Abstract
Introduction
Time to treatment is the key factor in stroke care. Although the initial medical assessment is usually made by a non-neurologist or a paramedic, it should ensure correct identification of all acute cerebrovascular accidents (CVAs). Our aim was to evaluate the accuracy of the physician-made prehospital diagnosis of acute CVA in patients referred directly to the neurological emergency department (ED), and to identify conditions mimicking CVAs.
Material and methods
This observational study included consecutive patients referred to our neurological ED by emergency physicians with a suspicion of CVA (acute stroke, transient ischemic attack (TIA) or a syndrome-based diagnosis) during 12 months. Referrals were considered correct if the prehospital diagnosis of CVA proved to be stroke or TIA.
Results
The prehospital diagnosis of CVA was correct in 360 of 570 cases. Its positive predictive value ranged from 100% for the syndrome-based diagnosis, through 70% for stroke, to 34% for TIA. Misdiagnoses were less frequent among ambulance physicians compared to primary care and outpatient physicians (33% vs. 52%, p < 0.001). The most frequent mimics were vertigo (19%), electrolyte and metabolic disturbances (12%), seizures (11%), cardiovascular disorders (10%), blood hypertension (8%) and brain tumors (5%). Additionally, 6% of all admitted CVA cases were referred with prehospital diagnoses other than CVA.
Conclusions
Emergency physicians appear to be sensitive in diagnosing CVAs but their overall accuracy does not seem high. They tend to overuse the diagnosis of TIA. Constant education and adoption of stroke screening scales may be beneficial for emergency care systems based both on physicians and on paramedics.
Keywords: stroke, transient ischemic attack, emergency medical services, prehospital management, misdiagnosis
Introduction
Time to treatment is a key factor for achieving a good outcome in patients suffering from acute cerebrovascular accidents (CVAs), especially if they are to receive thrombolysis or thrombectomy [1–3]. Effective communication between the prehospital emergency medical services (EMS) and the emergency department (ED) may improve the door-to-needle time [4]. It is also vital for strategies relying on a direct transfer of patients to comprehensive stroke centers, bypassing the ED of a local hospital [5]. Therefore, EMS should be able not only to identify all potential cases of stroke or transient ischemic attack (TIA), but also to differentiate CVAs from their frequent mimics [6, 7]. However, the first-line medical assessment is usually done in the prehospital setting by a paramedic or a physician who is not trained in neurology.
The aim of our study was to evaluate the accuracy of the prehospital diagnosis of an acute CVA made by EMS physicians in a cohort of patients referred directly to the neurological ED, and to identify conditions that are incorrectly suspected of being a stroke or a TIA.
Material and methods
Our neurological department with a stroke unit provides inpatient and outpatient neurological care for approximately 250 000 inhabitants of a highly urbanized area (Warsaw, Poland). During the study period the hospital profile was solely neuropsychiatric. Therefore, all patients presenting at the ED were supposed to suffer from either neurological or psychiatric conditions. Our catchment area overlapped with the non-neurological catchment area of a few other multi-profile hospitals. Patients could be referred to the ED by EMS physicians, primary care physicians (PCPs), and other specialists from outpatient clinics or non-neurological EDs. They could also report to the ED without any formal referral.
Neurological care in our ED was covered nonstop by a designated physician. During regular working hours it was a trainee neurologist with direct access to the stroke specialist. During out-of-office hours it was a trained neurologist. The annual volume of patients seen in the neurological ED is approximately 3000 and the number of confirmed strokes is approximately 300.
Patients referred to the hospital with a suspicion of CVA (defined as a new stroke or TIA) were given top priority and immediately qualified for intravenous thrombolysis according to the European license for alteplase [8]. Routine brain imaging included plain computed tomography (CT). Computed tomography angiography or magnetic resonance imaging (MRI) was done only in selected cases.
At the time of the study each emergency ambulance had a physician on board. The ambulance physician was a specialist or a trainee in one of the following: anesthesiology and intensive care, internal medicine, surgery, traumatology or pediatrics. He could administer necessary medical treatment at the scene and decide if the patient required transfer to a general ED or directly to a specialist ED. The prehospital diagnosis of stroke was based on his clinical judgment and was not supported by any stroke screening scales. Patients were not charged for the visit of the EMS team, even if the calls were clearly unjustified.
