Abstract
Introduction
To understand the effects of low-magnitude, high-frequency (LMHF) mechanical vibration at different intensities on human periodontal ligament stem cell (hPDLSC) proliferation and osteogenic differentiation.
Material and methods
The effect of vibration on hPDLSC proliferation, osteogenic differentiation, tenogenic differentiation and cytoskeleton was assessed at the cellular, genetic and protein level.
Results
The PDLSC proliferation was decreased after different magnitudes of mechanical vibration; however, there were no obvious senescent cells in the experimental and the static control group. Expression of osteogenesis markers was increased. The expression of alkaline phosphatase (ALP) and osteocalcin (OCN) mRNA was up-regulated at 0.1 g, 0.3 g, 0.6 g and 0.9 g magnitude, with the peak at 0.3 g. The type I collagen (Col-I) level was increased after vibration exposure at 0.1 g, 0.3 g, and 0.6 g, peaking at 0.3 g. The expression levels of both mRNA and protein of Runx2 and osterix (OSX) significantly increased at a magnitude of 0.1 g to 0.9 g, reached a peak at 0.3 g and then decreased slowly. The scleraxis, tenogenic markers, and mRNA expression decreased at 0.05 g, 0.1 g, and 0.3 g, and significantly increased at 0.6 g and 0.9 g. Compared with the static group, the F-actin stress fibers of hPDLSCs became thicker and clearer following vibration.
Conclusions
The LMHF mechanical vibration promotes PDLSC osteogenic differentiation and implies the existence of a magnitude-dependent effect of vibration on determining PDLSC commitment to the osteoblast lineage. Changes in the cytoskeleton of hPDLSCs after vibration may be one of the mechanisms of the biological effects.
Keywords: mechanical vibration, periodontal ligament stem cells, osteogenesis, proliferation, magnitude
Introduction
Human periodontal ligament stem cells (hPDLSCs) have been isolated and identified as mesenchymal stem cells (MSCs) since 2004 [1]. It has been shown that hPDLSCs are multipotent cells with features similar to bone marrow and dental pulp MSCs, which are capable of proliferating and producing different types of tissues such as bone, adipose and tooth associated tissues in specific media [1, 2]. As such, PDLSCs have a dynamic role in maintaining periodontal homeostasis, and are responsible for remodeling and regeneration of periodontal tissues.
It is well known that mechanical stimuli have an important role in bone tissue metabolism and periodontal tissue homeostasis [3–7]. Recently, low-magnitude, high-frequency (LMHF) vibration (e.g., acceleration less than < 1×g, where g = 9.81 m/s2, at 20–90 Hz) has gained much interest as studies have shown that such mechanical stimulation can positively influence skeletal homeostasis [8–11]. Studies have demonstrated that such kinds of mechanical stimuli exhibit anabolic effects on bone homeostasis in animals [12, 13], postmenopausal osteoporotic women [14], and children with musculoskeletal diseases [15]. However, the underlying mechanism of the anabolic role of LMHF mechanical vibration on bone remains unknown. It has been well accepted that mechanical signals may regulate the direction of stem cell differentiation [16, 17]. With specific regard to mechanical vibration, it has been shown that it has the ability to direct the lineage commitment of bone marrow stromal cells (BMSCs) to the osteoblast lineage [18, 19]. However, little is known about the response of hPDLSCs to any type of mechanical stimulus. While the effect of LMHF mechanical vibration on BMSCs has been established, its potential effect on the remodeling of paradental tissues, including alveolar bone and periodontal ligament (PDL), is currently unknown.
The PDL sensitively mediates the transmission of stress stimuli to the alveolar bone for periodontal tissue remolding. Derived from PDL, hPDLSCs are surmised to have a similar osteogenic response induced by strain. In this regard, our research group was particularly interested in whether LMHF mechanical vibration exerts an effect on hPDLSC proliferation and differentiation. According to findings from our previous experiments, LMHF mechanical vibration promotes PDLSC osteogenic differentiation and implies the existence of a frequency-dependent effect of vibration on determining PDLSC commitment to the osteoblast lineage. However, the magnitude of the mechanical vibration is vital for the therapy effect. Because mechanical stimuli have also been widely used to enhance the formation and properties of tissue-engineered bone [20], the optimal magnitude and frequency should be made clear. Only when these problems are figured out may it be possible to optimize the clinical application protocol of mechanical vibration to facilitate osteogenesis effectively and safely. Given the fact that the efficacy of mechanical stimulation is dependent not only on the frequency, but also the magnitude of the applied vibration stimulus [9, 21], we also wanted to establish the optimal parameters of the mechanical stimulus for promoting osteogenic differentiation. To test our hypothesis, we subjected hPDLSCs to LMHF mechanical vibration at a magnitude of 0.05 g to 0.9 g and a frequency of 50 Hz, based on our previous experiments showing that mechanical vibration at frequency of 50 Hz, with a 0.3 g magnitude, was more favorable for hPDLSC osteogenic differentiation. The effect of mechanical vibration on hPDLSC proliferation and osteogenic differentiation potential was assessed at the cellular, genetic and protein levels.
