Abstract
IMPORTANCE
Obesity affects nearly one sixth of U.S. children and results in alterations to body composition and physiology that can affect drug disposition, possibly leading to therapeutic failure or toxicity. The depth of available literature regarding obesity’s effect on drug safety, pharmacokinetics (PK) and dosing in obese children is unknown.
OBJECTIVE
To perform a systematic literature review describing the current evidence of the effect of obesity on drug disposition in children.
EVIDENCE REVIEW
We searched the Medline, Cochrane, and Embase databases (January 1970–December 2012) and included studies if they contained clearance, volume of distribution, or drug concentration data in obese children (age ≤18 years). We compared exposure and weight-normalized volume of distribution and clearance between obese and non-obese children. We explored the relationship between drug physicochemical properties and clearance and volume of distribution.
FINDINGS
Twenty studies met inclusion criteria and contained pharmacokinetic data for 21 drugs. The median number of obese children studied per drug was 10 (range 1–112), ages ranged from 0–29 years. Dosing schema varied and were based on a fixed dose (n=6, 29%), body weight (n=10, 48%), and body surface area (n=4, 19%). Clinically significant pharmacokinetic alterations were observed in obese children for 65% (11/17) of studied drugs. Pharmacokinetic alterations resulted in substantial differences in exposure between obese and non-obese children for 38% (5/13) of drugs. We found no association between drug lipophilicity or Biopharmaceutical Drug Disposition Classification System class and changes in volume of distribution or clearance due to obesity.
CONCLUSIONS AND RELEVANCE
Consensus is lacking on the most appropriate weight-based dosing strategy. Prospective pharmacokinetic trials in obese children are needed to ensure therapeutic efficacy and enhance drug safety.
The prevalence of childhood obesity has stabilized at epidemic proportions. Nearly 1 out of every 6 children or adolescents living in the U.S. has a body mass index (BMI) for age and sex above the 95th percentile and is considered obese.1 Obese children experience increased rates and severity of multiple disease states, require more frequent and more complex medical interventions,2–7 and use significantly more prescription medications than their non-obese peers.8
Relatively little is known about the impact of childhood obesity on drug pharmacokinetics (PK). Obesity demonstrates important alterations in physiology such as changes in tissue composition, increased circulating blood volume and cardiac output, altered regional flow distribution, and impaired liver and kidney function.9–11 All of these physiologic alterations can affect PK parameters including drug absorption, volume of distribution (V), metabolism, and elimination.12–14 Furthermore, physiochemical properties of a drug, such as lipid solubility or relative protein binding, might have differential effects on drug PK in obese versus non-obese children.14 To account for these physiologic and pharmacologic factors, some clinicians adjust weight-based dosing using various metrics of body size, such as ideal body weight (IBW). However, these dosing strategies are largely based on theoretical considerations or extrapolated from studies in adults.15 Currently, there is no comprehensive, evidence-based understanding of the impact of childhood obesity on drug PK.
To better understand the current evidence base, we performed a systematic review of published PK studies conducted over the preceding 4 decades in obese children and adolescents. We addressed the question of whether critical obesity-related physiologic parameters change drug PK in children and evaluated the impact on PK of important drug physiochemical properties including lipophilicity (logP) and Biopharmaceutical Drug Disposition Classification System (BDDCS) class, a classification based on drug permeability and solubility.16
METHODS
Study Identification
We performed a systematic literature review using the Medline, Cochrane, and Embase databases (January 1970–December 2012). The search strategy was defined in collaboration with librarians at Duke University Medical Center Library and the National Library of Medicine. Search terms included: pharmacokinetics, pharmacodynamics, PK/PD, medication, dosing, dose, dosage, overweight, obesity, and obese. Exact search strategies are displayed in Supplemental eTables 1 and 2. There were no language restrictions. We identified additional studies through pertinent review of article bibliographies and conference abstracts.
Study Selection
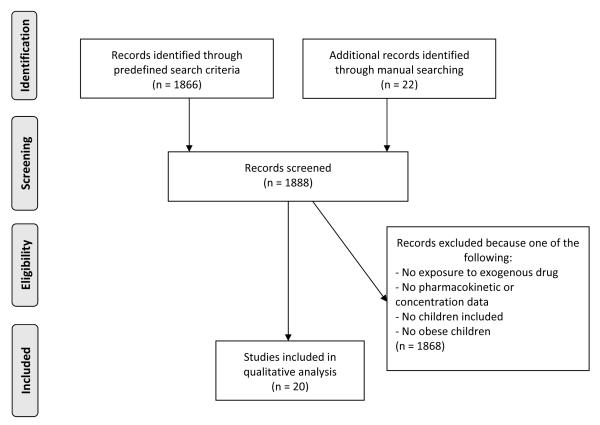
We compiled the final search results into a single library using Endnote X5 (Thomson Reuters, San Francisco, CA). We independently reviewed study abstracts for inclusion in the final analysis (M.G. and K.B.). If an abstract lacked sufficient detail, the full article was reviewed. We included studies if they contained any PK data for obese children (ages 2–18 years) including clearance (CL), V, area under the curve, half-life, or drug concentration data. Articles with only pharmacodynamics results were not included. Because definitions of obesity and overweight have varied over the years, we included all studies in which the authors used an accepted definition of obesity, regardless of the criteria used. Studies that included both obese and overweight children in the same analysis group were also included but are clearly identified.17–23 The different phases of systematic review are displayed in a flowchart, as described by the PRISMA 2009 statement24 (Figure 1).
