Abstract
Introduction
Multidrug-resistant tuberculosis (MDR-TB) is a hard-to-treat disease with a poor outcome of chemotherapy. In the present study, the efficacy and safety of recombinant human interleukin-2 (rhIL-2) were investigated in patients with MDR-TB.
Material and methods
Fifty culture-confirmed patients with MDR-TB were included. Twenty-five patients were randomly assigned to the trial group (injection of 500 000 IU of rhIL-2 once every other day at the first, third, fifth and seventh months in addition to standard multidrug therapy) and another 25 patients to the control group with standard multidrug therapy. All patients were monitored clinically, and T-cell subsets were analyzed by flow cytometry.
Results
The rates of sputum negative conversion and X-ray resolution in the trial group were higher than those of the control, and the improvements were significant by completion of treatment. In addition, CD4+CD25+ T cells in the controls rose gradually during treatment. The levels at the end of the seventh month were significantly higher than before, which were also significantly different when compared with those from the trial group at the same time. However, there were no such changes associated with treatment in the trial group. No significant differences appeared in other T cell subsets.
Conclusions
Exogenous IL-2 in the present regimen improves immunity status. Adjunctive immunotherapy with a long period of rhIL-2 is a promising treatment modality for MDR-TB.
Keywords: multidrug-resistant tuberculosis, immunotherapy, interleukin-2, CD4+CD25+ T cells
Introduction
Multidrug-resistant tuberculosis (MDR-TB) is a growing problem that poses a major threat to global tuberculosis (TB) control, especially in developed countries. Nearly 440 thousand people are infected with MDR-TB worldwide every year. As a high burden country, about 100 thousand MDR-TB cases appear in China annually [1]. Treatment of MDR-TB requires the use of toxic, expensive, and less effective second-line drugs for 2 years or more, leading to low patient compliance and poor treatment outcomes with high mortality. The recent situation demonstrates the urgent need to search for novel strategies to treat MDR-TB. Adjunctive immunotherapy, together with chemotherapy, has the potential to improve treatment outcomes of drug-resistant tuberculosis [2–4].
Protective immunity against Mycobacterium tuberculosis (Mtb) is based on cell-mediated immunity involving CD4+ and CD8+ T cells [5, 6]. Interleukin-2 (IL-2), mainly secreted by activated T cells, is central to the development of an adaptive immune response to infection, promoting differentiation and proliferation of lymphoid cells. The systemic immune response in peripheral blood is characterized by enhanced Th2 function and decreased Th1 function in TB pathogenesis [7]. These disturbances were remarkable in MDR-TB [8–10]. Recovery from TB depends, in part, on the generation of an effective cell-mediated immune response against the pathogen. Therefore, IL-2 injection has been deemed to be a natural adjunctive therapy for TB. Several studies have shown recombinant human interleukin-2 (rhIL-2) to be safe and well tolerated [11–13]. However, the only large-scale randomized trial evidenced that intradermal therapy with rhIL-2 did not produce an improvement in clinical symptoms and sputum bacillary clearance [13]. There has been a lack of more recent such clinical studies. Changes of T cell subsets in response to treatment were especially scarce in MDR-TB patients.
To further elucidate this issue, we conducted a randomized trial to examine the effects of rhIL-2 plus standard chemotherapy by following up the whole duration of therapy in MDR-TB patients. We also evaluated the frequencies of T cell subsets and CD4+CD25+ T cells at several time points in the peripheral blood of patients throughout the course of rhIL-2 administration, and aimed to explore the correlation between alterations in immune cells and treatment outcomes.
Material and methods
Patients
A total of 50 MDR-TB patients aged 18 to 70 years were recruited from six multicenter organizations: the Third Hospital of Zhenjiang City, the Fourth People's Hospital of Lianyungang, Taizhou People's Hospital, Nanjing Chest Hospital, the Sixth People's Hospital of Nantong, and Taixing Municipal Center for Disease Control and Prevention. Patients were identified according to guidelines for pulmonary TB diagnosis and therapy published by the Tuberculosis Branch Association of the Chinese Medical Association. All patients were HIV seronegative and had pulmonary MDR-TB, defined as culture-confirmed Mtb resistant to isoniazid and rifampicin. We excluded patients with serious medical comorbidities.
Approval was granted by the Hospital Ethics Committee of Jiangsu Province Hospital, and all participants provided informed consent.