Material and methods
Isolation and identification of human periodontal ligament stem cells
Human PDLSCs were isolated and cultured as previously described [22]. Periodontal ligament cells (PDLCs) were scraped from the healthy, noncarious premolar tooth roots, extracted from donors aged between 12 and 16 years old for orthodontic reasons with informed consent. The PDLCs at passages 2–4 were used for immunomagnetic microbead isolation of PDLSCs via the CD146 microbead kit. The sorted CD146(+) cells were identified as hPDLSCs by immunocytochemical staining using the following antibodies: STRO-1, CD146, CD271 and scleraxis [22]. To investigate the multipotency of hPDLSCs, the isolated cells were tested for the ability to undergo osteogenic and adipogenic differentiation [22]. The passages 3–4 of hPDLSCs were used in the following experiments. Collection and culture of hPDLSCs was approved by the Ethical Committee Board of the West China College of Stomatology, Sichuan University.
Study design
Cells seeded at 1 × 105/well in a six-well plate were randomly divided into mechanical vibration culture and static culture groups, both of which were cultured in a humidified atmosphere of 5% CO2 at 37°C. Cells were incubated overnight in αMEM with 10% FBS to promote cell attachment. After 24 h, the medium was replaced with αMEM with 2% FBS for 24 h in order to synchronize the cell cycle. Prior to application of the mechanical stimulus, the culture medium of both groups was replaced with fresh αMEM with 10% FBS.
Application of LMHF mechanical vibration strain
Six-well plates culturing hPDLSCs were mounted onto the platform, parallel to the ground, of a GJX-5 vibration sensor [22] (Beijing Sending Technology, Beijing, China). The hPDLSCs received sinusoidal LMHF mechanical stimuli (magnitude: 0.05 g to 0.9 g, frequency: 50 Hz) perpendicular to the ground for 30 min every 24 h.
RNA isolation and quantitative real-time RT-PCR
The total RNA of hPDLSCs under different culture conditions was isolated using Trizol reagent (Invitrogen) 2 h after the last stimulus of the fifth day. Following RNA isolation, real-time RT-PCR was carried out using the SYBR-PrimeScriptTM RT-PCR Kit (TaKaRa, China). The primer sequences for Runx2, osterix (OSX), Col-I, ALP, OCN, scleraxis and GAPDH were synthesized based on the GenBank database and are presented in Table I.
Table I.
qRT-PCR primers used in this study
| Target gene | Primers | Sequence | Fragment size [bp] |
|---|---|---|---|
| Runx2 | Forward | 5’-CAGATGGGACTGTGGTTACTGT-3’ | 169 |
| Reverse | 5’-GTGAAGACGGTTATGGTCAAGG-3’ | ||
| Osterix | Forward | 5’-CTGTGAAACCTCAAGTCCTATGGA-3’ | 69 |
| Reverse | 5’—GCTCTGCAGTCAAGGGAGATG -3’ | ||
| GAPDH | Forward | 5’- GGAAGGTGAAGGTCGGAGT -3’ | 229 |
| Reverse | 5’-TGGAAGATGGTGATGGGATT-3’ | ||
| ALP | Forward | 5’- CAGATGAAGTGGGAGTGCTTGT -3’ | 115 |
| Reverse | 5’-CTGATGTGGAGTATGAGAGTGACG-3’ | ||
| OCN | Forward | 5’- CTCACACTCCTCGCCCTATTG -3’ | 142 |
| Reverse | 5’- GCCTGGGTCTCTTCACTACCT -3’ | ||
| Col-I | Forward | 5’-CCACCTGCCTCTGGCTTCT -3’ | 68 |
| Reverse | 5’- AGCTGTGGAGGAGGGTTTCA -3’ | ||
| Scleraxis | Forward | 5’-GAACACCCAGCCCAAACAGAT-3’ | 63 |
| Reverse | 5’-TCCTTGCTCAACTTTCTCTGGTT-3’ |
Western blots
Total protein for western blot analysis were started to be extracted 12 h after the last stimulation cycle on the fifth day of culture. Forty μg protein extracts were separated on 10% SDS-PAGE gels and subsequently transferred to a PVDF membrane. After blocking, the membranes were probed with primary antibodies raised against osterix and Runx2 (Abcam, Cambridge, MA USA), followed by the addition of a horseradish peroxidase-conjugated secondary antibody (Zhongshan Bio Co., Beijing, China). Immunoreactive proteins were visualized using a chemiluminescence kit (Millipore, Billerica, MA, USA). Band intensities were determined using the ChemiDoc XRS Gel documentation system and Quantity One software (Bio-Rad, Hercules, CA, USA).
Cell proliferation assay
After a mechanical vibration stimulus for 3 days, cell proliferation in each group was measured using a cell counting kit (CCK-8; Dojindo Molecular Technologies Inc., Rockville, MD, USA) from 12 h after the last stimulus. The cell viability was shown in direct proportion to the absorbance at 450 nm; therefore, it was calculated as the OD value using a microplate reader (Varioskan Flash; Thermo Fisher Scientific, Waltham, MA, USA).
