Abstract
Introduction
Angiotensin II (Ang II) not only regulates systemic blood pressure through a vasoconstrictive effect, but also promotes bone resorption. We recently reported that Ang II (10–6 M) stimulated the production of matrix metalloproteinases via the AT1 receptor in osteoblastic ROS17/2.8 cells, but suppressed alkaline phosphatase activity. However, the roles of Ang II in osteoblastic differentiation and the function of osteogenesis in osteoblasts are unclear. Therefore, we examined the effect of Ang II on the expression of osteogenesis-related transcription factors and extracellular matrix (ECM) proteins, as well as mineralized nodule formation in ROS17/2.8 cells.
Material and methods
ROS17/2.8 cells were cultured with 0 (control) or 10–6 M Ang II in the presence or absence of the AT1 receptor blocker losartan. Mineralized nodule formation was detected by Alizarin Red staining. Gene and protein expression levels of transcription factors and ECM proteins were determined using real-time PCR and Western blotting, respectively.
Results
Runx2, Msx2, and osteocalcin expression significantly decreased with Ang II compared to the control, whereas AJ18 expression significantly increased. Osterix, Dlx5, type I collagen, bone sialoprotein, and osteopontin expression was unaffected. Mineralized nodule formation and calcium content in mineralized nodules decreased with Ang II. Losartan blocked suppressive or stimulatory effects of Ang II on Runx2, Msx2, osteocalcin, and AJ18 expression.
Conclusions
These results suggest that Ang II suppresses osteoblastic differentiation by altering the expression of osteogenesis-related transcription factors via the AT1 receptor and the function of osteogenesis in ROS17/2.8 cells.
Keywords: angiotensin, transcription factors, extracellular matrix proteins, osteoblast
Introduction
Bone is continuously destroyed and reformed in vertebrates in a stringently regulated equilibrium between osteoblastic bone formation and osteoclastic bone resorption, which maintains optimal bone mass and calcium homeostasis [1]. Osteoblasts perform a central role in bone formation via high alkaline phosphatase (ALPase) activity and the production of extracellular matrix (ECM) proteins, such as type I collagen, bone sialoprotein (BSP), osteopontin (OPN), and osteocalcin (OCN) [2–5]. Osteoblastic differentiation is controlled by multiple transcription factors at various stages of osteoblast development [6]. Two transcription factors, Runx2 and Osterix, are essential for osteoblastic differentiation during both intramembranous and endochondral bone formation [7–9]. Alterations in the function of various non-bone-specific transcription factors such as Msx2 and Dlx5 have also been shown to affect osteoblastic differentiation [10–13]. In contrast, the novel zinc-finger transcription factor AJ18 has been reported to regulate osteoblastic differentiation [14].
Angiotensin II (Ang II) plays a major role in the maintenance of extracellular fluid volume and blood pressure. Ang II becomes activated via the seven transmembrane G protein-coupled receptors [15], Ang II type 1 (AT1) and type 2 (AT2) receptors. Ang II is involved in osteoporosis, a systemic skeletal disease characterized by low bone mass and micro-architectural deterioration of bone tissue [16, 17]. Ang II promotes bone resorption via osteoclast AT1 receptors [18–21]. In addition, both animal and in vitro studies have indicated that Ang II may be related to osteoclast activation through cytokines and/or receptor activator of nuclear factor κB ligand (RANKL) production by osteoblasts [19–22]. We recently reported that Ang II stimulated the degradation process occurring during ECM turnover in osteoids through an increase in the production of matrix metalloproteinase (MMP)-3 and -13 via the AT1 receptor in osteoblastic ROS17/2.8 cells [23]. These findings suggest that Ang II induces not only osteoclast activation, but also the attachment of osteoclasts to the bone surface. However, the role of Ang II in osteoblastic differentiation through osteogenesis-related transcription factors and the function of osteogenesis in osteoblasts are unclear.
In the present study, we examined the effect of Ang II on the expression of osteogenesis-related transcription factors, such as Runx2, Osterix, Msx2, Dlx5, AJ18, and ECM proteins including type I collagen, BSP, OPN, and OCN in ROS17/2.8 cells. Additionally, the effects of Ang II on mineralized nodule formation by the cells and calcium content in mineralized nodules were examined.
Material and methods
Cell culture
The rat osteosarcoma cell line ROS17/2.8 was used in the present study as model osteoblasts. Cells were maintained in α-minimal essential medium (α-MEM; Gibco-BRL, Rockville, MD, USA) containing 10% (v/v) heat-inactivated fetal bovine serum (FBS; HyClone Laboratories, Logan, UT, USA) and 1% (v/v) penicillin/streptomycin (Sigma-Aldrich, St. Louis, MO, USA) at 37°C in a humidified atmosphere of 95% air and 5% CO2. Cells were seeded onto 6-well microplates at a density of 5.0 × 103 cells/cm2 and cultured in α-MEM with 0 or 10–6 M Ang II (Sigma-Aldrich) in the presence or absence of the AT1 receptor blocker losartan (5 µM; LKT Laboratories, Shenzhen, Longgang, China) for up to 7 days. Culture medium was changed every second or third day. The concentrations of Ang II and losartan were chosen based on our previous study [23].
Real-time PCR
Total RNA was isolated at the indicated time points using an RNeasy Mini Kit (Qiagen, Valencia, CA, USA). The mRNA was converted into complementary DNA (cDNA) using an RNA PCR kit (PrimScript; Takara Bio, Shiga, Japan). The resulting cDNA mixture was diluted 1: 2 in sterile, distilled water, and 2 µl of the diluted cDNA was subjected to real-time polymerase chain reaction (PCR) analysis using SYBR Green I. The reactions were performed in 25 µl of SYBR premixed Ex Taq solution (Takara Bio) containing 10 µM sense and antisense primers (Table I). Primers were designed using Primer3 software (Whitehead Institute for Biomedical Research, Cambridge, MA, USA). The PCR assays were performed using a Smart Cycler (Cepheid, Sunnyvale, CA, USA) and analyzed using the instrument's software. The protocol for Runx2, Osterix, Msx2, Dlx5, AJ18, type I collagen, BSP, OPN, and OCN PCRs consisted of 40 cycles at 95°C for 5 s and 60°C for 30 s. All real-time PCR experiments were performed in triplicate; the specificity of each product was verified through melting curve analysis. Calculated gene expression levels were normalized to the levels of glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
Table I.
