Abstract
O-GlcNAcylation is a reversible post-translational modification. O-GlcNAc addition and removal is catalyzed by O-GlcNAc transferase (OGT) and O-GlcNAcase (OGA), respectively. More recent evidence indicates that regulation of O-GlcNAcylation is important for inflammatory diseases and tumorigenesis. In this study, we revealed that O-GlcNAcylation was increased in the colonic tissues of dextran sodium sulfate (DSS)-induced colitis and azoxymethane (AOM)/DSS-induced colitis-associated cancer (CAC) animal models. Moreover, the O-GlcNAcylation level was elevated in human CAC tissues compared with matched normal counterparts. To investigate the functional role of O-GlcNAcylation in colitis, we used OGA heterozygote mice, which have an increased level of O-GlcNAcylation. OGA+/− mice have higher susceptibility to DSS-induced colitis than OGA+/+ mice. OGA+/− mice exhibited a higher incidence of colon tumors than OGA+/+ mice. In molecular studies, elevated O-GlcNAc levels were shown to enhance the activation of NF-κB signaling through increasing the binding of RelA/p65 to its target promoters. We also found that Thr-322 and Thr352 in the p65-O-GlcNAcylation sites are critical for p65 promoter binding. These results suggest that the elevated O-GlcNAcylation level in colonic tissues contributes to the development of colitis and CAC by disrupting regulation of NF-κB-dependent transcriptional activity.
Keywords: O-GlcNAcylation, O-GlcNAcase, colitis, colitis-associated cancer
INTRODUCTION
The addition of O-linked β-N-acetylglucosamine (O-GlcNAc) monosaccharides to Ser and Thr residues of nuclear, mitochondrial and cytoplasmic proteins is termed O-GlcNAcylation. The addition and removal of O-GlcNAc on target proteins is catalyzed by O-GlcNAc transferase (OGT) and O-GlcNAcase (OGA), respectively [1]. The O-GlcNAc cycling enzymes are expressed ubiquitously and are critical for development in vertebrates. Abnormal amounts of O-GlcNAcylation contribute to the etiology of chronic aging diseases, including cardiovascular disease, type 2 diabetes and Alzheimer's disease [2, 3]. O-GlcNAc-modified proteins play diverse roles in transcriptional regulation, the cell cycle, signaling, stress, and differentiation [1]. Because the cellular roles of O-GlcNAc-modified proteins are perturbed during tumorigenesis, aberrant O-GlcNAcylation was suggested to be closely linked to tumorigenesis [4]. OGT was reported to regulate transcriptional machinery by modifying the many transcription factors involved in cancer-relevant processes [4]. Notably, O-GlcNAc levels are significantly elevated in various cancer types, including those of the breast [5], prostate [6], colon [7], lung [8], pancreas [9] and chronic lymphocytic leukemia [10]. In vivo xenograft of breast [11], prostate [6] and pancreatic cancers [9] showed a critical role for O-GlcNAcylation in tumorigenesis or metastasis. However, the effect of O-GlcNAcylation in colorectal cancer (CRC) has not been demonstrated in vivo.
CRC is one of the most common malignancies, for which inflammation is an established risk factor [12]. Ulcerative colitis (UC), a common form of inflammatory bowel disease (IBD), is associated with an increased risk of CRC [13]. The NF-κB signaling pathway plays a pivotal role in the development and maintenance of intestinal inflammation [14]. Activation of NF-κB signaling was detected in mucosal IBD cells and colorectal carcinoma patients [15, 16]. Previous studies have suggested that NF-κB signaling plays a key role in linking intestinal inflammation with CRC development [17]. A number of genetic mouse models have demonstrated that aberrant NF-κB signaling in mucosal immune cells or intestinal epithelial cells leads to severe intestinal inflammation and tumors [18-20]. Many reports have shown that NF-κB signaling is affected by O-GlcNAcylation. However, no clear pattern in NF-κB activity in the presence of increased O-GlcNAc levels has been reported. O-GlcNAcylation of RelA/p65, a subunit of NF-κB, is critical for activation of NF-κB signaling in mesangial cells and T- and B-lymphocytes [21, 22]. p65 is O-GlcNAcylated on Thr-352, which is required for transcriptional activity under hyperglycemic conditions [23]. Mutation of two p65 O-GlcNAc sites (T322A and T352A) attenuated the growth of pancreatic ductal adenocarcinoma [9]. Furthermore, transforming growth factor-β-activated kinase1-binding protein (TAB1), an upstream signaling molecule of NF-κB, is O-GlcNAcylated and necessary for activation of NF-κB signaling during the innate immune response [24]. In contrast, several reports have suggested that elevated O-GlcNAcylation attenuates NF-κB signaling activation in rat aortic smooth muscle cells and primary cultured cardiomyocytes [25, 26]. Despite the increasing number of reports of a possible link between O-GlcNAcylation and inflammation, the pathogenetic role of O-GlcNAcylation in IBD and CRC, particularly when associated with chronic inflammation, has yet to be investigated. In this study we used OGA+/− mice with constitutively elevated colonic O-GlcNAc levels as an experimental model to investigate the role of O-GlcNAcylation in intestinal inflammation and colitis-associated cancer. Our results showed that elevated O-GlcNAcylation significantly enhanced colitis and CAC in the colon by modulating p65 DNA-binding activity.
RESULTS
Elevation of O-GlcNAcylation in colitis
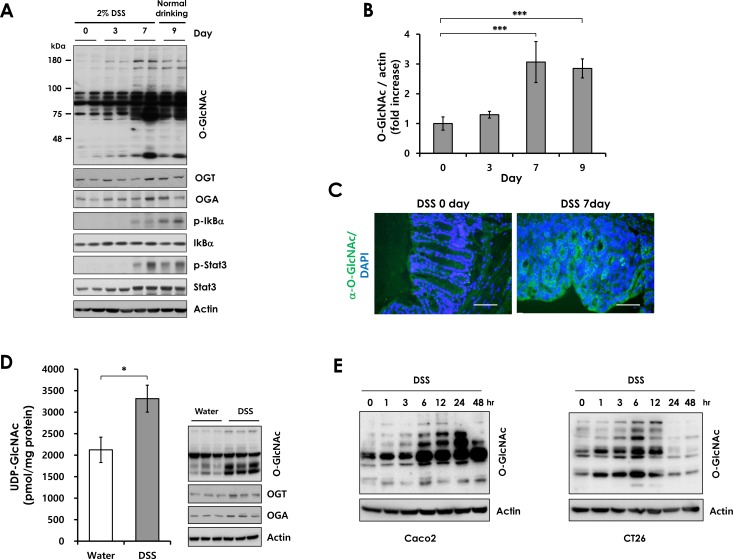
Although studies have suggested an association between O-GlcNAcylation and inflammation [24, 25], the involvement of O-GlcNAcylation in IBD has not been investigated. First, we examined whether O-GlcNAcylation is altered in colitis. Wild-type (WT) mice were treated with 2% dextran sulfate sodium (DSS) in drinking water for 7 days to induce colitis, and then provided with normal water. Immunoblotting revealed that global O-GlcNAcylation and O-GlcNAc cycling enzymes were elevated in colonic tissues during DSS-induced colitis (Figure 1A and 1B). OGT expression was consistent with that of OGA during DSS treatment. Apparently, increased OGT expression elevates O-GlcNAcylation, causing an increase in OGA expression to compensate for the increased O-GlcNAcylation. Consistent with previous reports, Iκb and STAT3 were phosphorylated during colitis, indicating development of inflammation [15, 27]. Interestingly, O-GlcNAcylation is increased during colitis, simultaneous with the activation of inflammatory signaling. We also confirmed increased O-GlcNAc levels in colonic tissue of DSS-treated mice using immunohistofluorescence (Figure 1C). To investigate whether UDP-GlcNAc, a donor substrate for O-GlcNACylation, is also elevated in DSS-treated colon tissues, we measured UDP-GlcNAc levels by HPLC analysis in colon tissues after loading control samples from colon tissues (Supplementary Figure 1). UDP-GlcNAc levels are increased in DSS-treated colon tissues compared with control tissues (Figure 1D). This result indicates that hyper-O-GlcNAcylation is caused by both increased flux of the hexosamine pathway and increased OGT expression.
