Abstract
Background
Sputum induction is a safe, well tolerated means of obtaining lower airway secretions from children with cystic fibrosis (CF), particularly for assessment of airway inflammation but the clinical value in diagnosing outpatient infections has not been extensively studied.
Objectives
Investigate the success rate and microbiologic yield of induced sputum (IS) compared to oropharyngeal swabs (OP) and expectorated sputum (ES) samples in children with CF, and determine if IS culture results impact treatment.
Methods
Two cohorts were included in this prospective, longitudinal comparative study. In one cohort, simultaneously collected OP, ES and IS specimens were obtained from 17 CF children at three visits over one year. In the second group, sputum induction was performed in 35 CF subjects at four annual visits and culture results were compared to their nearest respiratory culture within four months. Antimicrobial treatment regimens were captured retrospectively.
Results
Sputum induction was successful in 149 of 158 (94%) visit encounters. Polymicrobial infection (combined p=0.005) and gram negative organisms (combined p=0.003) were detected more frequently in IS samples compared to OP, as were the individual pathogens Pseudomonas aeruginosa (combined p=0.04) and Stenotrophomonas maltophilia (combined p= 0.05). The microbiologic yield of serial IS samples collected over one year was stable. IS culture results led to antibiotic changes in 6% of visit encounters. However, based on current practice 13% of visits could have resulted in treatment changes.
Conclusions
Sputum induction is feasible in the outpatient setting and appears to improve pathogen detection in children with CF.
Keywords: Cystic fibrosis, sputum induction, children, airway infection, Pseudomonas aeruginosa
INTRODUCTION
Chronic airway infection is a cardinal feature of CF–related lung disease1. In order for clinicians to prescribe appropriate antibiotic therapy, they must first be able to detect and accurately identify lower respiratory tract pathogens. OP swabs and bronchoalveolar lavage (BAL) are the specimens most commonly obtained to ascertain airway infection in non-expectorating children with CF. Although BAL can be used to assess lower respiratory tract microbiology, the feasibility of repeat sampling is limited by its risk, cost, and need for an experienced team to perform the procedure. OP swabs are commonly obtained in non-expectorating children with CF, but they may not reliably predict lower airway pathogens2–4. Additionally, OP swabs are generally inadequate for detection and isolation of fungi, nontuberculous mycobacteria (NTM) and Burkholderia cepacia complex5.
Sputum is the preferred specimen from those subjects who are capable of expectorating. In CF, there is a strong correlation between microbes detected in spontaneously expectorated sputum (ES) and those isolated in thoracotomy specimens6 and in BAL fluid7. However, ES requires children to have enough lung involvement and resultant respiratory secretions that allow regular sputum production. In CF, sputum induction has primarily been used in research settings and clinical trials to assess airway inflammation8–11. Only a few studies have considered the clinical value of sputum induction to diagnose infection in children with CF12–15. A recent pilot study demonstrated that the microbiological yield from induced sputum (IS) is similar to BAL16. While these studies demonstrated a higher diagnostic yield for identifying microbes in IS compared to other sampling techniques, sputum induction has not been widely adopted in routine clinical practice. In addition, longitudinal studies comparing IS to OP are limited15.
The objectives of this study were to determine the success rate of sputum induction in children with CF, evaluate the microbiological yield of IS samples compared to OP swabs and ES samples, examine the reproducibility of IS cultures by performing longitudinal measurements over 1 year, and explore whether IS culture results impact treatment. We hypothesized that IS samples would detect more pathogens than OP swabs and lead to treatment changes in children with CF.
MATERIALS AND METHODS
Study population and design
IS samples were collected from two prospective CF cohorts, historically diagnosed by sweat chloride >60 mEq/L and/or the presence of two known CF mutations. Pulmonary function testing and sputum induction were performed at each visit. Cohort 1 consisted of 17 patients, ages 8–20 years, currently free from a pulmonary exacerbation (based on clinical impression and no change in antibiotics within 30 days) and FEV1 > 40% predicted. In cohort 1, simultaneous OP, ES (if possible) and IS samples were collected in this order at three visits over one year (baseline, one month and one year) (Figure 1). OP swabs were obtained prior to sputum collection. Cohort 2 included 35 patients ages 6–15 years at the time of the first visit. In cohort 2, four outpatient clinic visits occurred annually over three years, when free of a pulmonary exacerbation, (defined by clinical impression and no recent hospitalizations and antibiotic changes within two weeks of each visit) (Figure 1). The patients in these cohorts were selected based on inclusion/exclusion criteria and the ability to complete the study. The full inclusion and exclusion criteria are listed in the online supplement. Sputum induction was performed and IS culture results were compared to the patient’s nearest surveillance respiratory culture, either OP or ES, obtained within four months of the sputum induction. The patients included in these two cohorts represented approximately 17% of all CF patients aged between 6 and 20 years that were being followed at Children’s Hospital Colorado at the start of specimen collection. The subjects included within the two cohorts had a similar frequency of pulmonary exacerbations when compared to the overall CF population at our center (Table E-1). The Colorado Multiple Institutional Review Board approved both studies. Informed consent and assent, when appropriate, was obtained from all patients. HIPAA standards were maintained during this study.