Assessment of senescence-associated β-galactosidase staining
The cell senescence of hPDLSCs was tested after mechanical vibration for 5 days, at the ideal intensity and frequency for enhancing PDLSCs’ osteodifferentiation based on the results of this and previous works (magnitude: 0.3 g, frequency: 50 Hz). Cell senescence was measured at 12 h after the last stimulus using a senescence-associated β-galactosidase staining kit (Beyotime, China). The senescent cells were observed in an optical microscope and counted from 5 random fields of vision.
Immunofluorescence analysis
We observed cytoskeletal changes after the mechanical vibration (magnitude: 0.3 g, frequency: 50 Hz), which was more conducive for the osteodifferentiation of PDLSCs, according to the results of our previous studies. Cells were subjected to mechanical vibration continuously for 2 h (magnitude: 0.3 g, frequency: 50 Hz), then fixed with 4% paraformaldehyde, rinsed with PBS 3 times, permeabilized with 0.25% Triton X-100 for 10 min, rinsed with PBS 3 times again, then incubated with 5% BSA at 37°C for 30 min. Next, the cells were incubated with 0.5 μM Alexa Fluor 488-conjugated phalloidin (Sigma, USA) at 37°C away from light for 1 h, rinsed twice with PBS, stained with DAPI (Sigma, USA) for 5 min, and finally rinsed twice with PBS. The cells on coverslips were observed using an epifluorescent microscope (Olympus IX70, Japan).
All assays were performed in triplicate, with three independent experiments.
Statistical analysis
All quantitative data are presented as the mean ± standard deviation. Statistical analysis to compare results between groups was carried out by one-way analysis of variance (ANOVA) using a multiple comparison Dunnett post-hoc test, with SPSS software, version 17.0 (SPSS Inc, Chicago, IL, USA). Statistically significant values were defined as p < 0.05.
Results
Effect of mechanical vibration on PDLSC proliferation and cellular senescence
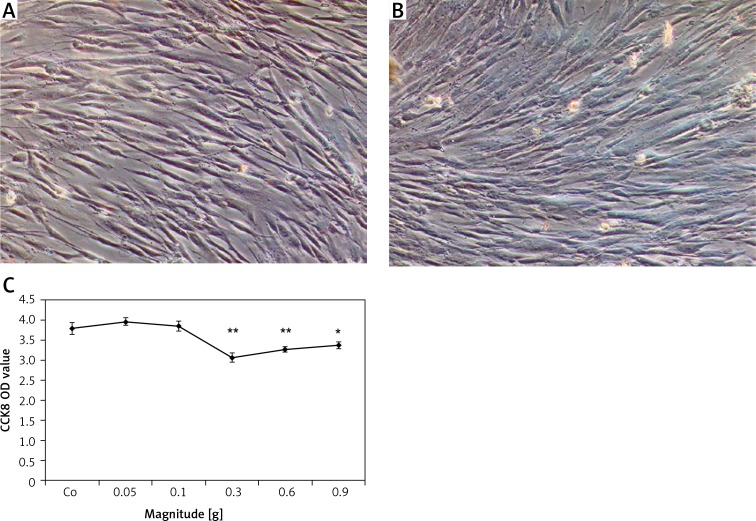
When the acceleration was set equal to or more than 0.3 g, PDLSC proliferation showed a significant decrease with the largest change in the 0.3 g group, compared to that in the control static group (Figure 1 C). To further confirm the effect of mechanical vibration on hPDLSC vitality, cell senescence was measured. As shown in Figures 1A and 1 B, no obvious SA-β-gal blue positive cell was observed either in the experimental or the control group.
Figure 1.
Effect of mechanical vibration on hPDLSC proliferation and senescence. There were no obvious SA--gal blue positive cells in the static control (A) and the experimental group (B). Scale bars = 200 μm for A and B. A trend toward decreased cell proliferation was evident with increased magnitude of stimulation, which was statistically significant at 0.3 g and above, when compared with the stationary control (C)
Bars represent the mean ± standard deviation (n = 3); *p < 0.05; **p < 0.01.
