Abstract
Introduction
The progesterone receptor (PR) gene plays an important role in reproduction-related events. Data on polymorphisms in the PR gene have revealed associations with cancer, particularly for the Alu insertion polymorphism, which has been suggested to affect progesterone receptor function and contribute to tumor promotion in the mammary gland.
Material and methods
We examined the role of the Alu insertion polymorphism in the PR gene by comparing the genotypes of 209 healthy Mexican women with those of 481 Mexican women with breast cancer (BC).
Results
The genotype frequencies observed in the controls and BC patients were 0% and 4% for T2/T2 (Alu insertion), 16% and 21% for T1/T2, and 84% and 75% for T1/T1 (Alu deletion), respectively. The obtained odds ratio (OR) was 1.7, with a 95% confidence interval (95% CI) of 1.1–2.6, p = 0.009, for the T1/T2–T2/T2 genotypes. The association was also evident when the distributions of the T1/T2–T2/T2 genotypes in patients in the following categories were compared: obesity grade II (OR = 1.81, 95% CI: 1.03–3.18, p = 0.039) and the chemotherapy response (OR = 1.91, 95% CI: 1.27–3.067, p = 0.002).
Conclusions
The T1/T2–T2/T2 genotypes of the Alu insertion polymorphism in the PR gene are associated with BC susceptibility in the analyzed Mexican population.
Keywords: PROGINS, progesterone receptor, polymorphism, breast cancer, Mexican population
Introduction
Breast cancer (BC) is one of the most common diseases in developing countries and around the world. It is estimated that there are millions of symptomatic women affected by BC and millions more who are currently asymptomatic who will develop cancer [1]. The incidence rates of cancer vary in different ethnic groups [2]. In many countries, particularity in Mexico, the incidence of BC has increased within the last 7 years, and it is now one of the main causes of death of working age women [3]. The state of Jalisco exhibits one of the highest mortalities associated with BC, and only 10% of all cases of BC are detected at stage I [4]. Breast cancer is considered to be a multifactorial disease and might result from a combination of abnormal gene interactions and environmental factors [5, 6]. Previous research has implicated a variety of risk factors in BC, including age, early or late menarche, menopause, oral contraceptive use, breastfeeding, ethnicity and genetics [7–9].
Therefore, elucidating genetic variants among different ethnic groups could contribute to explaining the progression of cancer as well as the chemotherapy response.
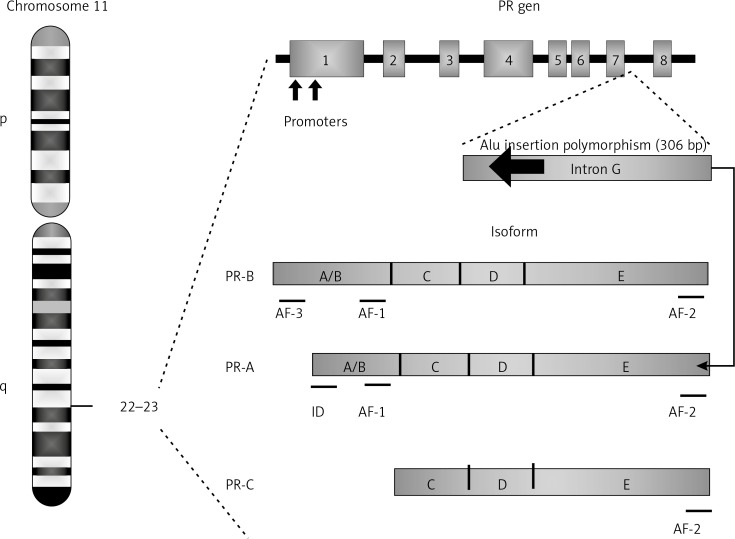
The human progesterone receptor (PR) gene, which is located on chromosome 11q22-23, comprises eight exons and seven introns (A–G). This gene encodes two isoforms, which are identical except for 165 additional amino acids found at the N-terminus of the B isoform. These isoforms regulate the biological action of progesterone: isoform A inhibits activation of the PR, while isoform B activates it [10, 11]. The PR gene exhibits several reported polymorphisms, one of which is designated the PROGINS haplotype and includes an Alu insertion in intron G, a G/T substitution in exon 4 (rs1042838), and a silent C/T substitution in exon 5 (rs1042839). These PROGINS polymorphisms are in complete linkage disequilibrium (Figure 1) [12]. The Alu polymorphism consists of a 306 bp insertion in the G intron located between exons 7 and 8 of isoform A of the PR gene in humans [13]. Although the biological impact of the Alu insertion polymorphism is not clear, it has been suggested that it might lead to aberrant gene transcription, resulting in an inability of the hormone receptor to bind progesterone and subsequently become activated, with a consequent reduction of the activity mediated by progesterone. Progesterone participates in the regulation of gene expression and affects cellular proliferation and differentiation in its target tissues. Therefore, PR gene deficiency may have a potential impact on oncogenesis [14].
Figure 1.