PCR primers used in the experiments
| Target | Forward primer | Reverse primer | Genbank acc No. |
|---|---|---|---|
| Runx2 | 5’-CGCATTCCTCATCCCAGTAT-3’ | 5’-GCCTGGGGTCTGTAATCTGA-3’ | NM_053470 |
| Osterix | 5’-AAGGCAGTTGGCAATAGTGG-3’ | 5’-CCGCTCTAGCTCCTGACAGT-3’ | AY_177399.1 |
| Dlx5 | 5’-GCGCTCAACCCATACCAGT-3’ | 5’-ACTCGGGACTCGGTTGTAGG-3’ | NM_005221 |
| Msx2 | 5’-TCACCACGTCCCAGCTTCTAG-3’ | 5’-AGCTTTTCCAGTTGCGCCTCC-3’ | NM_012982 |
| AJ18 | 5’-GTGATTGGCAAGCTGCAGAA-3’ | 5’-TAGCAGCCCACGAGATGGTC-3’ | AJ_833597 |
| Type I collagen | 5’-GCTGAGGGCAACAGCAGCAGATTC-3’ | 5’-GATGTCCAGAGGTGCAATGTCAA-3’ | NM_053356.1 |
| BSP | 5’-TGTGGAATGGTGCTACGGTCTC-3’ | 5’-CCTTCAATGACGCACCTGGA-3’ | RA_029413 |
| OPN | 5’-TCCTGCGGCAAGCATTCTC-3’ | 5’-CTGCCAAACTCAGCCACTTTCA-3’ | M_14656.1 |
| OCN | 5’-GGTGCAGACCTAGCAGACACCA-3’ | 5’-AGGTAGCGCCGGAGTCTCTATTCA-3’ | M_25490 |
| GAPDH | 5’-ATGGTGGTGAAGACGCCAGTA-3’ | 5’-GGCACAGTCAAGGCTGAGAATG-3’ | NM_017008 |
SDS-PAGE and Western blotting
Cells were lysed with extraction buffer containing 0.05% Triton X-100, 10 mM β-mercaptoethanol, 0.5 mM phenylmethylsulfonyl fluoride, 0.5 mM ethylenediaminetetraacetic acid, and 25 mM Tris-HCl (pH 7.4). Cell membranes were disrupted by sonication, and the samples were clarified by centrifugation. Supernatants containing 20 µg of intracellular protein were dissolved in 10 µl of sample buffer containing 1% sodium dodecyl sulfate (SDS), 2 M urea, 15 mg/ml dithiothreitol, and bromophenol blue, and then heated at 95°C for 5 min prior to loading. The proteins were resolved by 4–20% SDS-polyacrylamide gel electrophoresis (SDS-PAGE) with a discontinuous Tris-glycine buffer system [24], transferred to a polyvinylidene fluoride membrane using a semidry transfer apparatus, and probed with the indicated antibodies. Polyclonal or monoclonal IgG primary antibodies, including goat anti-Runx2, goat anti-Msx2, goat anti-OCN, mouse anti-β-tubulin (Santa Cruz Biotechnology, Santa Cruz, CA, USA), and mouse anti-AJ18, which was provided by Professor Naoto Suzuki at the Nihon University School of Dentistry [25], were used with the appropriate biotin-conjugated donkey anti-goat IgG (Chemicon International, Temecula, CA, USA) or goat anti-mouse IgG (Zymed, San Francisco, CA, USA) secondary antibodies. β-Tubulin was used as an internal standard. The membranes were labeled with streptavidin-horseradish peroxidase (streptavidin-HRP) and visualized using a commercial chemiluminescence kit (Amersham Life Sciences, Little Chalfont, Buckinghamshire, UK). Differences in protein expression were quantified by using a GS-800 calibrated imaging densitometer and Quantity One software (Bio-Rad Laboratories, Richmond, Calif, USA).
Mineralized nodule formation and calcium and protein measurements
Cells were plated in 24-well tissue culture plates at a density of 5.0 × 103 cells/cm2 and cultured in α-MEM with 50 mM β-glycerophosphate and 50 µg/ml ascorbic acid in the presence or absence of 10–6 M Ang II for 7 days. Culture medium was changed every second or third day. The condition of the cells and nodule formation were checked routinely by phase-contrast microscopy (Nikon, Tokyo, Japan). Mineralized nodules were detected by Alizarin Red staining (Wako Fine Chemical Industries, Osaka, Japan), as described previously [26–28].
Cells were plated in 6-well tissue culture plates at the same cell density and cultured in the aforementioned condition for 7 days. At the indicated time points, the medium was discarded, 300 µl 0.5 M HCl was added to each well, and the cells were incubated overnight to decalcify the mineralized nodules. The calcium content was determined quantitatively using the Calcium E-Test Kit (Wako Fine Chemical Industries). Protein content was determined quantitatively using the protein assay solution (Bio-Rad Laboratories, Hercules, CA, USA) after evaporation of HCl from the samples.
Statistical analysis
Each value is reported as the mean ± standard deviation (SD). Significant differences were determined using a one-way analysis of variance (ANOVA) followed by Tukey's multiple comparison test using GraphPad Prism software (ver. 5.0 San Diego, CA, USA). Differences with a p value < 0.05 were considered statistically significant.