Treatment allocation and anti-TB therapy
After screening, study participants were admitted to hospital, 25 MDR-TB patients were randomly assigned to standard chemotherapy (control), and another 25 MDR-TB patients were randomly assigned to standard chemotherapy plus intradermal injection of 500 000 IU rhIL-2 (Yuance, China) once every other day starting at the first, third, fifth and seventh months during the course of anti-TB treatment (rhIL-2 group). All patients received drug susceptibility testing, and were given 24 months of multidrug chemotherapy (6 months daily of pyrazinamide, kanamycin (amikacin or capreomycin), levofloxacin, protionamide, and p-aminosalicylic acid (ethambutol) and 18 months daily of pyrazinamide, levofloxacin, protionamide, and p-aminosalicylic acid (ethambutol)). The drugs in the brackets are the substitute for the prior medicine that cannot be applied for some reasons. The dosage of above drugs was adjusted for body weight. Treatment assignments were masked to clinical and laboratory staff.
Follow-up assessment
Bacteriology
Morning sputum specimens were collected monthly from patients for qualitative acid-fast bacilli smears and culture in improved Lowenstein Jensen (L-J) plates and p-nitrobenzoic acid (PNB) L-J media respectively. The rates of sputum smear and culture conversion were assessed at the time points of the third, sixth, twelfth, eighteenth, and twenty-fourth months during anti-TB treatment.
Radiology
Chest radiographs were read by two independent physicians who were blinded to the treatment assignation. Radiographic changes were evaluated by comparing baseline and follow-up chest X-rays after the end of the third, sixth, twelfth, eighteenth, and twenty-fourth months of anti-TB treatment. Changes were rated to the following four grades: marked absorption (meaning significant improvement of more than half of initial abnormalities), absorption (meaning definite improvement better than initial abnormalities but less than a half), no changes (no certain difference in films compared with original lesion), and deterioration (being worse than initial abnormalities or spreading to another area). Simultaneously, the cavity closure was observed.
Immunologic measurement of peripheral blood
Five milliliters of venous blood were obtained from patients on an empty stomach in the morning prior to study and after the first, third, and seventh months of treatment. Whole blood was collected in plastic tubes containing anticoagulant. One hundred μl of fresh blood was incubated in the dark at room temperature with 10 μl of antibodies conjugated with fluorescence for 15 min, including anti-human CD4-fluorescein isothiocyanate (FITC)/CD8-phycoerythrin (PE)/CD3-peridinin chlorophyll protein (PerCP) (BD company, USA), and anti-human CD4-FITC/CD25-PE (Immunotech, French). Ten μl of IgG-FITC/IgG-PE/IgG-Percy5 was placed into one tube as an isotype control. After incubating for 10 min, erythrocytes were removed using 1 ml of diluted lysing solution. Subsequently, the cells were washed with 1 ml of PBS, centrifuging at 1000 rpm for 5 min, and supernatant was removed. Then, the cells were resuspended in 500 μl of PBS, and run on the BD FACSCalibur (BD, USA). The samples were counted at 50,000 events, setting the lymphocyte gate using the CellQuest program. Finally, the percentage value of all the markers was calculated.
Safety
During anti-TB treatment, the adverse events were counted. Complete blood count, blood chemistry, serum electrolyte, and urinalysis were repeated monthly during treatment. Serum thyroid stimulating hormone was checked before the study and after 1 month of treatment. Subjects were followed for 2 years after the beginning of chemotherapy.
Statistical analysis
All results were analyzed using SPSS 11.1 (SPSS Inc, Chicago IL, USA). Numeric values were presented as mean ± standard deviation (SD). Bacillus negative and chest X-ray results were evaluated by the χ2 test. Differences between means of two groups were tested by the t test. Differences among multiple groups were evaluated by one-way analysis of variance (ANOVA). Pearson's test was used to analyze the correlation. Values of p less than 0.05 were considered significant.
Results
Patient clinical characteristics
Table I shows the characteristics of all subjects at the time of entry to the study. As can be seen in the Table, the base condition of participants was closely matched; the majority of patients were middle aged men, who had been treated many times, and were resistant to three or more drugs. One patient in the control group died of severe pneumonia at the 11th month of treatment, and there was loss of contact with 1 patient in the rhIL-2 group at the 10th month.
