Abstract
Purpose
The aim of this study was to compare coregistration of the bladder wall, bladder masses, and pelvic lymph nodes between sequential and simultaneous PET and MRI acquisitions obtained during hybrid 18F-FDG PET/MRI performed using a diuresis protocol in bladder cancer patients.
Methods
Six bladder cancer patients underwent 18F-FDG hybrid PET/MRI, including IV Lasix administration and oral hydration, before imaging to achieve bladder clearance. Axial T2-weighted imaging (T2WI) was obtained approximately 40 minutes before PET (“sequential”) and concurrently with PET (“simultaneous”). Three-dimensional spatial coordinates of the bladder wall, bladder masses, and pelvic lymph nodes were recorded for PET and T2WI. Distances between these locations on PET and T2WI sequences were computed and used to compare in-plane (x-y plane) and through-plane (z-axis) misregistration relative to PET between T2WI acquisitions.
Results
The bladder increased in volume between T2WI acquisitions (sequential, 176 [139]mL; simultaneous, 255 [146]mL). Four patients exhibited a bladder mass, all with increased activity (SUV, 9.5–38.4). Seven pelvic lymph nodes in 4 patients showed increased activity (SUV, 2.2–9.9). The bladder wall exhibited substantially less misregistration relative to PET for simultaneous, compared with sequential, acquisitions in in-plane (2.8 [3.1]mm vs 7.4 [9.1]mm) and through-plane (1.7 [2.2]mm vs 5.7 [9.6]mm) dimensions. Bladder masses exhibited slightly decreased misregistration for simultaneous, compared with sequential, acquisitions in in-plane (2.2 [1.4]mm vs 2.6 [1.9]mm) and through-plane (0.0 [0.0]mm vs 0.3 [0.8]mm) dimensions. FDG-avid lymph nodes exhibited slightly decreased in-plane misregistration (1.1 [0.8]mm vs 2.5 [0.6]mm), although identical through-plane misregistration (4.0 [1.9]mm vs 4.0 [2.8]mm).
Conclusions
Using hybrid PET/MRI, simultaneous imaging substantially improved bladder wall coregistration and slightly improved coregistration of bladder masses and pelvic lymph nodes.
Keywords: bladder cancer, PET/MRI, coregistration, staging, hybrid imaging
Bladder cancer is a common malignancy, with an estimate of nearly 75,000 new diagnoses in the United States in 2014.1 Although CT and MRI are the most widely used imaging modalities for bladder cancer evaluation, both of these modalities share common limitations. Mural inflammation from a series of interventions commonly applied in these patients, including transurethral bladder tumor resections, biopsies, and intravesical Bacillus Calmette-Guerin therapy, can contribute to misinterpretations on imaging in terms of the presence and stage of residual tumor as well as the differentiation of recurrent tumor from chronic reactive changes.2–4 In addition, both CT and MRI, including MRI with diffusion-weighted imaging (DWI), are limited in determining the presence of metastatic lymph nodes.5,6 Although PET has also received attention for bladder cancer evaluation,7,8 standard 18F-FDG PET is limited by free excretion of FDG into the urine and subsequent high accumulation within the bladder lumen.9 This accumulation obscures, if not entirely masks, focal bladder lesions. In addition, alignment of the bladder between the PET and CT acquisitions in PET/CT is recognized to be suboptimal.10 Therefore, FDG PET has historically had a very limited role in bladder cancer evaluation in clinical practice.9
Recent protocol advances suggest that FDG PET may in fact have greater potential for bladder cancer assessment than previously considered. A number of investigations have explored a forced diuresis protocol entailing, most commonly, oral hydration, IV diuretic administration, and subsequent voiding, performed between radiotracer administration and PET imaging.9,11,12 This approach is intended to achieve clearance of excreted FDG from the bladder lumen while allowing for persistent uptake within the bladder wall itself. Recent studies of PET/CT using this approach have shown that bladder tumors indeed are consistently FDG avid and can be well visualized by PET.12,13 In addition, small metastatic pelvic lymph nodes have been reported to exhibit increased activity using 18F-FDG PET/CT, further supporting the potential value of PET for bladder cancer assessment.14,15