The human PR gene contains eight coding exons and seven non-coding introns (A-G) encoding the PR-A and PR-B isoforms. The PR-A isoform is identical to PR-B, except that the PR-B isoform exhibits 165 amino acids in the amino-terminal region that form the third transactivation domain (AF-3). Exon 1 and part of 2 encode the A/B region, which contains the PR-B-specific transactivation domain AF-3, while AF-1 is found in both PR-B and PR-A. The inhibitory domain (ID) region is PR-A specific. The C region forms the DNA-binding domain (DBD); each of exons 2 and 3 encodes one zinc finger. The D region is encoded by exon 4 and part of exon 3 and forms the hinge region responsible for the nuclear location signal. The E region is encoded by exons 4 to 8 and contains AF-2 and the hormone (ligand)-binding domain. PR-C lacks the DBD, AF-3 and AF-1 regions. An amino-terminally deleted PR protein is predicted to result from the alternative initiation of translation at a methionine at position 595. The Alu insertion polymorphism interferes specifically with the PR-A isoform [14, 39]
Studies have found variability in the allelic frequency of the Alu insertion in the PR gene among different ethnic groups. In Caucasian Europeans from different regions (Finland, Sweden, Hungary, France, and Spain), a high frequency of 11–24% has been observed, and a frequency of 21% has been detected in US Caucasians. A low prevalence (3–9%) has been found in Asians and inhabitants of the Pacific Islands, Chukchi Coast, Bangladesh, Kungurtug, the Himalayas, and Pakistan as well as in Mexican Mayas from the state of Campeche (3–6%). The Alu allele was not observed in Africa (Cameroon, Senegal) or Oriental Asian countries (China, Japan) [12].
The PR gene Alu insertion has been studied in various pathologies, including endometriosis [13], and uterine, ovarian and breast cancers [15]. However, several of the studies examining the association between the Alu insertion and BC did not reveal any statistically significant association [16, 17].
Because the evidence is contradictory, the aim of this study was to determine whether there is an association between the Alu insertion polymorphism in the PR gene and BC in Mexican women.
Material and methods
DNA was extracted from peripheral blood lymphocytes from blood samples collected from 209 healthy women recruited as volunteer blood donors using standard protocols [18]. These volunteers were not matched by age with the patient group. Blood samples were also collected from 481 patients with a clinical and histological diagnosis of BC from June 2010 to October 2012. All of the individuals included in this study were residents of the metropolitan area of Guadalajara. All samples were obtained after a written informed consent form was signed, which was previously approved by ethical committee 1305 of the Centro de Investigación Biomédica de Occidente, Instituto Mexicano del Seguro Social (IMSS). This study was conducted respecting national and international ethical standards. Efforts were made to ensure that siblings of individuals who had already been sampled were excluded. Clinical and demographic data were obtained using written questionnaires. All of the patients were also interviewed to determine their occupational exposure and use of pharmacological therapies. The BC patient database and their DNA samples were examined for other polymorphisms [5, 6].
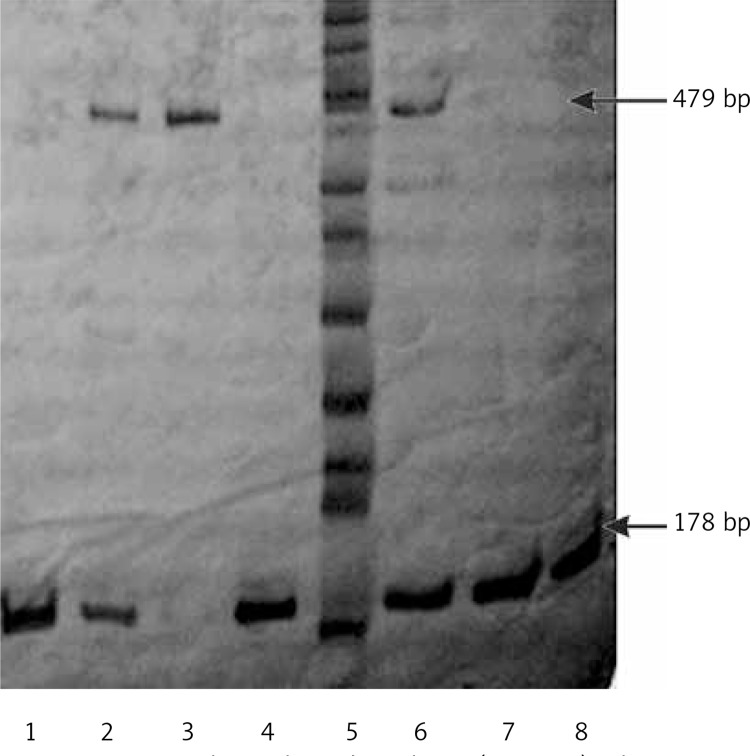
Amplification of the Alu insertion polymorphism in the PR gene was performed via PCR using the following primers: 5’-GGC AGA AAG CAA AAT AAA AAG A-3’ and 5’-AAA GTA TTT TCT TGC TAA ATG TC-3’ [10]. The PCR amplifications were performed in a total volume of 15 µl containing 0.2 mM dNTPs (Invitrogen, Carlsbad, CA USA), 5 pM of primers, 1.5 mM MgCl2, 2.5 U of Taq polymerase (Invitrogen, Carlsbad, CA USA), and 50 ng of genomic DNA. The PCR conditions were as follows: 95°C (4 min), followed by 35 cycles at 95°C (1 min), 55°C (1 min) and 72°C (1 min), with a final extension at 72°C (7 min). Using this procedure, two fragments of 178 and 479 bp were obtained. To allow allelic discrimination, the amplified products were separated in 6% polyacrylamide gels (29 : 1), followed by silver staining [19]. We determined that observing the 178 bp fragment alone represented the wild-type genotype (T1/T1, deletion), while two fragments at 178 and 479 bp indicated the heterozygous genotype (T1/T2), and a single fragment of 479 bp represented the homozygous insertion genotype (T2/T2) (Figure 2).
Figure 2.
Polyacrylamide gel 6% (29.1 : 1) silver nitrate stained. Gel. Lines 1, 4, 7, 8 homozygous T1T1 (178 bp); lines 2 and 6 heterozygous T1/T2 (178 and 479 bp); line 3 homozygous T2T2 (479 pb) and line 5 ladder (50 bp)
Statistical analysis
Allele frequencies were obtained by direct counting. Hardy-Weinberg equilibrium was tested using a goodness-of-fit χ2 test to compare the observed genotype frequencies to the expected frequencies among control subjects. Odds ratios and 95% confidence intervals (CI) were also calculated. A two-sided p < 0.05 was considered to be statistically significant. All statistical analyses were performed using PASW Statistic Base 18 software, 2009 (Chicago, IL).