Figure 1.
Study Outline of Systematic Literature Search and Inclusion of Identified Articles
Data Extraction
We extracted dosing and PK data and information regarding the body weight measurement used for dosing. Total body measurement (TBM) was defined as the actual total body weight or body surface area (BSA) of the child. IBW was defined as the weight at the 50th percentile of a weight for height on a sex-adjusted growth curve. Adjusted body measurement was defined as any measurement that relied on scaling between ideal and total body weight (e.g., IBW + 40%*[total body weight – IBW]) or adjusted BSA. We extracted TBM-normalized CL (ml/min/kg) or V (L/kg) values when they were reported or calculated TBM-normalized values by dividing CL and V with weights reported in the original source (individual or study mean weight values). Whenever possible, data were included for children only. We did not extract pharmacodynamics or safety data, as most studies did not report these data and were not powered to do so.
Comparison of PK Data in Obese and Non-Obese Control Children
We qualified exposure to the studied drug in obese children as sub-therapeutic, therapeutic, or supra-therapeutic based on target ranges provided in the original source (e.g., a target trough level or area under the curve). We compared exposure and weight-normalized PK parameters in obese children to non-obese controls within each study when available and expressed values in obese children as a percentage (%) of controls. To evaluate the association between changes in CL or V and the drug’s physicochemical properties, we plotted the ratio of CL in obese children to CL in control children, and the ratio of V in these 2 populations against the drug’s logP and BDDCS class. V and CL were normalized to actual body weight—either total body weight or BSA, depending on the weight metric used to dose the respective drug.
RESULTS
We identified 1888 unique publications, of which 1868 (99%) were excluded because they did not describe PK of an exogenous drug, they did not contain any PK data, or they did not include overweight or obese children (Figure 1). The remaining 20 publications contained PK data for 21 drugs, including 7 anti-neoplastic drugs, 4 anticonvulsants, 4 antibiotics, 3 analgesic/anesthetic drugs, 2 respiratory stimulants, and 1 immunosuppressant (Table 1). Six out of 21 (29%) drugs were not studied in a formal prospective PK trial (gentamicin29; vancomycin17,18,30; valproic acid19; divalproex sodium20; busulfan21; and cyclosporine36). PK data for these studies were collected following drug administration per standard of care, frequently with sparse sampling.
Table 1.
Pharmacokinetic Studies in Obese Children
| Drug | N | Mean age, years (SD or range) |
Definition of obesity |
|---|---|---|---|
| Analgesics/anesthetics | |||
| Acetaminophen25 | 12 | 15 (2) | BMI ≥ 95%ile |
| Antipyrine26 | 3 | 17 (0) | BMI ≥ 95%ile |
| Propofol27 | 20 | 16 (2) | BMI ≥ 95%ile |
| Antibiotics | |||
| Cefazolin28 | 5 | 7 (3) | BMI ≥ 95%ile |
| Gentamicin29 | 25 | 10 (4) | BMI ≥ 95%ile |
| Tobramycin28 | 5 | 7 (3) | BMI ≥ 95%ile |
| Vancomycin17,18,30 | 112 | (0.2, 18) | BMI ≥ 85%17,18, ≥ 95%ile30 |
| Anticonvulsants | |||
| Carbamazepine31,19 | 9 | (15, 29) | BMI ≥ 95%ile31, BMI ≥ 25 (study mean 30)19 |
| Divalproex sodium20 | 5 | 9 (5, 14) | > 115% IBWa |
| Midazolam32 | - | - | BMI ≥ 95%ile |
| Valproic acid19 | 5 | 21 (15, 29) | BMI ≥ 25 (study mean 27) |
| Antineoplastics | |||
| Busulfan21 | 22 | 7 (6) | BMI ≥ 85%ile |
| Cytarabine33 | 10 | 9 (2, 18) | BMI ≥ 95%ile |
| Doxorubicin34,35 | 4 | (9, 16) | BMI ≥ 95%ile |
| Etoposide33,35 | 25 | 9 (2, 18) | BMI ≥ 95%ile |
| Mercaptopurine22 | 9 | 7 (4) | 75% W/H |
| Methotrexate33 | 41 | 9 (2, 18) | BMI ≥ 95%ile |
| Teniposide33 | 10 | 9 (2, 18) | BMI ≥ 95%ile |
| Immunosuppressants | |||
| Cyclosporine36 | 30 + 72 | 15 (4) | BMI ≥ 95% + BMI ≥ 85%ile |
| Respiratory stimulants | |||
| Caffeine37 | 3 | 17 (0) | BMI ≥ 95%ile |
| Theophylline23 | 9 | - | >125% IBW |
BMI %, age- and sex-specific BMI percentile (≥85th considered overweight, ≥95th considered obese)38; % IBW, percentile of ideal body weight (>120% considered overweight)39; BMI ≥ 25 (considered moderate obesity)40; % W/H, percentile of weight for height.38
For divalproex sodium, the authors did not stratify based on obesity, but found an empiric difference in PK for children less than and greater than 115% IBW.
