Abstract
The aim of this review is to present an emerging zoonotic disease caused by Bartonella henselae. The wide spectrum of diseases connected with these bacteria varies from asymptomatic cases, to skin inflammation, fever of unknown origin, lymphadenopathy, eye disorders, encephalitis and endocarditis. The reservoirs of B. henselae are domestic animals like cats, guinea pigs, rabbits and occasionally dogs. Diagnosis is most often based on a history of exposure to cats and a serologic test with high titres of the immunoglobulin G antibody to B. henselae. Most cases of cat-scratch disease are self-limited and do not require antibiotic treatment. If an antibiotic is chosen, however, azithromycin has been shown to speed recovery.
Keywords: cat scratch disease, Bartonella henselae, child, lymphadenopathy
Introduction
Bartonella species are small Gram-negative bacteria which have been isolated from humans and mammals. Human infections due to Bartonella species are being increasingly reported all over the world. There are 25 species of Bartonella, with about half of them having been confirmed as human pathogens [1]. The wide spectrum of diseases connected with these bacteria ranges from asymptomatic cases, to skin inflammation, fever of unknown origin, lymphadenopathy, eye disorders, encephalitis and endocarditis. In immunocompromised patients, Bartonella sp. can cause opportunistic infections like bacillary angiomatosis and peliosis hepatitis. In immunocompetent individuals, B. henselae infection presents as cat-scratch disease (CSD) [2]. The connection between B. henselae and CSD was confirmed in 1989; however, the first description of disease was presented 40 years earlier based on histopathological examination of inflamed lymph nodes. The reservoirs of B. henselae are domestic animals like cats, guinea pigs, rabbits and occasionally dogs. In Poland, positive IgG antibodies for B. henselae are found in 50–90% of cats [3]. The cat flea Ctenocephalides felis (Siphonaptera: Pulicidae) is