Results
The comparative epidemiological data from the BC patients and the control individuals are shown in Table I. In the patient group, the average age was 53.64 years, ranging from 25 to 88 years. Menarche presented at a mean age of 12.67 years in the patients and 12.17 years in the controls. Oral contraceptive use (OR = 2.6, 95% CI: 1.6–4.3, p < 0.0001), abortion (OR = 3.2, 95% CI: 1.5–6.7, p = 0.002), breastfeeding > 6 months (OR = 3.1, 95% CI: 1.8–5.1, p < 0.0001), and menopause (OR = 15, 95% CI: 8.8–26.8, p < 0.0001), were observed to be risk factors.
Table I.
Demographic data for the study group
| Parameter | BC patients (n = 481) | Controls (n = 209) | OR (95% CI)* | Value of p | ||
|---|---|---|---|---|---|---|
| Age [years] | ||||||
| Mean (SD) | 53.64 | (11.8) | 33.11 | (9.2) | ||
| Menarche [years] | ||||||
| Mean (SD) | 12.67 | (1.6) | 12.17 | (1.0) | ||
| n | % | n | % | |||
| Menarche (range): | ||||||
| 7–10 | 35 | 7 | 1 | 1 | ||
| 11–13 | 310 | 64 | 189 | 90 | 0.36 (0.20–0.66) | 0.001 |
| 14–18 | 136 | 29 | 19 | 9 | ||
| Oral contraceptive use: | ||||||
| Yes | 200 | 42 | 48 | 23 | 2.6 (1.6–4.3) | < 0.0001 |
| No | 281 | 58 | 161 | 77 | ||
| Abortion: | ||||||
| Yes | 140 | 29 | 12 | 6 | 3.2 (1.5–6.7) | 0.002 |
| No | 341 | 71 | 197 | 94 | ||
| Breastfeeding: | ||||||
| = 6 months | 53 | 11 | 78 | 37 | 0.29 (0.16–0.53) | < 0.0001 |
| > 6 months | 267 | 56 | 44 | 21 | 3.1 (1.8–5.11) | < 0.0001 |
| No | 161 | 33 | 87 | 42 | ||
| Menopause: | ||||||
| Postmenopausal | 316 | 66 | 20 | 10 | 15 (8.8–26.8) | < 0.0001 |
| Premenopausal | 165 | 34 | 189 | 90 | ||
| Tobacco consumption: | ||||||
| Yes | 106 | 22 | 80 | 38 | NS | |
| No | 375 | 78 | 173 | 62 | ||
| Alcohol consumption: | ||||||
| Yes | 85 | 18 | 46 | 22 | NS | |
| No | 396 | 82 | 163 | 78 | ||
| Familial history (FH): | ||||||
| Yes | 311 | 65 | 45 | 22 | NS | |
| No | 170 | 35 | 164 | 78 | ||
| Disease type of FH: | ||||||
| No | 170 | 35 | 164 | 78 | NS | |
| BC | 56 | 12 | 5 | 2 | NS | |
| DM-AH | 129 | 27 | 24 | 11 | NS | |
| DM-AH-cancer** | 126 | 26 | 16 | 8 | NS | |
SD – Standard deviation, NS – no significant difference
OR (odds ratio) from the adjusted regression analysis
positive familial history of cancer and leukemia in first and second degree relatives of patients.
Table II provides the general clinical characteristics of the patient group. We observed that 25% of the patients exhibited diabetes mellitus (DM)-arterial hypertension (AH); 21% presented breast fibrosis-myomatosis-hysterectomy; 65% were positive for estrogen receptor; 89% displayed ductal histology; and 59% exhibited stage III–IV tumors. Additionally, approximately 19% of the patients presented high levels of serum glutamate oxaloacetate transaminase (SGOT), and more than 20% showed high levels of alkaline phosphatase (ALP), γ-glutamyl transpeptidase (GGT) and glucose.
Table II.
