Abstract
Betulinic acid (1) has been modified to ionic derivatives (2-5) to improve its water solubility and biological activities. The binding properties of these derivatives with respect to human serum albumin (HSA) was examined and found to be similar to current anti-HIV drugs. These compounds did not inhibit HIV reverse transcriptase, however, 1, 2 and 5 inhibited herpes simplex type 2 (HSV-2) replication at concentrations similar to those reported for acyclovir (IC50 ~0.1–10 μM) and with minimal cellular cytotoxicity. IC50 values for antiviral activity against HSV-2 186 were 1.6, 0.6, 0.9, 7.2, and 0.9 μM for compounds 1-5 respectively.
Keywords: Betulinic acid, HIV-1 reverse transcriptase, herpes simplex type 2 (HSV-2), inhibitor
New ionic derivatives of betulinic acid (1) such as 2 and 5 show strong inhibition against herpes simplex type 2 (HSV-2) replication with minimal cellular cytotoxicity.
Betulinic acid (1), also known as 3β-hydroxy-lup-20(29)-en-28-oic acid, is a natural pentacyclic lupane-type triterpene (Scheme 1) that can be extracted from certain plants including birch trees. This compound and many of its derivatives have a number of medically relevant biological properties such as anticancer, anti-HIV-1 (human immunodeficiency virus type-1), antibacterial, anti-malarial, anti-inflammatory, and anthelmintic activities.1-6 Betulinic acid and derivatives are of interest because of their anti-HIV-1 activity via several known mechanisms7 including inhibition of HIV-1 maturation,8-11 blocking viral infection at a post-binding stage, inhibition of an envelope-dependent step during fusion of the virus to the cell membrane,12-17 and inhibition of HIV-1 protease.18, 19 Due to mutation of HIV in response to most chemotherapeutic drugs, there is a constant demand for the development of novel anti-HIV compounds, particularly less expensive and less toxic agents.
Scheme 1.
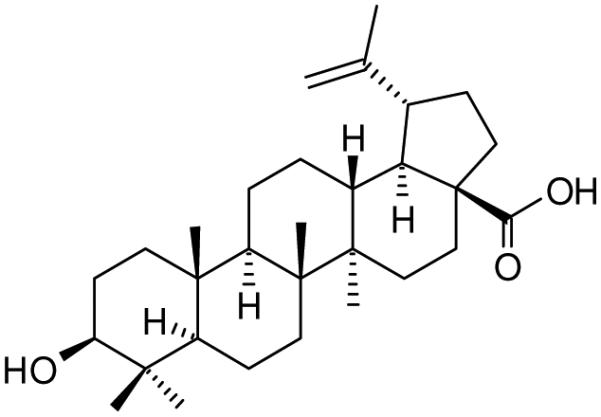
Structure of betulinic acid (1)
The inhibition of betulinic acid and derivatives against other viruses has not been extensively studied. Herpes simplex types 1 (HSV-1) and 2 (HSV-2) are enveloped, double stranded DNA viruses that initially infect mucosal epithelial cells and establish latency in trigeminal and sacral ganglia respectively. HSV-1 is the predominant cause of cold sores whereas HSV-2 primarily causes genital herpes. Genital herpes is a sexually transmitted disease transmitted through contact with genital or oral lesions and secretions. There are more than 700,000 new herpes infections annually in the United States (http://www.cdc.gov/std/Herpes/). The Center for Disease Control estimates that 20.9% and 11.5% of 14–49 year old women and men respectively are infected with HSV-2. Greater than 80% of infected individuals in this age bracket are asymptomatic or have mild symptoms and therefore have never received a HSV-2 positive diagnosis. The fact that virus can be transmitted via shedding from lesion-free skin might explain why infection rates stay high. The development of a HSV-2 vaccine remains a high priority but has been met with only limited success.20
Nucleoside analogues make up the majority of currently approved anti-herpesviral drugs (e.g. acyclovir). These drugs inhibit viral replication by targeting new viral DNA replication.21 Although critical in controlling infections caused by herpes simplex viruses, nucleoside analogues share a similar mechanism of action. Therefore, treatment options are limited once resistance develops, an important clinical concern for treatment of resistant infections, particularly in immunocompromised individuals. In addition, moderate to severe side effects of some nucleoside analogues make discovery of less toxic drugs desirable. Efforts over the last decade have focused on the identification and development of improved therapies with novel mechanisms of action.