Study design
We analyzed the data of all consecutive adult patients seen at our neurological ED during a 12-month period (September 2006 – September 2007), who reported with a formal referral having a physician-made prehospital diagnosis of stroke or TIA. Referrals with a descriptive diagnosis strongly suggestive of CVA (e.g. unilateral weakness or aphasia of abrupt onset) were also classified as suspected CVA. The referrals were considered correct if the prehospital diagnosis of CVA proved to be stroke or TIA at discharge from the hospital. We additionally included patients with referral diagnoses other than CVAs, who proved to suffer from a stroke or TIA after proper neurological assessment and brain imaging. Patients who presented to the ED without any prehospital referral were not included in the analysis.
The data about referrals were prospectively collected on a daily basis by one of the authors (M.G.) using a predefined questionnaire. Information about stroke/TIA cases not suspected of CVA by the referring physician were obtained from patients’ medical records. The study was conducted in concordance with the Declaration of Helsinki. Due to its observational design, we did not obtain patients’ written consent for participation.
Statistical analysis
Statistical analysis included basic descriptive and comparative statistics. Categorical variables were presented as a ratio and compared with the chi square test (or two-tailed Fisher's exact test if the expected value in at least one cell of a 2 × 2 contingency table was < 5). The accuracy of prehospital diagnosis was expressed as the positive predictive value (PPV) – the probability that a patient referred to the hospital as a CVA was confirmed to suffer from the CVA after proper neurological assessment. Calculations were carried out using Statistica 10.0 (StatSoft Inc., Tulsa, USA, 2011) and Confidence Interval Analysis (University of Southampton, Southampton, UK, 2009). Results were presented with 95% confidence intervals (95% CI). A value of p < 0.05 was considered statistically significant.
Results
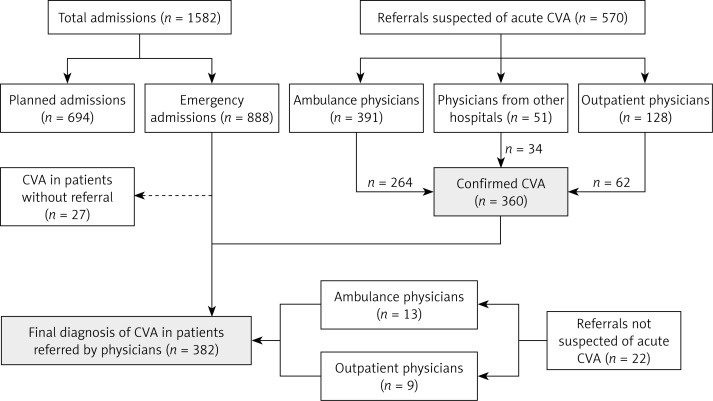
During the 12-month study period there were 570 referrals with a prehospital diagnosis of CVA (Figure 1). Patients were most frequently suspected of stroke (60.9%) or TIA (28.2%). The remaining 10.9% had a descriptive diagnosis classified as CVA (e.g. unilateral weakness or aphasia of abrupt onset) (Table I).
Figure 1.
Structure of admissions to the department and referrals suspected of acute cerebrovascular accident (CVA) during the study period
Table I.
Correct and incorrect prehospital diagnoses of acute cerebrovascular accidents (CVA) with the positive predictive values (PPV) for CVA
| Variable | Confirmed CVA | Non-confirmed CVA | PPV (95% CI) |
|---|---|---|---|
| Prehospital diagnosis of CVA: | 360 of 570 | 210 of 570 | 63% (59–67) |
| Stroke, n (%) | 243 (63.8) | 104 (49.5) | 70% (65–75) |
| TIA, n (%) | 55 (14.4) | 106 (50.5) | 34% (27–42) |
| Descriptive diagnosis, n (%) | 62 (16.3) | 0 (0.0) | 100% (94–100) |
Patients not suspected of stroke or TIA by the referring physician but discharged with a diagnosis of stroke or TIA (n = 22) were not included in the Table.
