Abstract
Background
Asthma in the mouse model spontaneously resolves after cessation of allergen exposure. We developed a mouse model where asthma features persisted for 6 months after cessation of allergen exposure.
Objective
To elucidate factors contributing to the persistence of asthma.
Methods
We utilized a combination of immunologic, genetic, microarray and pharmacologic approaches to dissect the mechanism of persistence of asthma.
Results
Elimination of T cells though antibody-mediated depletion or lethal irradiation and transplantation of Rag1−/− bone marrow in mice with chronic asthma resulted in resolution of airway inflammation but not airway hyperreactivity or remodeling. Elimination of T cells and ILC2 through lethal irradiation and transplantation of Rag2−/−γc−/− bone marrow or blockade of IL33 resulted in resolution of airway inflammation and hyperreactivity. Persistence of asthma required multiple interconnected feedback and feed forward circuits between ILC2 and epithelial cells. Epithelial IL33 induced ILC2, a rich source of IL13. The latter directly induced epithelial IL33 establishing a positive feedback circuit. IL33 auto-induced, generating another feedback circuit. IL13 upregulated IL33 receptors and facilitated IL33 auto-induction, thus establishing a feed forward circuit. Elimination of any component of these circuits resulted in resolution of chronic asthma. In agreement with the foregoing, IL33 and ILC2 were increased in the airways from asthmatic patients. IL33 correlated with disease severity.
Conclusions
We present a critical network of feedback and feed forward interactions between epithelial cells and ILC2 involved in maintaining chronic asthma. Although T cells contributed to the severity of chronic asthma they were redundant in maintaining airway hyperreactivity and remodeling.
Keywords: Type 2 innate lymphoid cells, IL33, T cells, feedback circuit, chronic asthma
Introduction
Asthma is a chronic illness (1, 2) with airway hyperreactivity and remodeling persisting in asymptomatic patients with normal pulmonary function (3, 4). Because of the perennial nature of some allergens it has been difficult to ascertain if continuous allergen exposure is necessary for persistence of asthma. Longitudinal observations in occupational asthma indicate that asthma persists in most patients years after removal from occupational exposures (5, 6). These results suggest that repetitive allergen exposure establishes a biochemical mechanism that sustains asthma in the absence of the inciting allergen. Tremendous progress has been made in uncovering the mechanisms surrounding inception and development of asthma through animal model studies (7, 8). Very little is known, however, about the mechanisms regulating the persistence of chronic asthma.
In the majority of mouse models asthma resolves spontaneously in 1–2 weeks (9–11. Repetitive allergen exposure in alternative models induces tolerance (9, 12, 13). In others, cessation of repetitive allergen exposure results in resolution of inflammation (14–16). In the longest study period to date, Johnson, et.al have demonstrated attenuated airway remodeling and airway hyperreactivity persisting for 9 weeks following cessation of repetitive dust mite antigen exposure (16).. The objective of the current study was to investigate the mechanisms of this persistence of asthma in the absence of the inciting allergens. To accomplish this we utilized our previously generated mouse model where asthma persisted for three weeks after cessation of repetitive dust mite, ragweed, and Aspergillus allergen (17)
Recent studies have identified a novel IL5/IL13-producing type-2 innate lymphoid cell population (ILC2) in mice (18–20) and in humans (21, 22). While numerous studies have established the importance of ILC2 in the initiation of airway eosinophilic inflammation (23–28) the role of ILC2 in maintenance of existing airway hyperreactivity has not been established, and their involvement in the airways from asthma patients has not been examined.
Results
Repetitive allergen exposure induces asthma that persists longer than 6 months after cessation of allergen exposure
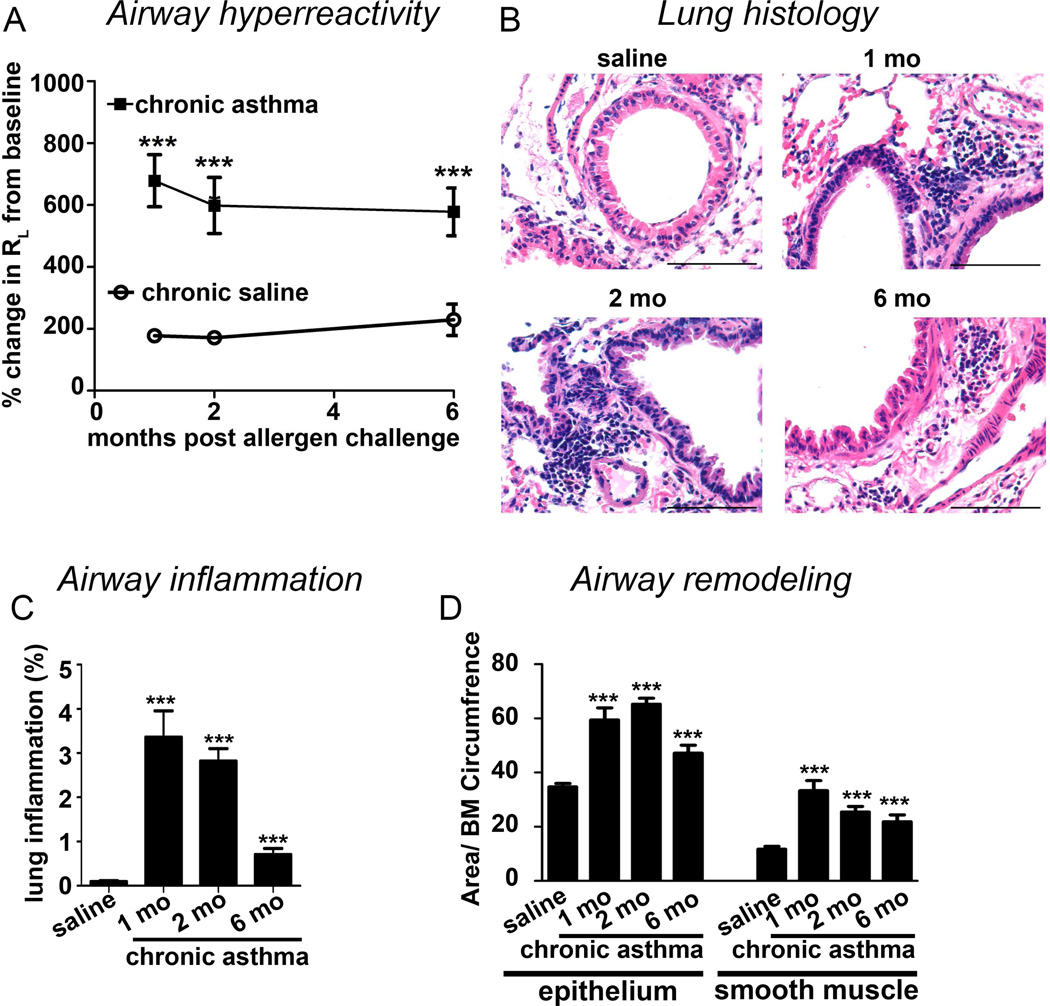
To establish experimental asthma in mice we administered intranasally three representative allergens—dust mite, ragweed and Aspergillus sp. without adjuvant twice a week for 6 weeks as described previously (17). In this model airway hyperreactivity persisted longer than 6 months (Figure 1A) after cessation of the allergen exposure. Airway inflammation (Figure 1B&C), and epithelial and peribronchial smooth muscle (Figure 1D) hypertrophy peaked 1–2 months post allergen cessation, but remained significantly elevated after 6 months. The previously reported increase in mucin and mast cells also persisted in chronic asthma (Repository Figure E1A–D).
Figure 1. Persistence of an asthma phenotype in a mouse model.
(A) Airway hyperreactivity (total lung resistance RL) measured 1, 2 and 6 months after cessation of the allergen exposure. (B) Corresponding histologic images of the lung from the mice with the chronic asthma model and saline controls. Saline control was from the 6 month time point. Scale bar is 100µm. (C) Quantification of peribronchial and perivascular inflammation as % total area in the field. (D) Quantification of epithelial and peribronchial smooth muscle. BM: Basement membrane. N=5–8 mice/ group, ***p<0.001.