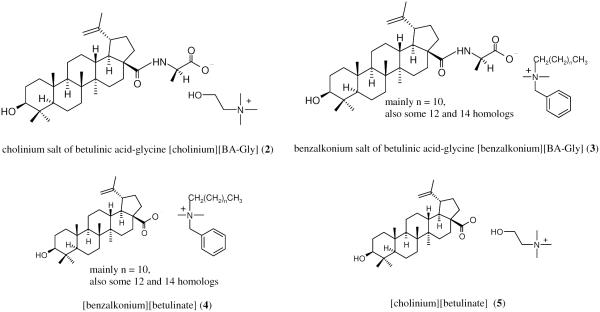
A major obstacle in maximizing the antiviral potency of betulinic acid is its poor solubility in aqueous solutions and, to a lesser extent, in many organic solvents including alcohols, ethers, and esters. The solubility of betulinic acid in water is only about 0.02 μg mL−1 at room temperature.22 Its solubility in common organic solvents at 25 °C is also fairly low; e.g., 1% (w/v) in ethanol and 5% (w/v) in DMSO.23 A limited number of derivatives of betulinic acid were reported to yield improved water solubility and biological activity compared to unmodified betulinic acid.1, 4, 24 Anticipating that ionic derivatives of betulinic acid may have improved water solubility, four ionic derivatives (2-5, Scheme 2) of betulinic acid (1, Scheme 1) were prepared previously by our group; their potentials as HIV-1 protease inhibitors and anti-cancer agents were examined.28, 29 Ionic derivatives had improved water solubilities and thus enhanced biological activity. In this study, the antiviral activities of these derivatives were explored as inhibitors of HIV-1 reverse transcriptase (RT) and herpes simplex virus type-2 (HSV-2) (see experimental procedures in Supplementary data).
Scheme 2.
Ionic derivatives (2-5) of betulinic acid
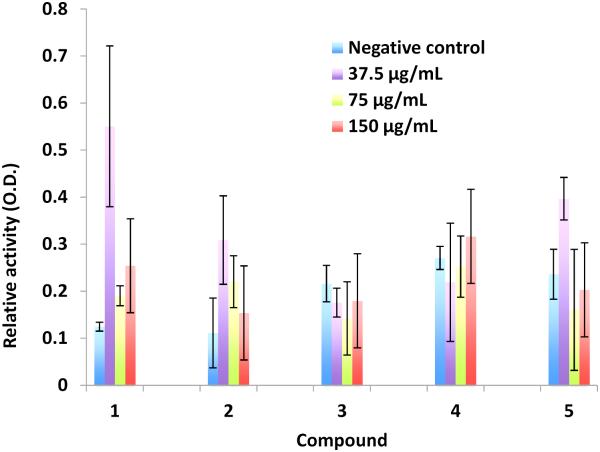
Anti-HIV activity was evaluated by infecting Jurkat cells with HIV-1 virus in the presence of ionic derivatives (at a final concentration of 1 μg/mL or 5 μg/mL), and measuring reverse transcriptase (RT) activity. Cell viability was determined by MTT assay while HIV replication was determined by RT assay. DMSO was used as the solvent control, therefore, the overall anti-HIV-1 activity was calculated as the ratio of [MTT activity/(RT activity with compound/RT activity in DMSO)]. A more active HIV-1 inhibitor is expected to have a larger ratio. When comparing with anti-HIV activity in DMSO (Table 1), only compound 2 at 5 μg/mL showed a slightly improved inhibition against HIV-1 RT. The other compounds including betulinic acid (1) showed no inhibitory activity of HIV-1 RT, when comparing with results (0.941 at 1 μM and 0.580 at 10 μM) of a known reverse-transcriptase inhibitor azidothymidine (AZT). RT activity was also evaluated using higher concentrations (37.5–150 μg/mL) of 1-5 with a Roche colorimetric assay (Figure 1), but no significant inhibition of RT was observed. RT activity was not found to be dose-dependent, which could be due to the interference of slight solution turbidity at high compound concentrations. The overall results are consistent with some earlier studies9, 12-14 where betulinic acid and derivatives (up to 219 μM 9) exhibited no inhibition of HIV-1 RT although some pentacyclic triterpenes25 and triterpenoids26, 27 were active against HIV-1 RT. Therefore, the anti-HIV properties of betulinic acid and derivatives do not appear to target RT.