Figure 1. O-GlcNAcylation levels during DSS treatment.
Wild-type mice were administered 2% DSS in drinking water for the indicated days. (A) Western blots of lysates from wild-type mice treated with DSS during a 9-day time course. Lysates for each time point were isolated from three separate mice. Two representative samples were loaded in each lane. (B) Densitometry was performed on immunoblots. The ratio of O-GlcNAc to β-actin was determined. Error bars represent the S.D. (n = 3) and (**) P < 0.005, (*) P < 0.05 (Student's t-test). (C) Immunofluorescence staining of the colon with O-GlcNAc mAb (RL2) in wild-type mice not treated or treated with DSS for 7 days (green, O-GlcNAc modified proteins; blue, nuclei). Bars =50 μm. (D) Measurement of Uridine diphosphate-N-acetylglucosamine (UDP-GlcNAc; B) levels in control (n=3) and DSS-treated (n=6) colon tissues by HPLC analysis (left). Matched O-GlcNAcylation levels (Immunoblots with RL2 antibody) (right). Error bars represent ± SEM. (*) P < 0.05 (Student's t-test). (E) Caco2 and CT26 cells were treated with 3% DSS for up to 48 hours. O-GlcNAcylation levels were analyzed by Western blot analysis.
To determine whether DSS affects directly the O-GlcNAc level in colonic epithelial cells, Caco2 and CT26 cells were treated with 3% DSS. DSS treatment increased O-GlcNAcylation in colon epithelial cells (Figure 1E). Notably, high levels of O-GlcNAcase expression was detected in colonic epithelial cells (Supplementary Figure 2). This finding suggests that O-GlcNAc modification might affect DSS-induced colitis progression in intestinal epithelial cells. Furthermore, we also tested whether DSS activates the NF-κB signaling in colonic epithelial cells and MEFs. However, p65 phosphorylation, IκBα phosphorylation, and IκBα degradation were not changed by DSS treatment (Supplementary Figure 3). These results suggest that O-GlcNAc cycling may play an important role in the pathogenesis of colitis.
Increased levels of O-GlcNAcylation in mouse and human CAC
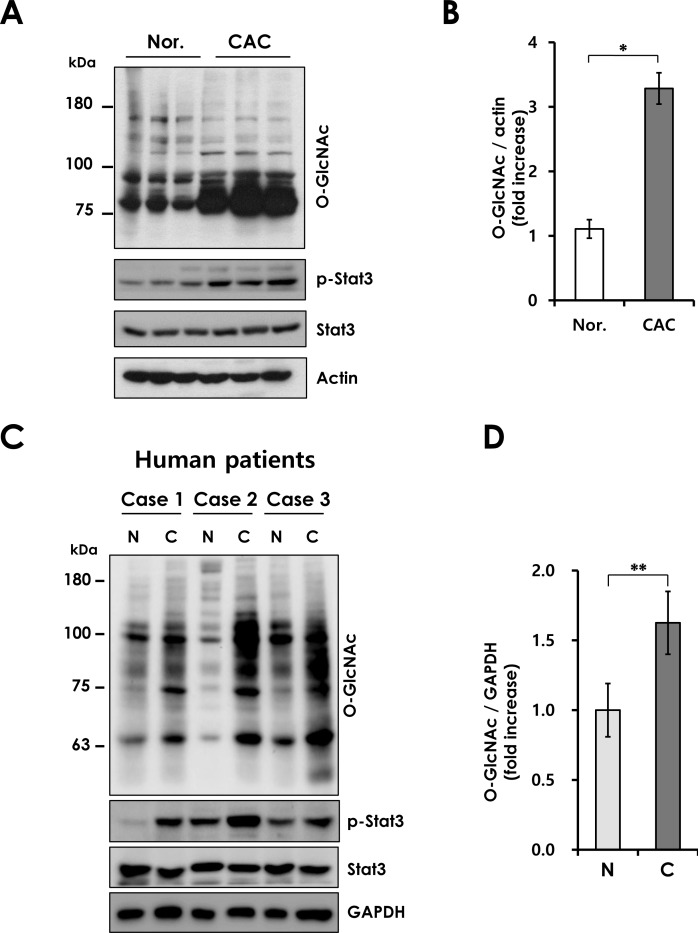
Next, we investigated whether O-GlcNAcylation is also increased in inflammation-associated tumorigenesis. O-GlcNAc levels were assayed in AOM/DSS-induced mouse colon tumors. We observed increased levels of O-GlcNAcylation in AOM/DSS-treated mice. O-GlcNAc levels were elevated in CAC tissues with increased STAT3 phosphorylation, a well-known marker of inflammation, in the AOM/DSS model. (Figure 2A and 2B). These results suggest that O-GlcNAcylation is closely correlated with CAC. To confirm the elevated O-GlcNAcylation in CAC, we used a matched pair of adenomas and uninvolved colonic mucosa from CAC patients. CAC samples show elevated levels of the O-GlcNAcylation and STAT3 phosphorylation compared to uninvolved colonic mucosa (Figure 2C and 2D). The elevated O-GlcNAcylation in CAC tissues might be a consequence of chronic inflammation, as observed in colitis. We speculate that increased O-GlcNAcylation may contribute to the development of CAC.
Figure 2. Elevated O-GlcNAcylation in mouse and human CAC.
(A) Western blot of lysates form wild-type mice not treated or treated with AOM/DSS for 12 weeks. (B) Densitometry was performed on immunoblots. The ratio of O-GlcNAc to β-actin was determined. Error bars represent the S.D. (n = 3), (*) P < 0.05 (Student's t-test). (C) Western blot analysis of O-GlcNAcylation level in 3 matched pairs of uninvolved colonic mucosa (N) and colitis-associated cancer (C) tissues. (D) Densitometry was performed on immunoblots. The ratio of O-GlcNAc to GAPDH was determined. Error bars represent the S.D. (n = 3), (**) P < 0.005 (Student's t-test).
