Abstract
Importance
Increases in testosterone use and mixed reports of adverse events have raised concerns about the cardiovascular safety of testosterone. Testosterone is available in several delivery mechanisms with varying pharmacokinetics; injections cause spikes in testosterone levels, while transdermal patches and gels cause more subtle but sustained increases. The comparative cardiovascular safety of gels, injections and patches has not been studied.
Objective
To determine the comparative cardiovascular safety of testosterone injections, patches, and gels.
Design
Retrospective cohort study.
Setting
Administrative claims from a commercially-insured and Medicare population in the United States, and general practitioner records from the United Kingdom, years 2000 – 2012
Participants
Adult (18+), male initiators of testosterone patches, gels, or injections following 180 days free of any testosterone use
Exposure
New initiation of a testosterone dosage form, followed for up to one year
Main Outcomes and Measures
In- or outpatient medical records, diagnoses, or claims for: cardio- and cerebrovascular events, including myocardial infarction (MI), unstable angina, stroke, composite acute event (MI, unstable angina, or stroke); venous thromboembolism (VTE); mortality, and all-cause hospitalization.
Results
We identified 431,687 testosterone initiators between the 3 datasets: 36% injection, 9% patch, 55% gel. Medicare had a majority of injection initiators (51%); the US commercially-insured population had majority gel initiators (56%); the United Kingdom had equal proportions of injections and gels (~41%). When compared to gels, injection initiators had higher hazards of CV events (MI, UA, and stroke) (HR=1.26, 95% CI: 1.18–1.35), hospitalization (HR=1.16, 95% CI: 1.13–1.18), and death (HR=1.34, 95% CI: 1.15–1.56), but not VTE (HR=0.92, 95% CI: 0.76–1.11). Patches did not confer increased hazards of CV events compared to gels (HR=1.10, 95% CI: 0.94–1.29), hospitalization (HR=1.04, 95% CI: 1.00–1.08), death (HR=1.02, 95% CI: 0.77–1.33), or VTE (HR=1.08, 95% CI: 0.79–1.47).
Conclusions and Relevance
Testosterone injections were associated with a greater risk of CV events, hospitalizations, and deaths compared with gels. Patches and gels had similar risk profiles. However, this study did not assess whether patients met criteria for use of testosterone and did not assess the safety of testosterone among users compared to non-users of the drug.
Background
Testosterone use has increased considerably in the United States (US), United Kingdom (UK) and other countries,1–5 and many initiators lack clear, documented indications for treatment1,4. Ongoing, unresolved concerns about cardiovascular safety have been raised by the halting of a randomized trial of testosterone gels in older men with limited mobility due to increased cardiovascular events6, and non-experimental studies reporting increased cardiovascular risk in older men with cardiovascular disease.7,8 Although the recent literature is mixed with some studies suggesting no harmful effects9–11, there has been considerable use contrary to recommended guidelines1,4, prompting interest and investigation into testosterone use and safety.
Testosterone is available in multiple dosage forms, including intramuscular injections, transdermal patches and gels, implantable pellets, intranasal sprays, and oral/buccal applications. While gels, injections, and patches all effectively raise testosterone levels, their pharmacokinetics differ; injections create spikes of super-normal testosterone levels which slowly decrease until a subsequent injection12; this cycling results in less time within normal ranges than with transdermal systems12. Gels and patches result in subtle, short-term increases in testosterone levels (24–48 hours), and daily reapplication can maintain consistent levels12. However, gels provide longer-lasting increases than patches13. As testosterone levels may influence short-term clotting and polycythemia, differing pharmacokinetics may result in varying safety profiles. We compared the cardiovascular risk of testosterone gels, injections, and patches in cohorts of real-world users drawn from large healthcare databases.
Methods
We conducted a new-user14 cohort study of testosterone injection, gel, and patch initiators in three secondary data sources: two from the US and one from the UK.
Data sources
The first US cohort consisted of commercially-insured men from the Truven MarketScan Commercial Claims and Encounters and Medicare Supplementary and Coordination of Benefit files (Truven Health Analytics, Inc., Ann Arbor, MI), 2000–2012. This database contains adjudicated insurance claims for in- and outpatient procedures and diagnoses and pharmacy-dispensed medications for those with employer-sponsored commercial insurance, spouses, dependents, and retirees with employer-sponsored Medicare supplementary plans from large, US employers. Supplementary laboratory test results were available for a subset whose labs were processed by a national laboratory testing company during the years 2007 – 2012. We included men aged 18+ years.
The Medicare cohort was drawn from a national, random 20% sample of the US Medicare fee-for-service population, 2007 – 2010. It contains billing claims for procedures, diagnoses, and dispensed medications for adults aged 65+ from throughout the US. No laboratory test results were available in this cohort.
The UK cohort was drawn from the Clinical Practice Research Datalink (CPRD), a compilation of general practitioner (GP) medical records from throughout the UK from January 2000 to June 2012, which contains outpatient clinical characteristics, diagnoses and procedures, reported hospital and specialist notes, and prescribed medications as recorded by GPs. Laboratory results were available for most tests performed. We included men aged 18+ years.
Exposure assessment
We identified men newly initiating testosterone following a 180-day washout period free of documented testosterone use. Only the first eligible new-use period per individual was included. Considered testosterone dosage forms included: pharmacy-dispensed transdermal gels and patches; pharmacy-dispensed injections; or in-office injections from procedure and supply codes. Exposure categories were grouped as gel, injection, and patch. Prior use of implanted pellets, oral/buccal testosterone, and oral methyltestosterone during washout were considered exclusion criteria, but due to rare, esoteric use and documented risks of methyltestosterone-induced liver problems, these forms were not considered as exposures for the comparative analysis. Patients with claims for two different forms on the index day were excluded because their exposure could not be accurately categorized.
The date of the first pharmacy prescription or injection procedure code following the washout period was considered the index date for the new-user cohorts.14 Due to potential for differential adherence and discontinuation between the dosage forms (injections are dosed every several weeks and patches/gels are dosed daily), we employed a first-exposure-carried-forward analysis where the patient was considered exposed continuously throughout follow-up (see eFigure 1).
Outcome assessment
We followed initiators for up to one year to observe outcomes, including: myocardial infarction (MI); unstable angina; stroke; composite acute events (MI, unstable angina, or stroke); all-cause hospitalization; mortality; and venous thromboembolism (VTE). The effects were estimated separately for each outcome, and those experiencing the outcome of interest during baseline were excluded to restrict to new-onset outcomes. Only the first occurrence of each outcome during follow-up was considered.
In MarketScan and Medicare, outcomes were based on International Classification of Diseases 9th Revision, Clinical Modification (ICD-9) diagnosis codes. MI, unstable angina, and stroke required an inpatient diagnosis of the condition with a hospital stay of at least one day.15,16 All-cause hospitalization was defined as hospitalization for any reason. VTE was defined an as inpatient diagnosis claim followed by a prescription for an antithrombotic drug in the following 30 days.17,18 Mortality was unavailable in MarketScan.