Table 1.
Anti-HIV activity in the ratio of [MTT activity/(RT activity with compound/RT activity in DMSO)]
| Compound | 1 μg/mL | 5 μg/mL |
|---|---|---|
| Negative control | 0.464 | 0.798 |
| Azidothymidine (AZT) | 0.941 (1 μM) | 0.580 (10 μM) |
| DMSO | 0.356 | 0.795 |
| 1 | 0.158 | 0.649 |
| 2 | 0.319 | 0.885 |
| 4 | 0.121 | 0.474 |
| 5 | 0.201 | 0.576 |
Figure 1.
Effect of betulinic acid (1) and derivatives (2-5) on reverse transcriptase (RT) activity
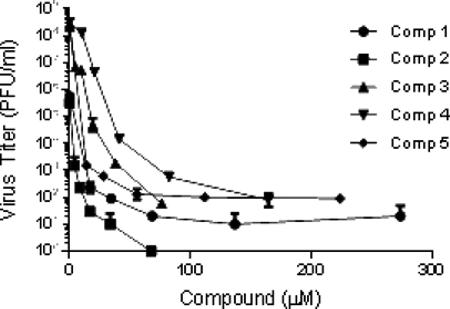
Betulin and betulinic derivatives (e.g. betulinic acid and betulonic acid) have been shown to possess anti-viral activity primarily against HSV-1, and in a single study against HSV-2 (Table 1).28-31 Activity against both acyclovir sensitive and acyclovir resistant HSV strains has been reported. Results from the current study show new betulinic acid derivatives with improved solubility retain significant anti-herpesviral activity against HSV-2 186 (Figure 2). Compounds 1-5 were examined for cytotoxicity in Vero cells (Figure S1 in Supplementary Data). As shown in Table 2, CC50s were greater than 100 μM for compounds 1, 2, and 5. Compounds 3 and 4 had CC50s of approximately 10.5 and 12 μM respectively. Compounds were tested for antiviral activity against HSV-2 186. IC50s were 1.6, 0.6, 0.9, 7.2, and 0.9 μM for compounds 1-5 respectively (Table 1). These results compare favorably to IC50s previously reported for acyclovir against multiple HSV-2 isolates, including HSV-2 186 (Table 1).32, 33 Selectivity indices calculated for compounds 1-5 were >62.5, >166.7, 11.6, 1.7, and >111.1 respectively. The IC50s (HSV-2) and SIs of compounds 1, 2, and 5 were as good as or better than those previously reported for related compounds (Table 1). The improved solubility of ionic derivatives 2 and 5 likely contributes to improved antiviral activity. The mechanism of action of these new derivatives against HSV-2 strains is currently being investigated.
Figure 2.
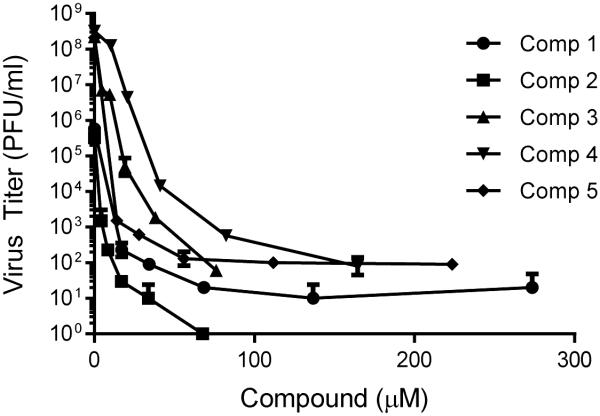
Anti-herpes viral activity of selected compounds against the HSV-2 strain 186. IC50 (µM) values were calculated from viral replication yield reduction assays. The data represent the average viral titer in quadruplicate samples at each indicated drug concentration. Compounds 1 and 2, and compounds 3, 4 and 5 were tested in two separate experiments.
Table 2.