The prehospital diagnosis of CVA was correct in 360 cases. Additionally, 22 patients referred as non-CVAs (mostly vertigo – 8 patients and headache – 3 patients) proved to suffer from a stroke or TIA. It gave a total of 382 final diagnoses of CVAs, including ischemic stroke (75.9%), TIA (13.6%) and hemorrhagic stroke (10.5%). Intravenous thrombolysis was administered in 12.4% of all ischemic strokes.
The overall PPV of the prehospital diagnosis of CVA was 63%. It differed between particular types of referrals, ranging from 100% for the syndrome-based diagnosis, through 70% for stroke, to 34% for TIA (Table I). The proportion of TIA referrals was significantly (p < 0.001) higher in patients incorrectly suspected of a CVA (50.5%) compared to patients with confirmed CVA (14.4%).
Patients incorrectly suspected of CVA had a median age of 73 years (IQR: 62–81) and were predominantly female (73.8%). The rate of incorrect CVA referrals did not differ (p = 0.884) between ambulance physicians (32.5%, 95% CI: 28.0–37.3%) and physicians from other EDs (33.3%, 95% CI: 22.0–47.0). However, it was significantly higher in the group of PCPs and other outpatient specialists (51.6%, 95% CI: 43.0–60.0, p < 0.001).
51.6% of patients incorrectly suspected of CVA suffered from other neurological disorders (mostly vertigo, seizures and brain tumor). In the remaining 48.4%, the disorders were non-neurological (mostly electrolyte and metabolic disturbances, cardiovascular disorders and hypertension). Patients incorrectly referred by emergency ambulance physicians were significantly older and more frequently had seizures in comparison to patients incorrectly referred by PCPs or other outpatient specialists. They also more frequently required admission, especially to the neurological ward (Table II).
Table II.
Conditions incorrectly diagnosed as an acute cerebrovascular accident (CVA) in the prehospital setting including comparison between referrals from the emergency ambulance physicians and general practitioners or other outpatient specialists
| Parameter | Overall (n = 210) |
Ambulance physicians (n = 127) |
Outpatient physicians (n = 66) |
Value of p |
|---|---|---|---|---|
| Female gender, n (%) | 134 (73.8) | 83 (65.4) | 40 (60.6) | 0.515 |
| Age, median (IQR) [years] | 73 (62–81) | 75 (67–81) | 68 (58–79) | 0.004 |
| Neurological disorders suspected of CVA, n (%): | 107 (51.0) | 69 (54.3) | 37 (56.1) | 0.819 |
| Vertigo | 39 (18.6) | 19 (15.0) | 16 (4.2) | 0.112 |
| Seizure | 24 (11.4) | 22 (17.3) | 1 (1.5) | 0.001 |
| Brain tumor | 11 (5.2) | 6 (4.7) | 5 (7.6) | 0.418 |
| Headache | 4 (1.9) | 3 (2.4) | 1 (1.5) | 1.000* |
| Other | 29 (13.8) | 19 (15.0) | 13 (19.7) | 0.401 |
| Non-neurological disorders suspected of CVA, n (%): | 103 (49.0) | 58 (45.7) | 29 (43.9) | 0.819 |
| Electrolyte and metabolic disturbances | 25 (11.9) | 19 (15.0) | 5 (7.6) | 0.140 |
| Cardiovascular disorders | 21 (10.0) | 14 (11.0) | 7 (10.6) | 0.930 |
| Hypertension | 17 (8.1) | 8 (6.3) | 7 (10.6) | 0.289 |
| Infections | 9 (4.3) | 7 (5.5) | 1 (1.5) | 0.190 |
| Other | 31 (14.8) | 10 (7.9) | 9 (13.6) | 0.202 |
| Subsequent admission of non-CVA, n (%): | 112 (53.3) | 84 (66.1) | 24 (36.4) | < 0.001 |
| Neurological ward | 52 (24.8) | 39 (30.7) | 10 (15.2) | 0.019 |
| Non-neurological ward | 62 (29.5) | 45 (35.4) | 14 (21.2) | 0.174 |
Two-tailed Fisher's exact test was used.