In the CPRD, outcomes were assessed using Read codes recorded by the GP or reported to the GP from hospitals or specialists.19,20
Covariates
We identified total serum testosterone tests performed during the baseline period with procedure codes. If a test result was available, the test result was categorized as ‘high,’ ‘normal,’ or ‘low’ according to assay-specific result flags or reference ranges. If flags or ranges were unavailable, we classified the result as: ‘low’, <300 mg/dL (10.4 nmol/L); ‘normal’, 300–849 ng/dL (10.4–25.4 nmol/L); ‘high’, ≥850 ng/dL (29.5 nmol/L).
Other covariates were assessed during the baseline period using diagnosis, procedure, and medication codes and included: calendar year; age; comorbidity; cardiovascular risk factors; healthcare utilization; preventive and screening care; indications for testosterone; and other medication use. Due to the medical records-based structure of the CPRD, body mass index (BMI) and smoking status were available in the CPRD cohort.
Statistical analysis
Hazard ratios (HR) and 95% confidence intervals (CI) were estimated using multivariable Cox proportional hazards models using days since initiation as the time scale; models were adjusted for a priori identified confounders. Censoring occurred at the first occurrence of: end of the study (MarketScan: December 31, 2012; Medicare: December 31, 2010; CPRD: June 30, 2012); one year post-index date; disenrollment; or death (except when death was the outcome of interest). Separate models for injections and patches were run using gels as the referent group as gels were the most commonly- and broadly-used form.
Additionally, we estimated the effects of testosterone dosage forms using propensity score (PS) matching. We estimated the predicted probability, or PS, of receiving the form of interest versus gels by modeling treatment received with the measured covariates as predictors in logistic regression models. Gel initiators were up-to-2:1 matched to initiators of injections or patches using a greedy matching algorithm which 1:1 matches without replacement to the 5th digit of the PS, if possible21; the algorithm then attempted to 1:1 match the remaining, unmatched gel initiators to the treatment group, resulting in 1 or 2 matched gel initiators for each injection or patch initiator. The HRs were then estimated in the matched datasets, estimating the treatment effect among those treated with the dosage form of interest.22
Due to differences in database structure and covariate availability, analyses were performed and results presented separately in the three databases. Summary estimates were obtained by meta-analyzing the three database-specific multivariable adjusted estimates with fixed effects models.23
Sensitivity analyses
We repeated the analyses separately in subgroups of those with documented low or normal baseline testosterone levels and those without recent measurements to account for potential for differential prescribing by testosterone level. Additionally, to remove contraindicated use in prostate cancer patients, we performed the analysis excluding patients with diagnoses of prostate or any cancer. As usage patterns changed over time1, we performed an analysis restricted to later years after gels had become the predominant therapy option: 2007–2012. Due to a study suggesting cardiovascular risk increased relatively soon after testosterone initiation,7 we performed an analysis following patients for only 6 months. We also performed an as-treated analysis in MarketScan where initiators’ follow-up time was censored at treatment discontinuation or switching from the index testosterone dosage form, or at one-year for continuous users. As there are multiple injectable testosterone formulations, we also separately considered injection testosterone cypionate, enanthate, and propionate formulations compared to gels.
Study logistics
We performed analyses using SAS 9.2 (SAS Institute, Cary, NC) and Episheet Spreadsheets for the Analysis of Epidemiologic Data. This project was approved by the Institutional Review Board of the University of North Carolina at Chapel Hill (IRB number 12–1490). The protocol was also approved by the Independent Scientific Advisory Committee of the CPRD, Medicines & Healthcare Products Regulatory Agency on May 17, 2013. Written consent was not required from study participants.
Results
We identified 487,131 eligible testosterone initiators in MarketScan (60% gel, 39% injection, 1% patch), 22,376 in Medicare (43% gel, 51% injection, 6% patch), and 6,607 in the CPRD (42% gel, 40% injection, 18% patch).
In MarketScan, gel, injection, and patch initiators tended to be similar, with a few notable exceptions. Mean age was similar between the three forms, but patch initiation was concentrated much earlier in the study period than other dosage forms, and gels were concentrated later. As testing became more common over the time period, gels had more total testosterone lab testing prior to gel initiation, but injections had more recorded ICD-9 or Read code diagnoses of hypogonadism. Additionally, gel initiators received more prostate-specific antigen (PSA) tests and lipid profiles (see Table 1 for selected covariates; full covariate distributions are shown in eTable 1). Of the testosterone injection initiators, 83% were testosterone cypionate, 9% testosterone enanthate, and 1% testosterone propionate; 55% of injections were in-office injections, 44% were pharmacy-dispensed (1% had both on the same day). In the 6 months post-initiation, 36% of gel users had a serum total testosterone test, 32% of injection users, and 28% of patch users.
Table 1.
Selected covariates of testosterone initiators by data source and initial formulation
| MarketScan | Medicare | CPRD | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Gel N=291,105 |
Injection N=189,186 |
Patch N=34,841 |
Gel N=9,600 |
Injection N=11,465 |
Patch N=1,311 |
Gel N=2,800 |
Injection N=2,614 |
Patch N=1,193 |
|
| Age (SD) | 52.60 (11.17) |
52.56 (12.42) |
54.50 (12.06) |
71.68 (5.88) |
72.52 (6.35) |
72.70 (6.69) |
55.0 (13.2) | 52.4 (15.1) | 55.2 (13.0) |
| Year (SD) | 2009.1 (2.8) | 2009.2 (3.0) | 2006.8 (3.2) | 2008.4 (1.1) |
2008.5 (1.2) |
2008.5 (1.1) |
2008.8 (2.3) |
2005.2 (4.0) |
2004.7 (3.4) |
| Had a recent testosterone lab, % |
65.48 | 60.09 | 53.88 | 71.91 | 61.29 | 66.21 | 56.88 | 37.13 | 48.82 |
| Number of testosterone labs during baseline, mean (SD) |
0.79 (0.70) | 0.71 (0.68) | 0.64 (0.68) | 0.92 (0.77) | 0.75 (0.75) | 0.79 (0.67) | 0.79 (0.87) | 0.49 (0.76) | 0.62 (0.75) |
| Total testo lab result available, % |
3.51 | 2.93 | 1.95 | -- | -- | -- | 56.07 | 36.42 | 48.03 |
| High*, % | 2.32 | 4.06 | 1.76 | -- | -- | -- | 0.44 | 0.63 | 0.17 |
| Normal*, % | 38.65 | 43.49 | 36.86 | -- | -- | -- | 14.59 | 14.08 | 16.75 |
| Low*, % | 59.04 | 52.45 | 61.38 | -- | -- | -- | 85.97 | 85.29 | 83.07 |
| Low testosterone / hypogonadism diagnosis, % |
37.72 | 54.74 | 26.56 | 49.24 | 35.39 | 41.42 | 13.61 | 11.55 | 9.05 |
| Fatigue, % | 24.08 | 27.82 | 18.52 | 33.23 | 35.35 | 36.38 | 0.96 | 1.11 | 0.84 |
| Sexual dysfunction, % |
0.17 | 0.20 | 0.15 | 31.29 | 32.39 | 24.26 | 25.79 | 21.31 | 25.90 |
| Baseline angina, % | 0.07 | 0.10 | 0.13 | 0.23 | 0.31 | 0.07 | 0.08 | 0.17 | |
| Baseline arrhythmia, % |
0.26 | 0.28 | 0.39 | 1.38 | 1.43 | 1.83 | 0.54 | 0.42 | 0.75 |
| Baseline fracture, % |
1.85 | 1.92 | 2.40 | 3.98 | 4.26 | 6.64 | 0.43 | 0.54 | 0.75 |
| Baseline hospitalization, % |
4.65 | 5.35 | 7.08 | 2.17 | 2.36 | 2.82 | 1.04 | 0.92 | 0.67 |
| Baseline MI, % | 0.13 | 0.18 | 0.18 | 0.24 | 0.31 | 0.18 | 0.19 | 0.25 | |
| Baseline revascularization, % |
0.46 | 0.55 | 0.50 | 0.97 | 1.19 | 0.00 | 0.04 | 0.17 | |
| Baseline stroke, % | 0.21 | 0.23 | 0.40 | 0.44 | 0.44 | 0.36 | 0.31 | 0.25 | |
| Baseline VTE, % | 0.05 | 0.06 | 0.07 | 0.00 | 0.00 | 0.00 | |||
| Baseline prostate cancer, % |
1.08 | 1.08 | 0.84 | 5.23 | 4.91 | 3.97 | 0.00 | 0.08 | 0.00 |
SD: Standard deviation; MI: myocardial infarction; VTE: venous thromboembolism; composite: MI, unstable angina, or stroke
The Medicare cohort was older on average than the MarketScan or CPRD cohorts. Characteristics were similar between the dosage forms, as the Medicare cohort was drawn from a narrower time period. However, patch initiators reported more heart failure and psychiatric disorders. Gel initiators had more recorded hypogonadism diagnoses and PSA screening, while patch initiators had fewer sexual dysfunction diagnoses. In the 6 months after initiation, more gel users (49%) had follow-up testosterone measurements than injections (39%) or patches (40%). Some characteristics were not displayed due to small cohort size restrictions in our data use agreement with the Centers for Medicare & Medicaid Services. See eTable 2 for complete covariate distributions.