Results
Effect of Ang II on transcription factor expression
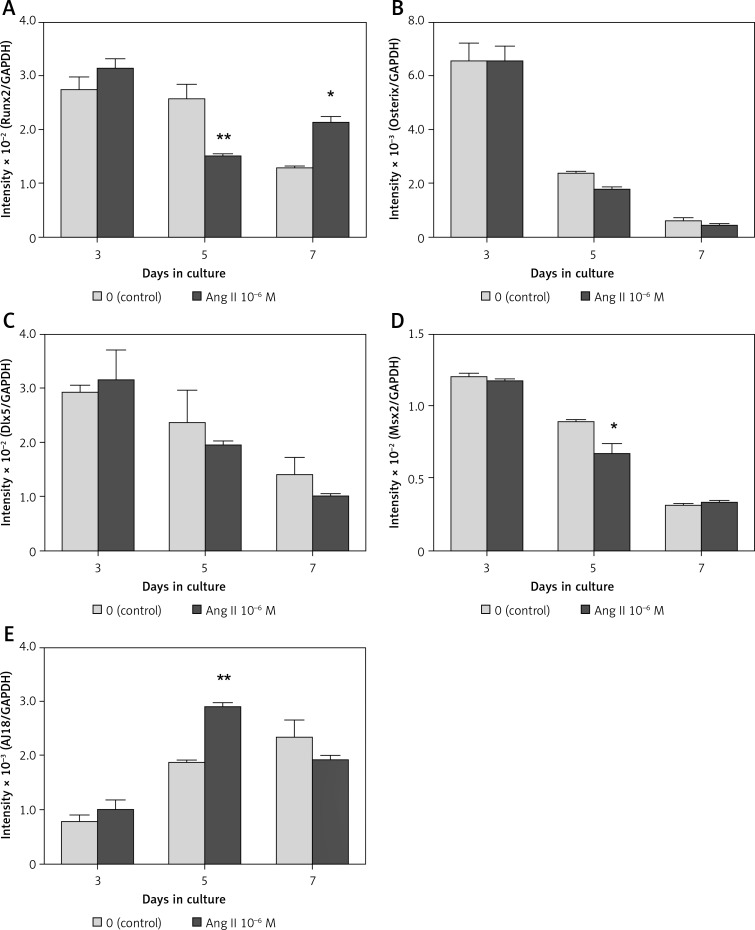
In both the presence and absence of 10–6 M Ang II, mRNA expression levels of Runx2, Osterix, Dlx5, and Msx2 were highest on day 3 of culture, whereas the expression level of AJ18 was lowest on day 3. In the presence of 10–6 M Ang II, mRNA expression levels of Runx2 and Msx2 decreased significantly by 0.58- and 0.75-fold, respectively, compared to the control (Figures 1 A, D) on day 5. In contrast, the expression levels of AJ18 on day 5 and Runx2 on day 7 increased significantly by 1.5- and 1.7-fold, respectively, compared to the control (Figures 1 A, E). The expression levels of Osterix and Dlx5 were not affected in the presence of Ang II during the 7-day culture period (Figures 1 B, C).
Figure 1.
Effect of Ang II on transcription factor mRNA expression levels. ROS17/2.8 cells were cultured with 0 (control) or 10–6 M Ang II for up to 7 days. The mRNA expression levels of Runx2 (A), Osterix (B), Dlx5 (C), Msx2 (D), and AJ18 (E) on days 3, 5, and 7 of culture were determined using real- time PCR
Each bar indicates the mean ± SD from three independent experiments. **p < 0.01, *p < 0.05 (Ang II treatment vs. control).
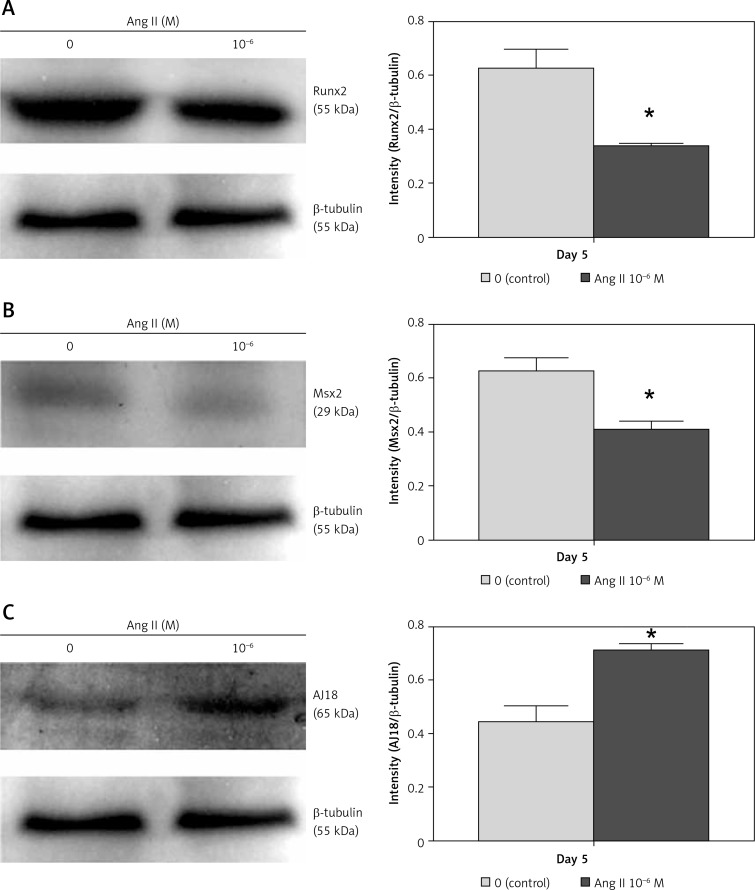
The Ang II-induced change in Runx2, Msx2, and AJ18 mRNA expression on day 5 correlated with protein expression. Protein expression levels of Runx2 and Msx2 decreased in cells cultured with 10–6 M Ang II, whereas the AJ18 expression level increased (Figure 2).
Figure 2.