Discussion
The final diagnosis of a stroke or TIA requires a detailed neurological assessment and brain imaging. Sometimes it is also necessary to have a period of clinical observation. However, signs and symptoms should be enough to make the initial diagnosis of CVA. To our best knowledge, this is the first prospective study addressing the accuracy of prehospital diagnosis of CVA made by emergency or outpatient physicians in a group of patients referred directly to the neurological ED. In many countries the regular ambulance staff does not include a physician. Polish EMS have also begun to evolve in this direction, but during the study period all emergency ambulances had a physician on board.
The decreasing trend for the time from stroke onset to hospital arrival has not been sufficiently matched by the reduction of in-hospital delays [9]. From the EMS perspective, specificity is usually not the key element in the chain of life. However, in stroke reperfusion therapy, a higher proportion of correct prehospital notifications would allow optimization of the use of resources and facilitate cooperation between EMS and stroke teams. In our study, all CVA patients were successfully identified by the neurologist at the ED. However, the initial diagnosis of stroke made by non-neurological ED staff may be incorrect in up to 30% of cases [10, 11]. Patients with stroke mimics may receive thrombolysis even in very experienced stroke centers. They account for up to 2% of all treated cases [12], which is similar to the proportion of patients treated despite international normalized ratio (INR) > 1.7 or blood pressure > 185/110 mm Hg [13]. Fortunately, stroke mimics are very unlikely to develop hemorrhagic complications after thrombolysis [12].
According to previous reports, the proportion of patients incorrectly suspected of stroke ranges from 19% to 48%, depending on the study setting, organization of the national emergency health care system and applied methodology [6, 14, 15]. It concurs with our findings, showing that 37% of patients were incorrectly suspected of CVA.
The diagnostic accuracy increases with the number of typical stroke signs, especially acute facial paresis, arm drift or abnormal speech [16]. Emergency calls reporting specifically stroke as a major problem are very often correct [17], but the overall PPV of dispatchers is usually below 60% [18–20]. Using an advanced medical priority dispatch software to support the telephone, triage significantly increases the negative predictive value (NPV), but does not improve the PPV [21]. On scene, assessment by the paramedics can be facilitated by one of several stroke screening scales [22–26]. These scales offer 60–90% sensitivity, specificity, and NPV, while their PPV ranges from 40% to 88% [20, 22–28]. In our study, emergency physicians were able to identify almost all CVAs, but their PPV seems relatively low. Even lower consistency between the prehospital diagnosis of CVA made by a physician and the discharge diagnosis was observed in Denmark [29]. We may not exclude that stroke screening scales allow the paramedics to match the PPV of emergency physicians [22, 23] and their adoption into everyday practice would reduce the number of stroke mimics. However, stroke screening scales are suitable only for conscious patients, and their actual sensitivity may be slightly overrated [30].
The initial diagnosis of TIA was reported to be incorrect in up to 60% of cases, if made by an ED non-neurologist or a general practitioner [21, 31–33]. The discrepancy between the PPV of prehospital diagnosis of stroke (70%) and the prehospital diagnosis of TIA (34%) found in our study may reflect the attitude of the EMS physicians. They probably tend to label less apparent cases as TIA to justify the patient's transfer to the neurological ED instead of choosing a general ED. It may be simply a combination of insufficient education and the feeling that patient needs to be seen at the hospital. However, it is also possible the EMS physicians prefer to start the hospital management with exclusion of life-threatening conditions such as stroke. Afterwards, the neurologist can always decide if other specialists should be involved. From the patient's perspective such an approach may seem acceptable, as the role of EMS is to identify all cases requiring immediate in-hospital care. Besides, 1 in 2 misdiagnosed patients suffered from another neurological condition, and 1 in 4 required admission to the neurological ward anyway, which is in line with other studies [10, 14, 22].