T cells contribute to severity but not persistence of asthma
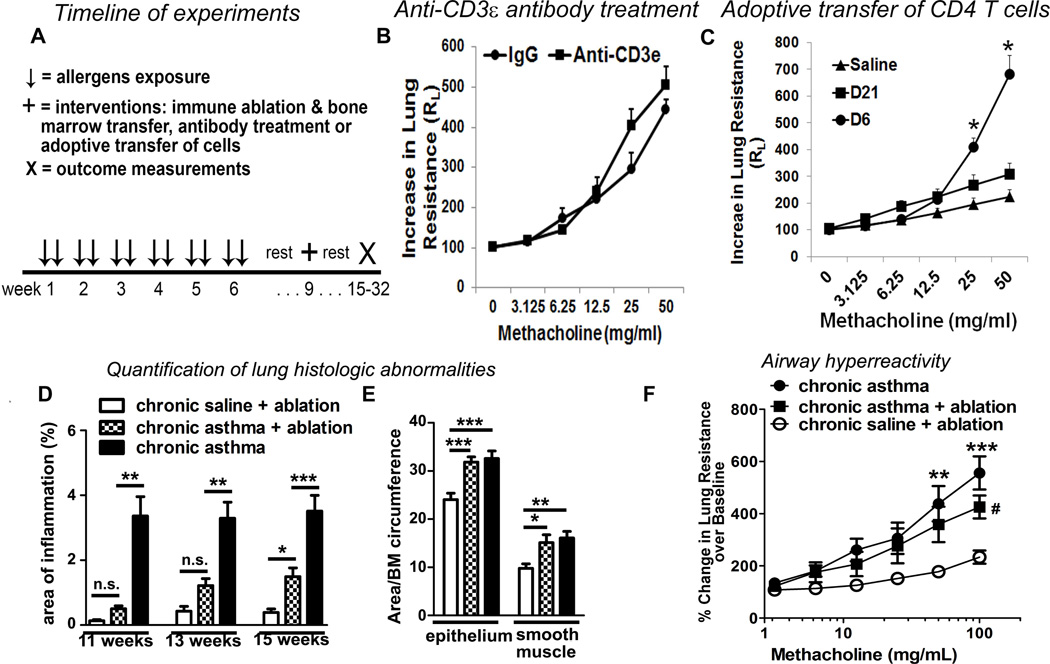
To examine the mechanism of asthma persistence we designed the following timeline for various interventions (Figure 2A). Previous studies have demonstrated resolution of acute asthma within 10–14 days after an allergen exposure (9–11). To demonstrate persistence of asthma we studied outcomes 3 weeks after the last allergen exposure. Nearly all interventions were carried out in week 10. Lethal irradiation of mice was done at the end of week 9. Lung inflammation and airway hyperreactivity was measured 6 weeks later in week 15 in order to provide sufficient time for marrow engraftment and restoration of immune response. We examined the contribution of T cells in asthma maintenance utilizing three different approaches. First, we treated mice with chronic asthma with an anti-CD3ε neutralizing antibody or an isotype control antibody in week 10. This treatment reduced T cell counts by 91% in the spleen (Repository Figure 1A) and inhibited airway inflammation as determined by quantification of lung histology by 70% (Repository Figure 1B). The anti-CD3ε antibody, however, failed to alter airway hyperreactivity (Figure 2B). Our results are, to some extent, similar to those reported by Doherty et al (29), who demonstrated that anti-CD4 T cell depletion inhibited airway inflammation but not smooth muscle mass, mucus metaplasia or airway fibrosis in a mouse model of chronic allergen exposure. Second, we adoptively transferred splenic CD4 T cells from chronic saline treated mice or from the chronic asthma model (isolated on week 10) to naïve mice and subsequently challenged the recipient mice intranasally with the sensitizing allergens on 3 consecutive days. Adoptively transferred CD4 T cells from mice with chronic asthma potently induced airway hyperreactivity in naïve mice 6 days after the transfer (Figure 2C) thus confirming the ability of CD4 T cells from mice with chronic asthma to induce acute asthma in naïve mice. Airway hyperreactivity did not persist, however, when examined on day 21. Third, we subjected mice with chronic asthma to lethal irradiation at the end of week 9 and transplanted bone marrow from naïve mice to sustain mouse viability. Splenic and mediastinal lymph node derived T cells collected 6 weeks after irradiation proliferated in response to anti-CD3/CD28 antibodies but failed to respond to the recall antigens dust mite (Repository Figure 2C), ragweed and Aspergillus (not shown). This indicates elimination of sensitized T cells in irradiated mice. In addition, antigen stimulation failed to increase the number of cytokine producing cells over the baseline (medium stimulation) after immune ablation. In contrast an increased number of T cells from the control chronic asthma mice (not subjected to lethal irradiation) produced IL2 (Repository Figure 2D) and IL4 (Repository Figure 2E) after dust mite stimulation. The results indicate that lethal irradiation has eliminated antigen specific T cells. This effect of lethal irradiation will henceforth be referred to as immune ablation.
Figure 2. Chronic asthma persists following T cell depletion.
(A) A timeline of intranasal allergen exposure for 6 weeks, the rest period and then irradiation, bone marrow transfer, antibody and inhibitor treatment in week 9–10 in the chronic asthma model. Outcomes were measured 3 days later unless otherwise stated. (B) Effect of an anti-CD3ε or an isotype control antibody on airway resistance. N=5, *: P=0.02. (C) Effect of adoptive transfer of spleen CD4 T cells from the chronic asthma model and saline control mice obtained in week 10 to naïve mice. Airway hyperreactivity was measured in chronic asthma CD4 recipient mice on day 6 and day 21 and in saline control CD4 recipient mice on day 6. N=4, *P<0.05. (D) Quantification of lung inflammation in weeks 11, 13, and 15 in mice following lethal irradiation and bone marrow transplantation. (E) Quantification of epithelial hypertrophy and peribronchial smooth muscle hypertrophy in week 15. All morphometric quantifications were performed in at least 5 independent fields per mouse. (F) Measurement of lung resistance in week 15. *: p<0.05, **: p<0.01, ***: p<0.001, #p<0.05, n.s.: not significant; N=5–6 mice/ group for (D–F).
We monitored airway inflammation in week 11, 13 and 15 (2, 4 and 6 weeks after immune ablation). Consistent with the absence of allergen-specific T cells, airway inflammation was nearly absent in week 11, but gradually increased, reaching statistical significance in week 15 (Figure 2D and Repository Figure E2F). Epithelial and smooth muscle hypertrophy (Figure 2E) and airway hyperreactivity (Figure 2F) persisted after immune ablation, although the degree of airway hyperreactivity was reduced. These results suggest that persistence of airway hyperreactivity and remodeling did not require the presence of sensitized T cells. The magnitude of airway hyperreactivity, however, was amplified by sensitized T cells.
