Abstract
Thyroid hormones and oxidative stress play significant roles in the normal functioning of the female reproductive system. Nitric oxide (NO), a free radical synthesized by nitric oxide synthases (NOS), participates in the regulation of thyroid function and is also a good biomarker for assessment of the oxidative stress status. Therefore, the purpose of this study was to investigate effects of thyroid hormones on uterine antioxidative status in young adult rats. Thirty immature female Sprague-Dawley rats were randomly divided into three groups: control, hypothyroid (hypo-T) and hyperthyroid (hyper-T). The results showed the body weights decreased significantly in both the hypo-T and hyper-T groups and that uterine weights were decreased significantly in the hypo-T group. The serum concentrations of total triiodothyronine (T3) and thyroxine (T4), as well as estradiol (E2), were significantly decreased in the hypo-T group, but increased in the hyper-T group. The progesterone (P4) concentrations in the hypo- and hyperthyroid rats markedly decreased. Immunohistochemistry results provided evidence that thyroid hormone nuclear receptor α/β (TRα/β) and three NOS isoforms were located in different cell types of rat uteri. The NO content and total NOS and inducible NOS (iNOS) activities were markedly diminished in the hypo-T group but increased in the hyper-T group. Moreover, the activities of both glutathione peroxidase (GSH-Px) and catalase (CAT) exhibited significant decreases and increases in the hypo-T and hyper-T groups, respectively. The malondialdehyde (MDA) contents in both the hypo-T and hyper-T groups showed a significant increase. Total superoxide dismutase (T-SOD) activity in the hypo- and hyper-T rats markedly decreased. In conclusion, these results indicated that thyroid hormones have an important influence on the modulation of uterine antioxidative status.
Keywords: Antioxidative status, Nitric oxide, Rat, Thyroid hormone, Uterus
Thyroid hormones are essential for normal mammalian development and are well known to play fundamental roles in the cardiovascular, nervous, immune and reproductive systems [1,2,3,4]. Indeed, thyroid dysfunction has been reported to exert various effects on the female reproductive capacity. In particular, both hypothyroidism (hypo-T) and hyperthyroidism (hyper-T) can lead to a variety of reproductive problems, causing menstrual disturbances, mainly hypomenorrhea and polymenorrhea in hyperthyroidism, and oligomenorrhea in hypothyroidism [5, 6]. Previous studies have found that thyrotoxicosis and hypothyroidism can disturb the menstrual cycle and ovulation [7]. Furthermore, we have previously reported that thyroid hormones play essential roles in follicular development in the ovary of postnatal and immature rats [8, 9]. Nevertheless, the mechanisms by which thyroid hormones affect reproductive capacity are poorly understood, and various factors may participate alone or in combination. Also, little is known about the effect of thyroid hormones on uterine development and function in young adult rats.
The action of thyroid hormones is mediated by binding to their specific thyroid hormone receptors, and there are two major thyroid hormone receptor (TR) isoforms, TRα and TRβ, that can mediate the biologic activities of triiodothyronine (T3) via transcriptional regulation [10, 11]. Results from previous investigations, strongly suggest the existence and expression of these receptors in different tissues [12]. Moreover, several studies have demonstrated that thyroid hormone receptors are expressed in female reproductive organs, such as the human endometrium [5], macaque uterus [13] and rat ovary [8], uterus and oviduct [14].
Reactive oxygen species (ROS) and antioxidants maintain a balance in a healthy body [15]. Oxidative stress is defined as a disturbance in the balance between the production of ROS and the body’s antioxidant defense capacity [16]. The oxidative stress status can be assessed by some endogenous antioxidants including catalase (CAT), glutathione peroxidase (GSH-Px) and total superoxide dismutase (T-SOD). Briefly, CAT, an iron-containing hemoprotein, converts hydrogen peroxide to water and oxygen. SOD catalyzes the dismutation of superoxide anion radicals to peroxide (H2O2) and molecular oxygen (O2). GSH-Px is an enzyme containing a selenium ion as a cofactor, and for the catalyzed reaction, it requires reduced glutathione (GSH), which is provided by glutathione reductase. Additionally, malondialdehyde (MDA) is a by-product of lipid peroxidation induced by free radicals and also widely used as a biomarker of oxidative stress [15, 17,18,19]. Recent studies have shown that oxidative stress plays a critical role in the normal functioning of the female reproductive system and in the pathogenesis of female infertility [15, 20, 21], and the role of oxidative stress in female fertility and subfertility is an area deserving of continued research. Moreover, some studies have shown that thyroid hormones are associated with the induction of oxidative stress in tissues, such as the brain, muscle, liver and testis [22,23,24,25]. Nevertheless, the effects of thyroid hormones on the uterine oxidative stress status in hyper- and hypo-T young adult rats are still unclear at present.
Nitric oxide (NO), a highly reactive free radical, is an important cellular signaling molecule involved in many physiological and pathological processes [26]. NO is synthesized from L-arginine by nitric oxide synthase (NOS), and is designated as neuronal NOS (nNOS), inducible NOS (iNOS), and endothelial NOS (eNOS). The nNOS and eNOS can also be called constitutive NOS (cNOS), and both are Ca2+ dependent [27]. NO is well recognized as a critical mediator of uterine function and development, including inhibition of myometrial contractions and endometrial platelet aggregation, and the initiation and control of menstrual bleeding [28,29,30,31,32]. Several studies have demonstrated that thyroid hormones can change NOS activity in heart, kidney and vascular myocytes [33, 34]. Our previous studies have revealed that abnormal thyroid hormones levels differentially regulated NOS activity in the ovary of neonatal and immature rats [8, 9]. In addition, NO plays a vital role in the defense against oxidative stress and is also a good biomarker for assessment of the oxidative stress status, because previous studies have demonstrated that NO brings about oxidation reactions that produce free radicals, thereby altering the antioxidative status [15, 35]. Indeed, study of the relationships among thyroid hormones, antioxidative status and nitric oxide in the female reproductive tract have attracted more attention, but currently knowledge concerning them is poorly understood.
To enrich our understanding of the effects of thyroid hormones on female reproduction, we examined the hypothesis that induction of both hyper- and hypo-T might alter thyroid hormone levels, and affect uterine development, as well as the antioxidative status, in the young adult rat uterus. Therefore, we first used propylthiouracil (PTU) and thyronine (T4) to induce rat models of hypo- and hyper-T, respectively, and then used the models to investigate whether altered thyroid hormone levels affect the antioxidative status in the uterus of hyper- and hypo-T rats.
Materials and Methods
Animals
Thirty immature female Sprague-Dawley rats at 21 days of age were obtained from Qinglongshan Experimental Animal Company (Nanjing, PR China). They were housed under the same laboratory conditions consisting of a 12-h light: 12-h dark cycle, a minimum relative humidity of 40% and a room temperature of 23 ± 2 C. Rats were fed standard rodent pellets and drinking water ad libitum. All the experimental protocols were approved in accordance with the Guide for the Care and Use of Laboratory Animals prepared by the Institutional Animal Care and Use Committee of Nanjing Agricultural University, PR China.