In our cohort, patients incorrectly suspected of CVA in the prehospital setting most frequently suffered from vertigo, seizures, electrolyte and metabolic disturbances, and cardiovascular disorders. Previous studies are not consistent in this matter, but seizures appear to be the most frequent misdiagnosis (9–28%) [10, 14, 22, 23, 34], which partially concurs with our findings. Other important conditions are infections (8–15%) [10, 14, 22, 23], brain tumors (4–10%) and electrolyte and metabolic disturbances (5–12%) [10, 14, 22, 23, 34]. Interestingly, previous studies report a low ratio of vertigo (2–6%), as well as a large variation in the frequency of headaches (3–15%) [10, 14, 22, 23, 34] and peripheral pareses (2–10%) [14]. Cardiovascular (6–11%) and psychiatric disorders (5–8%) are reported only in two studies [14, 22]. We found no psychiatric misdiagnoses, which is in agreement with the latter. However, the ratio of combined cardiovascular disorders and elevated blood pressure was relatively high (17.9%). As mentioned before, this may be explained by the need to justify referrals to the neurological ED for patients with malaise and other non-specific complaints, but without a relevant neurological deficit. It is also important to note that the profile of mimics referred by EMS was different than mimics referred by PCP and other outpatient specialists, favoring the accuracy of EMS. The higher rate of seizures in ambulance referral has been previously reported [22].
The analysis included only patients who arrived at the neurological ED of a neuropsychiatric hospital. As a consequence, it was not possible to calculate sensitivity and NPV of the emergency referrals because we had no information about the total number of dispatches and number of patients correctly diagnosed with non-CVAs. It is also possible that some patients living in our neurological catchment area were referred to another hospital as non-CVAs, but actually proved to suffer from a stroke or TIA. Therefore, to avoid bias we also refrained from stating the specificity, which according to our data may be approximated as not better than 94%.
However, due to the organization of the Polish health care system during the study period, we were able to collect a sample unbiased by the dispatcher's decision about sending an ambulance with a physician or with paramedics on board.
We used a combined discharge diagnosis of a new stroke or TIA to verify the accuracy of different prehospital diagnoses of CVA. This approach was not fully diagnosis-specific, but allowed a reliable evaluation in terms of the necessity of the referral.
In conclusion, Polish emergency physicians using clinical judgment for the pre-hospital diagnosis of patients transferred directly to the neurological ED miss only a small number of CVAs. However, their ability to correctly identify CVA does not seem high. They tend to overuse the prehospital diagnosis of TIA, probably to justify the choice of the neurological ED instead of a general ED in uncertain cases. This emphasizes the necessity for building stroke awareness in the EMS personnel and adopting stroke screening scales to improve the reliability of CVA referrals, both by physicians and paramedics. On the other hand, the emergency neurologist should remain vigilant, as from time to time even a non-CVA referral may require immediate cerebrovascular management.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Jauch EC, Saver JL, Adams HP, et al. Guidelines for the early management of patients with acute ischemic stroke. Stroke. 2013;44:870–947. doi: 10.1161/STR.0b013e318284056a. [DOI] [PubMed] [Google Scholar]
- 2.European Stroke Organization Guidelines. Available at: http://www.eso-stroke.org/pdf/ESO%20Guidelines_update_Jan_2009.pdf. Accessed May 30th, 2013.
- 3.Mikulík R, Kadlecová P, Czlonkowska A, et al. Factors influencing in-hospital delay in treatment with intravenous thrombolysis. Stroke. 2012;43:1578–83. doi: 10.1161/STROKEAHA.111.644120. [DOI] [PubMed] [Google Scholar]
- 4.Meretoja A, Strbian D, Mustanoja S, Tatlisumak T, Lindsberg PJ, Kaste M. Reducing in-hospital delay to 20 minutes in stroke thrombolysis. Neurology. 2012;79:306–13. doi: 10.1212/WNL.0b013e31825d6011. [DOI] [PubMed] [Google Scholar]
- 5.O'Brien W, Crimmins D, Donaldson W, et al. FASTER (Face, Arm, Speech, Time, Emergency Response): experience of Central Coast Stroke Services implementation of a prehospital notification system for expedient management of acute stroke. J Clin Neurosci. 2012;19:241–5. doi: 10.1016/j.jocn.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 6.Pope JV, Edlow J. Avoiding misdiagnosis in patients with neurological emergencies. Emerg Med Intern. 2012;2012:949275. doi: 10.1155/2012/949275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Easton JD, Saver JL, Albers GW, et al. Definition and evaluation of transient ischemic attack. Stroke. 2009;40:2276–93. doi: 10.1161/STROKEAHA.108.192218. [DOI] [PubMed] [Google Scholar]
- 8.Actilyse. The European Medicines Agency (EMEA) Available at: http://www.ema.europa.eu/pdfs/human/referral/Actilyse/334602en.pdf. Accessed May 30th, 2013.
