Abstract
Objective:
The U.S. Food and Drug Administration’s approval of buprenorphine in 2002 expanded options for treating opioid use disorder (OUD). Physicians who intend to treat OUD patients with buprenorphine must seek a waiver to prescribe it, which may contribute to state-by-state variation in the supply of waivered physicians.
Method:
This study integrates data extracted from the U.S. Drug Enforcement Agency’s database of waivered physicians with state-level indicators of the macro environment, health-related resources, and treatment demand.
Results:
In December 2013, the average state had 8.0 waivered physicians per 100,000 residents (SD = 5.2). Large regional differences between states in the Northeast relative to states in the Midwest, South, and West were observed. The percentage of residents covered by Medicaid as well as the population-adjusted availability of opioid treatment programs and substance use disorder treatment facilities were positively associated with buprenorphine physician supply. Buprenorphine physician supply was positively correlated with states’ rates of overdose deaths, suggesting that physicians may seek the waiver in response to the magnitude of the opioid problem in their state.
Conclusions:
States with greater health-related resources, particularly in terms of the supply of opioid treatment programs and substance use disorder treatment programs, had more waivered physicians in 2013. The finding regarding Medicaid coverage suggests that states implementing Medicaid expansion under health reform may experience additional growth in buprenorphine physician supply. However, large regional disparities in the supply of waivered physicians may impede access to care for many Americans with OUD.
Opioid use disorder (oud) is a significant public health problem in the United States, with nearly 2 million Americans meeting criteria for OUD in a given year (Substance Abuse and Mental Health Services Administration [SAMHSA], 2009). Opioid overdose deaths have increased dramatically since the mid-1990s (Paulozzi & Xi, 2008), and untreated OUDs are associated with substantial medical, social, and economic costs (Mark et al., 2001; Volkow et al., 2014). Before the approval of buprenorphine by the U.S. Food and Drug Administration (FDA) in 2002, opioid treatment programs (OTPs) delivering methadone maintenance were the primary setting for patients seeking a pharmacological approach to treatment (Jaffe & O’Keeffe, 2003). Historically, OTPs had been unable to meet the demand for pharmacotherapy because of their limited numbers and their concentration in large urban centers (Lewis, 1999; SAMHSA, 2002).
With the approval of buprenorphine, the range of evidence-based treatment practices for OUD has expanded (Ling & Wesson, 2003; Mattick et al., 2008). Clinical research has demonstrated its safety (Ling & Smith, 2002; Walsh & Eissenberg, 2003), its role in reducing opioid withdrawal symptoms (Bickel & Amass, 1995; Chadderton, 2000), and its effectiveness in reducing opioid use (Fiellin et al., 2008; Fudala et al., 2003). Buprenorphine is primarily marketed as Suboxone® (Reckitt Benckiser Pharmaceuticals, Richmond, VA) and, more recently, as Zubsolv® (Orexo US, Morristown, NJ); both products combine buprenorphine and naloxone in sublingual formulations. Generic buprenorphine-naloxone and mono-buprenorphine tablets are also available.
Buprenorphine offers a unique opportunity to consider the diffusion of an innovation that represents both a strategic choice of individual actors (i.e., physicians) as well as a broader movement toward the medicalization of substance use disorder (SUD) treatment. Physicians interested in prescribing buprenorphine for OUD treatment outside of OTP settings must apply for a waiver and submit documentation to SAMHSA demonstrating that they have completed 8 hours of approved training or that they hold specific board certifications (Center for Substance Abuse Treatment, 2004; West et al., 2004). In the first year, waivered physicians are limited to treating 30 patients at any one time; after the first year, physicians may request a waiver to treat up to 100 patients. Information regarding buprenorphine waivers is maintained in the Drug Enforcement Agency’s Controlled Substances Act (CSA) Registrants database. In the first year after FDA approval, about 2,000 physicians applied for the buprenorphine waiver (Kissin et al., 2006), which had increased to more than 20,000 physicians holding the waiver by 2011 (Stein et al., 2015).
Physicians’ decisions to seek the buprenorphine waiver are all the more notable given the long history of SUD treatment occurring outside of the traditional medical system (White, 1998). Historically, the core technology of SUD treatment has been psychosocial counseling delivered by counselors (Roman et al., 2000), and the majority of non-OTPs do not employ physicians (Knudsen et al., 2012). Most programs have been slow to integrate pharmacotherapy into their menu of services, with fewer than 40% of specialty organizations offering buprenorphine treatment (Abraham et al., 2013). However, the diffusion of buprenorphine among physicians represents an example of the integration of SUD treatment into the mainstream health care system that has been advocated in policy circles in recent years (Buck, 2011).
Health services research on the diffusion of buprenorphine has largely focused on organizations or individual physicians as the unit of analysis. Considerable work has examined its diffusion in SUD treatment programs and OTPs (Brigham et al., 2007; Ducharme & Abraham, 2008; Ducharme et al., 2007; Friedmann et al., 2010; Gordon et al., 2007; Knudsen et al., 2006, 2009; Koch et al., 2006; Kovas et al., 2007; Wallack et al., 2010). Several surveys of physicians have examined the nexus between attitudes and prescribing (Arfken et al., 2010; Kissin et al., 2006; Netherland et al., 2009; Reif et al., 2007; Thomas et al., 2008; WESTAT, 2006).
An understudied issue is the distribution of waivered physicians across U.S. states in terms of physician supply. Health services researchers typically measure physician supply as the number of physicians for a fixed unit of population (e.g., 100,000 residents) in a geographical unit, such as a county or a state (Cooper, 2009a). Prior work in general medical care has demonstrated that physician supply is associated with several health-related outcomes, such as patient-level health care utilization (Continelli et al., 2010), all-cause mortality (Macinko et al., 2007), health care quality (Cooper, 2009b), and state health rankings (Bigbee, 2008). Just as there are widespread geographic disparities in health and access to care (Koh et al., 2010; Radley & Schoen, 2012), there is also considerable geographic variability in the overall physician supply in the United States (Cooper, 2009b; Rosenthal et al., 2005; Wang & Luo, 2005). In part, this variability may reflect macrolevel differences between states as contexts in which medical professionals conduct their work (Declercq et al., 1998; Sekscenski et al., 1994).
To date, only two studies have considered the supply of buprenorphine physicians, and both focused on counties as the unit of analysis. Stein et al. (2015) examined the supply of waivered physicians in U.S. counties from 2008 to 2011 as a function of state policies and county characteristics. Rosenblatt et al. (2015) compared the supply of waivered physicians in counties by rural-urban status and then mapped the counties with at least one provider in mid-2012. In contrast, the present study examines state-level buprenorphine physician supply at the end of 2013 and considers three domains of correlates: the macro environment, health-related resources, and demand for treatment.
Key dimensions of the macro environment include economic, demographic, and political forces (Osborn & Hunt, 1974). Physician supply has been previously linked to state-level economic development (Cooper, 2009a), with per capita income being positively associated with the supply of specialist physicians (Cooper, 2009b). Prior work has also considered how the demographic composition of the population—particularly with regard to race, ethnicity, and age—may be associated with the distribution of physicians (Wang & Luo, 2005). Health disparities experienced by minority populations may be partly explained by geographic factors and the distribution of medical services (Baicker et al., 2005; Lackan et al., 2009; Weisfeld & Perlman, 2005). Although political control of state governments has not been examined in prior studies of physician supply, there is long-standing recognition of the connection between political control and social welfare policies (Dawson & Robinson, 1963). In the current American political context, there are substantial ideological differences between the two dominant parties regarding the role of the state in providing social services.
In addition to the macro environment, a state’s environmental munificence regarding health-related resources may be associated with its supply of buprenorphine physicians. Castrogiovanni (1991) defines environmental munificence as “the scarcity or abundance of critical resources needed by (one or more) firms operating within an environment” (p. 542). States vary in their overall scarcity or abundance of physicians (Mazurenko & Menachemi, 2012). Insurance coverage for the state’s residents is a crucial health-related resource. Higher rates of uninsured persons may represent a greater scarcity of the financial resources that support access to medical care (Cooper, 2009b). Greater rates of Medicaid coverage at the state level may actually provide crucial resources to help residents access needed services. SUDs are prevalent within the Medicaid population (Buck, 2011), and Medicaid is an increasingly significant source of payment for treatment services (Mark et al., 2011).
Other measures of the scarcity or abundance of health-related resources are more closely linked to behavioral health services. For example, a greater supply of treatment organizations, such as OTPs and SUD treatment programs, may be indicative of a more supportive state environment with greater resources allocated to treatment. Greater investment in related behavioral health services, such as state spending for mental health, might also point to a more abundant resource environment.
Finally, buprenorphine physician supply may reflect the demand for treatment related to the prevalence of OUD within the state. Published reports have demonstrated state-level variation in nonmedical use of prescription pain relievers (SAMHSA, 2013a) and rates of opioid overdose deaths (Centers for Disease Control and Prevention [CDC], 2011). Buprenorphine physician supply may be indicative of physicians seeking the waiver to address the health care needs of their state.