the best-recognised vector of B. henselae and transmission between cats and humans which mainly occurs through infected flea faeces. New potential vectors are confirmed to be capable of transmitting B. henselae, in particular Ixodes ricinus, the most widespread ixodid tick in Western Europe, which is frequently associated with bites in humans [4–6].
People become infected by being bitten or scratched by an infected animal [1, 7]. Bartonella infection in humans produces prolonged bacteraemia in blood.
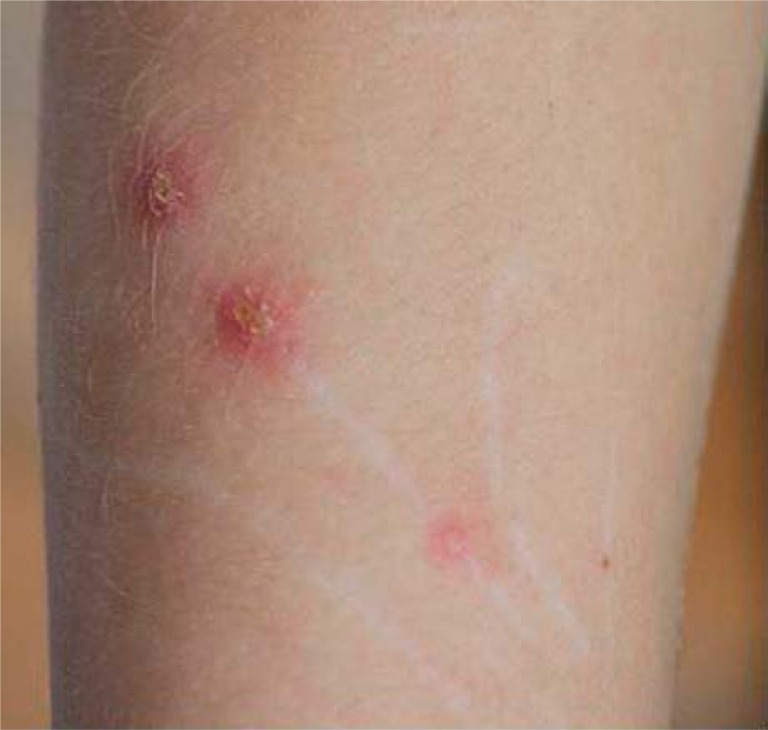
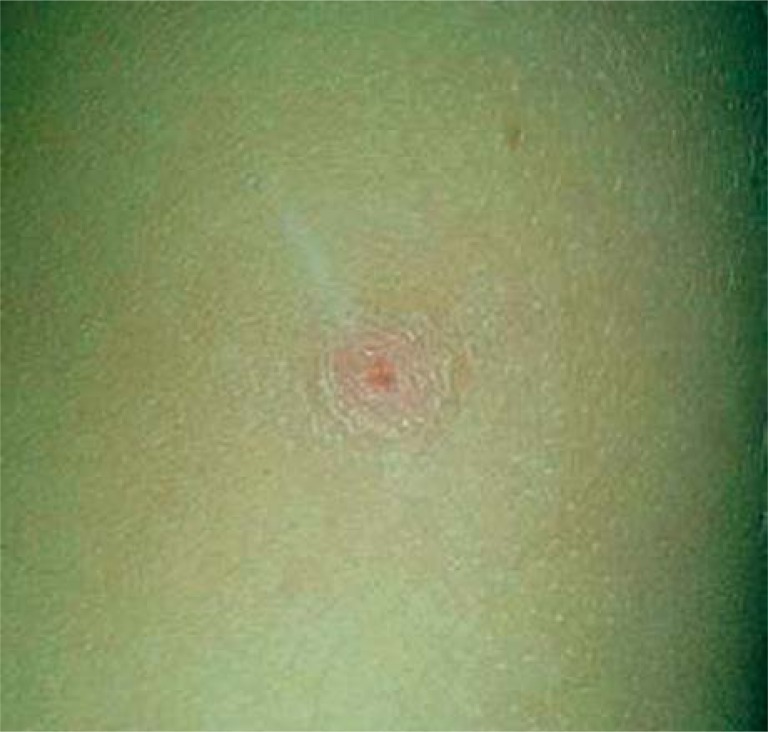
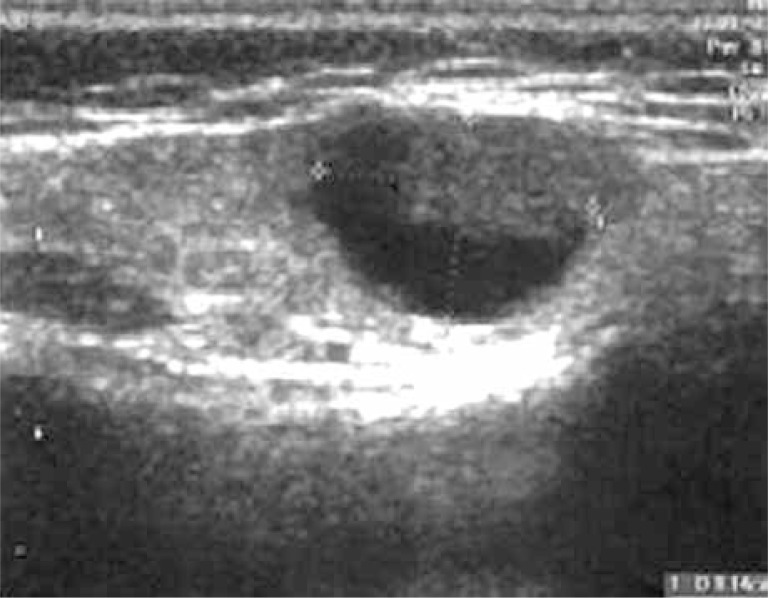
The disease begins with an erythematous papule (single or in the group) at the site of inoculation. Diagnosis can be easier if the doctor has all information about cat scratching in the patient's history or finds a visible sign of animal aggression (Figure 1) [2]. The papule appears 3 to 10 days after inoculation, and progresses through erythematous, vesicular, papular and crusted stages (Figure 2). In typical CSD, regional lymphadenopathy occurs 1 to 3 weeks after inoculation and lasts for up to several months. Eighty-five per cent of patients have only a single node involved. Asymmetric lymphadenopathy occurs most frequently in the axillary and epitrochlear nodes (46%), head and neck (26%), and the groin (17.5%). The lymph nodes are painful and movable with solid consistency (Figure 3). In 20–30% of patients, inflamed lymph nodes produce suppuration with purulent fistulas to the skin; approximately 10% of nodes require drainage. About 50% of patients present CSD with mild systemic symptoms like generalised aches, malaise, anorexia, nausea, and abdominal pain [8]. On ultrasound, nodes are multiple, hypoechoic, and highly vascularised with increased echogenicity of the surrounding soft tissues (Figure 4) [9]. Histopathological examination shows an aspecific granulomatous process, with microabscesses and local necrosis in the material taken from inflamed lymph nodes [10].