Although such methodological advances are encouraging, the ability to fully characterize bladder cancer is inherently limited using PET/CT. First, using the forced diuresis protocol, the bladder is actively reexpanding at the time of imaging, thus undergoing changes in shape and volume over time. Given that PET and CT are performed in sequential, rather than truly simultaneous, fashion using PET/CT, these dynamic changes of the bladder likely contribute to the recognized misregistration of the bladder between PET and CT acquisitions, even when obtained consecutively during a single examination.10 Patient motion may interfere with accurate coregistration as well, not just of the bladder itself, but also of other important pelvic structures such as small lymph nodes. Finally, the muscularis propria, a critical anatomic landmark in the staging of bladder cancer, is not well visualized as a distinct layer within the bladder wall on CT, further hindering comprehensive tumor evaluation by PET/CT.16
The advent of integrated PET/MRI scanners provides a novel imaging approach that may further improve upon recent advances in PET/CT in numerous respects.17 In PET/MRI, the acquisitions are spatially and temporal simultaneous, such that the degree of bladder filling and consequent bladder volume and shape are essentially identical between the PET and MRI image sets. This truly simultaneous acquisition is likely to also help compensate for the impact of patient motion on registration of the bladder and other pelvic structures. In addition, the higher contrast resolution of MRI, as compared with CT, allows improved delineation of the muscularis propria of the bladder wall and thereby improved local staging.16 Moreover, MRI offers a multiparametric approach that provides, along with anatomic assessment, quantitative metrics, including measures derived from DWI and dynamic contrast-enhanced (DCE) MRI. Such metrics have shown significant associations with bladder cancer aggressiveness in past studies.18,19 Furthermore, simultaneous PET allows for determination of SUV as an additional metric that can be incorporated into a multiparametric panel for bladder cancer assessment.
Although integrated PET/MRI systems have generated a large amoun of interest, these systems entail a large financial investment and are only being used clinically at a small number of centers. Centers may instead elect to separately perform conventional PET and MRI scans, with subsequent retrospective fusion. Therefore, the benefit of simultaneous imaging, as is only achievable with an integrated system, is important to investigate in order to ascertain such a system’s true value. Bladder cancer provides an appropriate setting for such an investigation given the dynamic changes in bladder size and shape during short time intervals over the course of a single scan. In this study, our aim was to compare the accuracy of coregistration of the bladder wall, bladder lesions, and pelvic lymph nodes between sequential and simultaneous acquisitions obtained during 18F-FDG PET/MRI performed using a forced diuresis protocol in bladder cancer patients.
PATIENTS AND METHODS
This prospective Health Insurance Portability and Accountability Act-compliant study received institutional review board approval. All subjects provided written informed consent. Six patients (mean [SD] age, 68 [8] years; 4 men, 2 women) with a history of biopsy-proven bladder cancer comprised the study participants. Three patients were awaiting treatment for recently diagnosed tumor, 1 patient had recurrent tumor after prior resection, and 2 patients were undergoing surveillance for recurrent tumor after prior therapy. All patients were scheduled to undergo clinically indicated MRI urography of the upper and lower tracts before recruitment for this study.
Patients fasted for 4 hours before receiving an IV injection of 10.0 mCi of FDG via antecubital vein. The patients then waited quietly within a dimly lit room. After approximately 45 minutes, the patients received an IV injection of 0.5 mg/kg furosemide (maximum dose of 40 mg) and drank 1 to 1.5 L of water. Next, patients were instructed to forcibly void twice. Finally, patients were asked to hold their urine while bladder refilling occurred during the remainder of the examination.
