Abstract
Aspirin-induced asthma (AIA) is a distinct clinical syndrome characterized by severe asthma exacerbations after ingestion of aspirin or other non-steroidal anti-inflammatory drugs. The exact pathomechanism of AIA remains unknown, though ongoing research has shed some light. Recently, more and more attention has been focused on the role of aspirin in the induction of oxidative stress, especially in cancer cell systems. However, it has not excluded the similar action of aspirin in other inflammatory disorders such as asthma. Moreover, increased levels of 8-isoprostanes, reliable biomarkers of oxidative stress in expired breath condensate in steroid-naïve patients with AIA compared to AIA patients treated with steroids and healthy volunteers, has been observed. This review is an attempt to cover aspirin-induced oxidative stress action in AIA and to suggest a possible related pathomechanism.
Keywords: aspirin-induced asthma, free radicals, isoprostanes, nasal polyps
Introduction
Acetylsalicylic acid (ASA) is a common drug in use today. So far, it is mainly known for its analgesic, antipyretic and anti-inflammatory action. However, when used daily, aspirin has also been seen to display antithrombotic properties which are helpful in lowering the risk of heart attack [1, 2], clot-related strokes [3, 4] and other blood flow problems. However, in the space of a few years there have been reports associating it with other completely new, very interesting properties.
Firstly, researchers have noticed that aspirin may intensify fat burning and reduce fatty liver in obese mice [5]. Many population studies have also shown an association between a reduced risk of Alzheimer's disease (AD) and use of non-steroidal anti-inflammatory drugs such as aspirin [6], which may well be related to alleviating inflammation associated with pathogenesis of AD [7].
For some time, aspirin has also been thought to possess anti-tumor properties. In a certain dose (75–100 mg) it helps to prevent certain cancers and reduces the risk of death from cancer by 40% for colorectal cancer, 60% for esophageal cancer, 30% for lung cancer and 10% for prostate cancer [8]. Direct inhibition of cyclooxygenase-2 (COX-2) activity is the main mechanism by which aspirin has been proposed to inhibit the development of certain cancers [9], although other mechanisms have also been hypothesized, some of which also indicate that aspirin may cause oxidative stress and thus induce cancer cell apoptosis.
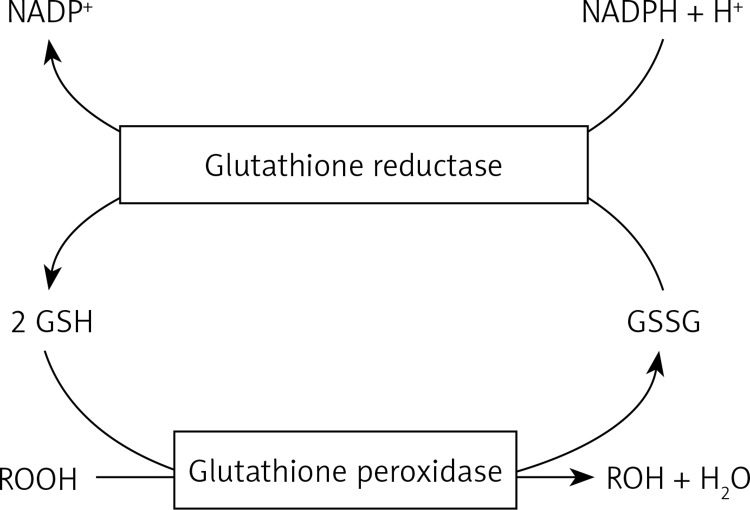
It is known that oxidative stress is the imbalance between the production of oxidants (reactive oxygen (ROS) and nitrogen (RNS) species including superoxide (O2 .–), hydrogen peroxide (H2O2), hypochlorite (ClO–), hydroxyl radical (OH–), nitric oxide (NO), and peroxynitrite (ONOO–)) and endogenous antioxidant defenses in cells. Antioxidant compounds constitute urate, glutathione, ubiquinone and thioredoxin; moreover, some proteins – ferritin, transferrin, lactoferrin, caeruloplasmin – acting as antioxidants, bind some transition metals that may start oxidative reactions. However, the antioxidant enzymes crucial for protection of the airway include, primarily, superoxide dismutases (SOD) and glutathione peroxidases (GPx). Superoxide dismutases convert superoxide to hydrogen peroxide, whereas GP removes hydrogen peroxide and lipid hydroperoxides. This GPx mechanism involves glutathione (GSH), which acts as a co-substrate and is itself oxidized to glutathione disulfide (GSSG) (Figure 1).
Figure 1.
Diagram of glutathione homeostasis
Allowedly mitochondria are the major site of intracellular ROS production due to electron leakage along the respiratory chain [10], but it may also arise from plasma membrane systems, endoplasmic reticulum, lysosomes, peroxisomes and cytosolic enzymes. However, exogenous sources of ROS production have been linked to asbestos, ozone, coal, diesel fuels and cigarette smoke [11, 12].
At low concentrations, ROS/RNS exert a number of beneficial effects including immune-mediated defense against pathogenic microorganisms [13]. In turn, high levels of these species can damage DNA, lipids, proteins and carbohydrates, leading to an enhanced inflammatory response in respiratory airways as seen in asthma [13, 14].
Adverse oxidative stress can be a consequence of inter alia chronic hypernutrition, unhealthy diet, sedentary lifestyle and environmental overexposure. Such activities may be additional factors that escalate mitochondria dysfunction, reduction of antioxidant resources and significantly stimulate intercellular pathways leading to oxidative stress. In some cases, an excessive oxidative burden leads to clinical signs, but it is ascribed to cumulative effects of multiple activities [15].
Oxidative stress in asthma
Oxidative stress is rapidly gaining recognition as a key phenomenon in chronic diseases and in the case of asthma, various environmental pollutants, oxidants and drugs may induce oxidative burden in mitochondria of airway epithelial cells, resulting in the release of proinflammatory mediators, which recruit various types of inflammatory cells including eosinophils, neutrophils, lymphocytes and macrophages [16]. Additionally, stimulated inflammatory cells release various kinds of ROS which damage surrounding tissues in the airway.
Based on a variety of studies, it is clear that pulmonary ROS formation is a component of the molecular mechanism of asthma. The framework of ROS generation is such that oxidants mediate inflammatory responses and activate pro-inflammatory cytokine (TNF-α, IL-1β, IL-8, IL-6) and chemokine genes that facilitate the up-regulation of adhesion molecules and the increased release of pro-inflammatory mediators [17]. Many known inflammatory target proteins such as matrix metalloproteinase-9 (MMP-9), intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), cyclooxygenase-2 (COX-2) and cytosolic phospholipase (cPLA2) are also associated with NADPH oxidase (NOX) activation and ROS overproduction [18–22]. Moreover, the morphological and functional properties of endothelial cells such as permeability and expression of adhesion molecules can be altered by ROS, leading to adhesive interaction between inflammatory and endothelial cells which may contribute to the expression of inflammatory symptoms [23]. More explicitly, ROS may act as signaling modifiers of such transcription factors as nuclear factor-κB (NF-κB) and activator protein-1 (AP-1) in epithelial cells, resulting in activation of many of the above-mentioned pro-inflammatory cytokines, enzymes and adhesion molecules [13, 24–27]. Oxidative stress has been proven to affect smooth muscle contraction [28], induce airway hyper-responsiveness [29], and increase mucus secretion and epithelial shedding within respiratory cells [30, 31]. In turn allergens, gaseous pollutants, bacteria and viruses activate inflammatory cells in asthmatic airways and cause respiratory burst releases of ROS to surrounding respiratory cells and tissues [32]. Many studies have revealed that patients with asthma demonstrated increased production of ROS, which correlates with severity of airflow limitation and the degree of airway hyperresponsiveness, as quantified by methacholine challenge [33–36]. Indeed, ROS induce direct contraction of airway smooth muscles, hyperresponsiveness, and this effect is enhanced when the epithelium is injured. Neutrophils isolated from peripheral blood of asthmatics generate greater amounts of ROS than cells from normal subjects, and their ability to produce ROS correlates with the degree of airway hyperresponsiveness to inhaled methacholine [37, 38]. Eosinophils derived from peripheral blood produce greater amounts of ROS after stimulation ex vivo in asthma, which also correlates with bronchial hyperresponsiveness [39–41]. Thus, this observation may suggest that ROS play a pivotal role in the pathogenesis of asthma and provide a link between epithelial injury arising from a variety of causes and airway hyperresponsiveness [42].
While much of the evidence for the involvement of ROS in the pathogenesis of asthma is indirect, numerous markers of oxidative burden have been measured [43]. Evaluation of levels of these markers is very beneficial in clinical investigations because it relates to severity of disease and provides a reproducible method for monitoring asthma. Exhaled air, exhaled breath condensate (EBC), induced sputum and bronchoalveolar lavage fluid (BAL) are biological samples that are often utilized for assessing oxidant burden in the airways.