Drawing on data from the 50 states and the District of Columbia, this study considers state-level variation in buprenorphine physician supply as a function of the macro environment, health-related resources, and demand for treatment. Regarding the macro environment, it is hypothesized that state economic indicators and Democratic political control are positively associated with buprenorphine physician supply. Demographic characteristics, such as greater minority representation as well as greater representation of children and the elderly in the population, are hypothesized to be negatively associated with buprenorphine physician supply. Furthermore, it is hypothesized that indicators of health-related resources and demand for opioid treatment are positively associated with buprenorphine physician supply.
Method
Study design
This study integrates data from secondary sources to examine state-level variation in the availability of waivered physicians who are authorized to prescribe buprenorphine. Numerous web-based searches were performed to identify sources of state-level data. Data regarding waivered physicians were purchased, and the remaining variables were publicly available from the Henry J. Kaiser Family Foundation, the U.S. Census Bureau, the CDC, SAMHSA, and publications, as described below. Attempts were made to find the most recent state-level data. Variation in publication dates resulted in independent variables that were measured at different points in time, but all independent variables temporally preceded or were concurrent with the measurement of the dependent variable.
Dependent variable
Buprenorphine physician supply.
The number of buprenorphine-waivered civilian physicians per 100,000 residents as of December 2013 constituted the measure of physician supply. The U.S. Drug Enforcement Agency maintains the CSA database on medical professionals who have registered to prescribe controlled substances (i.e., prescription medications with misuse potential). The CSA database includes codes indicating whether physicians are waivered to prescribe buprenorphine. Using the December 2013 database, the number of waivered physicians for each state and the District of Columbia was counted. Military physicians were excluded because they practice under the auspices of the federal government and are less likely to be affected by state contexts. The number of waivered physicians was then divided by the number of state residents in 2013 and multiplied by 100,000 (U.S. Census Bureau, 2014).
Independent variables
Macro environment: Economic indicators, political control, demography, and rurality.
Two economic measures were considered. First, the percentage of the total population living under 138% of the federal poverty line in 2011-2012 was measured (Henry J. Kaiser Foundation, 2014a). Second, real per capita personal income (in thousands) in 2011 was measured using data from the U.S. Department of Commerce’s Bureau of Economic Affairs (Aten et al., 2013).
Political control was measured by several variables. States were coded based on the political affiliation of the state governorship and control of the state legislature in 2013 (Henry J. Kaiser Foundation, 2013b), which yielded a typology of three mutually exclusive categories: Republican control of the governorship and majority control of both chambers of the state legislature (reference category); Democratic control of the governorship and majority control of both legislative chambers; and divided control in which neither party controls both the governorship and the state legislature. Nebraska has a unicameral legislature with members not elected by political affiliation; in 2013, Nebraska had a Republican governor, so it was included in the reference category. The District of Columbia was coded based on the affiliation of its mayor (i.e., Democrat) and the majority composition of its city council (i.e., Democrat). The duration of political control was measured using data published by Elliott and Balz (2013), in which twelve 2-year periods from 1990 to 2013 were counted for Democratic control of the governorship and dominance in the legislature, Republican control of the governorship and dominance in the legislature, and split control.
The demographic composition of the state with regard to race, ethnicity, and age was measured using U.S. Census data published on the Henry J. Kaiser Family Foundation’s website (2014d). The percentage of the state’s population who were African American and the percentage who were Hispanic/ Latino in 2011-2012 were measured. Age was measured by the percentage of the state’s population who were 18 years old or younger and the percentage who were 65 years or older in 2011-2012 (Henry J. Kaiser Foundation, 2014c).
The rurality of states, defined by the distribution of the population relative to geographic areas, was characterized by two measures. Population density was defined as the number of residents per square mile of land area (U.S. Census Bureau, 2013). Because the extraordinary density of the District of the Columbia skews this measure, population density was transformed by the natural log function. The second measure was the percentage of the state’s population who live in rural areas (U.S. Census Bureau, 2015a).
Health-related resources: Overall physician supply, insurance coverage, and behavioral health.
The number of nonwaivered physicians per 100,000 residents was measured using data from the December 2013 CSA database. After the database was restricted to nonmilitary practitioners, queries were conducted in the “Names” field to yield counts of the number of physicians who were credentialed with M.D. and D.O. degrees. Buprenorphine-waivered physicians were excluded from this measure. Each state count was then divided by state population and multiplied by 100,000 to yield the measure of overall physician supply.
Insurance coverage was measured using information published by the Henry J. Kaiser Family Foundation (2014b) that drew upon the U.S. Census Bureau’s Current Population Survey from 2012 to 2013. The two measures of insurance coverage were the percentage of the state population who were uninsured and the percentage of the total population covered by Medicaid.
The state context for behavioral health was measured by three indicators: (a) availability of OTPs offering methadone maintenance, (b) availability of non-OTP SUD treatment programs, and (c) state mental health agency expenditures for services. The number of OTPs in each state was counted in June 2013 based on queries of SAMHSA’s Treatment Locator (SAMHSA, 2013b) using methadone maintenance as “required” within the “Services Provided” field. Similar to the measure of OTPs, the number of facilities offering SUD treatment was counted by selecting “substance abuse treatment” as required in the “Services Provided” field. The raw counts of OTPs and SUD treatment facilities were transformed into the numbers of facilities per 100,000 state residents. State support for mental health was measured by per capita mental health service expenditures by the state mental health agency for fiscal year 2010 (Henry J. Kaiser Foundation, 2013a).
Demand for opioid treatment.
Four state-level variables addressed dimensions of opioid use within the state. First, the percentage of state residents (age 12 years and older) reporting any past-year nonmedical pain reliever drug use in 2011 was measured using data from the National Survey of Drug Use and Health (SAMHSA, 2013a). Two measures of opioid prescriptions were considered: the overall rate of opioid pain relievers prescribed and a measure of the summed rates of high-dose opioid pain relievers and extended-release opioid pain relievers prescribed per 100 persons in 2012 based on data captured by IMS Health’s National Prescription Audit database (Paulozzi et al., 2014). Finally, the rate of opioid overdose deaths per 100,000 residents in 2013 was measured using data from the CDC’s WONDER database (2015). Similar to the methodology described by Bachhuber et al. (2014), this measure included overdose deaths (International Statistical Classification of Diseases, 10th revision [World Health Organization, 1992], codes X40-X44, X60-X64, and Y10-Y14) where heroin and other opioids were coded (T40.0-T40.4).
Geographic region.
Using codes from the U.S. Census (2015b), states were categorized into four regions: Northeast (CT, MA, ME, NH, NJ, NY, PA, RI, and VT), Midwest (IA, IN, IL, KS, MI, MN, MO, NE, ND, OH, SD, and WI), South (AL, AR, DE, DC, FL, GA, KY, LA, MD, MS, NC, OK, SC, TN, TX, VA, and WV), and West (AK, AZ, CA, CO, HI, ID, MT, NM, NV, OR, UT, WA, and WY). States in the Northeast served as the reference category.
Analysis
Descriptive statistics were calculated for all study variables. A series of ordinary least squares regression models of buprenorphine physician supply were estimated. Initial unadjusted models examined each variable. All variables associated with the dependent variable at p < .05 (two-tailed) in the initial models were entered into the final multivariate model. All analyses were conducted in Stata version 13.1 (StataCorp LP, College Station, TX).