Gene expression profile shows increased ILC2 promoting molecules in chronic asthma
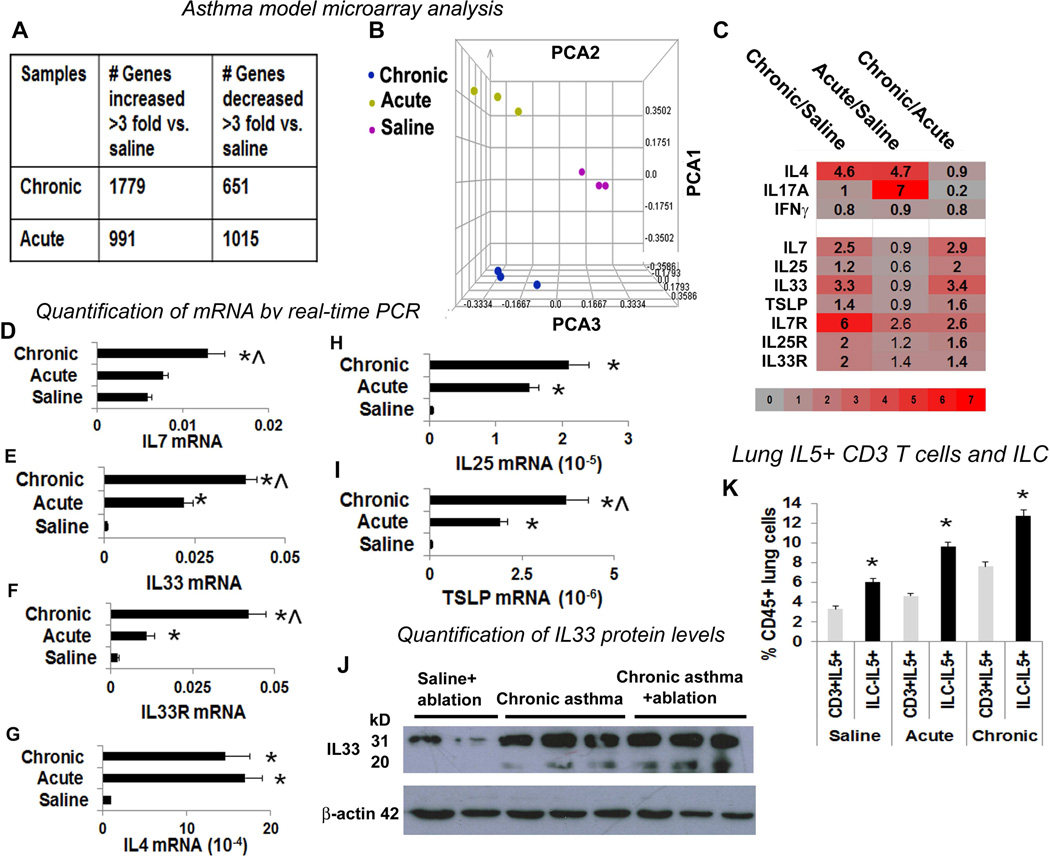
We compared gene expression in the lung tissue from chronic asthma by microarray with 2 different controls—acute asthma and a saline control as described previously (17; see Methods). We observed ~1000 genes up- and down-regulated in the acute model using a cut-off of 3 fold difference with the saline control, and nearly 3 times more genes upregulated than downregulated in the chronic model as compared to saline controls (Figure 3A). The principal component analysis (Figure 3B) derived positions of acute and chronic asthma do not overlap indicating a qualitative difference in gene expression. Because sensitized T cells were not essential for sustaining asthma, we analyzed ILC2-inducing cytokines (20, 27, 30). We observed increased expression of IL7, IL33 and their receptors in chronic asthma (Figure 3C), which was confirmed by real-time PCR (Figure 3D–F). IL4 but not IFNγ was increased in both acute and chronic asthma models relative to saline controls (Figure 3C). IL4 expression was confirmed by real time PCR (Figure 3G). In agreement with a recent study that demonstrated a central role for IL33as compared to IL25 and TSLP in dust mite and peanut allergic sensitization (31) IL25 and TSLP expression by real-time PCR but was 100–1000-fold lower than that of IL33 (Figure 3H&I) in acute and chronic asthma. We confirmed IL33 expression in the airways after immune ablation by western blotting (Figure 3J). The larger 31 kD form of IL33 undergoes proteolytic cleavage to produce a more active 20 kD form (32). The 20 kD form was present in chronic asthma but not in saline control and persisted following immune ablation.
Figure 3. Comparison of the gene expression profile between chronic and acute asthma by microarray.
(A) Microarray analysis of lung tissue obtained from mice with chronic and acute asthma models. N=3 mice/ group. The table shows differentially expressed genes in the asthma models in comparison to saline controls. (B) Principal component analysis (PCA) of the expressed genes demonstrating differences among the asthma models as reflected by their spatial positioning in the 3D space. (C) Heat map of select gene expression changes in chronic and acute asthma as compared to saline controls. (D–I) Measurement of mRNA for select genes by real-time PCR. The mRNA level was normalized to GAPDH mRNA expression. N=5 mice/group, *p<0.05. (J) IL33 levels were confirmed by western blotting to determine the presence of both full length IL33 and the mature cleaved product (N=3). (K) Comparison of lung IL5+CD3 T cells and IL5+ILC2 among the study groups. *P<0.05 as compared to CD3+IL5+ cells in each study group, N=5.
Chronic asthma is characterized by increased ILC2
Previous studies have demonstrated a crucial role for ILC2 in development of lung inflammation from Alternaria (25) and papain (26), airway hyperreactivity due to influenza (33), and allergic sensitization from dust mite and peanut (31). Our microarray data suggest a role for ILC2 in maintenance of chronic asthma. We studied ILC2 in the lung digest as described previously (19, 20, 26). First, we gated for live CD45+ cells (Repository Figure E3A–H). Next, we identified lineage (CD3, Ly-6G/Ly-6C, CD11b, B220, TER-119)- but CD25+ cells. This lin−CD25+ population was positive for ILC2 markers—c-Kit, sca-1, CRTH2, IL33R, and KLRG1, and negative for NK1.1 and FcεRI. About one fourth of these lin−CD25+ CRTH2+IL33R+ ILC2 cells were positive for IL5 and IL13 in non-immunized mice. This is in agreement with previous reports, which showed low basal expression of IL5 and IL13 and increased expression upon stimulation (18–20). These cells were negative for IL17 (Repository Figure E3I). The frequency of IL5+ ILC was consistently higher than that of IL5+ T cells in all study groups (Figure 3K).
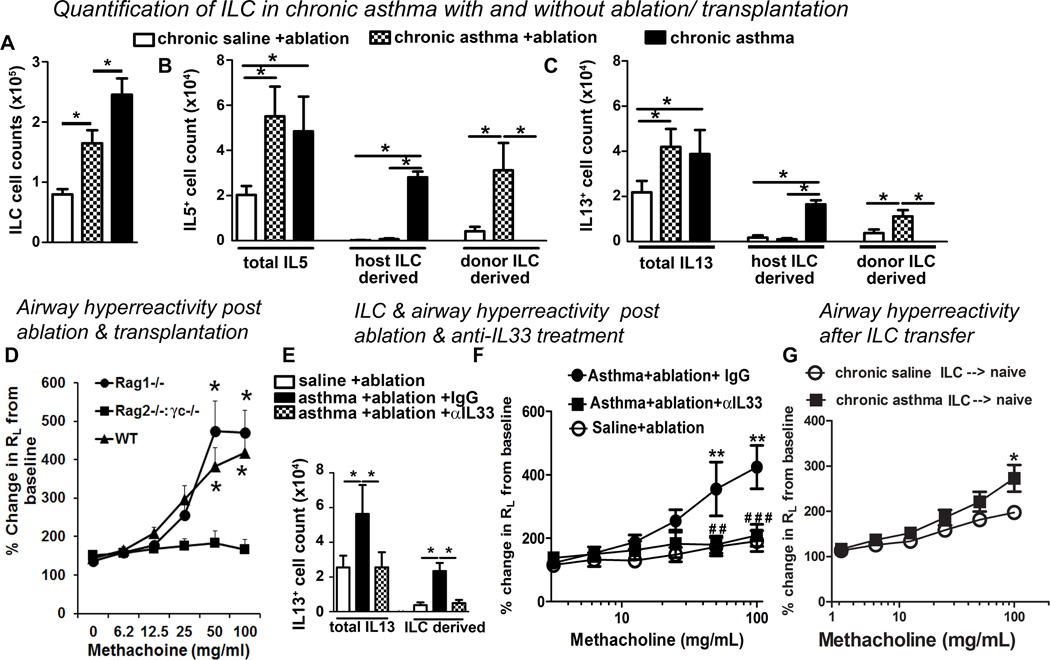
The total lin−CD25+ ILC population was increased in chronic asthma vs. saline control (Figure 4A and Repository Figure E3B, J&K). Following immune ablation and naïve bone marrow transplantation the ILC population decreased but remained elevated as compared to saline controls. IL5+ ILC2 constituted 61% of all IL5+ lung cells in the chronic asthma model (Figure 4B). Irradiation caused a near complete elimination of host-originated IL5+ and IL13+ ILC2 in both chronic asthma (Figure 4B&C) and saline controls (Repository Figure E4 D&E). This was replaced by donor-derived ILC. Interestingly, the number of donor-derived ILC increased 3-fold in recipients of marrow from the chronic asthma model as compared to the saline control suggesting a post-transfer expansion likely due to established elevated IL33 production present in chronic asthma.