Among the CPRD cohort, a much larger proportion of injection initiators did not have a recent testosterone measurement. Gel initiators tended to occur later in the time period, and gel initiators had more diagnoses of hypogonadism and PSA screening. There was also less statin use among the injection initiators (see eTable 3).
One-year incidence of cardiovascular events was low among the younger MarketScan and CPRD testosterone initiators, but more common in the older Medicare initiators (see Tables 2 and 3). Hospitalization rates were higher in the US databases than in the CPRD, and mortality was more frequent in Medicare. VTE rates were very low due to the restrictive definition meant to ensure true cases. In all databases, gel initiators tended to have lower crude rates of all outcomes than injection or patch initiators (see eFigure 2).
Table 2.
Comparative safety of injection versus topical gel testosterone among new testosterone initiators by data source
| Crude | Adjusted | PS Matched | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cohort | Outcome | Treatment | N | Rate / 1,000 PY |
HR | (95% CI) | HR | (95% CI) | N | Rate / 1,000 PY |
HR | (95% CI) |
| MarketScan | MI | Gel | 290,525 | 3.3 | -- | -- | -- | -- | 243,664 | 3.3 | -- | -- |
| Injection | 188,674 | 4.4 | 1.39 | (1.21, 1.49) | 1.30 | (1.17, 1.45) | 165,098 | 4.2 | 1.28 | (1.14, 1.43) | ||
| Unstable angina | Gel | 290,525 | 1.7 | -- | -- | -- | -- | 243,664 | 1.6 | -- | -- | |
| Injection | 188,674 | 1.9 | 1.17 | (1.00. 1.36) | 1.17 | (1.00, 1.37) | 165,098 | 1.9 | 1.24 | (1.04, 1.47) | ||
| Stroke | Gel | 290,494 | 4.2 | -- | -- | -- | -- | 243,975 | 4.1 | -- | -- | |
| Injection | 188,742 | 5.5 | 1.30 | (1.18, 1.43) | 1.18 | (1.07, 1.30) | 165,440 | 5.1 | 1.24 | (1.11, 1.37) | ||
| Composite | Gel | 289,931 | 8.8 | -- | -- | -- | -- | 243,349 | 8.7 | -- | -- | |
| Injection | 188,244 | 11.5 | 1.30 | (1.22, 1.39) | 1.23 | (1.15, 1.32) | 164,937 | 10.8 | 1.24 | (1.16, 1.33) | ||
| Hospitalization | Gel | 277,572 | 86.3 | -- | -- | -- | -- | 232,976 | 86.0 | -- | -- | |
| Injection | 179,064 | 101.8 | 1.18 | (1.15, 1.21) | 1.15 | (1.13, 1.18) | 157,046 | 98.7 | 1.15 | (1.12, 1.18) | ||
| VTE | Gel | 290,953 | 0.003 | -- | -- | -- | -- | 244,131 | 0.003 | -- | -- | |
| Injection | 189,076 | 0.003 | 0.88 | (0.72, 1.08) | 0.89 | (0.72, 1.09) | 165,483 | 0.003 | 0.95 | (0.77, 1.18) | ||
| Medicare | MI | Gel | 9,552 | 9.5 | -- | -- | -- | -- | 9,376 | 9.7 | -- | -- |
| Injection | 11,382 | 14.5 | 1.53 | (1.15, 2.02) | 1.37 | (1.02, 1.83) | 7,866 | 12.1 | 1.25 | (0.92, 1.71) | ||
| Unstable angina |
Gel | 9,552 | 3.9 | -- | -- | -- | -- | 9,376 | 3.9 | -- | -- | |
| Injection | 11,382 | 6.7 | 1.70 | (1.11, 2.60) | 1.57 | (1.01, 2.45) | 7,866 | 7.2 | 1.85 | (1.17, 2.91) | ||
| Stroke | Gel | 9,558 | 10.5 | -- | -- | -- | -- | 9,441 | 10.4 | -- | -- | |
| Injection | 11,414 | 16.2 | 1.54 | (1.18, 2.01) | 1.42 | (1.08, 1.88) | 7,918 | 16.7 | 1.56 | (1.17, 2.08) | ||
| Composite | Gel | 9,510 | 23.1 | -- | -- | -- | -- | 9,376 | 23.1 | -- | -- | |
| Injection | 11,333 | 36.6 | 1.58 | (1.32, 1.90) | 1.44 | (1.19, 1.73) | 7,866 | 34.9 | 1.44 | (1.19, 1.75) | ||
| Death | Gel | 9,600 | 32.2 | -- | -- | -- | -- | 9,478 | 32.7 | -- | -- | |
| Injection | 11,465 | 51.7 | 1.60 | (1.38, 1.86) | 1.32 | (1.13, 1.55) | 7,949 | 39.5 | 1.30 | (1.09, 1.53) | ||
| Hospitalization | Gel | 9,392 | 45.2 | -- | -- | -- | -- | 9,264 | 46.0 | -- | -- | |
| Injection | 11,194 | 65.9 | 1.56 | (1.28, 1.67) | 1.30 | (1.13, 1.49) | 7,775 | 59.5 | 1.29 | (1.12, 1.50) | ||
| VTE | Gel | 9,592 | 0.006 | -- | -- | -- | -- | 9,415 | 0.006 | -- | -- | |
| Injection | 11,458 | 0.007 | 1.36 | (0.74, 2.50) | 1.23 | (0.65, 2.32) | 7,914 | 0.008 | 1.43 | (0.75, 2.72) | ||
| CPRD | MI | Gel | 2,793 | 2.5 | -- | -- | -- | -- | 2,694 | 2.4 | -- | -- |
| Injection | 2,607 | 4.6 | 1.87 | (0.69, 5.04) | 2.08 | (0.35, 12.55) | 1,998 | 4.6 | 2.14 | (0.78, 5.89) | ||
| Unstable angina |
Gel | 2,793 | 0.4 | -- | -- | -- | -- | 2,694 | 0.5 | -- | -- | |
| Injection | 2,607 | 2.1 | 5.16 | (0.60, 44.17) | 1,998 | 1.5 | 5.22 | (0.58, 46.68) | ||||
| Stroke | Gel | 2,790 | 5.0 | -- | -- | -- | -- | 2,686 | 4.9 | -- | -- | |
| Injection | 2,606 | 10.5 | 1.94 | (0.99, 3.79) | 1.44 | (0.67, 3.10) | 1,993 | 10.0 | 1.29 | (0.58, 2.86) | ||