Results
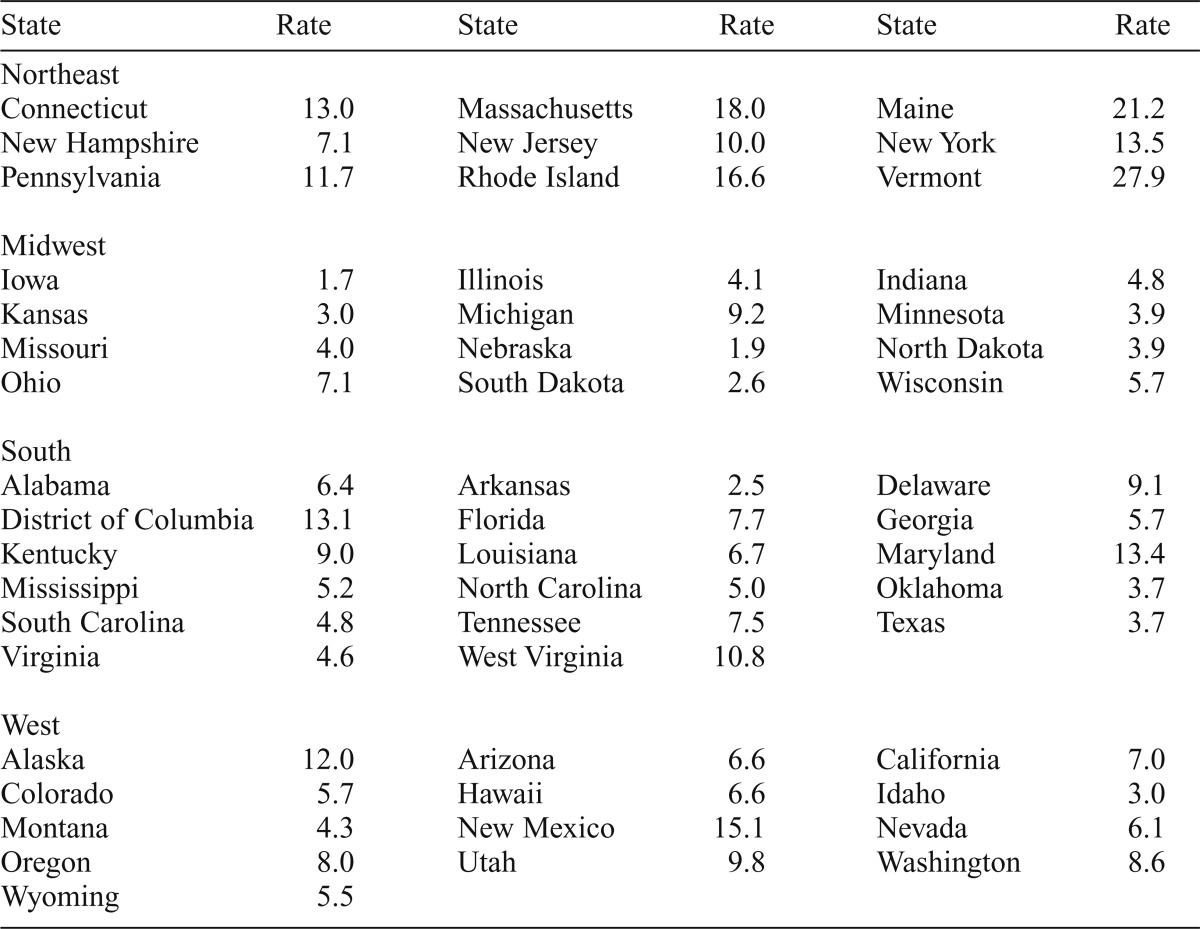
In December 2013, 23,629 physicians in the United States held the waiver to prescribe buprenorphine. Of these, 71.0% were waivered to treat only 30 patients, and 29.0% were waivered to treat up to 100 patients at any one time. Table 1 presents buprenorphine physician supply per 100,000 residents in each state and the District of Columbia. There was notable variation between states in the supply of waivered physicians. Iowa had the smallest supply of waivered physicians, whereas Vermont had the greatest supply.
Table 1.
Buprenorphine-waivered physicians per 100,000 residents by state in December 2013
| State | Rate | State | Rate | State | Rate |
| Northeast | |||||
| Connecticut | 13.0 | Massachusetts | 18.0 | Maine | 21.2 |
| New Hampshire | 7.1 | New Jersey | 10.0 | New York | 13.5 |
| Pennsylvania | 11.7 | Rhode Island | 16.6 | Vermont | 27.9 |
| Midwest | |||||
| Iowa | 1.7 | Illinois | 4.1 | Indiana | 4.8 |
| Kansas | 3.0 | Michigan | 9.2 | Minnesota | 3.9 |
| Missouri | 4.0 | Nebraska | 1.9 | North Dakota | 3.9 |
| Ohio | 7.1 | South Dakota | 2.6 | Wisconsin | 5.7 |
| South | |||||
| Alabama | 6.4 | Arkansas | 2.5 | Delaware | 9.1 |
| District of Columbia | 13.1 | Florida | 7.7 | Georgia | 5.7 |
| Kentucky | 9.0 | Louisiana | 6.7 | Maryland | 13.4 |
| Mississippi | 5.2 | North Carolina | 5.0 | Oklahoma | 3.7 |
| South Carolina | 4.8 | Tennessee | 7.5 | Texas | 3.7 |
| Virginia | 4.6 | West Virginia | 10.8 | ||
| West | |||||
| Alaska | 12.0 | Arizona | 6.6 | California | 7.0 |
| Colorado | 5.7 | Hawaii | 6.6 | Idaho | 3.0 |
| Montana | 4.3 | New Mexico | 15.1 | Nevada | 6.1 |
| Oregon | 8.0 | Utah | 9.8 | Washington | 8.6 |
| Wyoming | 5.5 | ||||
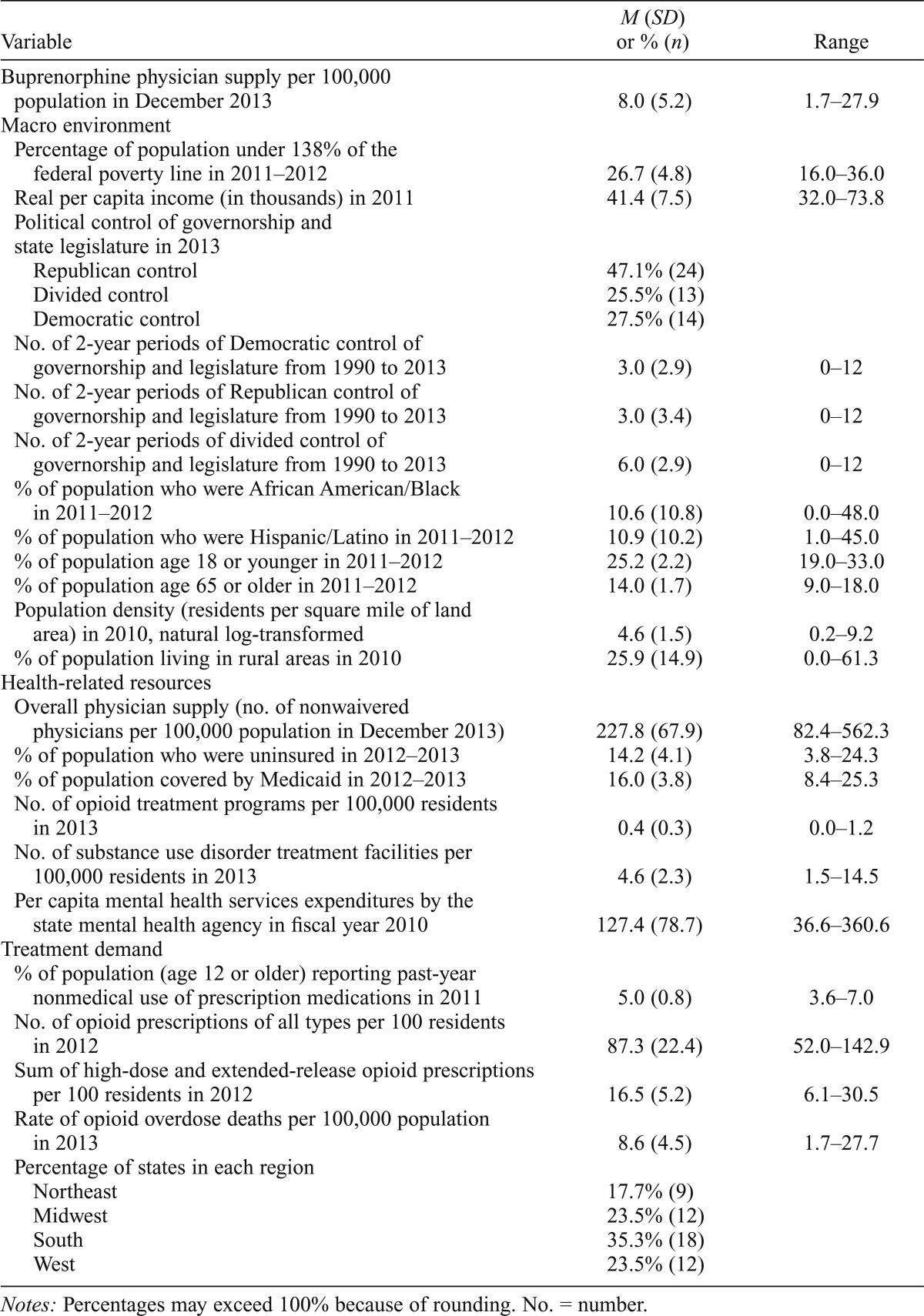
Descriptive statistics for the study variables are presented in Table 2. In the average state, about 463.3 physicians were waivered to prescribe buprenorphine. However, the standard deviation (SD = 574.8), which exceeded the mean, pointed to the extreme variability between states when the count was not adjusted for population. To some degree, this variability was reduced once the number of waivered physicians was converted into the measure of buprenorphine physician supply, or number of waivered physicians per 100,000 residents (M = 8.0, SD = 5.2).
Table 2.
