. Physiology (Bethesda) 2012;27(4):223–236. doi: 10.1152/physiol.00022.2012. [DOI] [PubMed] [Google Scholar]
- 16.Tojo A, Onozato ML, Ha H, Kurihara H, Sakai T, Goto A, et al. Reduced albumin reabsorption in the proximal tubule of early-stage diabetic rats. Histochemistry and cell biology. 2001;116(3):269–276. doi: 10.1007/s004180100317. [DOI] [PubMed] [Google Scholar]
- 17.Nielsen R, Christensen EI. Proteinuria and events beyond the slit. Pediatr Nephrol. 2010;25(5):813–822. doi: 10.1007/s00467-009-1381-9. [DOI] [PubMed] [Google Scholar]
- 18.Rodman JS, Seidman L, Farquhar MG. The membrane composition of coated pits, microvilli, endosomes, and lysosomes is distinctive in the rat kidney proximal tubule cell. J Cell Biol. 1986;102(1):77–87. doi: 10.1083/jcb.102.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hatae T, Fujita M, Sagara H, Okuyama K. Formation of apical tubules from large endocytic vacuoles in kidney proximal tubule cells during absorption of horseradish peroxidase. Cell and tissue research. 1986;246(2):271–278. doi: 10.1007/BF00215889. [DOI] [PubMed] [Google Scholar]
- 20.Hatae T, Ichimura T, Ishida T, Sakurai T. Apical tubular network in the rat kidney proximal tubule cells studied by thick-section and scanning electron microscopy. Cell and tissue research. 1997;288(2):317–325. doi: 10.1007/s004410050817. [DOI] [PubMed] [Google Scholar]
- 21.Birn H, Christensen EI, Nielsen S. Kinetics of endocytosis in renal proximal tubule studied with ruthenium red as membrane marker. Am J Physiol. 1993;264(2 Pt 2):F239–F250. doi: 10.1152/ajprenal.1993.264.2.F239. [DOI] [PubMed] [Google Scholar]
- 22.Nielsen S. Endocytosis in proximal tubule cells involves a two-phase membrane-recycling pathway. Am J Physiol. 1993;264(4 Pt 1):C823–C835. doi: 10.1152/ajpcell.1993.264.4.C823. [DOI] [PubMed] [Google Scholar]
- 23.Danielsen EM, Hansen GH. Lipid rafts in epithelial brush borders: atypical membrane microdomains with specialized functions. Biochim Biophys Acta. 2003;1617(1–2):1–9. doi: 10.1016/j.bbamem.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 24.Goligorsky MS, Hruska KA. Transcytosis in cultured proximal tubular cells. J Membr Biol. 1986;93(3):237–247. doi: 10.1007/BF01871178. [DOI] [PubMed] [Google Scholar]
- 25.Welling PA, Weisz OA. Sorting it out in endosomes: an emerging concept in renal epithelial cell transport regulation. Physiology (Bethesda) 2010;25(5):280–292. doi: 10.1152/physiol.00022.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mattila PE, Raghavan V, Rbaibi Y, Baty CJ, Weisz OA. Rab11a-positive compartments in proximal tubule cells sort fluid phase and membrane cargo. Am J Physiol Cell Physiol. 2013 doi: 10.1152/ajpcell.00236.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferrell N, Ricci KB, Groszek J, Marmerstein JT, Fissell WH. Albumin handling by renal tubular epithelial cells in a microfluidic bioreactor. Biotechnology and bioengineering. 2012;109(3):797–803. doi: 10.1002/bit.24339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Raghavan V, Rbaibi Y, Pastor-Soler NM, Carattino MD, Weisz OA. Shear stress-dependent regulation of apical endocytosis in renal proximal tubule cells mediated by primary cilia. Proc Natl Acad Sci U S A. 2014;111(23):8506–8511. doi: 10.1073/pnas.1402195111. This paper characterizes the endocytic response to flow in immortalized proximal tubule cells.