Imaging was performed using a whole-body integrated PET/MRI system (Siemens Biograph mMR) that entails a 3-T MRI scanner with an internal PET director. A pelvic phased-array coil was used for MRI image acquisition. As part of our institution’s routine MRI protocol for bladder cancer evaluation, T1-, T2-, and diffusion-weighted sequences were obtained from the abdomen and pelvis. The sequence of the pelvis evaluated in this report was a high-resolution axial T2-weighted imaging (T2WI) sequence of the bladder (TR/TE, 5120/118 milliseconds; FOV, 180 × 1 80 mm; matrix, 256 × 256; slice thickness, 3 mm; 3 averages; parallel imaging factor of 2). PET was performed in sinogram mode for a duration of 5 minutes (transaxial FOV, 600 mm; transaxial matrix, 172 × 172; axial FOV, 258 mm) and reconstructed using an iterative 3-dimensional algorithm at 3 iterations and 21 subsets. A breath-hold dual-echo Dixon-based acquisition was used for attenuation correction.20 To allow for a comparison of registration of sequential and simultaneous acquisitions, the axial T2WI sequence was performed both at the start of the examination as well as concurrently with the PET acquisition that was performed toward the end of the protocol before administration of gadolinium chelate. Using this approach, the 2 axial T2WI acquisitions were obtained approximately 40 minutes apart. After PET acquisition, 0.1 mmol/kg of gadobutrol (Bayer Healthcare Pharmaceuticals, Leverkusen, Germany) was administered intravenously at a rate of 3 mL/s, and DCE was performed using a radial golden angle compressed imaging sequence (spatial resolution, 1 × 1 × 3 mm; reconstructed temporal resolution of 2.3 seconds).21
Images were evaluated in conjunction by a radiologist with fellowship training in abdominal MRI and 6 years of experience as well as a nuclear medicine physician with 10 years of experience. MIM version 5.4 (MIM software; Cleveland, Ohio) was used for coregistration of MRI and PET data sets. For each patient, the x, y, and z spatial coordinates of various locations were determined for PET and each of the 2 axial T2WI acquisitions (Fig. 1). Assessed locations were the anterior, posterior, superior, and inferior walls of the urinary bladder; the visually estimated inner and outer wall of the primary bladder tumor; and the visually estimated center of any pelvic lymph nodes showing increased FDG activity. The distance of these locations between PET and each of the 2 axial T2WI (1 simultaneously acquired and 1 sequential) was calculated based on the method used in prior studies.22,23 The in-plane (x, y) error was calculated as [Δx2 + Δy2]0.5, and the through-plane (z) error was calculated as Δz. These differences were summarized separately for the bladder wall (pooling differences for each of the 4 evaluated aspects of the bladder wall into a single assessment), bladder masses (pooling differences for the inner and outer aspects of bladder masses into a single assessment), and pelvic lymph nodes. The readers also measured the SUV value within bladder masses and pelvic lymph nodes. In addition, in all patients, the volume of the bladder was computed for each of the 2 axial T2WI acquisitions using the prorate ellipsoid formula (product of anterior-posterior, left-right, and cranial-caudal diameters × 0.52); the cranial-caudal diameter of the bladder was estimated from the axial T2WI by multiplying the number of slices through the bladder lumen by the slice thickness.
FIGURE 1.
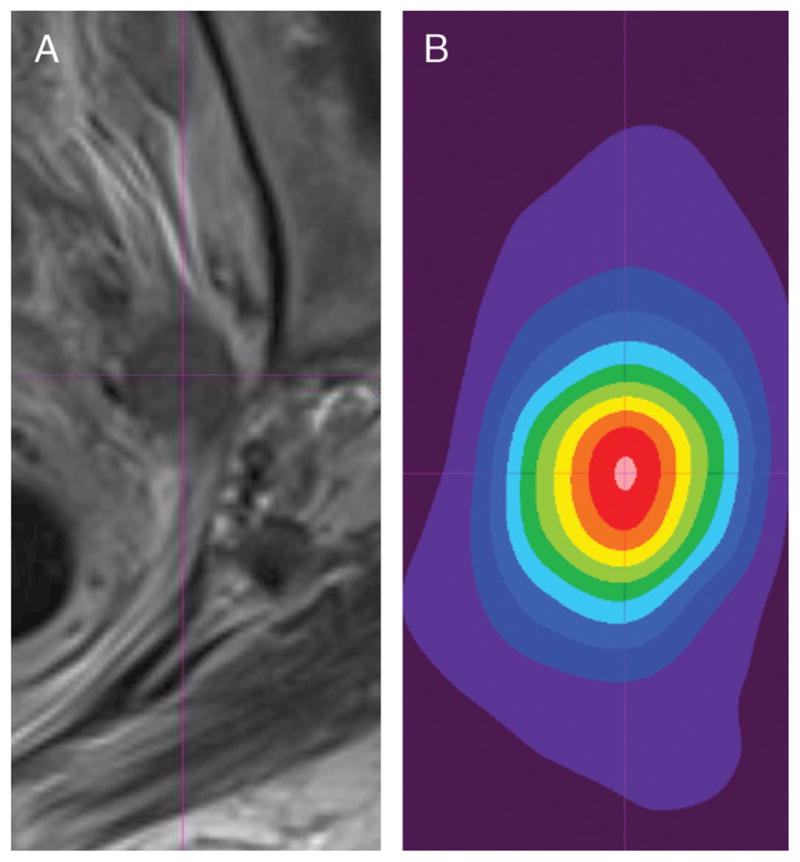
A 66-year-old woman with high-grade muscle-invasive bladder cancer and metastatic pelvic lymphadenopathy. A, Axial turbo spin-echo (TSE) T2WI and B, 18F-FDG PET image with 10-band rainbow color scale of abnormal left pelvic lymph node that shows increased FDG activity. Center of lymph node was visually estimated on each image set (center of thin purple crosshairs), and 3-dimensional spatial coordinates of center were recorded for each modality. These coordinates were then used to calculate misregistration between image sets.