Osteogenic-specific gene and protein expression levels in PDLSCs stimulated by mechanical vibration at different magnitudes
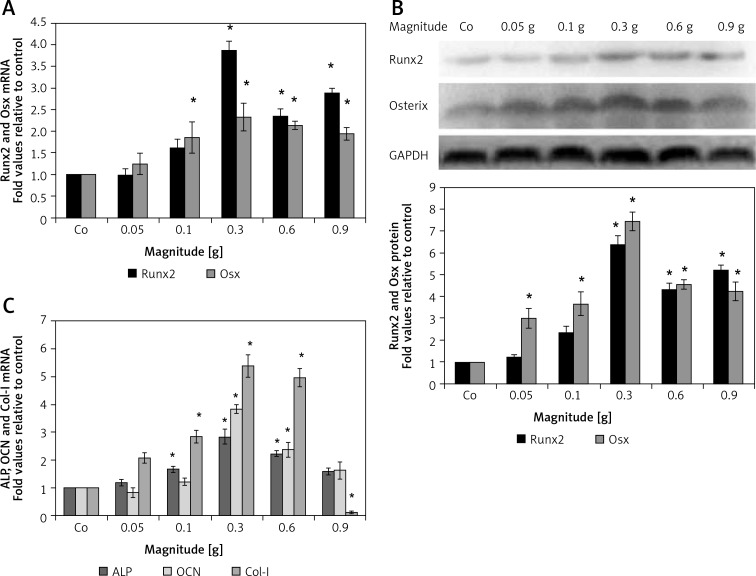
To investigate the effect of mechanical vibration at different magnitudes on osteogenic differentiation of hPDLSCs, genes associated with osteogenesis, including Runx2, Osx, Col-I, ALP and OCN, were measured by real-time RT-PCR. Compared to the control group, Runx2 mRNA expression slightly increased at 0.1 g, and peaked to approximately 4-fold versus control at 0.3 g (Figure 2 A); the other four mRNAs expression levels showed a common growing trend towards a significant peak at 0.3 g (Osx 2.3-fold, ALP 2.8-fold, OCN 3.8-fold, and Col-I 2.4-fold); then they decreased to a minimum at 0.9 g (Figures 2 A and C). The expression levels of bone specific proteins were further evaluated using western blotting (Figure 2 B). Similar to gene expression data, both Runx2 and Osx protein levels increased in magnitude-dependent manners, with significant peaks at 0.3 g (Runx2 6.4-fold and Osx 7.5-fold), respectively, then decreased to some extent. In summary, the increased protein levels of Runx2 and Osx at 0.1 g to 0.9 g magnitude stimulation were consistent with the changes in their respective mRNA levels.
Figure 2.
The effect of mechanical vibration at different magnitudes on osteogenic gene and protein expression in hPDLSCs. Quantitative PCR results indicate that Runx2 and osterix (Osx) mRNA levels were upregulated by mechanical vibration at 0.1–0.9 g magnitude, as compared to control cells (Co) (A); the mRNA expression levels of ALP, OCN, and Col-I were in a common growing trend towards a significant peak at 0.3 g, then decreased to a minimum at 0.9 g (B); Runx2 and osterix (Osx) protein expression levels increased with increasing magnitude, peaking at a magnitude of 0.3 g compared to control (C)
Each bar represents the mean ± standard deviation (n = 3); *p < 0.05.
Tenogenic-specific gene expression levels in PDLSCs stimulated by mechanical vibration at different magnitudes
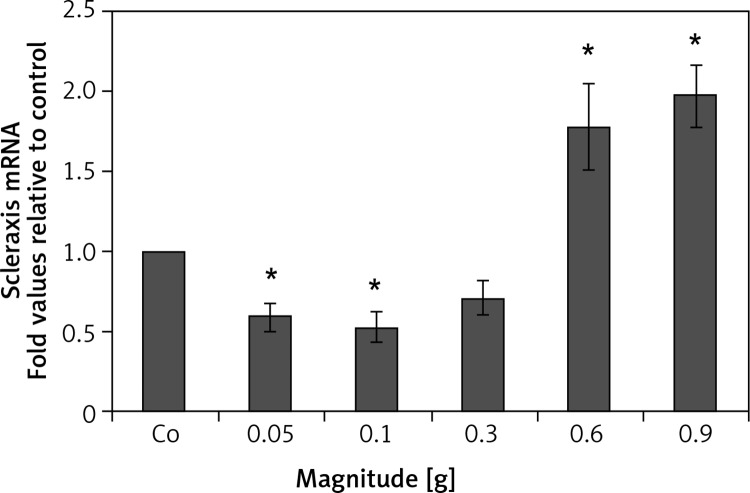
The PDLSCs express both osteogenic and tenogenic phenotypes. The mRNA expression level of scleraxis, a tendon-specific transcription factor, decreased at 0.05 g, 0.1 g, and 0.3 g, and significantly increased at 0.6 g and 0.9 g, with a peak increase at 0.9 g (1.97-fold increase versus control; p < 0.05) (Figure 3).
Figure 3.
The effect of mechanical vibration at different magnitudes on tenogenic gene expression in hPDLSCs. Quantitative PCR results indicate that the mRNA expression level of Scleraxis, a tendon specific transcription factor, decreased at 0.05 g, 0.1 g, and 0.3 g, and significantly increased at 0.6 g and 0.9 g, with a peak increase at 0.9 g
Each bar represents the mean ± standard deviation (n = 3); *p < 0.05.