Figure 1.
Cat-scratch disease – active skin lesions (1 week after inoculation)
Figure 2.
Cat-scratch disease – the crusted lesions after treatment
Figure 3.
Cat-scratch disease – local lymphadenopathy
Figure 4.
Hypoechoic lymph nodes in CSD – ultrasound picture
About 10% of patients present perinodal forms of bartonellosis, which are manifested by endocarditis, encephalitis, uveitis, conjunctivitis, hepatitis and tuberous sclerosis. Incidentally, inflammation in the musculoskeletal system, like osteitis, arthropathy and myalgia, have also been described [8].
Dermatologic manifestations
Skin lesions other than the papule seen at the site of inoculation are rare, occurring in only 5% of patients infected with B. henselae. These include maculopapular and urticarial eruptions, granuloma annulare, erythema nodosum, erythema marginatum, and leukocytoclastic vasculitis [11].
Extremely dangerous courses of Bartonella infection, such as bacillary angiomatosis, can be observed in immunocompromised patients, especially in those infected with HIV. Although this systemic disease can occur in various organ systems, skin lesions are most frequent, occurring in up to 90% of cases. Lesions are reddish-brown papules that are difficult to differentiate from Kaposi's sarcoma, epithelioid haemangioma, and pyogenic granuloma. An example of angioproliferation in immunocompromised individuals infected with B. henselae is shown by an accumulation of rounded blood vessels on biopsy, with plump epithelial cells and a mixed inflammatory infiltrate with neutrophil predominance [12].
Fever of unknown origin (FUO)
Bartonella henselae is identified as the third leading infectious cause of FUO, after Epstein-Barr virus infection and osteomyelitis [13]. There have been reported cases of B. henselae infection with abdominal lymphadenopathy, fever, and abdominal pain with or without hepatosplenomegaly [14].
Orthopaedic manifestations
Bone lesions are a rare complication of B. henselae infection and occur as osteomyelitis. Clinical manifestations include pain and tenderness over the affected bone and peripheral lymphadenopathy. Abnormalities on radiograph include lytic lesions, with occasional sclerosis or periosteal reaction [2]. The infection is described in different bones in the human body as separate or disseminated processes. Biopsy reveals necrotising granulomas in infected bones with adjacent abscesses. The prognosis for the treated patients is good [15].
Cardiac manifestations
Bartonella species account for 3% of cases of endocarditis. Presentation is insidious and subacute, with fever, dyspnoea, cardiac failure and cardiac murmur. The aortic valve is usually involved, and vegetation is found in 100% of patients [8].
Pseudomalignancy
Many reports describe B. henselae infection as mimicking different malignancies, especially lymphomas. Local lymphadenopathy, especially located in the neck and abdomen, with the clinical picture including the weight loss, prolonged fever and general weakness, induces wide-spectrum diagnostic procedures. According to American data, surgical nodal biopsy is performed in 24.5% of patients aged below 18 years, and 7.9% of patients undergo perinodal tissue biopsy [7].