Study Population
Study definitions of obesity and overweight varied. The majority of studies used the currently accepted Centers for Disease Control and Prevention definition for children of BMI percentile ≥95% for obesity and ≥85% for overweight (18/21 drugs). Other definitions included IBW percentile ≥125% and ≥115% (2/21), weight-for-height percentile ≥75% (1/21), and absolute BMI ≥ 25 (1/21). Thirteen out of 20 (65%) studies described PK parameters for obese children separately versus combining obese and overweight children in 1 analysis group. The median number of obese children studied per drug was 10 (range 1–112 subjects), with 12/21 (57%) studies including ≤10 obese children. Patient ages ranged from 0–29 years (1 study described PK in children and adults together).19
Studied Dosing Schedules and Exposure
Dosing schema showed considerable variability. Drugs were dosed using a fixed dose (n=6, 29%), based on body weight (n=10, 48%) or BSA (n=4, 19%), or based on body weight in 1 study and BSA in another study (n=1, 5%). When drugs were dosed by body weight or BSA, the body weight measurement used for dosing was as follows: TBM (n=7, 33%), adjusted body measurement (n=5, 24%), or both (n=3, 14%). No drug was dosed based on IBW (Table 2).
Table 2.
Drug Exposure in Obese Children by Dosing Method
| Drug | Dosed per | Body weight measurement |
Exposure in obesity |
Obese vs. control |
|---|---|---|---|---|
| Mercaptopurine22 | m2 | TBM | Subtherapeutic | ↓ |
| Vancomycin17,18,30 | kg | TBM | Subtherapeutic17,30 | ↔ 17,30 |
| TBM | Therapeutic18 | ↑ 18 | ||
| Teniposide33 | kg | TBM | Therapeutic | ↔ a |
| Methotrexate33 | m2 | TBM | Therapeutic | ↔ a |
| Cytarabine33 | m2 | TBM | Therapeutic | ↔ a |
| Theophylline23 | kg | TBM | Not available | Not available |
| Busulfan21 | kg | TBMb | Supratherapeutic | ↑ (124%) |
| Divalproex sodium20 | kg | TBM | Supratherapeutic | ↑ (156%) |
| ABM | Therapeutic | ↔ | ||
| Doxorubicin34,35 | kg34 | TBM | Therapeutic | Not available |
| m2 35 | ABM | Therapeutic | Not available | |
| Etoposide33,35 | m2 33 | TBM | Therapeutic | ↔ a |
| m2 35 | ABM | Therapeutic | Not available | |
| Tobramycin28 | kg | ABM | Therapeutic | Not available |
| Cefazolin28 | kg | ABM | Therapeutic | Not available |
| Gentamicin29 | kg | ABM | Therapeutic | ↔ |
| Cyclosporine36 | kg | ABM | Therapeutic | ↔ |
| Propofol27 | kg | ABM | Therapeutic | Not available |
| Acetaminophen25 | Fixed dosec | n/a | Therapeutic | ↑ (135%) |
| Carbamazepine19,31 | Fixed dose | n/a | Therapeutic19 | ↔ 19 |
| Valproic acid19 | Fixed dose | n/a | Therapeutic | ↔ |
| Antipyrine26 | Fixed dose | n/a | Not available | Not available |
| Midazolam32 | Fixed dose | n/a | Not available | Not available |
| Caffeine37 | Fixed dose | n/a | Not available | Not available |
TBM, total body measurement; ABM, adjusted body measurement; ↑, increased in comparison to controls; ↓, decreased in comparison to controls; ↔, equal to controls; (%), % of controls.
Comparison of exposure between obese and control subjects based on clinical outcomes (overall survival, event-free survival, and cumulative incidence of relapse).33
Test dose used for PK comparison.21
Acetaminophen single-dose regimen: 5 mg/kg, maximum 325 mg, the mean dose administered was 3.6 mg/kg (SD 0.8).25
Exposure data in obese children were available for 17 drugs, and a non-obese control comparison group was available for 13 of these drugs (Table 2). Compared with controls, obese children demonstrated meaningful differences in exposure for 5/13 drugs (38%), including 4/5 with increased exposure in the obese patients. Dosing by TBM demonstrated sub- or supra-therapeutic exposures for 4/10 drugs, while dosing strategies using various adjusted body measurement strategies resulted in appropriate exposures for 8/8 drugs (Table 2).
Pharmacokinetic Changes Due to Obesity
PK parameters were compared between obese and non-obese controls for 17 drugs, a slightly different set from the drugs with PK data (Table 3). As compared with controls, clinically significant PK alterations were seen in obese children for 11/17 (65%) of studied drugs, including decreased V (range 65–89% of controls) for 8 drugs, increased V (113%, 166%) for 2 drugs, decreased CL (range 30–84%) for 5 drugs, and increased CL (222%) for 1 drug (Table 3).
Table 3.
Observed PK Changes in Obese Children
| Drug | Volume of distributiona | Clearancea |
|---|---|---|
| Analgesics/anesthetics | ||
| Acetaminophen25 | ↓ (83%) | ↔ |
| Antipyrine26 | ↓ (76%) | ↓ (50%) |
| Antibiotics | ||
| Cefazolin28 | ↔ | ↔ |
| Gentamicin29 | ↓ (71%) b | |
| Tobramycin28 | ↓ (75%) b | ↔ |
| Vancomycin17 | ↓ (81%) | ↓ (80%) |
| Anticonvulsants | ||
| Carbamazepine31 | ↓ (89%) | ↓ (63%) |
| Midazolam32 | ↔ | |
| Antineoplastics | ||
| Busulfan21 | ↓ (84%) b | |
| Cytarabine33 | ↔ | |
| Doxorubicin34,35 | ↑ (113%) | ↔ |
| Etoposide35 | ↔ | |
| Mercaptopurine22 | ↑ (166%) b | ↑ (222%) b |
| Methotrexate33 | ↔ | |
| Teniposide33 | ↔ | |
| Respiratory stimulants | ||
| Caffeine37 | ↓ (65%) | ↓ (30%) |
| Theophylline23 | ↓ (69%) b |
V and CL are expressed as a percentage of mean values in non-obese controls. ↑, increased in comparison to controls; ↓, decreased in comparison to controls; ↔, equal to control.