Experimental design
Treatment was started on day 21 of weaning and lasted for four consecutive weeks. Rats were randomly divided into three groups, with ten rats in each group. The first group of rats served as the control. The second group (hypo-T) received 0.05% 6-propyl-2-thiouracil (PTU, Sigma-Aldrich, St. Louis, MO, USA) in drinking water for four weeks to induce hypothyroidism, and PTU was used to inhibit thyroxin secretion and induce hypothyroidism. The third group (hyper-T) was treated with L-thyroxin (T4, Sigma-Aldrich). T4 was dissolved in 0.1 mM sodium hydroxide (NaOH) solution and diluted in physiological saline, and then was administered to rats by daily subcutaneous injections of 20 µg/100 g body weights for the same period to induce hyperthyroidism. Notably, the doses of PTU and T4 were selected on the basis of our previous study [8].
Tissue preparation
After treatment for two weeks, vaginal smears were taken once daily (at 0800 h) to determine the estrous cycle, and were completed within 30 min for the three groups of rats. The stage of the estrous cycle was then determined by examining the type and abundance of cells present in the lavage. Rats were lavaged only once per day to minimize stress and other potential effects of handling. In the fourth week of treatment, we started to sacrifice rats and collect uterine samples after determining that the rats were in the estrus phase, and body weight was also recorded at this time. Notably, each rat in the three groups was used during the same period of the estrus phase. We used halothane to anesthetize the rats, then collected blood samples, centrifuged them at 4000 rpm for 10 min to retrieve sera and stored the isolated sera at −80°C until use. Subsequently, rats were sacrificed immediately, and uterine tissue was collected and weighed. One part of the uterine tissues was fixed in 4% paraformaldehyde for immunohistochemical examination, and the remaining uterine tissue was frozen and kept in −80 C until use.
Radioimmunoassay (RIA) for serum concentrations of triiodothyronine (T3), thyroxine (T4), estradiol (E2) and progesterone (P4)
Serum concentrations of total T3 and T4, as well as E2 and P4, were determined quantitatively using commercial RIA kits, respectively, at the General Hospital of the Nanjing Military Command, PR China. RIA kits were obtained from Shanghai University of Traditional Chinese Medicine, PR China. The sensitivities of the total T3, total T4, E2 and P4 determinations were 0.2 ng/ml, 5 ng/ml, 5 pg/ml and 0.05 ng/ml, respectively. Moreover, the intra-assay coefficients of variation were < 10% and inter-assay coefficients of variation were < 15% for total T3, total T4, E2 and P4.
Immunolocalization of TRα/β and three NOS isoforms in the rat uterus
Immunohistochemical staining was performed to examine the localization and expression of TRα/β, and three NOS isoforms (nNOS, iNOS, and eNOS) in the rat uterus. Uterine samples were in 4% paraformaldehyde at room temperature for 24 h and processed in a series of graded ethanol solutions. They were then embedded in paraffin, and then 6 μm sections were cut on a microtome, mounted on slides coated with APES (3-aminopropyl-triethoxysilane) and dried for 24 h at 37 C. Sections were deparaffinized and hydrated in a consecutive series of xylene and ethanol, and blocked with 5% bovine serum albumin (BSA) for one hour to avoid nonspecific staining. The sections were incubated overnight at room temperature with primary antibodies to nNOS (1:200), iNOS (1:200) and eNOS (1:200) purchased from Boster Biological Technology (Wuhan, PR China), as well as TRα/β (1:200) obtained from Santa Cruz Biotechnology. Then the sections were incubated with a secondary antibody (goat anti-rabbit IgG, diluted in PBS) for over 2 h in a humidified box at room temperature. Subsequently, the product was visualized by adding diaminobenzidine substrate (DAB; Sigma-Aldrich). The negative control sections were incubated with normal rabbit serum (NRS) instead of the primary antibody. Finally, the reacted sections were counterstained with hematoxylin solution and mounted with coverslips, and images were captured under a microscope. To assign the intensity of staining for TRα/β and the three NOS isoforms, three independent observers blinded to the experimental procedures were asked to rate the intensity of staining in the photomicrographs using a method previously described: −, no staining detected; +, weak staining; ++, moderate staining; +++, strong staining [36, 37]. Relative levels of immunostaining were assessed, and assessment was repeated at least four times.
Measurement of the uterine NO content and NOS activity
The content of NO in uterus homogenates was measured using a commercial NO reagent kit (Jiancheng Bioengineering Institute, Nanjing, PR China). Briefly, this method was based on the fact that nitrate reductase catalyzes the enzymatic conversion of nitrate to nitrite and determines the level of total nitric oxide. This step was followed by colorimetric measurement of nitrite as an azo dye product of the Griess reaction. A two-step diazotization reaction occurs during the Griess reaction, in which acidified nitrite produces a nitro sating agent, which reacts with sulfatic acid to produce the diazonium ion. It was then coupled with N-(1-naphthyl)ethylenediamine to form the chromophoric azo-derivative, and the optical density at 550 nm was detected using a microplate reader (BioTek Instruments, Winooski, VT, USA). In the present study, the NO contents of uterus homogenates were expressed as micromoles per gram of uterus proteins [36, 38]. The procedures indicated by the kits were performed strictly according to the manufacturer’s protocols.
Total NOS and iNOS activities were determined using a commercial reagent kit (Jiancheng Bioengineering Institute, Nanjing, PR China). Briefly, the NOS activity was determined by measuring the release of local NO generated via a five-electron oxidation of terminal guanidinium nitrogen on L-arginine by NOS. NO then bound to the nucleophilic materials and generated a colored compound. Afterwards, the reaction was terminated with citric acid. The optical density at 530 nm was detected using a microplate reader (BioTek Instruments). In addition, constitutive NOS activity was equal to the total NOS activity minus iNOS activity [8, 37, 39]. The procedures were performed strictly according to the manufacturer’s protocols.
Determination of the uterine CAT, T-SOD, GSH-PX and MDA levels
In order to further study the effect of thyroid hormones on oxidative stress status in the rat uterus, we also analyzed the CAT, T-SOD, GSH-PX and MDA levels. Firstly, uterus samples were homogenized and centrifuged at 3500 rpm for 10 min, and then the supernatant was collected. The uterine concentration of proteins was quantified by the classical Bradford method with Coomassie Brilliant Blue G-250 using a protein assay kit [40]. Subsequently, the supernatant was used to measure the activity of CAT, T-SOD and GSH-Px, as well as the MDA content, by using commercial reagent kits (Jiancheng Bioengineering Institute).