| Composite | Gel | 2,783 | 8.0 | -- | -- | -- | -- | 2,690 | 7.8 | -- | -- | |
| Injection | 2,600 | 16.9 | 2.03 | (1.19, 3.47) | 1.89 | (1.05, 3.40) | 1,996 | 16.2 | 1.85 | (1.05, 3.28) | ||
| Death | Gel | 2,800 | 13.7 | -- | -- | -- | -- | 2,689 | 13.7 | -- | -- | |
| Injection | 2,614 | 21.3 | 1.66 | (1.07, 2.58) | 1.51 | (0.94, 2.42) | 1,997 | 21.3 | 1.56 | (0.98, 2.49) | ||
| Hospitalization | Gel | 2,771 | 12.7 | -- | -- | -- | -- | 2,677 | 13.2 | -- | -- | |
| Injection | 2,590 | 19.5 | 1.54 | (0.97, 2.44) | 1.40 | (0.77, 2.51) | 1,989 | 16.2 | 1.23 | (0.70, 2.17) | ||
| VTE | Gel | 2,800 | 0.4 | -- | -- | -- | -- | 2,689 | 0.0 | -- | -- | |
| Injection | 2,614 | 0.0 | 1,997 | 0.0 | ||||||||
MI: myocardial infarction; VTE: venous thromboembolism; composite: MI, unstable angina, or stroke; HR: hazard ratio; CI: confidence interval
Covariates included in the final adjusted and PS models were: patient age; year; presence of serum tests for total and free testosterone levels; a diagnosis of low testosterone or hypogonadism; prior angina, arrhythmia, MI, revascularization, stroke, or VTE; fracture; prostate and any cancer; hospitalization; deficiency and blood loss anemia; peripheral arterial disease; arthritis; asthma; inflammatory bowel disease; chronic obstructive pulmonary disease; diabetes; fatigue; gout; heart failure; hypertension; other ischemic heart disease; liver disease of cirrhosis; lupus; obesity; osteoporosis; osteo- and rheumatoid arthritis; other heart disease; psoriasis; psychological disorders; percutaneous transluminal coronary angioplasty; pulmonary circulatory disease; peripheral vascular disease; rheumatic heart disease; sexual dysfunction; substance abuse; thyroid disease; peptic ulcer disease; screening tests and preventive health services including lipid tests, bone mineral density tests, colonoscopy, fecal occult blood test, flu vaccination; and prescription medication use, including angiotensin-converting-enzyme inhibitors, alpha blockers, anti-platelet drugs, anti-coagulant drugs, angiotensin II receptor blockers, beta blockers, calcium channel blockers, erectile dysfunction drugs, ezetimibe, fibrates, loop diuretics, niacin, nonsteroidal anti-inflammatory drugs, potassium sparing diuretics, proton-pump inhibitors, statins, and thiazide diuretics. Additional covariates included in the CPRD analysis include smoking status and BMI category.
Table 3.
Comparative safety of patch versus topical gel testosterone among new testosterone initiators by data source
| Crude | Adjusted | PS Matched | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Datasource | Outcome | Treatment | N | Rate / 1,000 PY |
HR | (95% CI) | HR | (95% CI) | N | Rate / 1,000 PY |
HR | (95% CI) |
| MarketScan | MI | Gel | 290,525 | 3.3 | -- | -- | -- | -- | 68,505 | 4.0 | -- | -- |
| Patch | 34,735 | 5.2 | 1.56 | (1.31, 1.85) | 1.20 | (1.00, 1.44) | 34,718 | 5.2 | 1.28 | (1.05, 1.57) | ||
| Unstable angina |
Gel | 290,525 | 1.7 | -- | -- | -- | -- | 68,505 | 2.9 | -- | -- | |
| Patch | 34,735 | 2.9 | 1.73 | (1.37, 2.19) | 1.04 | (0.82, 1.33) | 34,718 | 2.9 | 0.99 | (0.76, 1.28) | ||
| Stroke | Gel | 290,494 | 4.2 | -- | -- | -- | -- | 68,450 | 5.9 | -- | -- | |
| Patch | 34,701 | 6.8 | 1.61 | (1.38, 1.87) | 1.13 | (0.96, 1.32) | 34,683 | 6.8 | 1.15 | (0.96, 1.36) | ||
| Composite | Gel | 289,931 | 8.8 | -- | -- | -- | -- | 68,249 | 12.6 | -- | -- | |
| Patch | 34,596 | 14.1 | 1.60 | (1.44, 1.78) | 1.06 | (0.89, 1.27) | 34,579 | 14.1 | 1.13 | (1.00, 1.27) | ||
| Hospitalization | Gel | 277,572 | 86.3 | -- | -- | -- | -- | 63,974 | 105.5 | -- | -- | |
| Patch | 32,373 | 108.2 | 1.26 | (1.21, 1.31) | 1.04 | (1.00, 1.08) | 32,373 | 108.2 | 1.03 | (0.98, 1.07) | ||
| VTE | Gel | 290,953 | 0.003 | -- | -- | -- | -- | 68,677 | 0.003 | -- | -- | |
| Patch | 34,818 | 0.003 | 0.99 | (0.70, 1.42) | 1.03 | (0.71, 1.48) | 34,799 | 0.003 | 1.14 | (0.75, 1.74) | ||
| Medicare | MI | Gel | 9,552 | 9.5 | -- | -- | -- | -- | 2,610 | 10.6 | ||
| Patch | 1,305 | 15.9 | 1.67 | (0.99, 2.82) | 1.25 | (0.72, 2.18) | 1,305 | 15.9 | 1.50 | (0.80, 2.81) | ||
| Unstable angina |
Gel | 9,552 | 3.9 | -- | -- | -- | -- | 2,610 | 4.6 | |||