- 9.Rosamond D. A comprehensive review of prehospital and in-hospital delay times in acute stroke care. Int J Stroke. 2009;4:187–99. doi: 10.1111/j.1747-4949.2009.00276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hand PJ, Kwan J, Lindley RI, Dennis MS, Wardlaw JM. Distinguishing between stroke and mimic at the bedside: the Brain Attack Study. Stroke. 2006;37:769–75. doi: 10.1161/01.STR.0000204041.13466.4c. [DOI] [PubMed] [Google Scholar]
- 11.Morgenstern LB, Lisabeth LD, Mecozzi AC, et al. A population-based study of acute stroke and TIA diagnosis. Neurology. 2004;62:895–900. doi: 10.1212/01.wnl.0000115103.49326.5e. [DOI] [PubMed] [Google Scholar]
- 12.Zinkstok SM, Engelter ST, Gensicke H, et al. Safety of thrombolysis in stroke mimics: results from a multicenter cohort study. Stroke. 2013;44:1080–4. doi: 10.1161/STROKEAHA.111.000126. [DOI] [PubMed] [Google Scholar]
- 13.Karlinski M, Kobayashi A, Litwin T, et al. Intravenous thrombolysis for acute ischaemic stroke in patients not fully adhering to the European license in Poland. Neurol Neurochir Pol. 2012;46:3–14. doi: 10.5114/ninp.2012.27179. [DOI] [PubMed] [Google Scholar]
- 14.Rizos T, Ringleb PA, Huttner HB, Kohrmann M, Juttler E. Evolution of stroke diagnosis in the emergency room – a prospective observational study. Cerebrovasc Dis. 2009;28:448–53. doi: 10.1159/000235989. [DOI] [PubMed] [Google Scholar]
- 15.Libman RB, Wirkowski E, Alvir J, Rao TH. Conditions that mimic stroke in the emergency department. Implications for acute stroke trials. Arch Neurol. 1995;52:1119–22. doi: 10.1001/archneur.1995.00540350113023. [DOI] [PubMed] [Google Scholar]
- 16.Goldstein LB. Is this patient having a stroke? JAMA. 2005;293:2391–402. doi: 10.1001/jama.293.19.2391. [DOI] [PubMed] [Google Scholar]
- 17.Jones SP, Carter B, Ford GA, et al. The identification of acute stroke: an analysis of emergency calls. Int J Stroke. 2013;8:408–12. doi: 10.1111/j.1747-4949.2011.00749.x. [DOI] [PubMed] [Google Scholar]
- 18.Chenaitia H, Lefevre O, Ho V, et al. Emergency medical service in the stroke chain of survival. Eur J Emerg Med. 2013;20:39–44. doi: 10.1097/MEJ.0b013e32835015ac. [DOI] [PubMed] [Google Scholar]
- 19.Buck BH, Starkman S, Eckstein M, et al. Dispatcher recognition of stroke using the National Academy Medical Priority Dispatch System. Stroke. 2009;40:2027–30. doi: 10.1161/STROKEAHA.108.545574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramanujam P, Guluma KZ, Castillo EM, et al. Accuracy of stroke recognition by emergency medical dispatchers and paramedics-San Diego experience. Prehosp Emerg Care. 2008;12:307–13. doi: 10.1080/10903120802099526. [DOI] [PubMed] [Google Scholar]
- 21.Ferro JM, Falcão I, Rodrigues G, et al. Diagnosis of transient ischemic attack by the nonneurologist. A validation study. Stroke. 1996;27:2225–9. doi: 10.1161/01.str.27.12.2225. [DOI] [PubMed] [Google Scholar]
- 22.Harbison J, Hossain O, Jenkinson D, Davis J, Louw SJ, Ford GA. Diagnostic accuracy of stroke referrals from primary care, emergency room physicians, and ambulance staff using the face arm speech test. Stroke. 2003;34:71–6. doi: 10.1161/01.str.0000044170.46643.5e. [DOI] [PubMed] [Google Scholar]