Briefly, MDA content was estimated with the thiobarbituric acid (TBA) method according to the method described previously. This method was based on the reaction of MDA with TBA to form thiobarbituric acid-reactive substances (TBARS), and the result was expressed as nanomoles per milligram of uterine protein [41]. CAT activity was measured using the ammonium molybdate spectrophotometric method, which was based on the fact that ammonium molybdate can rapidly terminate the H2O2 degradation reaction catalyzed by CAT and react with the residual H2O2 to generate a yellow complex, which could be monitored by the absorbance at 405 nm [40]. The activity of GSH-Px in the rat uterus was measured by a kit based on principles described by previous reports in which GSH-Px degraded H2O2 in the presence of GSH, decreasing the GSH levels [42]. The remaining GSH was then measured using the reaction with DTNB. Absorbance was recorded at 412 nm. One unit of GSH-Px enzyme activity was defined as that capable of consuming 1µmol of GSH per minute. The activity of GSH-Px was expressed as U/mg of uterine protein [41]. Analyses of T-SOD activity were based on the fact that SOD inhibited the generation of nitrite from oxidation of hydroxylamine by superoxide anion (O2−) produced by the xanthine/xanthine oxidase system. The activity of T-SOD was expressed as units per milligram of protein and determined by measuring the reduction in optical density of the reaction solution at 550 nm with a spectrophotometer, and one unit of SOD was defined as the amount of SOD required to produce 50% inhibition of the rate of nitrite production [41]. Notably, all procedures were carried out following the manufacturer’s protocols.
Statistical analysis
All results are expressed as means ± SEM. Statistical analyses for pairs of groups were performed with a t-test, and analysis of variance followed by Tukey’s range test was performed for multiple comparisons. P < 0.05 was considered to be statistically significant.
Results
Effects of thyroid hormones on body weights and uterine weights
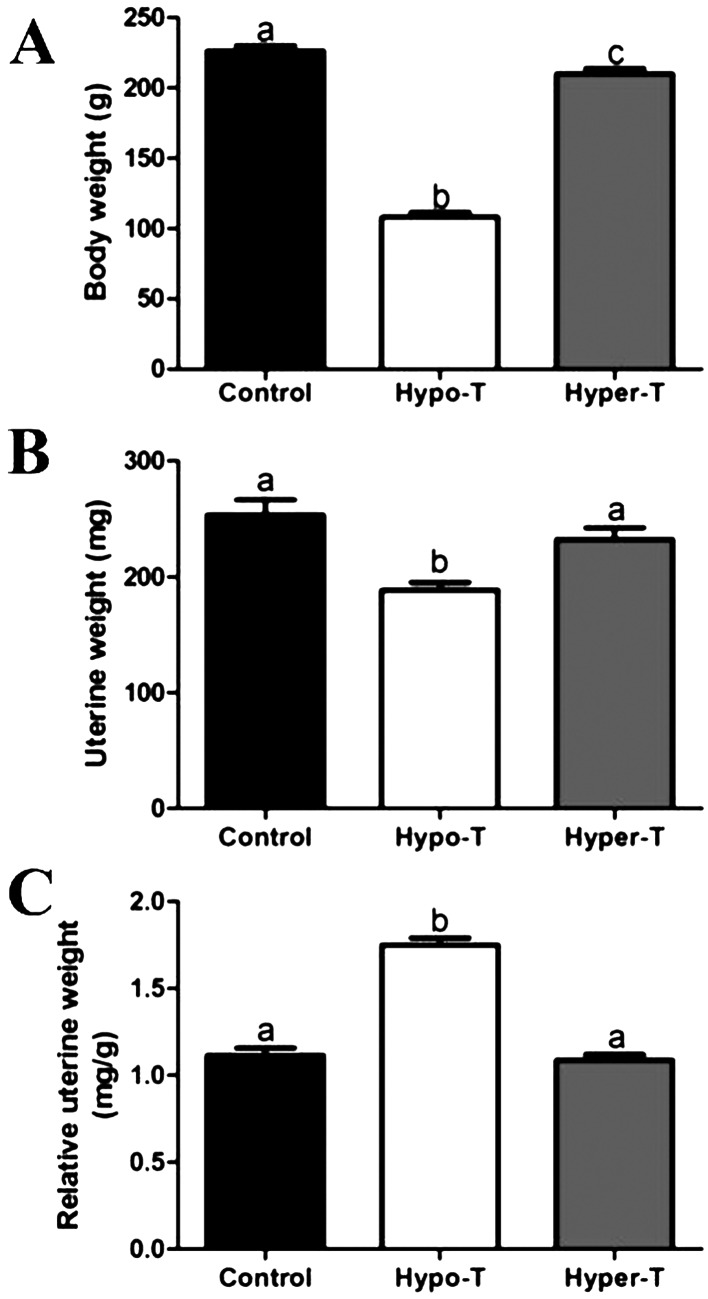
After treatments, the control group rats had good growth performance, and their activities were strong; they also had normal hair growth. However, rats with thyroid dysfunction had poor growth performance. Body weight showed a pronounced reduction in rats of both the hypo-T and hyper-T groups compared with the control group, and the body weight in the hypo-T group was the lowest among the three groups (Fig. 1A). These rats with thyroid dysfunction also showed some clinical symptoms. Hypo-T rats ate less food and drank less water compared with control rats, and they exhibited irritability and weakness, together with coarse and dry hair. While the feed intake of hyper-T rats was increased, they exhibited irritability and anxiety, and these rats had dry and brittle hair. In addition, a significant decrease in uterine weights was observed in hypo-T group rats compared with the rats in the other two groups, while no significant difference was observed in uterine weight between the hyper-T and control groups (Fig. 1B). Moreover, the relative uterine weight was increased in the hypo-T group compared with the control group, while the relative uterine weight was not statistically different in the hyper-T rats compared with the control rats (Fig. 1C).
Fig. 1.
Effects of thyroid hormones on body weight (A), uterine weight (B) and relative uterine weight (C) in the control and hypo-T and hyper-T rats (n=10, mean ± SEM). Relative uterine weight was calculated by dividing uterine weight (mg) by body weight (g). Different superscript letters indicate significant differences among groups (P < 0.05).
Serum hormone concentrations in the control and hypo- and hyper-T rats
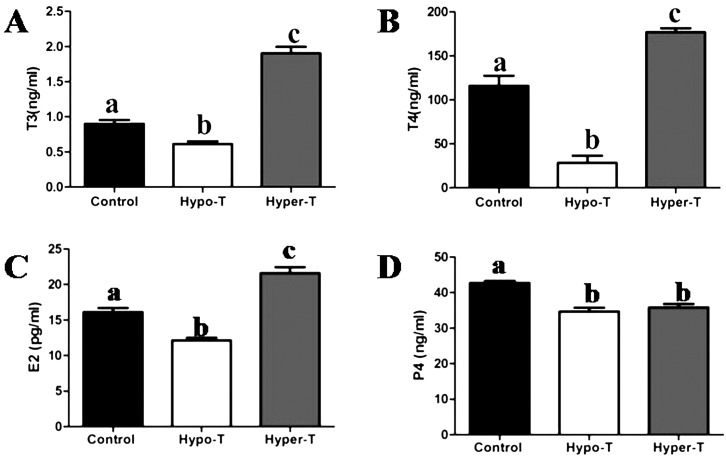
We quantified serum concentrations of total T3, total T4, E2 and P4 in the control and hypo- and hyper-T rats (Fig. 2). Compared with the control group, the serum concentrations of total T3, total T4 and E2 were markedly diminished in the hypo-T rats, whereas they were increased in the hyper-T group (Fig. 2A, 2B and 2C). Moreover, the P4 levels in the hypo-/hyper-T rats markedly decreased compared with the control rats (Fig. 2D).