| Patch | 1,305 | 4.6 | 1.18 | (0.46, 3.02) | 1.13 | (0.42, 3.04) | 1,305 | 4.6 | 1.01 | (0.35, 2.96) | ||
| Stroke | Gel | 9,558 | 10.5 | -- | -- | -- | -- | 2,599 | 15.7 | -- | -- | |
| Patch | 1,302 | 14.9 | 1.42 | (0.83, 2.43) | 1.25 | (0.72, 2.18) | 1,302 | 14.9 | 1.14 | (0.62, 2.10) | ||
| Composite | Gel | 9,510 | 23.1 | -- | -- | -- | -- | 2,590 | 28.9 | -- | -- | |
| Patch | 1,296 | 34.9 | 1.51 | (1.06, 2.15) | 1.22 | (0.84, 1.76) | 1,295 | 35.0 | 1.21 | (0.81, 1.82) | ||
| Death | Gel | 9,600 | 32.2 | -- | -- | -- | -- | 2,618 | 44.5 | -- | -- | |
| Patch | 1,311 | 58.2 | 1.81 | (1.37, 2.38) | 1.06 | (0.79, 1.42) | 1,311 | 58.2 | 1.24 | (0.90, 1.69) | ||
| Hospitalization | Gel | 9,392 | 45.2 | -- | -- | -- | -- | 2,546 | 60.0 | |||
| Patch | 1,274 | 57.4 | 1.24 | (0.94, 1.65) | 0.99 | (0.74, 1.33) | 1,273 | 57.4 | 0.98 | (0.72, 1.35) | ||
| VTE | Gel | 9,592 | 0.006 | -- | -- | -- | -- | 9,415 | 0.007 | -- | -- | |
| Patch | 11,458 | 0.008 | 1.36 | (0.74, 2.50) | 1.23 | (0.65, 2.32) | 7,914 | 0.008 | 1.43 | (0.75, 2.72) | ||
| CPRD | MI | Gel | 2,793 | 2.5 | -- | -- | -- | -- | 1,998 | 1.8 | -- | -- |
| Patch | 1,189 | 3.5 | 1.42 | (0.40, 5.02) | 18.62 | (0.07, 4706) |
1,132 | 3.2 | 1.07 | (0.30, 3.79) | ||
| Unstable angina |
Gel | 2,793 | 0.4 | -- | -- | -- | -- | 1,998 | 0.0 | -- | -- | |
| Patch | 1,189 | 2.6 | 6.62 | (0.69, 63.62) |
1,132 | 3.2 | 4.99 | (0.52, 47.95) |
||||
| Stroke | Gel | 2,790 | 5.0 | -- | -- | -- | -- | 1,976 | 4.4 | -- | -- | |
| Patch | 1,190 | 6.2 | 1.14 | (0.36, 2.86) | 1.50 | (0.52, 4.31) | 1,133 | 4.8 | 1.01 | (0.39, 2.60) | ||
| Composite | Gel | 2,783 | 8.0 | -- | -- | -- | -- | 1,977 | 6.2 | -- | -- | |
| Patch | 1,186 | 11.5 | 1.39 | (0.69, 2.79) | 1.43 | (0.67, 3.07) | 1,129 | 11.2 | 1.60 | (0.74, 3.45) | ||
| Death | Gel | 2,800 | 13.7 | -- | -- | -- | -- | 1,986 | 13.4 | -- | -- | |
| Patch | 1,193 | 10.5 | 0.79 | (0.41, 1.53) | 0.77 | (0.37, 1.61) | 1,137 | 16.0 | 0.86 | (0.43. 1.73) | ||
| Hospitalization | Gel | 2,771 | 12.7 | -- | -- | -- | -- | 2,000 | 11.8 | -- | -- | |
| Patch | 1,185 | 14.2 | 1.11 | (0.60, 2.03) | 1.30 | (0.57, 2.96) | 1,126 | 17.6 | 1.49 | (0.67, 3.33) | ||
| VTE | Gel | 2,800 | 0.4 | -- | -- | -- | -- | 1,986 | 0.0 | -- | -- | |
| Patch | 1,193 | 0.0 | 1,137 | 0.0 | ||||||||
MI: myocardial infarction; VTE: venous thromboembolism; composite: MI, unstable angina, or stroke; HR: hazard ratio; CI: confidence interval
Covariates included in the final adjusted and PS models were: patient age; year; presence of serum tests for total and free testosterone levels; a diagnosis of low testosterone or hypogonadism; prior angina, arrhythmia, MI, revascularization, stroke, or VTE; fracture; prostate and any cancer; hospitalization; deficiency and blood loss anemia; peripheral arterial disease; arthritis; asthma; inflammatory bowel disease; chronic obstructive pulmonary disease; diabetes; fatigue; gout; heart failure; hypertension; other ischemic heart disease; liver disease of cirrhosis; lupus; obesity; osteoporosis; osteo- and rheumatoid arthritis; other heart disease; psoriasis; psychological disorders; percutaneous transluminal coronary angioplasty; pulmonary circulatory disease; peripheral vascular disease; rheumatic heart disease; sexual dysfunction; substance abuse; thyroid disease; peptic ulcer disease; screening tests and preventive health services including lipid tests, bone mineral density tests, colonoscopy, fecal occult blood test, flu vaccination; and prescription medication use, including angiotensin-converting-enzyme inhibitors, alpha blockers, anti-platelet drugs, anti-coagulant drugs, angiotensin II receptor blockers, beta blockers, calcium channel blockers, erectile dysfunction drugs, ezetimibe, fibrates, loop diuretics, niacin, nonsteroidal anti-inflammatory drugs, potassium sparing diuretics, proton-pump inhibitors, statins, and thiazide diuretics. Additional covariates included in the CPRD analysis include smoking status and BMI category.
For the injection versus gel comparisons, we observed elevated crude HRs for most outcomes in all datasets. Upon adjustment, most effect measure estimates were attenuated, yet all remained elevated (see Table 2 and Figure 1), except for VTE. PS-matched estimates generally closely agreed with the adjusted estimates.
Figure 1.