Changes in the cytoskeleton of PDLSCs after mechanical vibration
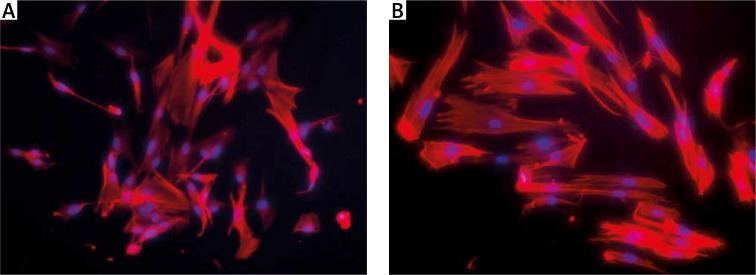
In order to examine the effect of mechanical vibration on the cytoskeleton of hPDLSCs as well as to investigate the potential role of the actin cytoskeleton in the osteogenic differentiation in response to mechanical vibration, we observed cytoskeletal changes after mechanical vibration. Compared to the static group, the F-actin stress fibers of hPDLSCs became thicker and clearer after vibration (Figure 4).
Figure 4.
Change in cytoskeleton of hPDLSCs in response to mechanical vibration. Compared with the static control group (A), the F-actin stress fibers of hPDLSCs became thicker and clearer following vibration stimulation (B)
Scale bars =100 μm for A and B.
Discussion
PDLSC proliferation and cellular senescence
In this work, we successfully isolated hPDLSCs using the CD146 microbead kit. The results of phenotypic detection of hPDLSCs showed that hPDLSCs have similar phenotypes to bone marrow stem cells (BMSCs), with their pluripotency also proven. This suggested that purified mesenchymal stem cells can be obtained from PDL, a readily available source for regenerative dentistry [22]. Subsequently, we examined the potential cellular and molecular regulation of hPDLSCs after mechanical vibration at a magnitude of 0.05 g to 0.9 g and a frequency 50 Hz, in order to test whether the efficacy of mechanical stimulation is dependent on the magnitude of the applied vibration stimulus. In our study, mechanical vibration caused a reduction of hPDLSC proliferation, which peaked at 0.3 g. Luu et al. [18] found that BMSC proliferation was positively influenced by mechanical vibration. Contrastingly, Zhou et al. [23] found that BMSC proliferation was decreased after vibration treatment. Senescence-associated β-galactosidase (SA–β-Gal) is an established biomarker associated with cellular aging. Senescent cells should be detected as cells showing a cytoplasmic blue precipitate [24]. In the present study, there were no obvious SA-β-gal positive cells in the experimental and the static control group. As such, although the decreased PDLSC proliferation observed in our study was confirmed by the cell counting assay, hPDLSCs maintained high vitality after mechanical vibration.
Osteogenic-specific gene and protein expression levels
Experimental studies have already affirmed the significance of mechanical strain on osteogenic differentiation of BMSCs. Jagodzinski et al. [25] found that cyclical stretching significantly up-regulated expression of ALP, OCN, Col-I and Runx2 of human BMSCs. Other studies with 3D-cultured BMSCs [26] also verified cyclic tensile strain-induced osteogenic differentiation. Tang found that hPDLSCs might be sensitive to cyclic tensile strain. The significant increase of Runx2, osterix and Satb2 expression levels may suggest an early response toward osteogenic orientation of hPDLSCs [27]. Studies have revealed that Runx2 and osterix are two essential transcription factors in the osteogenic pathway [28]. Our study aims to investigate the response of hPDLSCs subject to mechanical vibration at different magnitudes. In this study, the expression of both genes was increased by LMHF mechanical vibration in the range of 0.1 g to 0.9 g, which peaked at a magnitude of 0.3 g, albeit to different extents. Based upon the observed enhancement of Runx2 and osterix mRNA expression induced by mechanical vibration, we conclude that mechanical vibration can affect osteogenesis by increasing the commitment of PDLSCs to the osteogenic lineage. This conclusion is supported by our protein expression data, which were in parallel to mRNA data, implying that the efficacy of vibration was strongly dependent on the magnitude of the applied mechanical stimulation.
The increased matrix synthesis and maturation by mechanical vibration was shown by elevated expression levels of the middle (Col-I, ALP) and late (OCN) osteogenic markers. We interpret our data to indicate that LMHF mechanical vibration drives hPDLSCs to an osteogenic lineage.
The mRNA expression level of scleraxis decreased at 0.05 g, 0.1 g, and 0.3 g, and significantly increased at 0.6 g and 0.9 g. The differences in expression level between tenogenic differentiation markers and osteogenic differentiation markers may have arisen from their unique temporal-specific, frequency-specific and magnitude-specific regulation. Consistent with our data, Xie et al. [11] found that mechanical vibration at 45 Hz, 0.3 g can inhibit trabecular bone resorption, and maintain a high level of matrix quality in the growing skeleton. Lau et al. [8] found that when MLO-Y4 osteocytes were subjected to LMHF mechanical vibration, RANKL mRNA expression was decreased most significantly at 60 Hz, 0.3 g. Rubin et al. [19] and Luu [18] observed that mechanical vibration at 0.2 g, 90 Hz directed the lineage commitment of BMSCs to the osteoblast lineage. More recently, Zhou et al. [23] found that mechanical vibration at 40 Hz, 0.3 g promoted BMSC differentiation of BMSCs seeded on bone-derived scaffolds.
