Summary
Modulation of corticostriatal synaptic activity by dopamine is required for normal sensorimotor behaviors. After loss of nigrostriatal dopamine axons in Parkinson's disease, l-DOPA and dopamine D2-like receptor agonists are used as replacement therapy, although these drugs also trigger sensitized sensorimotor responses including dyskinesias and impulse control disorders. In mice, we lesioned dopamine projections to left dorsal striatum and assayed unilateral sensorimotor deficits with the corridor test as well as presynaptic corticostriatal activity with the synaptic vesicle probe, FM1-43. Sham-lesioned mice acquired food equivalently on both sides, while D2 receptor activation filtered the less active corticostriatal terminals, a response that required coincident co-activation of mGlu-R5 metabotropic glutamate and CB1 endocannabinoid receptors. Lesioned mice did not acquire food from their right, but overused that side following treatment with l-DOPA. Synaptic filtering on the lesioned side was abolished by either l-DOPA or a D2 receptor agonist, but when combined with a CB1 receptor antagonist, l-DOPA or D2 agonists normalized both synaptic filtering and behavior. Thus, high-pass filtering of corticostriatal synapses by the coordinated activation of D2, mGlu-R5, and CB1 receptors is required for normal sensorimotor response to environmental cues.
Introduction
While a variety of neurons die during the course of Parkinson's disease (PD) (Sulzer and Surmeier, 2013), the sensorimotor deficits associated with the disease are attributed to the death of dopamine (DA) neurons of the substantia nigra (SN) (Fahn and Sulzer, 2004), as demonstrated by the efficacy of treatment by the DA precursor, l-3,4-dihydroxyphenlalanine (l-DOPA) (Birkmayer and Hornykiewicz, 1961) and by D2-class DA receptor (D2-R) agonists. DA replacement therapies can however trigger excessive behavioral responses to environmental stimuli (Weintraub and Nirenberg, 2013) including dyskinesias (Fahn, 2005) and impulse control disorders (Voon et al., 2011). These responses increase in incidence and severity of these responses during prolonged therapy (Fahn, 2000), but sensitized responses to DA agonists occur immediately after the first administration of the drug to DA lesioned animals (Cenci et al., 1998; Morelli et al., 1989; Nadjar et al., 2009), and dyskinesias can be elicited from the first dose of l-DOPA in patients with inherited defects in DA synthesis (Pons et al., 2013). It is thus widely suspected that a stage is set for excessive behavioural responses by compensatory changes due to the loss of DA, and that DA agonists then trigger the activation of these undesired behaviors.
In normal motor striatum, DA participates in a “synaptic microcircuit” in which layer V/VI cortical pyramidal neurons, which fire at ~10Hz during phasic activity (Costa et al., 2006; Stern et al., 1997), and thalamic glutamatergic projections form classical excitatory synapses on the heads of dendritic spines of medium spiny neurons (MSNs). DA is released from nearby substantia nigra pars compacta (SNc) nigrostriatal axonal release sites (Nirenberg et al., 1996). This “synaptic microcircuit” modulates corticostriatal activity of striatonigral direct pathway MSNs that express D1 receptors (D1-Rs) and initiate specific motor signals by pausing tonic activity of substantia nigra reticulata output neurons (“go” signals) and striatopallidal indirect pathway MSNs that express D2-Rs and are thought to suppress competing motor networks (“no-go” signals) (Cui et al., 2013; Kravitz et al., 2010). DA depresses the corticostriatal excitation to D2-R expressing indirect pathway neurons, and has little or no direct effect on corticostriatal inputs to D1-R direct pathway neurons (Wang et al., 2013), but rather can exert a postsynaptic response (Yagishita et al., 2014) that appears to be due in part to activation of a circuit involving cholinergic receptors (Wang et al., 2013). D2-Rs on corticostriatal presynaptic terminals (Wang and Pickel, 2002) may also inhibit synaptic vesicle fusion (Bamford et al., 2008; Bamford et al., 2004b), although ascribing actions clearly to D2-R at particular sites within the striatum has been challenging.
In most studies, the D2-R mediated inhibition of excitatory corticostriatal transmission has been characterized as long-term depression (LTD), a form of long-lasting activity dependent plasticity implicated in motor learning and adaptive motor responses (Andre et al., 2010; Atwood et al., 2014; Cepeda et al., 2001; Hsu et al., 1995; Maura et al., 1988). LTD at corticostriatal synapses requires co-activation of D2-Rs and group-1 metabotropic glutamate receptors (mGlu-R1), encompassing mGlu-R1 and mGlu-R5 subtypes. In the most widely used LTD protocol, high frequency-evoked LTD (HFS: 100 Hz) engages convergent activity of D2-R and mGlu-R1 that depolarize MSN (Plotkin et al., 2013; Wang et al., 2012; Yin and Lovinger, 2006). However, LTD evoked by a more physiologically relevant stimulus pattern, which has been labelled low frequency stimulation (LFS: 10 Hz), requires D2-R but not mGluR-1 activation, and occurs in the absence of MSN depolarization (Ronesi and Lovinger, 2005). Both HFS and LFS LTD protocols require endocannabinoid (eCB) released from D2-R expressing MSNs to bind presynaptic corticostriatal CB1-Rs that presynaptically inhibit corticostriatal synaptic activity (Beltramo et al., 2000; Cadogan et al., 1997; Gerdeman and Lovinger, 2001; Giuffrida et al., 1999; Glass and Felder, 1997; Gubellini et al., 2002; Pertwee and Wickens, 1991).
In contrast to the regulation of LTD by DA in normal animals, the means by which DA replacement following DA lesion regulates cortico-basal ganglia circuits and motor response to environmental stimuli are much less understood. The ability to induce corticostriatal LTD at indirect pathway neurons following DA depletion is lost but reinstated by L-DOPA (Thiele et al., 2014), consistent with a role for DA replacement in inhibiting presynaptic corticostriatal transmission following DA lesion. To specifically examine such presynaptic changes at the corticostriatal synapse, we have adapted optical methods that measure synaptic vesicle fusion: our previous studies using this approach demonstrate that D2-R inhibition is not uniform at corticostriatal synapses, as both DA and D2-R agonists specifically inhibit the less active presynaptic terminals, while the most active terminals evade inhibition and are “passed”, allowing them to activate basal ganglia motor circuits, a response described as a high-pass filter (Bamford et al., 2008; Bamford et al., 2004b; Wang et al., 2012).
To determine how D2-R modulation of corticostriatal circuits is altered after DA lesion followed by DA replacement, we lesioned DA input in the left striatum, which provides a means to compare in the same animal presynaptic activity in the lesioned and corresponding intact striatum. Consistent with previous studies (Bamford 2004a), DA lesion caused a loss of D2-R circuit presynaptic filtering of corticostriatal activity along with a loss of sensorimotor behavior on the corresponding contralateral side. We find that corticostriatal filtering occurs via activation D2Rs and retrograde inhibition at CB1 endocannabinoid receptors, which is dependent on mGlu-R5 metabotropic glutamate receptors. Importantly, l-DOPA administration to hemi-lesioned mice elicited a clear over-compensation of sensorimotor behavior selectively on the previously neglected side, as well as excessive presynaptic inhibition and a consequent loss of normal presynaptic filtering by D2-Rs on the lesioned side. We further discovered that l-DOPA's over-compensation of both lateralized behavior and presynaptic inhibition in lesioned mice was normalized by a CB1-R antagonist.
Experimental Procedures
Animals
Experimental procedures were performed in accordance and approval by the Institutional Animal Care and Use Committee of Columbia University. C57BL/6J mice (n = 185), aged 12-16 weeks, were obtained from Jackson Labs (Bar Harbor, ME). To measure synaptic responses following behavioral tasks, mice were decapitated and 300-μm thick slices prepared on a vibratome on an angled agar block of 30° (Fig. 1A), producing a 60° section from horizontal after sectioning and allowed to recover for 1 h before optical analysis at room temperature in oxygenated [95% O2, 5% CO2] ACSF containing the following (in mM): 125 NaCl, 2.5 KCl, 26 NaHCO3, 0.3 KH2PO4, 2.4 CaCl2, 1.3 MgSO4, 0.8 NaH2PO4, 10 glucose (pH 7.2–7.4, 292–296 mOsm/L).
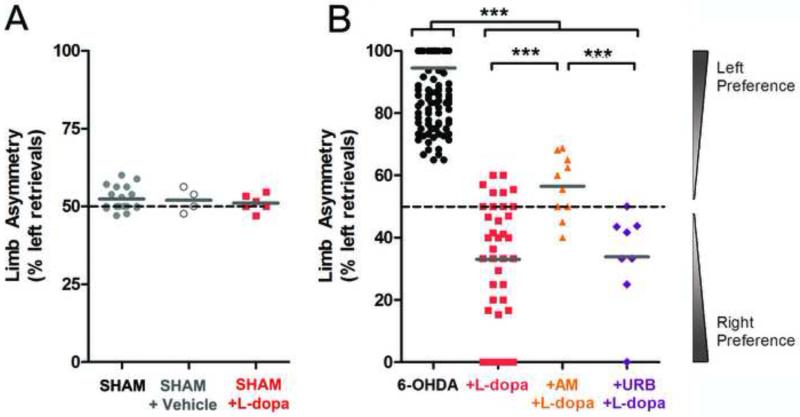
Figure 1. Behavioral response of sham and DA lesioned mice to l-DOPA and eCB receptor modulators.
(A) Sham control mice exhibited no preference for use of the left limb. Subsequent treatment with vehicle or l-DOPA did not affect behavioral response. (B) 6-OHDA injected mice exhibited a strong preference for use of the left limb (n = 68). The following day, mice were injected with l-DOPA i.p. (5 mg/kg, 30 min prior to retesting; n = 41) or L-DOPA with either AM251 (1 mg/kg, n = 10) or URB597 (3 mg/kg; n = 8) 15 min prior to l-DOPA. The mice treated with l-DOPA alone showed an overcompensation of use of the affected limb. The mice treated with AM251 prior to l-DOPA displayed a normalization of right limb use without overcompensation. Treatment with URB597 prior to l-DOPA had no effect on limb asymmetry. *** p < 0.001, Mann-Whitney test.
Drug administration
In some cases, animals were injected with the CB1-R antagonist, AM251 (1 mg/kg; Tocris Bioscience), or with the fatty acid amide hydrolase (FAAH) inhibitor, URB597 (3 mg/kg; Tocris Bioscience), to block the breakdown of the striatal cannabinoid, arachidonoylethanolamide (AEA) (Mackie and Stella, 2006) in 5% Tween-80 in saline and sonicated immediately prior to intraperitoneal (i.p.) injection. To provide saturation of the receptor, the level of AM251 administered was 10-fold higher than the reported ED50 in vivo (Gatley et al., 1996). Animals not receiving AM251 or URB597 were injected with equivalent amount of vehicle. Based on the drug's pharmacokinetic properties (Kathuria et al., 2003; Patel and Hillard, 2006), AM251 or URB597 were administered 15 min prior to treatment with l-DOPA methyl ester (5 mg/kg; Sigma-Aldrich) and benserazide (12 mg/kg; Sigma-Aldrich), to inhibit metabolism in peripheral tissues, in saline with 0.2% ascorbic acid. Mice not receiving l-DOPA received i.p. injection of benserazide in saline (vehicle).