Forrest plot of adjusted hazard ratios (aHR) for the risk of outcomes in injection versus gel testosterone initiators
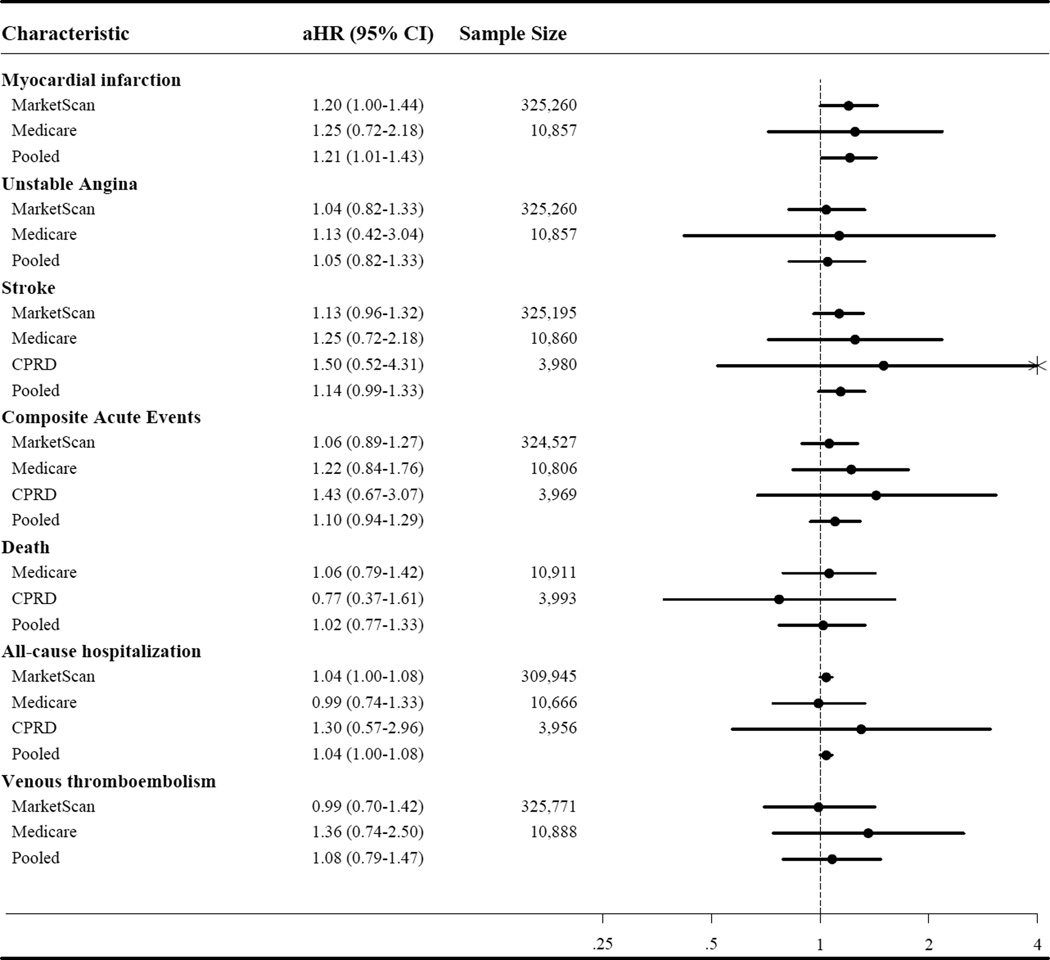
For the patch versus gel comparisons, the crude VTE HR estimates were null in all databases, and the crude CPRD death effect estimate was slightly protective; all other unadjusted estimates were above the null. Due to small sample sizes and limited number of events, some adjusted effect measures could not be estimated. Upon adjustment and PS-matching, the crude HR estimates attenuated toward the null, suggesting no or slight increases in risk in patch initiators compared to gel, although smaller sample sizes resulted in less precise effect measure estimates (see Table 3 and Figure 2). PS matched estimates and adjusted estimates were very similar.
Figure 2.
Forrest plot of adjusted hazard ratios (aHR) for the risk of outcomes in patch versus gel testosterone initiators
Results from the three databases generally agreed, although the MarketScan estimates tended to be closest to the null, followed by Medicare, with the CPRD estimates the most extreme (see Figures 1 and 2).
In analyses within testosterone level subgroups, small samples resulted in imprecise, unstable estimates (see eTables 4 and 5). Although they agreed with overall analyses in direction of effect, they should be interpreted cautiously. When excluding patients with prior cancers, results were similar to the overall estimates (see eTables 6 and 7). When considering initiation during the years 2007–2012, no differences were observed from the overall sample (see eTable 8), and when considering a 6-month follow-up period, results were also similar (see eTable 9). The as-treated analysis in MarketScan generally yielded HRs slightly higher than the estimates from the first-exposure-carried-forward analysis, but the conclusions generally agree with the primary analysis (see eTable 10). Mean treatment duration in days, (SD) was: gel, 122 (112); injection, 105 (104); and patch, 96 (91). When considering injection formulations separately, testosterone cypionate and enanthate showed effect estimates very similar to the overall results (see eTable 11); testosterone propionate was used rarely, and estimates were imprecise, but were consistent with the overall estimates.
Discussion
In this multi-cohort comparison of testosterone dosage forms, we observed consistent increases in the risk of cardio- and cerebrovascular events, hospitalization, and death among injection initiators compared with gels. When comparing patches with gels, we observed a slight increase in myocardial infarction among patch initiators, but all other outcomes were inconsistent. We did not observe any dosage form carrying a higher risk of VTE than others. While the increased risk of outcomes in injection initiators was consistent across databases, absolute incidences were small in this relatively short time period. In Medicare—the cohort with the oldest average population—one-year incidence of the composite MI, angina, and stroke outcome was 23.1 events / 1,000 person-years in gels, 36.6 in injections, and 34.9 in patches. In MarketScan and CPRD, outcome occurrence was lower, and even consistently increased relative risks translated into low absolute increases.
While prior safety research has investigated testosterone as a class,6–8,24,25 this study directly compared individual dosage forms. A prior CPRD study demonstrated that hypertension and polycythemia risk was higher in injection versus oral testosterone,26 suggesting that dose forms’ risk profiles do differ. A reanalysis of the Testosterone in Older Men with Mobility Limitations trial found that gel users who experienced adverse cardiovascular events had greater increases in serum free testosterone.27 Different dosage forms lead to different serum testosterone levels over time—injections result in spikes and periods of super-normal levels12—possibly accounting for observed CVD risk.
This analysis is subject to limitations inherent in the use of secondary healthcare data: unavailability of important patient characteristics; missing data; the non-randomized nature of the exposure; and potential outcome misclassification. Some predictors of cardiovascular events, such as smoking status and BMI, were unavailable in the US cohorts. However, they were available in the CPRD, and using a variety of cohorts allowed us to estimate the effect in settings with and without these potential confounders. The CPRD estimates generally agreed with the US-based estimates, and obesity was evenly-distributed between treatments (see eTable 3) and did not contribute substantially to the injection vs. gel outcome model for the composite event (β=-0.13, p=0.80). Smoking status was slightly imbalanced between treatments, and being a current smoker did contribute to the outcome model (β=0.78, p<0.0001). However, we compared models in the CPRD adjusted for obesity and smoking (HR=0.75, 95% CI: 0.42–1.33) to those not adjusted for them (HR=0.70, 95% CI: 0.39–1.24), and they did not vary substantially.
Additionally, hypogonadism symptoms can be diffuse and indistinct, and they were infrequently diagnosed and coded, often leaving us without the primary indications for testosterone treatment. However, by comparing new initiators of various forms, we restricted to those determined to require treatment by a clinician, and choice of form is likely not heavily influenced by indication or cardiovascular risk. However, important unmeasured behavioral, economic, or social differences between treatment groups may remain. All the included men have insurance coverage, but injections tend to be less expensive than branded gels and can be administered by healthcare providers; therefore, in-office injections may be prescribed more frequently to those with reduced personal disease-management skills or resources and subsequent higher cardiovascular risk. Gel initiators received more post-initiation follow-up serum testosterone tests, possibly indicating better health management and physician monitoring. However, patches had lower post-initiation monitoring similar to injections, yet similar increased event rates were not observed. In most measured clinical respects, initiators of various dosage forms were not meaningfully different.