One of them, exhaled nitric oxide (NO), has been identified as a potential marker to monitor airway inflammation and oxidative stress in asthma. Exhaled NO is increased in patients with asthma compared with normal subjects [44, 45], increased in allergen-induced late asthmatic reactions [46] and reduced when asthma exacerbations are treated with corticosteroids [47]. Is has also been proven that the level of FeNO positively correlates with measures of asthma control, as defined by recent symptoms or dyspnea, use of rescue medications, reversibility of airflow obstruction [48], level of sputum eosinophils and airway hyperresponsiveness [49], and the main site of origin of the increased levels of FeNO in asthma is the lower airways [45].
In the respiratory system, NO derived from constitutive NO synthase (cNOS) has homeostatic effects including dilatation of pulmonary blood vessels [50] and relaxation of airway smooth muscle. In turn, massively produced NO derived from the inducible type of NOS (iNOS, NOS2), which reacts with superoxide anion and produces reactive nitrogen species (RNS) such as peroxynitrite, has been reported to play a key role in airway and lung inflammation [51, 52]. Thus enhanced NOS2 expression in the airways of asthmatic patients is responsible for the increased NO levels in the exhaled breath [53, 54].
Other exhaled markers of oxidative burden in asthma are H2O2 and CO. It has been shown that the levels of exhaled CO are elevated in stable asthma and become reduced towards the normal value by administration of inhaled corticosteroids [55]. However, in the case of hydrogen peroxide, exhaled H2O2 concentrations are higher in smoking than nonsmoking subjects [56], intermittent, mild-moderate asthmatics than healthy subjects and steroid-untreated and steroid-treated asthmatics than healthy subjects [57]. Excessive smoking leads also to a further elevation in exhaled H2O2 levels in asthmatic patients, indicating that smoking contributes to an acute release of ROS in the airways [58].
The next important tool for reliably exploring oxidative stress in asthma is 8-iso-prostaglandin F2α (8-isoprostane). Elevated EBC 8-isoprostane concentrations have been reported in asthmatic children [59, 60] and adults with severe asthma in comparison to healthy controls [61], and these concentrations increase with asthma severity [61, 62]. EBC 8-isoprostane levels decrease after allergen avoidance in children with allergic asthma [62, 63], and smoking elicits an acute 50% increase in EBC 8-isoprostane levels within 15 min [64]. The level of 8-isoprostane seems to be higher in sputum than in EBC [65], and sputum concentrations of 8-isoprostane are also higher in adults with stable asthma than in healthy subjects [66]. However, the concentrations of inter alia 8-isoprostane do not coincide between EBC and BAL [67], but it is worth noting that BAL is too invasive and not sensitive enough to be utilized in the assessment of oxidative stress and airway inflammation in clinical practice.
It is also known that elevated levels of GSSG can be considered a marker of oxidative stress, whereas increased total or reduced GSH levels are thought to represent an adaptive response to the increased oxidative stress in the lungs. Patients experiencing an acute exacerbation of asthma revealed GSH levels lower than stable asthmatics, but still significantly higher than those of healthy controls [68]. Thus, from the clinical point of view, a decreased level of antioxidant enzymes, including systemic SOD, is related to airflow limitation and asthma aggravation [69].
Genetically determined host responses to oxidative stress also appear to be critical [70]. Genetic polymorphisms in genes encoding antioxidant enzymes may be significant risk factors for asthma. For example, the presence of polymorphisms of the glutathione-S-transferase gene was correlated with the response to second-hand smoking in asthmatic children [71].
Taken together, the evidence suggests that oxidative stress plays a major pathophysiological role in asthma and it is important for the severity of this condition. At the same time, either ROS generation or endogenous antioxidant mechanisms are out of balance, which causes that a more inflammatory state becomes apparent. Moreover, oxidative stress represents a greater predisposition to exacerbation of asthmatic symptoms after environmental exposure to various organic chemicals (diesel exhaust, cigarette smoke) that increase the secretion of ROS.
Aspirin-induced asthma
Three years after introducing aspirin to the market, the first case of asthma exacerbation by aspirin was reported. Nowadays, up to 20% of adult asthmatics with nasal polyps are sensitive to aspirin and other non-steroidal anti inflammatory drugs (NSAIDs) [72]. The first symptoms of aspirin-intolerant asthma (AIA), occurring within 2 h after ingestion of ASA, include bronchospasm accompanied by nasal congestion and/or rhinorrhoea. Generally AIA, also named as aspirin-exacerbated respiratory disease (AERD), ASA triad, and Samter's syndrome, is characterized by chronic rhinosinusitis, nasal polyps, severe bronchial asthma and intolerance to ASA or other NSAIDs.
So far, no unambiguous theory exists which provides a satisfactory explanation for the pathogenesis of AIA. One hypothesis explaining the pathogenesis of AIA is the cyclooxygenase theory. According to it, aspirin inhibits intracellular COX enzymes, inhibiting prostaglandin biosynthesis. In turn, inhibition of COX causes an imbalance between the synthesis of eicosanoids originating from the cyclooxygenase pathway, which have smooth muscle relaxant properties, in favor of the synthesis of lipoxygenase pathway eicosanoids contracting bronchitis (15-hydroxyeicosatetraenoic (15-HETE) acid [73], LTB4 [74], cysteinyl leukotrienes – cysLTs [75, 76]).
Alternatively, an equally important possibility explaining the pathogenesis of AIA is the diminished capacity for synthesis of lipoxins (LXs) [77]. The LXs are 15-lipoxygenase products that, in contrast to leukotrienes, have anti-inflammatory properties; they inhibit chemotaxis, transmigration across endothelial and epithelial monolayers, diapedesis and superoxide anion generation by polymorphonuclear leukocytes [78]. Thus, impairment of the balance between lipoxin and leukotriene production may be a key in the pathogenesis of aspirin hypersensitivity.
Apart from biochemical theories of aspirin-induced asthma, in the literature there is also a large amount of data concerning genetic mechanisms, suggesting the involvement of various genes. Some of them are associated with the arachidonic acid pathway (LTC4S [79], COX2 [80], cysLTR1 [81], cysLTR2 [82]), and some are related to eosinophilic inflammation (ACE [83], CCR3 [84], NLRP3 [85], TBX21 [86]) and pathogenesis of nasal polyps (TGF-β1 [87]). There are also gene polymorphisms whose relationship with AIA is not fully ascertained (FSIP1 [88], CEP68 [72], SLC22A2 [89, 90], ADAM33 [91], EMID2 [92], TLR3 [93], ADORA1 [94], CACNG6 [95], WDR46 [96], ZNRD1 [97], KIFC1 [98], CYP2C19 [99]).
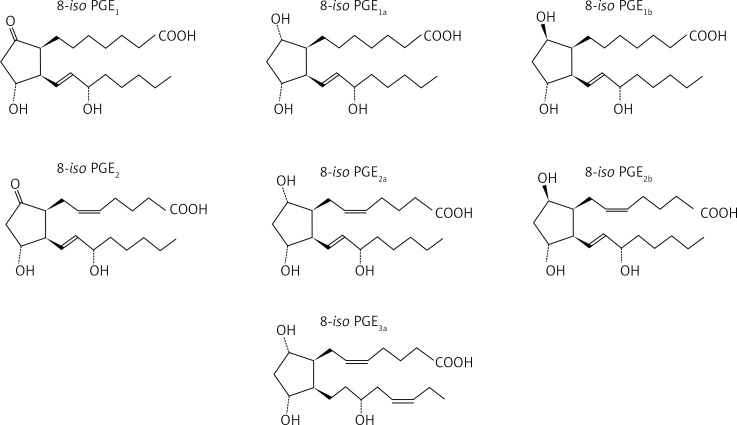
Recently, there has been a great deal of discussion about 8-isoprostanes, reliable biomarkers of lipid peroxidations which are stable prostaglandin-like arachidonate products formed on membrane phospholipids by the action of reactive oxygen species [100]. Structure-activity studies with 8-isoprostanes have revealed that E-ring compounds are more potent than F-ring compounds, doubly unsaturated compounds are more potent than singly unsaturated compounds, and the α configuration is more potent than the β configuration [101] (Figure 2).
Figure 2.
Chemical structures of ascertained isoprostanes
Moreover, the 8-isoprostanes have a higher contractile potency in human large airways compared with human small airways [102]. The two exceptions are 8-iso-PGE1, which have highly variable potency in both preparations, and 8-iso-PGF3α, which have powerful relaxant effects in human large airways [50]. Increased levels of 8-isoprostane have been found in expired breath condensate in steroid-naïve patients with AIA compared with steroid-treated patients with AIA and healthy control subjects [103]. Thus, the presence of 8-isoprostane in breath condensate appears to reflect oxidative stress and is progressively increased with the severity of asthma [61]. It is probable that the intensity of inflammation, as reflected by oxidative stress in the airways of patients with AIA, is greater than in subjects with ATA.