- 29.Chou CL, Marsh DJ. Measurement of flow rate in rat proximal tubules with a nonobstructing optical method. Am J Physiol. 1987;253(2 Pt 2):F366–F371. doi: 10.1152/ajprenal.1987.253.2.F366. [DOI] [PubMed] [Google Scholar]
- 30.Maunsbach AB, Giebisch GH, Stanton BA. Effects of flow rate on proximal tubule ultrastructure. Am J Physiol. 1987;253(3 Pt 2):F582–F587. doi: 10.1152/ajprenal.1987.253.3.F582. [DOI] [PubMed] [Google Scholar]
- 31.Dukes JD, Whitley P, Chalmers AD. The MDCK variety pack: choosing the right strain. BMC cell biology. 2011;12:43. doi: 10.1186/1471-2121-12-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gottlieb TA, Ivanov IE, Adesnik M, Sabatini DD. Actin microfilaments play a critical role in endocytosis at the apical but not the basolateral surface of polarized epithelial cells. J Cell Biol. 1993;120(3):695–710. doi: 10.1083/jcb.120.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boulant S, Kural C, Zeeh JC, Ubelmann F, Kirchhausen T. Actin dynamics counteract membrane tension during clathrin-mediated endocytosis. Nat Cell Biol. 2011;13(9):1124–1131. doi: 10.1038/ncb2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hyman T, Shmuel M, Altschuler Y. Actin is required for endocytosis at the apical surface of Madin-Darby canine kidney cells where ARF6 and clathrin regulate the actin cytoskeleton. Mol Biol Cell. 2006;17(1):427–437. doi: 10.1091/mbc.E05-05-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rodman JS, Kerjaschki D, Merisko E, Farquhar MG. Presence of an extensive clathrin coat on the apical plasmalemma of the rat kidney proximal tubule cell. J Cell Biol. 1984;98(5):1630–1636. doi: 10.1083/jcb.98.5.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ares GR, Ortiz PA. Dynamin2, clathrin, and lipid rafts mediate endocytosis of the apical Na/K/2Cl cotransporter NKCC2 in thick ascending limbs. The Journal of biological chemistry. 2012;287(45):37824–37834. doi: 10.1074/jbc.M112.386425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Altschuler Y, Barbas SM, Terlecky LJ, Tang K, Hardy S, Mostov KE, et al. Redundant and distinct functions for dynamin-1 and dynamin-2 isoforms. The Journal of cell biology. 1998;143(7):1871–1881. doi: 10.1083/jcb.143.7.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Howes MT, Kirkham M, Riches J, Cortese K, Walser PJ, Simpson F, et al. Clathrin-independent carriers form a high capacity endocytic sorting system at the leading edge of migrating cells. J Cell Biol. 2010;190(4):675–691. doi: 10.1083/jcb.201002119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prigent A. Monitoring renal function and limitations of renal function tests. Seminars in nuclear medicine. 2008;38(1):32–46. doi: 10.1053/j.semnuclmed.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 40.Praetorius HA, Spring KR. Removal of the MDCK cell primary cilium abolishes flow sensing. The Journal of membrane biology. 2003;191(1):69–76. doi: 10.1007/s00232-002-1042-4. [DOI] [PubMed] [Google Scholar]
- 41.Praetorius HA, Leipziger J. Released nucleotides amplify the cilium-dependent, flow-induced [Ca2+]i response in MDCK cells. Acta physiologica. 2009;197(3):241–251. doi: 10.1111/j.1748-1716.2009.02002.x. [DOI] [PubMed] [Google Scholar]
- 42.Berrout J, Jin M, Mamenko M, Zaika O, Pochynyuk O, O'Neil RG. Function of transient receptor potential cation channel subfamily V member 4 (TRPV4) as a mechanical transducer in flow-sensitive segments of renal collecting duct system. J Biol Chem. 2012;287(12):8782–8791. doi: 10.1074/jbc.M111.308411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kottgen M, Buchholz B, Garcia-Gonzalez MA, Kotsis F, Fu X, Doerken M, et al. TRPP2 and TRPV4 form a polymodal sensory channel complex. J Cell Biol. 2008;182(3):437–447. doi: 10.1083/jcb.200805124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pochynyuk O, Zaika O, O'Neil RG, Mamenko M. Novel insights into TRPV4 function in the kidney. Pflugers Archiv : European journal of physiology. 2013;465(2):177–186. doi: 10.1007/s00424-012-1190-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zaika O, Mamenko M, Berrout J, Boukelmoune N, O'Neil RG, Pochynyuk O. TRPV4 Dysfunction Promotes Renal Cystogenesis in Autosomal Recessive Polycystic Kidney Disease. J Am Soc Nephrol. 2013;24(4):604–616. doi: 10.1681/ASN.2012050442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tian W, Salanova M, Xu H, Lindsley JN, Oyama TT, Anderson S, et al. Renal expression of osmotically responsive cation channel TRPV4 is restricted to water-impermeant nephron segments. Am J Physiol Renal Physiol. 2004;287(1):F17–F24. doi: 10.1152/ajprenal.00397.2003. [DOI] [PubMed] [Google Scholar]
