Abstract
We report on a 61-year-old man with elevated serum prostate-specific antigen (PSA) level of 10.5 ng/mL who had undergone prior negative standard transrectal ultrasound (TRUS) biopsy at another institution. He was referred to our medical center for evaluation and underwent a clinical 3-T multiparametric magnetic resonance imaging (mpMRI) and a research protocol positron emission tomography-computed tomography (PET-CT) with the cellular proliferation radiotracer, 2′-deoxy-2′-[18F]fluoro-5-methyl-1-β-D-arabinofuranosyluracil (18F-FMAU). The PET-CT and mpMRI were fused with TRUS images for real-time hybrid-image based targeting of the biopsy needle. PET/CT with 18F-FMAU was helpful in localizing the non-standard biopsy sites that on histopathology revealed suspected tumor deposits.
Keywords: Prostate, Cancer, FMAU, PET, Ultrasound, MRI
None of the standard biopsy sites revealed carcinoma. The left base targeted biopsy site (with focal 18F-FMAU uptake) revealed atypical small acinar proliferation suspicious for early malignancy. Accurate prostate tumor localization and characterization allows for image-directed biopsy and focal therapy (1). PET in conjunction with radiotracers that track the thymidine salvage pathway of DNA synthesis has been studied for noninvasive imaging-based assessment of cellular proliferation in cancer (2). The radiolabeled thymidine analog, 18F-FMAU, is preferentially phosphorylated by the mitochondrial thymidine kinase 2 (TK2) and becomes incorporated into the DNA (3). Normal biodistribution of 18F-FMAU in human shows relatively high tracer uptake in the liver and the renal cortex, moderate uptake in the salivary glands, heart, and spleen and relatively low uptake in the bone marrow (2). We have previously shown that there may be an association between androgen signaling and thymidine metabolism and that 18F-FMAU PET may be useful in prostate tumor characterization (4). An automated cGMP-compliant radiosynthesis of 18F-FMAU has also been described (5, 6). Multi-modal image-guided localization, characterization and targeting of prostate tumors may alleviate the current overdiagnosis (and overtreatment) of indolent tumors and underdiagnosis (and loss of opportunity for delivery of appropriate treatment including focal therapy) of aggressive tumors (7, 8).
Figure 1.
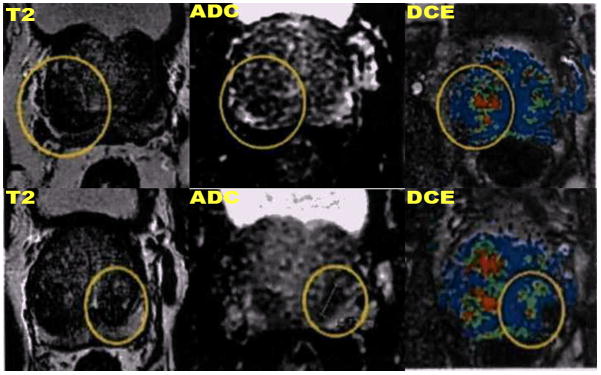
Axial T2-weighted (left column), diffusion-weighted imaging apparent diffusion coefficient map (ADC, mid column), dynamic contrast-enhanced (right column) demonstrate a heterogeneous enlarged prostate gland (6.3 × 4.4 × 5.5 cm, apex to base, AP and transverse, respectively, with volume of 79.3 cc) with focal regions of T2 hypointensity, mildly decreased ADC, and mild hyperperfusion (enclosed in circles) at the bilateral bases of the peripheral zone which were considered nonspecific in view of focal scar and chronic hematoma from prior biopsy procedure.
Figure 2.

Axial PET-CT with 18F-FMAU demonstrates diffuse increased tracer localization in the right base and focally increased tracer uptake in the left base of the prostate gland.
Figure 3.