Figure 1. Cohort Study Design.
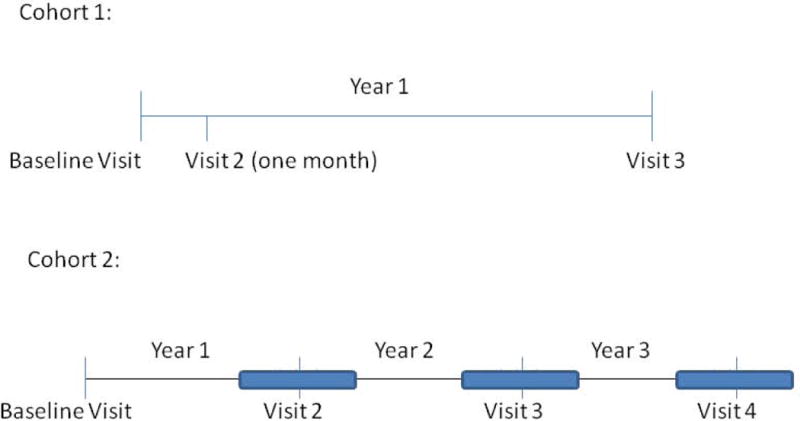
In cohort 1, concurrent oropharyngeal swabs, expectorated sputum (if possible) and induced sputum samples were obtained at baseline, 1 month and 1 year. In cohort 2, induced sputum was obtained at four annual visits and compared to the nearest oropharyngeal swab or expectorated sputum culture within 4 months.
Airway specimen collection, processing and culture
IS collection and processing procedures were the same in both cohorts. Sputum induction was performed according to a standard operating procedure as previously published17. Sputum induction was performed in a designated clinic room and equipment was cleaned between each patient. IS specimens were sent to the on-site clinical microbiology laboratory and were considered adequate if at least 0.5g was obtained. All respiratory specimens, including OP swabs and ES, were processed for comprehensive microbiology according to CF consensus guidelines18. OP samples underwent semi-quantitative bacterial cultures whereas ES and IS samples were processed for quantitative microbiology for CF-related bacterial pathogens. Fungal cultures and plating on Burkholderia cepacia selective agar were also performed on IS and ES, but not on OP samples per clinical laboratory guidelines.
Statistical analysis
Descriptive statistics were used to describe the patient demographics for the two cohorts. Matched sample types were compared for each organism using 2 × 2 tables. Corresponding p-values were calculated using the Obuchowski modified McNemar’s test for clustered paired 2×2 tables19. This test accounts for the intra-cluster correlation between the culture results for patients providing multiple samples over time and was performed using the CLUSTRPO SAS macro (SAS Version X, SAS Institute, Inc., Cary, NC)20. Each cohort was analyzed individually and then the p-values for each test were combined using Fisher’s combined probability test. A p-value less than 0.05 was considered significant.
RESULTS
Success rate of sputum induction
The baseline clinical characteristics of our study subjects are summarized in Table 1. In both cohorts, the use of hypertonic saline to induce sputum was well tolerated and an adequate sputum sample was obtained in 100% (Cohort 1) and 93% (Cohort 2) of patients. Combined, sputum induction was successful in 94% (95% CI: 90–98%) of visit encounters. At approximately half of the visits where sputum induction was not successful a subject was unable to expectorate and in the other half the quantity that was expectorated was insufficient to perform the testing (Table E-2). None of the subjects experienced adverse events during sputum induction and were monitored with peak flow measurements during the procedure and lung function testing at the conclusion which were compared to their pre-sputum induction lung function testing. Safety criteria for sputum induction are listed in the online supplement. In cohort 1, 13 of the 17 patients were able to spontaneously expectorate throughout the duration of the study whereas 4 of the patients initially were non-expectorating but could produce ES at later study visits. In cohort 2, OP swabs were obtained in 43% and an ES sample was provided in 57% of the outpatient visits within four months of the annual visit in which sputum induction was performed.
Table 1.