- 47.Rydholm S, Zwartz G, Kowalewski JM, Kamali-Zare P, Frisk T, Brismar H. Mechanical properties of primary cilia regulate the response to fluid flow. Am J Physiol Renal Physiol. 2010;298(5):F1096–F1102. doi: 10.1152/ajprenal.00657.2009. [DOI] [PubMed] [Google Scholar]
- 48.Liu W, Murcia NS, Duan Y, Weinbaum S, Yoder BK, Schwiebert E, et al. Mechanoregulation of intracellular Ca2+ concentration is attenuated in collecting duct of monocilium-impaired orpk mice. Am J Physiol Renal Physiol. 2005;289(5):F978–F988. doi: 10.1152/ajprenal.00260.2004. [DOI] [PubMed] [Google Scholar]
- 49.Besschetnova TY, Kolpakova-Hart E, Guan Y, Zhou J, Olsen BR, Shah JV. Identification of signaling pathways regulating primary cilium length and flow-mediated adaptation. Curr Biol. 2010;20(2):182–187. doi: 10.1016/j.cub.2009.11.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Delling M, DeCaen PG, Doerner JF, Febvay S, Clapham DE. Primary cilia are specialized calcium signalling organelles. Nature. 2013;504(7479):311–314. doi: 10.1038/nature12833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.DeCaen PG, Delling M, Vien TN, Clapham DE. Direct recording and molecular identification of the calcium channel of primary cilia. Nature. 2013;504(7479):315–318. doi: 10.1038/nature12832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jin X, Mohieldin AM, Muntean BS, Green JA, Shah JV, Mykytyn K, et al. Cilioplasm is a cellular compartment for calcium signaling in response to mechanical and chemical stimuli. Cellular and molecular life sciences : CMLS. 2014;71(11):2165–2178. doi: 10.1007/s00018-013-1483-1. This study documents ciliary Ca2+ changes in proximal tubule cells in response to changes in fluid shear stress and receptor activation.
- 53.Rodat-Despoix L, Hao J, Dandonneau M, Delmas P. Shear stress-induced Ca mobilization in MDCK cells is ATP dependent, no matter the primary cilium. Cell calcium. 2013;53(5–6):327–337. doi: 10.1016/j.ceca.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 54.Lazarowski ER, Sesma JI, Seminario-Vidal L, Kreda SM. Molecular mechanisms of purine and pyrimidine nucleotide release. Advances in pharmacology. 2011;61:221–261. doi: 10.1016/B978-0-12-385526-8.00008-4. [DOI] [PubMed] [Google Scholar]
- 55.Svenningsen P, Burford JL, Peti-Peterdi J. ATP releasing connexin 30 hemichannels mediate flow-induced calcium signaling in the collecting duct. Frontiers in physiology. 2013;4:292. doi: 10.3389/fphys.2013.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Haefliger JA, Krattinger N, Martin D, Pedrazzini T, Capponi A, Doring B, et al. Connexin43-dependent mechanism modulates renin secretion and hypertension. J Clin Invest. 2006;116(2):405–413. doi: 10.1172/JCI23327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hills CE, Kerr MI, Wall MJ, Squires PE. Visfatin reduces gap junction mediated cell-to-cell communication in proximal tubule-derived epithelial cells. Cell Physiol Biochem. 2013;32(5):1200–1212. doi: 10.1159/000354519. [DOI] [PubMed] [Google Scholar]
- 58.Sainio K, Gilbert SF, Lehtonen E, Nishi M, Kumar NM, Gilula NB, et al. Differential expression of gap junction mRNAs and proteins in the developing murine kidney and in experimentally induced nephric mesenchymes. Development. 1992;115(3):827–837. doi: 10.1242/dev.115.3.827. [DOI] [PubMed] [Google Scholar]
- 59.Stoessel A, Himmerkus N, Bleich M, Bachmann S, Theilig F. Connexin 37 is localized in renal epithelia and responds to changes in dietary salt intake. American journal of physiology Renal physiology. 2010;298(1):F216–F223. doi: 10.1152/ajprenal.00295.2009. [DOI] [PubMed] [Google Scholar]