Fig. 2.
Effects of thyroid hormones on serum concentrations of T3 (A), T4 (B), E2(C) and P4 (D) in the control and hypo-T and hyper-T rats (n = 10, mean ± SEM). Different superscript letters indicate significant differences among groups (P < 0.05).
Immunohistochemical staining of TRα/β and three NOS isoforms in the uterus
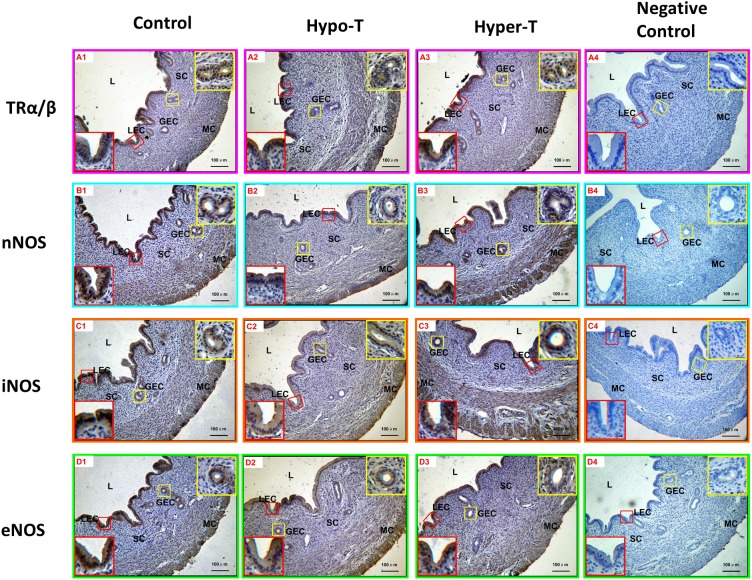
From the results of immunohistochemical staining, TRα/β was expressed more strongly in the uterine luminal epithelium and endometrial gland epithelium cells compared with other areas of rat uteri; as a result, the intensity of immunostaining was weak in myometrial smooth muscle cells, and immunostaining was not detected in stromal cells. The relative levels of immunostaining intensity in each treated group were different from those of the control group. The immunostaining intensity of TRα/β in the hyper-T group was increased, while it was decreased in the hypo-T group (Fig. 3-A1, -A2 and -A3).
Fig. 3.
Immunostaining of TRα/β and the three NOS isoforms (nNOS, iNOS and eNOS) in rat uteri. The staining intensities of TRα/β (A1-3), nNOS (B1-3), iNOS (C1-3) and eNOS (D1-3) were qualitatively different in various cell types of rat uterine. No specific staining was observed in the negative control sections (A4, B4, C4 and D4). NC, negative control sections; LEC, luminal epithelial cells; GEC, glandular epithelial cells; SC, stromal cells; MC, myometrial cells. Scale bar =100 µm.
Immunohistochemistry was also performed to determine the cell-specific localization of the three NOS isoforms in the young adult rat uterus. The results showed that nNOS was strongly immunolocalized in the uterine luminal and glandular epithelium but weakly immunolocalized in the myometrium. The immunostaining intensity of nNOS in the hypo-T group was decreased compared with those of the control and hyper-T groups, and the immunostaining intensity of nNOS in the myometrium of hyper-T rats was stronger than those of the control and hypo-T groups (Fig. 3-B1, -B2 and -B3). In addition, iNOS was strongly immunolocalized in the uterine luminal and glandular epithelium, whereas it was moderately immunolocalized in the myometrium and not detected in stromal cells. Compared with the control, the relative levels of immunostaining intensity in the hypo-T group were weak, while more extensive and stronger staining of iNOS was observed in the glandular epithelium in the hyper-T group (Fig. 3-C1, -C2 and -C3). Moreover, eNOS was expressed more strongly in the uterine luminal epithelium and endometrial gland epithelium cells compared with other areas of the rat uterus; as a result, the intensity of immunostaining was moderate in myometrial smooth muscle cells, and no staining was detected in stromal cells. Compared with the control, the relative levels of immunostaining intensity in both the hypo-T and hyper-T groups were weak (Fig. 3-D1, -D2 and -D3). No specific staining was observed in the negative control sections (Fig. 3-A4, -B4, -C4, and -D4). The relative levels of immunostaining of TRα/β and the three NOS isoforms in different cell types of the rat uteri are summarized in Table 1.
Table 1. Relative levels of immunostaining of TRα/β and the three NOS isoforms in different cell types of rat uterus.
| Uterine development | Staining intensity |
|||||||||||
| Control |
Hypo-T |
Hyper-T |
||||||||||
| TRα/β | nNOS | iNOS | eNOS | TRα/β | nNOS | iNOS | eNOS | TRα/β | nNOS | iNOS | eNOS | |
| Luminal epithelial cells | ++ | +++ | ++ | +++ | ++ | ++ | ++ | ++ | +++ | +++ | +++ | ++ |
| Glandular epithelial cells | ++ | ++ | ++ | ++ | + | + | ++ | + | ++ | +++ | +++ | + |
| Myometrial cells | + | + | ++ | ++ | + | + | ++ | + | ++ | ++ | ++ | + |
| Stromal cells | − | − | − | − | − | − | − | − | − | − | − | − |
Staining intensity: −, no staining detected; +, weak; ++, moderate; +++, strong staining.
Effects of thyroid hormones on the uterine NO content and NOS activity in uteri
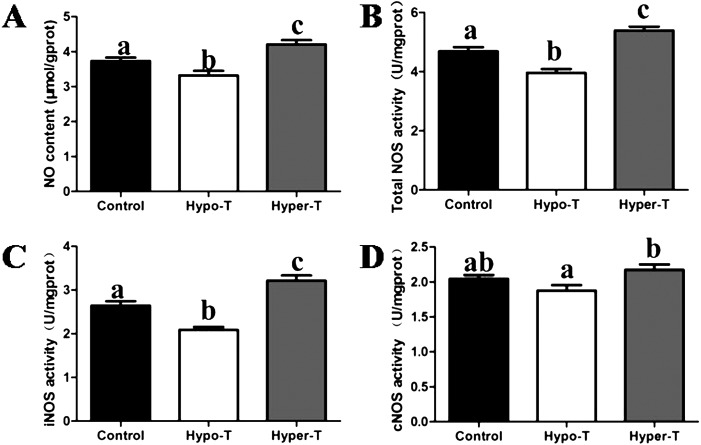
As shown in Fig. 4, we found that thyroid status significantly modulated uterine NO content and NOS activity in rats among the three groups. Compared with the control group, NO content, total NOS activity and iNOS activity were significantly decreased in the hypo-T group, whereas they were increased in hyper-T rats (Fig. 4A, B and C). In addition, constitutive NOS (cNOS) activity in the hypo-T group was significantly lower than that in the hyper-T group. However, neither the hypo-T nor hyper-T group differed significantly from the control group in cNOS activity (Fig. 4D).