6-OHDA lesion
We used 6-OHDA (Regis Technologies) to induce unilateral intrastriatal lesions, to compare intact and lesioned hemispheres in the same animal (Sauer and Oertel, 1994). All animals were able to eat, drink, and care for themselves (Cenci and Lundblad, 2007; Robinson et al., 2001). Thirty minutes prior to 6-OHDA, mice received desipramine (15 mg/kg, i.p.; Sigma-Aldrich) to protect noradrenergic terminals. Mice were anesthetized with ketamine (80 mg/kg) and xylazine (7 mg/kg) in saline (0.15-0.20 mL, i.p.) and positioned in a Kopf stereotaxic apparatus. A midline scalp incision was made, a burr hole (AP: +0.9, ML: +2.2 mm from bregma) marked, and a hole drilled with a Dremel tool. 6-OHDA (5 mg/mL) in saline and 0.02% ascorbic acid was infused by a mini-pump. A cannula was inserted into the striatum at AP: +0.9 mm; ML: +2.2 mm; DV: −2.5 mm in relation to dura. 6-OHDA solution was infused at 0.5 μl/min for 6 min (total dose: 15 μg). The cannula was left in place for 5 min to prevent backflow and reduce intracranial pressure before being slowly withdrawn. Sham control mice received the same injections in the absence of 6-OHDA. Mice recovered for 3 weeks before behavioral testing when dopaminergic cell loss and striatal terminal degeneration is maximal (Jeon et al., 1995).
Behavioral protocol
Forelimb asymmetries were quantified by corridor test (Dowd et al., 2005; Grealish et al., 2010), with mice placed individually in a corridor (60 cm long, 4 cm wide and 15 cm high) lined on both sides with 10 pairs of pots, each containing 3 sugar pellets. For two days prior to testing, mice were food-restricted (1.5 g/d) and habituated to the corridor apparatus, sugar pellets, and vehicle injections. On the test day, the number of sugar pellets retrieved from the left and right sides were counted over 10 min. The following week, the mice were re-habituated to the apparatus and received saline i.p. for two days. Limb preference to vehicle or l-DOPA was scored the following day. l-DOPA was administered as above 30 min prior to behavioural testing.
Imaging corticostriatal activity with FM1-43
Electrical stimulation was by Grass Stimulator (West Warwick, RI) through a stimulation isolator (AMPI, Jerusalem, Israel) and monitored by a Tektronix TDS 3014B digital oscilloscope (Beaverton, OR). FM1-43 (8 μM) was loaded into and released from corticostriatal terminals by electrical stimulation of layer V-VI in the M1 motor cortex (Fig. 1) using 350 μA, 200 μs pulses via a twisted tungsten bipolar electrode at 10 Hz for 10 min (Bamford et al., 2004a; Bamford et al., 2004b; Joshi et al., 2009; Wang et al., 2012).
Following terminal loading, slices were superfused with AD-7 (100 μM) for 20 min to remove adventitious staining. For stimulation-dependent destaining, pulse trains were again delivered to the cortex in the presence of AD-7 (100 μM) (Kay et al., 1999). A stimulus frequency of 10 Hz was used in all experiments, consistent with physiological firing (Stern et al., 1997). Stimulation-dependent destaining approximated 1st degree kinetics and was characterized by the destaining half-time of release (t1/2), the time required for fluorescent decay to half its original value.
To isolate effects of glutamate receptors and inhibit circuit responses, except where noted, we included the AMPA-R antagonist, NBQX (10 μM), the NMDA antagonist, AP-5 (50 μM), and the specific mGlu-R1 antagonist, CPCCOEt (40 μM) (Nicoletti et al., 2011; Niswender and Conn, 2010) in the ACSF superfusion during loading and unloading protocols. Drugs were applied by superfusion for 10 min prior to imaging: (+/−) quinpirole (D2-R agonist; 0.5 μM), (S)-sulpiride (D2-R antagonist; 10 μM), AM251 (CB1-R antagonist; 2 μM), WIN-55 212-2 (CB1-R agonist; 1 μM), and MPEP (mGlu-R5 antagonist; 40 μM) were obtained from Tocris Bioscience, Sigma-Aldrich, and AG Scientific.
Optical data analysis
Fluorescent corticostriatal terminals in the dorsolateral striatum were visualized using a Prairie multiphoton laser-scanning microscope used a titanium-sapphire laser (excitation 900 nm/emission 625 nm) and 40x water-immersion objective (Olympus). Images were captured in 16-bit, 75.2 x 75.2 μm ROI at 512 x 512 pixel resolution at 35 sec intervals using Prairie View 4.0.0.50 software. To compensate for z-axis shift, a z-series of five images, separated by 1 μm in the z-axis, was obtained for each imaging period. The time series of images was analyzed for changes in presynaptic terminal fluorescence using Image J (National Institutes of Health, Rockville, MD) (Bamford et al., 2004b; Zakharenko et al., 2001). Criteria for inclusion were (1) spherical shape, (2) fluorescence two standard deviations above background, (3) destaining greater than background fluorescence loss in non-stimulated controls. Image J aligned and combined the five image z-series for each time interval and the Multiple Thresholds plug-in (Damon Poburko, Stanford University) identified puncta of 0.5-1.5 μm in diameter. The intensity of the FM 1-43 fluorescence (excluding identified puncta) was measured over the course of the time series and Image J subtracted background fluorescence. The halftime of fluorescence intensity decay during destaining (t1/2) was determined using SigmaPlot software (SPSS, Chicago, IL). Nearness of fit to first-order kinetics was determined using A = 100*EXP (ln(0.5)*t/t1/2), an integrated form of the first-order kinetics equation, -d[A]/dt = k[A].
Electrophysiology and cyclic voltammetry
Cyclic voltammetry measurements of evoked DA release were as reported (Hernandez et al., 2012). Whole-cell patch clamp recordings were performed in slices perfused with normal ACSF (2 mL/min). Pipettes (3-5 MΩ) were filled with (in mM): 115 K-gluconate, 10 HEPES, 2 MgCl2, 20 KCl, 2 MgATP, 1 Na2-ATP, 0.3 GTP, pH = 7.3; 280 ± 5 mOsm. Current clamp recording were performed with a Axopatch 200B amplifier (Molecular Devices, Forster City, CA) and digitized at 10 kHz with a Digidata 1440A (Molecular Devices). Data were acquired using Clampex 10.2 software (Molecular Devices). In each cell, input resistance (measured by - 100 pA, 100 ms duration hyperpolarizing pulses) and resting membrane potential were monitored throughout the recording.
Western blot analysis
Following optical experiments, dorsal striata were removed from both hemispheres of each slice and sonicated in 75 μL of 1% SDS. Protein concentration was determined by the BCA method (Pierce). 15 μg were loaded in each well of a 15% bis-acrylamide gel and run at 180 V for 1 hr or until protein ladder markers (Fisher) were well separated and transferred to a polyvinylidene fluoride (PVDF) membrane at 40 V for 3 hr or at 10 V overnight. Membranes were washed in 1x Tris-buffered saline (TBS)/0.05% Tween-20, blocked in 5% milk and incubated with primary antibodies against the DA transporter (anti-rat DAT; 1:1000), tyrosine hydroxylase (anti-mouse TH; 1:5000), and β-actin (anti-mouse; 1:10000) (Millipore). Horseradish peroxidase (HRP)-conjugated goat secondary antibodies against mouse (Thermo Scientific) and rat (Novus) were used at 1:10000. Protein was detected by incubating membranes for 5 min with HRP substrate (Millipore), and Image J was used to determine the band intensity. Data are reported as average of mean ± SEM of signal intensity of the DA-lesioned hemisphere as percentage of signal intensity of the DA-intact hemisphere.
Dil Labeling
To observe corticostriatal axon projections, mice were anesthetized with ketamine/xylazine (80-100 mg/kg / 5-10mg/kg) and perfused with PBS for 4 mL/min for 3 min, followed by perfusion of 2% PFA for 3 min, post-fixed in 2% PFA for 24-48 h and stored in PBS. The brain was sectioned into 100 μm slices at the same angle as for the acute slice recording preparation, and collected in PBS.
Dil crystals (1,1'-dioctadecyl-3,3,3',3'-tetramethylindocarbocyanine perchlorate; Molecular Probes) were applied to the slices in layer V/VI under a dissecting microscope using a borosilicate glass micropipette (Kim et al., 2007). Slices were mounted using 4',6-diamidino-2-phenylindole (DAPI) fluoromount (Southern Bioscience). Dil crystals were allowed to diffuse at room temperature. Images were taken 5 days following deposition of Dil crystals using a Leica DM6000 confocal laser scanning microscope with 561 nm DPSS laser with a 20x oil-immersion lens at 1024 × 1024 pixel resolution. Serial stack images with 0.5 μm step size were projected to reconstruct images.
Statistical Analysis
For optical studies, the t1/2 value of each punctum was determined for each slice. Data are reported as median ± SEM, with n = number of slices. Each slice produced >15 puncta and 3 slices were obtained from each mouse. The ’box-and-whisker‘ indicate the distribution of median t1/2 values from each slice preparation, with the median of the median values indicated with a cross hatch, the ends of the box indicating the 25th and 75th percentiles, and the whiskers indicate the 10th and 90th percentiles.
Population distributions were compared using the non-parametric Mann-Whitney test and all data from each punctum within each experimental group are displayed in normal probability plots where the t1/2 of every punctum (y-axis) is plotted against the standard deviations (SD) from the median t1/2 (x-axis). As these distributions are often non-normal as determined by the Kolmogorov-Smirnov normality test, the distributions are analyzed in separate terciles: faster-destaining terminals (more than one SD below the median), intermediate- destaining terminals (less than one SD below and above the median), and slower- destaining terminals (more than one SD above the median). Faster- destaining terminals have a high probability of release and were considered “more active”, while slower- destaining terminals were “less active”. As multiple comparisons between groups require correction for increased risk of type I error, a Bonferroni adjustment to p-values was performed: comparisons between median values are made within each tercile/subgroup. In normal probability plots, we compare 3 to 4 groups within each tercile, and so the Bonferroni adjusted significance level is determined as 1 - (1 - α)1/n, which is approximated by α / n, required an p < 0.005 or p <0.004, with the number of independent statistical tests (n) was 1+(3*3)=10 or 1+(3*4)=13, respectively.