Descriptive statistics of state-level measures of buprenorphine physician supply and other state characteristics
| Variable | M (SD) or % (n) | Range |
| Buprenorphine physician supply per 100,000 population in December 2013 | 8.0 (5.2) | 1.7-27.9 |
| Macro environment | ||
| Percentage of population under 138% of the federal poverty line in 2011-2012 | 26.7 (4.8) | 16.0-36.0 |
| Real per capita income (in thousands) in 2011 | 41.4 (7.5) | 32.0-73.8 |
| Political control of governorship and state legislature in 2013 | ||
| Republican control | 47.1% (24) | |
| Divided control | 25.5% (13) | |
| Democratic control | 27.5% (14) | |
| No. of 2-year periods of Democratic control of governorship and legislature from 1990 to 2013 | 3.0 (2.9) | 0-12 |
| No. of 2-year periods of Republican control of governorship and legislature from 1990 to 2013 | 3.0 (3.4) | 0-12 |
| No. of 2-year periods of divided control of governorship and legislature from 1990 to 2013 | 6.0 (2.9) | 0-12 |
| % of population who were African American/Black in 2011-2012 | 10.6 (10.8) | 0.0-48.0 |
| % of population who were Hispanic/Latino in 2011-2012 | 10.9 (10.2) | 1.0-45.0 |
| % of population age 18 or younger in 2011-2012 | 25.2 (2.2) | 19.0-33.0 |
| % of population age 65 or older in 2011-2012 | 14.0 (1.7) | 9.0-18.0 |
| Population density (residents per square mile of land area) in 2010, natural log-transformed | 4.6 (1.5) | 0.2-9.2 |
| % of population living in rural areas in 2010 | 25.9 (14.9) | 0.0-61.3 |
| Health-related resources | ||
| Overall physician supply (no. of nonwaivered physicians per 100,000 population in December 2013) | 227.8 (67.9) | 82.4-562.3 |
| % of population who were uninsured in 2012-2013 | 14.2 (4.1) | 3.8-24.3 |
| % of population covered by Medicaid in 2012-2013 | 16.0 (3.8) | 8.4-25.3 |
| No. of opioid treatment programs per 100,000 residents in 2013 | 0.4 (0.3) | 0.0-1.2 |
| No. of substance use disorder treatment facilities per 100,000 residents in 2013 | 4.6 (2.3) | 1.5-14.5 |
| Per capita mental health services expenditures by the state mental health agency in fiscal year 2010 | 127.4 (78.7) | 36.6-360.6 |
| Treatment demand | ||
| % of population (age 12 or older) reporting past-year nonmedical use of prescription medications in 2011 | 5.0 (0.8) | 3.6-7.0 |
| No. of opioid prescriptions of all types per 100 residents in 2012 | 87.3 (22.4) | 52.0-142.9 |
| Sum of high-dose and extended-release opioid prescriptions per 100 residents in 2012 | 16.5 (5.2) | 6.1-30.5 |
| Rate of opioid overdose deaths per 100,000 population in 2013 | 8.6 (4.5) | 1.7-27.7 |
| Percentage of states in each region | ||
| Northeast | 17.7% (9) | |
| Midwest | 23.5% (12) | |
| South | 35.3% (18) | |
| West | 23.5% (12) |
Notes: Percentages may exceed 100% because of rounding. No. = number.
An analysis of variance with the Bonferroni correction of buprenorphine physician supply by region indicated significant differences, F(3, 47) = 16.31, p < .001. Of note, the Northeast (M = 15.5, SD = 6.3) had a significantly greater supply than the Midwest (M = 4.3, SD = 2.2), South (M = 6.9, SD = 3.1), and West (M = 7.7, SD = 3.3, all ps < .001). Pairwise differences between the Midwest, South, and West were not statistically significant. Given that Vermont had such a large supply, an additional analysis was conducted with Vermont excluded (not shown); the regional differences between the Northeast and the other three regions remained statistically significant, suggesting that Vermont did not unduly influence these regional differences.
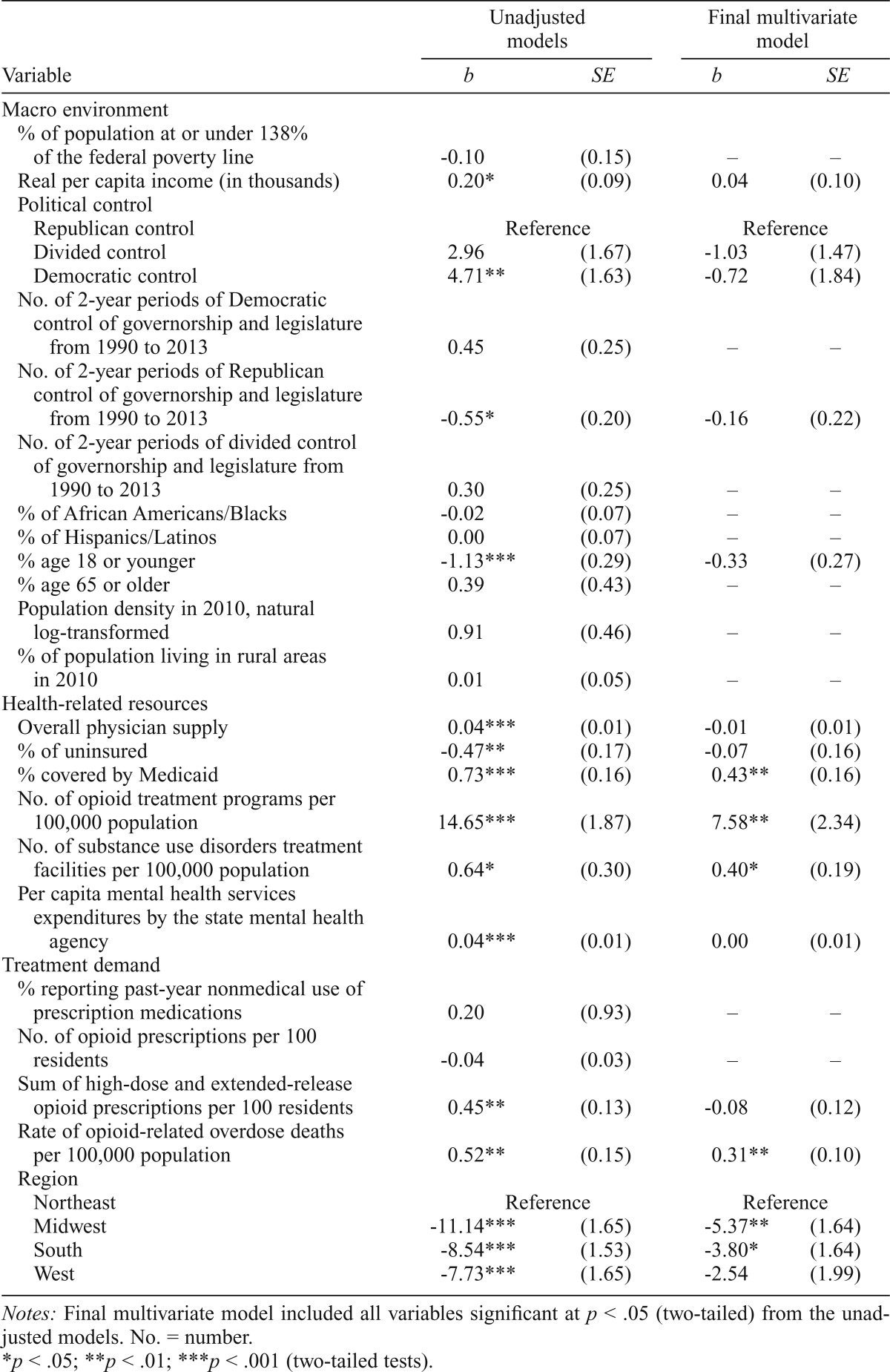
The first column of Table 3 presents results from a series of unadjusted ordinary least squares regression models of buprenorphine physician supply. There was modest support for the hypothesis that the macro environment was associated with buprenorphine physician supply. Real per capita income was positively associated with buprenorphine physician supply (standardized coefficient, β = .29). Of the demographic measures, the only significant measure was the percentage of the population who were younger than 18 years (β = -.49). Buprenorphine physician supply was greater in states under Democratic control in 2013 relative to states under Republican control (β = .41), but the difference between states with split political control and states under Republican control was not statistically significant. In addition, the association between the duration of Republican control and buprenorphine physician supply was negative (β = -.36).
Table 3.