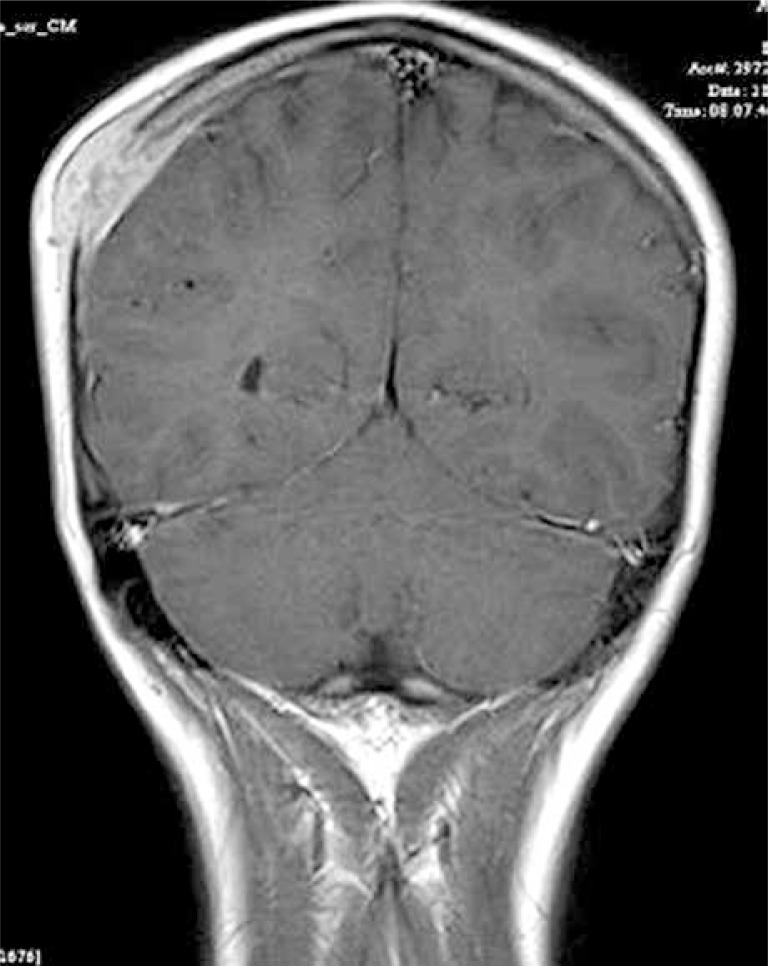
There are also reports in the literature of Bartonella infection presenting as solitary tumours in structures like the breast and bones. In their own practice, the authors have seen the unusual presentation of two patients with a solitary tissue mass overlying a skull lesion, both of which were suggestive of histiocytosis X (Figure 5). In both cases, bacteraemia was confirmed by polymerase chain reaction (PCR) of resected material.
Figure 5.
Cat-scratch disease – the solitary tissue mass overlying a skull lesion suggesting histiocytosis X
Diagnostic tests
The isolation of Bartonella sp. in culture is difficult, requiring a 2- to 6-week incubation for primary isolation. In addition, isolating B. henselae is usually unsuccessful if the patients lack systemic disease.
The most frequently used test for diagnosis is serology for B. henselae antibodies. There are two major serologic methods: indirect fluorescence assay (IFA) and enzyme immunoassay (EIA). The duration of serologic detection of antibodies is important for determining acute infection versus historical exposure. Positive IgM antibodies indicate acute disease, but their duration in the blood is approximately 100 days after exposure. The short duration of IgM means that they are identified in 50% of infected individuals [16]. IgG antibodies are detectable up to 22–28 weeks after inoculation. As 25% of patients remain seropositive for IgG after 1 year, it is difficult to diagnose active infection compared with the previous infection [7, 8].
The most advanced technique involves the detection of bacterial material in patient's tissues. There have been 3 main approaches to using PCR to diagnose Bartonella infection: amplification of the 16S rRNA gene, amplification of the citrate synthase gene (gltA), and amplification of the htrA gene of B. henselae. The specificity of PCR is nearly 100%, but sensitivity ranges from 43% to 76% [17]. In fact, the detection of Bartonella sp. in clinical material is equivalent to the level of isolation in culture [18].