OGA+/− mice exhibit increased susceptibility to acute and chronic DSS-induced colitis
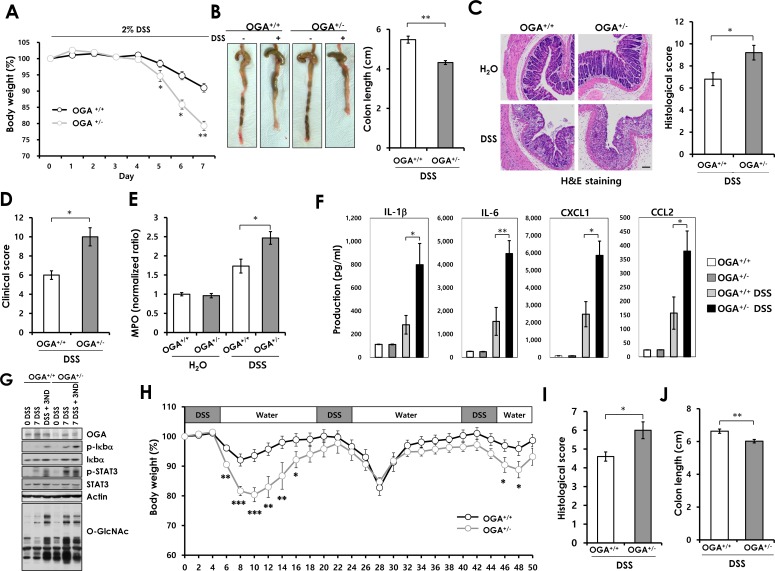
Emerging evidences suggest that O-GlcNAcylation regulates NF-κB signaling which is important for immune hemostasis and inflammation in the intestine. NF-κB signaling controls the expression of factors regulates apoptosis and proliferation during intestinal inflammation [14]. Previous studies show that disruption of NF-κB signaling in epithelium aggravates colitis with severe weight loss, tissue damage, and increased proinflammatory factors in the colonic mucosa [28]. These reports and the above results promoted us to investigate the involvement of O-GlcNAcylation in DSS-induced colitis and colonic inflammation. We used OGA+/−mice that have constantly elevated O-GlcNAcylation (Supplementary Figure 4A). Homozygous knockout of OGA is lethal perinatally [29]. Colitis was induced in OGA+/+and OGA+/−mice by administration of 2% DSS in drinking water for 7 days, followed by normal drinking water. During the DSS treatment period, OGA+/−mice exhibited more severe body weight loss, with an average weight loss of 8% in OGA+/+ versus 21% in OGA+/− mice (Figure 3A). The OGA+/− mice exhibited significantly higher mortality than OGA+/+ mice during the recovery phase (Supplementary Figure 4B). OGA+/− mice exhibited more severe colon shortening (Figure 3B). Histological analysis by H&E staining showed that colon tissue from DSS-treated OGA+/+mice had moderate inflammation and ulceration. However, OGA+/− mice displayed severe damage with extensive infiltration of neutrophils and a complete loss of crypt architecture (Figure 3C). In addition, OGA+/− mice showed rectal bleeding than OGA+/+ littermates, with a higher clinical score (Figure 3D). The extent of neutrophil infiltration was measured by MPO activity assay (Figure 3E). OGA+/− mice exhibited higher MPO activity than OGA+/+ mice, indicating increased neutrophil infiltration. Thus, these results suggest that increased O-GlcNAcylation aggravates DSS-induced mucosal damage, as indicated by the greater susceptibility of OGA+/−mice to DSS treatment. A number of proinflammatory cytokines mediate intestinal inflammation. Therefore, we compared cytokine production in colon samples from OGA+/+ and OGA+/−mice before and after DSS treatment. As shown in Figure 3F, OGA+/−mice exhibited a marked increase in DSS-induced colonic production of proinflammatory cytokines (IL-1β and IL-6) and chemokines (CXCL1 and CCL2). Consistent with these results, activation of the inflammatory pathway including NF-κB and STAT3 signaling, in the colon of DSS-treated OGA+/− mice was greater than in OGA+/+ mice due to the elevated O-GlcNAc levels of OGA+/−mice (Figure 3G).
Figure 3. OGA+/− mice exhibit increased susceptibility to acute DSS-induced colitis and chronic colitis.
Eight-week-old OGA+/+ and OGA+/− mice were treated with 2% DSS for 7 days and examined for clinical signs of colitis. (A) Weights of mice during the 7-day treatment period. n = 5 mice per group. (B) Photographs of representative colons and ceca from untreated and DSS-treated OGA+/+ and OGA+/− mice. Colon length, measured at day 7 after treatment. (C) H&E-stained distal colonic sections after 7 days of DSS treatment and histological scores. Bars=100 μm. (D) Clinical scores. (E) MPO activity as a measure of neutrophil infiltration. (F) Cytokines and chemokines measured by Luminex multiplex. n = 5 mice per group. Error bars represent ± SEM. (**) P < 0.005, (*) P < 0.05 (Student's t-test). (G) Colon tissue extracts from OGA+/+ and OGA+/− mice after 7 days of DSS treatment and after 3 days of recovery were immunoblotted with antibodies. (H) The mice were treat with 3 cycles of 3% DSS treatment followed by 14 days of water. The mice were analyzed on day 52. The mean changes in body weight of OGA+/+ and OGA+/− mice. n = 13 mice per group. (I) Histological scores (J) Colon length. n = 5 mice per group. Error bars represent ± SEM. (**) P < 0.005, (*) P < 0.05 (Student's t-test).
We next investigated the roles of O-GlcNAcylation in the DSS-induced chronic colitis. The chronic colitis was induced 3 cycles of 3% DSS treatment for 5 days by 14 days of water. Consistent with the results of acute colitis, OGA+/−mice showed increased susceptibility to chronic colitis compared with OGA+/+ mice. OGA+/−mice exhibited increased body weight loss (Figure 3H) and decreased survival rate during chronic colitis progression (Supplementary Figure 5A). Severe colitis of OGA+/−mice were reflected by histopathological image analysis (Supplementary Figure 5B) with higher histological scores (Figure 3I) and colonic shortening compared to OGA+/+ mice (Figure 3J). Overall, these results indicate that increased O-GlcNAcylation aggravates intestinal inflammation in a mouse model of colitis.
Increased O-GlcNAcylation contributes to colitis-associated colorectal cancer
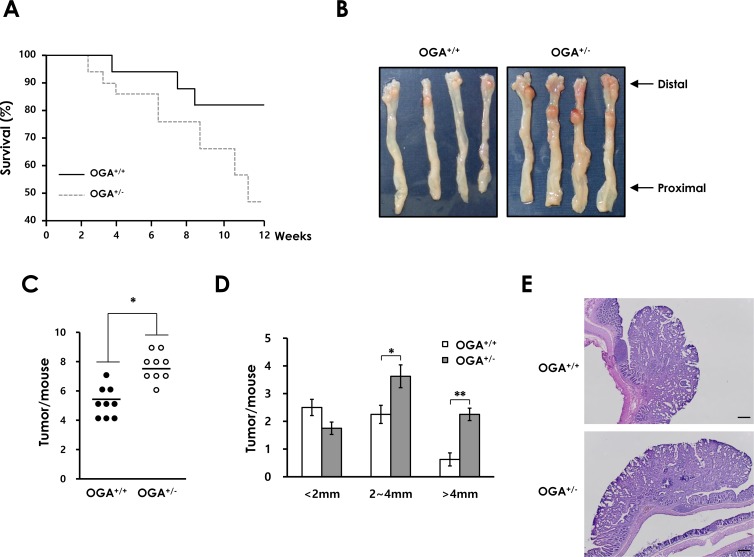
To further characterize the effects of elevated O-GlcNAcylation on DSS-induced colitis, we examined whether increased O-GlcNAcylation promotes the development of CAC. OGA+/+ and OGA+/− mice were assessed using the AOM/DSS model of CAC. As expected, OGA+/− mice exhibited a higher mortality rate than OGA+/+ mice (Figure 4A). The early death of OGA+/− mice might be associated with greater susceptibility to DSS-induced colitis as observed in Figure 3 and Supplementary Figure 4. Both OGA+/+ and OGA+/− mice developed tumors in the middle-to-distal portion of the colon (Figure 4B). OGA+/− mice developed a markedly greater number of tumors (Figure 4C). The average tumor size was also significantly greater in OGA+/− mice than OGA+/+ mice (Figure 4D). Histological examination consistently showed larger adenomas in colonic sections of OGA+/− mice compared with OGA+/+ mice (Figure 4E). These results reflect the result showing reduced survival rate of OGA+/+ mice. These results indicate that increased O-GlcNAcylation may accelerate tumor development.
Figure 4. Elevated O-GlcNAcylation contributes to colonic tumorigenesis in a mouse model of CAC.
(A) OGA+/+ and OGA+/− mice littermate survival (18 of 21 OGA+/+ mice 9 of 20 OGA+/− mice). (B) Representative colons at the end of the CAC protocol. (C) Number of tumors per mouse and (D) tumor size. Size distribution of tumors is shown. (E) Representative H&E-stained colonic tumors at the end of the CAC protocol. Bars =200 μm.