Figure 4. Role of innate lymphoid cells (ILC) in persistence of asthma.
(A) Quantification of lung ILC population in chronic asthma, and chronic asthma and saline control after immune ablation and bone marrow transplantation. (B&C) Total lung cytokine producing cell populations, and cytokine positive host CD45.2+ and transplanted donor CD45.1+ ILC populations (mean +/− SEM) were quantified for (B) IL5 and (C) IL13. *p<0.05 N=5 mice/group. (D) Bone marrow from either Rag1−/−, Rag2−/− γc−/− or wild-type mice was transplanted to chronic asthma mice following irradiation. Airway hyperreactivity was measured 6 weeks after irradiation. (E&F) Effect of IL33 blockade after immune ablation and bone marrow transplantation. (E) Total IL13+ lung cells and IL13+ ILC2. (F) Airway hyperreactivity (RL).* p<0.05, ** p<0.01, ***p<0.001. ##p< 0.05 and ###p<0.01 in αIL33 vs IgG. N=6–9 mice / group. Results shown are mean +/− SEM. (G) Effect of adoptive transfer of ILC from chronic asthma and saline control to naïve mice on airway hyperreactivity measured 21 days later. *p<0.05, N=5.
ILC2 but not antigen-specific T cells are essential for persistence of asthma
To determine the role of ILC2 in persistence of asthma we transferred bone marrow from Rag1−/−, Rag2−/− γc−/− and wild-type mice to irradiated mice with chronic asthma. Rag1−/− mice are deficient in T and B cells but have normal ILC whereas Rag2−/− γc−/− mice are additionally deficient in ILC (26). Mice that received marrow from Rag1−/− and wild-type mice maintained airway hyperreactivity (Figure 4D. In contrast, mice that received marrow from Rag2−/− γc−/− mice lost airway hyperreactivity. Airway inflammation and increased inflammatory cells in bronchoalveolar lavage fluid persisted in recipients of Rag1−/− but not Rag2−/− γc−/− marrow (Repository Figure E5A–C). The total number of ILC (lin−CD25+) was comparable between recipients of Rag1−/− marrow (Repository Figure E5D) and naïve marrow (Figure 4A). The ILC population was nearly absent in recipients of Rag2−/− γc−/− marrow (Repository Figure E5D). Despite the absence of ILC and T cells the recipients of Rag2−/− γc−/− marrow had a significant number of IL13+ cells in the lungs (Repository Figure E5E), which, however, was not sufficient to sustain asthma. This could be due to the difference in the quantity of IL13 or other cytokines/factors made by ILC2. The lack of asthma in Rag2−/− γc−/− marrow recipient mice eliminates the possibility that radio-resistant antigen-specific T cells or ILC2 that might have persisted after irradiation were responsible for the sustenance of asthma in mice with chronic asthma that received naïve marrow (Figure 2D–F).
IL33 blockade abolishes airway hyperreactivity, inflammation and IL5/IL13 producing cells
Persistent production of IL33 after immune ablation (Figure 3J) suggests that IL33-driven ILC2 may be critical for persistence of asthma. To test this hypothesis we administered 3 doses of an anti-IL33 antibody or goat IgG to immune ablated mice with chronic asthma 1 week before the outcome measures in week 15. Anti-IL33 treatment reduced the number of total lung ILC (CD45+lin−CD25+), IL5+ ILC2, total IL5+ lung cells (Repository Figure E6B), IL13+ ILC2 and total IL13+ lung cells to normal non-asthmatic level (Figure 4E). This was associated with a complete resolution of airway hyperreactivity (Figure 4F) and a significant reduction in airway inflammation (Repository Figure E6A). IL33 blockade also reduced total cell, lymphocyte and eosinophil counts in BAL (Repository Figure E6C).
Adoptive transfer of ILC induces sustained airway hyperreactivity
The foregoing experiments demonstrated that airway hyperreactivity could not be sustained in the absence of IL33 or ILC2. To demonstrate if activated ILC2 from asthmatic mice was sufficient to sustain airway hyperreactivity lung CD45+lin−CD25+ cells were sorted from the chronic asthma model and saline controls (both CD45.1+) and adoptively transferred (2× 105 cells) to naïve congenic CD45.2+ mice. We detected donor-derived ILC in the recipient lung 21 days after transfer (Repository Figure E7A&B). In contrast to adoptive transfer of CD T cells from mice with chronic asthma we observed significant airway hyperreactivity 21 days after adoptive transfer in recipients of ILC from the chronic asthma mice (Figure 4G).
Airway epithelial cells establish a positive feedback circuit through IL33 and ILC2
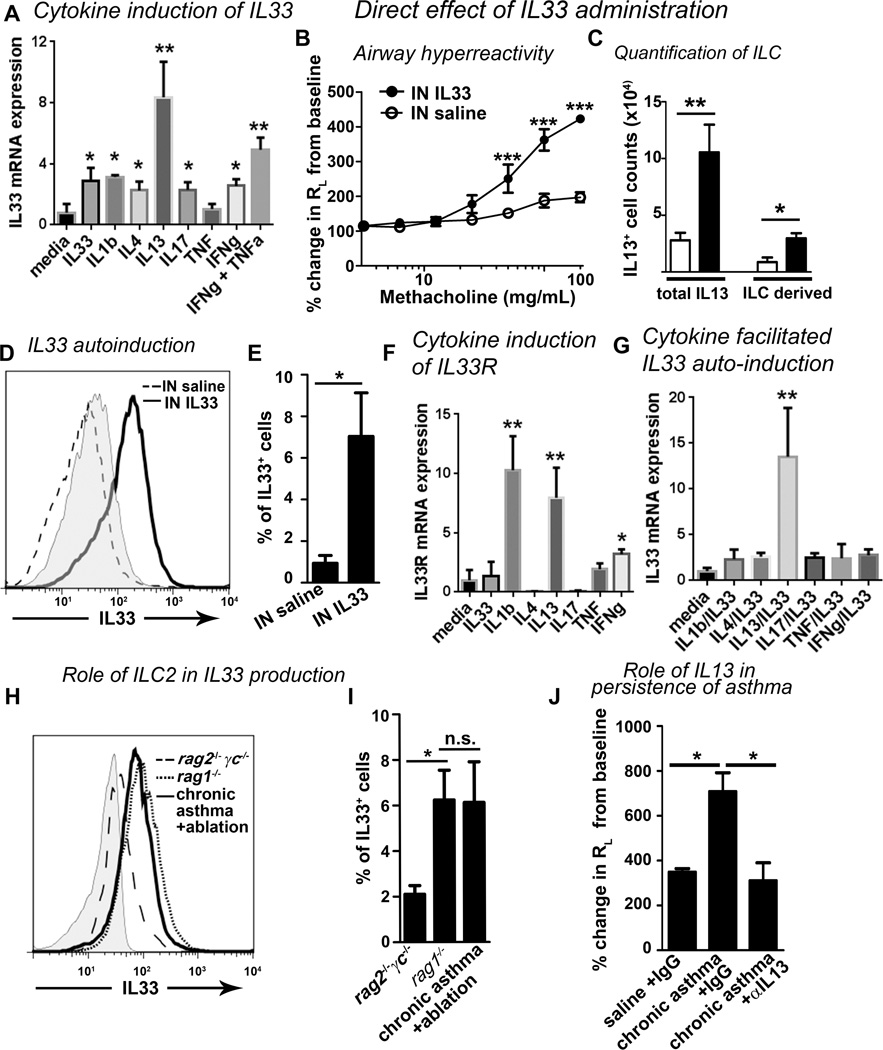
Self-sustenance of biological processes can be facilitated by development of a positive feedback circuit(s). We tested this putative mechanism by examining the effect of IL13, a major product of ILC2, on epithelial production of IL33 in the human lung epithelial cell line A549. IL13 was the most potent inducer of IL33 mRNA as compared to IL33, IL1β, IL4, IL17, TNF and IFNγ (Figure 5A). IL13 also stimulated the secretion of IL33 (Repository Figure E8A). Similar results were observed with the BEAS2B human bronchial epithelial cell line (not shown). These results suggest that IL13 can induce epithelial production of IL33 establishing a positive feedback circuit utilizingILC2.