Antiviral herpesviral activity of derivatives of betulinic acid a
| Compound | Cell Line |
Virus | CC50
μM |
IC50
μM |
SI | Reference |
|---|---|---|---|---|---|---|
| Acyclovir | Vero | Multiple HSV-2 (including 186) |
>10,000 | ~0.1–10 | > 1,000 | 32, 33 |
| 1 b | Vero | HSV-2 186 | > 100 | 1.6 | > 62.5 | this study |
| 2 | Vero | HSV-2 186 | > 100 | 0.6 | > 166.7 | this study |
| 3 | Vero | HSV-2 186 | 10.5 | 0.9 | 11.6 | this study |
| 4 | Vero | HSV-2 186 | 12 | 7.2 | 1.7 | this study |
| 5 | Vero | HSV-2 186 | > 100 | 0.9 | > 111.1 | this study |
| Betulin (Lup-20(29)-ene-3β,28-diol) | Vero | HSV-1 F | 165 | 0.9 | 183 | 29 |
|
| ||||||
| Betulin (Lup-20(29)-ene-3β,28-diol) | Vero | HSV-2 G | 165 | 9.4 | 17.6 | 29 |
| Betulin (Lup-20(29)-ene-3β,28-diol) | RC-37 (Vero) |
HSV-1 KOS | 49.7 | 0.7 | 71 | 31 |
| 3-α-hydroxylup-20(29)-ene-23,28-dioic acid | Vero | HSV-1 15577 | 246.9 | 64.4 | 3.8 | 30 |
| 3-epi-betulinic acid 3-O-sulfate | Vero | HSV-1 15578 | 228.3 | 45.7 | 5 | 30 |
| betulinic acid (1) | RC-37 (Vero) |
HSV-1 KOS | 10.9 | 0.7 | 15.6 | 31 |
| betulonic acid (3-oxolup-20(29)-en-28-oic acid) |
Vero | HSV-1 7401H | 35.6 | 5.7 | 6.2 | 28 |
All previously published value were converted from mg/ml to mM for comparison.
Values for compounds 1-5 are averages of triplicate samples.
Lastly, we investigated the binding characteristics of these derivatives with human serum albumin (HSA). HSA is a well-known plasma protein that is responsible for the binding and transport of many endogenous and exogenous substances (e.g. hormones and fatty acids), and drug molecules. Since the binding of drugs to plasma proteins is non-specific, only the unbound form of a drug is considered to interact with its receptor to produce a pharmacological effect.34 Therefore, a low binding constant or a high dissociation equilibrium constant (Kd) between drug molecules and HSA is desirable for achieving a maximum drug bioavailability. The fluorescence titration of betulinic acid or its derivative with HSA (with excitation at 285 nm 34 or 295 nm 35) was measured. The titration spectra and calculation plot for compound 5 is provided as an example (Figure S2), showing an increase in fluorescence intensity with an increase in concentration of 5 for up to 0.016 mM. Quenching of fluorescence was observed above this concentration. This could be due to the solubility issue of this compound as the solution appeared to be slightly turbid. The Kd values were further calculated for 1-5 and are in the range of 0.16−4.7×10−5 M for excitation at 285 nm, and 0.13−3.7×10−5 M for excitation at 295 nm (Table 3). The Kd values decrease in the order 2 > 3 >> 1 > 5 > 4, where ionic derivatives 2 and 3 have much improved Kd values than betulinic acid (1). The Kd values of our ionic derivatives are close to those for binding of common anti-HIV drugs to HSA (ranging between 4.4×10−5 M and 3.8×10−4 M).36 Subramanyam et al.34 determined the binding constant of betulinic acid (0.01−0.1 mM) with HSA (0.025 mM) as 1.685×106 M−1 (fluorescence excitation at 285 nm), which is equivalent to a Kd value of 0.593×10−6 M, approximately one magnitude lower than our Kd value of 0.45×10−5 M (Table 3).
Table 3.
Dissociation equilibrium constant (Kd) of betulinic acid and derivatives to HSA
| Compound | Kd/M(285 nm) a | Kd/M(295 nm) a | R |
|---|---|---|---|
| 1 | 0.45 × 10−5 | 0.13 × 10−5 | 0.93, 0.94 |
| 2 | 4.7 × 10−5 | 3.7 × 10−5 | 0.95, 0.97 |
| 3 | 1.9 × 10−5 | 1.9 × 10−5 | 0.95, 0.97 |
| 4 | 0.16 × 10−5 | 0.19 × 10−5 | 0.94, 0.95 |
| 5 | 0.39 × 10−5 | 0.40 × 10−5 | 0.96, 0.99 |
Fluorescence emission spectra were recorded on with excitation at 285 nm or 295 nm.