For behavioral studies, limb asymmetry is displayed as percentage of sugar pellet retrievals from the left in relation to the total retrievals from both sides. Sham or 6-OHDA injected mice were compared before and after in vivo treatments (vehicle, l-DOPA, URB597+ l-DOPA, or AM251+ l-DOPA) using the Mann-Whitney test. A Bonferroni adjustment to p-values was performed: we compare limb asymmetry from 3 to 4 groups so the number of independent statistical tests required p < 0.005 or p < 0.004, respectively.
Results
Treatment withl-DOPA and cannabinoid blockade produced normal behavior
To examine sensorimotor behavior in a mouse model for PD, we used the corridor test, in which mice acquire sugar pellets placed in wells on the left and right sides: this test has to date provided the most accurate motor trial for correlation of motor behavior with unilateral DA lesion (Grealish et al., 2010). Sham-lesioned mice showed equivalent pellet retrieval on both sides (left: 52 ± 1%; n = 20; Fig. 1A), but 6-OHDA-lesioned mice showed a very strong preference for food retrieval ipsilateral to the lesion (left: 95 ± 9%; n = 68 mice, p <0.0001, Mann-Whitney test; Fig. 1B), a level of side preference reported to correspond to >80% loss of SNc neurons (Grealish et al., 2010).
One week following these initial behavioral tests, we repeated the corridor following in vivo administration of l-DOPA (5 mg/kg, i.p., 30 min prior to behavioural trials). Both l-DOPA and sham-lesioned mice received benserazide (12 mg/kg), an inhibitor of peripheral DOPA decarboxylase clinically administered to increase CNS l-DOPA; in rats, 10 mg/kg benserazide does not significantly alter endogenous DA levels (Jonkers et al., 2001; Shen et al., 2003). CB1-Rs have been previously shown to filter corticostriatal activity (Wang et al., 2012; Yin and Lovinger, 2006), and so 15-min prior to l-DOPA, subsets of the mice were administered the CB1-R antagonist, AM251 (1 mg/kg), or the fatty acid amide hydrolase (FAAH) inhibitor URB597 (3 mg/kg), which blocks eCB catabolism and elevates extrasynaptic eCBs (Kathuria et al., 2003).
As expected, sham-lesioned mice (Fig. 1A) injected with vehicle (left: 52 ± 2%, n = 4) or with l-DOPA (5 mg/kg, i.p., 30 min prior to behavioral assays) (left: 51 ± 1%, n = 6) showed equivalent pellet retrieval on both sides (Fig. 1A). In marked contrast, l-DOPA in DA-lesioned mice (Fig. 1B) caused a behavioral over-compensation so that pellet retrievals were now much higher on the previously neglected right side (left: 33 ± 3%, n = 41, p < 0.001, Mann-Whitney). DA-lesioned mice treated with URB597 prior to l-DOPA, maintained this right retrieval preference (left: 34 ± 6%; n = 8 mice; p < 0.0001, Mann-Whitney). Blockade of CB1-Rs by AM251 prior to l-DOPA, however, normalized the frequency of left and right retrievals in DA-lesioned mice (left: 57 ± 3%; n = 10 mice; p < 0.0005 compared to l-DOPA alone, Mann Whitney). This finding indicates that l-DOPA administered to mice with unilateral DA lesion drove an overuse of the previously neglected side that was dependent on CB1-R transmission. We thus hypothesized that the combination of l-DOPA and AM251 in vivo could recover normal presynaptic filtering following DA lesion.
Acute corticostriatal slice preparation
To address this hypothesis, we used the endocytic probe, FM1-43, to measure presynaptic activity at corticostriatal synapses, an approach that provides a means to measure retrograde presynaptic modulation while blocking postsynaptic AMPA, NMDA, and metabotropic glutamate receptors. We prepared 300-μm thick acute slices 30° from the coronal plane (Fig. 2A), i.e., 60° from the horizontal plane, to favor preservation of axons in the M1 motor cortex to the motor striatum which course in a rostral-ventral direction (Cowan and Wilson, 1994; Smeal et al., 2007; Wilson, 1987). While many corticostriatal axons were severed in this preparation, Dil label deposited in layer V/ VI motor cortex demonstrate that intact axonal projections to the motor striatum were preserved even in the substantially thinner 100 μm thick sections, which were used to provide better Dil signal (Fig. 2B).
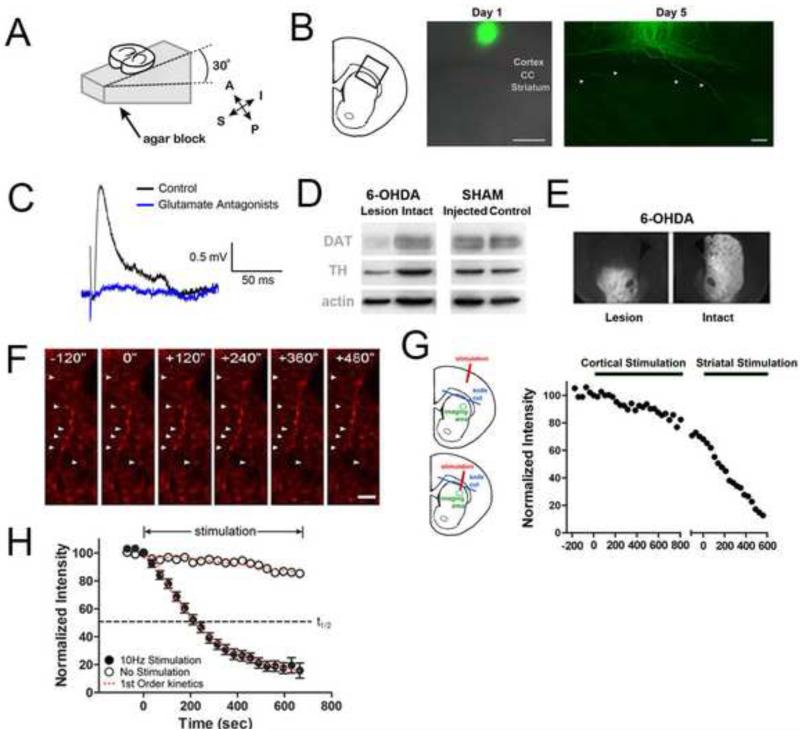
Figure 2. Loading and unloading of corticostriatal terminals with FM 1-43.
(A) Schematic of 30° angle coronal slice used to preserve cortical projections (A: anterior, P: posterior, S: superior, I: inferior). (B) Following deposition of Dil crystals in cortical layer V/VI neurons (center) in a 100 μm thick corticostriatal section, multiple intact cortical axons (arrows) display Dil label in dorsal striatum. Scale bar, 50 μm. (C) Excitatory postsynaptic potentials in motor striatal MSNs activated by a single pulse in layer V/VI prior to train stimuli used to load FM1-43 (black) and in the presence of the AMPA-R antagonist, NBQX (10 μM) and the NMDA antagonist, AP-5 (50 μM) (blue). (D) Representative western blot for DAT (70 kDA), TH (60 kDA), and β-actin (45 kDa) indicates that DA lesion by 6-OHDA produced a loss of 96 ± 4% of DAT expression and a loss of 82 ± 4% of TH expression (mean ± SEM, n = 39 paired slices). (E) Immunolabeling for DAT indicates that DA depletion is localized to dorsal striatum. (F) Electrical stimulation of motor cortex layer V in the presence of FM 1-43 labeled multiple puncta (arrows) in the dorsolateral striatum (left). Stimuli (10 Hz) beginning at t = 0 sec results in activity-dependent destaining. Scale bar, 5 μm. (G) Corticostriatal terminals were loaded with FM1-43 by 10 Hz stimuli in cortical layer V/VI. Following loading, a knife cut was made below the corpus callosum to destroy intact cortical axons that project to the dorsal striatum. Subsequent cortical layer V stimulation caused negligible FM1-43 destaining. Minor destaining was similar to that in unstimulated intact preparations, consistent with spontaneous release of FM1-43 from terminals or uncompensated bleaching (Wang 2012). Local striatal stimulation was required to unload FM1-43 from labeled corticostriatal terminals (right: striatal stimulation). (H) Averaged time-intensity analysis of fluorescent puncta shown in panel F. Stimulation of the motor cortex at 10 Hz (black) produced FM 1-43 destaining that followed first-order exponential kinetics. Minimal FM 1-43 destaining occurred when no stimuli (white) were applied.
We confirmed that there were functionally intact corticostriatal synaptic connections in the 300 μm slices by stimulating layer V/VI motor cortex and recording from MSNs in whole cell current clamp mode. Cortically-evoked EPSPs consistently arrived at the same short delay following a single pulse stimulus, consistent with a monosynaptic connection, and were abolished completely by NMDA and AMPA blockers (Fig. 2C), or by a knife cut made at the corpus callosum to sever corticostriatal fibers (not shown).
We conducted further tests to determine if there was current spread from the stimulus electrode in the cortex to the motor striatum in this preparation. In the presence of NMDA and AMPA blockers, the LFS used for presynaptic loading of FM1-43 (10 Hz for 10 min) produced no depolarization of the MSN resting potential (prior to train stimulation −82.4 ± 1.1 mV prior to train stimulation; −83.5 ± 1.4 mV after train stimulation, n = 10 neurons recorded in 10 different slices), indicating that there was no current spread from the stimulus electrode to MSNs. As previously described (Bamford et al., 2004b; Partridge et al., 2002), we also detected no evoked DA release in response to the loading stimulus in the cortex using cyclic voltammetry (not shown): this is in contrast to stimulus of the corpus callosum, where we have detected evoked DA release. In conclusion, the cortical LFS stimulus protocol stimulates corticostriatal presynaptic terminals of intact axons, but does not produce current spread that evokes either depolarization of MSNs or DA release.
Striatal DA lesions
To test the effects of DA depletion on behavior and synaptic selection, we unilaterally injected left striata with the DA neurotoxin, 6-OHDA, which destroys substantia nigra neurons that innervate that region (Ungerstedt, 1968), and injected the right striata with vehicle (sham-injections). We compared DAT and TH expression between injected and intact striata in sham and 6-OHDA-injected mice by western blot analysis of the dorsal striatum (Fig. 2D) on the same slices following the FM1-43 optical experiments described below. Immunoreactivity was not altered in sham control mice following injection of vehicle, but both DAT and TH were markedly reduced in the lesioned hemisphere of 6-OHDA-injected mice, with DAT levels reduced by 96 ± 4% and TH by 82 ± 4% (mean ± SEM, n = 39 paired slices). Immunofluorescence with anti-DAT antibodies revealed the specific localization of 6-OHDA-induced loss of DA to the injected dorsal striatum (Fig. 2E).