- 60.Zhang JT, Nicholson BJ. Sequence and tissue distribution of a second protein of hepatic gap junctions, Cx26, as deduced from its cDNA. The Journal of cell biology. 1989;109(6 Pt 2):3391–3401. doi: 10.1083/jcb.109.6.3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hanner F, Lam L, Nguyen MT, Yu A, Peti-Peterdi J. Intrarenal localization of the plasma membrane ATP channel pannexin1. Am J Physiol Renal Physiol. 2012;303(10):F1454–F1459. doi: 10.1152/ajprenal.00206.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bjaelde RG, Arnadottir SS, Overgaard MT, Leipziger J, Praetorius HA. Renal epithelial cells can release ATP by vesicular fusion. Front Physiol. 2013;4:238. doi: 10.3389/fphys.2013.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bailey MA, Imbert-Teboul M, Turner C, Marsy S, Srai K, Burnstock G, et al. Axial distribution and characterization of basolateral P2Y receptors along the rat renal tubule. Kidney Int. 2000;58(5):1893–1901. doi: 10.1111/j.1523-1755.2000.00361.x. [DOI] [PubMed] [Google Scholar]
- 64.Bailey MA, Imbert-Teboul M, Turner C, Srai SK, Burnstock G, Unwin RJ. Evidence for basolateral P2Y(6) receptors along the rat proximal tubule: functional and molecular characterization. Journal of the American Society of Nephrology : JASN. 2001;12(8):1640–1647. doi: 10.1681/ASN.V1281640. [DOI] [PubMed] [Google Scholar]
- 65.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50(3):413–492. [PubMed] [Google Scholar]
- 66.Filipovic DM, Adebanjo OA, Zaidi M, Reeves WB. Functional and molecular evidence for P2X receptors in LLC-PK1 cells. Am J Physiol. 1998;274(6 Pt 2):F1070–F1077. doi: 10.1152/ajprenal.1998.274.6.F1070. [DOI] [PubMed] [Google Scholar]
- 67.Turner CM, Vonend O, Chan C, Burnstock G, Unwin RJ. The pattern of distribution of selected ATP-sensitive P2 receptor subtypes in normal rat kidney: an immunohistological study. Cells Tissues Organs. 2003;175(2):105–117. doi: 10.1159/000073754. [DOI] [PubMed] [Google Scholar]
- 68.Goncalves RG, Gabrich L, Rosario A, Jr, Takiya CM, Ferreira ML, Chiarini LB, et al. The role of purinergic P2X7 receptors in the inflammation and fibrosis of unilateral ureteral obstruction in mice. Kidney Int. 2006;70(9):1599–1606. doi: 10.1038/sj.ki.5001804. [DOI] [PubMed] [Google Scholar]
- 69.Cureton DK, Massol RH, Whelan SP, Kirchhausen T. The length of vesicular stomatitis virus particles dictates a need for actin assembly during clathrin-dependent endocytosis. PLoS pathogens. 2010;6(9):e1001127. doi: 10.1371/journal.ppat.1001127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Russo LM, Sandoval RM, McKee M, Osicka TM, Collins AB, Brown D, et al. The normal kidney filters nephrotic levels of albumin retrieved by proximal tubule cells: retrieval is disrupted in nephrotic states. Kidney Int. 2007;71(6):504–513. doi: 10.1038/sj.ki.5002041. [DOI] [PubMed] [Google Scholar]
- 71.Tojo A, Endou H. Intrarenal handling of proteins in rats using fractional micropuncture technique. Am J Physiol. 1992;263(4 Pt 2):F601–F606. doi: 10.1152/ajprenal.1992.263.4.F601. [DOI] [PubMed] [Google Scholar]
- 72.Gekle M. Renal tubule albumin transport. Annu Rev Physiol. 2005;67:573–594. doi: 10.1146/annurev.physiol.67.031103.154845. [DOI] [PubMed] [Google Scholar]
- 73.Tenten V, Menzel S, Kunter U, Sicking EM, van Roeyen CR, Sanden SK, et al. Albumin is recycled from the primary urine by tubular transcytosis. Journal of the American Society of Nephrology : JASN. 2013;24(12):1966–1980. doi: 10.1681/ASN.2013010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dickson LE, Wagner MC, Sandoval RM, Molitoris BA. The proximal tubule and albuminuria: really! Journal of the American Society of Nephrology : JASN. 2014;25(3):443–453. doi: 10.1681/ASN.2013090950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schurman SJ, Scheinman SJ. Inherited cerebrorenal syndromes. Nature reviews Nephrology. 2009;5(9):529–538. doi: 10.1038/nrneph.2009.124. [DOI] [PubMed] [Google Scholar]
- 76.Rbaibi Y, Cui S, Mo D, Carattino M, Rohatgi R, Satlin LM, et al. OCRL1 Modulates Cilia Length in Renal Epithelial Cells. Traffic. 2012;13(9):1295–1305. doi: 10.1111/j.1600-0854.2012.01387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Luo N, West CC, Murga-Zamalloa CA, Sun L, Anderson RM, Wells CD, et al. OCRL localizes to the primary cilium: a new role for cilia in Lowe syndrome. Human molecular genetics. 2012;21(15):3333–3344. doi: 10.1093/hmg/dds163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Coon BG, Hernandez V, Madhivanan K, Mukherjee D, Hanna CB, Barinaga-Rementeria Ramirez I, et al. The Lowe syndrome protein OCRL1 is involved in primary cilia assembly. Human molecular genetics. 2012;21(8):1835–1847. doi: 10.1093/hmg/ddr615. [DOI] [PubMed] [Google Scholar]