Clinical data from patients with BC
| Variable | n | % |
|---|---|---|
| Personal medical history: | ||
| No | 243 | 51 |
| DM-AH | 122 | 25 |
| Breast, fibrosis, myomatosis | 102 | 21 |
| Depression, pregnancy, asthma | 14 | 3 |
| Body mass index (BMI) [kg/m2]*: | ||
| 18.5–24.9 (normal) | 88 | 18 |
| = 25–29.9 (overweight) | 157 | 32 |
| = 30–34.9 (obesity I) | 142 | 30 |
| = 35– > 40 (obesity II–IV) | 94 | 20 |
| Tumor localization: | ||
| Unilateral | 460 | 96 |
| Bilateral | 21 | 4 |
| Diagnostic time [years]: | ||
| 1–4 | 380 | 79 |
| 5–9 | 86 | 18 |
| 10–15 | 15 | 3 |
| Tumor markers : | ||
| HER2/neu | 124 | 26 |
| Estrogen receptor | 315 | 65 |
| Progesterone receptor | 279 | 58 |
| KI-167 | 117 | 24 |
| P53 | 119 | 25 |
| E-cadherin | 107 | 22 |
| Triple negative | 54 | 11 |
| No data | 12 | 2 |
| Histology: | ||
| Ductal | 430 | 89 |
| Lobular | 48 | 10 |
| Mixed | 3 | 1 |
| Tumor stage: | ||
| I–II | 196 | 41 |
| III–IV | 285 | 59 |
| Lymph node status: | ||
| Yes | 337 | 70 |
| No | 144 | 30 |
| Metastasis: | ||
| Yes | 160 | 33 |
| No | 321 | 67 |
| Chemotherapy response: | ||
| Yes | 319 | 66 |
| No | 162 | 34 |
| Chemotherapy type: | ||
| FEC | 362 | 75 |
| Other | 99 | 21 |
| No chemotherapy | 20 | 4 |
| Laboratory test | ||
| Hemoglobin [g/dl]: | ||
| < 11 | 99 | 21 |
| 11–16.4 | 382 | 79 |
| Hematocrit (%): | ||
| < 37 | 98 | 20 |
| 37–47 | 383 | 80 |
| Platelets [mm3]: | ||
| < 150,000 | 21 | 4 |
| 150,000–450,000 | 328 | 68 |
| > 450,000 | 132 | 28 |
| Leukocytes [mm3]: | ||
| < 150,000 | 51 | 11 |
| 150,000–500,000 | 430 | 89 |
| SGOT [µI/l]: | ||
| > 35 | 92 | 19 |
| 0–35 | 389 | 81 |
| SGPT [µI/l]: | ||
| > 45 | 52 | 11 |
| 5–45 | 429 | 89 |
| LDH [µI/l]: | ||
| > 333 | 75 | 16 |
| 105–333 | 406 | 84 |
| ALP [µI/l]: | ||
| > 45 | 110 | 23 |
| 5–45 | 371 | 77 |
| GGT [µI/l]: | ||
| > 45 | 124 | 26 |
| 5–45 | 357 | 74 |
| Glucose [µI/l]: | ||
| > 106 | 133 | 28 |
| 74–106 | 348 | 72 |
FEC – 5-fluorouracil, epirubicin, cyclophosphamide; others: paclitaxel, docetaxel, herceptin. SGOT – glutamate-oxaloacetate transaminase, SGPT – serum glutamic pyruvic transaminase, LDH – lactate dehydrogenase, ALP – alkaline phosphatase, GGT – γ-glutamyltransferase
according to OMS classifications. (Appropriate body mass index for Asian populations and its implications for policy and intervention strategies. Ginebra (Suiza): World Health Organization, 2004).
Table III summarizes the results of the multivariate logistic regression analysis, where the BC group was classified as presenting tumor stage I–II or III–IV as the dependent variable. An obesity grade of II–IV (OR = 2.4, 95% CI: 1.2–4.9, p = 0.012), metastatic nodules (OR = 2.6, 95% CI: 1.5–4.4, p < 0.001), non-response to chemotherapy (OR = 4.3, 95% CI: 1.5–12.3, p = 0.005), and elevated levels of lactate dehydrogenase (LDH) (OR = 3.0, 95% CI: 1.4–6.6, p = 0.004) were found to be risk factors associated with stage III–VI tumors.
Table III.
Binary logistic regression analysis of the patient group
| Variable | B | SD | Wald | df | Value of p | OR | 95% CI | |
|---|---|---|---|---|---|---|---|---|
| Low | Upper | |||||||
| Obesity grade II-IV | 0.896 | 0.358 | 6.271 | 1 | 0.012 | 2.449 | 1.215 | 4.937 |
| Lymph node status | 0.966 | 0.263 | 13.456 | 1 | < 0.001 | 2.626 | 1.568 | 4.400 |
| Metastasis | 4.368 | 1.055 | 17.157 | 1 | < 0.001 | 78.900 | 9.987 | 623.341 |
| Chemotherapy (non-response) | 1.475 | 0.530 | 7.755 | 1 | 0.005 | 4.370 | 1.548 | 12.340 |
| LDH (high level) | 1.128 | 0.392 | 8.284 | 1 | 0.004 | 3.088 | 1.433 | 6.655 |
| Constant | –1.332 | 0.238 | 31.198 | 1 | < 0.001 | 0.264 | ||
Variables included in the analysis: dependent: BC classified by tumor status as stage I-II or III-IV; independent: personal medical history, menarche in the range 7–10 years, 11–13 years, or 14–18 years; menopause, pregnancies, breastfeeding, oral contraceptive use, tobacco and alcohol consumption, a BMI of 18.5–24.9, = 25–29.9, = 30–34.9, or = 35– > 40 (obesity grade II–IV), lymph node status, metastasis, response to chemotherapy, laboratory tests (HB, HTO, platelets, leukocytes, urea, SGOT, SGPT, LDH, ALP, GGT and glucose).
The genotype and allele frequencies of the PR gene Alu insertion polymorphism were different in the control and patient groups (Table IV). The T1/T1 genotype was observed in 75% (360/481) of patients, compared to 84% (176/209) of the controls. The heterozygous genotype (T1/T2) was observed in 21% of the patients (103/481) and 16% (33/209) of the controls (OR = 1.4, 95% CI: 0.95–2.2, p = 0.08). The polymorphic genotype (T2/T2) was observed in 4% (18/481) of the patients and was not found in the control group (OR = 8.1, 95% CI: 1.08–61.2, p = 0.01). The genotype distribution in the control group was in Hardy-Weinberg equilibrium. All of the samples were analyzed, and all of the participants’ genotypes (for 209 controls and 481 BC patients) were obtained.
Table IV.