RESULTS
In the 6 patients, the bladder substantially increased in volume between the 2 acquisitions (mean [SD] volume of 255 [146]mL on the T2WI obtained simultaneously with PET in comparison with 176 [139]mL on the sequential T2WI obtained earlier in the PET/MRI examination). A focal bladder mass was present in 4 patients. In these 4 instances, the mass demonstrated visually increased FDG activity and elevated SUV (mean [SD], 22.6 [12.8]; range, 9.5–38.4). A total of 7 pelvic lymph nodes in 4 patients showed increased FDG activity (mean [SD], 6.3 [2.5]; range, 2.2–9.9). These nodes had a mean (SD) long axis diameter of 14 (8) mm (range, 9–31 mm) and mean (SD) short axis diameter of 10 (6) mm (range, 4–23 mm).
Table 1 summarizes the registration errors for sequential and simultaneous acquisitions. The bladder wall exhibited substantially less misregistration between PETand MRI for simultaneous, compared with sequential, acquisitions in both the in-plane (2.8 [3.1]mm vs 7.4 [9.1] mm, respectively) and through-plane (1.7 [2.2]mm vs 5.7 [9.6]mm, respectively) dimensions (Fig. 2). Bladder masses exhibited a slight decrease in misregistration for simultaneous, compared with sequential, acquisitions in both the in-plane (2.2 [1.4]mm vs 2.6 [1.9]mm, respectively) and through-plane (0.0 [0.0]mm vs 0.3 [0.8]mm, respectively) dimensions (Fig. 3). FDG-avid pelvic lymph nodes exhibited a slight decrease in misregistration in the in-plane dimension (1.1 [0.8]mm vs 2.5 [0.6]mm, respectively), although identical misregistration in the through-plane dimension (4.0 [1.9]mm vs 4.0 [2.8]mm, respectively) (Fig. 4).
TABLE 1.
Misregistration (Millimeters) Between Sequential and Simultaneous PET and MRI Image Sets Obtained During the Course of a Single PET/MRI Examination
| Structure | In-Plane Misregistration
|
Through-Plane Misregistration
|
||
|---|---|---|---|---|
| Sequential | Simultaneous | Sequential | Simultaneous | |
| Bladder wall | 7.4 (9.1) | 2.8 (3.1) | 5.7 (9.6) | 1.7 (2.2) |
| Bladder mass | 2.6 (1.9) | 2.2 (1.4) | 0.3 (0.8) | 0.0 (0.0) |
| Pelvic lymph nodes | 2.5 (0.6) | 1.1 (0.8) | 4.0 (2.8) | 4.0 (1.9) |
Data reports as mean (SD).
FIGURE 2.
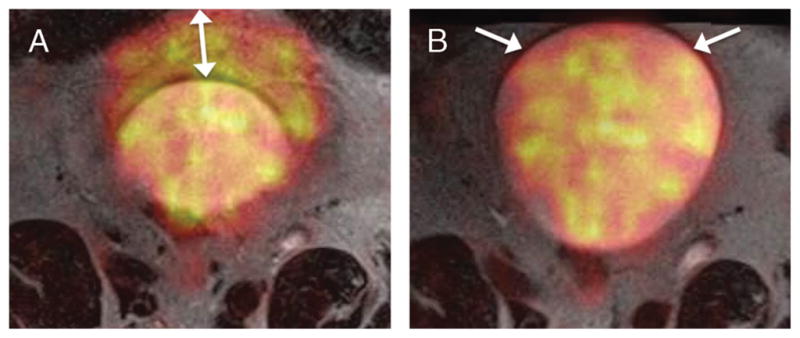
A 68-year-old man with prior transurethral resection of bladder cancer. A, Fused image from axial TSE T2WI and 18F-FDG PET acquisitions obtained sequentially show marked misregistration of anterior bladder wall (arrow) given active bladder filling and expansion between the 2 scans.
B, Fused image from simultaneously acquired axial TSE T2WI and 18F-FDG PET shows excellent registration of anterior bladder wall (arrows).
FIGURE 3.