Mechanisms underlying aspirin-induced oxidative stress and apoptosis
The majority of the research concerning mechanisms of aspirin action have mainly been carried out in cancer cell systems. However, it has not excluded the similar action of ASA in other inflammatory disorders.
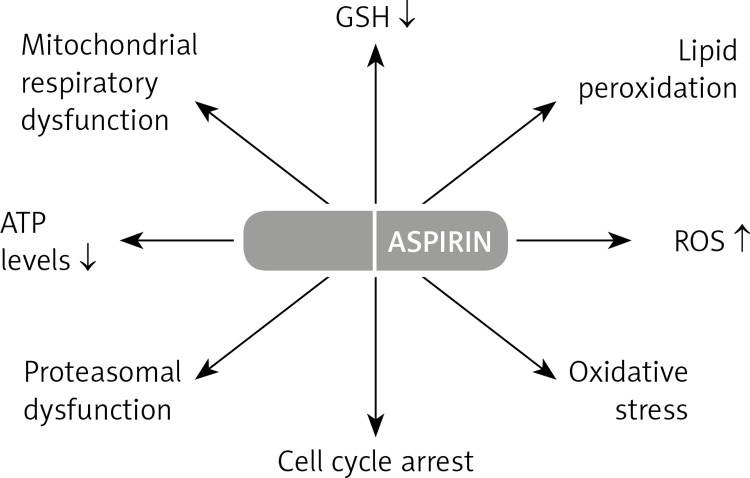
So far, numerous studies have demonstrated that aspirin treatment with 5 and 10 µmol/ml for 24 and 48 h induces oxidative stress, leading to apoptosis and alterations in signal transduction, mitochondrial respiratory function and cell cycle arrest [104–107].
In addition, other effects of ASA treatment include reduced expression of the anti-apoptotic protein Bcl-2, marked depletion of the GSH pool and increased reactive oxygen species production [107].
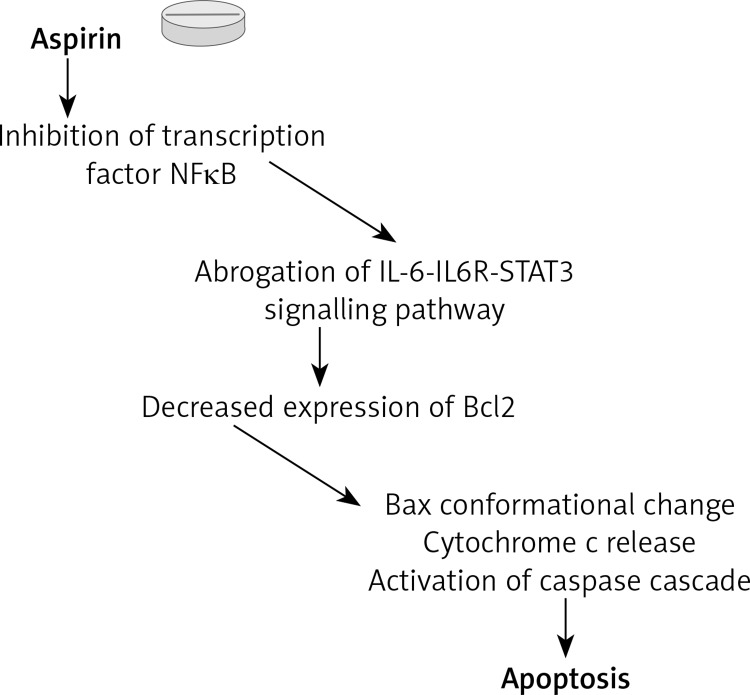
Bcl-2 plays a regulatory role in the transport of GSH into the mitochondria under oxidative stress conditions [107], and downregulation of this protein might also be the cause of mitochondrial membrane potential depletion. Thereafter, decreased expression of Bcl-2 may also cause TRAIL-induced apoptosis by activation of caspases, induction of conformational change, translocation of Bax and cytochrome c release during aspirin treatment [108–111]. Generally, tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is a cytotoxic molecule that has been shown to exert antitumor cytotoxic effects with minimal toxicity to normal tissues [112, 113]. In combination with NSAIDs, including aspirin, TRAIL may increase antitumor activity, leading to cell apoptosis [114, 115]. Both TRAIL and tumor necrosis factor (TNF)-α are known to bind to their cell surface receptors leading to caspase-8 activation. Activated caspase-8 multiplies the apoptotic signal either by activating other caspases or by cleaving BH3 domain-only proteins such as Bid in the Fas signaling pathway [116]. Bid translocates to the mitochondria from the cytosol to induce mitochondrial damage and activate Bax to form Bax multimers [117]. In a further response to apoptotic stimuli, Bax undergoes a conformational change and integrates into the mitochondrial membrane, inducing release of cytochrome c, and activation of caspase-9 and thus caspase-3 [118]. Caspase-3 is the most likely candidate for a mammalian cell death regulator induced by aspirin by cleaving many proteins, such as DNA repair enzyme poly(ADP-ribose) polymerase (PARP) and DNA fragmentation factor 45, leading to the typical 180 bp DNA strand breaks [119]. Cytochrome c release into the cytosol forms a complex with Apaf-1 and the proform of caspase-9 and, in the presence of ATP, this complex triggers a cascade by activating other caspases (3, 6 and 7) [120].
In turn, the factor that probably may be responsible for the reduced expression of Bcl-2 protein is NF-κB [121]. Previous studies have shown that aspirin inhibits the transcription factor NF-κB [106], which is mediated through preventing the phosphorylation and degradation of the inhibitory subunit IκB [122]. Furthermore, a more precise study has shown that aspirin-induced proteasomal dysfunction is responsible for prevention of the degradation of IκB and thereby blocking NF-κB activation [123]. The ubiquitin proteasome system (UPS) is the major lysosomal pathway of cells responsible for the short-lived, misfolded protein and the degradation of several transcription factors in eukaryotes [124]. It also includes proteins involved in cell survival, and it is believed that the dysfunction of this pathway will activate apoptotic signaling pathways and cell death [125, 126].
Another relative molecular mechanism by which aspirin may exert its apoptotic action is abrogation of the IL-6-IL-6R-STAT3 signaling pathway [127]. In general, IL-6 sends its signal into the cell by forming a complex with IL-6R and leads to activation of the receptor associated kinases of the JAK family. In turn, kinases phosphorylate the tyrosine residue (705) present in the transcription factor of the signal transducer and activator of transcription 3 (STAT3) [128]. Following this, STAT3 dimerizes and translocates into the nucleus, inducing the expression of such target genes as cyclin D1, XIAP and Bcl-2. It has been reported that the activation of NF-κB is crucial for the inducible expression of IL-6 expression [129], emphasizing once more participation of NF-κB in aspirin-induced apoptosis (Figure 3).
Figure 3.
Apoptotic mechanism of aspirin action. According to the results, aspirin leads to inhibition of NFκB and next reduces Bcl2 protein expression. In turn, Bcl2 protein reduction causes TRAIL and TNF-α-induced apoptosis by activation of various caspases, conformational change and translocation of Bax and cytochrome c release. Another relative molecular mechanism of aspirin is abrogation of IL-6-IL6R-STAT3 signalling pathway that may also result in reduction of Bcl2 protein expression
In addition, either a decreased level of GSH or elevated ROS number was found to be accompanied by an increase in lipid peroxidation (LPO), which presumably plays a substantial role in oxidative stress-induced mitochondrial dysfunction and apoptosis [107, 130]. On the other hand, treatment with NAC, a GSH synthesis precursor, resulted in partial protection from LPO, suggesting the protective role of mitochondrial GSH metabolism in membrane lipid peroxidation [131].
Oxidative stress-dependent mitochondrial permeability transition also leads to loss of membrane potential and inhibition of mitochondrial bioenergetics [107, 132]. It has been demonstrated that ASA causes a significant decrease in ATP levels accompanied by inhibition of the activities of the respiratory chain enzymes NADH:ubiquinone oxidoreductase (complex 1) and cytochrome c oxidase (complex IV), as well as the mitochondrial matrix enzyme aconitase as a marker for mitochondrial oxidative stress [107]. Application of NAC has also been found to attenuate the marked reduction of ATP level that was supported by the recovery in complex 1 activity in ASA-treated cells [131]. However, lack of recovery of cytochrome c oxidase and aconitase activity suggests that mitochondrial GSH is selectively involved in regulating the activities of the respiratory complexes. It may be attributed to their extremely sensitive responses towards oxidative stress, ROS, NO and glutathionylation of proteins [132].