Optical measurement of corticostriatal presynaptic activity
We measured exocytic activity at individual presynaptic terminals in the motor striatum using the endocytic probe FM1-43 (Bamford et al., 2004b; Wong et al., 2011) in the presence of AMPA, NMDA, and mGlu-R1 receptor inhibitors to block postsynaptic response and polysynaptic circuits within the striatum. Stimulation of layers V/VI of the M1 motor cortex in the presence of FM1-43 resulted in endocytosis of the probe into synaptic vesicles within axonal boutons (diameter = 0.5-1.5 μm) imaged by multi-photon laser scanning microscopy (Bamford et al., 2004b; Wang et al., 2012). Following quenching of background fluorescence by ADVASEP-7 (Kay et al., 1999), re-stimulation of the cortex revealed FM 1-43 exocytosis of recycling synaptic vesicles from presynaptic sites (Fig. 2F). To confirm that the loading of FM1-43 corticostriatal presynaptic sites was due to local stimulation of axons and somatodendritic regions in the cortex rather than current spread from the electrode (Tehovnik et al., 2006), we measured striatal FM1-43 destaining with a knife cut between the cortex and striatum at the corpus callosum: FM1-43 did not destain under these conditions (10 Hz for 10 min). As expected, local stimulation of the striatum proximal to the knife cut produced destaining (Fig. 2G).
The stimulus-dependent reduction in fluorescence intensity of the puncta due to release of FM1-43 into the extracellular space followed first-order kinetics characteristic of synaptic vesicle fusion (Fig. 2H) (Ahmed and Siegelbaum, 2009; Bamford et al., 2004b). Presynaptic corticostriatal activity was characterized by the half-time (t1/2) required for terminal fluorescence to destain to half its initial value. Destaining of terminal in control slices stimulated at 10 Hz (t1/2 = 267 ± 16 sec; n = 20 slices) was similar to previously reported values (Bamford et al., 2004b).
D2-Rs exert a sensitization of corticostriatal inhibition in 6-OHDA mice
Lesion of DA in animal models has long been known to drive a “supersensitive” response to the activation of DA receptors (Feltz and De Champlain, 1972; Schultz and Ungerstedt, 1978), including an increased D2-R inhibition of MSN activity in 6-OHDA lesioned rats that is independent of D1-Rs (Hu et al., 1990). Mice treated with reserpine or targeted deletion of the Th gene in DA neurons (Bamford et al., 2004b; Zhou and Palmiter, 1995) show sensitized corticostriatal presynaptic inhibition by D2-Rs (Bamford 2004a). The effect of D2-R activation on corticostriatal synaptic vesicle exocytosis, however, has not been examined following DA lesion. We compared FM1-43 destaining in the DA-lesioned and intact hemispheres (Fig. 3A-D) at a range of exposures to the D2-R agonist, quinpirole. Corticostriatal release in DA lesioned striata was more responsive to 0.5 μM quinpirole than the intact hemisphere (Fig. 3E), consistent with the prior findings of a sensitized D2-R response to the agonist (Bamford 2002b), while inhibition was saturated at 1.0 μM quinpirole: we therefore used 0.5 μM quinpirole for experimental protocols.
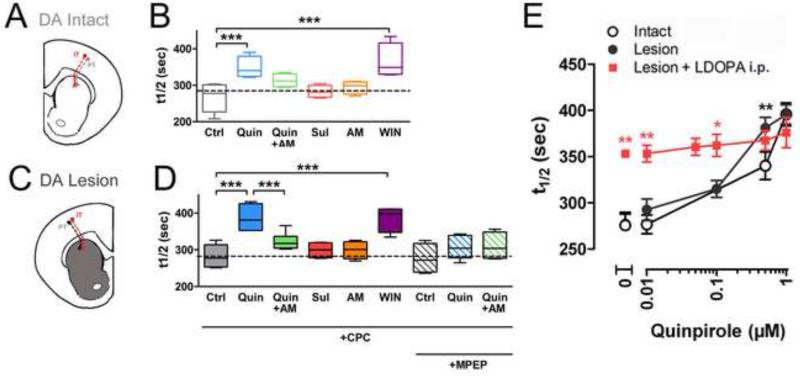
Figure 3. Chronic DA depletion sensitized responses to D2-R.
For box and whisker plots, the line within the box indicates average of median t1/2 values from the acute slices, the edges of the boxes represent 25th and 75th percentiles, and the whiskers indicate the 10th and 90th percentiles. (A) Diagram of corticostriatal projection on the intact side. (B) Distribution of median t1/2 values indicating corticostriatal presynaptic activity in slices from sham-lesioned striata (Ctrl) in the presence of AMPA, NDMA and the selective mGlu-R1 antagonist, CPCCOEt. The D2-R agonist, quinpirole (0.5 μM: dose response shown below) decreased release (i.e., increased t1/2 values), while the CB1-R antagonist, AM251 (AM) alone had no effect, but blocked the inhibition by quinpirole. The D2-R antagonist, sulpiride (Sul) had no effect. The CB1-R agonist, WIN55 212-2 (WIN) inhibited corticostriatal activity (n = 20 (Ctrl); 9 (Quin); 5 (Sul); 4 (WIN); 6 (AM); 7 (Quin+AM) * p < 0.05, ** p < 0.005, two-way ANOVA; *** p < 0.0001, Mann-Whitney test). (C) Diagram of corticostriatal projection on the lesioned side. (D) Distribution of median t1/2 values in slices from 6-OHDA-treated lesioned striata, in response to ligands as in panel B, and with the mGlu-R5 antagonist, MPEP (n = 4 (MPEP); 5 (Quin+MPEP); 4 (Quin+MPEP+AM), * p < 0.05, ** p < 0.005, two-way ANOVA; *** p < 0.0001, Mann-Whitney test). (E) Inhibition by quinpirole (0.5 μM) was enhanced in DA-depleted sections, indicating the induction of a hypersensitive D2-R response. l-DOPA administration in vivo (red) occluded the inhibitory effect of quinpirole at high concentrations of the agonist (n = 20 (Ctrl); 9 (Quin); 7 (L-dopa) * p < 0.05, ** p < 0.005, two-way ANOVA; *** p < 0.0001, Mann-Whitney test).
To investigate the role of CB1-R in D2-R responses, we compared the effects of CB1-R ligands in DA-lesioned and DA-intact hemispheres. The CB1-R agonist, WIN55 212-2 (1 μM), equivalently depressed corticostriatal release in both intact (t1/2 = 365 ± 11 sec; p <0.001) and lesioned hemispheres (t1/2 = 385 ± 16 sec; p <0.001). No significant effect was exerted by the CB1-R antagonist, AM251, at levels that would saturate CB-1R receptors (Gatley et al., 1997), (2 μM; DA intact: t1/2 = 294 ± 9 sec; DA lesion: t1/2 = 299 ± 12 sec), confirming a lack of CB1-R inhibition evoked by corticostriatal stimulation in the absence of D2-R activation (Wang et al., 2012).
To assess whether activation of CB1-Rs is required for presynaptic modulation by D2-Rs, we examined the combinatorial response of AM251 and quinpirole (0.5 μM). For the intact striata (Fig. 3B,D), quinpirole lost its ability to inhibit release in the presence of the CB1-R antagonist. On the lesioned side that exhibits a sensitized response to quinpirole (Fig. 3E), there was significant block of quinpirole's inhibition by AM251 (Fig. 3D), indicating that a large portion of quinpirole's inhibition of corticostriatal activity is mediated by activation of CB1-R. These data are consistent with the hypothesis that activation of presynaptic CB1-R receptors is driven by D2-R stimulation (Gerdeman and Lovinger, 2001; Wang et al., 2012).
We conducted the experiments on eCB/D2-R presynaptic inhibition in the presence of ionotropic glutamate receptor blockers (NBQX (10 μM), AP-5 (50 μM)) and the specific mGluR1 negative allosteric modulator, CPCCOEt (40 μM) (Nicoletti et al., 2011; Niswender and Conn, 2010), which does not block mGlu-R5 receptors. As presynaptic filtering occurred in the presence of these antagonists, we examined whether presynaptic inhibition by the CB1-R required mGlu-R5 receptors (Chen et al., 2011), which are highly expressed in MSNs (Nicoletti et al., 2011). We found that inhibition by quinpirole was blocked by the selective mGlu-R5 antagonist (Nicoletti et al., 2011), MPEP (40 μM; 307 ± 14 sec) (Fig. 3D). Thus, the effect of quinpirole on corticostriatal presynaptic inhibition in lesioned striata requires a combination of mGlu-R5 and D2-R activation, and when this is triggered by LFS of 10 Hz, it leads to activation of CB1 receptors that cause presynaptic inhibition. This response contrasts with quinpirole's effects on corticoaccumbens synapses, which were blocked by either mGlu-R1 or mGlu-R5 antagonists (Wang et al., 2012): the reason for the additional role for mGlu-R1 in the corticoaccumbens synapse is unknown, but D1-R can elicit presynaptic inhibition at those synapses, while in the dorsal striatum, presynaptic D1-R effects have only been observed following altered cholinergic signaling following in vivo exposure to psychostimulants (Bamford et al., 2008; Wang et al., 2012).
D2 and CB1 receptors selectively inhibit subsets of corticostriatal terminals
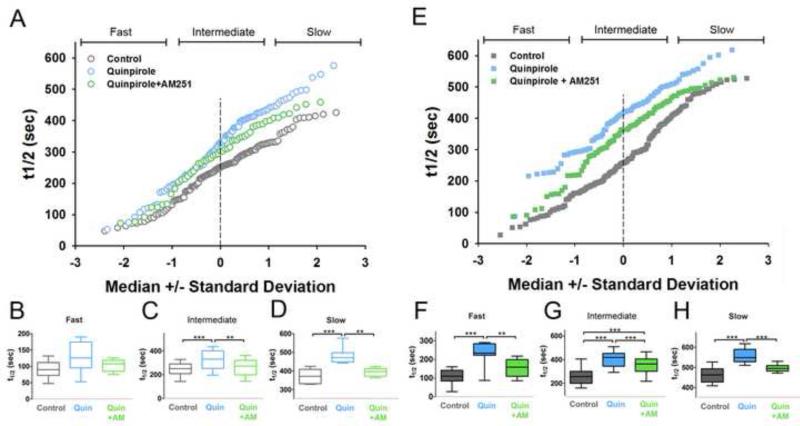
An important feature of the optical methods in these experimental designs is that they detect differences in presynaptic activity between individual synapses. The distribution of kinetics of individual corticostriatal terminals are represented in normal probability plots, in which the t1/2 of each punctum is plotted against its deviation from the median t1/2 value and normal distribution produces a straight line (Sulzer and Pothos, 2000; Van der Kloot, 1991).
In the DA-intact hemisphere, the D2-R agonist quinpirole exerted a “high-pass filter” by inhibiting intermediate- and slow-destaining terminals, while the fast-destaining (most active) terminals were unaffected (Fig. 4A-D), consistent with previous findings (Bamford et al., 2004a; Bamford et al., 2004b). In contrast, in the DA-lesioned hemisphere, quinpirole inhibited release from all terminals (Fig. 4E-H). Thus, similar to the D2-R sensitization in reserpine-treated and DA-deficient mice (Bamford et al., 2004b), DA lesion abolishes normal D2-R corticostriatal filtering.
Figure 4. DA depletion modifies D2 and CB1 receptor-modulation of corticostriatal release.