Treatment
The therapeutic approach to Bartonella infection varies on the basis of the clinical manifestations and the immune status of the patient. There is a significant divide in the literature between the in vitro and in vivo efficacy of antibiotics. In vitro, Bartonella species have been found to be susceptible to a number of antimicrobial agents like macrolides, aminoglycosides, β-lactams, third-generation cephalosporins, trimethoprim-sulfamethoxazole, rifampicin, and ciprofloxacin [18]. However, this broad spectrum of activity was not confirmed in clinical practice. In vitro, most of the antibiotics tested had bacteriostatic activity against Bartonella; only aminoglycosides demonstrated bactericidal activity. The weak cell membrane penetration of many antibiotics and their bacteriostatic activity are the main hypotheses for why these drugs fail to reach the intracellular Bartonella sp. [19].
Because of the natural history of uncomplicated CSD, antibiotics are not suggested for regional CSD. For mild-to-moderate infections in immunocompetent patients, management consists of reassurance, adequate follow-up and analgesics for pain. Nodes should be aspirated if they suppurate to relieve painful adenopathy; however, incision and drainage is not recommended due to the potential for chronic sinus tract formation. During aspiration, the needle should be moved around several different locations, because coalesced microabscesses often exist in multiple septated pockets [19, 20].
For patients with significant lymphadenopathy, azithromycin at doses of 10 mg/kg on day 1 and 5 mg/kg per day on days 2 to 5 can be considered. Other antibiotic options include rifampicin (20 mg/kg per day divided in 2 doses for 2–3 weeks), ciprofloxacin (20–30 mg/kg per day in 2 daily doses for 2–3 weeks), or trimethoprim-sulfamethoxazole (trimethoprim 8 mg/kg per day, sulfamethoxazole 40 mg/kg per day, in two divided doses). As the clinical spectrum of disease caused by B. henselae expands, choosing the proper treatment of these conditions becomes more difficult. The current knowledge of the treatment of neuroretinitis, encephalopathy, hepatosplenomegaly, endocarditis, and bacillary angiomatosis, as well as other disease processes, is derived from observational case studies. Limited data suggest that the treatment of hepatosplenic disease and prolonged fever in children should consist of a 10- to 14-day course of rifampicin. Due to the rapid development of rifampicin resistance, some experts recommend adding a second agent, such as gentamicin or azithromycin [20].
Prognosis
The prognosis for complete recovery in immunocompetent patients with CSD is excellent. Significant morbidity occurs in 5–10% of cases, usually because of involvement of the central or peripheral nervous system or because of multisystem disseminated disease. One episode of cat-scratch disease confers lifelong immunity to all patients.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Breitschwerdt EB, Kordick DL. Bartonella infection in animals: carriership, reservoir, potential, pathogenicity and zoonotic potential for human infection. Clin Microbiol Rev. 2000;13:428–38. doi: 10.1128/cmr.13.3.428-438.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mazur-Melewska K, Macedulski T, Prusinowska J, et al. Variable, clinical course of the cat scratch disease. Pediat Med Rodz. 2012;8:176–9. [Google Scholar]
- 3.Podsiadły E, Sokołowska E, Tylewska-Wierzbanowska S. Seroprevalence of Bartonella henselae and Bartonella quintana infections in Poland in 1998-2001. Przegl Epidemiol. 2002;56:399–407. [PubMed] [Google Scholar]
- 4.Boulouis HJ, Chang CH, Henn JB, et al. Factor associated with the rapid emergence of zoonotic Bartonella infections. Vet Res. 2005;36:383–410. doi: 10.1051/vetres:2005009. [DOI] [PubMed] [Google Scholar]