PK parameters are weight-normalized (e.g., volume of distribution in L/kg). If authors did not report weight-normalized values (antipyrine, carbamazepine, caffeine, and mercaptopurine), then weight-normalized parameters were calculated by dividing reported values by individual or study mean weight values.
Significant difference found in cited study.
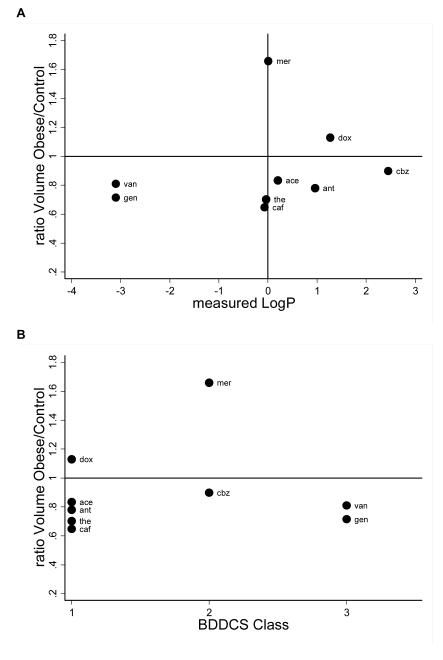
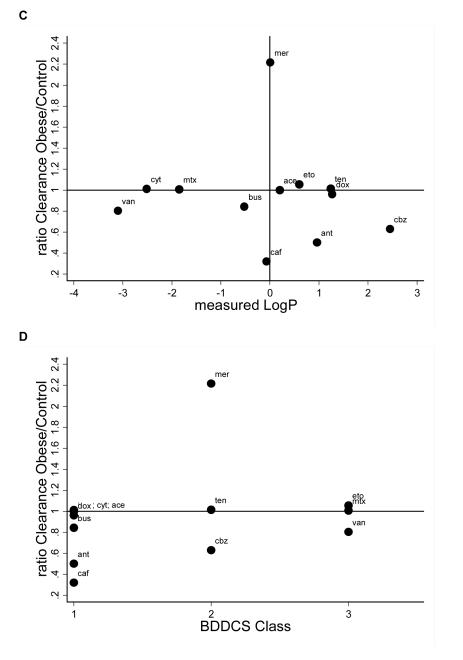
Figure 2 shows the ratios of V and CL for drugs with different logP and BDDCS class. A ratio of 1 indicates that weight-normalized V or CL were identical between obese and non-obese children, <1 indicates that obese children had a smaller V or CL, and >1 indicates that V or CL were higher in obese children. We did not identify any relationship between measured logP or BDDCS class and change in V or CL due to obesity.
Figure 2. Relationship Between Obesity-Related Changes in Pk and Drug Physicochemical Properties.
V and CL ratios plotted by measured logP and BDDCS class. Ratios displayed as obese/controls. CL and V are weight-normalized (e.g., V in L/kg). If authors did not report weight-normalized values (antipyrine, carbamazepine, and caffeine), then weight-normalized parameters were calculated by dividing reported values by individual or study mean weight values. A: V by measured logP; B: V by BDDCS class; C: CL by measured logP; D: CL by BDDCS class. V, volume of distribution; Cl, clearance; logP, lipophilicity; BDDCS, Biopharmaceutical Drug Disposition Classification System; ace, acetaminophen; ant, antipyrine; caf, caffeine; cbz, carbamazepine; dox, doxorubicin; eto, etoposide; gen, gentamicin; mer, mercaptopurine; mtx, methotrexate; ten, teniposide; the, theophylline; tob, tobramycin; van, vancomycin.
DISCUSSION
This is the first systematic review of PK studies conducted in obese children. Despite a comprehensive review strategy, we identified only 20 studies (evaluating 21 drugs) performed over the preceding 4 decades. Many of these studies identified important obesity-related changes in drug PK. However, the majority included small numbers of children, and 29% were conducted using therapeutic drug monitoring data and not as part of a formal PK trial. Also, many of the drugs that we highlight are not commonly prescribed agents.41,42 We found no data for several important drug classes for which obesity-related toxic overdosing or sub-therapeutic under-dosing have been previously described in adults, including acute care, cardiovascular, anesthetic agents, and contraception (including emergency contraception).43-50 For contraception, the lack of PK data in obese female adolescents is particularly concerning as evidenced by recent studies in obese female adults suggesting that higher doses are required to achieve therapeutic exposure and certain emergency contraceptive agents are less effective.49,50
Considering the prevalence and tremendous public health impact of childhood obesity, the relative paucity of drug PK data is concerning. Kendrick and colleagues completed the only prior review of PK studies in obese children (published in 2010), identifying just 10 drugs with available PK data. They concluded that clinicians may need to extrapolate from adult data while considering the effects of growth and development on PK.51 However, subsequent analyses have identified that simple extrapolation from studies in obese adults may give false predictions of CL and other PK values.15 These observed differences between obese children and adults might be explained by maturational differences in expression and activity of enzymatic pathways and/or drug transporters, by differences in elimination pathways, or by as yet unexplained differences in drug metabolism.15 Regardless, important differences exist and highlight the need for conducting PK studies specifically in obese children.