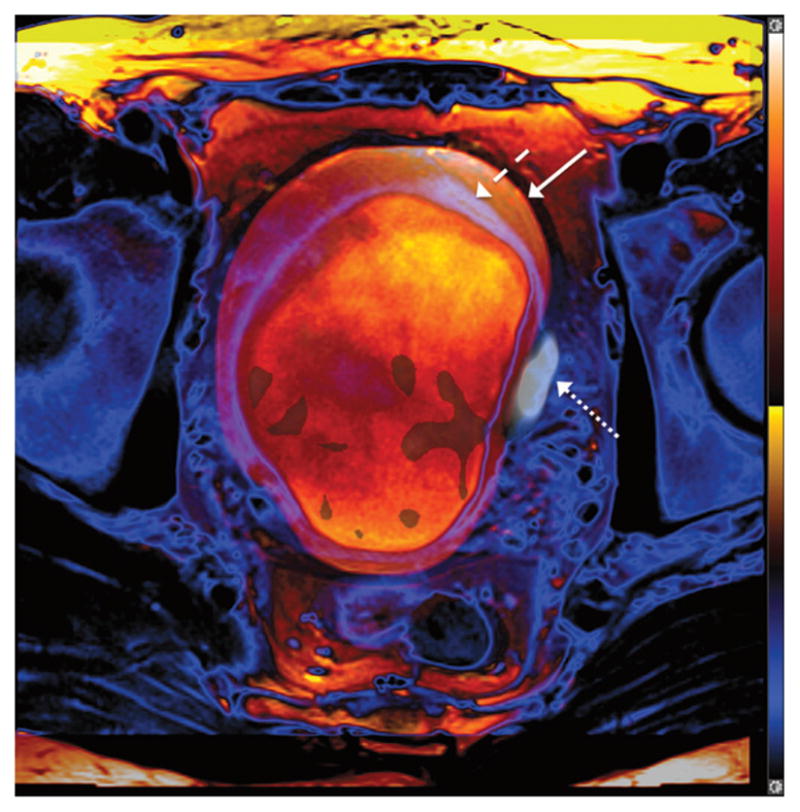
A 52-year-old man with muscle-invasive high-grade bladder cancer. Fusion of 2 axial TSE T2WIs obtained at separate points during the PET/MRI examination, with the bladder wall shaded in purple (dashed arrow) and blue (solid arrow) for the early and later acquisitions, respectively. Bladder has expanded between the 2 acquisitions, as indicated by the outer positioning of the bladder wall in the later image. Left lateral bladder wall tumor exhibits increased 18F-FDG activity (dotted arrow) on PET obtained simultaneously as later T2WI. Tumor shows improved coregistration with simultaneous, rather than sequential, T2WI.
FIGURE 4.
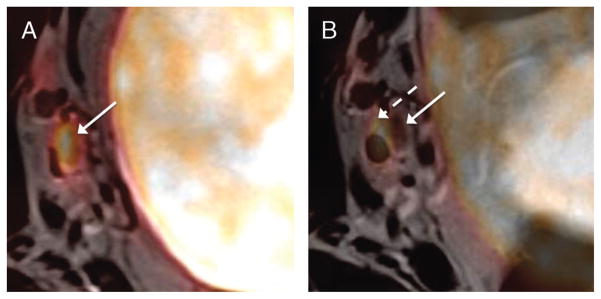
A 52-year-old man with muscle-invasive high-grade bladder cancer (same patient as in Figure 2). A, Fused image from axial TSE T2WI and simultaneously acquired 18F-FDG PET acquisition demonstrates small right pelvic side wall lymph node with increased FDG activity (solid arrow). Registration of the lymph node on the 2 images is excellent. B, Fused image from sequentially obtained axial T2WI and PET demonstrates misregistration between lymph node on MRI (dashed arrow) and PET (solid arrow). Also note improved coregistration of bladder wall on simultaneous image.