Genotype and allelic distribution of the Alu insertion polymorphism of the PR gene in healthy controls and BC patients
| Variable | Groups | ||||||
|---|---|---|---|---|---|---|---|
| Patients (N = 481) | Controls (N = 209)* | Patients vs. controls | |||||
| n | % | n | % | OR | 95% CI | Value of p*** | |
| Genotypes**: | 360 | 75 | 176 | 84 | 1 | ||
| T1/T1 | 360 | 75 | 176 | 84 | 1 | ||
| T1/T2 | 103 | 21 | 33 | 16 | 1.46 | 0.95–2.2 | 0.08 |
| T2/T2 | 18 | 4 | 0 | 8.1 | 1.08–61.2 | 0.01 | |
| T1/T2 and T2/T2 | 121 | 25 | 33 | 16 | 1.7 | 1.14–2.6 | 0.009 |
| Alleles: | |||||||
| T1 | 823 | 0.85 | 385 | 0.92 | 0.50 | 0.34–0.75 | 0.0007 |
| T2 | 139 | 0.15 | 33 | 0.08 | 1.9 | 1.3–2.9 | 0.0007 |
Hardy-Weinberg equilibrium in controls (χ2 test = 1.5; p = 0.215);
marker informativity of 0.84 assessed within a range of 0–1: markers with a score greater than 0.7 were considered to be highly informative, whereas markers with a value of 0.44 were considered to be moderately informative [5, 6].
Cochran-Armitage test.
Table V shows that the T1/T2 and T2/T2 genotypes were associated with obesity grade II (OR = 1.81, 95% CI: 1.03–3.188, p = 0.039) and non-response to chemotherapy (OR = 1.98, 95% CI: 1.27–3.06, p = 0.002), and the variables listed in Table I and II were found to be risk factors.
Table V.
Associations of the T1/T2 and T2/T2 genotypes of the Alu insertion polymorphism of the PR gene with more than one variable among the general characteristics of the BC patients
| Variable | B | SD | Wald | df | Value of p | OR | 95% CI | |
|---|---|---|---|---|---|---|---|---|
| Low | Upper | |||||||
| BMI (18.5–24.9 kg/m2) | –0.931 | 0.355 | 6.889 | 1 | 0.009 | 0.394 | 0.197 | 0.790 |
| Obesity grade II (35–39.9 kg/m2) | 0.594 | 0.289 | 4.239 | 1 | 0.039 | 1.81 | 1.03 | 3.188 |
| No chemotherapy response | 0.683 | 0.223 | 9.387 | 1 | 0.002 | 1.981 | 1.279 | 3.067 |
| Constant | –1.140 | 0.205 | 30.797 | 1 | < 0.001 | 0.320 | ||
Variables included in the analysis: dependent: BC patients classified by W/ins-Ins/Ins genotype; independent: personal medical history, menarche in the range 7–10 years, 11–13 years, of 14–18 years; menopause, pregnancies, breastfeeding, oral contraceptive use, tobacco and alcohol consumption, HF, HF type: BC, DM, AH, DM-AH-cancer, a BMI of 18.5–24.9, = 25–29.9, = 30–34.9, or = 35–> 40, lymph node status, metastasis, response to chemotherapy, laboratory tests (HB, HTO, platelets, leukocytes, urea, SGOT, SGPT, LDH, ALP, GGT and glucose).
Discussion
Breast cancer is a multifactorial disease with a complex etiology and is considered a major public health problem in industrialized countries. In Mexico, the incidence of BC has increased over the last decade, and it is currently one of the leading causes of death in working age women [3–6]. These facts are consistent with the observations made in the current study, where the average age of BC patients was 53.64 ±11.8 years, and oral contraceptive use, abortion, breastfeeding > 6 months and menopause were found to be risk factors. Multiple studies have previously observed these associations [5, 6, 20–22].
In this study, breastfeeding was recorded in 67% of the patients, in contrast with 58% of the controls, which made it a risk factor. However, when breastfeeding was classified by the duration of lactation, contradictory results were obtained, most likely due to differences in the lifestyles and mean age of the control group subjects [23].
When the group was stratified by tumor stage as showing either stage I–II or III–IV, followed by comparison with the clinical and biochemical characteristics of BC, an obesity grade of II–IV, metastasis to the lymph nodes, metastasis, non-response to chemotherapy, and high levels of LDH emerged as risk factors. In this context, genetic variants have been previously associated with prognostic markers such as tumor stage and metastatic nodules [24].
When we adjusted the study groups according to the body mass index (BMI) of the participants, we observed that obesity II–IV was a risk factor in the stage III–IV BC patients. Several theories have been put forth in an attempt to explain this association. These theories involve the roles of leptin, insulin and other molecules that mediate the inflammatory process independently of estrogen. In addition, peripheral circulating estrogens (arising from the aromatization of androgens) are elevated in obese postmenopausal women. Another current hypothesis proposes that obesity is associated with metabolic syndrome, which activates mitogenic molecular processes in breast epithelial cells and stimulates neoplasia. A third hypothesis suggests that adipocytes and the autocrine mechanisms involving proinflammatory cytokines in these cells are important for BC development [5, 6, 21–23, 25, 26].
Additionally, the presence of lymph node metastases and non-response to chemotherapy emerged as risk factors in stage III–IV BC patients. The chemotherapy response depends on several factors, including the presence of metastatic nodes, tumor markers, menopause, time of diagnosis, tumor stage, and treatment resistance. Adjuvant chemotherapy can induce persistent resistance to these drugs under longer exposure, and anthracyclines have achieved longer times to progression and survival times compared to cyclophosphamide/methotrexate/5-fluorouracil-based chemotherapy [5, 6, 27, 28].
Other associated factors included high LDH expression, which was indicative of a poor response in patients with later stages of BC, as previously reported in BC from Mexican, Caucasian (USA) and Serbian populations [5, 6, 29, 30]. Thus, hormone receptor-positive tumors are more likely to relapse to bone, showing a better outcome in comparison to estrogen receptor-negative tumors, which are more likely to relapse to the brain and exhibit unfavorable outcomes. Another factor that may influence the response to chemotherapy is menopause, which is associated with a less favorable response. Because each predictor can be useful in predicting survival, several studies have reported prognostic indices of survival for patients with BC [5, 6, 30].