Table I.
Baseline characteristics of study subjects in each group
| Characteristic | rhIL-2 (n = 25) | Control (n = 25) |
|---|---|---|
| Age [years] | 44.1 ±13 | 46.4 ±14 |
| Male, n (%) | 20 (80) | 21 (84) |
| Relapse patients | 24 | 23 |
| Treatment times | 3.1 ±1.1 | 3.1 ±1.7 |
| Resistant drugs | 3.2 ±0.9 | 3.3 ±1.2 |
| Cavity number | 1.3 ±0.9 | 1.5 ±0.9 |
| Disease lobe | 3.7 ±1.8 | 3.9 ±1.4 |
Values are presented as mean ± SD. rhIL-2 – recombinant human interleukin-2.
Microbiologic outcomes
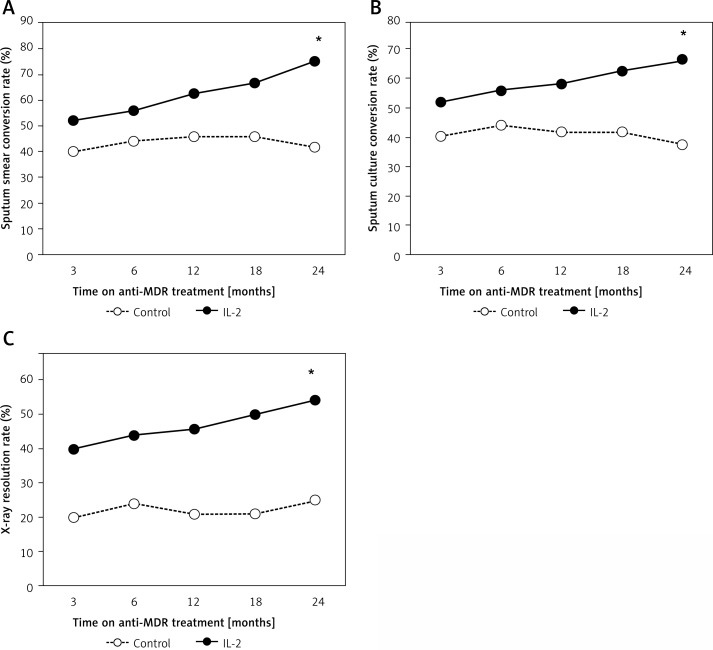
After initiation of treatment, patients from the rhIL-2 group demonstrated a trend in sputum increasingly converting to negative, the sputum smear negative rates changing from 52% at the third month to 75% at the end of therapy, and negative sputum cultures increased from 52% to 67%. By contrast, in the control group, the proportion of subjects who had their sputum change to negative was lower compared with the rhIL-2 group at each time point; the differences were significant by completion of the study (p = 0.019 for sputum smear rates; p = 0.043 for sputum culture rates) (Figure 1 A, B).
Figure 1.
Clinical outcomes in control and rhIL-2 trial group. A – Sputum smear conversion rates. B – Sputum culture conversion rates. C – Lesion absorption rates
*P < 0.05, significances determined by χ2 test. MDR-TB – multidrug-resistant tuberculosis, IL-2 – interleukin-2.
Radiographic outcomes
The rates of X-ray lesion absorption between the control and rhIL-2 group are shown in Figure 1. For patients comparing baseline and follow-up chest radiographs, individuals in the rhIL-2 group demonstrated more lesion absorption than those from the control group throughout the course of TB therapy. By the 24th month, the lesion absorption rate was 54% for the rhIL-2 arm, and only 25% for the control; the difference was significant (p = 0.039) (Figure 1 C). Next, increased cavity closure rates were observed among rhIL-2 immunotherapy patients. At the end of treatment, these rates were 17% for the control and 23% for the IL-2 group; there were no statistically significant differences between the two groups.
Immunologic changes during anti-MDR-TB treatment
To determine whether T cell subsets have a role in the immunological response to antituberculous treatment, we measured the percentage of CD3+, CD4+, and CD8+ T cells at several pre- and post-study time points. As shown in Table II, there were no differences in T cells; the levels of CD4+ and CD4+/CD8+ T cells tended to be decreased during MDR-TB treatment.
Table II.