Increased O-GlcNAcylation enhances LPS-induced activation of NF-κB signaling
Intestinal inflammation is caused by a dysregulated mucosal immune response to luminal bacteria. LPS and other bacterial products are recognized Toll-like receptors (TLRs), which lead to NF-κB signaling activation. TLR signaling is an important for luminal bacteria-induced intestinal inflammation [30, 31]. NF-κB signaling activation has been proposed as a link between inflammation and carcinogenesis by acting both in tumor and inflammatory cells to promote tumor development [32]. Notably, the key regulators of NF-κB signaling p65, IKKβ and TAK1, are O-glycosylated and regulated by this modification [23, 24, 33, 34].
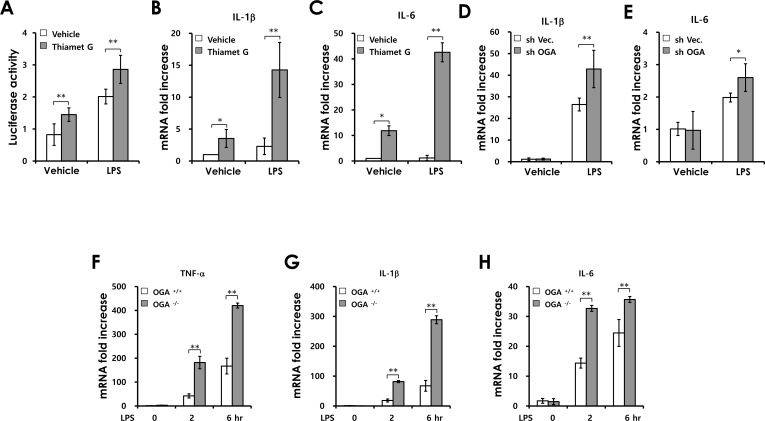
To investigate how O-GlcNAcylation affects NF-κB signaling in colonic epithelial cells, we examined the effect of increased O-GlcNAcylation on LPS-induced NF-κB activation. We used Thiamet G, a potent and selective inhibitor of OGA (Supplementary Figure 6). As shown in Figure 5A, Thiamet G enhanced LPS-induced NF-κB reporter gene activation in CT26 colonic epithelial cells. Consistent with this result, the expression of NF-κB target genes (IL-1β and IL-6) induced by LPS stimulation was increased in Thiamet G-treated cells (Figure 5B and 5C). We also observed that OGA knockdown promotes LPS-induced IL-1β and IL-6 mRNA production compared with control cells (Figure 5D and 5E). To further confirm the effect of increased O-GlcNAcylation, we used OGA−/− mouse embryonic fibroblasts (MEFs). Similarly, the expression of NF-κB target genes was significantly increased in OGA−/− MEFs after LPS treatment compared with in wild-type (WT) MEFs (Figure 5F-5H). These results indicate that increased O-GlcNAcylation promotes LPS-induced activation of NF-κB signaling in colonic epithelial cells and MEFs. These data suggest that dysregulation of O-GlcNAcylation contributes to the development of colitis and CAC through enhancing activation of inflammatory pathway in intestinal epithelial cells.
Figure 5. Increased O-GlcNAcylation enhances LPS induced-NF-κB signaling activation.
(A) CT26 cells were transfected with an NF-κB promoter–reporter construct before LPS (100 ng/mL) and/or Thiamet G (1μM) treatment; luciferase activity was measured at 6 h. (B and C) SYBR Green-based RT-PCR analysis was carried out after treatment with LPS (100 ng/mL) and/or Thiamet G (1 μM) and expression levels of IL-1β and IL-6 were determined by comparison with GAPDH. (D and E) Control and OGA knockdown Caco2 cell lines were stimulated with LPS and the mRNA levels of IL-1β and IL-6 were measured at 6h. (F-H) OGA+/+ and OGA−/− MEFs were stimulated by LPS (100 ng/ml) and the mRNA levels of TNF-α, IL-1β and IL-6 were measured at the indicated times post-stimulation. Error bars represent the S.D. (n = 3). (**) P < 0.005, (*) P < 0.05 (Student's t-test.
NF-κB p65 subunit binding to promoter regions is regulated by O-GlcNAcylation
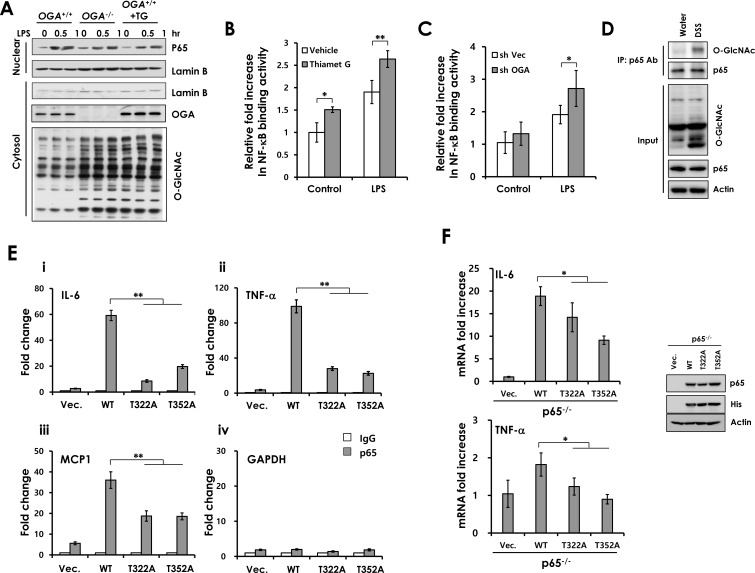
In subsequent experiments, we investigated the effect of O-GlcNAcylation on the NF-κB signaling pathway by western blot. LPS treatment rapidly induced Iκb phosphorylation, Iκb degradation and p65 phosphorylation in both control and OGA knockdown Caco2 cells (Supplementary Figure 7A). We observed the same results in OGA+/+ MEFs and OGA−/− MEFs (Supplementary Figure 7B). Since O-GlcNAcylation did not affect Iκb degradation and p65 phosphorylation, we next focused on p65 nuclear translocation. We found that elevated O-GlcNAcylation did not affect p65 nuclear translocation in OGA knockdown and Thiamet G-treated Caco2 cells (Supplementary Figure 7C) and found an identical result in OGA−/− MEFs and Thiamet G-treated OGA+/+ MEFs (Figure 6A).
Figure 6. The O-GlcNAcylation site of p65 is important for binding of p65 to the promoter elements.
(A) OGA+/+, OGA−/−, and Thiamet G-treated- OGA+/+ MEFs were stimulated with LPS. The nuclear extracts were prepared and subjected to Western blot analysis. (B) NF-κB p65 subunit DNA binding activity was analyzed with nuclear extracts isolated from control and Thiamet G pre-treated Caco2 or (C) control and OGA knockdown Caco2 cell lines. (D) Water-treated or DSS-treated colon homogenates were used for immunoprecipitation with a polyclonal p65 antibody and immunoblotted against O-GlcNAc. The levels of p65 expression and O-GlcNAcylation were detected in input homogenates. Recruitment of p65 on the (E) (i) IL-6, (ii) TNF-α, (iii) MCP1 promoter, and (iv) GAPDH (control) were measured by p65 ChIP assay in HEK-293 cells expressing p65 WT, p65T322A or p65T352A. (F) p65 KO MEFs were transfected with plasmids containing p65 WT, p65T322A, p65T352A, or empty vector. RT-PCRs were performed to detect the p65 target genes, IL-6 and TNF-α. The expression of these plasmids expressing WT or mutant p65 were determined by immunoblotting with anti p65, His, and β-actin antibodies (right). Error bars represent the S.D. (n = 3). (**) P < 0.005, (Student's t-test).