In the clinical setting, health care providers sometimes empirically adjust dosing in obese children based on perceived differences in PK (e.g., dosing by IBW). In the small number of obese children described in this systematic review, the PK differences we identified in obese children (CL was different in 6/15 drugs and V in 10/11 drugs) were not predicted by drug logP, and no relationship between BDDCS class and PK changes was observed. Given the paucity of systematic data investigating the impact of logP and BDDCS class in obese children, these drug characteristics should still be investigated in future studies.12,52 However, it is also possible that V and CL in obese children are affected by drug-specific factors other than logP or BDDCS class. Possible factors include route of absorption, metabolic pathway, and route of elimination. A recent study in obese adults reached a similar conclusion.14
Given the noted overweight-related alterations in drug exposure and PK, it would seem that optimal dosing regimens should be adjusted to account for obesity-related factors. Traditionally, a variety of adjustment methods have been proposed, including dosing regimens based on IBW, TBM, BSA, various adjusted body measurement formulae, or more complex physiologically based formulae, such as the ratio between V and body weight.14 In our analysis, there was little consistency in which adjustment methods were used. Evaluating exposure levels in obese children by dosing strategy, we found that dosing based on TBM resulted in inappropriate exposure for 4/10 drugs. When combined with the 8 drugs for which dosing using an adjusted body measurement achieved appropriate exposure, approximately two thirds of drugs in this review would result in inappropriate exposure if dosed by TBM. However, we cannot predict which drugs should be dosed by total or adjusted body measurement and which adjustment method to use to convert from total to adjusted body measurement.
The main limitations of our analysis are small study sizes, an overall small number of studied drugs, and the heterogeneity in study population and study design for the various PK studies that we identified. To maximize the power of this review, notwithstanding inconsistent weight categorizations and a small number of available studies, we reviewed all studies in obese children. Thirty-five percent included overweight as well as obese children, which may have caused underestimation of the effects of obesity. Many of the analyzed PK studies used sparse sampling strategies (e.g., therapeutic drug monitoring data) that limit the ability to analyze drug PK in a specific age group. For these reasons, we are cautious in drawing conclusions and avoid making specific dosing recommendations. Because the data are so sparse, we are collaborating with the National Institute of Child Health and Human Development in a systematic review of acute care and commonly used drugs to develop a PK database in obese children, normal weight children, and obese adults. Data generated from this review will be used to make dosing recommendations for obese children when possible and identify priority drugs in need of study in this population.
For future PK studies in obese children, we recommend including drugs of different therapeutic drug classes. Drug class prioritizing should be based on drug utilization, medical need, and expected PK alterations in obesity (based on adult studies).53 Based on our review of current PK studies in obese children, we recommend that future PK studies in children: 1) describe inclusion criteria, including the definitions of obesity (preferably age- and sex-adjusted BMI %), age, clinical diagnosis, and co-morbidities; 2) describe demographics of both obese and control subjects including age, weight, height, BMI, BMI %, diagnosis, and kidney and liver function; 3) provide detailed PK parameters including CL and V estimates by BMI group (>85%, >95%, >97%); and 4) report safety outcomes and, if possible, pharmacodynamic outcomes.
In conclusion, this systematic review describes PK changes due to obesity in children. We found that an evidence base is broadly lacking. Of the existing data, many of the studies were small PK studies or were conducted for drugs that are infrequently prescribed (e.g., anti-neoplastic drugs). The studies demonstrated considerable variability in weight-based dosing strategies, criteria for obesity, and type of PK analysis. We identified important but unpredictable differences in drug CL and V in obese children for two thirds of drugs. Furthermore, our analysis demonstrates that dosing based on TBM is often sub-optimal, as approximately two thirds of drugs studied demonstrated sub- or supra-therapeutic exposure when dosed using TBM. Therefore, given the increasing societal obesity-related morbidity and medical expenditure in children, there is an urgent need for formal PK studies in obese children to develop evidence-based dosing guidelines. With dedicated PK studies, we can determine PK parameters and use them to explore different dosing regimens using modeling and simulation. We have provided recommendations for the critical components of these future PK studies to standardize design and improve granularity of future structured reviews.
Supplementary Material
ACKNOWLEDGMENTS
Funding/Support: This work was funded under NICHD contract HHSN2752010000031 for the Pediatric Trials Network. Research reported in this publication was also supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number UL1TR001117. Dr Cohen-Wolkowiez receives support for research from the National Institutes of Health (NIH) (1K23HD064814), the National Center for Advancing Translational Sciences of the NIH (UL1TR001117), the Food and Drug Administration (1U01FD004858-01), the Biomedical Advanced Research and Development Authority (BARDA) (HHSO100201300009C), the nonprofit organization Thrasher Research Fund (www.thrasherresearch.org), and from industry for drug development in adults and children (www.dcri.duke.edu/research/coi.jsp). Dr Gonzalez is funded by training grant T32GM086330 from the National Institute of General Medical Sciences. Dr Benjamin receives support from the United States government for his work in pediatric and neonatal clinical pharmacology (1R01HD057956-05, 1K24HD058735-05, UL1TR001117, and NICHD contract HHSN275201000003I) and the nonprofit organization Thrasher Research Fund for his work in neonatal candidiasis (www.thrasherresearch.org); he also receives research support from industry for neonatal and pediatric drug development (www.dcri.duke.edu/research/coi.jsp). Dr Watt receives support from NIGMS (1T32GM086330-01A1) and the Thrasher Research Fund (www.thrasherresearch.org) for his work in pediatric clinical pharmacology.