Advances in molecular and genetic epidemiology have increased our knowledge of the mechanisms underlying breast carcinogenesis and the relationships between exposure to carcinogens, diet, and individual genetic variations in relation to susceptibility. Gene polymorphisms with a low penetrance have also been found to be risk factors in BC [5, 6, 31, 32].
Progesterone metabolism has been proposed to be a contributing factor via progesterone binding to specific DNA sequences and acting as a transcriptional factor for target genes. The action of progesterone is mediated by the PR gene. Binding of progesterone to its receptor results in a complex activation cascade, beginning with conformational changes, protein phosphorylation, dissociation from heat shock proteins, and dimer formation, finally leading to nuclear transport of the active protein-progesterone complex [33]. The insertion of an Alu sequence in intron 7 of the PR gene was first described as part of the PROGINS complex, and it has been observed that this variant acts as a risk factor in several types of progesterone-dependent cancer, such as endometrial, ovarian and BC [7, 10, 11, 13, 15].
We observed similar allele frequencies of the Alu insertion polymorphism in our control group to those previously reported in Mexico (Mayas from the state of Campeche, Yucatan Peninsula) [34, 35]. However, little is known about the association of this polymorphism with BC. In this study, the allelic frequency (T2) of the Alu insertion polymorphism was 0.08 in controls and 0.15 in BC patients with associated risk factors. Several authors have studied the PROGINS region, although they have not analyzed the same markers, and the studied groups have had different compositions (e.g., regarding menopause, tumor stages, ethnic backgrounds, ages). Consequently, there are conflicting results regarding this association, with several studies observing a decreased risk of BC in association with the Alu insertion polymorphism [36–39], whereas Chambo et al. [36] detected a trend towards an association between the PROGINS polymorphism and dense breasts in 123 BC postmenopausal Brazilian patients who were not on hormone therapy and displayed no clinical or mammographic breast alterations. Additionally, it has been found that the presence of both a wild-type PROGINS and mutated CYP17 genotype resulted in a 4.87 times higher risk of exhibiting dense breasts (p = 0.030).
Wang-Gohrke et al. [37] reported that risk was decreased in women carrying the PROGINS allele in 554 BC patients and 559 age-matched controls with ages of 51 years or younger in the Rhein-Neckar-Odenwald and Freiburg study regions (Germany), suggesting a gene dosage effect of the A2 allele. Furthermore, there is suggestive evidence of differential effects based on menopausal status and family history of BC.
Wasserman et al. [38] detected an association of PROGINS A1/A1 and other AIB1 LG genotypes in postmenopausal, obese patients with BC in the Women's Healthy Eating and Living (WHEL) study of a Caucasian population.
Govindan et al. [15] found an association of the T2 Alu insertion polymorphism of the PROGINS gene in 157 cases of breast cancer in an Indian population. However, other studies on BC in Caucasian populations (New Orleans and State of California) have found no such association [34].
It has been suggested that the Alu insertion polymorphism may affect ligand and hormone binding properties and hence increase transcription activity for mutated transcripts and reduce the response to progesterone by affecting gene expression and mRNA stability. These effects could repress estrogen receptor activation and contribute to estrogen-related tumor promotion in the mammary gland and may have an impact on BC oncogenesis [33, 37].
In this study, we also observed an association of the T1/T2-T2/T2 genotype as a risk factor in patients showing obesity grade II (BMI 35–39 kg/m2) and no chemotherapy response. Weight gain in women with postmenopausal BC in industrialized countries has a significant impact on health [5, 6]. The relationship between obesity and BC is complex and has been associated with factors including genetic predisposition, social class, exercise, alcohol consumption and diet [5, 6, 38]. Several studies have also observed that obesity is associated with an increased risk of developing BC, including showing associations with postmenopausal status, increased mortality when the BMI is increased by > 40 kg/m2 [39, 40], the presence of lymph node metastasis and developed metastases [6], and poor prognosis in both pre- and post-menopausal BC patients [39]. Esfahlan et al. [39] studied in an Iranian population the relationship between steroid receptor status and body weight in 70 BC patients and concluded that obesity could play a significant role in estrogen receptor gene expression as well as affecting the progression and proliferation of BC cells. Several hypotheses have been proposed to attempt to explain this association, which influences tumor growth and BC prognosis. It has been suggested that the aromatization of estrogen and androgens is greater in obese postmenopausal women than in non-obese women, and this increase could stimulate tumor growth and adversely affect patient prognosis. The adipose tissue of obese women is known to secrete high levels of active estrogen, stimulating mammary epithelial cell mitosis and promoting the development of tumors. In fact, the most likely scenario is that all of these mechanisms may work together to explain the relationship linking menopause and subsequent weight gain in BC [5, 6, 39, 40]. The results observed in this study are most likely due to both prolonged exposure to estrogens and nutritional status [38–41].
We also observed an association of the T1/T2-T2/T2 genotypes of BC patients and non-response to chemotherapy. However, there are no available studies addressing the influence of the T1/T2-T2/T2 Alu insertion of the PR genotype and the response to chemotherapy in BC patients. Progesterone metabolism can likely influence the chemotherapy response, in addition to other factors, such as the known presence of metastatic nodes, tumor markers, menopause, the time of diagnosis, tumor stage, and treatment resistance [5, 6, 28]. This response may be affected by polymorphisms in the PR gene that can cause an increase in PR activity, producing changes in DNA and subsequently participating in neoplastic progression.