Clinical Characteristics of Study Cohorts at Enrollment
| Cohort 1 (n=17)
|
Cohort 2 (n=35)
|
|
|---|---|---|
| Females | 7 (41%) | 15 (43%) |
| Age (Years – Median, Range) | 13 (8 to 20) | 12 (6 to 15) |
| CF Genotype | ||
| ΔF508 homozygous | 7 (44%) | 14 (40%) |
| ΔF508 heterozygous | 6 (38%) | 15 (43%) |
| Other/Other | 3 (19%) | 6 (17%) |
| FEV1, % predicted (mean +/− SD) | 99.3 ± 19.1 | 95.3 ± 15.0 |
| Total number of study visits | 48 | 134 |
| Number of IS samples collected | 48 (100%) | 101 (93%) |
| Number of ES samples collected | 38 (79%) | 58 (57%) |
| Number of OP samples collected | 47 (98%) | 43 (43%) |
Microbiological yield of induced sputum samples compared with OP swabs and ES samples
Cohort 1: Comparison of Concurrently Obtained Respiratory Samples
We first compared results from matched respiratory samples from the 17 patients that comprised this cohort over 3 visits (n=48). Compared to OP samples, IS samples tended to more often detect Pseudomonas aeruginosa (51% vs 40%, p=0.07), Stenotrophomonas maltophilia (15% vs 4%, p=0.21) and Achromobacter xylosoxidans (11% vs 2%, p=0.11), although these differences were not statistically significant (Figure 2). There was also a trend to detect Staphylococcus aureus more frequently in OP swabs compared to IS samples (47% vs 32%, p=0.10) (Figure 2). There was no difference in the detection of polymicrobial bacterial infections (defined as two or more CF bacterial pathogens) or in the detection of gram negative organisms combined when comparing IS and OP samples in this cohort. Aspergillus species were identified in 32% of IS and 29% of ES samples; OP swabs were not processed for fungal culture. Similar pathogen (bacterial and fungal) detection rates seen when comparing IS and ES culture results.
Figure 2. Microbiologic Yield of Induced Sputum compared to Oropharyngeal Swab in Concurrent Samples (Cohort 1).
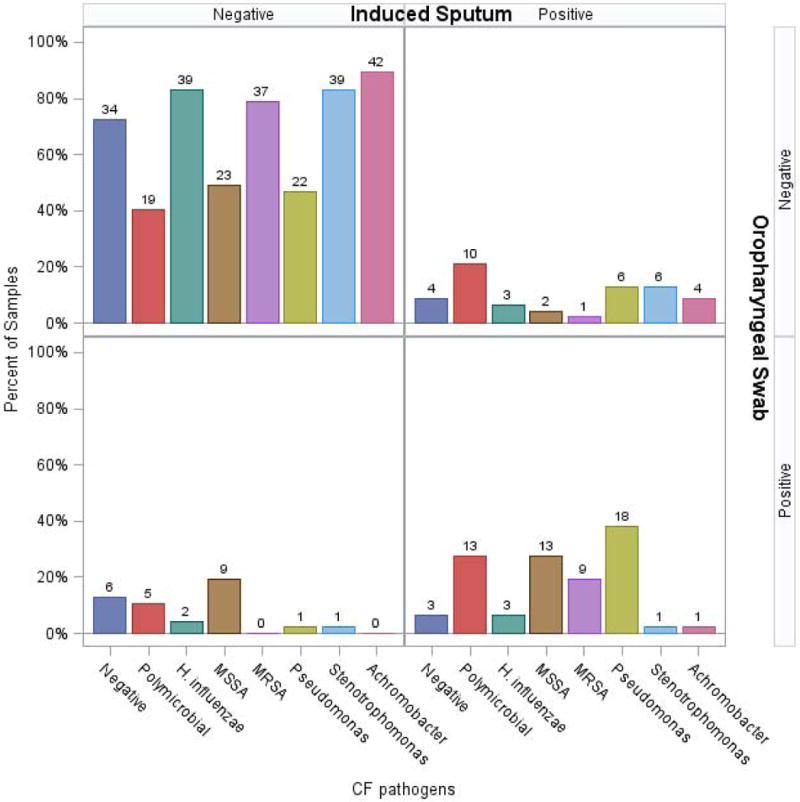
Concurrent induced sputum and oropharyngeal swab cultures were obtained. There was a trend towards the increased detection of polymicrobial infections, gram negative organisms including P. aeruginosa, S. maltophilia and A. xylosoxidans. There was also a trend to identify to identify S. aureus more frequently in OP swabs but no statistically significant differences were noted.
Cohort 2: Annual Induced Sputum Compared to Nearest Surveillance Culture Results
In this cohort, we compared IS culture results (n=125) that were successfully obtained at annual visits to the nearest ES or OP sample. Twenty-four visits in which IS was obtained did not have a surveillance culture obtained within four months of the IS collection. These IS samples were excluded, resulting in a total of 101 visits available for analysis (Table 1).
Compared to OP swabs, IS samples were more likely to detect H. influenzae (23% vs 5%, p =0.01) and S. maltophilia (16% vs 2%, p=0.04) (Figure 3). There was also a trend to detect P. aeruginosa more frequently (21% vs 9%, p=0.10). Similar detection rates between OP and IS samples were seen for A. xylosoxidans (2% vs 0%, p=0.33) and Staphylococcus species (40% vs 47%, p=0.38). IS samples identified polymicrobial bacterial infections more frequently than OP swabs (33% vs 2%, p=0.002) (Figure 3). IS samples were also more likely to detect gram negative organisms (H. influenzae, S. maltophilia, P. aeruginosa, A. xylosoxidans) in this cohort, (53% vs 16%, p=0.001). Aspergillus species were identified in 29% of IS samples, but as noted previously, OP swabs were not processed for fungal culture. Similar pathogen (bacterial and fungal) detection rates seen when comparing IS and ES culture results.
