To the Editor
We report the results of an observational cohort study examining atopic dermatitis (AD) disease control by age. AD is the most common chronic inflammatory skin disease and is characterized by an episodic course. Conventionally thought to remit by adolescence, increasing data suggest heterogeneity in disease courses, though detailed prospective studies are lacking.(1)
Methods
To examine AD disease control by age, we used longitudinal data from the Pediatric Eczema Elective Registry (PEER). It is a cohort study designed to test whether there is an increased risk of malignancy associated with the use of pimecrolimus, and includes a patient-reported measure of disease control every 6 months.(2, 3) To enroll, patients must have AD diagnosed by a physician and used 1% topical pimecrolimus cream for at least 42 out of the last 180 days.
Our primary outcome, disease control, is a repeating composite variable based on self-reported control (complete, good, limited or poor) and treatment use (any prescription treatments for AD over the same 6-month period). Those who reported complete control were sub-categorized into two groups: complete control without treatment (i.e. apparent remission) and complete control with treatment.
We examined the data graphically. Then, to account for the longitudinal nature of our data and repeated outcome, we created a separate binary generalized linear latent and mixed model or GLLAMM for each level of disease control. This enabled us to calculate the subject-specific odds of better or worse control for each additional year of age while controlling for potential confounders specified a priori including age at onset, enrollment age, sex, race, family income and history of atopic disease at enrollment. The number of subjects with at least one follow-up visit by 10/13 determined the sample size. Additional methodological details are provided in the online supplement. This study was approved by the IRB at the University of Pennsylvania.
Results
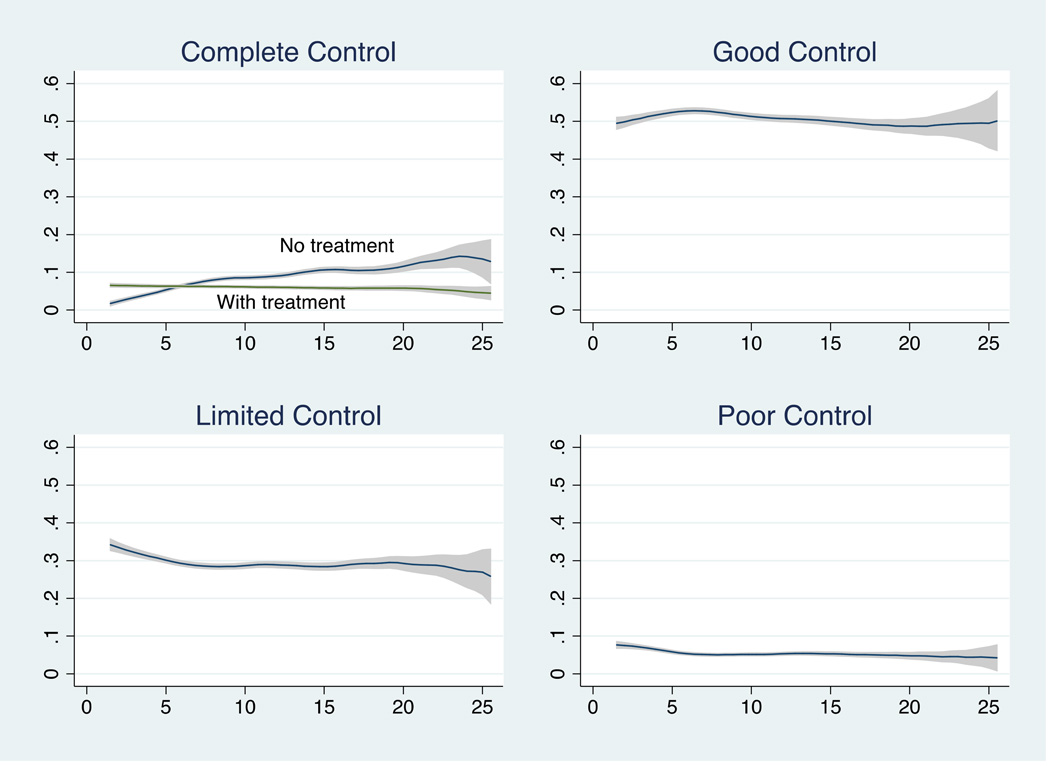
There were 5,798 participants ages 2–26 who returned 49,840 surveys. They were 47% male, 44% white, the mean age of enrolment was 7.2 years, and the mean duration of follow up was 4.2 years (see supplementary Table 1 for additional cohort characteristics). Complete control without treatment was reported on 8% of all surveys while good or limited disease control was reported 80% of the time. The largest change with age occurred among the complete control with no treatment group (Figure 1).