Effect of Ang II on Runx2, Msx2, and AJ18 protein expression levels. ROS17/2.8 cells were cultured with 0 (control) or 10–6 M Ang II for 5 days, and the protein expression levels of Runx2 (A), Msx2 (B), and AJ18 (C) were examined with Western blotting
Effect of Ang II on mineralized nodule formation
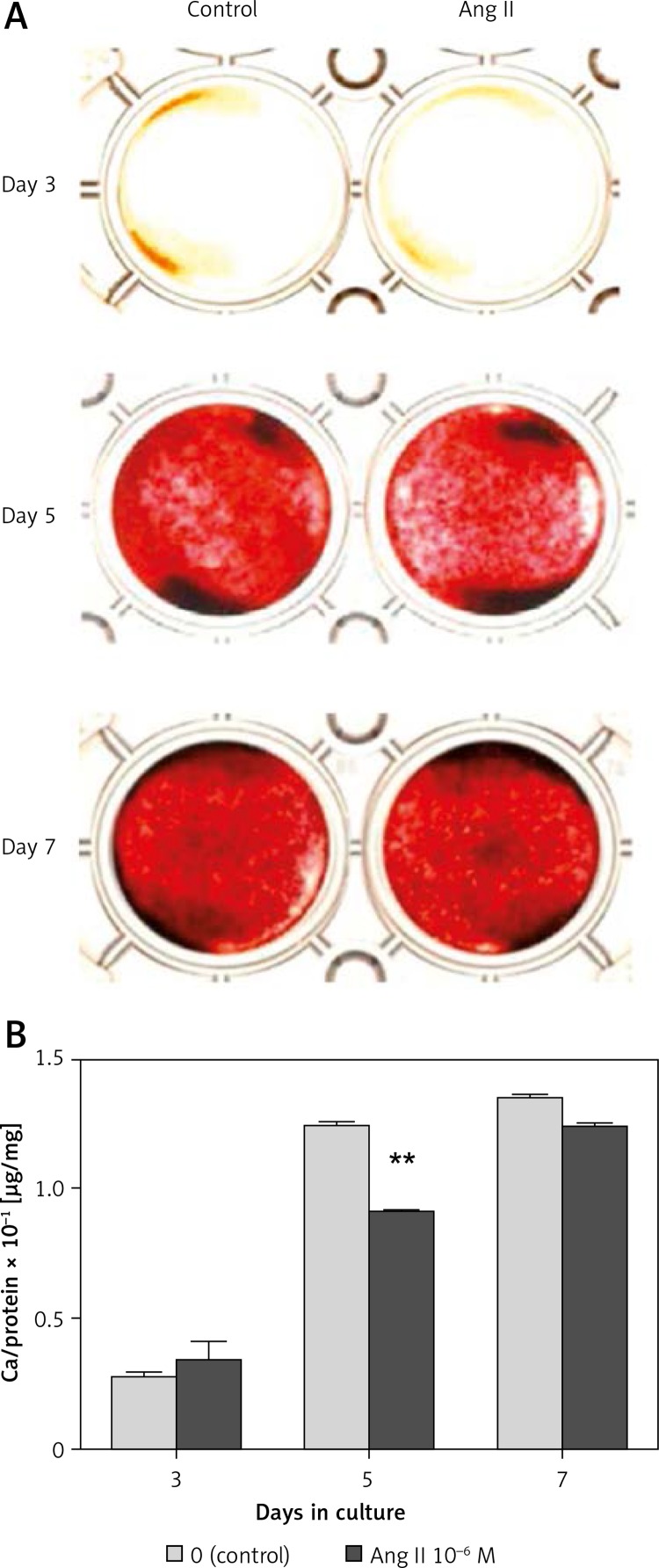
In both the presence and absence of 10–6 M Ang II, the intensity of Alizarin Red staining in mineralized nodules increased gradually until day 7 of culture (Figure 3 A). The intensity on day 5 was slightly suppressed in the presence of 10–6 M Ang II compared to the control. The calcium content on day 3 was not affected by the addition of 10–6 M Ang II, whereas that on day 5 significantly decreased 0.74-fold in the presence of Ang II compared to the control (Figure 3 B). The calcium content on day 7 decreased slightly in the presence of Ang II compared to the control, but a significant difference was not observed.
Figure 3.
Effect of Ang II on mineralized nodule formation. ROS17/2.8 cells were cultured with 0 (control) or 10–6 M Ang II for up to 7 days. Mineralized nodule formation (A) was examined by Alizarin Red staining on days 3, 5, and 7 of culture, and the calcium content in mineralized nodules (B) was determined using a calcium assay kit
Each bar indicates the mean ± SD from three independent experiments. **p < 0.01 (Ang II treatment vs. control).
Effect of Ang II on ECM expression
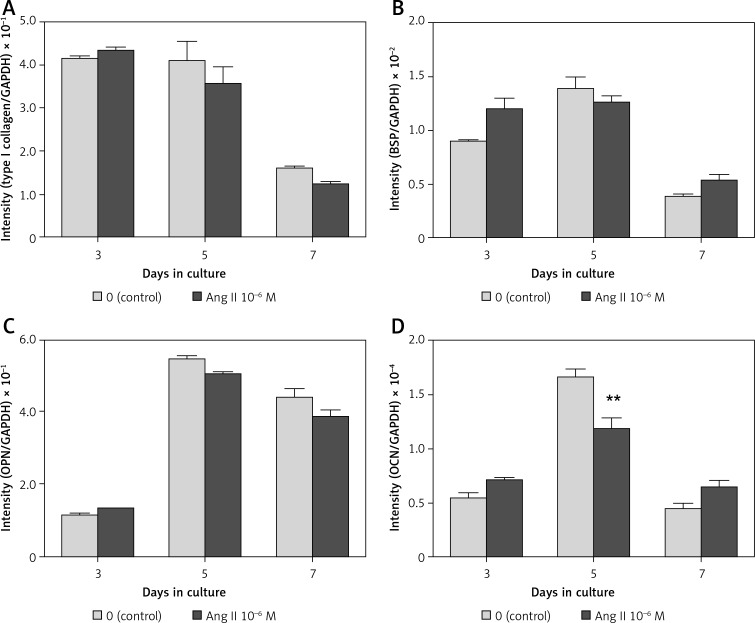
In both the presence and absence of 10–6 M Ang II, the mRNA expression level of type I collagen was highest on day 3 of culture, whereas the expression levels of BSP, OPN, and OCN were highest on day 5 (Figures 4 A–D). In the presence of 10–6 M Ang II, the mRNA expression level of OCN decreased significantly 0.71-fold compared to the control on day 5 of culture; the expression level was not affected on days 3 and 7 (Figure 4 D). The expression levels of type I collagen, BSP, and OPN were not affected by the addition of Ang II during the 7-day culture period (Figure 4 A–C).
Figure 4.