Figure 5. Role of IL33 in persistence of asthma.
(A) A549 cells were cultured in media alone or with IL33 (10 ng/mL), IL1β (2 ng/mL), IL4 (10 ng/mL), IL13 (20 ng/mL), IL17 (10 ng/mL), TNF (2 ng/mL), IFNγ (10 ng/mL) or IFNγ + TNF (same concentrations) for 72 hours and then mRNA was analyzed for IL33 normalized to GAPDH. * p<0.05, ** p<0.01, N=4–8 per group. (B–E) Direct effect of IL33 on airway hyperreactivity, lung ILC and autoinduction. Mice were treated intranasally for 3 consecutive days with 400ng/ dose of IL33. Methacholine induced airway hyperreactivity (RL) (B), lung IL13+ILC (C), and IL33 autoinduction (D: fluorescence intensity, grey: goat IgG control; E: IL33+ cells) were measured after 15 days. * p<0.05, ** p<0.01, ***p<0.001. N=5 mice/ group. (F) Cytokine induction of IL33R mRNA in A549 cells. * p<0.05, ** p<0.01, N=4 per group. (G) A549 cells were pre-treated for 24 hours with either media or the cytokines as in (A) and then treated for 72 hours with/without IL33 (20ng/mL). ** p<0.01, N=4. (H–I) Persistence of IL33+ cells (fluorescence intensity in H and IL33+ cell number in I) in chronic asthma mice following irradiation and transfer of bone marrow from naïve, Rag1−/− and Rag2−/−γc−/− mice. *p<0.05, N=5 per group. (J) Effect of 3 doses of an anti-IL13 antibody on total lung resistance (RL). The change in RL with the 25 mg dose of methacholine is shown. *p<0.05, N= 5 per group.
IL33auto-induction represents another positive feedback circuit in chronic asthma
Previous studies have shown that IL33 induces acute asthma in mice when examined 24 hours after the ultimate dose (24, 34–36). To extend these findings to chronic asthma, we examined the persistence of asthma 15 days after intranasal IL33 administration. IL33 induced airway hyperreactivity (Figure 5B) and mild airway inflammation (Repository Figure E8 B&C) that persisted for 2 weeks. IL33 also increased the number of lung IL13+ ILC2 (Figure 5C), the total number of IL5+ and IL13+ lung cells, and IL5+ ILC2 (Repository Figure E8 D&E). These experiments suggest that IL33 induces a sustained effect in the airways. To address the mechanism for this sustained effect, we investigated IL33 auto-induction. Hardman et al (36) initially described IL33 auto-induction 24 hours after intranasal delivery of IL33. To extend these findings we observed persistent IL33 production (Figure 5 D&E) in pro-surfactant protein C + epithelial cells (Repository Figure E9A) and CD11b+ hematopoietic cells 2 weeks after intranasal IL33 administration.. These results are in agreement with the data from an IL33 reporter mouse model (36) and our previous in vitro experiments (Figure 5A) indicating that IL33 was capable of auto-induction.
IL13 generates a feed forward mechanism for IL33 auto-induction
Airway epithelial cells express low basal levels of mRNA for IL33 receptor (37), thus permitting IL33 auto-induction. IL13 and IL1β but not IL4 or IL17 strongly induced IL33R (Figure 5F). Next, we asked whether preincubation with the IL33-inducing cytokines facilitated IL33 auto-induction. Only IL13 but not IL1β, IL4 or IL17 synergistically enhanced IL33 auto-induction (Figure 5G). Although this synergy was modest, nonetheless, the result suggests that IL13 was mechanistically connected with IL33 auto-induction. Given that IL33 stimulates ILC2 which subsequently produces IL13 capable of stimulating additional IL33 release, the result suggests an IL33-driven feed forward mechanism. Although IL1β was a strong inducer of IL33R, it was unable to facilitate IL33 auto-induction. We speculate that this is likely due to competition for the shared IL1 receptor accessory protein (IL1RAcP) between IL33R and IL1β. To confirm a role for our triple allergen cocktail utilized in the induction of the chronic asthma model, we tested their effect on IL33 production in vitro. The dust mite, ragweed, and Aspergillus sp. directly stimulated IL33 mRNA transcription 24 hours after exposure in A549 epithelial cells (Repository Figure E9B). This is in agreement with direct induction of IL33 by other allergens in previous studies (24, 25, 36). IL33 production persisted equally in recipients of ILC-sufficient naïve and Rag1−/− bone marrow, but not ILC-deficient Rag2−/−γc−/− marrow (Figure 5H&I). This suggests that ILC2 was essential for sustained IL33 production in vivo during chronic asthma.
IL13 is important for persistence of asthma
We previously reported that a single administration of an anti-IL13 antibody inhibited acute but not chronic asthma (17). As this study uncovered that IL13 is likely to be involved in establishing a positive feedback mechanism, we reasoned that repetitive IL13 blockade would be required to override the feedback mechanism. To test this hypothesis we administered 3 doses of an anti-IL13 antibody i.p. to mice with chronic asthma in week 10. The airway hyperreactivity resolved three days after the anti-IL13 antibody treatment (Figure 5J).