Proportions of T cell subsets in two groups with MDR-TB over the course of treatment (x ± SD)
| Study point | n | CD3+ (%) | CD4+ (%) | CD8+ (%) | CD4+/CD8+ | |
|---|---|---|---|---|---|---|
| Control | 0 month | 24 | 62.67 ±14.58 | 34.64 ±11.05 | 24.26 ±11.00 | 1.73 ±0.92 |
| 1 month | 21 | 61.55 ±13.94 | 33.32 ±9.70 | 25.99 ±12.70 | 1.52 ±0.58 | |
| 3 month | 22 | 62.74 ±13.77 | 34.15 ±11.89 | 25.00 ±10.11 | 1.43 ±0.67 | |
| 7 month | 21 | 63.07 ±14.16 | 35.22 ±11.33 | 24.58 ±9.83 | 1.61 ±0.76 | |
| rhIL-2 | 0 month | 22 | 59.85 ±16.09 | 35.41 ±9.99 | 23.48 ±7.20 | 1.66 ±0.77 |
| 1 month | 21 | 63.93 ±11.15 | 36.13 ±8.66 | 24.47 ±7.72 | 1.63 ±0.70 | |
| 3 month | 22 | 57.04 ±15.94 | 31.01 ±9.90 | 24.63 ±8.42 | 1.47 ±0.87 | |
| 7 month | 22 | 58.82 ±12.29 | 31.58 ±9.28 | 24.27 ±7.49 | 1.41 ±0.61 |
Data are expressed as mean ± standard deviation. The proportions of CD3+, CD4+, CD8+, and CD4+/CD8+ are assessed in different numbers of patients prior to the study and during TB treatment. MDR-TB – multidrug-resistant tuberculosis. rhIL-2 – recombinant human interleukin-2.
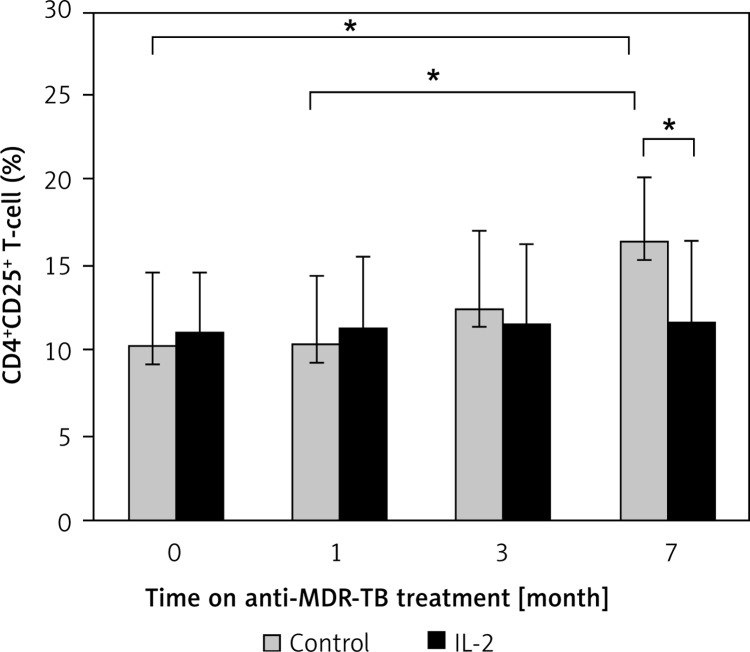
We next assessed whether both groups were different in frequencies of CD4+CD25+ T cells. The levels of CD4+CD25+ T cells from the control were found to be elevated after treatment, and were significantly higher at the 7th month as compared with the levels of pretreatment and after one month of anti-TB treatment (p = 0.017). When compared with patients from the rhIL-2 group, the levels of CD4+CD25+ T cells from the control were also significantly higher (p = 0.017). In contrast, the administered rhIL-2 regimen did not induce obvious changes in the number of CD4+CD25+ T cells (Figure 2).
Figure 2.
Quantities of CD4+CD25+ T cells in different groups before and after treatment
*P < 0.05, Significances determined by t test. MDR-TB – multidrug-resistant tuberculosis, IL-2 – interleukin-2.
We then tried to investigate the interaction of CD4+CD25+ T cells with other T cells using Spearman's nonparametric test. CD4+CD25+ T cells were negatively correlated with CD8+ and CD4+CD25– T cells (r = –0.214, p = 0.007; r = –0.192, p = 0.016 respectively).