Ordinary least squares regression models of state-level buprenorphine physician supply in December 2013
| Variable | Unadjusted models |
Final multivariate model |
||
| b | SE | b | SE | |
| Macro environment | ||||
| % of population at or under 138% of the federal poverty line | -0.10 | (0.15) | – | – |
| Real per capita income (in thousands) | 0.20* | (0.09) | 0.04 | (0.10) |
| Political control | ||||
| Republican control | Reference |
Reference |
||
| Divided control | 2.96 | (1.67) | -1.03 | (1.47) |
| Democratic control | 4.71** | (1.63) | -0.72 | (1.84) |
| No. of 2-year periods of Democratic control of governorship and legislature from 1990 to 2013 | 0.45 | (0.25) | – | – |
| No. of 2-year periods of Republican control of governorship and legislature from 1990 to 2013 | -0.55* | (0.20) | -0.16 | (0.22) |
| No. of 2-year periods of divided control of governorship and legislature from 1990 to 2013 | 0.30 | (0.25) | – | – |
| % of African Americans/Blacks | -0.02 | (0.07) | – | – |
| % of Hispanics/Latinos | 0.00 | (0.07) | – | – |
| % age 18 or younger | -1.13*** | (0.29) | -0.33 | (0.27) |
| % age 65 or older | 0.39 | (0.43) | – | – |
| Population density in 2010, natural log-transformed | 0.91 | (0.46) | – | – |
| % of population living in rural areas in 2010 | 0.01 | (0.05) | – | – |
| Health-related resources Overall physician supply | 0.04*** | (0.01) | -0.01 | (0.01) |
| % of uninsured | -0.47** | (0.17) | -0.07 | (0.16) |
| % covered by Medicaid | 0.73*** | (0.16) | 0.43** | (0.16) |
| No. of opioid treatment programs per 100,000 population | 14.65*** | (1.87) | 7.58** | (2.34) |
| No. of substance use disorders treatment facilities per 100,000 population | 0.64* | (0.30) | 0.40* | (0.19) |
| Per capita mental health services expenditures by the state mental health agency | 0.04*** | (0.01) | 0.00 | (0.01) |
| Treatment demand | ||||
| % reporting past-year nonmedical use of prescription medications | 0.20 | (0.93) | – | – |
| No. of opioid prescriptions per 100 residents | -0.04 | (0.03) | – | – |
| Sum of high-dose and extended-release opioid prescriptions per 100 residents | 0.45** | (0.13) | -0.08 | (0.12) |
| Rate of opioid-related overdose deaths per 100,000 population | 0.52** | (0.15) | 0.31** | (0.10) |
| Region | ||||
| Northeast | Reference |
Reference |
||
| Midwest | -11.14*** | (1.65) | -5.37** | (1.64) |
| South | -8.54*** | (1.53) | -3.80* | (1.64) |
| West | -7.73*** | (1.65) | -2.54 | (1.99) |
Notes: Final multivariate model included all variables significant at p < .05 (two-tailed) from the unadjusted models. No. = number.
p < .05;
p < .01;
p < .001 (two-tailed tests).
Initial unadjusted models examining health-related resources revealed that all six variables were significantly associated with buprenorphine physician supply at the bivariate level. There was a strong positive correlation between overall physician supply and buprenorphine physician supply (β = .53). States with greater percentages of uninsured persons had lower rates of buprenorphine supply (β = -.37), whereas the association for Medicaid-covered persons was positive (β = .54). There were positive associations for the supply of OTPs (β = .75), the supply of SUD treatment programs (β = .29), and state spending on mental health services (β = .54) in these unadjusted models.
Of the four indicators of treatment demand, two variables were statistically significant in the unadjusted models. The state-level rate of overdose deaths was positively associated with buprenorphine physician supply (β = .46). There was also a positive association between the rate of high-dose and extended-release opioid pain reliever prescriptions and buprenorphine physician supply (β = .45). Buprenorphine physician supply was not associated with the percentage of the state population reporting past-year nonmedical prescription drug use or the rate for all opioid prescriptions.
In addition, there were large differences in buprenorphine physician supply between the Northeast and the other three regions at the bivariate level. States in the Midwest (β = -.92), South (β = -.80), and West (β = -.64) had significantly smaller supplies of waivered physicians than states in the Northeast.
The final model in Table 3 included all variables that were significant at p < .05 in the unadjusted models. The percentage of the population covered by Medicaid was positively associated with the rate of waivered physicians (β = .32). The supply of OTPs (β = .39) and SUD treatment facilities (β = .18) were positively associated with buprenorphine physician supply. Finally, states with higher rates of opioid overdose deaths in 2013 had greater supplies of waivered buprenorphine physicians (β = .27). Regional differences between the Northeast and Midwest (β = -.44) and South (β = -.35) remained statistically significant. This final model explained about 77.7% of the variance in buprenorphine physician supply (adjusted R2 = .777).
Discussion
Buprenorphine has been approved for the treatment of OUD for more than a decade, but the supply of waivered physicians in U.S. states has been minimally studied. This analysis from late 2013 revealed that buprenorphine physician supply varies considerably between states, with much of this variance attributable to regional differences between the Northeast and other parts of the country. Four additional state characteristics—the proportion of the population covered by Medicaid, the supply of OTPs, the supply of SUD treatment programs, and the rate of overdose deaths—were associated with buprenorphine physician supply in the final multivariate model.
The large regional differences in buprenorphine physician supply have substantial implications for treatment access. Northeastern states have a much greater supply of waivered physicians than other regions, which may increase access for individuals in those states. There is also a significantly greater supply of OTPs for states in the Northeast relative to the other three regions; such programs predominantly offer methadone maintenance, which is another effective method of treating OUD. Furthermore, northeastern states are smaller in geographic terms, which means that the supply of waivered physicians is concentrated in a much smaller geographic area. This geographic concentration may be advantageous for patients in the Northeast in that barriers posed by geographic distance and transportation may be less substantial than in other parts of the country. The differences between the Northeast and the other three regions were somewhat reduced by the addition of other state characteristics, but still considerable. In contrast to the two studies of county-level supply (Rosenblatt et al., 2015; Stein et al., 2015), this state-level analysis did not find significant differences by population density or the percentage of residents living in rural areas. It may be that county-level differences between rural and urban areas do not extrapolate to macrolevel differences between states.
Regarding the hypotheses about state characteristics, the final multivariate model provided the greatest support for indicators of health-related resources. In addition to the environmental richness indicated by the supplies of OTPs and other SUD treatment programs, Medicaid coverage was positively associated with buprenorphine physician supply. It may seem counterintuitive that Medicaid coverage is viewed as an indicator of environmental richness, given its role as a safety net for low-income individuals. However, Medicaid is an increasingly significant funder of treatment services for SUDs (Buck, 2011; Mark et al., 2011), and by 2013, all state Medicaid programs included at least some coverage for buprenorphine treatment (Rinaldo & Rinaldo, 2013).
The finding about Medicaid coverage may have important implications in the current era of health reform. A major element of the Affordable Care Act is the expansion of Medicaid to cover more individuals, particularly childless adults, who historically have had limited access to Medicaid coverage (Hill et al., 2014; Price & Eibner, 2013). The Supreme Court ruled in 2012 in the National Federation of Independent Business v. Sebelius case that states can decline to expand Medicaid. In 2013, about half of U.S. states were implementing the Medicaid expansion, and these states are receiving additional federal funding to cover the expanded Medicaid population. By the end of 2014, 10 Republican governors, 7 of whom represented states that supported the Supreme Court challenge to the Affordable Care Act, had announced plans to expand Medicaid, although those plans still required federal and state legislative approval (Altman, 2014). An important direction for future research is examination of whether state-level decisions to expand Medicaid attract more physicians into providing buprenorphine treatment. The combination of a larger pool of insured individuals, coupled with the Affordable Care Act’s greater parity for SUD treatment and the inclusion of SUD treatment as an essential health benefit in insurance plans (Garfield & Druss, 2012; Pating et al., 2012), should provide the economic impetus for more physicians to provide buprenorphine treatment. Monitoring whether the supply of buprenorphine physicians changes over time, particularly in expansion states, is an important direction for future research.
Although the supply of OTPs was conceptualized as representing munificence of health-related resources, an alternative explanation may be drawn from institutional theory (DiMaggio & Powell, 1991). A greater density of OTPs delivering methadone maintenance services indicates greater normative support for the medicalization of SUDs within the state. At the same time, some OTPs rely heavily on governmental funding (Ducharme & Roman, 2009), which also supports viewing them as indicators of environmental richness. Also of note was that the supply of other SUD treatment facilities was also positively associated with buprenorphine physician supply. Although the slow diffusion of buprenorphine and other medications within this sector of the treatment system would seemingly suggest resistance to medicalization, the positive correlation suggests that more treatment-rich states may be better positioned to attract physicians into applying for the buprenorphine waiver. It should be noted that the CSA database does not provide information about practice setting (i.e., office, OTP, or SUD treatment program), so it cannot speak to the distribution of physicians across these settings. However, surveys of waivered physicians indicate that the majority are delivering buprenorphine in office-based settings (Arfken et al., 2010). In addition, some of the state-level variation in buprenorphine supply may reflect contagion effects where physicians begin delivering buprenorphine treatment because other local physicians are offering this service. Variations in professional norms that are rooted in differences in medical school curricula about SUDs may also explain additional variation in physician supply. Certainly, these interpretations are not mutually exclusive, and more research is needed to test these various explanations.