Effect of Ang II on mRNA expression levels of ECM proteins. ROS17/2.8 cells were cultured with 0 (control) or 10–6 M Ang II for up to 7 days, and the mRNA expression levels of type I collagen (A), BSP (B), OPN (C), and OCN (D) on days 3, 5, and 7 of culture were determined using real-time PCR
Each bar indicates the mean ± SD from three independent experiments. **p < 0.01 (Ang II treatment vs. control).
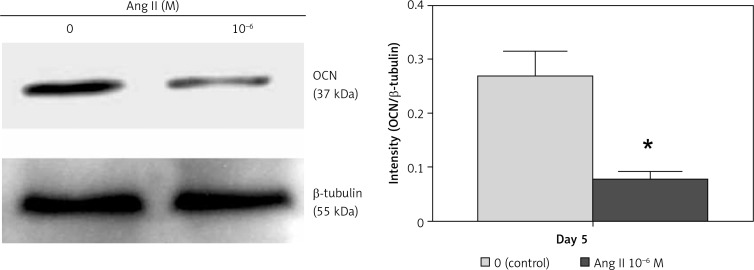
The Ang II-induced change in OCN mRNA expression on day 5 correlated with protein expression. The OCN protein expression levels also decreased in cells cultured with 10–6 M Ang II compared to the control on day 5 (Figure 5).
Figure 5.
Effect of Ang II on OCN protein expression levels. ROS17/2.8 cells were cultured with 0 (control) or 10–6 M Ang II for 5 days, and the protein expression level of OCN were examined with Western blot analysis
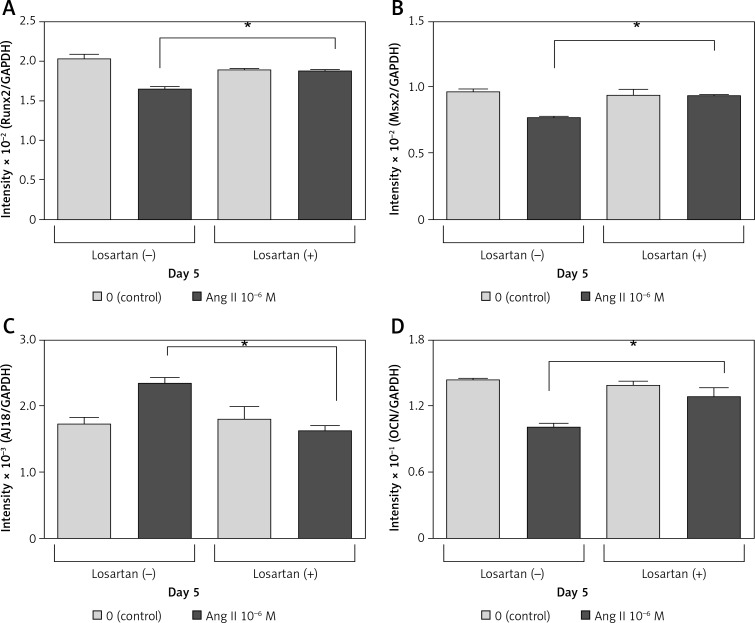
Effect of losartan on Ang II-induced change in Runx2, Msx2, AJ18, and OCN mRNA expression
Losartan (an AT1 receptor blocker) completely blocked the suppressive effects of 10–6 M Ang II on Runx2, Msx2, and OCN mRNA expression on day 5 of culture (Figures 6 A, B, D). Losartan also blocked the stimulatory effects of 10–6 M Ang II on AJ18 mRNA expression on day 5 (Figure 6 C).
Figure 6.
Effect of losartan on Runx2, Msx2, AJ18, and osteocalcin mRNA expression. ROS17/2.8 cells were cultured with 0 (control) or 10–6 M Ang II in the presence or absence of the AT1 receptor blocker (losartan) for 5 days. mRNA expression levels of Runx2 (A), Msx2 (B), AJ18 (C), and OCN (D) were determined using real-time PCR
Each bar indicates the mean ± SD from three independent experiments. *p < 0.05.
Discussion
Based on previous studies reporting that Ang II suppresses ALPase activity and mineralized nodule formation in rat calvarial osteoblasts, Ang II has been considered to suppress osteoblastic differentiation [29, 30]. To the best of our knowledge, no studies had examined the effects of Ang II on the expression of osteogenesis-related transcription factors in osteoblasts. Recently, we reported that ALPase activity decreased when ROS17/2.8 cells were stimulated with 10–6 M Ang II during the growth stage until confluence [23]. Therefore, we investigated the effects of Ang II on osteoblastic differentiation by examining the expression of osteogenesis-related transcription factors using ROS17/2.3 cells as osteoblasts. Previous studies indicated that ROS17/2.8 cells are typical osteoblasts; the cells had high ALPase activities and formed mineralized nodules in culture for 7 days [26–28].
In the present study, the expression of Runx2 and Msx2 decreased significantly in the presence of 10–6 M Ang II compared to the control, whereas AJ18 expression increased significantly. In addition, Ang II suppressed mineralized nodule formation by ROS17/2.8 cells. Runx2 is essential for osteoblast differentiation of mesenchymal stem cells. Furthermore, mesenchymal stem cells differentiate into mature osteoblasts, which express high levels of OCN [31]. In the present study, Runx2 expression, as well as OCN expression, decreased in the presence of 10–6 M Ang II in ROS17/2.8 cells.
Msx2 plays an important role in regulating bone development. Liu et al. [11] suggested that overexpression of Msx2 transiently inhibits osteoblast differentiation, resulting in an increase in the osteoblast pool and ultimately in an increase in bone growth. Moreover, other studies have demonstrated that Msx2 promotes osteoblast differentiation and/or proliferation [32, 33]. Our present results also indicated that the function of Msx2 is to promote osteoblastic differentiation. Osterix, which contains a three zinc-finger motif, is a second transcription factor essential for osteoblast differentiation [9]. Dlx5 expression is correlated with osteoblast differentiation, and maximal Dlx5 expression occurs during the final stages of in vitro osteoblast differentiation, suggesting that Dlx5 may be involved in the maturation of the bone cell phenotype [34]. AJ18 may downregulate osteoblast differentiation by binding to osteoblast-specific element 2 and modulate transactivation by Runx2 [14, 25]. In light of these findings, the results of the present study suggest that 10–6 M Ang II suppresses osteoblastic differentiation by decreasing Runx2 and Msx2 expression, and increasing AJ18 expression.