Figure 3. Microbiologic Yield of an Annual Induced Sputum compared to Nearest Oropharyngeal Swab (Cohort 2).
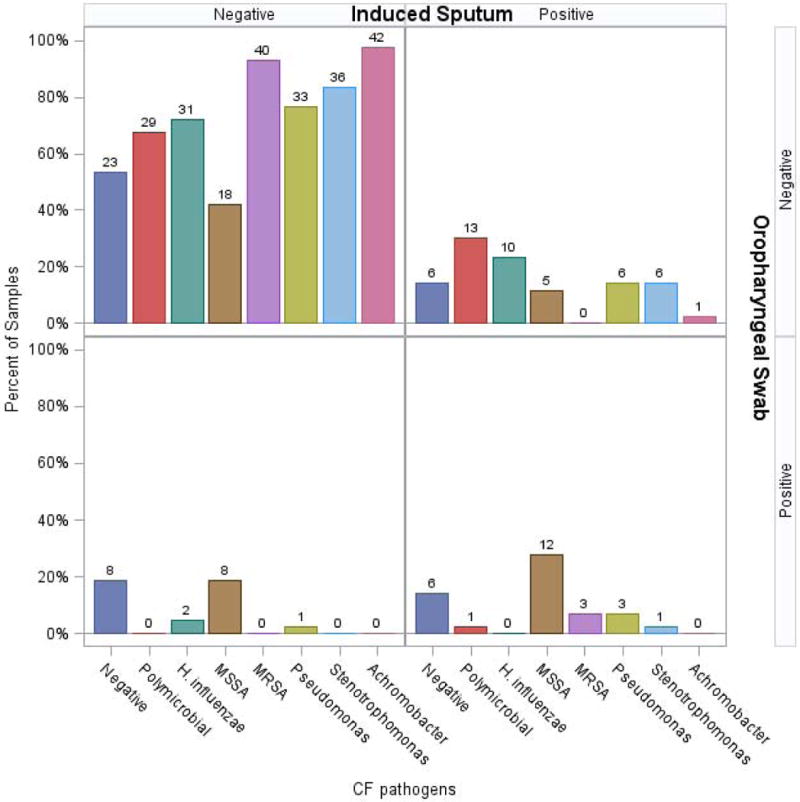
Annual induced sputum culture was compared to the nearest oropharyngeal swab culture within 4 months. Induced sputum yielded more polymicrobial infections and a statistically significant increase in the detection of gram negative organisms (H. influenzae, S. maltophilia, P. aeruginosa, A. xylosoxidans).
Combined induced sputum data from both cohorts
The combined p-values from cohort 1 and 2 indicate that the individual pathogens S. maltophilia (p = 0.05) and P. aeruginosa (p=0.04) were detected more frequently in IS samples compared to OP swabs, as were polymicrobial bacterial infections (p=0.005). Additionally, the overall rate of identifying gram negative organisms (H. influenzae, P. aeruginosa, S. maltophilia, A. xylosoxidans) was higher in the IS samples compared to OP swabs (combined p=0.003).
Serial stability of induced sputum culture results over one year in Cohort 1
The IS culture results at baseline in cohort 1 were compared to the results at the 1 month and 1 year visits. Similar microbiology results were obtained at the three visits, demonstrating good concordance for IS cultures over time (Figure 4). There were no statistically significant differences between any of the organisms identified in IS at the baseline visit compared to the one month and one year visits. The concordance between organisms at baseline and the 1 year visit was 87.6% for IS samples. This was similar to the concordance seen for ES (85.7%) and OP (87.8%) samples.
Figure 4. Microbiologic Yield of Induced Sputum Collected at 3 Visits over 1 Year (Cohort 1).