Additionally, time trends in testosterone testing and treatment exist, with both becoming more common later in the study period.1 The proportion of initiators using a gel as their index prescription has increased, with injection and patch use decreasing over the study period.1 Increased cardiovascular risk in injections versus gels may be a function of comparing an older, sicker patient case mix early in the study period to healthier gel initiators. However, we do not see the same patterns when comparing patches to gels, and patches were prescribed even earlier, on average, suggesting that the observed effects are not merely time trends. We adjusted for calendar year, and sensitivity analyses considering only the later years of the study did not show different effects.
Many patients initiated testosterone without recorded serum testosterone tests or relevant diagnoses, and we did not require evidence of usage according to guidelines28 for inclusion in the cohort. Due to inadequate information on pre- and post-initiation testosterone levels, we could not measure the impact of achieved blood levels. We did not adjust for baseline testosterone levels in the primary analyses. Baseline testosterone levels were unavailable in Medicare and for the majority of MarketScan initiators. Additionally, many individuals did not have a serum testosterone level measured in the 6 months prior to initiation (MarketScan 37%, Medicare 34%, CPRD 54%); some baseline tests may be missing due to out-of-pocket payment, use of other insurance coverage, or failure to bill/record, although treatment in men without levels or with normal levels has been observed1. However, treatment choice could not be influenced by baseline testosterone levels in those without measurements, and levels were not strongly associated with choice of dosage form in those with baseline measurements. Selection bias may be introduced by restricting only to those with tests performed and results available, therefore, we adjusted for having a baseline test performed, but not for the result.
Different routes of testosterone administration may lead to differential non-adherence. Gel and patch use were assessed through pharmacy dispensing (US) or written prescriptions (UK), while injections were assessed from both pharmacy information and in-office procedure codes. We could not measure whether the written prescriptions (UK) were actually dispensed or the dispensed prescriptions (US) were used, whereas codes for in-office injections have much lower potential for non-receipt than pharmacy prescriptions leading to potential differences in misclassification due to non-adherence between dosage forms. However, 45% of injection users in MarketScan initiated with pharmacy-dispensed injections, allowing for greater non-adherence than in-office injections.
Additionally, claims-based studies have the potential for outcome misclassification as claims are generated for billing rather than research. In the US cohorts, we utilized restrictive claims-based definitions for MI, angina, stroke, and VTE to avoid inclusion of rule-out diagnoses. Similar definitions in other studies have demonstrated very high specificity,15,18 and thus we estimated relative measures of effect rather than absolute which will be unbiased in the presence of nondifferential outcome misclassification.29 However, differences in diagnosing, reporting, and recording between the databases may have resulted in the different magnitudes of effect estimates observed between databases.
While the study was limited by the non-randomized nature of the exposure, this study benefits greatly from the large, diverse patient sample representing patients across age groups, populations, treatment and practice patterns, and healthcare systems. MarketScan is representative of those with employer-sponsored commercial insurance in the US, and our Medicare cohort came from a random 20% sample of Medicare beneficiaries from throughout the US. The CPRD is widely representative of primary medical practice in the UK where testosterone use is more restrained,1 pharmaceutical advertising is limited, and there is less disparity in healthcare access. Despite of these differences, effect measure estimates generally agreed across cohorts, suggesting robustness of the results.
Our analysis suggests that testosterone injections may increase the short-term risk of cardiovascular events, stroke, death, and hospitalization compared with gels. The risks from patches and gels appeared similar, and lower than injections. With potential long-term effects of testosterone on lipids, further exploration of longer-term testosterone treatment on cardiovascular risk is warranted. With continuing concern about the safety and effectiveness of testosterone treatment in men with primary and age-related hypogonadism and the trend of treatment of men with normal testosterone levels or without recent baseline testing, it is important to understand the potential hazards of testosterone treatment.
Supplementary Material
Acknowledgments
The authors would like to acknowledge Dongmei Li, MS (University of North Carolina at Chapel Hill) and Pascal Egger (University of Basel) for their programming and database assistance.
This work was funded by the US National Institute on Aging (Grant 5 R01 AG042845 02), and the database infrastructure was partially funded by the Department of Epidemiology, UNC Gillings School of Global Public Health; the Cecil G. Sheps Center for Health Services Research, UNC; the CER Strategic Initiative of UNC’s Clinical Translational Science Award (1 ULI RR025747); and The UNC School of Medicine. The funding sources had no role in the: design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
TS receives investigator-initiated research funding and support as Principal Investigator (R01 AG023178) and Co-Investigator (R01 AG042845) from the National Institute on Aging (NIA), and as Co-Investigator (R01 CA174453) from the National Cancer Institute (NCI) at the National Institutes of Health (NIH), and as Principal Investigator of a Pilot Project from the Patient Centered Outcomes Research Institute (PCORI). Dr. Stürmer does not accept personal compensation of any kind from any pharmaceutical company, though he receives salary support from the Center for Pharmacoepidemiology (current members: GlaxoSmithKline, UCB BioSciences, Merck) and research support from pharmaceutical companies (Amgen, Merck) to the Department of Epidemiology, University of North Carolina at Chapel Hill.
MAB also receives investigator-initiated research funding from the National Institutes of Health (R01 AG042845, R21 HD080214, R01 AG023178) and through contracts with the Agency for Healthcare Research and Quality’s DEcIDE program and the Patient Centered Outcomes Research Institute. He has received research support from Amgen for unrelated projects and has served as a scientific advisor for Amgen, Merck, GSK (honoraria/payment received by the institution). He has received consulting fees from RxAnte and World Health Information Consultants.
JBL had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
The authors contributed to the work in the following ways:
JBL had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. JBL designed the study protocol, obtained funding, performed the data analysis, and drafted the manuscript.
CRM refined the study protocol, provided access to the CPRD data, interpreted results, and critically revised the manuscript.
JLS provided input on the study protocol, interpreted results, and critically revised the manuscript.
TS refined the study protocol, provided access to the Medicare data, and critically revised the manuscript.
SSJ provided access to the CPRD data, interpreted results, and critically revised the manuscript.
MAB designed the study protocol, obtained funding, provided access to the MarketScan data, performed the data analysis, and critically revised the manuscript.
The authors state the following financial disclosures:
JBL, CRM, JLS, SSJ: None
Presentation: Preliminary results of this work have been presented at the International Convention on Pharmacoepidemiology and Therapeutic Risk Management (ICPE) in Taipei, October 26, 2014.