- 23.Nor AM, Mcallister C, Louw SJ, et al. Agreement between ambulance paramedic- and physician-recorded neurological signs with Face Arm Speech Test (FAST) in acute stroke patients. Stroke. 2004;35:1355–9. doi: 10.1161/01.STR.0000128529.63156.c5. [DOI] [PubMed] [Google Scholar]
- 24.Kidwell CS, Starkman S, Eckstein M, Weems K, Saver JL. Identifying stroke in the field: prospective validation of the Los Angeles Prehospital Stroke Screen (LAPSS) Stroke. 2000;31:71–6. doi: 10.1161/01.str.31.1.71. [DOI] [PubMed] [Google Scholar]
- 25.Kothari RU, Pancioli A, Liu T, Brott T, Broderick J. Cincinnati Prehospital Stroke Scale: reproducibility and validity. Ann Emerg Med. 1999;33:373–8. doi: 10.1016/s0196-0644(99)70299-4. [DOI] [PubMed] [Google Scholar]
- 26.Bray JE, Coughlan K, Barger B, Bladin C. Paramedic diagnosis of stroke: examining long-term use of the Melbourne Ambulance Stroke Screen (MASS) in the field. Stroke. 2010;41:1363–6. doi: 10.1161/STROKEAHA.109.571836. [DOI] [PubMed] [Google Scholar]
- 27.Nor AM, Davis J, Sen B, et al. The Recognition of Stroke in the Emergency Room (ROSIER) scale: development and validation of a stroke recognition instrument. Lancet Neurol. 2005;4:727–34. doi: 10.1016/S1474-4422(05)70201-5. [DOI] [PubMed] [Google Scholar]
- 28.Frendl DM, Strauss DG, Underhill BK, Goldstein LB. Lack of impact of paramedic training and use of the Cincinnati prehospital stroke scale on stroke patient identification and on-scene time. Stroke. 2009;40:754–6. doi: 10.1161/STROKEAHA.108.531285. [DOI] [PubMed] [Google Scholar]
- 29.Fischer CE, Barnung S, Nielsan SL, Rasmusen LS. Prehospital identification of stroke – room for improvement. Eur J Neurol. 2008;15:792–6. doi: 10.1111/j.1468-1331.2008.02141.x. [DOI] [PubMed] [Google Scholar]
- 30.Whiteley WN, Wardlaw JM, Dennis MS, Sandercock PA. Clinical scores for the identification of stroke and transient ischaemic attack in the emergency department: a cross-sectional study. J Neurol Neurosurg Psychiatry. 2011;82:1006–10. doi: 10.1136/jnnp.2010.235010. [DOI] [PubMed] [Google Scholar]
- 31.Dennis MS, Bamford JM, Sandercock PA, Warlow CP. Incidence of transient ischemic attacks in Oxfordshire, England. Stroke. 1989;20:333–9. doi: 10.1161/01.str.20.3.333. [DOI] [PubMed] [Google Scholar]
- 32.Sheehan OC, Merwick A, Kelly LA, et al. Diagnostic usefulness of the ABCD2 score to distinguish transient ischemic attack and minor ischemic stroke from noncerebrovascular events: The North Dublin TIA Study. Stroke. 2009;40:3449–54. doi: 10.1161/STROKEAHA.109.557074. [DOI] [PubMed] [Google Scholar]
- 33.Prabhakaran S, Silver AJ, Warrior L, Mcclenathan B, Lee VH. Misdiagnosis of transient ischemic attacks in the emergency room. Cerebrovasc Dis. 2008;26:630–5. doi: 10.1159/000166839. [DOI] [PubMed] [Google Scholar]
- 34.Weir NU, Buchan AM. A study of the workload and effectiveness of a comprehensive acute stroke service. J Neurol Neurosurg Psychiatry. 2005;76:863–5. doi: 10.1136/jnnp.2004.053462. [DOI] [PMC free article] [PubMed] [Google Scholar]