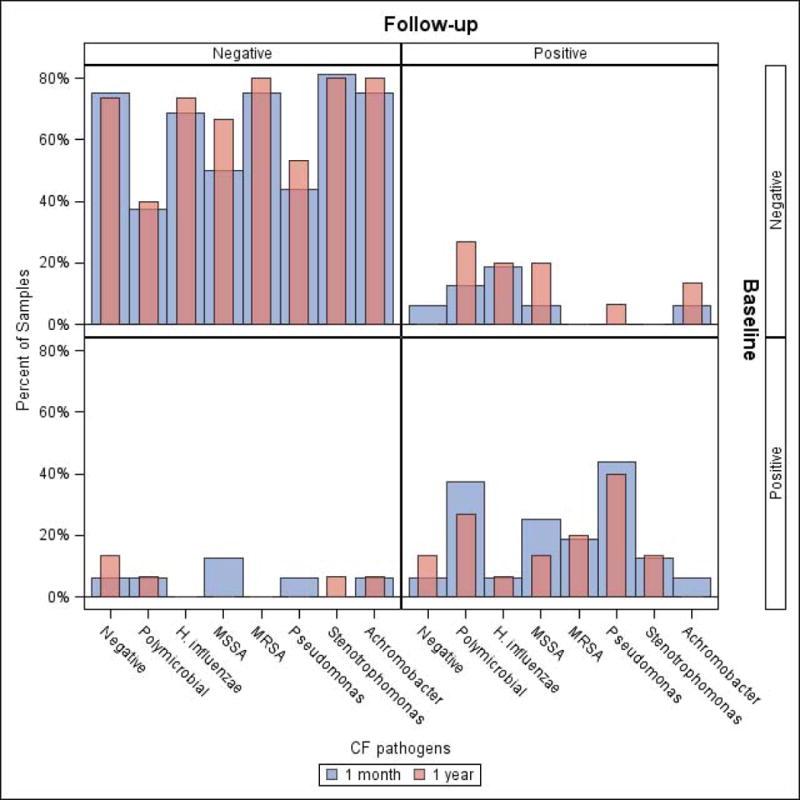
Induced sputum culture results at baseline were compared to the one month and one year visit. Similar microbiology results were obtained at each visit suggesting good concordance for induced sputum over one year. The concordance rate for organisms identified at the baseline visit compared to the 1 year visit was 87.6%.
Impact of induced sputum culture results on treatment
Antibiotic history was retrospectively reviewed for patients in both cohorts. In Cohort 1, no antibiotic changes were made due to the IS culture results. In cohort 2, on six occasions in four patients, IS identified Pseudomonas not identified in their nearest clinical culture resulting in the initiation of anti-Pseudomonal treatment (6% of visit encounters). However, on review of culture results there were 5 additional patients who had grown Pseudomonas on an induced sputum culture (one in cohort 1 and four in cohort 2) that did not grow Pseudomonas on either an expectorated sputum or oropharyngeal culture. These patients either grew Pseudomonas for the first time or had regrown it after several negative interim cultures. These patients would have treatment changes based on our current treatment practices. Furthermore, three patients were noted to have Stenotrophomonas on induced sputum cultures that were not identified on ES or OP cultures. Two of these patients had not grown it previously whereas one had not grown it in 2 years. These patients would have changes in their isolation in the event of an admission based on our center’s current isolation policy. Therefore, we believe that induced sputum culture results had the potential to impact at least 13% of the subjects within the study. Additionally, 32% and 29% of subjects, in cohort 1 and 2 respectively, grew Aspergillus on an induced sputum culture. At our center OP swabs cannot be processed for fungal culture. The increased identification of Aspergillus could have implications for the detection of allergic bronchopulmonary aspergillosis (ABPA) contributing to respiratory symptoms and further investigation of IS in the evaluation of ABPA is warranted. This could further increase the potential clinical benefit of induced sputum samples.
DISCUSSION
In this study, sputum induction was highly successful in children with CF and improved detection of polymicrobial infections, P. aeruginosa, S. maltophilia and H. influenzae compared to OP swabs. Sputum induction also allowed detection of additional CF pathogens, specifically fungi (e.g. Aspergillus) that cannot be reliably identified in OP swabs. The microbiologic yield of IS was similar to ES samples and repeat IS culture results were generally stable in longitudinal samples collected over one year. IS culture results led to an antimicrobial treatment change in 6% of visit encounters; however the potential benefit of induced sputum is likely greater based on our retrospective review of this patient population.
Similar to previous studies, this study demonstrates that the use of sputum induction is both safe and effective and can provide additional microbiologic information in children with CF12–15,21. In our study a high percentage of patients were able to expectorate, especially in Cohort 1. This may have increased the success rate of sputum induction in our study and further studies in non-expectorating children are warranted. Furthermore, studies of younger, preschool aged children are also needed. Two smaller studies of sputum induction in pediatric CF patients found similar high rates of success. For example, DeBoeck and colleagues demonstrated, in their cohort of 19 patients, 4 to 15 years of age, that all were able to expectorate after sputum induction21. Mussaffi and coworkers had success in obtaining an induced sputum culture in 46 out of 48 attempts in non-expectorating children aged 8 months to 8 years15. In addition, induced sputum has been successfully obtained in patients, including children, with pneumonia, tuberculosis and Pneumocystis carinii infections22–24. Other groups have examined the microbiologic yield of IS samples. Al-Saleh and colleagues demonstrated that IS samples generated a higher microbiologic yield compared to OP and ES samples13. In their cohort, differences in culture results were seen in 27% of samples when IS was compared to ES plus OP samples, with 80% of the additional microbiologic yield being found in IS samples13. Similarly, Ho and colleagues demonstrated an increase in microbiologic yield with the use of IS compared to OP cough swabs which resulted in changes in isolation and antibiotic use for 8 out of 15 non-expectorating patients who were able to perform induced sputum14. Mussaffi and coworkers examined longitudinal data in 19 patients over a two year period15. In this cohort, P. aeruginosa was identified more frequently with IS samples (11 positive cultures) compared to OP swabs (5 positive cultures) but this difference was not statistically significant. Although some patients in this cohort underwent repeat cultures, the frequency of IS cultures was variable ranging between 1 and 5 collections15. We analyzed longitudinal data using serially collected airway samples over a one year period (cohort 1) and a three year period (cohort 2). Our study demonstrated that over a one year period, CF pathogens detected in IS were generally stable, which was not specifically evaluated in the aforementioned studies.