(A) The t1/2 value for each punctum is displayed on the y-axis as a normal probability plot: a straight line indicates a normal distribution. Values are shown for controls (grey), and in the presence of quinpirole (blue), and quinpirole + AM251 (green). (B) No significant change occurred in fast-destaining terminals. (C-D) The inhibitory effect of quinpirole (blue) on intermediate- and slow-destaining terminals was blocked by quinpirole + AM251 (green) (n = 20 (Ctrl); 7 (Quin); 7 (Quin+AM) ** p < 0.001, *** p < 0.0001, Mann-Whitney test). (E) t1/2 values in DA-depleted striatum (n = 14 (Ctrl); 9 (Quin); 7 (Quin+AM) ** p < 0.001, *** p < 0.0001, Mann-Whitney test). (F) Quinpirole inhibited destaining of fast, (G) intermediate, and (H) slow-destaining terminals. All terminal populations were blocked by AM251. (n = 6 to 8 slices; ** p < 0.001, *** p < 0.0001, Mann-Whitney test).
The CB1-R antagonist, AM251, which had no effect on its own (Fig. 3B, D), partially occluded quinpirole's inhibition in intact striata, as seen in the intermediate and slower terminals: consistent with a lack of effect by D2-R on faster terminals, there was no occlusion by AM251 (Fig. 4A-D). In contrast, in lesioned hemispheres while AM251 continued to partially block some inhibition by D2-Rs (Fig. 4E-H), it also effectively blocked D2-R-mediated inhibition of the faster terminals that was particular to the lesioned side. Thus, a portion of quinpirole's effects are due to CB1-R in both intact and DA lesioned hemispheres, and the combination of quinpirole and AM251 allowed the more active terminals to “pass”, restoring the normal high-pass characteristics of D2-R activation in intact striata to the lesioned side.
L-DOPA effects on corticostriatal presynaptic activity
To confirm if the behavioral overcompensation following l-DOPA was consistent with D2-R sensitization and loss of normal presynaptic filtering, some mice were immediately sacrificed for optical recordings following the corridor test. As expected, l-DOPA in vivo exerted no effect on presynaptic release in sham-lesioned mice or on the intact side of DA-lesioned mice (not shown). On the depleted side, l-DOPA administered in vivo decreased stimulus-dependent release from corticostriatal terminals (t1/2 = 349 ± 7 sec; p < 0.001, Mann-Whitney test), and sensitized the presynaptic inhibition by quinpirole (0.5 μM) (Fig. 3E, red squares), indicating that l-DOPA in vivo effectively activated D2-R.
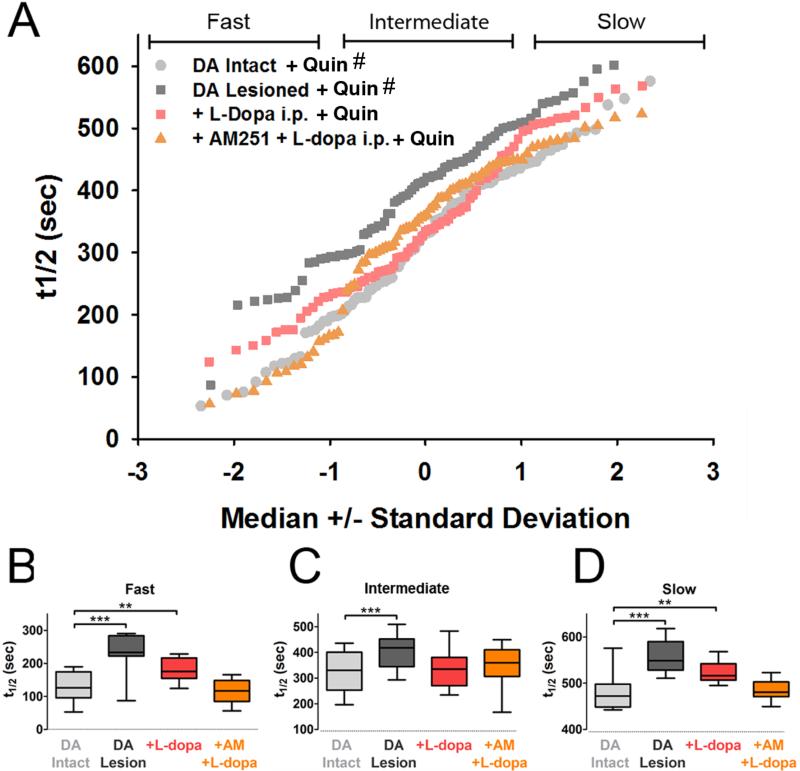
Consistent with the sensitized D2-R behavioral response on the lesioned side, quinpirole inhibited corticostriatal release more on the lesioned side than the intact side (Fig. 5, t1/2 = 360 ± 14 sec: compare dark and light grey). In lesioned striata, l-DOPA in vivo partially occluded quinpirole inhibition (Fig. 5, compare red and dark grey), indicating that the response to systemic l-DOPA was long-lasting and persisted in the slice preparation. Administration of l-DOPA in vivo did not reinstate normal quinpirole-induced D2-R high-pass filtering in the lesioned striata (Fig. 5: compare red points and light grey). However, animals treated with the combination of AM251 and l-DOPA in vivo (t1/2 = 343 ± 15 sec) displayed a complete restoration of normal D2-R high-pass filtering on the lesioned side (Fig. 5; compare orange and light grey), in which the fast-destaining terminals were no longer inhibited by D2-R activation (Fig. 5B).
Figure 5. l-DOPA in vivo modifies corticostriatal activity.
(A) Individual corticostriatal terminal kinetics. D2Rs were activated by quinpirole in vitro throughout the experiments. Destaining was slower in lesioned than sham-treated striatum. Pre-treatment with l-DOPA in vivo (red), enhanced corticostriatal destaining in lesioned striata, particularly by increasing synaptic vesicle fusion from the fast and slow-destaining terminals. (B-D), but in contrast to the intact side, quinpirole slowed the faster terminals in the lesioned side (B), so that normal high-pass filtering was absent. The activity in the lesioned hemisphere of mice administered AM251 prior to l-DOPA (orange) displayed a full recovery of quinpirole response identical to normal quinpirole response on the intact side. (B) t1/2 values of FM1-43 release from kinetics from fast, (C) intermediate, and (D) slow-destaining terminals. ** p < 0.01, *** p < 0.001, Mann-Whitney test. # indicates that the population is also displayed in Figure 4 and is redisplayed here for purposes of comparison.
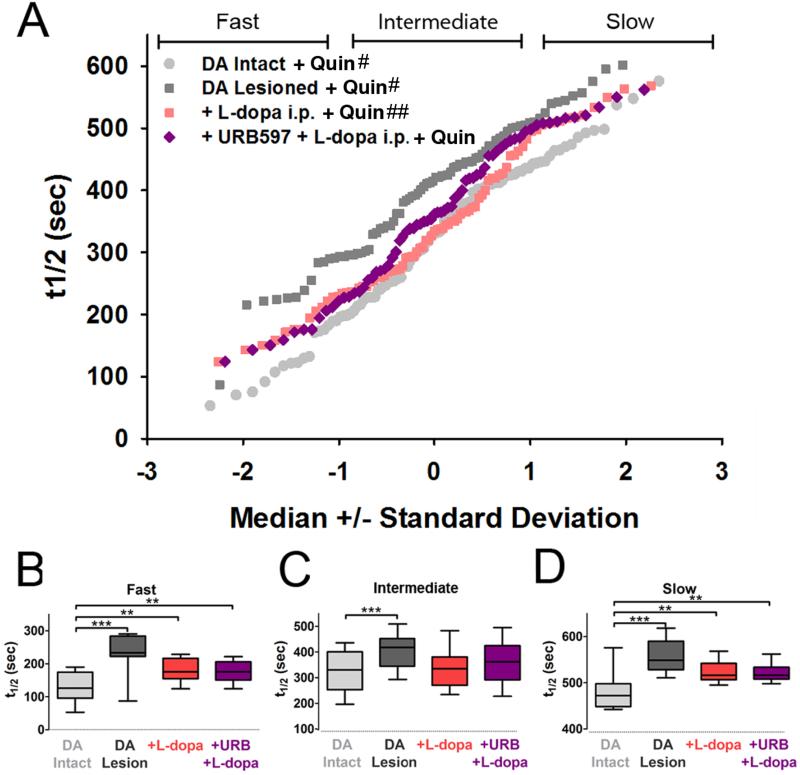
We examined a possible role for increased eCB levels in corticostriatal plasticity following 6-OHDA by treating mice with either l-DOPA alone or with a combination of URB597 and l-DOPA. URB597 had no effect following l-DOPA in vivo or quinpirole in vitro (t1/2 = 371 ± 10 sec; Fig. 6; compare purple and red). Thus, while eCB transmission was required for normal D2-R presynaptic filtering, the heightened response to l-DOPA following DA lesion was due to sensitized responses by D2-R and CB1-R rather that altered eCB degradation, and additional eCB availability did not further exacerbate the response to l-DOPA.
Figure 6. l-DOPA effects on corticostriatal terminal kinetics in the presence of D2-R activation and inhibition of endocannabinoid breakdown.
(A) Individual corticostriatal terminal kinetics. (B) t1/2 values for fast, (C) intermediate, and (D) slow-destaining terminals with treatments indicated (n = 20 (Ctrl-Nonlesion); 14 (Ctrl-Lesion) 7 (L-dopa) 6 (L-Dopa+URB); **p<0.005, ***p < 0.0001, Mann-Whitney test). # and ## indicates that the population is also displayed in Figure 4 or Figure 5 respectively and is redisplayed here for purposes of comparison.
Discussion
A central goal in neuroscience has been to reveal relationships between synaptic plasticity and behavior. Recent work, for example, indicates that LTD can mediate fear-conditioned learning (Nabavi et al., 2014). To elucidate the relationship between sensorimotor behavior and synaptic plasticity, we examined filtering of corticostriatal presynaptic activity by DA in normal mice and in a classical model of parkinsonism. Lesion of striatal DA in PD results in a loss of ability to engage in appropriate motor response to environmental cues. While substitution of DA by l-DOPA or D2-R agonists provides effective therapy, they also trigger undesired excessive behaviors in patients (Weintraub and Nirenberg, 2013). Here, we find parallel phenomena in DA hemilesioned mice, in that l-DOPA elicited an overactive sensorimotor response on the side corresponding to the DA lesion: differences between the two sides would in principle identify DA-regulated corticostriatal synaptic properties that mediate DA-dependent behavioral responses to environmental stimuli.