References
- 1.Layton JB, Li D, Meier CR, et al. Testosterone Lab Testing and Initiation in the United Kingdom and the United States, 2000 to 2011. The Journal of Clinical Endocrinology & Metabolism. 2014;99(3):835–842. doi: 10.1210/jc.2013-3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gan EH, Pattman S, S HSP, Quinton R. A UK epidemic of testosterone prescribing, 2001–2010. Clinical endocrinology. 2013 Mar 10; doi: 10.1111/cen.12178. [DOI] [PubMed] [Google Scholar]
- 3.Handelsman DJ. Pharmacoepidemiology of testosterone prescribing in Australia, 1992–2010. The Medical journal of Australia. 2012 Jun 4;196(10):642–645. doi: 10.5694/mja11.11277. [DOI] [PubMed] [Google Scholar]
- 4.Baillargeon J, Urban RJ, Ottenbacher KJ, Pierson KS, Goodwin JS. Trends in androgen prescribing in the united states, 2001 to 2011. JAMA Internal Medicine. 2013:1–2. doi: 10.1001/jamainternmed.2013.6895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walsh TJ, Shores MM, Fox AE, et al. Recent trends in testosterone testing, low testosterone levels, and testosterone treatment among Veterans. Andrology. 2015 Feb 13; doi: 10.1111/andr.12014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Basaria S, Coviello AD, Travison TG, et al. Adverse events associated with testosterone administration. The New England journal of medicine. 2010 Jul 8;363(2):109–122. doi: 10.1056/NEJMoa1000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finkle WD, Greenland S, Ridgeway GK, et al. Increased risk of non-fatal myocardial infarction following testosterone therapy prescription in men. PloS one. 2014;9(1):e85805. doi: 10.1371/journal.pone.0085805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vigen R, O’Donnell CI, Barón AE, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA : the journal of the American Medical Association. 2013;310(17):1829–1836. doi: 10.1001/jama.2013.280386. [DOI] [PubMed] [Google Scholar]
- 9.Shores MM, Smith NL, Forsberg CW, Anawalt BD, Matsumoto AM. Testosterone treatment and mortality in men with low testosterone levels. The Journal of clinical endocrinology and metabolism. 2012 Jun;97(6):2050–2058. doi: 10.1210/jc.2011-2591. [DOI] [PubMed] [Google Scholar]
- 10.Muraleedharan V, Marsh H, Kapoor D, Channer KS, Jones TH. Testosterone deficiency is associated with increased risk of mortality and testosterone replacement improves survival in men with type 2 diabetes. European journal of endocrinology / European Federation of Endocrine Societies. 2013 Dec;169(6):725–733. doi: 10.1530/EJE-13-0321. [DOI] [PubMed] [Google Scholar]
- 11.Baillargeon J, Urban RJ, Kuo Y-F, et al. Risk of Myocardial Infarction in Older Men Receiving Testosterone Therapy. Annals of Pharmacotherapy. 2014 Jul 2; doi: 10.1177/1060028014539918. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dobs AS, Meikle AW, Arver S, Sanders SW, Caramelli KE, Mazer NA. Pharmacokinetics, efficacy, and safety of a permeation-enhanced testosterone transdermal system in comparison with bi-weekly injections of testosterone enanthate for the treatment of hypogonadal men. The Journal of clinical endocrinology and metabolism. 1999 Oct;84(10):3469–3478. doi: 10.1210/jcem.84.10.6078. [DOI] [PubMed] [Google Scholar]
- 13.Swerdloff RS, Wang C, Cunningham G, et al. Long-term pharmacokinetics of transdermal testosterone gel in hypogonadal men. The Journal of clinical endocrinology and metabolism. 2000 Dec;85(12):4500–4510. doi: 10.1210/jcem.85.12.7045. [DOI] [PubMed] [Google Scholar]
- 14.Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. American journal of epidemiology. 2003 Nov 1;158(9):915–920. doi: 10.1093/aje/kwg231. [DOI] [PubMed] [Google Scholar]
- 15.Lakshminarayan K, Larson JC, Virnig B, et al. Comparison of Medicare Claims Versus Physician Adjudication for Identifying Stroke Outcomes in the Women’s Health Initiative. Stroke; a journal of cerebral circulation. 2014 Mar 1;45(3):815–821. doi: 10.1161/STROKEAHA.113.003408. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kiyota Y, Schneeweiss S, Glynn RJ, Cannuscio CC, Avorn J, Solomon DH. Accuracy of medicare claims-based diagnosis of acute myocardial infarction: estimating positive predictive value on the basis of review of hospital records. American heart journal. 2004;148(1):99–104. doi: 10.1016/j.ahj.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 17.Willey VJ, Bullano MF, Hauch O, et al. Management patterns and outcomes of patients with venous thromboembolism in the usual community practice setting. Clinical therapeutics. 2004 Jul;26(7):1149–1159. doi: 10.1016/s0149-2918(04)90187-7. [DOI] [PubMed] [Google Scholar]
- 18.Tamariz L, Harkins T, Nair V. A systematic review of validated methods for identifying venous thromboembolism using administrative and claims data. Pharmacoepidemiology and drug safety. 2012 Jan;21(Suppl 1):154–162. doi: 10.1002/pds.2341. [DOI] [PubMed] [Google Scholar]
- 19.Hammad TA, McAdams MA, Feight A, Iyasu S, Dal Pan GJ. Determining the predictive value of Read/OXMIS codes to identify incident acute myocardial infarction in the General Practice Research Database. Pharmacoepidemiology and drug safety. 2008 Dec;17(12):1197–1201. doi: 10.1002/pds.1672. [DOI] [PubMed] [Google Scholar]
- 20.Jick H, Jick SS, Derby LE. Validation of information recorded on general practitioner based computerised data resource in the United Kingdom. BMJ. 1991 Mar 30;302(6779):766–768. doi: 10.1136/bmj.302.6779.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parsons LS. Reducing bias in a propensity score matched-pair sample using greedy matching techniques. 2001 [Google Scholar]
- 22.Brookhart MA, Wyss R, Layton JB, Stürmer T. Propensity Score Methods for Confounding Control in Nonexperimental Research. Circulation: Cardiovascular Quality and Outcomes. 2013 Sep 1;6(5):604–611. doi: 10.1161/CIRCOUTCOMES.113.000359. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fleiss JL. The statistical basis of meta-analysis. Stat Methods Med Res. 1993;2(2):121–145. doi: 10.1177/096228029300200202. [DOI] [PubMed] [Google Scholar]
- 24.Glueck CJ, Richardson-Royer C, Schultz R, et al. Testosterone Therapy, Thrombophilia-Hypofibrinolysis, and Hospitalization for Deep Venous Thrombosis-Pulmonary Embolus: An Exploratory, Hypothesis-Generating Study. Clinical and applied thrombosis/hemostasis : official journal of the International Academy of Clinical and Applied Thrombosis/Hemostasis. 2013 Aug;7 doi: 10.1177/1076029613499819. [DOI] [PubMed] [Google Scholar]
- 25.Eisenberg ML, Li S, Herder D, Lamb DJ, Lipshultz LI. Testosterone therapy and mortality risk. International journal of impotence research. 2014 Jul 31; doi: 10.1038/ijir.2014.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jick SS, Hagberg KW. The risk of adverse outcomes in association with use of testosterone products: a cohort study using the UK-based general practice research database. British journal of clinical pharmacology. 2013 Jan;75(1):260–270. doi: 10.1111/j.1365-2125.2012.04326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Basaria S, Davda MN, Travison TG, Ulloor J, Singh R, Bhasin S. Risk factors associated with cardiovascular events during testosterone administration in older men with mobility limitation. The journals of gerontology. Series A, Biological sciences and medical sciences. 2013 Feb;68(2):153–160. doi: 10.1093/gerona/gls138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. The Journal of clinical endocrinology and metabolism. 2010 Jun;95(6):2536–2559. doi: 10.1210/jc.2009-2354. [DOI] [PubMed] [Google Scholar]
- 29.Greenland S, Lash TL. Bias Analysis. In: Rothman KJ, Greenland S, Lash TL, editors. Modern Epidemiology. 3rd ed. Baltimore: Lippincott Williams & Wilkins; 2008. pp. 345–380. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.