Safety
Adjunct immunotherapy with rhIL-2 was safe and generally well tolerated. The systemic adverse events between the control and rhIL-2 group were similar (p > 0.05, Table III). No subjects required dose reduction or discontinuation of rhIL-2 due to its side effects. Adverse events were usually mild and local, such as local pain and tenderness on palpation, erythema, hyperpigmentation, etc.
Table III.
Number of systemic adverse events in control and rhIL-2 trial group
| Systemic adverse events | Control (n) | rhIL-2 (n) |
|---|---|---|
| Hepatic injury | 4 | 2 |
| Kidney injury | 3 | 3 |
| Gastrointestinal reaction | 4 | 2 |
| Blood system influence | 0 | 3 |
| Auditory nerve damage | 0 | 0 |
| Optic nerve damage | 0 | 0 |
| Drug allergy | 0 | 0 |
| Nervous-mental system | 0 | 0 |
| Muscle and joint pain | 4 | 2 |
| Electrolyte abnormalities | 2 | 3 |
| Thyroid dysfunction | 1 | 0 |
| Total | 18 | 15 |
Fisher's exact test comparing rhIL-2 and control group. rhIL-2 – recombinant human interleukin-2.
Discussion
Interleukin-2 has been a standard option for treatment of some malignant tumors; therefore, it could be moved rapidly into a clinical setting for treatment of TB if benefits could be demonstrated. Indeed, we found that adjunctive immunotherapy with rhIL-2 enhanced sputum conversion negative rates, and improved the chest radiograph. Although rhIL-2 administration only lasted for 7 months, the successful effects from rhIL-2 were maintained during the whole duration of antituberculosis treatment, suggesting that the immune regulatory effects were sustained long term. Intradermal therapy with rhIL-2 was generally safe and well tolerated.
Our results are consistent with the serial studies of Johnson et al. In 1995, they firstly reported the beneficial results of rhIL-2 for TB of 20 patients with drug-sensitive tuberculosis (DS-TB), or MDR-TB with optimal multidrug chemotherapy [14]. Subsequently, in 1997, a clinical trial in 35 patients with MDR-TB comparing daily or pulsed IL-2 therapy with placebo identified improved sputum clearance with daily treatment, and it was also noted that there were strikingly different results with variation in the rhIL-2 treatment schedule [11]. However, the earlier studies followed up only short term, and not the full course of treatment. Furthermore, there are three different changes in our long period of rhIL-2 treatment protocol: 1) single dose: 500 000 IU per time in our study, 225 000 IU used by Johnson; 2) dosing schedule: once every other day scheme, compared to twice daily rhIL-2 treatment regimen; 3) duration: a total of four courses applied at the first, third, fifth and seventh month of treatment vs. the first month of anti-TB treatment. The reason for novel treatment modalities in our study could be described as follows. The MDR-TB patients included in our study mostly belonged to the form of relapse, having experienced over three times multidrug therapy due to chronic and severe infection. Because of the gravity and refractory profile, we made the treatment program of rhIL-2 prolonged to cover the enhancement period of MDR-TB therapy. Our results extended previously published reports, and further supported findings that a long period of rhIL-2 had favorable effects on MDR-TB patients [15].
These improvements were undoubtedly associated with rhIL-2-induced changes of immune status. Interleukin-2 signals influence the differentiation of effector CD4+ T cells, promoting the generation of Th1, Th2, and regulatory T (Treg) cells [16]. Recent insight into the crucial role of IL-2 is not only for protective immunity but also necessary for the development and homeostasis of Treg cells [16, 17]. Experimental studies found that IL-2 was needed to efficiently activate Treg cells, and also demonstrated that Treg cells, which did not produce IL-2, competed for IL-2 secreted by responder T cells [18, 19]. These findings indicated that IL-2 designed to enhance the immune response could induce suppressive activity, which might explain the detrimental effects of rhIL-2 reported by Johnson [13]. To understand when and how IL-2 plays its distinct function is crucial for the development of safe and efficient therapeutic approaches for protective immunity. A recent review indicated that the dichotomous role of IL-2 depended on different doses of IL-2 [16, 18]. Interleukin-2 at low doses may be used to expand Treg cell numbers in conditions of autoimmune diseases [19], while high-dose IL-2 administration can abolish Treg suppression of responder T cells, having a therapeutic role in some kinds of cancer [16, 18].