In conclusion, betulinic acid (1) and ionic derivatives (2-5) showed no appreciable inhibition against HIV-1 reverse transcriptase, but had significant activity against HSV-2. Compounds 1, 2, and 5 had low cytotoxicity with good selectivity indices. The compounds had antiviral activities similar to those reported previously for acyclovir. The binding properties of these compounds with HSA were similar to common anti-HIV drugs with compounds 2 and 3 having relatively higher dissociation equilibrium constants (Kd).
Supplementary Material
Acknowledgements
The author acknowledges the supports by the Henry Dreyfus Teacher-Scholar Award (2012), NIH BRS-RISE grant (1R25GM096956), NIH NIBIB contract award (HHSN268201200011C), and the National Natural Science Foundation of China (21328601).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary data
Supplementary data associated with this article can be found, in the online version, at xxx.
References and notes
- 1.Baglin I, Mitaine-Offer A-C, Nour M, Tan K, Cave C, Lacaille-Dubois M-A. Mini Rev. Med. Chem. 2003;3:525. doi: 10.2174/1389557033487917. [DOI] [PubMed] [Google Scholar]
- 2.Cichewicz RH, Kouzi SA. Med. Res. Rev. 2004;24:90. doi: 10.1002/med.10053. [DOI] [PubMed] [Google Scholar]
- 3.Eiznhamer DA, Xu ZQ. IDrugs. 2004;7:359. [PubMed] [Google Scholar]
- 4.Yogeeswari P, Sriram D. Curr. Med. Chem. 2005;12:657. doi: 10.2174/0929867053202214. [DOI] [PubMed] [Google Scholar]
- 5.Krasutsky PA. Nat. Prod. Rep. 2006;23:919. doi: 10.1039/b606816b. [DOI] [PubMed] [Google Scholar]
- 6.Mullauer FB, Kessler JH, Medema JP. Anti-Cancer Drugs. 2010;21:215. doi: 10.1097/CAD.0b013e3283357c62. [DOI] [PubMed] [Google Scholar]
- 7.Aiken C, Chen CH. Trends Mol. Med. 2005;11:31. doi: 10.1016/j.molmed.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Fujioka T, Kashiwada Y, Kilkuskie RE, Cosentino LM, Ballas LM, Jiang JB, Janzen WP, Chen I-S, Lee K-H. J. Nat. Prod. 1994;57:243. doi: 10.1021/np50104a008. [DOI] [PubMed] [Google Scholar]
- 9.Kashiwada Y, Hashimoto F, Cosentino LM, Chen C-H, Garrett PE, Lee K-H. J. Med. Chem. 1996;39:1016. doi: 10.1021/jm950922q. [DOI] [PubMed] [Google Scholar]
- 10.Kashiwada Y, Wang H-K, Nagao T, Kitanaka S, Yasuda I, Fujioka T, Yamagishi T, Cosentino LM, Kozuka M, Okabe H, Ikeshiro Y, Hu C-Q, Yeh E, Lee K-H. J. Nat. Prod. 1998;61:1090. doi: 10.1021/np9800710. [DOI] [PubMed] [Google Scholar]
- 11.Kashiwada Y, Chiyo J, Ikeshiro Y, Nagao T, Okabe H, Cosentino LM, Fowke K, Morris-Natschke SL, Lee K-H. Chem. Pharm. Bull. 2000;48:1387. doi: 10.1248/cpb.48.1387. [DOI] [PubMed] [Google Scholar]
- 12.Mayaux J-F, Bousseau A, Pauwels R, Huet T, Hénin Y, Dereu N, Evers M, Soler F, Poujade C, De Clercq E, Le Pecq J-B. Proc. Natl. Acad. Sci. USA. 1994;91:3564. doi: 10.1073/pnas.91.9.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evers M, Poujade C, Soler F, Ribeill Y, James C, Lelièvre Y, Gueguen J-C, Reisdorf D, Morize I, Pauwels R, De Clercq E, Hénin Y, Bousseau A, Mayaux J-F, Le Pecq J-B, Dereu N. J. Med. Chem. 1996;39:1056. doi: 10.1021/jm950670t. [DOI] [PubMed] [Google Scholar]
- 14.Soler F, Poujade C, Evers M, Carry J-C, Hénin Y, Bousseau A, Huet T, Pauwels R, De Clercq E, Mayaux J-F, Le Pecq J-B, Dereu N. J. Med. Chem. 1996;39:1069. doi: 10.1021/jm950669u. [DOI] [PubMed] [Google Scholar]