A recent study has also shown that HepG2 cells were arrested in the G0/G1 phase followed by apoptosis after ASA treatment [107]. It is likely that downregulation of Bcl-2 [133], or interference in signaling via prostaglandin E2-dependent epidermal growth factor receptors and prostaglandin E2- dependent cell surface receptors may contribute to G0/G1 cell cycle arrest [134, 135] (Figure 4).
Figure 4.
Various aspirin actions in cell
The NSAIDs-induced endoplasmic reticulum stress also seems to be connected with the mechanism of apoptosis. The accumulation of unfolded protein in the endoplasmic reticulum (ER) induces what is known as the unfolded protein response (UPR) [77] transduced by three transmembrane proteins: inositol-requiring enzyme 1 (IRE1), PKR-like ER kinase (PERK) and activating transcription factor 6 (ATF6). Cells initially adapt to the accumulation of unfolded proteins by inducing ER-resident stress proteins (chaperons) such as glucose-regulated protein GPR78 that refold the unfolded proteins. These mentioned UPR components are bound to the ER chaperone GPR78 in nonstressed conditions. However, during ER stress GPR78 is separated, leading to their activation. However, if this adaption is not sufficient, the apoptotic response is initiated by CHOP (CCAAT/enhancer-binding protein homologous protein). Treatment with NSAIDs has demonstrated induction of GRP78 and CHOP protein [136]. Studies have also shown that oxidative stress is a strong inducer of UPR in nasal polyps (one of the main symptoms of AIA) cells [137]. It is suggested that ROS trigger UPR by modifying the redox state of the ER lumen, which generates impaired disulfide bonds in maturing proteins [138]. What is more, UPR seems to be linked with the lipoxygenase signaling pathway, because increased LTB4 secretion under UPR induction in nasal polyp cells has already been proven [137]. Therefore UPR may be related to chronic inflammation observed in nasal polyps enforcing recruitment of neutrophils.
As well as high levels of malondialdehyde (MDA), one of the metabolites of free radical-mediated lipid peroxidation was also demonstrated in nasal polyp tissues [139, 140].
Conclusions
The lungs are directly exposed to a higher oxygen concentration than most other tissues, so they develop appropriate mechanisms to be protected against oxidants. However, excessive exposure to oxidizing agents may cause oxidative stress, resulting in the release of proinflammatory mediators, which then recruit inflammatory cells. Additionally, stimulated inflammatory cells release ROS that damage surrounding tissues in the airway, which positively correlates with the direct contraction of airway smooth muscles and greater hyperresponsiveness. Additionally, impairment in antioxidant defenses might be a critical point for asthmatic patients to aggravate lung functions in response to various factors as additional sources of free radicals such as exhaust fumes, cigarette smoke, radiation, certain chemical compounds and drugs. Regarding acetylsalicylic acid, a literature review has revealed half a dozen articles devoted to the aspirin-induced oxidative burden in cancer systems, but there is a lack of research explaining this discrepancy in asthma (possibly due to the enormous research effort invested in oncology at present and the urgent need to find safe and efficient cures). However, confirmation that aspirin may exacerbate oxidative stress in asthma will be very helpful and beneficial in understanding why some asthmatic patients have developed hypersensitivity to aspirin and why they are preponderantly adults. According to this theory, patients with acquired lung mitochondrial dysfunction as a pathophysiological mechanism in this disorder would have a tendency to develop AIA. Moreover, aspirin would constitute a factor that provokes oxidative stress in cells, which would be additionally supported by the inflammatory milieu.
To sum up aforesaid results, it cannot be excluded that oxidative stress might play a pivotal role in pathogenesis of AIA, but it will require a lot of research. So, we hope that this review may contribute to a greater interest in discovering new signaling pathways in aspirin-induced asthma by conducting multiple studies to confirm the aforementioned correlation.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Hayden M, Pignone M, Phillips C, Mulrow C. Aspirin for the primary prevention of cardiovascular events: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;136:161–72. doi: 10.7326/0003-4819-136-2-200201150-00016. [DOI] [PubMed] [Google Scholar]
- 2.Antithrombotic Trialists C, Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849–60. doi: 10.1016/S0140-6736(09)60503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilterdink JL, Bendixen B, Adams HP, Jr, Woolson RF, Clarke WR, Hansen MD. Effect of prior aspirin use on stroke severity in the trial of Org 10172 in acute stroke treatment (TOAST) Stroke. 2001;32:2836–40. doi: 10.1161/hs1201.099384. [DOI] [PubMed] [Google Scholar]
- 4.Kalra L, Perez I, Smithard DG, Sulch D. Does prior use of aspirin affect outcome in ischemic stroke? Am J Med. 2000;108:205–9. doi: 10.1016/s0002-9343(99)00431-3. [DOI] [PubMed] [Google Scholar]
- 5.Hawley SA, Fullerton MD, Ross FA, et al. The ancient drug salicylate directly activates AMP-activated protein kinase. Science. 2012;336:918–22. doi: 10.1126/science.1215327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGeer PL, Schulzer M, McGeer EG. Arthritis and anti-inflammatory agents as possible protective factors for Alzheimer's disease: a review of 17 epidemiologic studies. Neurology. 1996;47:425–32. doi: 10.1212/wnl.47.2.425. [DOI] [PubMed] [Google Scholar]
- 7.Eikelenboom P, Rozemuller JM, van Muiswinkel FL. Inflammation and Alzheimer's disease: relationships between pathogenic mechanisms and clinical expression. Exp Neurol. 1998;154:89–98. doi: 10.1006/exnr.1998.6920. [DOI] [PubMed] [Google Scholar]
- 8.Rothwell PM, Fowkes FG, Belch JF, Ogawa H, Warlow CP, Meade TW. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet. 2011;377:31–41. doi: 10.1016/S0140-6736(10)62110-1. [DOI] [PubMed] [Google Scholar]
- 9.Thun MJ, Henley SJ, Patrono C. Nonsteroidal anti-inflammatory drugs as anticancer agents: mechanistic, pharmacologic, and clinical issues. J Natl Cancer Instit. 2002;94:252–66. doi: 10.1093/jnci/94.4.252. [DOI] [PubMed] [Google Scholar]
- 10.Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552:335–44. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hussain S, Laumbach R, Coleman J, et al. Controlled exposure to diesel exhaust causes increased nitrite in exhaled breath condensate among subjects with asthma. J Occup Environ Med. 2012;54:1186–91. doi: 10.1097/JOM.0b013e31826bb64c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morrow JD, Frei B, Longmire AW, et al. Increase in circulating products of lipid peroxidation (F2-isoprostanes) in smokers. Smoking as a cause of oxidative damage. N Engl J Med. 1995;332:1198–203. doi: 10.1056/NEJM199505043321804. [DOI] [PubMed] [Google Scholar]
- 13.Henricks PA, Nijkamp FP. Reactive oxygen species as mediators in asthma. Pulm Pharmacol Ther. 2001;14:409–20. doi: 10.1006/pupt.2001.0319. [DOI] [PubMed] [Google Scholar]
- 14.Suski JM, Lebiedzinska M, Bonora M, Pinton P, Duszynski J, Wieckowski MR. Relation between mitochondrial membrane potential and ROS formation. Meth Mol Biol. 2012;810:183–205. doi: 10.1007/978-1-61779-382-0_12. [DOI] [PubMed] [Google Scholar]