Less support was found for demand indicators, and no support was found for the measures of the macro environment in the final model. Notably, the rate of opioid overdoses was positively associated with waivered physician supply in the final model. Overdose deaths are perhaps a stronger measure of the prevalence of OUD than the measures of opioid medications prescribed and rates of nonmedical use. One possible interpretation of this finding is that physicians may have been responding to the size of the opioid epidemic in their state, such that greater overdose rates may prompt some physicians to seek the buprenorphine waiver to address this substantial public health problem. However, the analyses cannot establish causality, in part because the CSA database does not track the year in which physicians obtained their waivers.
There are numerous limitations to this research. First, the measures of other state characteristics were drawn from secondary sources that reflect data from varying years. Although the most recent data were sought, it is unknown whether state characteristics have changed in the intervening years and, if so, what impact those changes may have on these results. Prior research on the supply of other types of medical professionals informed the selection of state characteristics, but other variables may explain variance in buprenorphine physician supply. For example, because state-level rates have not been published, the study was unable to examine whether the prevalence of heroin use disorder was associated with buprenorphine physician supply. State policy related to Medicaid reimbursement for buprenorphine services is also likely to be associated with physician supply. The county-level analyses by Stein and colleagues (2015) demonstrated that state policies, at least in earlier years, were correlated with buprenorphine physician supply. Although all state Medicaid programs now provide some coverage for buprenorphine treatment (Rinaldo & Rinaldo, 2013), the complexities of state Medicaid policies regarding prior authorization, treatment duration, and so forth are another direction for future research. There are also inherent limitations in statistical power that come from the relatively small sample size of state-level analyses. Finally, physician supply is an imperfect measure of treatment access because not all physicians who are waivered actually treat patients with OUD (Arfken et al., 2010).
The U.S. approach to buprenorphine differs from that of other countries in notable ways. For example, France and England have no special training requirements related to buprenorphine and allow any physician to prescribe it (Auriacombe et al., 2004; Fatséas & Auriacombe, 2007; Strang et al., 2007). Italy has focused on the delivery of buprenorphine in specialty treatment centers, whereas Germany relies on a mix of primary care and specialty centers coupled with a high degree of patient supervision and limited take-home doses (Carrieri et al., 2006). In Australia, community pharmacists play a central role in delivering buprenorphine treatment (Nielsen et al., 2007). These differences, as well as the absence of a single-payer system in the United States, likely mean that the present findings cannot generalize to other contexts.
This examination of the supply of physicians waivered to prescribed buprenorphine in the United States suggests patterns of health care inequality based on regional variations. Furthermore, state-level indicators of health-related resources were associated with buprenorphine physician supply. Future research should continue to consider alternative explanations for variability in the physician supply between states. The implementation of the Affordable Care Act offers a unique opportunity to examine whether state differences in Affordable Care Act implementation, particularly with regard to state policies on expanding Medicaid, have an impact on the supply of buprenorphine-waivered physicians across the United States.
Footnotes
This study was supported by National Institute on Drug Abuse (NIDA) Grant R33DA035641. NIDA had no further role in study design; in the collection, analysis, or interpretation of data; or in the writing of this article. The content is solely the responsibility of the author and does not represent the official views of the National Institutes of Health or NIDA.
References
- Abraham A. J., Knudsen H. K., Rieckmann T., Roman P. M. Disparities in access to physicians and medications for the treatment of substance use disorders between publicly and privately funded treatment programs in the United States. Journal of Studies on Alcohol and Drugs. 2013;74:258–265. doi: 10.15288/jsad.2013.74.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman D. Medicaid expansion in red states. The Wall Street Journal. 2014, December 18 Retrieved from http://blogs.wsj.com/washwire/2014/12/18/medicaid-expansion-in-red-states. [Google Scholar]
- Arfken C. L., Johanson C. E., di Menza S., Schuster C. R. Expanding treatment capacity for opioid dependence with office-based treatment with buprenorphine: National surveys of physicians. Journal of Substance Abuse Treatment. 2010;39:96–104. doi: 10.1016/j.jsat.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Aten B. H., Figueroa E. B., Martin T. M. Real personal income and regional price parities for states and metropolitan areas, 2007–2011. Survey of Current Business. 2013;93:89–103. [Google Scholar]
- Auriacombe M., Fatséas M., Dubernet J., Daulouède J. P., Tignol J. French field experience with buprenorphine. American Journal on Addictions, 13, Supplement. 2004:S17–S28. doi: 10.1080/10550490490440780. [DOI] [PubMed] [Google Scholar]
- Bachhuber M. A., Saloner B., Cunningham C. O., Barry C. L. Medical cannabis laws and opioid analgesic overdose mortality in the United States, 1999–2010. JAMA Internal Medicine. 2014;174:1668–1673. doi: 10.1001/jamainternmed.2014.4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baicker K., Chandra A., Skinner J. S. Geographic variation in health care and the problem of measuring racial disparities. Perspectives in Biology and Medicine, 48, Supplement. 2005:S42–S53. [PubMed] [Google Scholar]
- Bickel W. K., Amass L. Buprenorphine treatment for opioid dependence: A review. Experimental and Clinical Psychopharmacology. 1995;3:477–489. [Google Scholar]
- Bigbee J. L. Relationships between nurse- and physician-to-population ratios and state health rankings. Public Health Nursing. 2008;25:244–252. doi: 10.1111/j.1525-1446.2008.00701.x. [DOI] [PubMed] [Google Scholar]
- Brigham G. S., Amass L., Winhusen T., Harrer J. M., Pelt A. Using buprenorphine short-term taper to facilitate early treatment engagement. Journal of Substance Abuse Treatment. 2007;32:349–356. doi: 10.1016/j.jsat.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Buck J. A. The looming expansion and transformation of public substance abuse treatment under the Affordable Care Act. Health Affairs. 2011;30:1402–1410. doi: 10.1377/hlthaff.2011.0480. [DOI] [PubMed] [Google Scholar]
- Carrieri M. P., Amass L., Lucas G. M., Vlahov D., Wodak A., Woody G. E. Buprenorphine use: the international experience. Clinical Infectious Diseases, 43, Supplement. 2006:S197–S215. doi: 10.1086/508184. [DOI] [PubMed] [Google Scholar]
- Castrogiovanni G. J. Environmental munificence: A theoretical assessment. Academy of Management Review. 1991;16:542–565. [Google Scholar]
- Center for Substance Abuse Treatment. Clinical guidelines for the use of buprenorphine in the treatment of opioid addiction (Treatment Improvement Protocol #40) Rockville, MD: Substance Abuse and Mental Health Services Administration; 2004. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Vital signs: Overdoses of prescription opioid pain relievers—United States, 1999–2008. Morbidity and Mortality Weekly Report. 2011;60:1487–1492. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Multiple cause of death 1999–2013. 2015. CDC WONDER online database. Retrieved from http://wonder.cdc.gov/mcd-icd10.html. [Google Scholar]
- Chadderton A. Clinical issues in using buprenorphine in the treatment of opiate dependence. Drug and Alcohol Review. 2000;19:329–335. [Google Scholar]
- Continelli T., McGinnis S., Holmes T. The effect of local primary care physician supply on the utilization of preventive health services in the United States. Health & Place. 2010;16:942–951. doi: 10.1016/j.healthplace.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Cooper R. A. Regional variation and the affluence-poverty nexus. JAMA. 2009a;302:1113–1114. doi: 10.1001/jama.2009.1222. [DOI] [PubMed] [Google Scholar]
- Cooper R. A. States with more physicians have better-quality health care. Health Affairs. 2009b;28:w91–w102. doi: 10.1377/hlthaff.28.1.w91. [DOI] [PubMed] [Google Scholar]
- Dawson R. E., Robinson J. A. Inter-party competition, economic variables, and welfare policies in the American states. Journal of Politics. 1963;25:265–289. [Google Scholar]
- Declercq E. R., Paine L. L., Simmes D. R., DeJoseph J. F. State regulation, payment policies, and nurse-midwife services. Health Affairs. 1998;17:190–200. doi: 10.1377/hlthaff.17.2.190. [DOI] [PubMed] [Google Scholar]
- DiMaggio P J., Powell W. W. The iron cage revisited: Institutional isomorphism and collective rationality in organizational fields. In: Powell W. W., DiMaggio P J., editors. The new institutionalism in organizational analysis. Chicago, IL: University of Chicago Press; 1991. pp. 63–82. [Google Scholar]
- Ducharme L. J., Abraham A. J. State policy influence on the early diffusion of buprenorphine in community treatment programs. Substance Abuse Treatment, Prevention, and Policy. 2008;3:17. doi: 10.1186/1747-597X-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducharme L. J., Knudsen H. K., Roman P M., Johnson J. A. Innovation adoption in substance abuse treatment: Exposure, trialability, and the Clinical Trials Network. Journal of Substance Abuse Treatment. 2007;32:321–329. doi: 10.1016/j.jsat.2006.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducharme L. J., Roman P. M. Opioid treatment programs in the Clinical Trials Network: Representativeness and buprenorphine adoption. Journal of Substance Abuse Treatment. 2009;37:90–94. doi: 10.1016/j.jsat.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott K., Balz D. Party control by state. The Washington Post. 2013, December 28 Retrieved from http://www.washingtonpost.com/wp-srv/special/national/red-blue. [Google Scholar]
- Fatséas M., Auriacombe M. Why buprenorphine is so successful in treating opiate addiction in France. Current Psychiatry Reports. 2007;9:358–364. doi: 10.1007/s11920-007-0046-2. [DOI] [PubMed] [Google Scholar]
- Fiellin D. A., Moore B. A., Sullivan L. E., Becker W. C., Pantalon M. V., Chawarski M. C., Schottenfeld R. S. Long-term treatment with buprenorphine/naloxone in primary care: Results at 2–5 years. American Journal on Addictions. 2008;17:116–120. doi: 10.1080/10550490701860971. [DOI] [PubMed] [Google Scholar]
- Friedmann P. D., Jiang L., Alexander J. A. Top manager effects on buprenorphine adoption in outpatient substance abuse treatment programs. Journal of Behavioral Health Services & Research. 2010;37:322–337. doi: 10.1007/s11414-009-9169-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fudala P J., Bridge T. P., Herbert S., Williford W. O., Chiang C. N., Jones K., Tusel D. the Buprenorphine/Naloxone Collaborative Study Group. Office-based treatment of opiate addiction with a sublingual-tablet formulation of buprenorphine and naloxone. The New England Journal of Medicine. 2003;349:949–958. doi: 10.1056/NEJMoa022164. [DOI] [PubMed] [Google Scholar]
- Garfield R. L., Druss B. G. Health reform, health insurance, and mental health care. American Journal of Psychiatry. 2012;169:675–677. doi: 10.1176/appi.ajp.2012.12040506. [DOI] [PubMed] [Google Scholar]
- Gordon A. J., Trafton J. A., Saxon A. J., Gifford A. L., Goodman F., Calabrese V. S., Liberto J. the Buprenorphine Work Group of the Substance Use Disorders Quality Enhancement Research Initiative. Implementation of buprenorphine in the Veterans Health Administration: results of the first 3 years. Drug and Alcohol Dependence. 2007;90:292–296. doi: 10.1016/j.drugalcdep.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Henry J. Kaiser Foundation. State mental health agency (SMHA), per capita mental health services expenditures. 2013a Retrieved from https://web.archive.org/web/20130921134959/ http://kff.org/other/state-indicator/smha-expenditures-per-capita.