Fig. 4.
Effects of thyroid hormones on the levels of NO (A), total NOS (B), iNOS (C) and cNOS (D) in the uterus of young adult rats (n = 10, mean ± SEM). Different superscript letters indicate significant differences among groups (P < 0.05).
Effects of thyroid hormones on CAT, T-SOD, GSH-PX and MDA levels in uteri
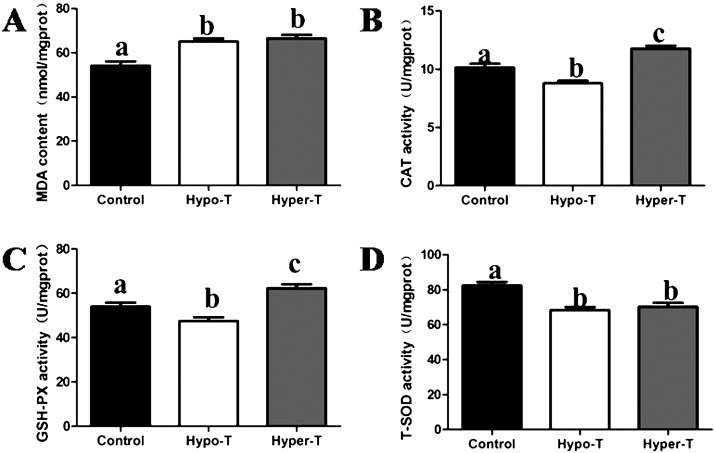
To evaluate the effect of thyroid hormones on the antioxidative status in rat uteri, we investigated the activities of CAT, T-SOD and GSH-PX, as well as the content of MDA, which were key parameters of oxidative stress. Our results showed that the uterine MDA contents in both the hypo-T and hyper-T groups were consistently higher than those in the control group and showed significant differences (Fig. 5A). Compared with the control group, there was a significant decrease in CAT and GSH-Px activities in the hypo-T group and a significant in CAT and GSH-Px activities in the hyper-T group (Fig. 5B and C). Additionally, T-SOD activity in both the hypo-T and hyper-T groups showed a decrease in the uterus compared with the control rats, whereas there was almost no significant difference between the hypo-T and hyper-T groups (Fig. 5D).
Fig. 5.
Effects of thyroid hormones on the levels of MDA (A), CAT (B), GSH-Px (C), and T-SOD (D) in the uterus of young adult rats (n = 10, mean ± SEM). Different superscript letters indicate significant differences among groups (P < 0.05).
Discussion
Our present study provides a new understanding of the possible relationship between thyroid hormones and oxidative stress in uteri. To the best of our knowledge, this is the first report to investigate the effects of hypo- and hyperthyroidism on the uterine antioxidative status in the young adult rat.
In the current study, it was observed that rats with thyroid dysfunction had poor growth performance. This finding was consistent with previous reports of decreased body weight in hypo- and hyper-T rats [8, 43]. In addition, the serum concentrations of total T3 and T4 were significantly decreased in the hypo-T group, but increased in the hyper-T group, similar to previous studies [8, 44]. Thyroid hormone alterations can change body weights, as body growth and organ development are tightly governed by thyroid hormones. Moreover, in this study, we found that serum estradiol levels exhibited significant decreases in the hypo-T rats. It is known that estrogen plays an essential role in uterine development. Thus, such a change in estrogen can explain the smaller uterine size in the hypo-T rats. We also found that the relative uterine weight was increased in the Hypo-T group. This must be because growth was retarded. Importantly, both growth performance and thyroid hormone levels are significant hallmarks of hypo- and hyperthyroidism, indicating that our experimental design is effective.
Thyroid hormones were reported to have significant influences on reproductive hormones in both sexes [6, 45]. In women, both hyper- and hypothyroidism were associated with menstrual disorders and reduced fertility [45]. The results of the present study showed that the serum E2 levels exhibited significant decreases and increases in the hypo-T and hyper-T groups, respectively, while the P4 levels in the hypo- and hyper-T rats markedly decreased after the treatments. These findings were in accordance with those of previous studies. Women with hyperthyroidism have total estradiol levels two to three times the normal values [6], and woman with thyroid hormone deficiency have decreased estradiol levels [46]. Another study showed that estrogen was significantly increased but progesterone was unchanged in hyper-T women who were still menstruating [47]. Furthermore, an in vitro study indicated that thyroid hormones greatly stimulated P4 release from human corpus luteum cells indirectly [48]. Both E2 and P4 levels play essential roles in uterine development and the menstrual cycle. Therefore, it was indicated that thyroid hormones might cause menstrual irregularities and influence uterine development by altering the reproductive hormone levels under different thyroid statuses.
It was reported that thyroid hormone could improve uterine epithelial morphology in hypothyroid rats [49]. It is also known that estrogen can stimulate endometrial growth. In the present study, we found that the serum concentrations of estradiol decreased significantly in the hypo-T group. These results were consistent with previous reports and suggested that thyroid hormones might play an important role with respect to the normal structure of uterine endometrium epithelial cells.
The roles of thyroid hormones in uterine physiology and function have received more attentions since the identification of functional thyroid receptors in uteri [5, 13, 14]. From the results of immunohistochemistry, we confirmed that TRα/β was present and located in different cell types of rat uteri, which suggested that there was an important relationship between thyroid hormones and uterine development. The results were in good agreement with the findings of previous studies [5, 13]. The binding of thyroid hormones to the thyroid hormone receptors mediates the biological activities of T3 via transcriptional regulation through transcription factors present in the female reproductive tract [50]. The relative levels of immunostaining intensity in each treated group were different from those of the control group, which may be because of the changes in thyroid hormones in different thyroid states. This indicates that an increase in thyroid hormone levels could upregulate the expression of TRα/β, while a decrease in thyroid hormones levels could downregulate the expression.
Thyroid hormones are implicated in the regulation of oxidative metabolism, and any alteration of thyroid hormones is suspected to induce cellular oxidative stress [51]. Enzymatic antioxidants include SOD, CAT, GSH, and GSH-PX, which are known to serve as protective responses for elimination of reactive free radicals [15]. Generation of superoxide radicals takes place due to incomplete reduction of oxygen molecules during cellular respiration [52]. Superoxide radicals are converted to hydrogen peroxide by the enzyme SOD [53]. From the present study, it is evident that T-SOD activity in rats with hypo-/hyperthyroidism markedly decreased after PTU and T4 treatments. The decrease in T-SOD activity may be due to increased endogenous production of ROS as evidenced by increased lipid hydroperoxides. In support of this observation, the uterine MDA content was found to be negatively correlated with T-SOD activity in the hypo- and hyper-T rats. These results suggested that thyroid hormone might cause downregulation of uterine SOD activity.