Our study points to a number of potential benefits of sputum induction to ascertain infection in non-expectorating CF children. The use of sputum induction has been used to study airway inflammation in CF9–11,25, but is not routinely used in most CF care centers. We found that induced sputum improves the detection of CF pathogens and may lead to antibiotic changes when P. aeruginosa is identified. Given the importance of early P. aeruginosa eradication, improved detection using IS may have significant clinical implications. Furthermore, fungi, NTM, and B. cepacia complex cannot be reliably identified from OP swabs; yet, these organisms are known to cause clinically significant symptoms. Detection of pathogens, including B. cepacia complex and NTM, also has important implications for clinic and hospital isolation precautions. Additionally in this era of CFTR modulation, airway infection is likely to persist but pathogens and microbial communities may shift in response to this therapy. Patients using CFTR modulators have demonstrated improvement in lung function26. It is unclear if this improved lung function will also lead to decreased spontaneous sputum expectoration. Therefore, sputum induction may prove valuable in this group of patients.
There were some limitations in our study. Sputum induction cultures obtained in Cohort 2 were compared to previously obtained OP or expectorated sputum; therefore, there may have been a change in microbiology during the interval between the two samples. Additionally the methodology in the collection of samples was different between the two cohorts. Therefore we first analyzed the two cohorts separately and then performed a secondary analysis of the combined cohorts. A further limitation is that the antibiotic treatment changes were evaluated using a retrospective review. Therefore the true prevalence in antibiotic changes may not be identified if documentation as to the reasons for antibiotic changes was incomplete. Another limitation of our study is the small sample sizes of our cohorts. This could have limited our ability to detect certain, less common pathogens. For example, we did not detect B. cepacia in any of the patients in our study cohorts; larger studies are likely needed to determine the role of sputum induction in detecting B. cepacia given the overall low prevalence of this pathogen in the CF population. This study was not designed to evaluate if induced sputum would be helpful in the detection of mycobacteria and therefore we cannot make any conclusions about the detection and identification of NTM from IS samples. Further evaluation as to the role of routine sputum induction for NTM cultures is indicated. Finally, it is unknown if the use of IS would change long term clinical outcomes even if new pathogens were identified and treated earlier.
In comparing the two cohorts in this study, Cohort 2, which had a higher percentage of non-expectorating patients, had a greater difference in the detection of CF pathogens in IS versus OP samples compared to Cohort 1. In Cohort 1, a larger portion of patients were routine expectorators. It could therefore be hypothesized that if these patients are frequently expectorating, their oropharynx could be colonized with lower airway pathogens as sputum passes through the oropharynx prior to being expectorated. Therefore, among non-expectorating children, the detection rate with IS, and thus, the potential clinical benefit, may be greater than in those who routinely expectorate. More studies of sputum induction focusing primarily or exclusively in a non-expectorating CF population are required. This may also explain why changes in antibiotics were not seen in Cohort 1 with a high rate of ES since new bacteria were less likely to be found on IS samples compared to ES.
In this study of CF children, free from a pulmonary exacerbation, IS improved detection of CF pathogens compared to OP samples, particularly in non-expectorating children, and could allow screening for fungi, NTM and B. cepacia complex. These results led to changes in treatment for a subset of patients due to the identification of P. aeruginosa. Repeated IS culture results from individual patients did not change substantially over one year. For patients who do not routinely expectorate, the use of IS may lead to the identification of new pathogens in the lower airways that are not detected with OP swabs. Based on our data, we recommend annual sputum induction in school-age non-expectorating children with CF.
Supplementary Material
Figure E-1: Microbiologic Yield of Oropharyngeal Swabs Collected at 3 Visits over 1 Year (Cohort 1). Oropharyngeal culture results at baseline were compared to the one month and one year visit. Similar microbiology results were obtained at each visit suggesting good concordance for induced sputum over one year. The concordance rate for organisms identified at the baseline visit compared to the 1 year visit was 87.8%.
Figure E-2: Microbiologic Yield of Expectorate Sputum Collected at 3 Visits over 1 Year (Cohort 1). Expectorated sputum culture results at baseline were compared to the one month and one year visit. Similar microbiology results were obtained at each visit suggesting good concordance for induced sputum over one year. The concordance rate for organisms identified at the baseline visit compared to the 1 year visit was 85.7%.