Role of the Sponsor: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, which had no role in study design; the collection, analysis, and interpretation of data; the writing of the report; or the decision to submit the manuscript for publication.
APPENDIX. The Pediatric Trials Network Administrative Core Committee
Katherine Y. Berezny, Duke Clinical Research Institute, Durham, NC; Edmund Capparelli, University of California–San Diego, San Diego, CA; Gregory L. Kearns, Children’s Mercy Hospital, Kansas City, MO; Matthew Laughon, University of North Carolina at Chapel Hill, Chapel Hill, NC; Andre Muelenaer, Virginia Tech Carilion School of Medicine, Roanoke, VA; T. Michael O’Shea, Wake Forest Baptist Medical Center, Winston Salem, NC; Ian M. Paul, Penn State College of Medicine, Hershey, PA; John van den Anker, George Washington University School of Medicine and Health, Washington, DC; Kelly Wade, Children’s Hospital of Philadelphia, Philadelphia, PA; Thomas J. Walsh, MD, Weill Cornell Medical College of Cornell University, New York, NY.
The Eunice Kennedy Shriver National Institute of Child Health and Human Development: Perdita Taylor-Zapata, Anne Zajicek, Alice Pagan
The EMMES Corporation (Data Coordinating Center): Ravinder Anand, Traci Clemons, Gina Simone
Footnotes
Conflict of interest: The authors have no conflicts of interest to disclose.
Author Contributions: Drs Harskamp-van Ginkel and Watt had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Harskamp-van Ginkel, Becker, Cohen-Wolkowiez, Barrett, Benjamin, Siegel, Banks, Watt
Analysis and interpretation of data: Harskamp-van Ginkel, Hill, Becker, Testoni, Gonzalez, Benjamin, Siegel, Watt
Drafting of the manuscript: Harskamp-van Ginkel, Becker
Critical revision of the manuscript for important intellectual content: Hill, Testoni, Cohen-Wolkowiez, Gonzalez, Barrett, Benjamin, Siegel, Banks, Watt
Study supervision: Watt, Barrett, Siegel
Additional Contribution: The authors would like to thank Florence Chang of the National Library of Medicine, National Institutes of Health, for advice and guidance with the literature search.
REFERENCES
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. 2014;311(8):806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bechard LJ, Rothpletz-Puglia P, Touger-Decker R, Duggan C, Mehta NM. Influence of obesity on clinical outcomes in hospitalized children: a systematic review. JAMA Pediatr. 2013;167(5):476–482. doi: 10.1001/jamapediatrics.2013.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buescher PA, Whitmire JT, Plescia M. Relationship between body mass index and medical care expenditures for North Carolina adolescents enrolled in Medicaid in 2004. Prev Chronic Dis. 2008;5(1):A04. [PMC free article] [PubMed] [Google Scholar]
- 4.Hampl SE, Carroll CA, Simon SD, Sharma V. Resource utilization and expenditures for overweight and obese children. Arch Pediatr Adolesc Med. 2007;161(1):11–14. doi: 10.1001/archpedi.161.1.11. [DOI] [PubMed] [Google Scholar]
- 5.Hering E, Pritsker I, Gonchar L, Pillar G. Obesity in children is associated with increased health care use. Clin Pediatr. 2009;48(8):812–818. doi: 10.1177/0009922809336072. [DOI] [PubMed] [Google Scholar]
- 6.Wenig CM, Knopf H, Menn P. Juvenile obesity and its association with utilisation and costs of pharmaceuticals—results from the KiGGS study. BMC Health Serv Res. 2011;11:340. doi: 10.1186/1472-6963-11-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ingelfinger JR. Bariatric surgery in adolescents. N Engl J Med. 2011;365(15):1365–1367. doi: 10.1056/NEJMp1109981. [DOI] [PubMed] [Google Scholar]
- 8.Kuhle S, Fung C, Veugelers PJ. Medication use in normal weight and overweight children in a nationally representative sample of Canadian children. Arch Dis Child. 2012;97(9):842–847. doi: 10.1136/archdischild-2011-301195. [DOI] [PubMed] [Google Scholar]
- 9.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346(16):1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 10.Gunta SS, Mak RH. Is obesity a risk factor for chronic kidney disease in children? Pediatr Nephrol. 2013;28(10):1949–1956. doi: 10.1007/s00467-012-2353-z. [DOI] [PubMed] [Google Scholar]
- 11.Tanner RM, Brown TM, Muntner P. Epidemiology of obesity, the metabolic syndrome, and chronic kidney disease. Curr Hypertens Rep. 2012;14(2):152–159. doi: 10.1007/s11906-012-0254-y. [DOI] [PubMed] [Google Scholar]
- 12.Cheymol G. Effects of obesity on pharmacokinetics implications for drug therapy. Clin Pharmacokinet. 2000;39(3):215–231. doi: 10.2165/00003088-200039030-00004. [DOI] [PubMed] [Google Scholar]
- 13.Henegar JR, Bigler SA, Henegar LK, Tyagi SC, Hall JE. Functional and structural changes in the kidney in the early stages of obesity. J Am Soc Nephrol. 2001;12(6):1211–1217. doi: 10.1681/ASN.V1261211. [DOI] [PubMed] [Google Scholar]
- 14.Jain R, Chung SM, Jain L, et al. Implications of obesity for drug therapy: limitations and challenges. Clin Pharmacol Ther. 2011;90(1):77–89. doi: 10.1038/clpt.2011.104. [DOI] [PubMed] [Google Scholar]
- 15.Brill MJ, Diepstraten J, van Rongen A, van Kralingen S, van den Anker JN, Knibbe CA. Impact of obesity on drug metabolism and elimination in adults and children. Clin Pharmacokinet. 2012;51(5):277–304. doi: 10.2165/11599410-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 16.Wu CY, Benet LZ. Predicting drug disposition via application of BCS: transport/absorption/elimination interplay and development of a biopharmaceutics drug disposition classification system. Pharm Res. 2005;22(1):11–23. doi: 10.1007/s11095-004-9004-4. [DOI] [PubMed] [Google Scholar]