Different regimens of frequencies and intensity of mechanical vibration have been used in cells, animals and human beings, with different results [12, 13, 29]. The ideal frequency and intensity of the stimulus is still not established; although it is known that frequencies that range from 15 Hz to 90 Hz can be strongly anabolic [12], the range of appropriate intensity has not yet been determined. In this regard, our results are in agreement with some previous studies [8, 11, 23], but there are discrepancies with other researchers which we believe reflect the use of different cell types and research models [9, 18, 19]. Some researchers have suggested that the efficacy of mechanical signals that stimulate bone formation are dependent on the applied frequency, rather than the strain magnitude [9, 21]; however, it has been suggested that the degree of cellular response is determined not only by the frequency of the load, but also by the magnitude, rate, and even cycle number of the load [30].
Changes in the cytoskeleton
In our study, we determined the effect of magnitude at a constant frequency (50 Hz) in order to look for an optimal magnitude to stimulate PDLSCs toward osteogenic differentiation. From our results, mechanical vibration at a magnitude of 0.3 g to 0.6 g was more conducive for the osteodifferentiation of PDLSCs, implying that there is also a magnitude-dependent effect of vibration on determining PDLSCs’ commitment to different lineages. What remains unclear is how the mechanical force is recognized by the cells and transduced into a cellular signal that controls transcriptional activity. The physical mechanisms modulating the vibration-induced response have not been identified. Regardless of the physical mechanism involved in transmitting mechanical signals to the cell, the cytoskeleton as the continuous structure between chromosome and cell membrane is likely involved in transmitting mechanical cues within a cell [31]. This hypothesis that the cytoskeleton is intimately involved in transmitting and amplifying the oscillatory mechanical signal was corroborated by the up-regulation of genes important for cytoskeletal remodeling [32]. It was reported that mechanical force was transferred through the cytoskeleton to the nucleus [33], leading to a biochemical cascade. Previous studies [34] have shown that unidirectional steady flow induced actin stress fiber formation. The purpose of this project was to examine the effect of mechanical vibration on the actin cytoskeleton of hPDLSCs as well as to determine the potential role of the actin cytoskeleton in the osteogenic differentiation in response to vibration. In contrast, the development of thick actin fibers, consistent with the formation of stress fibers, was apparent in cells exposed to a mechanical stimulus. Consistent with Judex [32], the mechanically driven osteogenic commitment of undifferentiated PDLSCs was influenced by the level of cytoskeletal remodeling, which was correlated with the magnitude of applied acceleration. We postulate that the actin cytoskeleton plays a pivotal role in determining the PDLSC mechanical properties and modulation of cellular mechanics at the early stage of stem-cell osteodifferentiation. However, there is a discrepancy in the relationship between osteogenic differentiation of stem cells and skeletal changes. Rodríguez et al. [35] reported that the actin cytoskeleton changed from a large number of thin, parallel microfilament bundles extending across the entire cytoplasm in undifferentiated MSCs to a few thick actin filament bundles located at the outermost periphery in differentiated cells. Titushkin discovered that the actin cytoskeleton changed from thick stress fibers in hMSCs into the thinner filamentous network in osteoblasts [36]. Even so, changes in the cytoskeleton of hPDLSCs after vibration may be one of the mechanisms of the biological effects.
In conclusion, our study provides a first glimpse at how hPDLSCs respond to LMHF mechanical vibration at the level of cell proliferation, gene transcription and protein expression. Specifically, we observed a decrease in PDLSC proliferation and an increase in markers of osteogenesis after mechanical vibration stimulation. From our previous results, mechanical vibration at a frequency of 40 Hz to 90 Hz was more conducive for the osteodifferentiation of PDLSCs, implying the existence of a frequency-dependent effect of vibration on determining PDLSC commitment to the osteoblast lineage. This work indicated that mechanical vibration at a magnitude of 0.3 g to 0.6 g with a 50 Hz frequency was more favorable for PDLSC osteogenic differentiation, implying that the osteogenic efficacy of mechanical stimulation is also dependent on the magnitude. In addition, changes in the cytoskeleton of hPDLSCs after vibration may be one of the mechanisms of the biological effects. Therefore, our data provided a range of parameters for in vivo testing and clinical applications in the future.
More long-term studies in vitro and in vivo are needed to determine whether vibration at different magnitudes and frequencies than those tested in the current study could become a suitable therapy to enhance periodontal regeneration and accelerate periodontal tissue remodeling.
Acknowledgments
This work was supported by a grant from the National Natural Science Foundation of China (No. 81070867).