Profound immune suppression was shown in MDR-TB [8, 20], whereas different studies presented various performances. Our findings disagreed with the results of Chu et al. [12], who found that there were higher levels of CD4+ and CD4+/CD8+ ratio in the rhIL-2 group after treatment, but agreed with the study by Johnson et al. [11], who found no significant changes in the size of leukocyte subpopulations during the study in any patient group. Moreover, in our study, while not statistically significant, the value of CD4+ and CD4+/CD8+ ratio declined with rhIL-2 administered. The mechanism involved might be related to the successful control of inflammation and down-regulation of the immune response after rhIL-2 therapy. Alternatively, there is increasing evidence for the importance of CD8+ T cytotoxic cells, which play a major role in the late phases of TB disease [21, 22]. It was further proposed that efficient immunotherapy might seek to increase CD8+ activity [22]. Thus, it could be speculated that the underlying clinical response to rhIL-2 was not only simply associated with boosting Th1 protective immunity, but might be linked to an additional mechanism.
Here, we tested the overall frequencies of CD4+CD25+ T cells. This subpopulation contains Treg and effector T cells, as CD25+ is also a marker of activation of T cells [23, 24]. It is becoming evident that CD4+CD25+ Treg cells are expanded in DS-TB and MDR-TB patients [10, 25–27], and may contribute to inhibit effector functions of Th1 and CD8+ T cells. We observed that CD4+CD25+ T cells were negatively correlated with CD4+CD25– T and CD8+ T cells, which indicated that CD4+CD25+ T cells in the present study might include many Treg cells, though we did not measure FOXP3 expression. Interestingly, we found an increase in the proportion of CD4+CD25+ T cells after standard chemotherapy. One possible explanation was that the immune reaction to mycobacterial antigens was very strong due to the TB bacillary persistence. Similar observations were made in extra-pulmonary TB patients [28]. Another possible explanation might be the low level of IL-2. As evidenced in our previous study [8], MDR-TB patients produced lower levels of IL-2 in their serum compared to DS-TB and healthy controls. Such a low level of IL-2 has been proved to drive expansion of Treg cells, which by competition for IL-2 resulted in further inhibition of responder T cells [18]. In patients with active TB, frequencies of CD4+CD25+ Treg cells were increased, being maintained even at completion of chemotherapy [29]. We did not identify CD4+CD25+FoxP3+ T cells; it would be of great interest to measure this subpopulation using CD4, high expression of CD25 and low expression of CD127 antigen in our next investigation [30]. Collectively, the increase of CD4+CD25+ T cells may represent an increase of both activated T cells and Treg cells, which are associated with high level inflammatory status, plausibly correlating with the fact that the clinical outcomes were suboptimal in the multidrug arm.
In contrast to previous reports [11, 13], the important finding was that rhIL-2 induced only slight but no significant increase of CD4+CD25+ T cells. The reasons underlying the differing results between our studies and earlier studies might be related to the rhIL-2 treatment regimen. As we have mentioned, in the current study, the single dosage of each injection was higher, and the duration of 4 months of rhIL-2 application was a long period, which might represent high-dose administration. In the presence of high-dose IL-2, the function of effector T cells was enhanced, and the expansion of Treg cells was abrogated [16, 18]. It was probably that the numbers of CD4+CD25+ Treg cells were limited, caused by the IL-2 regimen used in the present study, which contributed to the lack of significant change of CD4+CD25+ T cells in the rhIL-2 group. A recent review concluded that pathogen clearance is achieved through a careful balance between effector and suppressor response [23]. The success of rhIL-2 therapies might in part originate from such a balance, thereby increasing clearance of TB.
In conclusion, we demonstrated that adjunctive immunotherapy with rhIL-2 showed protective efficacy in MDR-TB patients. Using rhIL-2 for a long period did not induce significant expansion of CD4+CD25+ T cells. By contrast, persistence of increased frequencies of CD4+CD25+ T cells might be related to infection progression in MDR-TB patients receiving only multidrug chemotherapy. However, TB immunology is considerably complex, and further clinical and laboratory studies are warranted to explore the interaction between IL-2 and T cells.
Acknowledgments
This study was funded by a grant from the Important National Science and Technology Specific Projects of China (Approval No. 2008zx100 03-014).