DISCUSSION
Past studies have aimed to assess the benefit of simultaneous acquisitions in PET/MRI by comparing to PET/CT 23 or to separate PET and MRI examinations with subsequent retrospective fusion.10 In either of these contexts, additional patient- and imaging-related factors aside from solely the timing of the acquisitions vary between the comparisons. In our study, by comparing sequential and simultaneous acquisitions obtained over the course of a single PET/MRI examination, we more directly assess the impact of simultaneous imaging on fusion accuracy, essentially eliminating other potential confounding sources of misregistration. Precise coregistration is important for improving the accuracy of clinical staging, which could have significant impact on clinical management (such as surgical, medical, and radiation planning) and achieving accurate attenuation correction and quantification of metrics across the modalities.23
Registration of the bladder wall itself improved substantially in both the in-plane and through-plane directions. This improvement is expected given the active filling and associated expansion of the bladder over short periods. Although bladder masses also exhibited improved registration in both dimensions with simultaneous imaging, this improvement was very small in comparison with the improved alignment of the bladder wall. It is possible that the diseased segment of the bladder wall was held largely fixed in place over time given the presence of tumor, despite expansion and motion of the remainder of the bladder wall that was free of tumor. Because the 4 tumors present in this cohort were all bulky lesions, it is possible that a greater benefit would have been observed for smaller lesions. In addition, pelvic lymph nodes were slightly better aligned in the in-plane direction using simultaneous imaging, possibly due to better compensation for patient motion using simultaneous imaging. On the other hand, the essentially identical through-plane misregistration of pelvic lymph nodes for sequential and simultaneous scans may relate to the thicker slices of both PET and MRI in the through-plane registration, as well as a possibly greater impact of patient motion in this direction.
The forced diuresis was an important component of our protocol for achieving clearance of excreted tracer from the bladder, thereby facilitating visualization of increased activity and quantification of SUV within bladder lesions. This was a noninvasive and easily implemented approach that was well tolerated by patients. An alternate approach entailing bladder irrigation after urinary catheter insertion has been described in the literature.24,25 However, such a technique is invasive, uncomfortable, and entails greater time and cost.9 Although the diuresis protocol incorporating oral hydration and furosemide was successful in our protocol, the logistics for technique have been variable across studies in the literature,11–13 and standardization remains required. For instance, the optimal amount of hydration and furosemide to be administered and the optimal agents between radiotracer administration and diuresis and between diuresis and imaging are not yet established.
Integrated PET/MRI is hoped to provide a comprehensive evaluation of bladder cancer that is not currently achievable with any single imaging modality. As previously described, the forced diuresis protocol allowed identification of focal increased FDG activity in the 4 bladder masses, in comparison with a lack of FDG activity in the bladder wall in 2 patients being monitored for recurrent tumor after prior treatment. In addition, increased FDG activity was well visualized within pelvic lymph nodes. Although not evaluated in this work, the combination of T2WI and DWI has shown high performance in past studies for determining the local T stage of bladder cancer.26–28 Moreover, PET/MRI provides a panel of quantitative metrics that can further aid risk assessment. For instance, apparent diffusion coefficient values of bladder cancer have previously been shown to correlate with tumor grade, stage, likelihood of neoadjuvant chemotherapy response, as well as likelihood of recurrence after treatment,18,29,30 whereas parameters from DCE have shown significant associations with grade and likelihood of recurrence.19 By combining these metrics with SUV, it may be possible to provide more precise prognostic information that will aid treatment selection. Ultimately, by achieving lesion detection, T staging, N staging, and a multiparametric quantitative assessment of tumor aggressiveness, PET/MRI using the described protocol has potential to serve as a powerful tool for guiding bladder cancer management.
This study has a number of limitations. First, the sample size was small. However, this was a pilot study to assess for a potential impact of simultaneous imaging on registration accuracy, as well as to evaluate the effectiveness of our PET/MRI protocol using forced diuresis to allow visualization of FDG activity within bladder masses. In addition, the evaluated structures were subjectively localized on the included image sets. This methodology is similar to that of earlier studies,22,23 and we are unaware of an alternate automated approach. In addition, although the bladder tumors were all histologically proven, we lack histological confirmation that the evaluated lymph nodes were metastatic in nature. In view of these limitations, larger studies with clinical and pathological outcomes clearly remain necessary. Such studies are currently underway at our institution.
In conclusion, in this pilot study, integrated 18F-FDG PET/MRI using a forced diuresis protocol achieved clearance of radiotracer from the bladder lumen and subsequent visualization of marked increased radiotracer activity in bladder masses. Simultaneously acquired PET and MRI acquisitions exhibited substantially improved registration of the bladder wall in comparison with sequentially acquired image sets, as well as slight improvement in registration of bladder masses and pelvic lymph nodes. On this basis, PET/MRI using this protocol seems to have clinical potential to assist risk assessment and treatment decisions for bladder cancer. However, future studies remain needed.
Acknowledgments
Sources of funding: Supported by the Society of Abdominal Radiology Howard S. Stern Research Grant.