Hydrogen peroxide is neutralized by two enzymes, CAT and GSH-Px [53]. In the current study, the CAT and GSH-Px activities exhibited significant increases in the hyper-T group but significant decreases in the in hypo-T group. These observed changes pointed to different antioxidant defense properties in different thyroid statuses and also strongly suggested that the hydrogen peroxide content in uteri was under tight regulation of thyroid hormones. Previous studies have reported that thyroid hormones induce alterations in CAT and GSH-Px activities [23, 35]. It is worth noting that PTU-induced hypothyroidism might have significantly decreased the uterine antioxidant activities of CAT, GSH-Px and T-SOD in our current study. On the other hand, T4-induced hyperthyroidism decreased uterine T-SOD activity but increased CAT and GSH-Px activities. The reason why the variation tendencies of the three antioxidative enzymes in uteri were not consistent under different thyroid conditions was difficult to ascertain in the present study. More evidence is needed to clarify this phenomenon in the future. In any case, the changes in enzyme levels might be due to alteration of the tissue oxidant state. Therefore, the results of the current investigation indicated that any alteration in thyroid state of the body would affect the antioxidant defense of young adult rat uteri, thereby impairing reproductive system functions.
Lipid peroxidation (LPx) is an autocatalytic mechanism leading to oxidative destruction of cellular membranes, and their destruction can lead to cell death and to the production of toxic and reactive aldehyde free radical metabolites [54, 55]. The extent of cellular peroxidative processes depends on the tissue antioxidant defense capacity. It was reported that ROS might propagate the initial attack on lipid membranes to cause LPx [56]. As an end product of LPx, MDA is commonly used to monitor lipid oxidation status in the body [57]. In this work, MDA content was also evaluated under different thyroid conditions, and both hypo- and hyper-T rats showed a significant increase in uterine MDA contents, which probably led to alteration of the uterine antioxidative status. Indeed, it has been previously demonstrated that thyroid dysfunction induced LPx in various tissues including the liver, kidneys and testes [23, 35]. Therefore, based on the evidence from this and previous studies, it is reasonable to propose that the observed changes in uterine MDA contents might be considered as adaptative changes to the modulated metabolic rate caused by the thyroid status in order to protect the uterus from oxidative damage.
NO is the intracellular and intercellular gas message molecule and is also a strong gas molecule free radical [26]. Under normal physiological conditions, NO plays a vital role in the defense against oxidative stress and is well recognized as a critical mediator of uterine function and development [15, 29]. In the present study, it was observed that abnormal thyroid hormone levels could differentially regulate uterine NO content and NOS activity, which might lead to alteration of the uterine antioxidative status and affect uterine development and function. These results indicate hyper- and hypothyroidism could increase and decrease the expression of NOS and synthesis of NO, respectively. Our findings are in accordance with previous studies showing that NOS activity was higher in the hypothalamus, cerebral cortex, heart, vessels and kidney of hyper-T rats and significantly decreased in the same tissues in rats with hypothyroidism [33, 58, 59]. Our immunohistochemistry results provided evidence indicating that the cellular expression and localization patterns of nNOS, iNOS and eNOS varied in the uterus of young adult rats, which was consistent with our previous study, which showed that the three NOS isoforms exhibited cell-specific expression in postnatal porcine uteri [39]. Recently, several studies have demonstrated that a decrease in E2 levels could downregulate the expression of nNOS and synthesis of NO [60, 61]. The results of the present study showed that serum E2 levels exhibited significant decreases and increases in the hypo-T and hyper-T groups, respectively. Therefore, these results can help to explain the differences in immunostaining intensity levels of the three NOS isoforms among the three groups. Moreover, it was also reported that oxidative stress and perturbation of the redox equilibrium in the endothelium are of central importance for NOS activity and NO production [62]. Cells within different tissues display varying responses to NO, which might be related to the presence of cellular antioxidants [63]. Therefore, these data indicated that the levels of uterine NO and NOS could be induced by thyroid hormones, which might cause oxidative stress to damage uterine development and function. However, this needs to be corroborated by more evidences.
In conclusion, the results of the present study using hypo- and hyper-T rat models provide the evidence that thyroid hormones have an important influence on the modulation of uterine antioxidative status in the young adult rat. Nevertheless, the roles of NOS signaling and oxidative stress in thyroid hormone-induced reproductive problems still need further research. Clearly, we believed that there might be significant relationships between thyroid hormones and uterine antioxidative status, and these findings might be helpful in clarifying the effects of thyroid dysfunction on female reproduction overall.
Acknowledgments
This work was supported in part by the National Natural Science Foundation of China (No. 31172206).