Hagiwara et al. [29] previously reported that Ang II suppressed mineralized nodule formation and mRNA expression of OCN in rat calvarial osteoblasts on day 14 of culture. Their previous report is in accordance with our present results using ROS17/2.8 cells. We think that Ang II suppressed osteocalcin expression when its expression was highest in both our present study and the previous study by Hagiwara et al. However, they did not examine the effects of Ang II on the expression of other ECM proteins such as BSP, OPN, and type I collagen. In the present study, we examined the effect of Ang II on the expression of these ECM proteins to clarify the mechanism by which Ang II suppresses mineralized nodule formation. As a result, OCN expression decreased with the addition of Ang II; in contrast, type I collagen, BSP, and OPN expression was not affected. Non-collagenous matrix proteins are believed to be important in the organization of the collagen matrix and regulating the formation and growth of hydroxyapatite crystals [3–5, 35]. As described above, we clarified that Ang II suppressed ALPase activity in ROS17/2.8 cells [23]. ALPase, which hydrolyzes the ester bond of organic phosphate compounds under alkaline conditions, plays an important role in bone calcification as well as ECM proteins. Not only does the enzyme hydrolyze substances that inhibit calcification, such as pyrophosphate and adenosine triphosphate (ATP), it is also indispensable for producing the increased phosphate concentration required for hydroxyapatite crystallization [2]. These findings indicate that Ang II suppresses mineralized nodule formation by decreasing ALPase activity and OCN expression in osteoblasts. Animal and epidemiological evidence suggests that high blood pressure is associated with abnormalities of calcium metabolism, leading to an increase in calcium loss, thereby increasing the risk of osteoporosis [36, 37]. Moreover, there are previous studies indicating that Ang II induced osteoclastic bone resorption, and antihypertensive drugs, such as angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers, increased bone mass [18, 19, 38, 39]. These previous findings and our present results indicated that Ang II might play a role in osteoporosis by not only activating osteoclastic bone resorption [40], but also suppressing osteoblastic matrix calcification.
In the present study, the calcium content in mineralized nodules on days 5 and 7 of culture decreased in the presence of Ang II compared to the control. However, a significant difference was not observed on day 7. Thus, the suppressive effect of Ang II on nodule calcification was attenuated on day 7 rather than day 5. This phenomenon conformed to the effects of Ang II on Msx2, AJ18, and OCN expression. Significantly decreased expression of Msx2 and OCN, and significantly increased AJ18 expression, were observed on day 5 but not day 7 of culture. In contrast, mRNA expression of Runx2 increased significantly in Ang II-treated cells compared with untreated control cells on day 7. This phenomenon may be independent of the direct effect of Ang II, because our previous study using ROS17/2.8 cells indicated that the expression of AT1 and AT2 receptors markedly decreased on day 7 of culture compared with days 3 and 5 [23]. Growth factors, such as bone morphogenetic protein 2 and fibroblast growth factor 2 (FGF2), and inflammatory cytokines, such as interleukin (IL)-1β and IL-6, induce Runx2 expression [41–45]. Moreover, in vitro studies have reported that Ang II induces the production of IL-6 and FGF2 in mouse calvarial osteoblast [46] and skeletal muscle cells [47], respectively. In the present study, we did not examine the effects of Ang II on the expression of growth factors and cytokines in ROS17/2.8 cells. However, Ang II may induce cytokines including IL-6 and/or growth factors such as FGF2 in the early culture period, and the autocrine action of these factors might be related to increasing Runx2 expression during the late culture period. Based on the present results of mineralized nodule formation, nodule calcium content, and ECM protein expression, we believe that upregulation of Runx2 in the late culture period is not crucial for the function of osteogenesis in ROS17/2.8 cells.
Previous studies have indicated that Ang II induces RANKL and MMP expression via AT1 receptors [19, 23]. In the present study, losartan, an AT1 receptor blocker, completely inhibited the decreasing expression of Runx2, Msx2, and OCN, and the increasing expression of AJ18 in Ang II-treated cells. Moreover, another previous study [29] reported that the suppressive effect of Ang II on ALPase activity and OCN expression was attenuated by an AT1 receptor blocker, but not an AT2 receptor blocker in rat calvarial osteoblasts. These findings suggest that Ang II binds to the AT1 receptor and alters ALPase activity and Runx2, Msx2, AJ18, and OCN expression in osteoblasts.
In conclusion, Ang II suppresses osteoblastic differentiation through decreasing Runx2 and Msx2 expression and increasing AJ18 expression via the AT1 receptor in ROS17/2.8 cells. In addition, Ang II suppresses mineralized nodule formation by decreasing ALPase activity and OCN production via AT1 receptors in ROS17/2.8 cells.