Figure 1. Proportion of surveys with each level of disease control by year of age.
X axis represents the proportion of surveys reporting the specified level of disease control at each age; y axis represents age in years. Proportion of surveys with each level of disease control by year of age displayed as Epanechnikov kernel-weighted local polynomial smoothed plots with 95% confidence intervals.
With multivariate models, we found an elevated subject specific odds of complete control for each additional year of age, and a reduced subject specific odds of limited or poor control for each additional year of age (Table 1). When we split those who reported complete control into treatment groups, we found the subject-specific odds of complete control without treatment for each additional year of age compared to good, limited or poor control was 1.78 (95%CI 1.72–1.83, see Table 1). This outcome was infrequent, however. Only 3,967 (8% of all surveys), and 1,205 (21% of all patients) ever reported a 6-month period of complete control without treatment. Of these patients, 546 (45%) subsequently reported medication use or less than complete control, suggesting a minority of patients “outgrew” their disease while being followed in the PEER cohort.
Table 1.
Results of binomial logistic GLLAMM regression models for the subject-specific odds of each level of disease control for each additional year of age
| Disease Control Level | Number of surveys (% of total) |
Subject-specific odds of disease control level for each additional year of agea |
|---|---|---|
| Complete | 7,022 (14%) | 1.37 (1.34–1.39)b |
| No treatment/apparent remission | 3,967 (8%) | 1.78 (1.72–1.83)b |
| With treatment | 3, 055 (6%) | 1.07 (1.05–1.10)b |
| Good | 25,521 (51%) | 1.13 (1.11–1.14)c |
| Limited | 14,588 (29%) | 0.87 (0.6–0.88)d |
| Poor | 2,709 (5%) | 0.85 (0.83–0.88)e |
| Not reported | 104 (0%) | N/A |
Notes:
Models adjusted for enrollment age, age of onset, sex, race, family income at enrollment, and history of atopic disease. In all models age at onset, male sex, income greater $50,000 US dollars, and white race were positively associated with periods of complete control without treatment, and age at enrollment and atopic history were negatively associated with periods of complete control without treatment.
Compared to those with good, limited or poor control.
Compared to those with limited or poor control.
Compared to those with complete or good control.
Compared to those with complete, good, or limited control. Results were robust to sensitivity analyses examining the impact of duration of follow up, loss to follow up, enrollment before the FDA’s Black Box Warning in January 2006 regarding the potential cancer risk with pimecrolimus, and identity of the individual filling out the survey (i.e. patient or parent).
In a secondary analysis, we also tested whether the subject-specific odds of a dermatologist visit during each 6-month period of follow up changed with age. Regardless of the level of disease control, the odds of seeing a dermatologist declined for all patients as they aged (supplementary Table 2).
Discussion
Our findings add to the sparse literature on AD disease control over time and imply that most patients in this cohort had active disease at all ages. Additionally, we found that the decline in the odds of a dermatologist visit was similar whether a subject reported complete control or poor control, suggesting that all patients tend to see their providers less as they get older, possibly because they learn to manage their disease on their own or are simply less likely to visit a physician. This finding could help to explain why some dermatologists may perceive that AD tends to resolve as children age.
This is one of the largest longitudinal studies of AD with good representation of minority groups, data on treatment use, and repeated measures of disease control every 6 months. Because of the enrollment criteria, the results are most generalizable to patients who have used pimecrolimus for at least 6 weeks. The criteria may have selected for patients with more severe or persistent disease, though the AAD recommends topical pimecrolimus based on the highest level of evidence,(7) and data suggest it is not typically used as a second line treatment.(8) After enrollment, subjects were not required to continue to use pimecrolimus, and in fact most stopped.(2) It is possible that the use of pimecrolimus reflects more about the prescribing tendencies of providers than the patient’s disease characteristics.