- 5.Boushira E, Franca M, Boulouis HJ, et al. Assessment of persistence of Bartonella henselae in Ctenocephalides felis. Appl Environ Microbiol. 2013;79:7439–52. doi: 10.1128/AEM.02598-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cotte V, Bonnet S, Le Rhun D, et al. Transmission of Bartonella henselae by Ixodes ricinus. Emerg Infect Dis. 2008;14:1074–80. doi: 10.3201/eid1407.071110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reynolds MG, Holman RC, Curns AT, et al. Epidemiology of cat-scratch disease hospitalizations among children in the United States. Ped Infect Dis J. 2005;24:700–4. doi: 10.1097/01.inf.0000172185.01939.fc. [DOI] [PubMed] [Google Scholar]
- 8.Florin TA, Zaoutis TE, Zaoutis LB. Beyond cat scratch disease: widening spectrum of Bartonella henselae. Pediatrics. 2008;121:e1413–25. doi: 10.1542/peds.2007-1897. [DOI] [PubMed] [Google Scholar]
- 9.Rohr A, Saettele MR, Patel SA, et al. Spectrum of radiological manifestations of paediatric cat-scratch disease. Pediatr Radiol. 2012;42:1380–4. doi: 10.1007/s00247-012-2451-x. [DOI] [PubMed] [Google Scholar]
- 10.Mancino P, Ucciferri C, Falasca K, et al. Inguinal lymphadenopathy due to Bartonella henselae. Le Infezioni in Medicina. 2008;2:91–3. [PubMed] [Google Scholar]
- 11.Landau M, Kletter Y, Avidor B, et al. Unusual eruption as a presenting symptom of cat scratch disease. J Am Acad Dermatol. 1999;41:833–6. doi: 10.1016/s0190-9622(99)70337-3. [DOI] [PubMed] [Google Scholar]
- 12.Chian CA, Arrese JE, Pierard GE. Skin manifestations of Bartonella infections. Int J Dermatol. 2002;41:461–6. doi: 10.1046/j.1365-4362.2002.01489.x. [DOI] [PubMed] [Google Scholar]
- 13.Jacobs RF, Schutze GE. Bartonella henselae as a cause of prolonged fever and fever of unknown origin in children. Clin Infect Dis. 1998;26:80–4. doi: 10.1086/516256. [DOI] [PubMed] [Google Scholar]
- 14.Arisoy ES, Correa AG, Wagner ML, Kaplan SL. Hepatosplenic cat-scratch disease in children: selected clinical features and treatment. Clin Infect Dis. 1999;28:778–84. doi: 10.1086/515197. [DOI] [PubMed] [Google Scholar]
- 15.Hipp SJ, O'Shields A, Fordham LA, et al. Multifocal bone marrow involvement in cat scratch disease. Ped Infect Dis J. 2005;4:472–4. doi: 10.1097/01.inf.0000160993.52059.3a. [DOI] [PubMed] [Google Scholar]
- 16.Podsiadły E, Sapiejka E, Dąbrowska-Bień J, et al. Diagnostics of cat scratch disease and present methods of bartonellosis recognition – a case report. Pol Merk Lek. 2009;26:152–61. [PubMed] [Google Scholar]
- 17.Hansmann Y, DeMartino S, Piemont Y, et al. Diagnosis of cat scratch disease with detection of Bartonella henselae by PCR: a study of patients with lymph node enlargement. J Clin Microbiol. 2005;43:3800–6. doi: 10.1128/JCM.43.8.3800-3806.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ives TJ, Manzewitsch P, Regnery RL, et al. In vitro susceptibilities of Bartonella henselae, B. quintana, B. elizabethae, Rickettsia rickettsii, R. conorii, R. akari and R. prowazekii to macrolide antibiotics as determined by immunofluorescent antibody analysis of infected vero cell monolayers. Antimicrob Agents Chemother. 1997;41:578–82. doi: 10.1128/aac.41.3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rolain JM, Brouqui P, Koehler JE, et al. Recommendations for treatment of human infections caused by Bartonella species. Antimicrob Agents Chemother. 2004;48:1921–33. doi: 10.1128/AAC.48.6.1921-1933.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Margileth AM. Recent advances in diagnosis and treatment of cat scratch disease. Curr Infect Dis Rep. 2000;2:141–6. doi: 10.1007/s11908-000-0026-8. [DOI] [PubMed] [Google Scholar]