- Henry J. Kaiser Foundation. State political parties. 2013b. Retrieved from https://web.archive.org/web/20130511115506/ http://kff.org/other/state-indicator/state-political-parties. [Google Scholar]
- Henry J. Kaiser Foundation. Distribution of total population by federal poverty level. 2014a Retrieved from https://web.archive.org/web/20140516085839/ http://kff.org/other/state-indicator/distribution-by-fpl">http://kff.org/other/state-indicator/distribution-by-fpl.
- Henry J. Kaiser Foundation. Health insurance coverage of the total population. 2014b Retrieved from https://web.archive.org/web/20140712184316/ http://kff.org/other/state-indicator/total-population.
- Henry J. Kaiser Foundation. Population distribution by age. 2014c Retrieved from https://web.archive.org/web/20140727105245/ http://kff.org/other/state-indicator/distribution-by-age.
- Henry J. Kaiser Foundation. Population distribution by race/ethnicity. 2014d Retrieved from https://web.archive.org/web/20140906092720/ http://kff.org/other/state-indicator/distribution-by-raceethnicity.
- Hill S. C., Abdus S., Hudson J. L., Selden T. M. Adults in the income range for the Affordable Care Act’s Medicaid expansion are healthier than pre-ACA enrollees. Health Affairs. 2014;33:691–699. doi: 10.1377/hlthaff.2013.0743. [DOI] [PubMed] [Google Scholar]
- Jaffe J. H., O’Keeffe C. From morphine clinics to buprenorphine: Regulating opioid agonist treatment of addiction in the United States. Drug and Alcohol Dependence, 70, Supplement. 2003:S3–S11. doi: 10.1016/s0376-8716(03)00055-3. [DOI] [PubMed] [Google Scholar]
- Kissin W., McLeod C., Sonnefeld J., Stanton A. Experiences of a national sample of qualified addiction specialists who have and have not prescribed buprenorphine for opioid dependence. Journal of Addictive Diseases. 2006;25:91–103. doi: 10.1300/J069v25n04_09. [DOI] [PubMed] [Google Scholar]
- Knudsen H. K., Abraham A. J., Johnson J. A., Roman P. M. Buprenorphine adoption in the National Drug Abuse Treatment Clinical Trials Network. Journal of Substance Abuse Treatment. 2009;37:307–312. doi: 10.1016/j.jsat.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen H. K., Ducharme L. J., Roman P. M. Early adoption of buprenorphine in substance abuse treatment centers: Data from the private and public sectors. Journal of Substance Abuse Treatment. 2006;30:363–373. doi: 10.1016/j.jsat.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Knudsen H. K., Oser C. B., Abraham A. J., Roman P. M. Physicians in the substance abuse treatment workforce: Understanding their employment within publicly funded treatment organizations. Journal of Substance Abuse Treatment. 2012;43:152–160. doi: 10.1016/j.jsat.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch A. L., Arfken C. L., Schuster C. R. Characteristics of U.S. substance abuse treatment facilities adopting buprenorphine in its initial stage of availability. Drug and Alcohol Dependence. 2006;83:274–278. doi: 10.1016/j.drugalcdep.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Koh H. K., Oppenheimer S. C., Massin-Short S. B., Emmons K. M., Geller A. C., Viswanath K. Translating research evidence into practice to reduce health disparities: A social determinants approach. American Journal of Public Health, 100, Supplement 1. 2010:S72–S80. doi: 10.2105/AJPH.2009.167353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovas A. E., McFarland B. H., McCarty D. J., Boverman J. F, Thayer J. A. Buprenorphine for acute heroin detoxification: Diffusion of research into practice. Journal of Substance Abuse Treatment. 2007;32:199–206. doi: 10.1016/j.jsat.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Lackan N. A., Eschbach K., Stimpson J. P., Freeman J. L., Goodwin J. S. Ethnic differences in in-hospital place of death among older adults in California: Effects of individual and contextual characteristics and medical resource supply. Medical Care. 2009;47:138–145. doi: 10.1097/MLR.0b013e3181844dba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis D. C. Access to narcotic addiction treatment and medical care: Prospects for the expansion of methadone maintenance treatment. Journal of Addictive Diseases. 1999;18:5–21. doi: 10.1300/J069v18n02_02. [DOI] [PubMed] [Google Scholar]
- Ling W., Smith D. Buprenorphine: Blending practice and research. Journal of Substance Abuse Treatment. 2002;23:87–92. doi: 10.1016/s0740-5472(02)00257-x. [DOI] [PubMed] [Google Scholar]
- Ling W., Wesson D. R. Clinical efficacy of buprenorphine: Comparisons to methadone and placebo. Drug and Alcohol Dependence, 70, Supplement. 2003:S49–S57. doi: 10.1016/s0376-8716(03)00059-0. [DOI] [PubMed] [Google Scholar]
- Macinko J., Starfield B., Shi L. Quantifying the health benefits of primary care physician supply in the United States. International Journal of Health Services. 2007;37:111–126. doi: 10.2190/3431-G6T7-37M8-P224. [DOI] [PubMed] [Google Scholar]
- Mark T. L., Levit K. R., Vandivort-Warren R., Buck J. A., Coffey R. M. Changes in US spending on mental health and substance abuse treatment, 1986–2005, and implications for policy. Health Affairs. 2011;30:284–292. doi: 10.1377/hlthaff.2010.0765. [DOI] [PubMed] [Google Scholar]
- Mark T. L., Woody G. E., Juday T., Kleber H. D. The economic costs of heroin addiction in the United States. Drug and Alcohol Dependence. 2001;61:195–206. doi: 10.1016/s0376-8716(00)00162-9. [DOI] [PubMed] [Google Scholar]
- Mattick R. P., Kimber J., Breen C., Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database of Systematic Reviews. 2008 doi: 10.1002/14651858.CD002207.pub3. Art. No. CD002207. [DOI] [PubMed] [Google Scholar]
- Mazurenko O., Menachemi N. Environmental market factors associated with physician career satisfaction. Journal of Healthcare Management. 2012;57:307–322. [PubMed] [Google Scholar]
- Netherland J., Botsko M., Egan J. E., Saxon A. J., Cunningham C. O., Finkelstein R., Fiellin D. A. the BHIVES Collaborative. Factors affecting willingness to provide buprenorphine treatment. Journal of Substance Abuse Treatment. 2009;36:244–251. doi: 10.1016/j.jsat.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen S., Dietze P., Dunlop A., Muhleisen P., Lee N., Taylor D. Buprenorphine supply by community pharmacists in Victoria, Australia: Perceptions, experiences and key issues identified. Drug and Alcohol Review. 2007;26:143–151. doi: 10.1080/09595230601146645. [DOI] [PubMed] [Google Scholar]
- Osborn R. N., Hunt J. G. Environment and organizational effectiveness. Administrative Science Quarterly. 1974;19:231–246. [Google Scholar]
- Pating D. R., Miller M. M., Goplerud E., Martin J., Ziedonis D. M. New systems of care for substance use disorders: Treatment, finance, and technology under health care reform. Psychiatric Clinics of North America. 2012;35:327–356. doi: 10.1016/j.psc.2012.03.004. [DOI] [PubMed] [Google Scholar]
- Paulozzi L. J., Mack K. A., Hockenberry J. M. the Division of Unintentional Injury Prevention, National Center for Injury Prevention and Control, CDC. Vital signs: variation among states in prescribing of opioid pain relievers and benzodiazepines - United States, 2012. Morbidity and Mortality Weekly Report. 2014;63:563–568. [PMC free article] [PubMed] [Google Scholar]