To determine whether elevated O-GlcNAcylation enhances DNA binding of p65, we performed a p65-binding activity assay using nuclear extracts isolated from control Caco2 cells and Thiamet G-treated Caco2 cells. LPS significantly increased the binding activity of p65 in the control extract. LPS-induced p65 binding activity was significantly enhanced in extracts both Thiamet G-treated cells and OGA knockdown Caco2 cells (Figure 6B and 6C). These results indicate that O-GlcNAcylation regulates p65 DNA-binding activity. Accordingly, we tested whether p65 was highly O-GlcNAcylated in DSS-treated colonic extracts. We detected elevated p65 O-GlcNAcylation in DSS-treated colon compared with control (Figure 6D).
Next, we investigated whether p65 O-GlcNAcylation affects promoter recruitment of p65 using chromatin immunoprecipitation (ChIP) assay. A previous study reported that Thr-322 and Thr-352 of p65 are O-GlcNAcylated [23]. We determined whether Thr-322 or Thr-352 mutation disrupts p65 binding to target promoters. HEK293 cell were transfected with WT p65 or His-p65 mutants (T322A and T352A). The O-GlcNAc level of p65 mutants was reduced slightly in the presence of identical amounts of each expression plasmid (Supplementary Figure 8). WT p65 was recruited to IL-6, TNF-α and MCP-1 promoter elements. However, mutation of Thr-322 or 352 to alanine reduced p65 binding to target promoters (IL-6, TNF-α and MCP1; Figure 6E). Consistent with these data, overexpression of p65 O-GlcNAcylation mutants in p65 KO MEFs exhibited reduced mRNA expression of IL-6 and TNF-α (Figure 6F). These findings indicate that p65 O-GlcNAcylation on Thr-322 and Thr-352 is required for transcriptional activation of p65 and suggest that elevated p65 O-GlcNAcylation promotes NF-κB signaling by modulating promoter recruitment of p65.
DISCUSSION
Many studies have suggested that NF-κB signaling is a critical link between inflammation and carcinogenesis. Although the involvement of O-GlcNAcylation in NF-κB signaling has been suggested, the molecular mechanism and physiological roles have not been elucidated. In this study, we showed that O-GlcNAcylation is elevated in intestinal inflammation. Notably, O-GlcNAcylation levels were elevated in mouse and human CAC, suggesting that O-GlcNAcylation contributes to chronic inflammation and tumor development. We showed that increased O-GlcNAcylation enhances sensitivity to DSS-induced colitis and promotes CAC by modulating NF-κB signaling. O-GlcNAcylation of p65 affects its binding to target promoters. These results suggest a role for O-GlcNAc cycling in the pathogenesis of inflammatory bowel diseases and colorectal cancer.
Previous studies showed that NF-κB signaling is regulated by O-GlcNAcylation differently. O-GlcNAcylation of the Iκ-B kinase subunit β at Ser733, an inactivating phosphorylation site, is required for catalytic activity in response to high-glucose conditions [33]. The NF-κB p65 subunit is O-GlcNAcylated at Thr-305, Thr-322 and Thr-352. Point mutation of these sites revealed that O-GlcNAc modification at Thr-352 regulates p65 transcriptional activity [23]. In addition, Thr-305 is essential for p65 transcriptional activity by potentiating p300-mediated acetylation [35]. These studies demonstrated that O-GlcNAcylation of both IKK and NF-κB p65 enhances the transcriptional activity of NF-κB signaling under hyperglycemic conditions. In contrast, other studies have reported that elevated O-GlcNAcylation suppressed NF-κB signaling. Glucosamine (GlcN), a major product of the hexosamine biosynthesis pathway, or PUGNAC (OGA inhibitor) treatment inhibits TNF-α–induced NF-κB signaling in vascular smooth-muscle cells by blocking NF-κB p65 Ser 536 phosphorylation [25]. In rat trauma hemorrhage, increased O-GlcNAc modification in the heart, liver and kidney improved cardiac function and organ perfusion with reduced activation of NF-κB signaling [26, 36]. Although reported in many studies, the role of O-GlcNAcylation in NF-κB signaling is dependent on the experimental design and conditions used. In the present study, we showed that increased O-GlcNAcylation caused by OGA deletion or knockdown did not affect Iκb degradation or p65 phosphorylation and nuclear translocation (Supplementary Figure 7). However, elevated O-GlcNAcylation affected NF-κB p65 transcriptional activity by modulating p65 DNA binding (Figure 6B). Regarding phosphorylation of Iκb, this in vitro result is inconsistent with the in vivo data shown in Figure 3G. We speculate that elevated p65 promoter-binding activity eventually aggravate colonic inflammation in DSS-treated OGA+/− mice. Consequently, OGA+/− mice exhibited increased Iκb and STAT3 phosphorylation compared with WT mice during colitis. Our finding is supported by reports of involvement of many O-GlcNAc–modified proteins in transcriptional regulation: c-myc [37], FoxO-1 [38], PGC-1α [39], and HCF-1 [40].
Recent studies have suggested that dysregulation of O-GlcNAcylation is associated with tumor growth and metastasis. Correlatively, aberrant O-GlcNAcylation and protein levels of O-GlcNAc cycling enzymes are observed in tumors. O-GlcNAcylation levels are elevated in breast, lung, chronic lymphocytic leukemia, and colon tumors [5, 8, 10]. Consistent with these reports, we found that O-GlcNAcylation was elevated in the colon tissues of CAC mice, and OGA+/− mice showed an increased incidence of colorectal tumors compared to control mice. Evidently, NF-κB signaling contributes to cancer development and progression. Colon cancer cell lines and human tumor samples show increased activity of NF-κB, which is a crucial mediator of inflammation-induced tumor growth and progression [41, 42]. Based on these results, we suggest that elevation of O-GlcNAcylation aggravates chronic inflammation by enhancing NF-κB signaling activation, which contributes to CAC development. In contrast, OGA+/− mice were protected from tumor development in Apcmin/+ model of sporadic CRC [43]. This finding suggests that increased O-GlcNAcylation suppresses colorectal tumorigenesis. This observation indicates that O-GlcNAcylation differentially affects sporadic and inflammatory-driven tumorigenesis. We suggest that colitis-associated cancer which is different state of inflammation compared with sporadic cancer requires hyper-O-GlcNAyclation for enhancing NF-κB signaling in tumor growth and progression. Previous studies also suggest that the opposite effects on colorectal tumorigenesis in different types of colorectal cancer models is responsible for involvement of different signaling pathway [44, 45].
In addition to O-GlcNAcylation modulating NF-κB signaling, the effects of the many transcriptional mediators that are O-GlcNAcylated in colorectal tumorigenesis cannot be disregarded. Dysregulation of O-GlcNAcylation alters the activity of transcriptional mediators that contribute to tumor growth and metastasis [4, 46]. Consistent with these reports, reduced O-GlcNAcylation of FoxM1, an oncogenic transcription factor, via OGT knockdown in breast cancer cells leads to inhibition of tumor growth both in vitro and in vivo [11]. Furthermore, phosphofructokinase 1 (PFK1) is O-GlcNAcylated. Inhibition of PFK1 O-GlcNAcylation blocks tumor growth in vivo [47]. Additionally, a proteomic study newly identified aberrant O-GlcNAcylated proteins involved in the stress response, biosynthesis, RNA metabolism, gene expression, cytoskeleton, and primary breast malignant tumors [48].
In summary, we demonstrated the role of O-GlcNAcylation in colitis and CAC using OGA+/− mice. Our results indicate that O-GlcNAc cycling is critical for regulation of NF-κB signaling in colitis and CAC. Therefore, we propose that dysregulation of O-GlcNAcylation is involved in diverse inflammatory diseases, and thus pharmacological modulation of O-GlcNAc cycling enzymes could be a novel therapeutic strategy for such conditions.