Table E-1: Frequency of Pulmonary Exacerbations. The overall frequency of pulmonary exacerbations in Cohort 1, Cohort 2 and our CF center demonstrates that Cohort 1 and 2 are similar to the CF population at this center. Amongst patients with at least 1 exacerbation, Cohort 1 and 2 the rate of exacerbations compared to the overall CF center was not increased.
Table E-2: Reasons for failure with induced sputum. At approximately half of the visits where sputum induction was not successful, a subject was unable to expectorate and in the other half the quantity that was expectorated was insufficient to perform the testing. None of these patients experienced adverse events during the sputum induction process.
Acknowledgments
The authors of this manuscript have reported grant funding from the NIH, the Cystic Fibrosis Foundation and the Colorado Department of Public Health and Environment as potential conflicts of interest
Sources of Support: Cystic Fibrosis Foundation (ELLWAY12B0 [Hoppe], ZEMANI08A0, ACCURSO3Y02), K23 RR018611 (NCRR/NIH, Sagel), K23 HL114883-01A1 (NHLBI/NIH, Zemanick), NIH T32 Training Grant (5T32HL007670, Hoppe), and NIH/NCATS Colorado CTSI Grant Number UL1 TR001082 – Contents are the authors’ sole responsibility and do not necessarily represent official NIH views.
Footnotes
Prior conference presentation: Preliminary data included in this paper was presented as an abstract at the 2011 American Thoracic Society Meeting: Ellway JE*, Zemanick ET, Towler E, Sagel SD. Clinical Value of Sputum Induction to Diagnose Infection in Cystic Fibrosis. Am J Respir Crit Care Med 2011; 183: A1129).
*Published under maiden name (Dr. Hoppe)
References
- 1.Gibson RL, Burns JL, Ramsey BW. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am J Respir Crit Care Med. 2003;168:918–951. doi: 10.1164/rccm.200304-505SO. [DOI] [PubMed] [Google Scholar]
- 2.Ramsey BW, Wentz KR, Smith AL, et al. Predictive value of oropharyngeal cultures for identifying lower airway bacteria in cystic fibrosis patients. Am Rev Respir Dis. 1991;144:331–337. doi: 10.1164/ajrccm/144.2.331. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong DS, Grimwood K, Carlin JB, et al. Bronchoalveolar lavage or oropharyngeal cultures to identify lower respiratory pathogens in infants with cystic fibrosis. Pediatr Pulmonol. 1996;21:267–275. doi: 10.1002/(SICI)1099-0496(199605)21:5<267::AID-PPUL1>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 4.Rosenfeld M, Emerson J, Accurso F, et al. Diagnostic accuracy of oropharyngeal cultures in infants and young children with cystic fibrosis. Pediatr Pulmonol. 1999;28:321–328. doi: 10.1002/(sici)1099-0496(199911)28:5<321::aid-ppul3>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 5.Burns JL, Rolain JM. Culture-based diagnostic microbiology in cystic fibrosis: can we simplify the complexity? J Cyst Fibros. 2014;13:1–9. doi: 10.1016/j.jcf.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Thomassen MJ, Klinger JD, Badger SJ, et al. Cultures of thoracotomy specimens confirm usefulness of sputum cultures in cystic fibrosis. J Pediatr. 1984;104:352–356. doi: 10.1016/s0022-3476(84)81094-x. [DOI] [PubMed] [Google Scholar]
- 7.Gilljam H, Malmborg AS, Strandvik B. Conformity of bacterial growth in sputum and contamination free endobronchial samples in patients with cystic fibrosis. Thorax. 1986;41:641–646. doi: 10.1136/thx.41.8.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henig NR, Tonelli MR, Pier MV, et al. Sputum induction as a research tool for sampling the airways of subjects with cystic fibrosis. Thorax. 2001;56:306–311. doi: 10.1136/thorax.56.4.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ordonez CL, Henig NR, Mayer-Hamblett N, et al. Inflammatory and microbiologic markers in induced sputum after intravenous antibiotics in cystic fibrosis. Am J Respir Crit Care Med. 2003;168:1471–1475. doi: 10.1164/rccm.200306-731OC. [DOI] [PubMed] [Google Scholar]
- 10.Sagel SD, Chmiel JF, Konstan MW. Sputum biomarkers of inflammation in cystic fibrosis lung disease. Proc Am Thorac Soc. 2007;4:406–417. doi: 10.1513/pats.200703-044BR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sagel SD, Kapsner R, Osberg I, et al. Airway inflammation in children with cystic fibrosis and healthy children assessed by sputum induction. Am J Respir Crit Care Med. 2001;164:1425–1431. doi: 10.1164/ajrccm.164.8.2104075. [DOI] [PubMed] [Google Scholar]
- 12.Suri R, Marshall LJ, Wallis C, et al. Safety and use of sputum induction in children with cystic fibrosis. Pediatr Pulmonol. 2003;35:309–313. doi: 10.1002/ppul.10226. [DOI] [PubMed] [Google Scholar]