IL33 and ILC2 are increased in human asthma
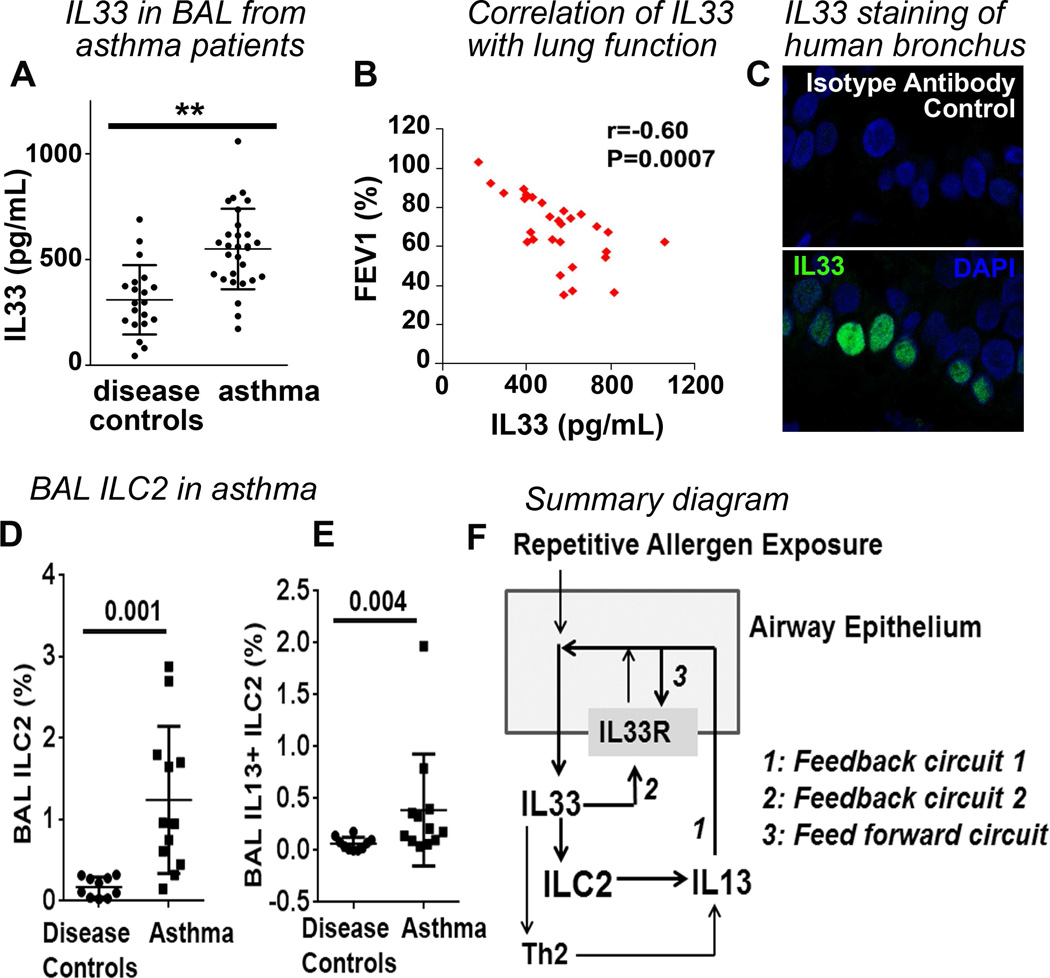
Next, we examined the clinical relevance of IL33 and ILC2. The IL33 level was significantly increased in BAL from asthmatic patients as compared to disease controls (median 560 pg/mL in asthma vs. 295 pg/mL in disease control, Figure 6A). The demographic and clinical characteristics of the study subjects are provided in Repository Table-E1. BAL IL33 negatively correlated (r=−0.60, P=0.0007) with airway flow volume (Figure 6B) and asthma control test (ACT) scores, which reflect symptoms (r=−0.56, P=0.04). In agreement with previous reports (38, 39), IL33 was localized to the nucleus of the basal cell layer of airway epithelium (Figure 6C). Elevated IL33 predicted increased ILC2 in the airways. We identified human ILC2 as lineage−FcεRI−CD127+IL33R+cells (Repository Figure E10) as reported previously (21, 40). The majority of these cells expressed IL5 and IL13. The frequency of ILC2 in BAL was significantly elevated in asthmatic patients as compared to disease controls (median 1.2% in asthma vs. 0.24% in disease controls, Figure 6D). The number of IL5+ ILC2 was also elevated in asthma (Figure 6E). Human asthma is heterogeneous. Expectedly, there were high and low expressers of IL33 and ILC2. The results suggest that IL33 and ILC2 are involved in a subgroup of asthmatic patients.
Figure 6. Role of IL33 and ILC2 in human asthma.
(A) IL33 level (ELISA) in bronchoalveolar lavage (BAL) from asthma patients and disease controls. **p<0.005. (B) Correlation between BAL IL33 and forced expiration volume in 1 sec (FEV1), a measure of airway obstruction. (C) IL33 immunostaining of an endobronchial biopsy sample from an asthmatic patient. Green: IL33; Blue: DAPI for nuclear staining. N=9. (D&E) Frequency of ILC2 (Lin−IL7Rα+FcεRI−IL33R+) and IL13+ ILC2 in BAL from asthmatic patients and disease controls. (F). A summary diagram depicting the mechanism of sustained IL33 production and persistent of asthma. Repetitive allergen exposure induces IL33 production by epithelial cells. IL33 induces ILC2 and Th2, and stimulates IL13 production. IL13 directly induces IL33 production, which establishes the feedback circuit 1. IL33 auto-induces, which establishes the feedback circuit 2. IL13 augments IL33R expression and enhances IL33 auto-induction, thus generating a feed forward circuit.
Discussion
Persistence of asthma is generally considered to be mediated by allergen-specific memory T cells (41). The depletion of allergen-specific memory T cells in our model did not eliminate airway hyperreactivity and remodeling. However, their absence reduced airway hyperreactivity and, especially, inflammation. The depletion of ILC2, on the other hand, led to resolution of all features of asthma. Although many previous studies demonstrated the ability of ILC2 to induce airway eosinophilic inflammation independent of T cells, this is the first demonstration of ILC2 inducing sustained airway hyperreactivity. We substantiated the role of ILC2 through gain-in-function (adoptive transfer) and loss of function (lethal irradiation followed by Rag−/−γc−/− marrow transplantation and anti-IL33 treatment) approaches.
Epithelial cells and ILC2 established 3 distinct positive feedback and feed forward mechanisms to sustain asthma as summarized in Figure 6F. The outcome of these interconnected positive feedback and feed forward circuits was persistent IL33 production and development of chronic asthma. The importance of the ILC2-driven feedback and feed forward circuits was illustrated by the loss of IL33 production in irradiated mice that received Rag2−/−γc−/− but not Rag1−/− marrow. The interdependence of IL33 and IL13 was recently reported in a mouse model of virus-induced COPD (42).
An important feature of these positive feedback and feed forward circuits is that they are interconnected. There is strong mathematical and experimental evidence that interconnected positive feedback circuits induce ultrasensitivity and bistability (43, 44). Ultrasensitivity is manifested when a linear input generates a sigmoidal output. Ultrasensitivity induces system bistability. A system is considered bistable when it is “on” (active) in the absence of any input (45–47). The effect of interconnected feed forward circuits is illustrated by the differential effect of IL13 vs IL4 or IL17 on IL33 auto-induction. While all three cytokines directly induced IL33, only IL13 produced synergy through its feed forward effect on IL33 receptor expression. Previously, we demonstrated that repetitive stimulation of epithelial cells with IL13 led to ERK1/2 bistability through the establishment of a signaling feedback circuit (48). ERK1/2 bistability primed epithelial cells for heightened cytokine production. ERK1/2 signaling is likely relevant as its phosphorylation is increased pERK1/2 in airway epithelial cells from asthmatic patients (49). Thus, IL13 could establish multiple feedback and feed forward circuits for sustained IL33 production.
We demonstrated the biological relevance of our findings in human asthma. In agreement with previous reports (50, 51) we showed increased IL33 in BAL from asthmatic patients. The IL33 level negatively correlated with the airway flow volume. We demonstrated for the first time a significant increase in ILC2 in BAL from asthmatic patients. Previously, increased ILC2 was reported in nasal polyps from patients with chronic rhinosinusitis (21, 40) and in the peripheral blood from asthmatic patients (52). The frequency in the polyps ranged from 0.1–3.6% of CD45+ cells and that in the blood ranged from 0.01–0.03% of mononuclear cells. The frequency of ILC2 in BAL from asthmatic patients was similar to that in the polyps but was higher than that in peripheral blood. The findings of this paper have implications for chronic illnesses in general. Our results imply that recurrent episodes of any acute illness can establish feedback and feed forward circuits. Once established, these feedback circuits sustain the disease process during intervals between the acute episodes, which facilitates the transition of an acute illness into a chronic one.
Material and methods are described in the online repository materials.
Supplementary Material
Key messages.
A network of interaction between epithelium and ILC2 generates a mechanism for sustained IL33 production and persistence of asthma.
ILC2 is essential for persistence of asthma.
T cells, although redundant, contribute the magnitude of inflammation and airway hyperreactivity.
Acknowledgments
Funding Support: This work was supported by NIH grants RO1 AI68088, AI091614, RO1 AI102943, U19 AI100275 & PPG HL36577.