References
- 1.American Cancer Society. Cancer Facts & Figures 2015. Atlanta, GA: American Cancer Society; 2015. [Google Scholar]
- 2.El-Assmy A, Abou-El-Ghar ME, Refaie HF, et al. Diffusion-weighted magnetic resonance imaging in follow-up of superficial urinary bladder carcinoma after transurethral resection: initial experience. BJU Int. 2012;110:E622–E627. doi: 10.1111/j.1464-410X.2012.11345.x. [DOI] [PubMed] [Google Scholar]
- 3.Wang HJ, Pui MH, Guo Y, et al. Diffusion-weighted MRI in bladder carcinoma: the differentiation between tumor recurrence and benign changes after resection. Abdom Imaging. 2014;39:135–141. doi: 10.1007/s00261-013-0038-0. [DOI] [PubMed] [Google Scholar]
- 4.Sadow CA, Silverman SG, O’Leary MP, et al. Bladder cancer detection with CT urography in an Academic Medical Center. Radiology. 2008;249:195–202. doi: 10.1148/radiol.2491071860. [DOI] [PubMed] [Google Scholar]
- 5.Roy C, Bierry G, Matau A, et al. Value of diffusion-weighted imaging to detect small malignant pelvic lymph nodes at 3T. Eur Radiol. 2010;20:1803–1811. doi: 10.1007/s00330-010-1736-4. [DOI] [PubMed] [Google Scholar]
- 6.Giannarini G, Petralia G, Thoeny HC. Potential and limitations of diffusion-weighted magnetic resonance imaging in kidney, prostate, and bladder cancer including pelvic lymph node staging: a critical analysis of the literature. Eur Urol. 2012;61:326–340. doi: 10.1016/j.eururo.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 7.Ho L, Quan V, Henderson R, et al. High-grade urothelial carcinoma of the prostate on FDG PET-CT. Clin Nucl Med. 2007;32:746–747. doi: 10.1097/RLU.0b013e318123f83e. [DOI] [PubMed] [Google Scholar]
- 8.Kibel AS, Dehdashti F, Katz MD, et al. Prospective study of [18F]fluorodeoxyglucose positron emission tomography/computed tomography for staging of muscle-invasive bladder carcinoma. J Clin Oncol. 2009;27:4314–4320. doi: 10.1200/JCO.2008.20.6722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fang N, Wang YL, Zeng L, et al. Feasible method to enable clear visualization of suspected bladder cancer with 18F-FDG PET/CT. Clin Imaging. 2014;38:704–709. doi: 10.1016/j.clinimag.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 10.Brendle CB, Schmidt H, Fleischer S, et al. Simultaneously acquired MR/PET images compared with sequential MR/PET and PET/CT: alignment quality. Radiology. 2013;268:190–199. doi: 10.1148/radiol.13121838. [DOI] [PubMed] [Google Scholar]
- 11.Coquan E, Lasnon C, Joly F, et al. Diuretic F-FDG PET/CT for therapy monitoring in urothelial bladder cancer. Eur J Nucl Med Mol Imaging. 2014;41:1818–1819. doi: 10.1007/s00259-014-2800-0. [DOI] [PubMed] [Google Scholar]
- 12.Nayak B, Dogra PN, Naswa N, et al. Diuretic 18F-FDG PET/CT imaging for detection and locoregional staging of urinary bladder cancer: prospective evaluation of a novel technique. Eur J Nucl Med Mol Imaging. 2013;40:386–393. doi: 10.1007/s00259-012-2294-6. [DOI] [PubMed] [Google Scholar]
- 13.Harkirat S, Anand S, Jacob M. Forced diuresis and dual-phase F-fluorodeoxyglucose-PET/CT scan for restaging of urinary bladder cancers. Indian J Radiol Imaging. 2010;20:13–19. doi: 10.4103/0971-3026.59746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mertens LS, Fioole-Bruining A, van Rhijn BW, et al. FDG-positron emission tomography/computerized tomography for monitoring the response of pelvic lymph node metastasis to neoadjuvant chemotherapy for bladder cancer. J Urol. 2013;189:1687–1691. doi: 10.1016/j.juro.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 15.Goodfellow H, Viney Z, Hughes P, et al. Role of fluorodeoxyglucose positron emission tomography (FDG PET)-computed tomography (CT) in the staging of bladder cancer. BJU Int. 2014;114:389–395. doi: 10.1111/bju.12608. [DOI] [PubMed] [Google Scholar]