In conclusion, our results show that the frequencies of the homozygous and heterozygous genotypes of the Alu insertion polymorphism in the PR gene are significantly different in control vs. BC patients. The differences were most evident in patients showing obesity grade II, and the chemotherapy response may contribute significantly to BC susceptibility in the analyzed sample from a Mexican population. Nevertheless, further studies are required to confirm or reject these observations.
Acknowledgments
We thank Dr. Efrain Salas and nurses from UMAE Hospital de Gineco-Obstetricia, CMNO, IMSS for facilitating sample collection.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Siegel R, Desantis C, Virgo K, et al. Cancer treatment and survivor ship statistics, 2012. CA Cancer J Clin. 2012;62:220–41. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- 2.Miller JW, King JB, Joseph DA, et al. Breast cancer screening among adult women – behavioral risk factor surveillance system, United States, 2010. MMWR Morb Mortal Wkly Rep. 2012;61:46–50. [PubMed] [Google Scholar]
- 3.Chávarri-Guerra Y, Villarreal-Garza C, Liedke PE, et al. Breast cancer in Mexico: a growing challenge to health and the health system. Lancet Oncol. 2012;13:335–43. doi: 10.1016/S1470-2045(12)70246-2. [DOI] [PubMed] [Google Scholar]
- 4.Knaul FM, Nigenda G, Lozano R, Arreola-Ornelas H, Langer A, Frenk J. Breast cancer in Mexico: an urgent priority. Salud Publica Mex. 2009;51:335–44. doi: 10.1590/s0036-36342009000800026. [DOI] [PubMed] [Google Scholar]
- 5.Gómez-Flores L, Escoto A, Puebla AM, et al. Association of the tumor necrosis factor-alpha –308G>A polymorphism with breast cancer in Mexican women. Genet Mol Res. 2013;12:5680–93. doi: 10.4238/2013.November.18.17. [DOI] [PubMed] [Google Scholar]
- 6.Gallegos MP, Figuera LE, Ramos MC, et al. The association between the 844ins68 polymorphism in the CBS gene and breast cancer. Arch Med Sci. 2014;10:1214–24. doi: 10.5114/aoms.2014.47830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdulrahman GO, Jr, Rahman GA. Epidemiology of breast cancer in Europe and Africa. J Cancer Epidemiol. 2012;2012:915610. doi: 10.1155/2012/915610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu YL, Sun Q, Shan GL, et al. A case-control study on risk factors of breast cancer in China. Arch Med Sci. 2012;8:303–9. doi: 10.5114/aoms.2012.28558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mojgan H, Massoud H, Ahmad E. ERCC1 intron 1 was associated with breast cancer risk. Arch Med Sci. 2012;8:655–8. doi: 10.5114/aoms.2012.30289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gimenes C, Bianco B, Mafra FA, Rosset V, Christofolini DM, Barbosa CP. The progins progesterone receptor gene polymorphism is not related to endometriosis-associated infertility or to idiopathic infertility. Clinics. 2010;65:1073–6. doi: 10.1590/S1807-59322010001100002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mani SK, Oyola MG. Progesterone signaling mechanisms in brain and behavior. Front Endocrinol (Lausanne) 2012;3:7. doi: 10.3389/fendo.2012.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rockwell LC, Rowe EJ, Arnson K, et al. Worldwide distribution of allelic variation at the progesterone receptor locus and the incidence of female reproductive cancers. Am J Hum Biol. 2012;24:42–51. doi: 10.1002/ajhb.21233. [DOI] [PubMed] [Google Scholar]
- 13.Costa IR, Silva RC, Frare AB, et al. Polymorphism of the progesterone receptor gene associated with endometriosis in patients from Goiás, Brazil. Genet Mol Res. 2011;10:1364–70. doi: 10.4238/vol10-3gmr913. [DOI] [PubMed] [Google Scholar]
- 14.Romano A, Delvoux B, Fischer DC, Groothuis P. The PROGINS polymorphism of the human progesterone receptor diminishes the response to progesterone. J Mol Endocrinol. 2007;38:331–50. doi: 10.1677/jme.1.02170. [DOI] [PubMed] [Google Scholar]
- 15.Govindan S, Ahmad SN, Vedicherla B, et al. Association of progesterone receptor gene polymorphism (PROGINS) with endometriosis, uterine fibroids and breast cancer. Cancer Biomark. 2007;3:73–8. doi: 10.3233/cbm-2007-3201. [DOI] [PubMed] [Google Scholar]
- 16.Johnatty SE, Spurdle AB, Beesley J, et al. Progesterone receptor polymorphisms and risk of breast cancer: results from two Australian breast cancer studies. Breast Cancer Res Treat. 2008;109:91–9. doi: 10.1007/s10549-007-9627-3. [DOI] [PubMed] [Google Scholar]
- 17.Runnebaum IB, Wang-Gohrke S, Vesprini D, et al. Progesterone receptor variant increases ovarian cancer risk in BRCA1 and BRCA2 mutation carriers who were never exposed to oral contraceptives. Pharmacogenetics. 2001;11:635–8. doi: 10.1097/00008571-200110000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;6:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanguinetti CJ, Dias NE, Simpson AJ. Rapid silver staining and recovery of PCR products separated on polyacrylamide gels. Biotechniques. 1994;17:914–21. [PubMed] [Google Scholar]
- 20.Naieni KH, Ardalan A, Mahmoodi M, Motevalian A, Yahyapoor Y, Yazdizadeh B. Risk factors of breast cancer in north of Iran: a case-control in Mazandaran Province. Asian Pac J Cancer Prev. 2007;8:395–8. [PubMed] [Google Scholar]