Conflict of Interest: RA is funded by grants from NIH. CC was supported by the NLB fellowship of NJH. MMG was supported by grants from AAFA, CCTSI and Children’s Environmental Health Center of NIEHS and EPA. RJM was supported by MedImmune, NIH, Teva, AstraZeneca, MedImmune, and Merck.
Abbreviations
- BAL
bronchoalveolar lavage
- γc
common γc chain of IL2 receptor
- IL33R
IL33 receptor
- ILC
innate lymphoid cells
- IN
intranasal
- RAG
recombinase activating gene
- RL
total lung resistance
- ST2
suppressor of tumorigenicity (IL33 receptor)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The manuscript has two repository tables and ten repository figures. The microarray data was submitted to NIH Gene Expression Omnibus (GEO) repository. The accession number is GSE54297.
References
- 1.Jackson DJ, Sykes A, Mallia P, Johnston SL. Asthma exacerbations: origin, effect, and prevention. J Allergy Clin Immunol. 2011;128:1165–1174. doi: 10.1016/j.jaci.2011.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lemanske RF, Busse WW. Asthma: clinical expression and molecular mechanisms. J Allergy Clin Immunol. 2010;125:S95–S102. doi: 10.1016/j.jaci.2009.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Townley RG, Ryo YU, Kolotkin BM, Kang B. Bronchial sensitivity to methacholine in current and former asthmatic and allergic rhinitis patients and control subjects. J Allergy Clin Immunol. 1975;56:429–442. doi: 10.1016/0091-6749(75)90061-5. [DOI] [PubMed] [Google Scholar]
- 4.Shapiro GG, Furukawa CT, Pierson PE, Bierman CW. Methacholine bronchial challenge in children. J Allergy Clin Immunol. 1982;69:365–369. doi: 10.1016/0091-6749(82)90147-6. [DOI] [PubMed] [Google Scholar]
- 5.Malo JL, Ghezzo H, D'Aquino C, L'Archeveque J, Cartier A, Chan-Yeung M. Natural history of occupational asthma: relevance of type of agent and other factors in the rate of development of symptoms in affected subjects. J Allergy Clin Immunol. 1992;90:937–944. doi: 10.1016/0091-6749(92)90466-f. [DOI] [PubMed] [Google Scholar]
- 6.Moller DR, McKay RT, Bernstein IL, Brooks SM. Persistent airways disease caused by toluene diisocyanate. Am Rev Respir Dis. 1986;134:175–176. doi: 10.1164/arrd.1986.134.1.175. [DOI] [PubMed] [Google Scholar]
- 7.Kips JC, Anderson GP, Fredberg JJ, Herz U, Inman MD, Jordana M, et al. Murine models of asthma. Eur Respir J. 2003;22:374–382. doi: 10.1183/09031936.03.00026403. [DOI] [PubMed] [Google Scholar]
- 8.Kumar RK, Foster PS. Modeling allergic asthma in mice: pitfalls and opportunities. Am J Respir Cell Mol Biol. 2002;27:267–272. doi: 10.1165/rcmb.F248. [DOI] [PubMed] [Google Scholar]
- 9.Duez C, Tomkinson A, Shultz LD, Bratton DL, Gelfand EW. Fas deficiency delays the resolution of airway hyperresponsiveness after allergen sensitization and challenge. J Allergy Clin Immunol. 2001;108:547–556. doi: 10.1067/mai.2001.118288. [DOI] [PubMed] [Google Scholar]
- 10.Haworth O, Cernadas M, Levy BD. NK cells are effectors for resolvin E1 in the timely resolution of allergic airway inflammation. J Immunol. 2011;186:6129–6135. doi: 10.4049/jimmunol.1004007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leech MD, Benson RA, De Vries A, Fitch PM, Howie SE. Resolution of Der p1-induced allergic airway inflammation is dependent on CD4+CD25+Foxp3+ regulatory cells. J Immunol. 2007;179:7050–7058. doi: 10.4049/jimmunol.179.10.7050. [DOI] [PubMed] [Google Scholar]
- 12.Kumar RK, Herbert C, Kasper M. Reversibility of airway inflammation and remodelling following cessation of antigenic challenge in a model of chronic asthma. Clin Exp Allergy. 2004;34:1796–1802. doi: 10.1111/j.1365-2222.2004.02097.x. [DOI] [PubMed] [Google Scholar]
- 13.Schramm CM, Puddington L, Wu C, Guernsey L, Gharaee-Kermani M, Phan SH, et al. Chronic inhaled ovalbumin exposure induces antigen-dependent but not antigen-specific inhalational tolerance in a murine model of allergic airway disease. Am J Pathol. 2004;164:295–304. doi: 10.1016/S0002-9440(10)63119-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen ZG, Zhang TT, Li HT, Chen FH, Zou XL, Ji JZ, Chen H. Neutralization of TSLP inhibits airway remodeling in a murine model of allergic asthma induced by chronic exposure to house dust mite. PloS one. 2013;8:e51268. doi: 10.1371/journal.pone.0051268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henderson WR, Jr, Tang LO, Chu SJ, Tsao SM, Chiang GK, Jones F, et al. A role for cysteinyl leukotrienes in airway remodeling in a mouse asthma model. Am J Respir Crit Care Med. 2002;165:108–116. doi: 10.1164/ajrccm.165.1.2105051. [DOI] [PubMed] [Google Scholar]
- 16.Johnson JR, Wiley RE, Fattouh R, Swirski FK, Gajewska BU, Coyle AJ, et al. Continuous exposure to house dust mite elicits chronic airway inflammation and structural remodeling. Am J Respir Crit Care Med. 2004;69:378–385. doi: 10.1164/rccm.200308-1094OC. [DOI] [PubMed] [Google Scholar]
- 17.Goplen N, Karim MZ, Liang Q, Gorska MM, Rozario S, Guo L, Alam R. Combined sensitization of mice to extracts of dust mite, ragweed, and Aspergillus species breaks through tolerance and establishes chronic features of asthma. J Allergy Clin Immunol. 2009;123:925–932. e911. doi: 10.1016/j.jaci.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, et al. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 19.Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saenz SA, Siracusa MC, Perrigoue JG, Spencer SP, Urban JF, Jr, Tocker JE, et al. IL25 elicits a multipotent progenitor cell population that promotes T(H)2 cytokine responses. Nature. 2010;464:1362–1366. doi: 10.1038/nature08901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mjösberg JM, Trifari S, Crellin NK, Peters CP, van Drunen CM, Piet B, et al. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nature Immunol. 2011;12:1055–1062. doi: 10.1038/ni.2104. [DOI] [PubMed] [Google Scholar]
- 22.Spits H, Di Santo JP. The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nature Immunol. 2011;12:21–27. doi: 10.1038/ni.1962. [DOI] [PubMed] [Google Scholar]
- 23.Barlow JL, Peel S, Fox J, Panova V, Hardman CS, Camelo A, et al. IL-33 is more potent than IL-25 in provoking IL-13-producing nuocytes (type 2 innate lymphoid cells) and airway contraction. J Allergy Clin Immunol. 2013;132:933–941. doi: 10.1016/j.jaci.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 24.Bartemes KR, Iijima K, Kobayashi T, Kephart GM, McKenzie AN, Kita H. IL-33-responsive lineage- CD25+ CD44(hi) lymphoid cells mediate innate type 2 immunity and allergic inflammation in the lungs. J Immunol. 2012;88:1503–1513. doi: 10.4049/jimmunol.1102832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doherty TA, Khorram N, Chang JE, Kim HK, Rosenthal P, Croft M, Broide DH. STAT6 regulates natural helper cell proliferation during lung inflammation initiated by Alternaria. Amer J Physiol. Lung cell Mol Physiol. 2012;303:L577–L588. doi: 10.1152/ajplung.00174.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Halim TY, Krauss RH, Sun AC, Takei F. Lung natural helper cells are a critical source of Th2 cell-type cytokines in protease allergen-induced airway inflammation. Immunity. 2012;36:451–463. doi: 10.1016/j.immuni.