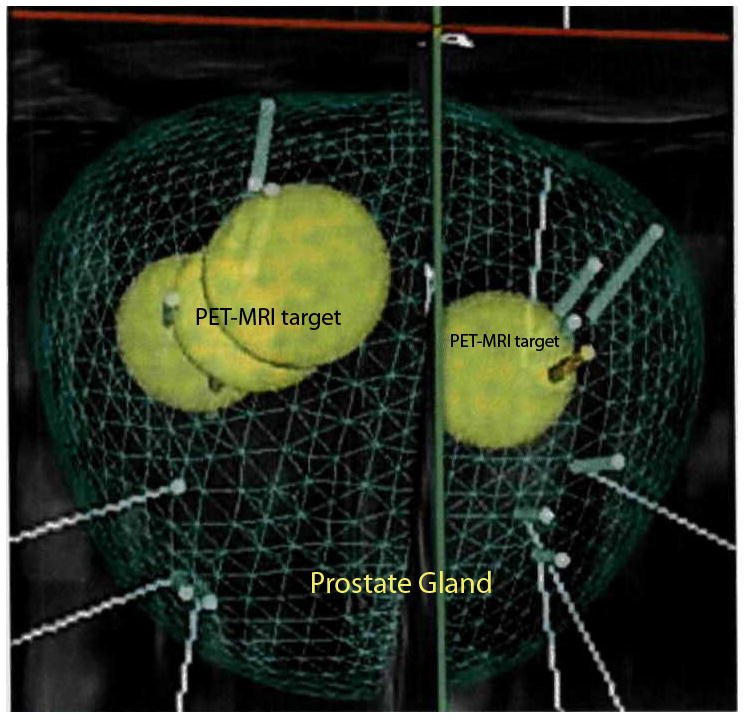
D biopsy mapping trajectories constructed from the fused 18F-FAMU PET-CT, mpMRI, and TRUS images. The yellow spheres denote the locations of the PET and mpMRI abnormities within the gland represented in a volumetric matrix mesh denoting the prostate gland boundaries. Green and orange poles portray standard biopsy and PET-mpMRI directed biopsy sites, respectively.
Acknowledgments
Supported by grant from the Whittier Foundation (PI: H. Jadvar) and NIH/NCI P30-CA014089. The authors would like to thank Bhushan Desai, MBBS, MS, for his help in patient enrollment and Madlen Aladadyan, MPH, for her administrative help.
Footnotes
Conflict of Interest: The authors declare no conflicts of interest.
References
- 1.Jadvar H. Imaging cellular proliferation in prostate cancer with positron emission tomography. Asia Oceania J Nucl Med Biol. 2015 Epub Ahead of Publication( http://aojnmb.mums.ac.ir/article_3873_0.html. [PMC free article] [PubMed]
- 2.Bading JR, Shields AF. Imaging of cell proliferation: status and prospects. J Nucl Med. 2008;49 (Suppl 2):64S–80S. doi: 10.2967/jnumed.107.046391. [DOI] [PubMed] [Google Scholar]
- 3.Tehrani OS, Shields AF. PET imaging of proliferation with pyrimdines. J Nucl Med. 2013;54:903–912. doi: 10.2967/jnumed.112.112201. [DOI] [PubMed] [Google Scholar]
- 4.Jadvar H, Yap LP, Park R, et al. [18F]-2′-fluoro-5-methyl-1-beta-D-arabinofuranosyluracil (18F-FMAU) in prostate cancer: initial preclinical observations. Mol Imaging. 2012;11:426–432. [PMC free article] [PubMed] [Google Scholar]
- 5.Li Z, Cai H, Conti PS. Automated synthesis of 2′-deoxy-2′-[18F]fluoro-5-methyl- 1-β-D-arabinofuranosyluracil ([18F]-FMAU) using a one reactor radiosynthesis module. Nucl Med Biol. 2011;38:201–206. doi: 10.1016/j.nucmedbio.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 6.Chen K, Conti PS. Radiosynthesis of 2′-deoxy-2′-[18F]fluoro-5-methyl-1-β-D-arabinofuranosyluracil ([18F]FMAU) In: Scott PJH, editor. Radiochemical Syntheses. Vol. 2. John Wiley & Sons Publisher; 2015. pp. 53–61. [Google Scholar]
- 7.Ukimura O, Faber K, Gill IS. Intraprostatic targeting. Curr Opin Urol. 2012;22:97–103. doi: 10.1097/MOU.0b013e32835017fa. [DOI] [PubMed] [Google Scholar]
- 8.Ukimura O, Coleman J, de la Taille A, et al. Contemporary role of systematic prostate biopsies: indications, technique, implications on patient care. Eur Urol. 2013;63:214–30. doi: 10.1016/j.eururo.2012.09.033. [DOI] [PubMed] [Google Scholar]