Conflict of interest
The authors declare no conflict of interest.
References
- 1.World Health Organization. Multidrug and extensively drug-resistant TB (M/XDR-TB): 2010 global report on surveillance and response; Geneva, Switzerland: WHO; 2010. http://www.who.int/tb/publications/2010/mdrtb2010report_executive_summary.pdf. Accessed July 2012.m, WHO/HTM/TB/2010.3. [Google Scholar]
- 2.Mercedes GJ. Immunity to TB and targets for immunotherapy. Immunotherapy. 2012;4:187–99. doi: 10.2217/imt.11.168. [DOI] [PubMed] [Google Scholar]
- 3.Uhlin M, Andersson J, Zumla A, Maeurer M. Adjunct immunotherapies for tuberculosis. J Infect Dis. 2012;205:S325–34. doi: 10.1093/infdis/jis197. [DOI] [PubMed] [Google Scholar]
- 4.Churchyard GJ, Kaplan G, Fallows D, Wallis RS, Onyebujoh P, Rook GA. Advances in immunotherapy for tuberculosis treatment. Clin Chest Med. 2009;30:769–82. doi: 10.1016/j.ccm.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 5.Philips JA, Ernst JD. Tuberculosis pathogenesis and immunity. Ann Rev Pathol Mech Dis. 2012;7:353–84. doi: 10.1146/annurev-pathol-011811-132458. [DOI] [PubMed] [Google Scholar]
- 6.Dheda K, Schwander SK, Zhu B, van Zyl-Smit RN, Zhang Y. The immunology of tuberculoisis: from bench to beside. Respirogy. 2010;15:433–50. doi: 10.1111/j.1440-1843.2010.01739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lienhardt C, Azzurri A, Amedei A, et al. Active tuberculosis in Africa is associated with reduced Th1 and increased Th2 activity in vivo. Eur J Immunol. 2002;32:1605–13. doi: 10.1002/1521-4141(200206)32:6<1605::AID-IMMU1605>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 8.Tan Q, Xie WP, Min R, et al. Characterization of Th1- and Th2-type immune response in human multidrug-resistant tuberculosis. Eur J Clin Microbiol Infect Dis. 2012;31:1233–42. doi: 10.1007/s10096-011-1434-4. [DOI] [PubMed] [Google Scholar]
- 9.Shahemabadi AS, Hosseini AZ, Shaghsempour S, Rayani M, Pouramiri M. Evaluation of T cell immune responses in multi-drug-resistant tuberculosis (MDR-TB) patients to Mycobacterium tuberculosis total lipid antigens. Clin Exp lmmunol. 2007;149:285–94. doi: 10.1111/j.1365-2249.2007.03406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geffner L, Yokobori N, Basile J, et al. Patients with multidrug-resistant tuberculosis display impaired Thl responses and enhanced regulatory T-cell levels in response to an outbreak of multidrug-resistant Mycobacterium tuberculosis M and Ra strains. Infect lmmun. 2009;77:5025–34. doi: 10.1128/IAI.00224-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson BJ, Bekker LG, Rickman R, et al. rhuIL-2 adjunctive therapy in multidrug resistant tuberculosis: a comparison of two treatment regimens and placebo. Tuber Lung Dis. 1997;78:195–203. doi: 10.1016/s0962-8479(97)90026-5. [DOI] [PubMed] [Google Scholar]
- 12.Chu NH, Zhu LZ, Yie ZZ, et al. A controlled clinical study on the efficacy of recombinant human interleukin-2 in the treatment of pulmonary tuberculosis. Chin J Tuberc Respir Dis. 2003;26:548–51. [PubMed] [Google Scholar]
- 13.Johnson JL, Ssekasanvu E, Okwera A, et al. Randomized trial of adjunctive interleukin-2 in adults with pulmonary tuberculosis. Am J Respir Crit Care Med. 2003;168:185–91. doi: 10.1164/rccm.200211-1359OC. [DOI] [PubMed] [Google Scholar]
- 14.Johnson BJ, Ress SR, Willcox P, et al. Clinical and immune responses of tuberculosis patients treated with low- lose IL-2 and multidrug therapy. Cytokines Mol Ther. 1995;1:185–96. [PubMed] [Google Scholar]