- 17.Nassar L, Hadad S, Gefen A, et al. Prospective evaluation of the dosing regimen of vancomycin in children of different weight categories. Curr Drug Saf. 2012;7(5):375–381. doi: 10.2174/157488612805076606. [DOI] [PubMed] [Google Scholar]
- 18.Miller M, Miller JL, Hagemann TM, Harrison D, Chavez-Bueno S, Johnson PN. Vancomycin dosage in overweight and obese children. Am J Health Syst Pharm. 2011;68(21):2062–2068. doi: 10.2146/ajhp110107. [DOI] [PubMed] [Google Scholar]
- 19.Suemaru K, Kawasaki H, Yasuhara K, et al. Steady-state serum concentrations of carbamazepine and valproic acid in obese and lean patients with epilepsy. Acta Med Okayama. 1998;52(3):139–142. doi: 10.18926/AMO/31328. [DOI] [PubMed] [Google Scholar]
- 20.Good CR, Feaster CS, Krecko VF. Tolerability of oral loading of divalproex sodium in child psychiatry inpatients. J Child Adolesc Psychopharmacol. 2001;11(1):53–57. doi: 10.1089/104454601750143447. [DOI] [PubMed] [Google Scholar]
- 21.Browning B, Thormann K, Donaldson A, Halverson T, Shinkle M, Kletzel M. Busulfan dosing in children with BMIs >=85% undergoing HSCT: a new optimal strategy. Biol Blood Marrow Transplant. 2011;17(9):1383–1388. doi: 10.1016/j.bbmt.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 22.Zuccaro P, Guandalini S, Pacifici R, et al. Fat body mass and pharmacokinetics of oral 6-mercaptopurine in children with acute lymphoblastic leukemia. Ther Drug Monit. 1991;13(1):37–41. doi: 10.1097/00007691-199101000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Yaffe SJ, Danish M. Problems of drug administration in the pediatric patient. Drug Metab Rev. 1978;8(2):303–318. doi: 10.3109/03602537808993790. [DOI] [PubMed] [Google Scholar]
- 24.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barshop NJ, Capparelli EV, Sirlin CB, Schwimmer JB, Lavine JE. Acetaminophen pharmacokinetics in children with nonalcoholic fatty liver disease. J Pediatr Gastroenterol Nutr. 2011;52(2):198–202. doi: 10.1097/MPG.0b013e3181f9b3a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caraco Y, Zylber-Katz E, Berry EM, Levy M. Antipyrine disposition in obesity: evidence for negligible effect of obesity on hepatic oxidative metabolism. Eur J Clin Pharmacol. 1995;47(6):525–530. doi: 10.1007/BF00193706. [DOI] [PubMed] [Google Scholar]
- 27.Diepstraten J, Chidambaran V, Sadhasivam S, et al. Propofol clearance in morbidly obese children and adolescents: influence of age and body size. Clin Pharmacokinet. 2012;51(8):543–551. doi: 10.2165/11632940-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 28.Koshida R, Nakashima E, Taniguchi N, Tsuji A, Benet LZ, Ichimura F. Prediction of the distribution volumes of cefazolin and tobramycin in obese children based on physiological pharmacokinetic concepts. Pharm Res. 1989;6(6):486–491. doi: 10.1023/a:1015968407226. [DOI] [PubMed] [Google Scholar]
- 29.Choi JJ, Moffett BS, McDade EJ, Palazzi DL. Altered gentamicin serum concentrations in obese pediatric patients. Pediatr Infect Dis J. 2011;30(4):347–349. doi: 10.1097/INF.0b013e3181ff023e. [DOI] [PubMed] [Google Scholar]
- 30.Moffett BS, Kim S, Edwards MS. Vancomycin dosing in obese pediatric patients. Clin Pediatr. 2011;50(5):442–446. doi: 10.1177/0009922810393500. [DOI] [PubMed] [Google Scholar]
- 31.Caraco Y, Zylber-Katz E, Berry EM, Levy M. Carbamazepine pharmacokinetics in obese and lean subjects. Ann Pharmacother. 1995;29(9):843–847. doi: 10.1177/106002809502900902. [DOI] [PubMed] [Google Scholar]
- 32.Vaughns JD, Rongen AV, Finkel J, et al. Population PK: single dose midazolam in obese children. Clin Pharmacol Ther. 2012;91(S1):S129–30. [Google Scholar]
- 33.Hijiya N, Panetta JC, Zhou Y, et al. Body mass index does not influence pharmacokinetics or outcome of treatment in children with acute lymphoblastic leukemia. Blood. 2006;108(13):3997–4002. doi: 10.1182/blood-2006-05-024414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thompson PA, Rosner GL, Matthay KK, et al. Impact of body composition on pharmacokinetics of doxorubicin in children: a Glaser Pediatric Research Network study. Cancer Chemother Pharmacol. 2009;64(2):243–251. doi: 10.1007/s00280-008-0854-z. [DOI] [PubMed] [Google Scholar]
- 35.Ritzmo C, Söderhäll S, Karlén J, Nygren H, Eksborg S. Pharmacokinetics of doxorubicin and etoposide in a morbidly obese pediatric patient. Pediatr Hematol Oncol. 2007;24(6):437–445. doi: 10.1080/08880010701451343. [DOI] [PubMed] [Google Scholar]
- 36.Kasap B, Soylu A, Turkmen M, Kavukcu S, Bora S, Gulay H. Effect of obesity and overweight on cyclosporine blood levels and renal functions in renal adolescent recipients. Transplant Proc. 2006;38(2):463–465. doi: 10.1016/j.transproceed.2005.12.054. [DOI] [PubMed] [Google Scholar]
- 37.Caraco Y, Zylber-Katz E, Berry EM, Levy M. Caffeine pharmacokinetics in obesity and following significant weight reduction. Int J Obes Relat Metab Disord. 1995;19(4):234–239. [PubMed] [Google Scholar]
- 38.Centers for Disease Control and Prevention [Accessed on January 5, 2015];Basics About Childhood Obesity. www.cdc.gov/obesity/childhood/basics.html.