MATERIALS AND METHODS
Mice
OGA+/− mice (C57BL/SV129) were generated as described previously [29]. OGA+/− mice were backcrossed with C57BL/6J wild-type mice. Mouse strains were bred and housed in the Animal Research Facility at POSTECH and UNIST.
Human patient samples
The biospecimens for this study were provided by the Pusan National University Hospital, a member of the National Biobank of Korea, which is supported by the Ministry of Health and Welfare. All samples derived from the National Biobank of Korea were obtained with informed consent under institutional review board-approved protocols. All patients were included based on clinical, endoscopical, and pathological proven by gastroenterologists. Four tissue specimens were obtained from patients with CAC. A part of the colon with the cancer and a small segment of normal colon were obtained from patients with CAC.
Colitis and CAC induction
To induce colitis, 7–8-week-old sex-matched OGA+/+ and OGA+/− mice were provided with 2% DSS (molecular weight, 35,000–50,000; MP Biomedicals, Solon, OH) in drinking water ad libitum for 7 days. CAC was induced as described previously [49], with some modifications since C57Bl/6J mice show low susceptibility to AOM/DSS-induced CAC [50]. Mice were intraperitoneally injected with AOM (10 mg/kg body weight) and maintained on a regular diet and water for 7 days (OGA+/+ and OGA+/− mice). Mice were then subjected to five cycles of DSS treatment, in which each cycle consisted of 1.5% DSS for 7 days (OGA+/+ and OGA+/− mice) followed by a 7-day recovery period with regular water.
Clinical activity score
Body weight loss, stool consistency and the presence of occult/gross blood were assessed daily for each mouse as described previously [51], with some modifications. Body weight change was scored as follows: 0, no change; 1, 1–5% weight loss; 2, 5–10% weight loss; 3, 10–20% weight loss; 4, >20% weight loss. Stool character was scored as follows: 0, normal; 1, soft with well-formed pellets; 2, soft without pellets; 3, diarrhea. Occult blood was scored as follows: 0, no blood; 2, blood; 3, gross bleeding. These scores were added to generate a clinical activity score ranging from 0 to 10.
Myeloperoxidase (MPO) activity
MPO activity was determined using the EnzChek MPO Activity Assay Kit (Invitrogen) according to the manufacturer's instructions. Briefly, the colon was homogenized in MPO assay buffer and the homogenate was centrifuged at 13000× g for 15 min at 4°C. Supernatants were used for assay. The protein concentrations in all tissue extracts were adjusted to 1 mg/ml. The absorbance at 412 nm was measured using a spectrophotometer.
Cytokine measurements
To assay cytokine levels in colon tissue, a part of the colon was homogenized mechanically in PBS containing 0.1% Triton-X100 and a complete protease inhibitor mixture. Mouse cytokines and chemokines in colon homogenates were determined by Luminex assay (Bio-Rad, Hercules, CA).
Measurement of UDP-GlcNAc
UDP-GlcNAc levels were determined using by HPLC analysis of extracts of colon tissue samples as previously described [52]. Briefly, colon tissues are homogenized in 70% ice-cold ethanol. Then the lysates are further lysed on the ice using a handy sonic disruptor. The extract was centrifuged at 16,000 × g for 10 min at 4°C in order to remove insoluble material, and the supernatant was lyophilized. Purification of the extracted nucleotide sugars, and separation and quantification by ion pair reversed phase HPLC, have already been reported in detail previously [52].
Cell culture and generation of MEFs
The human (Caco-2) and mouse (CT26) colonic epithelial cell lines, and RAW264.7 cell lines were purchased from the American Type Culture Collection (ATCC); (Caco2, 2010), (CT26, 2010), and Cell lines were cultured in the recommended ATCC media. Cell line authentication was done by ATCC. Cells were daily checked by morphology and tested to be Mycoplasma free by PCR 1 weeks after recovery from frozen stocks in culture. These cell lines were routinely cultured at 37°C in 5% CO2. The cultures were maintained for no longer than 8 weeks after recovery from frozen stocks in culture. Primary MEFs were isolated from 12.5–14.5 dpc embryos from heterozygous mice breeding. After removal of the intestinal organs and head, embryos were washed with phosphate-buffered saline (PBS), minced and trypsinized. After centrifugation, the dissociated cells were plated in medium. The medium was composed of DMEM glutamax, 10% FBS, Pen/Strep and 1% non-essential amino acids. Genotyping was done by both PCR and Western blot. Early passage (P<4) MEFs were used for all experiments.
Western blotting
Tissue and cell lysates were prepared using standard procedures. Samples of 20–30 μg of protein were separated on an SDS-polyacrylamide gel and visualized. Western blotting antibodies used were anti-phospho-IκB-α (9246; Cell Signaling Technology), anti-IκB-α (4812; Transduction Laboratories), anti-Stat3 (Transduction Laboratories), anti-phospho-Stat3 (9131; Cell Signaling Technology), anti-phoshpo-p65 (3036; Cell Signaling Technology), anti-p65 (ab7970; Abcam), anti-Lamin B (6216; Santa Cruz Biotechnology Inc.), anti-O-GlcNAc (CTD110.6)(9875; Cell Signaling Technology), anti-O-GlcNAc (RL2)(MA1-072; Thermo), and β-actin (691001; MP Biomedicals). Anti-OGA polyclonal antibodies had been previously generated and were used as previously described.[53] The peroxidase-labeled goat anti-rabbit IgG and the goat anti-mouse IgA, IgM, and IgG used as the secondary antibodies were obtained from Kirkegaard & Perry Laboratories (KPL, Gaithersburg).
NF-κB transcriptional activity assay
To assay NF-κB transcriptional activity, colonic epithelial cells were seeded (1×104/well) in a 24-well plate. After reaching 60% confluence, the cells were transiently transfected with 0.5 μg of NF-κB-luciferase promoter-reporter construct (pGL4.32 [luc2P/NF-κB -RE/Hygro]) and 0.1 μg of control reporter plasmid (pRL-TK), containing the Renilla reniformis luciferase gene downstream of the TK promoter. Transfections were carried out using Lipofectamine (Invitrogen) as a transfection reagent. Twenty-four hours after transfection, the cells were treated with LPS, washed with ice-cold PBS and harvested in reporter lysis buffer. Luciferase activity was measured using the Dual Luciferase Assay System. All experiments were performed in triplicate and relative luciferase activity was reported as the fold induction after normalization for transfection efficiency.
Statistical analysis
Data are presented as means ± standard errors of the means (SEMs) or standard deviation (SD) as indicated in the figure legends. Comparisons between two groups were made by unpaired two-tailed Student's t tests. P values of <0.05 were considered statistically significant. Microsoft Excel was used for statistical calculations.
Histological assessment of colitis, NF-κB binding activity assay, ChIP assay, LPS injection and mouse model of endotoxin shock and shRNA knockdown and stable cell line generation.
SUPPLEMENTARY MATERIAL FIGURES AND TABLES
Acknowledgments
The biospecimens for this study were provided by the Pusan National University Hospital, a member of the National Biobank of Korea, which is supported by the Ministry of Health and Welfare. All samples derived from the National Biobank of Korea were obtained with informed consent under institutional review board-approved protocols. These specimen were provided by national biobank of Korea, PNUH. We thank Dr. Jin Won Cho for kindly providing DNA constructs, p65 WT, p65T322A mutant and p65T352A mutant and Dr. Alexander Hoffmann (University of California at San Diego, La Jolla, CA) for the p65 null MEFs.
Footnotes
Grant support
This work was supported by the National Research Foundation of Korea (NRF) Grant funded by the Korean Government (MSIP) (No. 2010-0028684), the Korean Government (MOE) (2013R1A1A2064434).
Disclosure of Potential Conflicts of Interest
The authors have no conflict of interest to disclose.