- 13.Al-Saleh S, Dell SD, Grasemann H, et al. Sputum induction in routine clinical care of children with cystic fibrosis. J Pediatr. 2010;157:1006–1011 e1001. doi: 10.1016/j.jpeds.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 14.Ho SA, Ball R, Morrison LJ, et al. Clinical value of obtaining sputum and cough swab samples following inhaled hypertonic saline in children with cystic fibrosis. Pediatr Pulmonol. 2004;38:82–87. doi: 10.1002/ppul.20035. [DOI] [PubMed] [Google Scholar]
- 15.Mussaffi H, Fireman EM, Mei-Zahav M, et al. Induced sputum in the very young: a new key to infection and inflammation. Chest. 2008;133:176–182. doi: 10.1378/chest.07-2259. [DOI] [PubMed] [Google Scholar]
- 16.Blau H, Linnane B, Carzino R, et al. Induced sputum compared to bronchoalveolar lavage in young, non-expectorating cystic fibrosis children. J Cyst Fibros. 2014;13:106–110. doi: 10.1016/j.jcf.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 17.Sagel SD, Wagner BD, Anthony MM, et al. Sputum biomarkers of inflammation and lung function decline in children with cystic fibrosis. Am J Respir Crit Care Med. 2012;186:857–865. doi: 10.1164/rccm.201203-0507OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burns JL, Emerson J, Stapp JR, et al. Microbiology of sputum from patients at cystic fibrosis centers in the United States. Clin Infect Dis. 1998;27:158–163. doi: 10.1086/514631. [DOI] [PubMed] [Google Scholar]
- 19.Obuchowski NA. On the comparison of correlated proportions for clustered data. Stat Med. 1998;17:1495–1507. doi: 10.1002/(sici)1097-0258(19980715)17:13<1495::aid-sim863>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 20.Lieber MCA. A SAS Macro Implementing an Extension of McNemar’s Test for Clustered Data. SUGI. 1998;23 Paper 204. [Google Scholar]
- 21.De Boeck K, Alifier M, Vandeputte S. Sputum induction in young cystic fibrosis patients. Eur Respir J. 2000;16:91–94. doi: 10.1034/j.1399-3003.2000.16a16.x. [DOI] [PubMed] [Google Scholar]
- 22.Lahti E, Peltola V, Waris M, et al. Induced sputum in the diagnosis of childhood community-acquired pneumonia. Thorax. 2009;64:252–257. doi: 10.1136/thx.2008.099051. [DOI] [PubMed] [Google Scholar]
- 23.Moore HA, Apolles P, de Villiers PJ, et al. Sputum induction for microbiological diagnosis of childhood pulmonary tuberculosis in a community setting. Int J Tuberc Lung Dis. 2011;15:1185–1190. i. doi: 10.5588/ijtld.10.0681. [DOI] [PubMed] [Google Scholar]
- 24.Zar HJ, Dechaboon A, Hanslo D, et al. Pneumocystis carinii pneumonia in South African children infected with human immunodeficiency virus. Pediatr Infect Dis J. 2000;19:603–607. doi: 10.1097/00006454-200007000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Ordonez CL, Kartashov AI, Wohl ME. Variability of markers of inflammation and infection in induced sputum in children with cystic fibrosis. J Pediatr. 2004;145:689–692. doi: 10.1016/j.jpeds.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 26.Ramsey BW, Davies J, McElvaney NG, et al. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med. 2011;365:1663–1672. doi: 10.1056/NEJMoa1105185. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure E-1: Microbiologic Yield of Oropharyngeal Swabs Collected at 3 Visits over 1 Year (Cohort 1). Oropharyngeal culture results at baseline were compared to the one month and one year visit. Similar microbiology results were obtained at each visit suggesting good concordance for induced sputum over one year. The concordance rate for organisms identified at the baseline visit compared to the 1 year visit was 87.8%.
Figure E-2: Microbiologic Yield of Expectorate Sputum Collected at 3 Visits over 1 Year (Cohort 1). Expectorated sputum culture results at baseline were compared to the one month and one year visit. Similar microbiology results were obtained at each visit suggesting good concordance for induced sputum over one year. The concordance rate for organisms identified at the baseline visit compared to the 1 year visit was 85.7%.
Table E-1: Frequency of Pulmonary Exacerbations. The overall frequency of pulmonary exacerbations in Cohort 1, Cohort 2 and our CF center demonstrates that Cohort 1 and 2 are similar to the CF population at this center. Amongst patients with at least 1 exacerbation, Cohort 1 and 2 the rate of exacerbations compared to the overall CF center was not increased.
Table E-2: Reasons for failure with induced sputum. At approximately half of the visits where sputum induction was not successful, a subject was unable to expectorate and in the other half the quantity that was expectorated was insufficient to perform the testing. None of these patients experienced adverse events during the sputum induction process.