The experimental procedure was approved by the Ethical Committee Board of West China College of Stomatology, Sichuan University. The Judgment's reference number is 2009026.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Seo BM, Miura M, Gronthos S, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149–55. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- 2.Trubiani O, Orsini G, Zini N, et al. Regenerative potential of human periodontal ligament derived stem cells on three-dimensional biomaterials: a morphological report. J Biomed Mat Res Part A. 2008;87:986–93. doi: 10.1002/jbm.a.31837. [DOI] [PubMed] [Google Scholar]
- 3.Gardner MJ, van der Meulen MCH, Demetrakopoulos D, Wright TM, Myers ER, Bostrom MP. In vivo cyclic axial compression affects bone healing in the mouse tibia. J Orthop Res. 2006;24:1679–86. doi: 10.1002/jor.20230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Omar H, Shen G, Jones AS, Zoellner H, Petocz P, Darendeliler MA. Effect of low magnitude and high frequency mechanical stimuli on defects healing in cranial bones. J Oral Maxillofac Surg. 2008;66:1104–11. doi: 10.1016/j.joms.2008.01.048. [DOI] [PubMed] [Google Scholar]
- 5.Rubin C, Turner AS, Bain S, Mallinckrodt C, McLeod K. Anabolism – low mechanical signals strengthen long bones. Nature. 2001;412:603–4. doi: 10.1038/35088122. [DOI] [PubMed] [Google Scholar]
- 6.McCulloch CAG, Lekic P, McKee MD. Role of physical forces in regulating the form and function of the periodontal ligament. Periodontology. 2000;24:56–72. doi: 10.1034/j.1600-0757.2000.2240104.x. [DOI] [PubMed] [Google Scholar]
- 7.Kaneko S, Ohashi K, Soma K, Yanagishita M. Occlusal hypofunction causes changes of proteoglycan content in the rat periodontal ligament. J Periodont Res. 2001;36:9–17. doi: 10.1034/j.1600-0765.2001.00607.x. [DOI] [PubMed] [Google Scholar]
- 8.Lau E, Al-Dujaili S, Guenther A, Liu D, Wang L, You L. Effect of low-magnitude, high-frequency vibration on osteocytes in the regulation of osteoclasts. Bone. 2010;46:1508–15. doi: 10.1016/j.bone.2010.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Judex S, Lei X, Han D, Rubin C. Low-magnitude mechanical signals that stimulate bone formation in the ovariectomized rat are dependent on the applied frequency but not on the strain magnitude. J Biomech. 2007;40:1333–9. doi: 10.1016/j.jbiomech.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 10.Oxlund BS, Ørtoft G, Andreassen TT, Oxlund H. Low-intensity, high-frequency vibration appears to prevent the decrease in strength of the femur and tibia associated with ovariectomy of adult rats. Bone. 2003;32:69–77. doi: 10.1016/s8756-3282(02)00916-x. [DOI] [PubMed] [Google Scholar]
- 11.Xie L, Jacobson JM, Choi ES, et al. Low-level mechanical vibrations can influence bone resorption and bone formation in the growing skeleton. Bone. 2006;39:1059–66. doi: 10.1016/j.bone.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 12.Rubin C, Turner AS, Mallinckrodt C, Jerome C, McLeod K, Bain S. Mechanical strain, induced noninvasively in the high-frequency domain, is anabolic to cancellous bone, but not cortical bone. Bone. 2002;30:445–52. doi: 10.1016/s8756-3282(01)00689-5. [DOI] [PubMed] [Google Scholar]
- 13.Rubin C, Turner AS, Muller R, et al. Quantity and quality of trabecular bone in the femur are enhanced by a strongly anabolic, noninvasive mechanical intervention. J Bone Miner Res. 2002;17:349–57. doi: 10.1359/jbmr.2002.17.2.349. [DOI] [PubMed] [Google Scholar]
- 14.Rubin C, Recker R, Cullen D, Ryaby J, McCabe J, McLeod K. Prevention of postmenopausal bone loss by a low©\magnitude, high©\frequency mechanical stimuli: a clinical trial assessing compliance, efficacy, and safety. J Bone Miner Res. 2004;19:343–51. doi: 10.1359/JBMR.0301251. [DOI] [PubMed] [Google Scholar]
- 15.Ward K, Alsop C, Caulton J, Rubin C, Adams J, Mughal Z. Low magnitude mechanical loading is osteogenic in children with disabling conditions. J Bone Miner Res. 2004;19:360–9. doi: 10.1359/JBMR.040129. [DOI] [PubMed] [Google Scholar]
- 16.Pelaez D, Charles Huang CY, Cheung HS. Cyclic compression maintains viability and induces chondrogenesis of human mesenchymal stem cells in fibrin gel scaffolds. Stem Cells and Development. 2009;18:93–102. doi: 10.1089/scd.2008.0030. [DOI] [PubMed] [Google Scholar]
- 17.Thorpe SD, Buckley CT, Vinardell T, O'Brien FJ, Campbell VA, Kelly DJ. The response of bone marrow-derived mesenchymal stem cells to dynamic compression following TGF-beta3 induced chondrogenic differentiation. Annals of Biomedical Engineering. 2010;38:2896–909. doi: 10.1007/s10439-010-0059-6. [DOI] [PubMed] [Google Scholar]