Our results indicate that that normal sensorimotor behavior relies on D2-R high-pass presynaptic filtering that in turn requires mGlu-R5 and CB1-R signalling. Consistent with previous studies in mice with intact or sham-lesioned striata, D2-R activation attenuated the excitatory drive in the majority of cortical inputs, but the most active cortical inputs were “passed” (Bamford et al., 2004b). We found that when corticostriatal filtering is normal, the presence of food as a sensory stimulus elicited equivalent acquisition on both sides. When DA was lesioned in the left striata, the D2-R inhibition of cortical inputs was absent, and as expected, mice neglected food presented on the corresponding right side. Consistent with a sensitized D2-R response following chronic DA depletion, unilaterally lesioned mice exhibited a sensitized presynaptic D2-R response on the lesioned left striata, so that DA reinstatement by l-DOPA elicited a preference for the previously neglected side. Similar to D2-R presynaptic filtering in prefrontal cortical terminals that synapse in the nucleus accumbens (Wang et al., 2012), in the intact striatum, D2-R mediated high-pass filtering of corticostriatal presynaptic terminals was blocked by CB1-R antagonists. Presynaptic inhibition by DA was sensitized and filtering was lost in DA lesioned striatum. However, when lesioned mice were administered l-DOPA with a CB1-R antagonist, normal corticostriatal filtering by D2-Rs was reinstated, and food retrieval again became symmetrical as in control or sham-lesioned mice.
To our knowledge, a behavioral consequence of corticostriatal synaptic plasticity has not previously been identified. Our data suggest that normal behavioral responses to environmental stimuli requires DA-mediated high-pass filtering of corticostriatal signalling that allows transmission of circuits driven by the most active cortical inputs. Conversely, excessive behavioral responses to environmental stimuli following DA depletion are due to excessive inhibitory responses driven by D2-R. This high-pass filtering of cortical inputs may explain at least in part how DA blocks synchronous activity of striatal neurons that otherwise occurs in PD models (Costa et al., 2006). We note, however, that while DA replacement and D2-R drive the sensitized responses, this apparently requires additional synaptic alterations that are established in sensorimotor circuitry in compensation to DA depletion, and that there are multiple candidates for such alterations, particularly via changes in D1-R signalling (Heiman et al., 2014).
Interaction of D2, mGlu-R5 and CB1 receptor activation regulates corticostriatal activity
A remarkable feature of presynaptic D2-R filtering noted in previous studies (Bamford et al., 2004a; Bamford et al., 2004b; Yin and Lovinger, 2006) is that it requires a coincidence of DA release and cortical activity driven at 10 Hz, while no filtering occurs if the cortical activity is driven at 1 Hz. The reason for this dependence on the stimulus frequency has not been clear. Surprisingly, we now find that D2-R mediated presynaptic filtering occurred in the presence of NMDA, AMPA, and mGlu-R1 antagonists. Rather, D2-R filtering required activation of mGlu-R5 receptors, which is mostly found extrasynaptically on MSNs (Paquet and Smith, 2003). As D2-Rs are also extrasynaptic receptors (Yung et al., 1995), both mGlu-R5 and D2-R on MSNs, as well as any D2-Rs found presynaptically on corticostriatal synapses (Wang and Pickel, 2002), would be activated by extrasynaptic transmission. While glutamate release from cortical axon terminals occurs at classical synapses, glutamate “spillover” occurs in the striatum when the cortex is driven at 10 Hz, as such LFS of motor cortex, or glutamate uptake blockers, decreases DA release by activating mGlu-R1 receptors on DA axons (Zhang and Sulzer, 2003). Our results suggest that DA affects corticostriatal presynaptic activity when corticostriatal synapses are highly active and coincident phasic DA neuron firing, which occurs with unexpected reward (Mirenowicz and Schultz, 1996), acts to drive extrasynaptic overflow by saturating the DA uptake transporter (Schmitz et al., 2003; Sulzer et al., 2010). Importantly, this plasticity occurs at cortical activity firing rates (~10 Hz) that occur during voluntary motor behavior (Costa et al., 2006) and also drive glutamate overflow (Zhang and Sulzer, 2003).
We find that the role for CB1-Rs in corticostriatal presynaptic inhibition is downstream from activation of mGlu-R5 and D2-R, as CB1-R antagonists block D2-R effects, while CB1-R agonists inhibit this activity without D2-R activation. In addition to any direct effects of DA on cortical terminal presynaptic D2-R, activation of D2-Rs on striatopallidal MSNs rapidly increases striatal eCB release, which inhibits corticostriatal synapses (Beltramo et al., 2000; Brown et al., 1990; Ferrer et al., 2003; Gerdeman and Lovinger, 2001; Giuffrida et al., 1999; Gubellini et al., 2002; Herkenham et al., 1991; Meschler and Howlett, 2001; Wang et al., 2012). Our results are consistent with evidence that activation of D2-R-expressing MSNs and mGlu-Rs elicit eCB release that acts as a retrograde messenger to produce LTD at corticostriatal synapses (Adermark et al., 2009; Plotkin et al., 2013). Most studies, in contrast, have used high frequency stimuli (generally 100 Hz) to induce a form of LTD that requires depolarization of MSNs by ionotropic glutamate receptors to open l-type calcium channels. The presynaptic filtering we report with 10 Hz stimuli is strikingly similar to corticostriatal LTD evoked by 10 Hz stimuli, which requires eCB transmission but not postsynaptic depolarization, NMDA, AMPA, or mGlu-R1 activation (Ronesi and Lovinger, 2005). It is possible that corticostriatal LTD is related to the inhibition of low-probability release (“slow”) terminals by D2-R, mGlu-R5, and CB1-R, a feature revealed by our optical methods.
As mGlu-R5 and D2-R are receptors that are located extrasynaptically, and as high corticostriatal activity can lead to spillover of glutamate that can activate extrasynaptic metabotropic glutamate receptors (Zhang and Sulzer, 2003), we propose that when glutamate and DA both engage in extrasynaptic or “social” neurotransmission, the combination allows transmission from the most active corticostriatal terminals while simultaneously inhibiting the less active synapses. This requirement for combinatorial synaptic activity could provide a “coincidence detector” sensorimotor learning mechanism, as synaptic selection would only occur during convergent extrasynaptic transmission of DA activated by environmental stimuli and extrasynaptic activation of mGlu-R5 during physiologically relevant levels of cortical activity. The means by which active terminals evade inhibition remains unknown, but a possibility is that that high calcium or cAMP levels may override eCB signalling. The sensitized D2-Rs and behavioral responses following DA lesion also suggest a mechanism whereby l-DOPA or D2-R agonists may trigger dyskinesias, impulse control disorders, or DA dysregulation syndrome in PD patients (Voon et al., 2011). If so, adjunctive therapies that modulate CB1-R or mGlu-R5 may be clinically beneficial.
highlights Wong et al.
Corticostriatal synaptic filtering is absent following dopamine lesion
Filtering is not reinstated by l-DOPA or D2 agonist, which overactivate behavior
With a CB1 receptor antagonist, l-DOPA or D2 agonists normalize synaptic filtering and behavior
filtering by D2, mGlu-R5, and CB1 receptors is required for normal sensorimotor behavior
Acknowledgements
Supported by the National Institute of Neurological Disorders and Stroke–National Institutes of Health (Grant #R01NS060803 to N.S.B. and #R01NS075222 to E.M.), Eunice Kennedy Shriver National Institute of Child Health and Human Development–National Institutes of Health (Grant # U54HD083091 to N.S.B.), Seattle Children's Hospital, Seattle, WA (N.S.B.), the National Institute on Neurological Disorders Udall Center of Excellence (NS38370 to D.S.), the National Institute on Drug Abuse (Grants DA07418 and DA10154 to D.S.) and the Parkinson's Disease and the JPB Foundations (D.S.). A.B. received fellowships from Swedish Research Council and Sweden America Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions: MW conducted the optical, western blot, and behavioral experiments. AB contributed to surgeries and experimental planning and analysis. SC and EVM conducted the patch clamp and cyclic voltammetry experiments. MW, NB, and DS designed and interpreted the experiments and wrote the manuscript.