- 15.Labrosse B, Pleskoff O, Sol N, Jones C, Henin Y, Alizon M. J. Virol. 1997;71:8230. doi: 10.1128/jvi.71.11.8230-8236.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holz-Smith SL, Sun I-C, Jin L, Matthews TJ, Lee K-H, Chen CH. Antimicrob. Agents Chemother. 2001;45:60. doi: 10.1128/AAC.45.1.60-66.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bär S, Alizon M. J. Virol. 2004;78:811. doi: 10.1128/JVI.78.2.811-820.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma C, Nakamura N, Miyashiro H, Hattori M, Shimotohno K. Chem. Pharm. Bull. 1999;47:141. doi: 10.1248/cpb.47.141. [DOI] [PubMed] [Google Scholar]
- 19.Quéré L, Wenger T, Schramm HJ. Biochem. Biophys. Res. Commun. 1996;227:484. doi: 10.1006/bbrc.1996.1533. [DOI] [PubMed] [Google Scholar]
- 20.Johnston C, Koelle DM, Wald A. Vaccine. 2014;32:1553. doi: 10.1016/j.vaccine.2013.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Visalli RJ, van Zeijl M. Antiviral Res. 2003;59:73. doi: 10.1016/s0166-3542(03)00108-6. [DOI] [PubMed] [Google Scholar]
- 22.Jäger S, Winkler K, Pfüller U, Scheffler A. Planta Med. 2007;73:157. doi: 10.1055/s-2007-967106. [DOI] [PubMed] [Google Scholar]
- 23.Zhao H, Jones CL, Cowins JV. Green Chem. 2009;11:1128. [Google Scholar]
- 24.Mukherjee R, Kumar V, Srivastava SK, Agarwal SK, Burman AC. Anti-Cancer Agents Med. Chem. 2006;6:271. doi: 10.2174/187152006776930846. [DOI] [PubMed] [Google Scholar]
- 25.Pengsuparp T, Cai L, Fong HHS, Kinghorn AD, Pezzuto JM, Wani MC, Wall ME. J. Nat. Prod. 1994;57:415. doi: 10.1021/np50105a017. [DOI] [PubMed] [Google Scholar]
- 26.Akihisa T, Ogihara J, Kato J, Yasukawa K, Ukiya M, Yamanouchi S, Oishi K. Lipids. 2001;36:507. doi: 10.1007/s11745-001-0750-4. [DOI] [PubMed] [Google Scholar]
- 27.Sun H, Qiu S, Lin L, Wang Z, Lin Z, Pengsuparp T, Pezzuto JM, Fong HHS, Cordell GA, Farnsworth NR. J. Nat. Prod. 1996;59:525. doi: 10.1021/np960149h. [DOI] [PubMed] [Google Scholar]
- 28.Kurokawa M, Basnet P, Ohsugi M, Hozumi T, Kadota S, Namba T, Kawana T, Shiraki K. J. Pharmacol. Exp. Ther. 1999;289:72. [PubMed] [Google Scholar]
- 29.Gong Y, Raj KM, Luscombe CA, Gadawski I, Tam T, Chu J, Gibson D, Carlson R, Sacks SL. Antiviral Res. 2004;64:127. doi: 10.1016/j.antiviral.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 30.Li Y, Jiang R, Ooi LSM, But PPH, Ooi VEC. Phytother. Res. 2007;21:466. doi: 10.1002/ptr.1962. [DOI] [PubMed] [Google Scholar]
- 31.Heidary Navida M, Laszczyk-Lauer MN, Reichling J, Schnitzler P. Phytomedicine. 2014;21:1273. doi: 10.1016/j.phymed.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 32.Sangdara A, Bhattarakosol P. J. Med. Assoc. Thai. 2008;91:908. [PubMed] [Google Scholar]
- 33.Piret J, Boivin G. Antimicrob. Agents Chemother. 2011;55:459. doi: 10.1128/AAC.00615-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Subramanyam R, Gollapudi A, Bonigala P, Chinnaboina M, Amooru DG. J. Photochem. Photobiol. B: Biol. 2009;94:8. doi: 10.1016/j.jphotobiol.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 35.Montero MT, Hernández J, Estelrich J. Biochem. Edu. 1990;18:99. [Google Scholar]
- 36.Bocedi A, Notaril S, Narciso P, Bolli A, Fasano M, Ascenzi P. IUBMB Life. 2004;56:609. doi: 10.1080/15216540400016286. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.