REFERENCES
- 1.Hart GW, Slawson C, Ramirez-Correa G, Lagerlof O. Cross Talk Between O-GlcNAcylation and Phosphorylation: Roles in Signaling, Transcription, and Chronic Disease. Annu Rev Biochem. 2011;80:825–858. doi: 10.1146/annurev-biochem-060608-102511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lazarus BD, Love DC, Hanover JA. O-GlcNAc cycling: implications for neurodegenerative disorders. Int J Biochem Cell Biol. 2009;41:2134–2146. doi: 10.1016/j.biocel.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang X, Ongusaha PP, Miles PD, Havstad JC, Zhang F, So WV, Kudlow JE, Michell RH, Olefsky JM, Field SJ, Evans RM. Phosphoinositide signalling links O-GlcNAc transferase to insulin resistance. Nature. 2008;451:964–969. doi: 10.1038/nature06668. [DOI] [PubMed] [Google Scholar]
- 4.Slawson C, Hart GW. O-GlcNAc signalling: implications for cancer cell biology. Nat Rev Cancer. 2011;11:678–684. doi: 10.1038/nrc3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gu Y, Mi W, Ge Y, Liu H, Fan Q, Han C, Yang J, Han F, Lu X, Yu W. GlcNAcylation plays an essential role in breast cancer metastasis. Cancer Res. 2010;70:6344–6351. doi: 10.1158/0008-5472.CAN-09-1887. [DOI] [PubMed] [Google Scholar]
- 6.Lynch TP, Ferrer CM, Jackson SR, Shahriari KS, Vosseller K, Reginato MJ. Critical role of O-Linked beta-N-acetylglucosamine transferase in prostate cancer invasion, angiogenesis, and metastasis. J Biol Chem. 2012;287:11070–11081. doi: 10.1074/jbc.M111.302547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yehezkel G, Cohen L, Kliger A, Manor E, Khalaila I. O-linked beta-N-acetylglucosaminylation (O-GlcNAcylation) in primary and metastatic colorectal cancer clones and effect of N-acetyl-beta-D-glucosaminidase silencing on cell phenotype and transcriptome. J Biol Chem. 2012;287:28755–28769. doi: 10.1074/jbc.M112.345546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mi W, Gu Y, Han C, Liu H, Fan Q, Zhang X, Cong Q, Yu W. O-GlcNAcylation is a novel regulator of lung and colon cancer malignancy. Biochimica et biophysica acta. 2011;1812:514–519. doi: 10.1016/j.bbadis.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 9.Ma Z, Vocadlo DJ, Vosseller K. Hyper-O-GlcNAcylation is anti-apoptotic and maintains constitutive NF-kappaB activity in pancreatic cancer cells. J Biol Chem. 2013;288(21):15121–15130. doi: 10.1074/jbc.M113.470047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi Y, Tomic J, Wen F, Shaha S, Bahlo A, Harrison R, Dennis JW, Williams R, Gross BJ, Walker S, Zuccolo J, Deans JP, Hart GW, Spaner DE. Aberrant O-GlcNAcylation characterizes chronic lymphocytic leukemia. Leukemia. 2010;24:1588–1598. doi: 10.1038/leu.2010.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caldwell SA, Jackson SR, Shahriari KS, Lynch TP, Sethi G, Walker S, Vosseller K, Reginato MJ. Nutrient sensor O-GlcNAc transferase regulates breast cancer tumorigenesis through targeting of the oncogenic transcription factor FoxM1. Oncogene. 2010;29:2831–2842. doi: 10.1038/onc.2010.41. [DOI] [PubMed] [Google Scholar]
- 12.Itzkowitz SH, Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am J Physiol Gastrointest Liver Physiol. 2004;287:G7–17. doi: 10.1152/ajpgi.00079.2004. [DOI] [PubMed] [Google Scholar]
- 13.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wullaert A, Bonnet MC, Pasparakis M. NF-kappaB in the regulation of epithelial homeostasis and inflammation. Cell research. 2011;21:146–158. doi: 10.1038/cr.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schreiber S, Nikolaus S, Hampe J. Activation of nuclear factor kappa B inflammatory bowel disease. Gut. 1998;42:477–484. doi: 10.1136/gut.42.4.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kojima M, Morisaki T, Sasaki N, Nakano K, Mibu R, Tanaka M, Katano M. Increased nuclear factor-kB activation in human colorectal carcinoma and its correlation with tumor progression. Anticancer Res. 2004;24:675–681. [PubMed] [Google Scholar]
- 17.Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E, Ben-Neriah Y. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461–466. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- 18.Karrasch T, Kim JS, Muhlbauer M, Magness ST, Jobin C. Gnotobiotic IL-10(−/−);NF-kappa B-EGFP mice reveal the critical role of TLR/NF-kappa B signaling in commensal bacteria-induced colitis. Journal of Immunology. 2007;178:6522–6532. doi: 10.4049/jimmunol.178.10.6522. [DOI] [PubMed] [Google Scholar]
- 19.Hoshi N, Schenten D, Nish SA, Walther Z, Gagliani N, Flavell RA, Reizis B, Shen Z, Fox JG, Iwasaki A, Medzhitov R. MyD88 signalling in colonic mononuclear phagocytes drives colitis in IL-10-deficient mice. Nat Commun. 2012;3:1120. doi: 10.1038/ncomms2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Asquith MJ, Boulard O, Powrie F and, Maloy KJ. Pathogenic and protective roles of MyD88 in leukocytes and epithelial cells in mouse models of inflammatory bowel disease. Gastroenterology. 2010;139:519–529, 529. e511–512. doi: 10.1053/j.gastro.2010.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.James LR, Tang D, Ingram A, Ly H, Thai K, Cai L, Scholey JW. Flux through the hexosamine pathway is a determinant of nuclear factor kappa B-dependent promoter activation. Diabetes. 2002;51:1146–1156. doi: 10.2337/diabetes.51.4.1146. [DOI] [PubMed] [Google Scholar]
- 22.Golks A, Tran TTT, Goetschy JF, Guerini D. Requirement for O-linked N-acetylglucosaminyltransferase in lymphocytes activation. Embo J. 2007;26:4368–4379. doi: 10.1038/sj.emboj.7601845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang WH, Park SY, Nam HW, Kim DH, Kang JG, Kang ES, Kim YS, Lee HC, Kim KS, Cho JW. NF kappa B activation is associated with its O-GlcNAcylation state under hyperglycemic conditions. P Natl Acad Sci USA. 2008;105:17345–17350. doi: 10.1073/pnas.0806198105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pathak S, Borodkin VS, Albarbarawi O, Campbell DG, Ibrahim A, van Aalten DM. O-GlcNAcylation of TAB1 modulates TAK1-mediated cytokine release. Embo Journal. 2012;31:1394–1404. doi: 10.1038/emboj.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xing D, Gong K, Feng W, Nozell SE, Chen YF, Chatham JC, Oparil S. O-GlcNAc modification of NFkappaB p65 inhibits TNF-alpha-induced inflammatory mediator expression in rat aortic smooth muscle cells. Plos One. 2011;6:e24021. doi: 10.1371/journal.pone.0024021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zou L, Yang S, Champattanachai V, Hu S, Chaudry IH, Marchase RB, Chatham JC. Glucosamine improves cardiac function following trauma-hemorrhage by increased protein O-GlcNAcylation and attenuation of NF-{kappa}B signaling. Am J Physiol Heart Circ Physiol. 2009;296:H515–523. doi: 10.1152/ajpheart.01025.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suzuki A, Hanada T, Mitsuyama K, Yoshida T, Kamizono S, Hoshino T, Kubo M, Yamashita A, Okabe M, Takeda K, Akira S, Matsumoto S, Toyonaga A, Sata M, Yoshimura A. CIS3/SOCS3/SSI3 plays a negative regulatory role in STAT3 activation and intestinal inflammation. J Exp Med. 2001;193:471–481. doi: 10.1084/jem.193.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steinbrecher KA, Harmel-Laws E, Sitcheran R, Baldwin AS. Loss of epithelial RelA results in deregulated intestinal proliferative/apoptotic homeostasis and susceptibility to inflammation. Journal of immunology. 2008;180:2588–2599. doi: 10.4049/jimmunol.180.4.2588. [DOI] [PubMed] [Google Scholar]