References
- Adermark L, Talani G, Lovinger DM. Endocannabinoid-dependent plasticity at GABAergic and glutamatergic synapses in the striatum is regulated by synaptic activity. Eur J Neurosci. 2009;29:32–41. doi: 10.1111/j.1460-9568.2008.06551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed MS, Siegelbaum SA. Recruitment of N-Type Ca(2+) channels during LTP enhances low release efficacy of hippocampal CA1 perforant path synapses. Neuron. 2009;63:372–385. doi: 10.1016/j.neuron.2009.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andre VM, Cepeda C, Cummings DM, Jocoy EL, Fisher YE, William Yang X, Levine MS. Dopamine modulation of excitatory currents in the striatum is dictated by the expression of D1 or D2 receptors and modified by endocannabinoids. Eur J Neurosci. 2010;31:14–28. doi: 10.1111/j.1460-9568.2009.07047.x. [DOI] [PubMed] [Google Scholar]
- Atwood BK, Lovinger DM, Mathur BN. Presynaptic long-term depression mediated by G-coupled receptors. Trends Neurosci. 2014 doi: 10.1016/j.tins.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamford NS, Robinson S, Palmiter RD, Joyce JA, Moore C, Meshul CK. Dopamine modulates release from corticostriatal terminals. J Neurosci. 2004a;24:9541–9552. doi: 10.1523/JNEUROSCI.2891-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamford NS, Zhang H, Joyce JA, Scarlis CA, Hanan W, Wu NP, Andre VM, Cohen R, Cepeda C, Levine MS, et al. Repeated exposure to methamphetamine causes long-lasting presynaptic corticostriatal depression that is renormalized with drug readministration. Neuron. 2008;58:89–103. doi: 10.1016/j.neuron.2008.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamford NS, Zhang H, Schmitz Y, Wu NP, Cepeda C, Levine MS, Schmauss C, Zakharenko SS, Zablow L, Sulzer D. Heterosynaptic dopamine neurotransmission selects sets of corticostriatal terminals. Neuron. 2004b;42:653–663. doi: 10.1016/s0896-6273(04)00265-x. [DOI] [PubMed] [Google Scholar]
- Beltramo M, de Fonseca FR, Navarro M, Calignano A, Gorriti MA, Grammatikopoulos G, Sadile AG, Giuffrida A, Piomelli D. Reversal of dopamine D(2) receptor responses by an anandamide transport inhibitor. J Neurosci. 2000;20:3401–3407. doi: 10.1523/JNEUROSCI.20-09-03401.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkmayer W, Hornykiewicz O. Der L-3,4-dioxyphenylalanin (DOPA) Effekt bei der Parkinson-akinesia. Wien Klin Wochenschr. 1961;73:787–788. [PubMed] [Google Scholar]
- Brown SJ, James S, Reddington M, Richardson PJ. Both A1 and A2a purine receptors regulate striatal acetylcholine release. J Neurochem. 1990;55:31–38. doi: 10.1111/j.1471-4159.1990.tb08817.x. [DOI] [PubMed] [Google Scholar]
- Cadogan AK, Alexander SP, Boyd EA, Kendall DA. Influence of cannabinoids on electrically evoked dopamine release and cyclic AMP generation in the rat striatum. J Neurochem. 1997;69:1131–1137. doi: 10.1046/j.1471-4159.1997.69031131.x. [DOI] [PubMed] [Google Scholar]
- Cenci MA, Lee CS, Bjorklund A. L-DOPA-induced dyskinesia in the rat is associated with striatal overexpression of prodynorphin- and glutamic acid decarboxylase mRNA. Eur J Neurosci. 1998;10:2694–2706. [PubMed] [Google Scholar]
- Cenci MA, Lundblad M. Ratings of L-DOPA-induced dyskinesia in the unilateral 6-OHDA lesion model of Parkinson's disease in rats and mice. Curr Protoc Neurosci. 2007 doi: 10.1002/0471142301.ns0925s41. Chapter 9, Unit 9 25. [DOI] [PubMed] [Google Scholar]
- Cepeda C, Hurst RS, Altemus KL, Flores-Hernandez J, Calvert CR, Jokel ES, Grandy DK, Low MJ, Rubinstein M, Ariano MA, et al. Facilitated glutamatergic transmission in the striatum of D2 dopamine receptor-deficient mice. J Neurophysiol. 2001;85:659–670. doi: 10.1152/jn.2001.85.2.659. [DOI] [PubMed] [Google Scholar]
- Chen M, Wan Y, Ade K, Ting J, Feng G, Calakos N. Sapap3 deletion anomalously activates short-term endocannabinoid-mediated synaptic plasticity. J Neurosci. 2011;31:9563–9573. doi: 10.1523/JNEUROSCI.1701-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa RM, Lin SC, Sotnikova TD, Cyr M, Gainetdinov RR, Caron MG, Nicolelis MA. Rapid alterations in corticostriatal ensemble coordination during acute dopamine-dependent motor dysfunction. Neuron. 2006;52:359–369. doi: 10.1016/j.neuron.2006.07.030. [DOI] [PubMed] [Google Scholar]
- Cowan RL, Wilson CJ. Spontaneous firing patterns and axonal projections of single corticostriatal neurons in the rat medial agranular cortex. J Neurophysiol. 1994;71:17–32. doi: 10.1152/jn.1994.71.1.17. [DOI] [PubMed] [Google Scholar]
- Cui G, Jun SB, Jin X, Pham MD, Vogel SS, Lovinger DM, Costa RM. Concurrent activation of striatal direct and indirect pathways during action initiation. Nature. 2013;494:238–242. doi: 10.1038/nature11846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd E, Monville C, Torres EM, Dunnett SB. The Corridor Task: a simple test of lateralised response selection sensitive to unilateral dopamine deafferentation and graft-derived dopamine replacement in the striatum. Brain Res Bull. 2005;68:24–30. doi: 10.1016/j.brainresbull.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Fahn S. The spectrum of levodopa-induced dyskinesias. Ann Neurol. 2000;47:S2–9. discussion S9-11. [PubMed] [Google Scholar]
- Fahn S. Does levodopa slow or hasten the rate of progression of Parkinson's disease? Journal of neurology. 2005;252(Suppl 4):IV37–IV42. doi: 10.1007/s00415-005-4008-5. [DOI] [PubMed] [Google Scholar]
- Fahn S, Sulzer D. Neurodegeneration and neuroprotection in Parkinson disease. NeuroRx. 2004;1:139–154. doi: 10.1602/neurorx.1.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltz P, De Champlain J. Enhanced sensitivity of caudate neurones to microiontophoretic injections of dopamine in 6-hydroxydopamine treated cats. Brain Res. 1972;43:601–605. doi: 10.1016/0006-8993(72)90414-3. [DOI] [PubMed] [Google Scholar]
- Ferrer B, Asbrock N, Kathuria S, Piomelli D, Giuffrida A. Effects of levodopa on endocannabinoid levels in rat basal ganglia: implications for the treatment of levodopa-induced dyskinesias. Eur J Neurosci. 2003;18:1607–1614. doi: 10.1046/j.1460-9568.2003.02896.x. [DOI] [PubMed] [Google Scholar]
- Gatley SJ, Gifford AN, Volkow ND, Lan R, Makriyannis A. 123I-labeled AM251: a radioiodinated ligand which binds in vivo to mouse brain cannabinoid CB1 receptors. Eur J Pharmacol. 1996;307:331–338. doi: 10.1016/0014-2999(96)00279-8. [DOI] [PubMed] [Google Scholar]
- Gatley SJ, Lan R, Pyatt B, Gifford AN, Volkow ND, Makriyannis A. Binding of the non-classical cannabinoid CP 55,940, and the diarylpyrazole AM251 to rodent brain cannabinoid receptors. Life Sci. 1997;61:Pl 191–197. doi: 10.1016/s0024-3205(97)00690-5. [DOI] [PubMed] [Google Scholar]
- Gerdeman G, Lovinger DM. CB1 cannabinoid receptor inhibits synaptic release of glutamate in rat dorsolateral striatum. J Neurophysiol. 2001;85:468–471. doi: 10.1152/jn.2001.85.1.468. [DOI] [PubMed] [Google Scholar]
- Giuffrida A, Parsons LH, Kerr TM, Rodriguez de Fonseca F, Navarro M, Piomelli D. Dopamine activation of endogenous cannabinoid signaling in dorsal striatum. Nat Neurosci. 1999;2:358–363. doi: 10.1038/7268. [DOI] [PubMed] [Google Scholar]
- Glass M, Felder CC. Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors augments cAMP accumulation in striatal neurons: Evidence for a Gs linkage to CB1 receptor. The journal of neuroscience : the official jour. 1997;17:5327. doi: 10.1523/JNEUROSCI.17-14-05327.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grealish S, Mattsson B, Draxler P, Bjorklund A. Characterisation of behavioural and neurodegenerative changes induced by intranigral 6-hydroxydopamine lesions in a mouse model of Parkinson's disease. Eur J Neurosci. 2010;31:2266–2278. doi: 10.1111/j.1460-9568.2010.07265.x. [DOI] [PubMed] [Google Scholar]
- Gubellini P, Picconi B, Bari M, Battista N, Calabresi P, Centonze D, Bernardi G, Finazzi-Agro A, Maccarrone M. Experimental parkinsonism alters endocannabinoid degradation: implications for striatal glutamatergic transmission. J Neurosci. 2002;22:6900–6907. doi: 10.1523/JNEUROSCI.22-16-06900.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiman M, Heilbut A, Francardo V, Kulicke R, Fenster RJ, Kolaczyk ED, Mesirov JP, Surmeier DJ, Cenci MA, Greengard P. Molecular adaptations of striatal spiny projection neurons during levodopa-induced dyskinesia. Proc Natl Acad Sci U S A. 2014;111:4578–4583. doi: 10.1073/pnas.1401819111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, de-Costa BR, Richfield EK. Neuronal localization of cannabinoid receptors in the basal ganglia of the rat. Brain Res. 1991;547:267–274. doi: 10.1016/0006-8993(91)90970-7. [DOI] [PubMed] [Google Scholar]
- Hernandez D, Torres CA, Setlik W, Cebrian C, Mosharov EV, Tang G, Cheng HC, Kholodilov N, Yarygina O, Burke RE, et al. Regulation of presynaptic neurotransmission by macroautophagy. Neuron. 2012;74:277–284. doi: 10.1016/j.neuron.2012.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu KS, Huang CC, Yang CH, Gean PW. Presynaptic D2 dopaminergic receptors mediate inhibition of excitatory synaptic transmission in rat neostriatum. Brain Res. 1995;690:264–268. doi: 10.1016/0006-8993(95)00734-8. [DOI] [PubMed] [Google Scholar]
- Hu X-T, Wachtel SR, Galloway MP, White FJ. Lesions of the nigrostriatal dopamine projection increase the inhibitory effects of D1 and D2 dopamine agonists on caudate-putamen neurons and relieve D2 receptors from the necessity of D1 receptor stimulation. Journal of Neuroscience. 1990;10:2318–2329. doi: 10.1523/JNEUROSCI.10-07-02318.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon BS, Jackson-Lewis V, Burke RE. 6-Hydroxydopamine lesion of the rat substantia nigra: time course and morphology of cell death. Neurodegeneration. 1995;4:131–137. doi: 10.1006/neur.1995.0016. [DOI] [PubMed] [Google Scholar]
- Jonkers N, Sarre S, Ebinger G, Michotte Y. Benserazide decreases central AADC activity, extracellular dopamine levels and levodopa decarboxylation in striatum of the rat. J Neural Transm. 2001;108:559–570. doi: 10.1007/s007020170056. [DOI] [PubMed] [Google Scholar]
- Joshi PR, Wu NP, Andre VM, Cummings DM, Cepeda C, Joyce JA, Carroll JB, Leavitt BR, Hayden MR, Levine MS, et al. Age-dependent alterations of corticostriatal activity in the YAC128 mouse model of Huntington disease. J Neurosci. 2009;29:2414–2427. doi: 10.1523/JNEUROSCI.5687-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathuria S, Gaetani S, Fegley D, Valino F, Duranti A, Tontini A, Mor M, Tarzia G, La Rana G, Calignano A, et al. Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med. 2003;9:76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- Kay AR, Alfonso A, Alford S, Cline HT, Holgado AM, Sakmann B, Snitsarev VA, Stricker TP, Takahashi M, Wu LG. Imaging synaptic activity in intact brain and slices with FM1-43 in C. elegans, lamprey, and rat. Neuron. 1999;24:809–817. doi: 10.1016/s0896-6273(00)81029-6. [DOI] [PubMed] [Google Scholar]