- 15.DiMauro S, Schon EA. Mitochondrial disorders in the nervous system. Ann Rev Neurosci. 2008;31:91–123. doi: 10.1146/annurev.neuro.30.051606.094302. [DOI] [PubMed] [Google Scholar]
- 16.Levine SJ. Bronchial epithelial cell-cytokine interactions in airway inflammation. J Investig Med. 1995;43:241–9. [PubMed] [Google Scholar]
- 17.Rahman I. Oxidative stress, chromatin remodeling and gene transcription in inflammation and chronic lung diseases. J Biochem Mol Biol. 2003;36:95–109. doi: 10.5483/bmbrep.2003.36.1.095. [DOI] [PubMed] [Google Scholar]
- 18.Barbieri SS, Zacchi E, Amadio P, et al. Cytokines present in smokers’ serum interact with smoke components to enhance endothelial dysfunction. Cardiovasc Res. 2011;90:475–83. doi: 10.1093/cvr/cvr032. [DOI] [PubMed] [Google Scholar]
- 19.Lee IT, Luo SF, Lee CW, et al. Overexpression of HO-1 protects against TNF-alpha-mediated airway inflammation by down-regulation of TNFR1-dependent oxidative stress. Am J Pathol. 2009;175:519–32. doi: 10.2353/ajpath.2009.090016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee CW, Lin CC, Lee IT, Lee HC, Yang CM. Activation and induction of cytosolic phospholipase A2 by TNF-alpha mediated through Nox2, MAPKs, NF-kappaB, and p300 in human tracheal smooth muscle cells. J Cell Physiol. 2011;226:2103–14. doi: 10.1002/jcp.22537. [DOI] [PubMed] [Google Scholar]
- 21.Lin CP, Huang PH, Tsai HS, et al. Monascus purpureus-fermented rice inhibits tumor necrosis factor-alpha-induced upregulation of matrix metalloproteinase 2 and 9 in human aortic smooth muscle cells. J Pharmacy Pharmacol. 2011;63:1587–94. doi: 10.1111/j.2042-7158.2011.01364.x. [DOI] [PubMed] [Google Scholar]
- 22.Luo SF, Chang CC, Lee IT, et al. Activation of ROS/NF-kappaB and Ca2 + /CaM kinase II are necessary for VCAM-1 induction in IL-1beta-treated human tracheal smooth muscle cells. Toxicol Appl Pharmacol. 2009;237:8–21. doi: 10.1016/j.taap.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 23.Nagata M. Inflammatory cells and oxygen radicals. Curr Drug Targets Inflamm Allergy. 2005;4:503–4. doi: 10.2174/1568010054526322. [DOI] [PubMed] [Google Scholar]
- 24.Adcock IM, Brown CR, Kwon O, Barnes PJ. Oxidative stress induces NF kappa B DNA binding and inducible NOS mRNA in human epithelial cells. Biochem Biophys Res Commun. 1994;199:1518–24. doi: 10.1006/bbrc.1994.1403. [DOI] [PubMed] [Google Scholar]
- 25.Haddad JJ, Olver RE, Land SC. Antioxidant/pro-oxidant equilibrium regulates HIF-1alpha and NF-kappa B redox sensitivity. Evidence for inhibition by glutathione oxidation in alveolar epithelial cells. J Biol Chem. 2000;275:21130–9. doi: 10.1074/jbc.M000737200. [DOI] [PubMed] [Google Scholar]
- 26.Krishna MT, Chauhan AJ, Frew AJ, Holgate ST. Toxicological mechanisms underlying oxidant pollutant-induced airway injury. Rev Environ Health. 1998;13:59–71. [PubMed] [Google Scholar]
- 27.Barnes PJ, Karin M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066–71. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- 28.Zuo L, Nogueira L, Hogan MC. Reactive oxygen species formation during tetanic contractions in single isolated Xenopus myofibers. J Appl Physiol. 2011;111:898–904. doi: 10.1152/japplphysiol.00398.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zuo L, Clanton TL. Reactive oxygen species formation in the transition to hypoxia in skeletal muscle. Am J Physiol Cell Physiol. 2005;289:C207–16. doi: 10.1152/ajpcell.00449.2004. [DOI] [PubMed] [Google Scholar]
- 30.Doelman CJ, Leurs R, Oosterom WC, Bast A. Mineral dust exposure and free radical-mediated lung damage. Exp Lung Res. 1990;16:41–55. doi: 10.3109/01902149009064698. [DOI] [PubMed] [Google Scholar]
- 31.Nabe T, Ikedo A, Hosokawa F, et al. Regulatory role of antigen-induced interleukin-10, produced by CD4(+) T cells, in airway neutrophilia in a murine model for asthma. Eur J Pharmacol. 2012;677:154–62. doi: 10.1016/j.ejphar.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 32.Wood LG, Gibson PG, Garg ML. Biomarkers of lipid peroxidation, airway inflammation and asthma. Eur Respir J. 2003;21:177–86. doi: 10.1183/09031936.03.00017003a. [DOI] [PubMed] [Google Scholar]
- 33.Riedl MA, Nel AE. Importance of oxidative stress in the pathogenesis and treatment of asthma. Curr Opin Allergy Clin Immunol. 2008;8:49–56. doi: 10.1097/ACI.0b013e3282f3d913. [DOI] [PubMed] [Google Scholar]
- 34.Comhair SA, Bhathena PR, Dweik RA, Kavuru M, Erzurum SC. Rapid loss of superoxide dismutase activity during antigen-induced asthmatic response. Lancet. 2000;355:624. doi: 10.1016/S0140-6736(99)04736-4. [DOI] [PubMed] [Google Scholar]
- 35.Smith LJ, Shamsuddin M, Sporn PH, Denenberg M, Anderson J. Reduced superoxide dismutase in lung cells of patients with asthma. Free Radic Biol Med. 1997;22:1301–7. doi: 10.1016/s0891-5849(96)00550-3. [DOI] [PubMed] [Google Scholar]
- 36.Comhair SA, Xu W, Ghosh S, et al. Superoxide dismutase inactivation in pathophysiology of asthmatic airway remodeling and reactivity. Am J Pathol. 2005;166:663–74. doi: 10.1016/S0002-9440(10)62288-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seltzer J, Bigby BG, Stulbarg M, et al. O3-induced change in bronchial reactivity to methacholine and airway inflammation in humans. J Appl Physiol. 1986;60:1321–6. doi: 10.1152/jappl.1986.60.4.1321. [DOI] [PubMed] [Google Scholar]
- 38.Hiltermann TJ, Peters EA, Alberts B, et al. Ozone-induced airway hyperresponsiveness in patients with asthma: role of neutrophil-derived serine proteinases. Free Radic Biol Med. 1998;24:952–8. doi: 10.1016/s0891-5849(97)00381-x. [DOI] [PubMed] [Google Scholar]
- 39.Sanders SP, Zweier JL, Harrison SJ, Trush MA, Rembish SJ, Liu MC. Spontaneous oxygen radical production at sites of antigen challenge in allergic subjects. Am J Respir Crit Care Med. 1995;151:1725–33. doi: 10.1164/ajrccm.151.6.7767513. [DOI] [PubMed] [Google Scholar]
- 40.Vachier I, Damon M, Le Doucen C, et al. Increased oxygen species generation in blood monocytes of asthmatic patients. Am Rev Respir Dis. 1992;146:1161–6. doi: 10.1164/ajrccm/146.5_Pt_1.1161. [DOI] [PubMed] [Google Scholar]
- 41.Foreman RC, Mercer PF, Kroegel C, Warner JA. Role of the eosinophil in protein oxidation in asthma: possible effects on proteinase/antiproteinase balance. Int Arch Allergy Immunol. 1999;118:183–6. doi: 10.1159/000024061. [DOI] [PubMed] [Google Scholar]
- 42.Hulsmann AR, Raatgeep HR, den Hollander JC, et al. Oxidative epithelial damage produces hyperresponsiveness of human peripheral airways. Am J Respir Crit Care Med. 1994;149:519–25. doi: 10.1164/ajrccm.149.2.8306055. [DOI] [PubMed] [Google Scholar]
- 43.Bucchieri F, Puddicombe SM, Lordan JL, et al. Asthmatic bronchial epithelium is more susceptible to oxidant-induced apoptosis. Am J Respir Cell Mol Biol. 2002;27:179–85. doi: 10.1165/ajrcmb.27.2.4699. [DOI] [PubMed] [Google Scholar]
- 44.Salome CM, Roberts AM, Brown NJ, Dermand J, Marks GB, Woolcock AJ. Exhaled nitric oxide measurements in a population sample of young adults. Am J Respir Crit Care Med. 1999;159:911–6. doi: 10.1164/ajrccm.159.3.9802108. [DOI] [PubMed] [Google Scholar]
- 45.Kharitonov SA, Chung KF, Evans D, O'Connor BJ, Barnes PJ. Increased exhaled nitric oxide in asthma is mainly derived from the lower respiratory tract. Am J Respir Crit Care Med. 1996;153:1773–80. doi: 10.1164/ajrccm.153.6.8665033. [DOI] [PubMed] [Google Scholar]
- 46.Kharitonov SA, O'Connor BJ, Evans DJ, Barnes PJ. Allergen-induced late asthmatic reactions are associated with elevation of exhaled nitric oxide. Am J Respir Crit Care Med. 1995;151:1894–9. doi: 10.1164/ajrccm.151.6.7767537. [DOI] [PubMed] [Google Scholar]