Acknowledgments
This study was supported by the following: Grants-in-Aid for Scientific Research (C) (nos. 24592842 and 25462942) and a Grant-in-Aid for Young Scientists (B) (no. 24792008) from the Japanese Society for the Promotion of Science; the Strategic Research Base Development Program for Private Universities, subsidized by MEXT, 2010 (S1001024); and the Promotion and Mutual Aid Corporation for Private Schools of Japan; the Sato fund and the Uemura fund, the Nihon University School of Dentistry; and the Nihon University Multidisciplinary Research Grant for 2012.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Katagiri T, Takahashi N. Regulatory mechanisms of osteoblast and osteoclast differentiation. Oral Dis. 2002;8:147–59. doi: 10.1034/j.1601-0825.2002.01829.x. [DOI] [PubMed] [Google Scholar]
- 2.Anderson HC. Mechanism of mineral formation in bone. Lab Invest. 1989;60:320–30. [PubMed] [Google Scholar]
- 3.Young MF, Kerr JM, Ibaraki K, Heegaard AM, Robey PG. Structure, expression, and regulation of the major noncollagenous matrix proteins of bone. Clin Orthop Relat Res. 1992;281:275–94. [PubMed] [Google Scholar]
- 4.Robey PG, Fedarko NS, Hefferan TE, et al. Structure and molecular regulation of bone matrix proteins. J Bone Miner Res. 1993;(Suppl. 2):S483–7. doi: 10.1002/jbmr.5650081310. [DOI] [PubMed] [Google Scholar]
- 5.Mundlos S, Olsen BR. Heritable diseases of the skeleton. Part II: Molecular insights into skeletal development-matrix components and their homeostasis. FASEB J. 1997;11:227–33. [PubMed] [Google Scholar]
- 6.Yang X, Karsenty G. Transcription factors in bone: developmental and pathological aspects. Trends Mol Med. 2002;8:340–5. doi: 10.1016/s1471-4914(02)02340-7. [DOI] [PubMed] [Google Scholar]
- 7.Otto F, Thornell AP, Crompton T, et al. CBFA1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell. 1997;89:765–71. doi: 10.1016/s0092-8674(00)80259-7. [DOI] [PubMed] [Google Scholar]
- 8.Karsenty G. Role of CBFA1 in osteoblast differentiation and function. Semin Cell Dev Biol. 2000;11:343–6. doi: 10.1006/scdb.2000.0188. [DOI] [PubMed] [Google Scholar]
- 9.Nakashima K, Zhou X, Kunkel G, et al. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108:17–29. doi: 10.1016/s0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- 10.Acampora D, Merlo GR, Paleari L, et al. Craniofacial, vestibular and bone defects in mice lacking the Distal-less-related gene Dlx5. Development. 1999;126:3795–809. doi: 10.1242/dev.126.17.3795. [DOI] [PubMed] [Google Scholar]
- 11.Liu YH, Tang Z, Kundu RK, et al. Msx2 gene dosage influences the number of proliferative osteogenic cells in growth centers of the developing murine skull: a possible mechanism for MSX2-mediated craniosynostosis in humans. Dev Biol. 1999;205:260–74. doi: 10.1006/dbio.1998.9114. [DOI] [PubMed] [Google Scholar]
- 12.Miyama K, Yamada G, Yamamoto TS, et al. A BMP-inducible gene, Dlx5, regulates osteoblast differentiation and mesoderm induction. Dev Biol. 1999;208:123–33. doi: 10.1006/dbio.1998.9197. [DOI] [PubMed] [Google Scholar]
- 13.Satokata I, Ma L, Ohshima H, et al. Msx2 deficiency in mice causes pleiotropic defects in bone growth and ectodermal organ formation. Nat Genet. 2000;244:391–5. doi: 10.1038/74231. [DOI] [PubMed] [Google Scholar]
- 14.Jheon AH, Ganss B, Cheifetz S, Sodek J. Characterization of a novel KRAB/C2H2 zinc finger transcription factor involved in bone development. J Biol Chem. 2001;276:18282–9. doi: 10.1074/jbc.M010885200. [DOI] [PubMed] [Google Scholar]
- 15.Senbonmatsu T, Saito T, Landon EJ, et al. A novel angiotensin II type 2 receptor signaling pathway: possible role in cardiac hypertrophy. EMBO J. 2003;22:6471–82. doi: 10.1093/emboj/cdg637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guiglia R, Di Fede O, Lo Russo L, Sprini D, Rini GB, Campisi G. Osteoporosis, jawbones and periodontal disease. Med Oral Patol Oral Cir Bucal. 2013;18:e93–9. doi: 10.4317/medoral.18298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Redlich K, Smolen JS. Inflammatory bone loss: pathogenesis and therapeutic intervention. Nat Rev Drug Discov. 2012;11:234–50. doi: 10.1038/nrd3669. [DOI] [PubMed] [Google Scholar]
- 18.Hatton R, Stimpel M, Chambers TJ. Angiotensin II is generated from angiotensin I by bone cells and stimulates osteoclastic bone resorption in vitro. J Endocrinol. 1997;152:5–10. doi: 10.1677/joe.0.1520005. [DOI] [PubMed] [Google Scholar]
- 19.Shimizu H, Nakagami H, Osako MK, et al. Angiotensin II accelerates osteoporosis by activating osteoclasts. FASEB J. 2008;22:2465–75. doi: 10.1096/fj.07-098954. [DOI] [PubMed] [Google Scholar]
- 20.Guan XX, Zhou Y, Li JY. Reciprocal Roles of angiotensin II and angiotensin II receptors blockade (ARB) in regulating Cbfa1/RANKL via cAMP signaling pathway: possible mechanism for hypertension-related osteoporosis and antagonistic effect of ARB on hypertension-related osteoporosis. Int J Mol Sci. 2011;12:4206–13. doi: 10.3390/ijms12074206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaneko K, Ito M, Fumoto T, et al. Physiological function of the angiotensin AT1a receptor in bone remodeling. J Bone Miner Res. 2011;26:2959–66. doi: 10.1002/jbmr.501. [DOI] [PubMed] [Google Scholar]
- 22.Bandow K, Nishikawa Y, Ohnishi T, et al. Low-intensity pulsed ultrasound (LIPUS) induces RANKL, MCP-1, and MIP-1b expression in osteoblasts through the angiotensin II type 1 receptor. J Cell Physiol. 2007;211:392–8. doi: 10.1002/jcp.20944. [DOI] [PubMed] [Google Scholar]