- Kim BG, Dai HN, McAtee M, Vicini S, Bregman BS. Labeling of dendritic spines with the carbocyanine dye DiI for confocal microscopic imaging in lightly fixed cortical slices. J Neurosci Methods. 2007;162:237–243. doi: 10.1016/j.jneumeth.2007.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz AV, Freeze BS, Parker PR, Kay K, Thwin MT, Deisseroth K, Kreitzer AC. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;466:622–626. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie K, Stella N. Cannabinoid receptors and endocannabinoids: evidence for new players. The AAPS journal. 2006;8:E298–306. doi: 10.1007/BF02854900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maura G, Giardi A, Raiteri M. Release-regulating D-2 dopamine receptors are located on striatal glutamatergic nerve terminals. J Pharmacol Exp Ther. 1988;247:680–684. [PubMed] [Google Scholar]
- Meschler JP, Howlett AC. Signal transduction interactions between CB1 cannabinoid and dopamine receptors in the rat and monkey striatum. Neuropharmacology. 2001;40:918–926. doi: 10.1016/s0028-3908(01)00012-0. [DOI] [PubMed] [Google Scholar]
- Mirenowicz J, Schultz W. Preferential activation of midbrain dopamine neurons by appetitive rather than aversive stimuli. Nature. 1996;379:449–451. doi: 10.1038/379449a0. [DOI] [PubMed] [Google Scholar]
- Morelli M, Fenu S, Garau L, Di Chiara G. Time and dose dependence of the ‘priming’ of the expression of dopamine receptor supersensitivity. Eur J Pharmacol. 1989;162:329–335. doi: 10.1016/0014-2999(89)90296-3. [DOI] [PubMed] [Google Scholar]
- Nabavi S, Fox R, Proulx CD, Lin JY, Tsien RY, Malinow R. Engineering a memory with LTD and LTP. Nature. 2014 doi: 10.1038/nature13294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadjar A, Gerfen CR, Bezard E. Priming for l-dopa-induced dyskinesia in Parkinson's disease: a feature inherent to the treatment or the disease? Prog Neurobiol. 2009;87:1–9. doi: 10.1016/j.pneurobio.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoletti F, Bockaert J, Collingridge GL, Conn PJ, Ferraguti F, Schoepp DD, Wroblewski JT, Pin JP. Metabotropic glutamate receptors: from the workbench to the bedside. Neuropharmacology. 2011;60:1017–1041. doi: 10.1016/j.neuropharm.2010.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nirenberg MJ, Vaughan RA, Uhl GR, Kuhar MJ, Pickel VM. The dopamine transporter is localized to dendritic and axonal plasma membranes of nigrostriatal dopaminergic neurons. Jones, SR. 1996;15:436–447. doi: 10.1523/JNEUROSCI.16-02-00436.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niswender CM, Conn PJ. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol. 2010;50:295–322. doi: 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquet M, Smith Y. Group I metabotropic glutamate receptors in the monkey striatum: subsynaptic association with glutamatergic and dopaminergic afferents. J Neurosci. 2003;23:7659–7669. doi: 10.1523/JNEUROSCI.23-20-07659.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge JG, Apparsundaram S, Gerhardt GA, Ronesi J, Lovinger DM. Nicotinic acetylcholine receptors interact with dopamine in induction of striatal long-term depression. J Neurosci. 2002;22:2541–2549. doi: 10.1523/JNEUROSCI.22-07-02541.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Hillard CJ. Pharmacological evaluation of cannabinoid receptor ligands in a mouse model of anxiety: further evidence for an anxiolytic role for endogenous cannabinoid signaling. J Pharmacol Exp Ther. 2006;318:304–311. doi: 10.1124/jpet.106.101287. [DOI] [PubMed] [Google Scholar]
- Pertwee RG, Wickens AP. Enhancement by chlordiazepoxide of catalepsy induced in rats by intravenous or intrapallidal injections of enantiomeric cannabinoids. Neuropharmacology. 1991;30:237–244. doi: 10.1016/0028-3908(91)90150-a. [DOI] [PubMed] [Google Scholar]
- Plotkin JL, Shen W, Rafalovich I, Sebel LE, Day M, Chan CS, Surmeier DJ. Regulation of dendritic calcium release in striatal spiny projection neurons. J Neurophysiol. 2013;110:2325–2336. doi: 10.1152/jn.00422.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pons R, Syrengelas D, Youroukos S, Orfanou I, Dinopoulos A, Cormand B, Ormazabal A, Garzia-Cazorla A, Serrano M, Artuch R. Levodopa-induced dyskinesias in tyrosine hydroxylase deficiency. Mov Disord. 2013;28:1058–1063. doi: 10.1002/mds.25382. [DOI] [PubMed] [Google Scholar]
- Robinson S, Krentz L, Moore C, Meshul CK. Blockade of NMDA receptors by MK-801 reverses the changes in striatal glutamate immunolabeling in 6-OHDA-lesioned rats. Synapse. 2001;42:54–61. doi: 10.1002/syn.1099. [DOI] [PubMed] [Google Scholar]
- Ronesi J, Lovinger DM. Induction of striatal long-term synaptic depression by moderate frequency activation of cortical afferents in rat. J Physiol. 2005;562:245–256. doi: 10.1113/jphysiol.2004.068460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer H, Oertel WH. Progressive degeneration of nigrostriatal dopamine neurons following intrastriatal terminal lesions with 6-hydroxydopamine: a combined retrograde tracing and immunocytochemical study in the rat. Neuroscience. 1994;59:401–415. doi: 10.1016/0306-4522(94)90605-x. [DOI] [PubMed] [Google Scholar]
- Schmitz Y, Benoit-Marand M, Gonon F, Sulzer D. Presynaptic regulation of dopaminergic neurotransmission. J Neurochem. 2003;87:273–289. doi: 10.1046/j.1471-4159.2003.02050.x. [DOI] [PubMed] [Google Scholar]
- Schultz W, Ungerstedt U. Striatal cell supersensitivity to apomorphine in dopaminelesioned rats correlated to behaviour. Neuropharmacology. 1978;17:349–353. doi: 10.1016/0028-3908(78)90005-9. [DOI] [PubMed] [Google Scholar]
- Shen H, Kannari K, Yamato H, Arai A, Matsunaga M. Effects of benserazide on L-DOPA-derived extracellular dopamine levels and aromatic L-amino acid decarboxylase activity in the striatum of 6-hydroxydopamine-lesioned rats. The Tohoku journal of experimental medicine. 2003;199:149–159. doi: 10.1620/tjem.199.149. [DOI] [PubMed] [Google Scholar]
- Smeal RM, Gaspar RC, Keefe KA, Wilcox KS. A rat brain slice preparation for characterizing both thalamostriatal and corticostriatal afferents. J Neurosci Methods. 2007;159:224–235. doi: 10.1016/j.jneumeth.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Stern EA, Kincaid AE, Wilson CJ. Spontaneous subthreshold membrane potential fluctuations and action potential variability of rat corticostriatal and striatal neurons in vivo. J Neurophysiol. 1997;77:1697–1715. doi: 10.1152/jn.1997.77.4.1697. [DOI] [PubMed] [Google Scholar]
- Sulzer D, Pothos EN. Regulation of quantal size by presynaptic mechanisms. Rev Neurosci. 2000;11:159–212. doi: 10.1515/revneuro.2000.11.2-3.159. [DOI] [PubMed] [Google Scholar]
- Sulzer D, Surmeier DJ. Neuronal vulnerability, pathogenesis, and Parkinson's disease. Mov Disord. 2013;28:41–50. doi: 10.1002/mds.25095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D, Zhang H, Benoit-Marand M, Gonon F. Handbook of Basal Ganglia Structure and Function: A Decade of Progress. H. Elsevier Press; Amsterdam: 2010. Regulation of extracellular dopamine: release and reuptake. pp. 297–312. [Google Scholar]
- Tehovnik EJ, Tolias AS, Sultan F, Slocum WM, Logothetis NK. Direct and indirect activation of cortical neurons by electrical microstimulation. J Neurophysiol. 2006;96:512–521. doi: 10.1152/jn.00126.2006. [DOI] [PubMed] [Google Scholar]
- Thiele SL, Chen B, Lo C, Gertler TS, Warre R, Surmeier JD, Brotchie JM, Nash JE. Selective loss of bi-directional synaptic plasticity in the direct and indirect striatal output pathways accompanies generation of parkinsonism and l-DOPA induced dyskinesia in mouse models. Neurobiol Dis. 2014;71:334–344. doi: 10.1016/j.nbd.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerstedt U. 6-Hydroxy-dopamine induced degeneration of central monoamine neurons. Eur J Pharmacol. 1968;5:107–110. doi: 10.1016/0014-2999(68)90164-7. [DOI] [PubMed] [Google Scholar]
- Van der Kloot W. The regulation of quantal size. Progress in Neurobiology. 1991;36:93–130. doi: 10.1016/0301-0082(91)90019-w. [DOI] [PubMed] [Google Scholar]
- Voon V, Mehta AR, Hallett M. Impulse control disorders in Parkinson's disease: recent advances. Curr Opin Neurol. 2011;24:324–330. doi: 10.1097/WCO.0b013e3283489687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Pickel VM. Dopamine D2 receptors are present in prefrontal cortical afferents and their targets in patches of the rat caudate-putamen nucleus. J Comp Neurol. 2002;442:392–404. doi: 10.1002/cne.10086. [DOI] [PubMed] [Google Scholar]
- Wang W, Darvas M, Storey GP, Bamford IJ, Gibbs JT, Palmiter RD, Bamford NS. Acetylcholine encodes long-lasting presynaptic plasticity at glutamatergic synapses in the dorsal striatum after repeated amphetamine exposure. J Neurosci. 2013;33:10405–10426. doi: 10.1523/JNEUROSCI.0014-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Dever D, Lowe J, Storey GP, Bhansali A, Eck EK, Nitulescu I, Weimer J, Bamford NS. Regulation of prefrontal excitatory neurotransmission by dopamine in the nucleus accumbens core. J Physiol. 2012;590:3743–3769. doi: 10.1113/jphysiol.2012.235200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub D, Nirenberg MJ. Impulse control and related disorders in Parkinson's disease. Neurodegener Dis. 2013;11:63–71. doi: 10.1159/000341996. [DOI] [PubMed] [Google Scholar]
- Wilson CJ. Morphology and synaptic connections of crossed corticostriatal neurons in the rat. J Comp Neurol. 1987;263:567–580. doi: 10.1002/cne.902630408. [DOI] [PubMed] [Google Scholar]
- Wong MY, Sulzer D, Bamford NS. Imaging presynaptic exocytosis in corticostriatal slices. Methods Mol Biol. 2011;793:363–376. doi: 10.1007/978-1-61779-328-8_24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagishita S, Hayashi-Takagi A, Ellis-Davies GC, Urakubo H, Ishii S, Kasai H. A critical time window for dopamine actions on the structural plasticity of dendritic spines. Science. 2014;345:1616–1620. doi: 10.1126/science.1255514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Lovinger DM. Frequency-specific and D2 receptor-mediated inhibition of glutamate release by retrograde endocannabinoid signaling. Proc Natl Acad Sci U S A. 2006;103:8251–8256. doi: 10.1073/pnas.0510797103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung KK, Bolam JP, Smith AD, Hersch SM, Ciliax BJ, Levey AI. Immunocytochemical localization of D1 and D2 dopamine receptors in the basal ganglia of the rat: light and electron microscopy. Neuroscience. 1995;65:709–730. doi: 10.1016/0306-4522(94)00536-e. [DOI] [PubMed] [Google Scholar]
- Zakharenko SS, Zablow L, Siegelbaum SA. Visualization of changes in presynaptic function during long-term synaptic plasticity. Nat Neurosci. 2001;4:711–717. doi: 10.1038/89498. [DOI] [PubMed] [Google Scholar]
- Zhang H, Sulzer D. Glutamate spillover in the striatum depresses dopaminergic transmission by activating group I metabotropic glutamate receptors. J Neurosci. 2003;23:10585–10592. doi: 10.1523/JNEUROSCI.23-33-10585.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou QY, Palmiter RD. Dopamine-deficient mice are severely hypoactive, adipsic, and aphagic. Cell. 1995;83:1197–1209. doi: 10.1016/0092-8674(95)90145-0. [DOI] [PubMed] [Google Scholar]