Our findings suggest heterogeneity in AD disease trajectories. We found that age is predictive of disease control, inpendent of sex, race, income, history of atopic disease, age at onset and age at enrollment. Examination of the interaction between age and each of these factors is the focus of ongoing work. Along with increasing data about the role of immune and epidermal barrier dysfunction in AD,(9, 10) our results argue for a shift in the general characterization from predominantly a childhood disease to a heterogeneous chronic disease with intermittent periods of disease activity.
Supplementary Material
Capsule summary.
Most patients report good or limited disease control into their teens and early adult years. The odds of complete control not requiring medications increased with age, but only 21% of patients ever achieved this outcome.
Acknowledgments
Funding sources: This work was supported by T32-AR-0007465-3 and the Dermatology Foundation (KA), K24-AR064310 (JMG), and AR056755 (DM). The PEER study was funded by Valeant Pharmaceuticals International through a grant to the Trustees of the University of Pennsylvania and DM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Bieber T. Atopic dermatitis 2.0: from the clinical phenotype to the molecular taxonomy and stratified medicine. Allergy. 2012;67(12):1475–1482. doi: 10.1111/all.12049. [DOI] [PubMed] [Google Scholar]
- 2.Kapoor R, Hoffstad O, Bilker W, Margolis DJ. The frequency and intensity of topical pimecrolimus treatment in children with physician-confirmed mild to moderate atopic dermatitis. Pediatric dermatology. 2009;26(6):682–687. doi: 10.1111/j.1525-1470.2009.01013.x. [DOI] [PubMed] [Google Scholar]
- 3.Margolis JS, Abuabara K, Bilker W, Hoffstad O, Margolis DJ. Persistence of mild to moderate atopic dermatitis. JAMA dermatology. 2014;150(6):593–600. doi: 10.1001/jamadermatol.2013.10271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kapp A, Papp K, Bingham A, Folster-Holst R, Ortonne JP, Potter PC, et al. Long-term management of atopic dermatitis in infants with topical pimecrolimus, a nonsteroid anti-inflammatory drug. The Journal of allergy and clinical immunology. 2002;110(2):277–284. doi: 10.1067/mai.2002.126500. [DOI] [PubMed] [Google Scholar]
- 5.Vissing NH, Jensen SM, Bisgaard H. Validity of information on atopic disease and other illness in young children reported by parents in a prospective birth cohort study. BMC medical research methodology. 2012;12:160. doi: 10.1186/1471-2288-12-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goodman SN, Berlin JA. The use of predicted confidence intervals when planning experiments and the misuse of power when interpreting results. Annals of internal medicine. 1994;121(3):200–206. doi: 10.7326/0003-4819-121-3-199408010-00008. [DOI] [PubMed] [Google Scholar]
- 7.Hanifin JM, Cooper KD, Ho VC, Kang S, Krafchik BR, Margolis DJ, et al. Guidelines of care for atopic dermatitis, developed in accordance with the American Academy of Dermatology (AAD)/American Academy of Dermatology Association "Administrative Regulations for Evidence-Based Clinical Practice Guidelines". J Am Acad Dermatol. 2004;50(3):391–404. doi: 10.1016/j.jaad.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Manthripragada AD, Pinheiro SP, MaCurdy TE, Saneinejad S, Worrall CM, Kelman JA, et al. Off-label topical calcineurin inhibitor use in children. Pediatrics. 2013;132(5):e1327–e132. doi: 10.1542/peds.2013-0931. [DOI] [PubMed] [Google Scholar]
- 9.Leung DY, Guttman-Yassky E. Deciphering the complexities of atopic dermatitis: shifting paradigms in treatment approaches. The Journal of allergy and clinical immunology. 2014;134(4):769–779. doi: 10.1016/j.jaci.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abuabara K, Margolis DJ. Do children really outgrow their eczema, or is there more than one eczema? The Journal of allergy and clinical immunology. 2013;132(5):1139–1140. doi: 10.1016/j.jaci.2013.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.