- 47.Kharitonov SA, Yates DH, Barnes PJ. Inhaled glucocorticoids decrease nitric oxide in exhaled air of asthmatic patients. Am J Respir Crit Care Med. 1996;153:454–7. doi: 10.1164/ajrccm.153.1.8542158. [DOI] [PubMed] [Google Scholar]
- 48.Sippel JM, Holden WE, Tilles SA, et al. Exhaled nitric oxide levels correlate with measures of disease control in asthma. J Allergy Clin Immunol. 2000;106:645–50. doi: 10.1067/mai.2000.109618. [DOI] [PubMed] [Google Scholar]
- 49.Jatakanon A, Lim S, Kharitonov SA, Chung KF, Barnes PJ. Correlation between exhaled nitric oxide, sputum eosinophils, and methacholine responsiveness in patients with mild asthma. Thorax. 1998;53:91–5. doi: 10.1136/thx.53.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hampl V, Herget J. Role of nitric oxide in the pathogenesis of chronic pulmonary hypertension. Physiol Rev. 2000;80:1337–72. doi: 10.1152/physrev.2000.80.4.1337. [DOI] [PubMed] [Google Scholar]
- 51.Rahman I, Biswas SK, Kode A. Oxidant and antioxidant balance in the airways and airway diseases. Eur J Pharmacol. 2006;533:222–39. doi: 10.1016/j.ejphar.2005.12.087. [DOI] [PubMed] [Google Scholar]
- 52.van der Vliet A, Eiserich JP, Shigenaga MK, Cross CE. Reactive nitrogen species and tyrosine nitration in the respiratory tract: epiphenomena or a pathobiologic mechanism of disease? Am J Respir Crit Care Med. 1999;160:1–9. doi: 10.1164/ajrccm.160.1.9807044. [DOI] [PubMed] [Google Scholar]
- 53.Hansel TT, Kharitonov SA, Donnelly LE, et al. A selective inhibitor of inducible nitric oxide synthase inhibits exhaled breath nitric oxide in healthy volunteers and asthmatics. FASEB J. 2003;17:1298–300. doi: 10.1096/fj.02-0633fje. [DOI] [PubMed] [Google Scholar]
- 54.Brindicci C, Ito K, Torre O, Barnes PJ, Kharitonov SA. Effects of aminoguanidine, an inhibitor of inducible nitric oxide synthase, on nitric oxide production and its metabolites in healthy control subjects, healthy smokers, and COPD patients. Chest. 2009;135:353–67. doi: 10.1378/chest.08-0964. [DOI] [PubMed] [Google Scholar]
- 55.Zayasu K, Sekizawa K, Okinaga S, Yamaya M, Ohrui T, Sasaki H. Increased carbon monoxide in exhaled air of asthmatic patients. Am J Respir Crit Care Med. 1997;156:1140–3. doi: 10.1164/ajrccm.156.4.96-08056. [DOI] [PubMed] [Google Scholar]
- 56.Nowak D, Antczak A, Krol M, et al. Increased content of hydrogen peroxide in the expired breath of cigarette smokers. Eur Respir J. 1996;9:652–7. doi: 10.1183/09031936.96.09040652. [DOI] [PubMed] [Google Scholar]
- 57.Teng Y, Sun P, Zhang J, et al. Hydrogen peroxide in exhaled breath condensate in patients with asthma: a promising biomarker? Chest. 2011;140:108–16. doi: 10.1378/chest.10-2816. [DOI] [PubMed] [Google Scholar]
- 58.Horvath I, Donnelly LE, Kiss A, Balint B, Kharitonov SA, Barnes PJ. Exhaled nitric oxide and hydrogen peroxide concentrations in asthmatic smokers. Respiration. 2004;71:463–8. doi: 10.1159/000080630. [DOI] [PubMed] [Google Scholar]
- 59.Baraldi E, Ghiro L, Piovan V, et al. Increased exhaled 8-isoprostane in childhood asthma. Chest. 2003;124:25–31. doi: 10.1378/chest.124.1.25. [DOI] [PubMed] [Google Scholar]
- 60.Shahid SK, Kharitonov SA, Wilson NM, Bush A, Barnes PJ. Exhaled 8-isoprostane in childhood asthma. Respir Res. 2005;6:79. doi: 10.1186/1465-9921-6-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Montuschi P, Corradi M, Ciabattoni G, Nightingale J, Kharitonov SA, Barnes PJ. Increased 8-isoprostane, a marker of oxidative stress, in exhaled condensate of asthma patients. Am J Respir Crit Care Med. 1999;160:216–20. doi: 10.1164/ajrccm.160.1.9809140. [DOI] [PubMed] [Google Scholar]
- 62.Baraldi E, Carraro S, Alinovi R, et al. Cysteinyl leukotrienes and 8-isoprostane in exhaled breath condensate of children with asthma exacerbations. Thorax. 2003;58:505–9. doi: 10.1136/thorax.58.6.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bodini A, Peroni D, Vicentini L, et al. Exhaled breath condensate eicosanoids and sputum eosinophils in asthmatic children: a pilot study. Pediatr Allergy Immunol. 2004;15:26–31. doi: 10.1046/j.0905-6157.2003.00097.x. [DOI] [PubMed] [Google Scholar]
- 64.Montuschi P, Collins JV, Ciabattoni G, et al. Exhaled 8-isoprostane as an in vivo biomarker of lung oxidative stress in patients with COPD and healthy smokers. Am J Respir Crit Care Med. 2000;162:1175–7. doi: 10.1164/ajrccm.162.3.2001063. [DOI] [PubMed] [Google Scholar]
- 65.Simpson JL, Wood LG, Gibson PG. Inflammatory mediators in exhaled breath, induced sputum and saliva. Clin Exp Allergy. 2005;35:1180–5. doi: 10.1111/j.1365-2222.2005.02327.x. [DOI] [PubMed] [Google Scholar]
- 66.Wood LG, Garg ML, Simpson JL, et al. Induced sputum 8-isoprostane concentrations in inflammatory airway diseases. Am J Respir Crit Care Med. 2005;171:426–30. doi: 10.1164/rccm.200408-1010OC. [DOI] [PubMed] [Google Scholar]
- 67.Jackson AS, Sandrini A, Campbell C, Chow S, Thomas PS, Yates DH. Comparison of biomarkers in exhaled breath condensate and bronchoalveolar lavage. Am J Respir Crit Care Med. 2007;175:222–7. doi: 10.1164/rccm.200601-107OC. [DOI] [PubMed] [Google Scholar]
- 68.Deveci F, Ilhan N, Turgut T, Akpolat N, Kirkil G, Muz MH. Glutathione and nitrite in induced sputum from patients with stable and acute asthma compared with controls. Ann Allergy Asthma Immunol. 2004;93:91–7. doi: 10.1016/S1081-1206(10)61452-4. [DOI] [PubMed] [Google Scholar]
- 69.Comhair SA, Ricci KS, Arroliga M, et al. Correlation of systemic superoxide dismutase deficiency to airflow obstruction in asthma. Am J Respir Crit Care Med. 2005;172:306–13. doi: 10.1164/rccm.200502-180OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.London SJ. Gene-air pollution interactions in asthma. Proc Am Thorac Soc. 2007;4:217–20. doi: 10.1513/pats.200701-031AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gilliland FD, Li YF, Gong H, Jr, Diaz-Sanchez D. Glutathione s-transferases M1 and P1 prevent aggravation of allergic responses by secondhand smoke. Am J Respir Crit Care Med. 2006;174:1335–41. doi: 10.1164/rccm.200509-1424OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim JH, Park BL, Cheong HS, et al. Genome-wide and follow-up studies identify CEP68 gene variants associated with risk of aspirin-intolerant asthma. PloS one. 2010;5:e13818. doi: 10.1371/journal.pone.0013818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kowalski ML, Ptasinska A, Bienkiewicz B, Pawliczak R, DuBuske L. Differential effects of aspirin and misoprostol on 15-hydroxyeicosatetraenoic acid generation by leukocytes from aspirin-sensitive asthmatic patients. J Allergy Clin Immunol. 2003;112:505–12. doi: 10.1016/s0091-6749(03)01716-0. [DOI] [PubMed] [Google Scholar]
- 74.Ono E, Taniguchi M, Higashi N, et al. Increase in salivary cysteinyl-leukotriene concentration in patients with aspirin-intolerant asthma. Allergol Int. 2011;60:37–43. doi: 10.2332/allergolint.09-OA-0166. [DOI] [PubMed] [Google Scholar]
- 75.Christie PE, Tagari P, Ford-Hutchinson AW, et al. Urinary leukotriene E4 concentrations increase after aspirin challenge in aspirin-sensitive asthmatic subjects. Am Rev Respir Dis. 1991;143:1025–9. doi: 10.1164/ajrccm/143.5_Pt_1.1025. [DOI] [PubMed] [Google Scholar]
- 76.Sladek K, Szczeklik A. Cysteinyl leukotrienes overproduction and mast cell activation in aspirin-provoked bronchospasm in asthma. Eur Respir J. 1993;6:391–9. [PubMed] [Google Scholar]
- 77.Kupczyk M, Antczak A, Kuprys-Lipinska I, Kuna P. Lipoxin A4 generation is decreased in aspirin-sensitive patients in lysine-aspirin nasal challenge in vivo model. Allergy. 2009;64:1746–52. doi: 10.1111/j.1398-9995.2009.02047.x. [DOI] [PubMed] [Google Scholar]