- Paulozzi L. J., Xi Y. Recent changes in drug poisoning mortality in the United States by urban-rural status and by drug type. Pharmacoepidemiology and Drug Safety. 2008;17:997–1005. doi: 10.1002/pds.1626. [DOI] [PubMed] [Google Scholar]
- Price C. C., Eibner C. For states that opt out of Medicaid expansion: 3.6 million fewer insured and $8.4 billion less in federal payments. Health Affairs. 2013;32:1030–1036. doi: 10.1377/hlthaff.2012.1019. [DOI] [PubMed] [Google Scholar]
- Radley D. C., Schoen C. Geographic variation in access to care—the relationship with quality. The New England Journal of Medicine. 2012;367:3–6. doi: 10.1056/NEJMp1204516. [DOI] [PubMed] [Google Scholar]
- Reif S., Thomas C. P., Wallack S. S. Factors determining how early adopter physicians use buprenorphine in treatment. Journal of Addiction Medicine. 2007;1:205–212. doi: 10.1097/ADM.0b013e31814c3fa8. [DOI] [PubMed] [Google Scholar]
- Rinaldo S. G., Rinaldo D. W. Availability without accessibility? State Medicaid coverage and authorization requirements for opioid dependence medications. 2013. Retrieved from https://web.archive.org/web/20150304192426/ http://www.asam.org/docs/default-source/advocacy/aaam_implications-for-opioid-addiction-treatment_final. [Google Scholar]
- Roman P. M., Johnson J. A., Blum T. C. The transformation of private alcohol problem treatment: Results from a national study. Advances in Medical Sociology. 2000;7:321–342. [Google Scholar]
- Rosenblatt R. A., Andrilla C. H. A., Catlin M., Larson E. H. Geographic and specialty distribution of US physicians trained to treat opioid use disorder. Annals of Family Medicine. 2015;13:23–26. doi: 10.1370/afm.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal M. B., Zaslavsky A., Newhouse J. P. The geographic distribution of physicians revisited. Health Services Research. 2005;40:1931–1952. doi: 10.1111/j.1475-6773.2005.00440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekscenski E. S., Sansom S., Bazell C., Salmon M. E., Mullan F. State practice environments and the supply of physician assistants, nurse practitioners, and certified nurse-midwives. The New England Journal of Medicine. 1994;331:1266–1271. doi: 10.1056/NEJM199411103311905. [DOI] [PubMed] [Google Scholar]
- Stein B. D., Gordon A. J., Dick A. W., Burns R. M., Pacula R. L., Farmer C. M., Sorbero M. Supply of buprenorphine waivered physicians: The influence of state policies. Journal of Substance Abuse Treatment. 2015;48:104–111. doi: 10.1016/j.jsat.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strang J., Manning V., Mayet S., Ridge G., Best D., Sheridan J. Does prescribing for opiate addiction change after national guidelines? Methadone and buprenorphine prescribing to opiate addicts by general practitioners and hospital doctors in England, 1995–2005. Addiction. 2007;102:761–770. doi: 10.1111/j.1360-0443.2007.01762.x. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. The DASIS report: Facilities providing methadone/LAAM treatment to clients with opiate addiction. 2002. Retrieved from https://web.archive.org/web/20150304194334/ http://files.ireta.org/opiates2005/04.pdf. [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2008 National Survey of Drug Use and Health: National findings. Rockville, MD: Office of Applied Studies; 2009. Retrieved from https://web.archive.org/web/20150304193804/ https://dpft.org/resources/NSDUHresults2008.pdf. [Google Scholar]
- Substance Abuse and Mental Health Services Administration. The NSDUH report: State estimates of nonmedical use of prescription pain relievers. 2013a. Retrieved from https://web.archive.org/web/20140907004941/ http://www.samhsa.gov/data/2k12/NSDUH115/sr115-nonmedical-use-pain-relievers.htm. [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Substance abuse treatment facility locator. 2013b Retrieved from http://find-treatment.samhsa.gov/TreatmentLocator/faces/geographicSearch.jspx.
- Thomas C. P., Reif S., Haq S., Wallack S. S., Hoyt A., Ritter G. A. Use of buprenorphine for addiction treatment: Perspectives of addiction specialists and general psychiatrists. Psychiatric Services. 2008;59:909–916. doi: 10.1176/ps.2008.59.8.909. [DOI] [PubMed] [Google Scholar]
- U.S. Census Bureau. Table 14. State population–Rank, percent change, and population density: 1980 to 2010. 2013 Retrieved from https://web.archive.org/web/20131026105030/ http://www.census.gov/compen-dia/statab/2012/tables/12s0014.pdf. [Google Scholar]
- U.S. Census Bureau. State totals: Vintage 2013. 2014 Retrieved from https://web.archive.org/web/20141008170740/ http://www.census.gov/popest/data/state/totals/2013/index.html.
- U.S. Census Bureau. 2010 Census urban and rural classification and urban area criteria. 2015a. Retrieved from https://web.archive.org/web/20150226211106/ http://www.census.gov/geo/reference/ua/urban-rural-2010.html. [Google Scholar]
- U.S. Census Bureau. Census regions and divisions of the United States. 2015b Retrieved from https://web.archive.org/web/20150226213756/ http://www.census.gov/geo/maps-data/maps/pdfs/reference/us_regdiv.pdf.
- Volkow N. D., Frieden T. R., Hyde P. S., Cha S. S. Medication-assisted therapies—tackling the opioid-overdose epidemic. The New England Journal of Medicine. 2014;370:2063–2066. doi: 10.1056/NEJMp1402780. [DOI] [PubMed] [Google Scholar]
- Wallack S. S., Thomas C. P., Martin T. C., Chilingerian J., Reif S. Substance abuse treatment organizations as mediators of social policy: Slowing the adoption of a congressionally approved medication. Journal of Behavioral Health Services & Research. 2010;37:64–78. doi: 10.1007/s11414-008-9132-4. [DOI] [PubMed] [Google Scholar]
- Walsh S. L., Eissenberg T. The clinical pharmacology of buprenorphine: Extrapolating from the laboratory to the clinic. Drug and Alcohol Dependence, 70, Supplement. 2003:S13–S27. doi: 10.1016/s0376-8716(03)00056-5. [DOI] [PubMed] [Google Scholar]
- Wang F., Luo W. Assessing spatial and nonspatial factors for healthcare access: Towards an integrated approach to defining health professional shortage areas. Health & Place. 2005;11:131–146. doi: 10.1016/j.healthplace.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Weisfeld A., Perlman R. L. Disparities and discrimination in health care: An introduction. Perspectives in Biology and Medicine, 48, Supplement. 2005:S1–S9. [PubMed] [Google Scholar]
- West J. C., Kosten T. R., Wilk J., Svikis D., Triffleman E., Rae D. S., Regier D. A. Challenges in increasing access to buprenorphine treatment for opiate addiction. American Journal on Addictions, 13, Supplement. 2004:S8–S16. doi: 10.1080/10550490490440753. [DOI] [PubMed] [Google Scholar]
- WESTAT. The SAMHSA evaluation of the impact of the DATA waiver program: Summary report (Task Order 277-00-6111) 2006 Retrieved from http://buprenorphine.samhsa.gov/FOR_FINAL_summaryreport_colorized.pdf.
- White W. Slaying the dragon: The history of addiction treatment and recovery in America. Bloomington, IL: Chestnut Health Systems; 1998. [Google Scholar]
- World Health Organization. 10th revised ed. Geneva, Switzerland: Author; 1992. ICD-10: International statistical classification of diseases and related health problems. [Google Scholar]