- 39.National Heart, Lung, and Blood Institute . Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults. National Heart, Lung and Blood Institute; Bethesda, MD: 1998. [Google Scholar]
- 40.Centers for Disease Control and Prevention [Accessed on January 5, 2015];Defining Overweight and Obesity. www.cdc.gov/obesity/adult/defining.html.
- 41.Lawless SH, Hildebrand JR, III, Frank G. The Prevalence of Inpatient Pediatrics Prescribed and Administered Medications. National Institute for Child Health and Human Development, U.S. Department of Health and Human Services; Rockville, MD: 2005. [Google Scholar]
- 42.Korelitz JB, Bethel J, Chang D, Xu Y. Frequency of Medication Usage in the Pediatric Population. National Institute for Child Health and Human Development, U.S. Department of Health and Human Services; Rockville, MD: 2005. [Google Scholar]
- 43.Cheymol G, Poirier JM, Carrupt PA, et al. Pharmacokinetics of beta-adrenoceptor blockers in obese and normal volunteers. Br J Clin Pharmacol. 1997;43(6):563–570. doi: 10.1046/j.1365-2125.1997.00609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fisher DG, Schwartz PH, Davis AL. Pharmacokinetics of exogenous epinephrine in critically ill children. Crit Care Med. 1993;21(1):111–117. doi: 10.1097/00003246-199301000-00021. [DOI] [PubMed] [Google Scholar]
- 45.Puhringer FK, Keller C, Kleinsasser A, Giesinger S, Benzer A. Pharmacokinetics of rocuronium bromide in obese female patients. Eur J Anaesthesiol. 1999;16(8):507–510. doi: 10.1046/j.1365-2346.1999.00523.x. [DOI] [PubMed] [Google Scholar]
- 46.Steinberg C, Notterman DA. Pharmacokinetics of cardiovascular drugs in children. Inotropes and vasopressors. Clin Pharmacokinet. 1994;27(5):345–367. doi: 10.2165/00003088-199427050-00003. [DOI] [PubMed] [Google Scholar]
- 47.Edelman AB, Carlson NE, Cherala G, et al. Impact of obesity on oral contraceptive pharmacokinetics and hypothalamic–pituitary–ovarian activity. Contraception. 2009;80(2):119–127. doi: 10.1016/j.contraception.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Westhoff CL, Torgal AH, Mayeda ER, Pike MC, Stanczyk FZ. Pharmacokinetics of a combined oral contraceptive in obese and normal weight women. Contraception. 2010;81(6):474–480. doi: 10.1016/j.contraception.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Edelman AB, Cherala G, Munar MY, McInnis M, Stanczyk FZ, Jensen JT. Correcting oral contraceptive pharmacokinetic alterations due to obesity: a randomized controlled trial. Contraception. 2014;90(5):550–556. doi: 10.1016/j.contraception.2014.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Glasier A, Cameron ST, Blithe D, et al. Can we identify women at risk of pregnancy despite using emergency contraception? Data from randomized trials of ulipristal acetate and levonorgestrel. Contraception. 2011;84(4):363–367. doi: 10.1016/j.contraception.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 51.Kendrick JG, Carr RR, Ensom MH. Pharmacokinetics and drug dosing in obese children. J Pediatr Pharmacol Ther. 2010;15(2):94–109. [PMC free article] [PubMed] [Google Scholar]
- 52.Benet LZ. Predicting drug disposition via application of a Biopharmaceutics Drug Disposition Classification System. Basic Clin Pharmacol Toxicol. 2010;106(3):162–167. doi: 10.1111/j.1742-7843.2009.00498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barrett JS, Patel D, Jayaraman B, Narayan M, Zuppa A. Key performance indicators for the assessment of pediatric pharmacotherapeutic guidance. J Pediatr Pharmacol Ther. 2008;13(3):141–155. doi: 10.5863/1551-6776-13.3.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.