- 21.Macciò A, Madeddu C. Obesity, inflammation, and postmenopausal breast cancer: therapeutic implications. Sci World J. 2011;11:2020–36. doi: 10.1100/2011/806787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin AM, Weber BL. Genetic and hormonal risk factors in breast cancer. J Natl Cancer Inst. 2000;92:1126–35. doi: 10.1093/jnci/92.14.1126. [DOI] [PubMed] [Google Scholar]
- 23.Lazcano EC, Tovar V, Alonso P, Romieu I, Lopez L. Breast cancer. A historical theme, present and future. J Public Health. 1996;38:139–52. [PubMed] [Google Scholar]
- 24.Romanowicz-Makowska H, Brys M, Forma E, et al. Single nucleotide polymorphism (SNP) Thr241Met in the XRCC3 gene and breast cancer risk in Polish women. Pol J Pathol. 2012;63:121–5. [PubMed] [Google Scholar]
- 25.Henderson BE, Roos RK, Judd HL. Regular ovulatory cycles do increase breast cancer risk? Cancer. 1985;56:1206–8. doi: 10.1002/1097-0142(19850901)56:5<1206::aid-cncr2820560541>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 26.Coelho M, Oliveira T, Fernandes R. Biochemistry of adipose tissue: an endocrine organ. Arch Med Sci. 2013;9:191–200. doi: 10.5114/aoms.2013.33181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hwang RF, Gonzalez-Angulo AM, Yi M, et al. Low locoregional failure rates in selected breast cancer patients with tumor-positive sentinel lymph nodes who do not undergo completion axillary dissection. Cancer. 2007;110:723–30. doi: 10.1002/cncr.22847. [DOI] [PubMed] [Google Scholar]
- 28.Pompella A, De Tata V, Paolicchi A, Zunino F. Expression of gamma-glutamyltransferase in cancer cells and its significance in drug resistance. Biochem Pharmacol. 2006;71:231–8. doi: 10.1016/j.bcp.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 29.Dennison JB, Molina JR, Mitra S, et al. Lactate dehydrogenase B: a metabolic marker of response to neoadjuvant chemotherapy in breast cancer. Clin Can Res. 2013;19:3703–13. doi: 10.1158/1078-0432.CCR-13-0623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Radenkovic S, Milosevic Z, Konjevic G, et al. Lactate dehydrogenase, catalase, and superoxide dismutase in tumor tissue of breast cancer patients in respect to mammographic findings. Cell Biochem Biophys. 2013;66:287–95. doi: 10.1007/s12013-012-9482-7. [DOI] [PubMed] [Google Scholar]
- 31.Smolarz B, Zadrożny M, Duda-Szymańska J, et al. RAD51 genotype and triple-negative breast cancer (TNBC) risk in Polish women. Pol J Pathol. 2013;64:39–43. doi: 10.5114/pjp.2013.34602. [DOI] [PubMed] [Google Scholar]
- 32.Mojgan H, Massoud H, Ahmad E. ERCC1 intron 1 was associated with breast cancer risk. Arch Med Sci. 2012;8:655–8. doi: 10.5114/aoms.2012.30289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Su MT, Lee IW, Chen YC, Kuo PL. Association of progesterone receptor polymorphism with idiopathic recurrent pregnancy loss in Taiwanese Han population. J Assist Reprod Genet. 2011;28:239–43. doi: 10.1007/s10815-010-9510-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Donaldson C, Crapazano J, Watson J, Levine E, Batzer M. PROGINS Alu insertion and human genomic diversity. Mutat Res. 2002;501:137–41. doi: 10.1016/s0027-5107(02)00015-5. [DOI] [PubMed] [Google Scholar]
- 35.Antunez-de-Mayolo A, Antunez-de-Mayolo G, Thomas E, Reategui EP, Brown MD, Herrera RJ. Worldwide distribution of a polymorphism Alu insertion in the progesterone receptor gene. In: Papiha SS, Deka R, Chakraborty R, editors. In: Genomic diversity: applications inhuman population genetics. New York: Kluwar Academic/Plenum Publishers; 1999. pp. 213–22. [Google Scholar]
- 36.Chambo D, Kemp C, Costa AM, Souza NC, Guerreiro da Silva ID. Polymorphism in CYP17, GSTM1 and the progesterone receptor genes and its relationship with mammographic density. Braz J Med Biol Res. 2009;42:323–9. doi: 10.1590/s0100-879x2009000400003. [DOI] [PubMed] [Google Scholar]
- 37.Wang-Gohrke S, Chang-Claude J, Becher H, Kieback DG, Runnebaum IB. Progesterone receptor gene polymorphism is associated with decreased risk for breast cancer by age 50. Cancer Res. 2000;60:2348–50. [PubMed] [Google Scholar]
- 38.Wasserman L, Flatt SW, Natarajan L, et al. Correlates of obesity in postmenopausal women with breast cancer: comparison of genetic, demographic, disease-related, life history and dietary factors. Int J Obes Relat Metab Disord. 2004;28:49–56. doi: 10.1038/sj.ijo.0802481. [DOI] [PubMed] [Google Scholar]
- 39.Esfahlan RJ, Zarghami N, Esfahlan AJ, Mollazadeh M, Nejati K, Nasiri M. The possible impact of obesity on androgen, progesterone and estrogen receptors (ERalpha and ERbeta) gene expression in breast cancer patients. Breast Cancer Auckl. 2011;5:227–37. doi: 10.4137/BCBCR.S7707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kelesidis I, Kelesidis T, Mantzoros CS. Adiponectin and cancer: a systematic review. Br J Cancer. 2006;94:1221–5. doi: 10.1038/sj.bjc.6603051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cork DM, Lennard TW, Tyson-Capper AJ. Alternative splicing and the progesterone receptor in breast cancer. Breast Cancer Res. 2008;10:207. doi: 10.1186/bcr2097. [DOI] [PMC free article] [PubMed] [Google Scholar]