- 78.Bonnans C, Levy BD. Lipid mediators as agonists for the resolution of acute lung inflammation and injury. Am J Respir Cell Mol Biol. 2007;36:201–5. doi: 10.1165/rcmb.2006-0269TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pillinger MH, Capodici C, Rosenthal P, et al. Modes of action of aspirin-like drugs: salicylates inhibit erk activation and integrin-dependent neutrophil adhesion. Proc Natl Acad Sci USA. 1998;95:14540–5. doi: 10.1073/pnas.95.24.14540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Picado C, Fernandez-Morata JC, Juan M, et al. Cyclooxygenase-2 mRNA is downexpressed in nasal polyps from aspirin-sensitive asthmatics. Am J Respir Crit Care Med. 1999;160:291–6. doi: 10.1164/ajrccm.160.1.9808048. [DOI] [PubMed] [Google Scholar]
- 81.Kim SH, Oh JM, Kim YS, et al. Cysteinyl leukotriene receptor 1 promoter polymorphism is associated with aspirin-intolerant asthma in males. Clin Exp Allergy. 2006;36:433–9. doi: 10.1111/j.1365-2222.2006.02457.x. [DOI] [PubMed] [Google Scholar]
- 82.Early SB, Barekzi E, Negri J, Hise K, Borish L, Steinke JW. Concordant modulation of cysteinyl leukotriene receptor expression by IL-4 and IFN-gamma on peripheral immune cells. Am J Respir Cell Mol Biol. 2007;36:715–20. doi: 10.1165/rcmb.2006-0252OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim TH, Chang HS, Park SM, et al. Association of angiotensin I-converting enzyme gene polymorphisms with aspirin intolerance in asthmatics. Clin Exp Allergy. 2008;38:1727–37. doi: 10.1111/j.1365-2222.2008.03082.x. [DOI] [PubMed] [Google Scholar]
- 84.Kim SH, Yang EM, Lee HN, Choi GS, Ye YM, Park HS. Association of the CCR3 gene polymorphism with aspirin exacerbated respiratory disease. Respir Med. 2010;104:626–32. doi: 10.1016/j.rmed.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 85.Hitomi Y, Ebisawa M, Tomikawa M, et al. Associations of functional NLRP3 polymorphisms with susceptibility to food-induced anaphylaxis and aspirin-induced asthma. J Allergy Clin Immunol. 2009;124:779–85 e6. doi: 10.1016/j.jaci.2009.07.044. [DOI] [PubMed] [Google Scholar]
- 86.Sutterwala FS, Ogura Y, Szczepanik M, et al. Critical role for NALP3/CIAS1/Cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity. 2006;24:317–27. doi: 10.1016/j.immuni.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 87.Minshall EM, Leung DY, Martin RJ, et al. Eosinophil-associated TGF-beta1 mRNA expression and airways fibrosis in bronchial asthma. Am J Respir Cell Mol Biol. 1997;17:326–33. doi: 10.1165/ajrcmb.17.3.2733. [DOI] [PubMed] [Google Scholar]
- 88.Kim JY, Kim JH, Park TJ, et al. Positive association between aspirin-intolerant asthma and genetic polymorphisms of FSIP1: a case-case study. BMC Pulm Med. 2010;10:34. doi: 10.1186/1471-2466-10-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gosens R, Zaagsma J, Grootte Bromhaar M, Nelemans A, Meurs H. Acetylcholine: a novel regulator of airway smooth muscle remodelling? Eur J Pharmacol. 2004;500:193–201. doi: 10.1016/j.ejphar.2004.07.025. [DOI] [PubMed] [Google Scholar]
- 90.Park TJ, Kim JH, Bae JS, et al. Possible association of SLC22A2 polymorphisms with aspirin-intolerant asthma. Int Arch Allergy Immunol. 2011;155:395–402. doi: 10.1159/000321267. [DOI] [PubMed] [Google Scholar]
- 91.Sakagami T, Jinnai N, Nakajima T, et al. ADAM33 polymorphisms are associated with aspirin-intolerant asthma in the Japanese population. J Human Genet. 2007;52:66–72. doi: 10.1007/s10038-006-0081-6. [DOI] [PubMed] [Google Scholar]
- 92.Pasaje CF, Kim JH, Park BL, et al. A possible association of EMID2 polymorphisms with aspirin hypersensitivity in asthma. Immunogenetics. 2011;63:13–21. doi: 10.1007/s00251-010-0490-8. [DOI] [PubMed] [Google Scholar]
- 93.Palikhe NS, Kim SH, Kim JH, Losol P, Ye YM, Park HS. Role of Toll-like receptor 3 variants in aspirin-exacerbated respiratory disease. Allergy Asthma Immunol Res. 2011;3:123–7. doi: 10.4168/aair.2011.3.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kim SH, Kim YK, Park HW, et al. Adenosine deaminase and adenosine receptor polymorphisms in aspirin-intolerant asthma. Respir Med. 2009;103:356–63. doi: 10.1016/j.rmed.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 95.Lee JS, Kim JH, Bae JS, et al. Association of CACNG6 polymorphisms with aspirin-intolerance asthmatics in a Korean population. BMC Med Genet. 2010;11:138. doi: 10.1186/1471-2350-11-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pasaje CF, Bae JS, Park BL, et al. WDR46 is a genetic risk factor for aspirin-exacerbated respiratory disease in a Korean population. Allergy Asthma Immunol Res. 2012;4:199–205. doi: 10.4168/aair.2012.4.4.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pasaje CF, Bae JS, Park BL, et al. A possible association between ZNRD1 and aspirin-induced airway bronchoconstriction in a Korean population. J Investig Allergol Clin Immunol. 2012;22:193–200. [PubMed] [Google Scholar]
- 98.Pasaje CF, Bae JS, Park BL, et al. Genetic variations in KIFC1 and the risk of aspirin exacerbated respiratory disease in a Korean population: an association analysis. Mol Biol Rep. 2012;39:5913–9. doi: 10.1007/s11033-011-1403-0. [DOI] [PubMed] [Google Scholar]
- 99.Kohyama K, Abe S, Kodaira K, et al. Polymorphisms of the CYP2C19 gene in Japanese patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2011;128:1117–20. doi: 10.1016/j.jaci.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 100.Dworski R, Murray JJ, Roberts LJ, 2nd, et al. Allergen-induced synthesis of F(2)-isoprostanes in atopic asthmatics. Evidence for oxidant stress. Am J Respir Crit Care Med. 1999;160:1947–51. doi: 10.1164/ajrccm.160.6.9903064. [DOI] [PubMed] [Google Scholar]
- 101.Oliveira L, Stallwood NA, Crankshaw DJ. Effects of some isoprostanes on the human umbilical artery in vitro. Br J Pharmacol. 2000;129:509–14. doi: 10.1038/sj.bjp.0703083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Janssen LJ, Premji M, Netherton S, et al. Excitatory and inhibitory actions of isoprostanes in human and canine airway smooth muscle. J Pharmacol Exp Therap. 2000;295:506–11. [PubMed] [Google Scholar]
- 103.Antczak A, Montuschi P, Kharitonov S, Gorski P, Barnes PJ. Increased exhaled cysteinyl-leukotrienes and 8-isoprostane in aspirin-induced asthma. Am J Respir Crit Care Med. 2002;166:301–6. doi: 10.1164/rccm.2101021. [DOI] [PubMed] [Google Scholar]
- 104.Goel A, Chang DK, Ricciardiello L, Gasche C, Boland CR. A novel mechanism for aspirin-mediated growth inhibition of human colon cancer cells. Clin Cancer Res. 2003;9:383–90. [PubMed] [Google Scholar]
- 105.Husain SS, Szabo IL, Tamawski AS. NSAID inhibition of GI cancer growth: clinical implications and molecular mechanisms of action. Am J Gastroenterol. 2002;97:542–53. doi: 10.1111/j.1572-0241.2002.05528.x. [DOI] [PubMed] [Google Scholar]
- 106.Kopp E, Ghosh S. Inhibition of NF-kappa B by sodium salicylate and aspirin. Science. 1994;265:956–9. doi: 10.1126/science.8052854. [DOI] [PubMed] [Google Scholar]
- 107.Raza H, John A, Benedict S. Acetylsalicylic acid-induced oxidative stress, cell cycle arrest, apoptosis and mitochondrial dysfunction in human hepatoma HepG2 cells. Eur J Pharmacol. 2011;668:15–24. doi: 10.1016/j.ejphar.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 108.Li M, Lotan R, Levin B, Tahara E, Lippman SM, Xu XC. Aspirin induction of apoptosis in esophageal cancer: a potential for chemoprevention. Cancer Epidemiol Biomarkers Prevent. 2000;9:545–9. [PubMed] [Google Scholar]