- 23.Nakai K, Kawato T, Morita T, et al. Angiotensin II induces the production of MMP-3 and MMP-13 through the MAPK signaling pathways via the AT1 receptor in osteoblasts. Biochimi. 2013;95:922–33. doi: 10.1016/j.biochi.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 24.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 25.Yanagisawa M, Suzuki N, Mitsui N, Koyama Y, Otsuka K, Shimizu N. Compressive force stimulates the expression of osteogenesis-related transcription factors in ROS 17/2.8 cells. Arch Oral Biol. 2008;53:214–9. doi: 10.1016/j.archoralbio.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 26.Mikami Y, Omoteyama K, Kato S, Takagi M. Inductive effects of dexamethasone on the mineralization and the osteoblastic gene expression in mature osteoblast-like ROS17/2.8 cells. Biochem Biophys Res Commun. 2007;362:368–73. doi: 10.1016/j.bbrc.2007.07.192. [DOI] [PubMed] [Google Scholar]
- 27.Kimura A, Kawato T, Katono-Tani , et al. Hydrogen sulfide suppresses mineralized nodule formation by osteoblastic ROS17/2.8 Cells. J Hard Tissue Biol. 2012;21:219–24. [Google Scholar]
- 28.Kuwabara A, Tanabe N, Kawato T, et al. Interleukin-17A induces extracellular matrix protein expression in osteoblastic ROS17/2.8 cells. J Hard Tissue Biol. 2011;20:247–58. [Google Scholar]
- 29.Hagiwara H, Hiruma Y, Inoue A, Yamaguchi A, Hirose S. Deceleration by angiotensin II of the differentiation and bone formation of rat calvarial osteoblastic cells. J Endocrinol. 1998;156:543–50. doi: 10.1677/joe.0.1560543. [DOI] [PubMed] [Google Scholar]
- 30.Lamparter S, Kling L, Schrader M, Ziegler R, Pfeilschifter J. Effects of angiotensin II on bone cells in vitro. J Cell Physiol. 1998;175:89–98. doi: 10.1002/(SICI)1097-4652(199804)175:1<89::AID-JCP10>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 31.Komori T. Regulation of osteoblast differentiation by transcription factors. J Cell Biochem. 2006;99:1233–9. doi: 10.1002/jcb.20958. [DOI] [PubMed] [Google Scholar]
- 32.Ishii M, Merrill AE, Chan YS, et al. Msx2 and Twist cooperatively control the development of the neural crest-derived skeletogenic mesenchyme of the murine skull vault. Development. 2003;130:6131–42. doi: 10.1242/dev.00793. [DOI] [PubMed] [Google Scholar]
- 33.Ichida F, Nishimura R, Hata K, et al. Reciprocal roles of Msx2 in regulation of osteoblast and adipocyte differentiation. J Biol Chem. 2004;279:34015–22. doi: 10.1074/jbc.M403621200. [DOI] [PubMed] [Google Scholar]
- 34.Hassan MQ, Javed A, Morasso MI, et al. Dlx3 transcriptional regulation of osteoblast differentiation: temporal recruitment of Msx2, Dlx3, and Dlx5 homeodomain proteins to chromatin of the osteocalcin gene. Mol Cell Biol. 2004;24:9248–61. doi: 10.1128/MCB.24.20.9248-9261.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ganss B, Kim RH, Sodek J. Bone sialoprotein. Crit Rev Oral Biol Med. 1999;10:79–98. doi: 10.1177/10454411990100010401. [DOI] [PubMed] [Google Scholar]
- 36.Resnick LM, Laragh JH, Sealey JE, Alderman MH. Divalent cations in essential hypertension: relations between serum ionized calcium, magnesium, and plasma renin activity. N Engl J Med. 1983;309:888–91. doi: 10.1056/NEJM198310133091504. [DOI] [PubMed] [Google Scholar]
- 37.Cappuccio FP, Kalaitzidis R, Duneclift S, Eastwood JB. Unravelling the links between calcium excretion, salt intake, hypertension, kidney stones and bone metabolism. J Nephrol. 2000;13:169–77. [PubMed] [Google Scholar]
- 38.Lynn H, Kwok T, Wong SY, Woo J, Leung PC. Angiotensin converting enzyme inhibitor use is associated with higher bone mineral density in elderly Chinese. Bone. 2006;38:584–88. doi: 10.1016/j.bone.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 39.Rejnmark L, Vestergaard P, Mosekilde L. Treatment with beta-blockers, ACE inhibitors, and calcium-channel blockers is associated with a reduced fracture risk: a nationwide case-control study. J Hypertens. 2006;24:581–9. doi: 10.1097/01.hjh.0000203845.26690.cb. [DOI] [PubMed] [Google Scholar]
- 40.Maeno M, Tanaka H, Zhang F, Kitami S, Nakai K, Kawato T. Direct and indirect effects of IL-17A on RANKL-induced osteoclastogenesis. J Hard Tissue Biol. 2013;22:287–92. [Google Scholar]
- 41.Koch FP, Weinbach C, Hustert E, Al-Nawas B, Wagner W. GDF-5 and BMP-2 regulate bone cell differentiation by gene expression of MSX1, MSX2, Dlx5, and Runx2 and influence OCN gene expression in vitro. Int J Periodontics Restorative Dent. 2012;32:285–93. [PubMed] [Google Scholar]
- 42.Nakayama Y, Yang L, Takai H, Kaneko H, Abiko Y, Ogata Y. Fibroblast growth factor 2 and forskolin induce mineralization-associated genes in two kinds of osteoblast-like cells. J Oral Sci. 2012;54:251–89. doi: 10.2334/josnusd.54.251. [DOI] [PubMed] [Google Scholar]
- 43.Sonomoto K, Yamaoka K, Oshita K, et al. Interleukin-1beta induces differentiation of human mesenchymal stem cells into osteoblasts via the Wnt-5a/receptor tyrosine kinase-like orphan receptor 2 pathway. Arthritis Rheum. 2012;64:3355–63. doi: 10.1002/art.34555. [DOI] [PubMed] [Google Scholar]
- 44.Li Y, Bäckesjö CM, Haldosén LA, Lindgren U. IL-6 receptor expression and IL-6 effects change during osteoblast differentiation. Cytokine. 2008;43:165–73. doi: 10.1016/j.cyto.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 45.Iwasaki K, Komaki M, Mimori K, Leon E, Izumi Y, Ishikawa I. IL-6 induces osteoblastic differentiation of periodontal ligament cells. J Dent Res. 2008;87:937–42. doi: 10.1177/154405910808701002. [DOI] [PubMed] [Google Scholar]
- 46.Guo L, Wang M, Zhang ZY, et al. Angiotensin II induces interleukin-6 synthesis in osteoblasts through ERK1/2 pathway via AT1 receptor. Arch Oral Biol. 2011;56:205–11. doi: 10.1016/j.archoralbio.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 47.Tang W, Wei Y, Le K, et al. Mitogen-activated protein kinases ERK 1/2- and p38-GATA4 pathways mediate the Ang II-induced activation of FGF2 gene in neonatal rat cardiomyocytes. Biochem Pharmacol. 2011;81:518–25. doi: 10.1016/j.bcp.2010.11.012. [DOI] [PubMed] [Google Scholar]