References
- 1.Choksi NY, Jahnke GD, St Hilaire C, Shelby M. Role of thyroid hormones in human and laboratory animal reproductive health. Birth Defects Res B Dev Reprod Toxicol 2003; 68: 479–491. [DOI] [PubMed] [Google Scholar]
- 2.Jannini EA, Ulisse S, D’Armiento M. Thyroid hormone and male gonadal function. Endocr Rev 1995; 16: 443–459. [DOI] [PubMed] [Google Scholar]
- 3.Metz LD, Seidler FJ, McCook EC, Slotkin TA. Cardiac alpha-adrenergic receptor expression is regulated by thyroid hormone during a critical developmental period. J Mol Cell Cardiol 1996; 28: 1033–1044. [DOI] [PubMed] [Google Scholar]
- 4.Krassas GE. Thyroid disease and female reproduction. Fertil Steril 2000; 74: 1063–1070. [DOI] [PubMed] [Google Scholar]
- 5.Aghajanova L, Stavreus-Evers A, Lindeberg M, Landgren BM, Sparre LS, Hovatta O. Thyroid-stimulating hormone receptor and thyroid hormone receptors are involved in human endometrial physiology. Fertil Steril 2011; 95: 230–237: 237.e1–e2. [DOI] [PubMed] [Google Scholar]
- 6.Dittrich R, Beckmann MW, Oppelt PG, Hoffmann I, Lotz L, Kuwert T, Mueller A. Thyroid hormone receptors and reproduction. J Reprod Immunol 2011; 90: 58–66. [DOI] [PubMed] [Google Scholar]
- 7.Doufas AG, Mastorakos G. The hypothalamic-pituitary-thyroid axis and the female reproductive system. Ann N Y Acad Sci 2000; 900: 65–76. [DOI] [PubMed] [Google Scholar]
- 8.Fedail JS, Zheng K, Wei Q, Kong L, Shi F. Roles of thyroid hormones in follicular development in the ovary of neonatal and immature rats. Endocrine 2014; 46: 594–604. [DOI] [PubMed] [Google Scholar]
- 9.Zheng K, Sulieman FJ, Li J, Wei Q, Xu M, Shi F. Nitric oxide and thyroid hormone receptor alpha 1 contribute to ovarian follicular development in immature hyper- and hypo-thyroid rats. Reprod Biol 2015; 15: 27–33. [DOI] [PubMed] [Google Scholar]
- 10.Cheng SY, Leonard JL, Davis PJ. Molecular aspects of thyroid hormone actions. Endocr Rev 2010; 31: 139–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yen PM. Physiological and molecular basis of thyroid hormone action. Physiol Rev 2001; 81: 1097–1142. [DOI] [PubMed] [Google Scholar]
- 12.Shahrara S, Drvota V, Sylvén C. Organ specific expression of thyroid hormone receptor mRNA and protein in different human tissues. Biol Pharm Bull 1999; 22: 1027–1033. [DOI] [PubMed] [Google Scholar]
- 13.Hulchiy M, Zhang H, Cline JM, Hirschberg AL, Sahlin L. Receptors for thyrotropin-releasing hormone, thyroid-stimulating hormone, and thyroid hormones in the macaque uterus: effects of long-term sex hormone treatment. Menopause 2012; 19: 1253–1259. [DOI] [PubMed] [Google Scholar]
- 14.Öner J, Oner H. Immunodetection of thyroid hormone receptor (alpha1/alpha2) in the rat uterus and oviduct. Acta Histochem Cytochem 2007; 40: 77–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agarwal A, Gupta S, Sharma RK. Role of oxidative stress in female reproduction. Reprod Biol Endocrinol 2005; 3: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Betteridge DJ. What is oxidative stress? Metabolism 2000; 49(Suppl 1): 3–8. [DOI] [PubMed] [Google Scholar]
- 17.Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ J 2012; 5: 9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurasaki M, Saito T, Kaji H, Kojima Y, Saito K. Increased erythrocyte catalase activity in patients with hyperthyroidism. Horm Metab Res 1986; 18: 56–59. [DOI] [PubMed] [Google Scholar]
- 19.Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. Selenium: biochemical role as a component of glutathione peroxidase. Science 1973; 179: 588–590. [DOI] [PubMed] [Google Scholar]
- 20.Ruder EH, Hartman TJ, Goldman MB. Impact of oxidative stress on female fertility. Curr Opin Obstet Gynecol 2009; 21: 219–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruder EH, Hartman TJ, Blumberg J, Goldman MB. Oxidative stress and antioxidants: exposure and impact on female fertility. Hum Reprod Update 2008; 14: 345–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Das K, Chainy GB. Thyroid hormone influences antioxidant defense system in adult rat brain. Neurochem Res 2004; 29: 1755–1766. [DOI] [PubMed] [Google Scholar]
- 23.Choudhury S, Chainy GB, Mishro MM. Experimentally induced hypo- and hyper-thyroidism influence on the antioxidant defence system in adult rat testis. Andrologia 2003; 35: 131–140. [DOI] [PubMed] [Google Scholar]
- 24.Shinohara R, Mano T, Nagasaka A, Hayashi R, Uchimura K, Nakano I, Watanabe F, Tsugawa T, Makino M, Kakizawa H, Nagata M, Iwase K, Ishizuki Y, Itoh M. Lipid peroxidation levels in rat cardiac muscle are affected by age and thyroid status. J Endocrinol 2000; 164: 97–102. [DOI] [PubMed] [Google Scholar]
- 25.Huh K, Kwon TH, Kim JS, Park JM. Role of the hepatic xanthine oxidase in thyroid dysfunction: effect of thyroid hormones in oxidative stress in rat liver. Arch Pharm Res 1998; 21: 236–240. [DOI] [PubMed] [Google Scholar]
- 26.Hou YC, Janczuk A, Wang PG. Current trends in the development of nitric oxide donors. Curr Pharm Des 1999; 5: 417–441. [PubMed] [Google Scholar]
- 27.Welter H, Bollwein H, Weber F, Rohr S, Einspanier R. Expression of endothelial and inducible nitric oxide synthases is modulated in the endometrium of cyclic and early pregnant mares. Reprod Fertil Dev 2004; 16: 689–698. [DOI] [PubMed] [Google Scholar]
- 28.Cameron IT, Campbell S. Nitric oxide in the endometrium. Hum Reprod Update 1998; 4: 565–569. [DOI] [PubMed] [Google Scholar]
- 29.Rosselli M. Nitric oxide and reproduction. Mol Hum Reprod 1997; 3: 639–641. [DOI] [PubMed] [Google Scholar]
- 30.Chwalisz K, Garfield RE. Role of nitric oxide in the uterus and cervix: implications for the management of labor. J Perinat Med 1998; 26: 448–457. [DOI] [PubMed] [Google Scholar]
- 31.Andronowska A, Wasowska B, Całka J, Doboszyńska T. Localization and correlation between NADPH-diaphorase and nitric oxide synthase isoforms in the porcine uterus during the estrous cycle. Cell Tissue Res 2005; 321: 243–250. [DOI] [PubMed] [Google Scholar]
- 32.Cella M, Aisemberg J, Sordelli MS, Billi S, Farina M, Franchi AM, Ribeiro ML. Prostaglandins modulate nitric oxide synthase activity early in time in the uterus of estrogenized rat challenged with lipopolysaccharide. Eur J Pharmacol 2006; 534: 218–226. [DOI] [PubMed] [Google Scholar]
- 33.Quesada A, Sainz J, Wangensteen R, Rodriguez-Gomez I, Vargas F, Osuna A. Nitric oxide synthase activity in hyperthyroid and hypothyroid rats. Eur J Endocrinol 2002; 147: 117–122. [DOI] [PubMed] [Google Scholar]