- 109.Kim KY, Seol JY, Jeon GA, Nam MJ. The combined treatment of aspirin and radiation induces apoptosis by the regulation of bcl-2 and caspase-3 in human cervical cancer cell. Cancer Letters. 2003;189:157–66. doi: 10.1016/s0304-3835(02)00519-0. [DOI] [PubMed] [Google Scholar]
- 110.Kim KM, Song JJ, An JY, Kwon YT, Lee YJ. Pretreatment of acetylsalicylic acid promotes tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis by down-regulating BCL-2 gene expression. J Biol Chem. 2005;280:41047–56. doi: 10.1074/jbc.M503713200. [DOI] [PubMed] [Google Scholar]
- 111.Pique M, Barragan M, Dalmau M, Bellosillo B, Pons G, Gil J. Aspirin induces apoptosis through mitochondrial cytochrome c release. FEBS Lett. 2000;480:193–6. doi: 10.1016/s0014-5793(00)01922-0. [DOI] [PubMed] [Google Scholar]
- 112.Ashkenazi A, Dixit VM. Apoptosis control by death and decoy receptors. Curr Opin Cell Biol. 1999;11:255–60. doi: 10.1016/s0955-0674(99)80034-9. [DOI] [PubMed] [Google Scholar]
- 113.Ashkenazi A, Pai RC, Fong S, et al. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Investig. 1999;104:155–62. doi: 10.1172/JCI6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.de Jong S, Timmer T, Heijenbrok FJ, de Vries EG. Death receptor ligands, in particular TRAIL, to overcome drug resistance. Cancer Metastasis Rev. 2001;20:51–6. doi: 10.1023/a:1013112624971. [DOI] [PubMed] [Google Scholar]
- 115.Wajant H, Pfizenmaier K, Scheurich P. TNF-related apoptosis inducing ligand (TRAIL) and its receptors in tumor surveillance and cancer therapy. Apoptosis. 2002;7:449–59. doi: 10.1023/a:1020039225764. [DOI] [PubMed] [Google Scholar]
- 116.Kruidering M, Evan GI. Caspase-8 in apoptosis: the beginning of “the end”? IUBMB Life. 2000;50:85–90. doi: 10.1080/713803693. [DOI] [PubMed] [Google Scholar]
- 117.Roucou X, Montessuit S, Antonsson B, Martinou JC. Bax oligomerization in mitochondrial membranes requires tBid (caspase-8-cleaved Bid) and a mitochondrial protein. Biochem J. 2002;368:915–21. doi: 10.1042/BJ20020972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Antonsson B, Montessuit S, Sanchez B, Martinou JC. Bax is present as a high molecular weight oligomer/complex in the mitochondrial membrane of apoptotic cells. J Biol Chem. 2001;276:11615–23. doi: 10.1074/jbc.M010810200. [DOI] [PubMed] [Google Scholar]
- 119.Boulares AH, Yakovlev AG, Ivanova V, et al. Role of poly(ADP-ribose) polymerase (PARP) cleavage in apoptosis. Caspase 3-resistant PARP mutant increases rates of apoptosis in transfected cells. J Biol Chem. 1999;274:22932–40. doi: 10.1074/jbc.274.33.22932. [DOI] [PubMed] [Google Scholar]
- 120.Pan G, Humke EW, Dixit VM. Activation of caspases triggered by cytochrome c in vitro. FEBS Letters. 1998;426:151–4. doi: 10.1016/s0014-5793(98)00330-5. [DOI] [PubMed] [Google Scholar]
- 121.Bui NT, Livolsi A, Peyron JF, Prehn JH. Activation of nuclear factor kappaB and Bcl-x survival gene expression by nerve growth factor requires tyrosine phosphorylation of IkappaBalpha. J Cell Biol. 2001;152:753–64. doi: 10.1083/jcb.152.4.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yin MJ, Yamamoto Y, Gaynor RB. The anti-inflammatory agents aspirin and salicylate inhibit the activity of I(kappa)B kinase-beta. Nature. 1998;396:77–80. doi: 10.1038/23948. [DOI] [PubMed] [Google Scholar]
- 123.Dikshit P, Chatterjee M, Goswami A, Mishra A, Jana NR. Aspirin induces apoptosis through the inhibition of proteasome function. J Biol Chem. 2006;281:29228–35. doi: 10.1074/jbc.M602629200. [DOI] [PubMed] [Google Scholar]
- 124.Glickman MH, Ciechanover A. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev. 2002;82:373–428. doi: 10.1152/physrev.00027.2001. [DOI] [PubMed] [Google Scholar]
- 125.Jana NR, Dikshit P, Goswami A, Nukina N. Inhibition of proteasomal function by curcumin induces apoptosis through mitochondrial pathway. J Biol Chem. 2004;279:11680–5. doi: 10.1074/jbc.M310369200. [DOI] [PubMed] [Google Scholar]
- 126.Jana NR, Zemskov EA, Wang G, Nukina N. Altered proteasomal function due to the expression of polyglutamine-expanded truncated N-terminal huntingtin induces apoptosis by caspase activation through mitochondrial cytochrome c release. Human Mol Genet. 2001;10:1049–59. doi: 10.1093/hmg/10.10.1049. [DOI] [PubMed] [Google Scholar]
- 127.Kim SR, Bae MK, Kim JY, Wee HJ, Yoo MA, Bae SK. Aspirin induces apoptosis through the blockade of IL-6-STAT3 signaling pathway in human glioblastoma A172 cells. Biochem Biophys Res Commun. 2009;387:342–7. doi: 10.1016/j.bbrc.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 128.Heinrich PC, Behrmann I, Muller-Newen G, Schaper F, Graeve L. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem J. 1998;334:297–314. doi: 10.1042/bj3340297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Beetz A, Peter RU, Oppel T, et al. NF-kappaB and AP-1 are responsible for inducibility of the IL-6 promoter by ionizing radiation in HeLa cells. Int J Radiat Biol. 2000;76:1443–53. doi: 10.1080/09553000050176207. [DOI] [PubMed] [Google Scholar]
- 130.Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial ROS-induced ROS release: an update and review. Biochim Biophys Acta. 2006;1757:509–17. doi: 10.1016/j.bbabio.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 131.Raza H, John A. Implications of altered glutathione metabolism in aspirin-induced oxidative stress and mitochondrial dysfunction in HepG2 cells. PloS One. 2012;7:e36325. doi: 10.1371/journal.pone.0036325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hanif R, Pittas A, Feng Y, et al. Effects of nonsteroidal anti-inflammatory drugs on proliferation and on induction of apoptosis in colon cancer cells by a prostaglandin-independent pathway. Biochem Pharmacol. 1996;52:237–45. doi: 10.1016/0006-2952(96)00181-5. [DOI] [PubMed] [Google Scholar]
- 133.Li P, Zhang ST, Yu ZL, et al. Effects of cyclooxygenase-2 non-selective and selective inhibitors on proliferation inhibition and apoptosis induction of esophageal squamous carcinoma cells. Dis Esoph. 2009;22:21–31. doi: 10.1111/j.1442-2050.2008.00836.x. [DOI] [PubMed] [Google Scholar]
- 134.Dajani OF, Meisdalen K, Guren TK, et al. Prostaglandin E2 upregulates EGF-stimulated signaling in mitogenic pathways involving Akt and ERK in hepatocytes. J Cell Physiol. 2008;214:371–80. doi: 10.1002/jcp.21205. [DOI] [PubMed] [Google Scholar]
- 135.Doherty GA, Byrne SM, Molloy ES, et al. Proneoplastic effects of PGE2 mediated by EP4 receptor in colorectal cancer. BMC Cancer. 2009;9:207. doi: 10.1186/1471-2407-9-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tsutsumi S, Gotoh T, Tomisato W, et al. Endoplasmic reticulum stress response is involved in nonsteroidal anti-inflammatory drug-induced apoptosis. Cell Death Different. 2004;11:1009–16. doi: 10.1038/sj.cdd.4401436. [DOI] [PubMed] [Google Scholar]
- 137.Jeanson L, Kelly M, Coste A, et al. Oxidative stress induces unfolding protein response and inflammation in nasal polyposis. Allergy. 2012;67:403–12. doi: 10.1111/j.1398-9995.2011.02769.x. [DOI] [PubMed] [Google Scholar]
- 138.Chen W, Shang WH, Adachi Y, Hirose K, Ferrari DM, Kamata T. A possible biochemical link between NADPH oxidase (Nox) 1 redox-signalling and ERp72. Biochem J. 2008;416:55–63. doi: 10.1042/BJ20071259. [DOI] [PubMed] [Google Scholar]
- 139.Dogru H, Delibas N, Doner F, Tuz M, Uygur K. Free radical damage in nasal polyp tissue. Otolaryngol Head Neck Surgery. 2001;124:570–2. doi: 10.1067/mhn.2001.115086. [DOI] [PubMed] [Google Scholar]
- 140.Dagli M, Eryilmaz A, Besler T, Akmansu H, Acar A, Korkmaz H. Role of free radicals and antioxidants in nasal polyps. Laryngoscope. 2004;114:1200–3. doi: 10.1097/00005537-200407000-00013. [DOI] [PubMed] [Google Scholar]