- 18.Luu YK, Capilla E, Rosen CJ, et al. Mechanical stimulation of mesenchymal stem cell proliferation and differentiation promotes osteogenesis while preventing dietary©\induced obesity. J Bone Miner Res. 2009;24:50–61. doi: 10.1359/JBMR.080817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rubin C, Capilla E, Luu Y, et al. Adipogenesis is inhibited by brief, daily exposure to high-frequency, extremely low-magnitude mechanical signals. Proc Natl Acad Sci. 2007;104:17879. doi: 10.1073/pnas.0708467104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ignatius A, Blessing H, Liedert A, et al. Tissue engineering of bone: effects of mechanical strain on osteoblastic cells in type I collagen matrices. Biomaterials. 2005;26:311–8. doi: 10.1016/j.biomaterials.2004.02.045. [DOI] [PubMed] [Google Scholar]
- 21.Rubin CT, McLeod KJ. Promotion of bony in growth by frequency-specific, low-amplitude mechanical strain. Clin Orthop Rel Res. 1994;298:165. [PubMed] [Google Scholar]
- 22.Zhang C, Li J, Zhang L, et al. Effects of mechanical vibration on proliferation and osteogenic differentiation of human periodontal ligament stem cells. Arch Oral Biol. 2012;57:1395–407. doi: 10.1016/j.archoralbio.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 23.Zhou Y, Guan X, Zhu Z, et al. Osteogenic differentiation of bone marrow-derived mesenchymal stromal cells on bone-derived scaffolds: effect of microvibration and role of ERK1/2 activation. Eur Cells Mater. 2011;22:12–25. doi: 10.22203/ecm.v022a02. [DOI] [PubMed] [Google Scholar]
- 24.Chen Y, Xu X, Tan Z, Ye C, Zhao Q, Chen Y. Age-related BMAL1 change affects mouse bone marrow stromal cell proliferation and osteo-differentiation potential. Arch Med Sci. 2012;8:30–8. doi: 10.5114/aoms.2012.27277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jagodzinski M, Drescher M, Zeichen J, et al. Effects of cyclic longitudinal mechanical strain and dexamethasone on osteogenic differentiation of human bone marrow stromal cells. Eur Cell Mater. 2004;7:C41. doi: 10.22203/ecm.v007a04. [DOI] [PubMed] [Google Scholar]
- 26.Kearney E, Farrell E, Prendergast P, Campbell V. Tensile strain as a regulator of mesenchymal stem cell osteogenesis. Ann Biomed Eng. 2010;38:1767–79. doi: 10.1007/s10439-010-9979-4. [DOI] [PubMed] [Google Scholar]
- 27.Tang N, Zhao Z, Zhang L, et al. Up-regulated osteogenic transcription factors during early response of human periodontal ligament stem cells to cyclic tensile strain. Arch Med Sci. 2012;8:422–30. doi: 10.5114/aoms.2012.28810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakashima K, Zhou X, Kunkel G, et al. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108:17–29. doi: 10.1016/s0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- 29.Rubin C, Xu G, Judex S. The anabolic activity of bone tissue, suppressed by disuse, is normalized by brief exposure to extremely low-magnitude mechanical stimuli. FASEB J. 2001;15:2225. doi: 10.1096/fj.01-0166com. [DOI] [PubMed] [Google Scholar]
- 30.Bacabac RG, Smit TH, Van Loon J, Doulabi BZ, Helder M, Klein-Nulend J. Bone cell responses to high-frequency vibration stress: does the nucleus oscillate within the cytoplasm? FASEB J. 2006;20:858–64. doi: 10.1096/fj.05-4966.com. [DOI] [PubMed] [Google Scholar]
- 31.Dahl KN, Booth-Gauthier EA, Ladoux B. In the middle of it all: mutual mechanical regulation between the nucleus and the cytoskeleton. J Biomech. 2010;43:2–8. doi: 10.1016/j.jbiomech.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 32.Uzer G, Pongkitwitoon S, Ete Chan M, Judex S. Vibration induced osteogenic commitment of mesenchymal stem cells is enhanced by cytoskeletal remodeling but not fluid shear. J Biomech. 2013;46:2296–302. doi: 10.1016/j.jbiomech.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang N, Butler JP, Ingber DE. Mechanotransduction across the cell surface and through the cytoskeleton. Science. 1993;260:1124–7. doi: 10.1126/science.7684161. [DOI] [PubMed] [Google Scholar]
- 34.Piekarski K. Transport mechanism operating between blood supply and osteocytes in long bones. Nature. 1977;269:80–2. doi: 10.1038/269080a0. [DOI] [PubMed] [Google Scholar]
- 35.Rodríguez JP, González M, Ríos S, Cambiazo V. Cytoskeletal organization of human mesenchymal stem cells (MSC) changes during their osteogenic differentiation. J Cell Biochem. 2004;93:721–31. doi: 10.1002/jcb.20234. [DOI] [PubMed] [Google Scholar]
- 36.Titushkin I, Cho M. Modulation of cellular mechanics during osteogenic differentiation of human mesenchymal stem cells. Biophys J. 2007;93:3693–702. doi: 10.1529/biophysj.107.107797. [DOI] [PMC free article] [PubMed] [Google Scholar]