- 15.Barnes PF. Immunotherapy for tuberculosis: wave of the future or tilting at windmills? Am J Resp Crit Care. 2003;168:142–3. doi: 10.1164/rccm.2305001. [DOI] [PubMed] [Google Scholar]
- 16.Boyman O, Sprent J. The role of interleukin-2 during homeostasis and activation of the immune system. Nat Rev Immun. 2012;12:180–90. doi: 10.1038/nri3156. [DOI] [PubMed] [Google Scholar]
- 17.Malek TR. The main function of IL-2 is to promote the development of T regulatory cells. J Leukoc Biol. 2003;74:961–5. doi: 10.1189/jlb.0603272. [DOI] [PubMed] [Google Scholar]
- 18.de la Rosa M, Rutz S, Dominger H, Scheffold A. Interleukin-2 is essential for CD4+CD25+ regulatory T cell function. Eur J Immunol. 2004;34:2480–8. doi: 10.1002/eji.200425274. [DOI] [PubMed] [Google Scholar]
- 19.Brandenburg S, Takahashi T, de la Rosa M, et al. IL-2 induces in vivo suppression by CD4+CD25+Foxp3+regulatory T cells. Eur J Immunol. 2008;38:1643–53. doi: 10.1002/eji.200737791. [DOI] [PubMed] [Google Scholar]
- 20.Kiran B, Cagatay T, Clark P, et al. Can immune parameters be used as predictors to distinguish between pulmonary multidrug-resistant and drug-sensitive tuberculosis? Arch Med Sci. 2010;6:77–82. doi: 10.5114/aoms.2010.13511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith SM, Dockrell HM. Role of CD8+ T cells in mycobacterial infections. Immunol Cell Biol. 2000;78:325–33. doi: 10.1046/j.1440-1711.2000.00936.x. [DOI] [PubMed] [Google Scholar]
- 22.Rook GA, Lowrie DB, Hernandez-Pando R. Immunotherapeutics for tuberculosis in experimental animals: is there a common pathway activated by effective protocols? J Infect Dis. 2007;196:191–8. doi: 10.1086/518937. [DOI] [PubMed] [Google Scholar]
- 23.Mills-Kingston HG. Regulatory T cvells: friend or foe in immunity to infection? Nat Rev Immunol. 2004;4:841–55. doi: 10.1038/nri1485. [DOI] [PubMed] [Google Scholar]
- 24.Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+CD25high regulatory cells in human peripheral blood. J Immunol. 2001;167:1245–53. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- 25.Guyot-Revol V, Innes JA, Hackforth S, Hinks T, Lalvani A. Regulatory T cells are expanded in blood and disease sites in patients with tuberculosis. Am J Respir Crit Care Med. 2006;173:803–10. doi: 10.1164/rccm.200508-1294OC. [DOI] [PubMed] [Google Scholar]
- 26.Hougardy JM, Place S, Hildebrand M, et al. Regulatory T cells depress immune responses to protective antigens in active tuberculosis. Am J Respir Crit Care Med. 2007;176:409–16. doi: 10.1164/rccm.200701-084OC. [DOI] [PubMed] [Google Scholar]
- 27.Xu CC, Xie WP, Dai GQ, Tan Q, Min R, Wang H. Change of the peripheral blood CD4 CD25 Foxp3 T cells in patients with multidrug-resistant tuberculosis. Acta Univ Med Nanjing. 2011;31:419–21. [Google Scholar]
- 28.He XY, Xiao L, Chen HB, et al. T regulatory cells and Th1/Th2 cytokines in peripheral blood from tuberculosis patients. Eur J Clin Microbiol Infect Dis. 2010;29:643–50. doi: 10.1007/s10096-010-0908-0. [DOI] [PubMed] [Google Scholar]
- 29.Ribeiro-Rodrigues R, Resende Co T, Rojas R, et al. A role for CD4+CD25+ T cells in regulation of the immune response during human tuberculosis. Clin Exp Immunol. 2006;144:25–34. doi: 10.1111/j.1365-2249.2006.03027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ciebiada M, Kasztalska K, Gorska-Ciebiada M, Barylski M, Gorski P. Expression of IL-7 receptor in human peripheral regulatory T cells. Arch Med Sci. 2013;9:555–60. doi: 10.5114/aoms.2012.31387. [DOI] [PMC free article] [PubMed] [Google Scholar]