- 29.Yang YR, Song M, Lee H, Jeon Y, Choi EJ, Jang HJ, Moon HY, Byun HY, Kim EK, Kim DH, Lee MN, Koh A, Ghim J, Choi JH, Lee-Kwon W, Kim KT, et al. O-GlcNAcase is essential for embryonic development and maintenance of genomic stability. Aging Cell. 2012;11:439–448. doi: 10.1111/j.1474-9726.2012.00801.x. [DOI] [PubMed] [Google Scholar]
- 30.Fukata M, Michelsen KS, Eri R, Thomas LS, Hu B, Lukasek K, Nast CC, Lechago J, Xu RL, Naiki Y, Soliman A, Arditi M, Abreu MT. Toll-like receptor-4 is required for intestinal response to epithelial injury and limiting bacterial translocation in a murine model of acute colitis. Am J Physiol-Gastr L. 2005;288:G1055–G1065. doi: 10.1152/ajpgi.00328.2004. [DOI] [PubMed] [Google Scholar]
- 31.Cario E, Rosenberg IM, Brandwein SL, Beck PL, Reinecker HC, Podolsky DK. Lipopolysaccharide activates distinct signaling pathways in intestinal epithelial cell lines expressing Toll-like receptors. Journal of immunology. 2000;164:966–972. doi: 10.4049/jimmunol.164.2.966. [DOI] [PubMed] [Google Scholar]
- 32.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 33.Kawauchi K, Araki K, Tobiume K, Tanaka N. Loss of p53 enhances catalytic activity of IKKbeta through O-linked beta-N-acetyl glucosamine modification. Proc Natl Acad Sci U S A. 2009;106:3431–3436. doi: 10.1073/pnas.0813210106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma ZY, Vocadlo DJ, Vosseller K. Hyper-O-GlcNAcylation Is Anti-apoptotic and Maintains Constitutive NF-kappa B Activity in Pancreatic Cancer Cells. J Biol Chem. 2013;288:15121–15130. doi: 10.1074/jbc.M113.470047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Allison DF, Wamsley JJ, Kumar M, Li D, Gray LG, Hart GW, Jones DR, Mayo MW. Modification of RelA by O-linked N-acetylglucosamine links glucose metabolism to NF-kappaB acetylation and transcription. Proc Natl Acad Sci U S A. 2012;109:16888–16893. doi: 10.1073/pnas.1208468109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Not LG, Marchase RB, Fulop N, Brocks CA, Chatham JC. Glucosamine administration improves survival rate after severe hemorrhagic shock combined with trauma in rats. Shock. 2007;28:345–352. doi: 10.1097/shk.0b013e3180487ebb. [DOI] [PubMed] [Google Scholar]
- 37.Chou TY, Hart GW. O-linked N-acetylglucosamine and cancer: messages from the glycosylation of c-Myc. Adv Exp Med Biol. 2001;491:413–418. doi: 10.1007/978-1-4615-1267-7_26. [DOI] [PubMed] [Google Scholar]
- 38.Kuo M, Zilberfarb V, Gangneux N, Christeff N, Issad T. O-glycosylation of FoxO1 increases its transcriptional activity towards the glucose 6-phosphatase gene. FEBS Lett. 2008;582:829–834. doi: 10.1016/j.febslet.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 39.Housley MP, Udeshi ND, Rodgers JT, Shabanowitz J, Puigserver P, Hunt DF, Hart GW. A PGC-1alpha-O-GlcNAc transferase complex regulates FoxO transcription factor activity in response to glucose. J Biol Chem. 2009;284:5148–5157. doi: 10.1074/jbc.M808890200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruan HB, Han X, Li MD, Singh JP, Qian K, Azarhoush S, Zhao L, Bennett AM, Samuel VT, Wu J, Yates JR, 3rd, Yang X. O-GlcNAc transferase/host cell factor C1 complex regulates gluconeogenesis by modulating PGC-1alpha stability. Cell Metab. 2012;16:226–237. doi: 10.1016/j.cmet.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lind DS, Hochwald SN, Malaty J, Rekkas S, Hebig P, Mishra G, Moldawer LL, Copeland EM, MacKay S. Nuclear factor-kappa B is upregulated in colorectal cancer. Surgery. 2001;130:363–369. doi: 10.1067/msy.2001.116672. [DOI] [PubMed] [Google Scholar]
- 42.Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 43.Yang YR, Jang HJ, Yoon S, Lee YH, Nam D, Kim IS, Lee H, Kim H, Choi JH, Kang BH, Ryu SH, Suh PG. OGA heterozygosity suppresses intestinal tumorigenesis in Apc(min/+) mice. Oncogenesis. 2014;3:e109. doi: 10.1038/oncsis.2014.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salcedo R, Worschech A, Cardone M, Jones Y, Gyulai Z, Dai RM, Wang E, Ma W, Haines D, O'HUigin C, Marincola FM, Trinchieri G. MyD88-mediated signaling prevents development of adenocarcinomas of the colon: role of interleukin 18. J Exp Med. 2010;207:1625–1636. doi: 10.1084/jem.20100199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rakoff-Nahoum S, Medzhitov R. Regulation of spontaneous intestinal tumorigenesis through the adaptor protein MyD88. Science. 2007;317:124–127. doi: 10.1126/science.1140488. [DOI] [PubMed] [Google Scholar]
- 46.Ozcan S, Andrali SS, Cantrell JE. Modulation of transcription factor function by O-GlcNAc modification. Biochim Biophys Acta. 2010;1799:353–364. doi: 10.1016/j.bbagrm.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yi W, Clark PM, Mason DE, Keenan MC, Hill C, Goddard WA, 3rd, Peters EC, Driggers EM, Hsieh-Wilson LC. Phosphofructokinase 1 glycosylation regulates cell growth and metabolism. Science. 2012;337:975–980. doi: 10.1126/science.1222278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Champattanachai V, Netsirisawan P, Chaiyawat P, Phueaouan T, Charoenwattanasatien R, Chokchaichamnankit D, Punyarit P, Srisomsap C, Svasti J. Proteomic analysis and abrogated expression of O-GlcNAcylated proteins associated with primary breast cancer. Proteomics. 2013 doi: 10.1002/pmic.201200126. [DOI] [PubMed] [Google Scholar]
- 49.Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, Kagnoff MF, Karin M. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 50.Van Der Kraak L, Meunier C, Turbide C, Jothy S, Gaboury L, Marcus V, Chang SY, Beauchemin N, Gros P. A two-locus system controls susceptibility to colitis-associated colon cancer in mice. Oncotarget. 2010;1:436–446. doi: 10.18632/oncotarget.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238–249. [PubMed] [Google Scholar]
- 52.Nakajima K, Kitazume S, Angata T, Fujinawa R, Ohtsubo K, Miyoshi E, Taniguchi N. Simultaneous determination of nucleotide sugars with ion-pair reversed-phase HPLC. Glycobiology. 2010;20:865–871. doi: 10.1093/glycob/cwq044. [DOI] [PubMed] [Google Scholar]
- 53.Song M, Kim HS, Parka JM, Kim SH, Kim IH, Ryu SH, Suh PG. O-GlcNAc transferase is activated by CaMKIV-dependent phosphorylation under potassium chloride-induced depolarization in NG-108-15 cells. Cell Signal. 2008;20:94–104. doi: 10.1016/j.cellsig.2007.09.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.