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 27.Kim HY, Chang YJ, Subramanian S, Lee HH, Albacker LA, Matangkasombut P, et al. Innate lymphoid cells responding to IL-33 mediate airway hyperreactivity independently of adaptive immunity. J Allergy Clin Immunol. 2012;129:216–227. e211–e216. doi: 10.1016/j.jaci.2011.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petersen BC, Budelsky AL, Baptist AP, Schaller MA, Lukacs NW. Interleukin-25 induces type 2 cytokine production in a steroid-resistant interleukin-17RB+ myeloid population that exacerbates asthmatic pathology. Nature Med. 2012;18:751–758. doi: 10.1038/nm.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doherty TA, Soroosh P, Broide DH, Croft M. CD4+ cells are required for chronic eosinophilic lung inflammation but not airway remodeling. Am J Physiol Lung Cell Mol Physiol. 2009;296:L229–L235. doi: 10.1152/ajplung.90543.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wong SH, Walker JA, Jolin HE, Drynan LF, Hams E, Camelo A, et al. Transcription factor RORalpha is critical for nuocyte development. Nature Immunol. 2012;13:229–236. doi: 10.1038/ni.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chu DK, Llop-Guevara A, Walker TD, Flader K, Goncharova S, Boudreau JE, et al. IL-33, but not thymic stromal lymphopoietin or IL-25, is central to mite and peanut allergic sensitization. J Allergy Clin Immunol. 2013;131:187–200. e181–e188. doi: 10.1016/j.jaci.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 32.Lefrançais E, Roga S, Gautier V, Gonzalez-de-Peredo A, Monsarrat B, Girard JP, Cayrol C. IL-33 is processed into mature bioactive forms by neutrophil elastase and cathepsin G. Proc Natl Acad Sci (USA) 2012;109:1673–1678. doi: 10.1073/pnas.1115884109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang YJ, Kim HY, Albacker LA, Baumgarth N, McKenzie AN, Smith DE, et al. Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nat Immunol. 2011;12:631–638. doi: 10.1038/ni.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kondo Y, Yoshimoto T, Yasuda K, Futatsugi-Yumikura S, Morimoto M, Hayashi N, et al. Administration of IL-33 induces airway hyperresponsiveness and goblet cell hyperplasia in the lungs in the absence of adaptive immune system. Int Immunol. 2008;20:791–800. doi: 10.1093/intimm/dxn037. [DOI] [PubMed] [Google Scholar]
- 35.Kurowska-Stolarska M, Kewin P, Murphy G, Russo RC, Stolarski B, Garcia CC, et al. IL-33 induces antigen-specific IL-5+ T cells and promotes allergic-induced airway inflammation independent of IL-4. J Immunol. 2008;181:4780–4790. doi: 10.4049/jimmunol.181.7.4780. [DOI] [PubMed] [Google Scholar]
- 36.Hardman CS, Panova V, McKenzie AN. IL-33 citrine reporter mice reveal the temporal and spatial expression of IL-33 during allergic lung inflammation. Eur J Immunol. 2013;43:488–498. doi: 10.1002/eji.201242863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yagami A, Orihara K, Morita H, Futamura K, Hashimoto N, Matsumoto K, Saito H, Matsuda A. IL-33 mediates inflammatory responses in human lung tissue cells. J Immunol. 2010;185:5743–5750. doi: 10.4049/jimmunol.0903818. [DOI] [PubMed] [Google Scholar]
- 38.Masamune A, Watanabe T, Kikuta K, Satoh K, Kanno A, Shimosegawa T. Nuclear expression of interleukin-33 in pancreatic stellate cells. Am J Physiol. Gastrointest Liver Physiol. 2010;299:G821–G832. doi: 10.1152/ajpgi.00178.2010. [DOI] [PubMed] [Google Scholar]
- 39.Zhang L, Lu R, Zhao G, Pflugfelder SC, Li DQ. TLR-mediated induction of pro-allergic cytokine IL-33 in ocular mucosal epithelium. Int J Biochem Cell Biol. 2011;43:1383–1391. doi: 10.1016/j.biocel.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shaw JL, Fakhri S, Citardi MJ, Porter PC, Corry DB, Kheradmand F, et al. IL-33-responsive innate lymphoid cells are an important source of IL-13 in chronic rhinosinusitis with nasal polyps. Am J Respir Crit Care Med. 2013;188:432–439. doi: 10.1164/rccm.201212-2227OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Corry DB, Grünig G, Hadeiba H, Kurup VP, Warnock ML, Sheppard D, et al. Requirements for allergen-induced airway hyperreactivity in T and B cell-deficient mice. Mol Med. 1998;4:344–355. [PMC free article] [PubMed] [Google Scholar]
- 42.Byers DE, Alexander-Brett J, Patel AC, Agapov E, Dang-Vu G, Jin X, et al. Long-term IL-33-producing epithelial progenitor cells in chronic obstructive lung disease. J Clin Invest. 2013;123:3967–3982. doi: 10.1172/JCI65570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chang DE, Leung S, Atkinson MR, Reifler A, Forger D, Ninfa AJ. Building biological memory by linking positive feedback loops. Proc Natl Acad Sci (USA) 2010;107:175–180. doi: 10.1073/pnas.0908314107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shin SY, Rath O, Zebisch A, Choo SM, Kolch W, Cho KH. Functional roles of multiple feedback loops in extracellular signal-regulated kinase and Wnt signaling pathways that regulate epithelial-mesenchymal transition. Cancer Res. 2010;70:6715–6724. doi: 10.1158/0008-5472.CAN-10-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xiong W, Ferrell JE., Jr A positive-feedback-based bistable 'memory module' that governs a cell fate decision. Nature. 2003;426:460–465. doi: 10.1038/nature02089. [DOI] [PubMed] [Google Scholar]
- 46.Markevich NI, Hoek JB, Kholodenko BN. Signaling switches and bistability arising from multisite phosphorylation in protein kinase cascades. J Cell Biol. 2004;164:353–359. doi: 10.1083/jcb.200308060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Srividhya J, Li Y, Pomerening JR. Open cascades as simple solutions to providing ultrasensitivity and adaptation in cellular signaling. Phys Biol. 2011;8:046005. doi: 10.1088/1478-3975/8/4/046005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu W, Tundwal K, Liang Q, Goplen N, Rozario S, Quayum N, et al. Establishment of extracellular signal-regulated kinase 1/2 bistability and sustained activation through Sprouty 2 and its relevance for epithelial function. Mol Cell Biol. 2010;30:1783–1799. doi: 10.1128/MCB.01003-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu W, Liang Q, Balzar S, Wenzel S, Gorska MM, Alam R. Cell-specific activation profile of extracellular signal-regulated kinase 1/2, Jun N-terminal kinase, and p38 mitogen-activated protein kinases in asthmatic airways. J Allergy Clin Immunol. 2008;121:893–902. e892. doi: 10.1016/j.jaci.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 50.Préfontaine D, Lajoie-Kadoch S, Foley S, Audusseau S, Olivenstein R, Halayko AJ, et al. Increased expression of IL-33 in severe asthma: evidence of expression by airway smooth muscle cells. J Immunol. 2009;183:5094–5103. doi: 10.4049/jimmunol.0802387. [DOI] [PubMed] [Google Scholar]
- 51.Préfontaine D, Nadigel J, Chouiali F, Audusseau S, Semlali A, Chakir J, et al. Increased IL-33 expression by epithelial cells in bronchial asthma. J Allergy Clin Immunol. 2010;125:752–754. doi: 10.1016/j.jaci.2009.12.935. [DOI] [PubMed] [Google Scholar]
- 52.Bartemes KR, Kephart GM, Fox SJ, Kita H. Enhanced innate type 2 immune response in peripheral blood from patients with asthma. J Allergy Clin Immunol. 2014 Sep;134(3):671–678.e4. doi: 10.1016/j.jaci.2014.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.