- 16.Verma S, Rajesh A, Prasad SR, et al. Urinary bladder cancer: role of MR imaging. Radiographics. 2012;32:371–387. doi: 10.1148/rg.322115125. [DOI] [PubMed] [Google Scholar]
- 17.Catalano OA, Rosen BR, Sahani DV, et al. Clinical impact of PET/MR imaging in patients with cancer undergoing same-day PET/CT: initial experience in 134 patients—a hypothesis-generating exploratory study. Radiology. 2013;269:857–869. doi: 10.1148/radiol.13131306. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi S, Koga F, Yoshida S, et al. Diagnostic performance of diffusion-weighted magnetic resonance imaging in bladder cancer: potential utility of apparent diffusion coefficient values as a biomarker to predict clinical aggressiveness. Eur Radiol. 2011;21:2178–2186. doi: 10.1007/s00330-011-2174-7. [DOI] [PubMed] [Google Scholar]
- 19.Tuncbilek N, Kaplan M, Altaner S, et al. Value of dynamic contrast-enhanced MRI and correlation with tumor angiogenesis in bladder cancer. AJR Am J Roentgenol. 2009;192:949–955. doi: 10.2214/AJR.08.1332. [DOI] [PubMed] [Google Scholar]
- 20.Eiber M, Martinez-Moller A, Souvatzoglou M, et al. Value of a Dixon-based MR/PET attenuation correction sequence for the localization and evaluation of PET-positive lesions. Eur J Nucl Med Mol Imaging. 2011;38:1691–1701. doi: 10.1007/s00259-011-1842-9. [DOI] [PubMed] [Google Scholar]
- 21.Rosenkrantz AB, Geppert C, Grimm R, et al. Dynamic contrast-enhanced MRI of the prostate with high spatiotemporal resolution using compressed sensing, parallel imaging, and continuous golden-angle radial sampling: preliminary experience. JMRI-J Magn Reson Im. 2014 doi: 10.1002/jmri.24661. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohade C, Osman M, Marshall LN, et al. PET-CT: accuracy of PET and CT spatial registration of lung lesions. Eur J Nucl Med Mol Imaging. 2003;30:721–726. doi: 10.1007/s00259-002-1055-3. [DOI] [PubMed] [Google Scholar]
- 23.Rakheja R, DeMello L, Chandarana H, et al. Comparison of the accuracy of PET/CT and PET/MRI spatial registration of multiple metastatic lesions. AJR Am J Roentgenol. 2013;201:1120–1123. doi: 10.2214/AJR.13.11305. [DOI] [PubMed] [Google Scholar]
- 24.Leisure GP, Vesselle HJ, Faulhaber PF, et al. Technical improvements in fluorine-18-FDG PET imaging of the abdomen and pelvis. J Nucl Med Technol. 1997;25:115–119. [PubMed] [Google Scholar]
- 25.Lin WY, Wang KB, Tsai SC, et al. Unexpected accumulation of F-18 FDG in the urinary bladder after bladder irrigation and retrograde filling with sterile saline: a possible pitfall in PET examination. Clin Nucl Med. 2009;34:560–563. doi: 10.1097/RLU.0b013e3181b06c41. [DOI] [PubMed] [Google Scholar]
- 26.El-Assmy A, Abou-El-Ghar ME, Mosbah A, et al. Bladder tumour staging: comparison of diffusion- and T2-weighted MR imaging. Eur Radiol. 2009;19:1575–1581. doi: 10.1007/s00330-009-1340-7. [DOI] [PubMed] [Google Scholar]
- 27.Takeuchi M, Sasaki S, Ito M, et al. Urinary bladder cancer: diffusion-weighted MR imaging—accuracy for diagnosing T stage and estimating histologic grade. Radiology. 2009;251:112–121. doi: 10.1148/radiol.2511080873. [DOI] [PubMed] [Google Scholar]
- 28.Watanabe H, Kanematsu M, Kondo H, et al. Preoperative T staging of urinary bladder cancer: does diffusion-weighted MRI have supplementary value? AJR Am J Roentgenol. 2009;192:1361–1366. doi: 10.2214/AJR.08.1430. [DOI] [PubMed] [Google Scholar]
- 29.Yoshida S, Koga F, Kobayashi S, et al. Role of diffusion-weighted magnetic resonance imaging in predicting sensitivity to chemoradiotherapy in muscle-invasive bladder cancer. Int J Radiat Oncol Biol Phys. 2012;83:e21–e27. doi: 10.1016/j.ijrobp.2011.11.065. [DOI] [PubMed] [Google Scholar]
- 30.Funatsu H, Imamura A, Takano H, et al. Can pretreatment ADC values predict recurrence of bladder cancer after transurethral resection? Eur J Radiol. 2012;81:3115–3119. doi: 10.1016/j.ejrad.2012.06.009. [DOI] [PubMed] [Google Scholar]