- 34.Carrillo-Sepúlveda MA, Ceravolo GS, Fortes ZB, Carvalho MH, Tostes RC, Laurindo FR, Webb RC, Barreto-Chaves ML. Thyroid hormone stimulates NO production via activation of the PI3K/Akt pathway in vascular myocytes. Cardiovasc Res 2010; 85: 560–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Venditti P, Di Meo S. Thyroid hormone-induced oxidative stress. Cell Mol Life Sci 2006; 63: 414–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang W, Wei QW, Wang ZC, Ding W, Wang W, Shi FX. Cell-specific expression and immunolocalization of nitric oxide synthase isoforms and the related nitric oxide/cyclic GMP signaling pathway in the ovaries of neonatal and immature rats. J Zhejiang Univ Sci B 2011; 12: 55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wei Q, Li J, Li X, Zhang L, Shi F. Reproductive toxicity in acrylamide-treated female mice. Reprod Toxicol 2014; 46: 121–128. [DOI] [PubMed] [Google Scholar]
- 38.Gong Y, Liu L, Xie B, Liao Y, Yang E, Sun Z. Ameliorative effects of lotus seedpod proanthocyanidins on cognitive deficits and oxidative damage in senescence-accelerated mice. Behav Brain Res 2008; 194: 100–107. [DOI] [PubMed] [Google Scholar]
- 39.Li Y, Zhou X, Wei QW, Huang RH, Shi FX. Cell-specific expression and immunolocalization of nitric oxide synthase isoforms and soluble guanylyl cyclase α and β subunits in postnatal porcine uteri. Acta Histochem 2014; 116: 466–473. [DOI] [PubMed] [Google Scholar]
- 40.Jiang SZ, Yang ZB, Yang WR, Gao J, Liu FX, Broomhead J, Chi F. Effects of purified zearalenone on growth performance, organ size, serum metabolites, and oxidative stress in postweaning gilts. J Anim Sci 2011; 89: 3008–3015. [DOI] [PubMed] [Google Scholar]
- 41.Yang Y, Fan F, Zhuo R, Ma F, Gong Y, Wan X, Jiang M, Zhang X. Expression of the laccase gene from a white rot fungus in Pichia pastoris can enhance the resistance of this yeast to H2O2-mediated oxidative stress by stimulating the glutathione-based antioxidative system. Appl Environ Microbiol 2012; 78: 5845–5854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hafeman DG, Sunde RA, Hoekstra WG. Effect of dietary selenium on erythrocyte and liver glutathione peroxidase in the rat. J Nutr 1974; 104: 580–587. [DOI] [PubMed] [Google Scholar]
- 43.Tohei A, Imai A, Watanabe G, Taya K. Influence of thiouracil-induced hypothyroidism on adrenal and gonadal functions in adult female rats. J Vet Med Sci 1998; 60: 439–446. [DOI] [PubMed] [Google Scholar]
- 44.Carlé A, Knudsen N, Pedersen IB, Perrild H, Ovesen L, Rasmussen LB, Laurberg P. Determinants of serum T4 and T3 at the time of diagnosis in nosological types of thyrotoxicosis: a population-based study. Eur J Endocrinol 2013; 169: 537–545. [DOI] [PubMed] [Google Scholar]
- 45.Krassas GE, Poppe K, Glinoer D. Thyroid function and human reproductive health. Endocr Rev 2010; 31: 702–755. [DOI] [PubMed] [Google Scholar]
- 46.Longcope C, Abend S, Braverman LE, Emerson CH. Androstenedione and estrone dynamics in hypothyroid women. J Clin Endocrinol Metab 1990; 70: 903–907. [DOI] [PubMed] [Google Scholar]
- 47.Akande EO, Hockaday TD. Plasma concentration of gonadotrophins, oestrogens and progesterone in thyrotoxic women. Br J Obstet Gynaecol 1975; 82: 541–551. [DOI] [PubMed] [Google Scholar]
- 48.Datta M, Roy P, Banerjee J, Bhattacharya S. Thyroid hormone stimulates progesterone release from human luteal cells by generating a proteinaceous factor. J Endocrinol 1998; 158: 319–325. [DOI] [PubMed] [Google Scholar]
- 49.Inuwa IM, Williams MA. A morphometric study on the endometrium of rat uterus in hypothyroid and thyroxine treated hypothyroid rats. Ups J Med Sci 2006; 111: 215–225. [DOI] [PubMed] [Google Scholar]
- 50.Stavreus Evers A. Paracrine interactions of thyroid hormones and thyroid stimulation hormone in the female reproductive tract have an impact on female fertility. Front Endocrinol (Lausanne) 2012; 3: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Villanueva I, Alva-Sánchez C, Pacheco-Rosado J. The role of thyroid hormones as inductors of oxidative stress and neurodegeneration. Oxid Med Cell Longev 2013; 2013: 218145. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cadenas E, Davies KJ. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic Biol Med 2000; 29: 222–230. [DOI] [PubMed] [Google Scholar]
- 53.Sahoo DK, Roy A, Bhanja S, Chainy GB. Experimental hyperthyroidism-induced oxidative stress and impairment of antioxidant defence system in rat testis. Indian J Exp Biol 2005; 43: 1058–1067. [PubMed] [Google Scholar]
- 54.Şener G, Sehirli O, Velioğlu-Oğünç A, Ercan F, Erkanli G, Gedik N, Yeğen BC. Propylthiouracil (PTU)-induced hypothyroidism alleviates burn-induced multiple organ injury. Burns 2006; 32: 728–736. [DOI] [PubMed] [Google Scholar]
- 55.Messarah M, Saoudi M, Boumendjel A, Boulakoud MS, Feki AE. Oxidative stress induced by thyroid dysfunction in rat erythrocytes and heart. Environ Toxicol Pharmacol 2011; 31: 33–41. [DOI] [PubMed] [Google Scholar]
- 56.Nasiadek M, Skrzypińska-Gawrysiak M, Daragó A, Zwierzyńska E, Kilanowicz A. Involvement of oxidative stress in the mechanism of cadmium-induced toxicity on rat uterus. Environ Toxicol Pharmacol 2014; 38: 364–373. [DOI] [PubMed] [Google Scholar]
- 57.Sumida S, Tanaka K, Kitao H, Nakadomo F. Exercise-induced lipid peroxidation and leakage of enzymes before and after vitamin E supplementation. Int J Biochem 1989; 21: 835–838. [DOI] [PubMed] [Google Scholar]
- 58.Ueta Y, Levy A, Chowdrey HS, Lightman SL. Hypothalamic nitric oxide synthase gene expression is regulated by thyroid hormones. Endocrinology 1995; 136: 4182–4187. [DOI] [PubMed] [Google Scholar]
- 59.Serfözö Z, Kiss PB, Kukor Z, Lontay B, Palatka K, Varga V, Erdodi F, Elekes K. Thyroid hormones affect the level and activity of nitric oxide synthase in rat cerebral cortex during postnatal development. Neurochem Res 2008; 33: 569–578. [DOI] [PubMed] [Google Scholar]
- 60.Arnal JF, Fontaine C, Billon-Galés A, Favre J, Laurell H, Lenfant F, Gourdy P. Estrogen receptors and endothelium. Arterioscler Thromb Vasc Biol 2010; 30: 1506–1512. [DOI] [PubMed] [Google Scholar]
- 61.Ravella K, Al-Hendy A, Sharan C, Hale AB, Channon KM, Srinivasan S, Gangula PR. Chronic estrogen deficiency causes gastroparesis by altering neuronal nitric oxide synthase function. Dig Dis Sci 2013; 58: 1507–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Elahi MM, Naseem KM, Matata BM. Nitric oxide in blood. The nitrosative-oxidative disequilibrium hypothesis on the pathogenesis of cardiovascular disease. FEBS J 2007; 274: 906–923. [DOI] [PubMed] [Google Scholar]
- 63.Hogg N, Singh RJ, Kalyanaraman B. The role of glutathione in the transport and catabolism of nitric oxide. FEBS Lett 1996; 382: 223–228. [DOI] [PubMed] [Google Scholar]