Abstract
Introduction
CCN4, also termed WNT-inducible signaling pathway protein-1 (WISP-1), has important roles in inflammation and tissue injury. This study aimed to investigate the effect of CCN4 inhibition using monoclonal anti-CCN4 antibody (CCN4mAb) on the liver injury and fibrosis in a mouse model of liver fibrosis.
Material and methods
The mouse liver fibrosis model was induced by carbon tetrachloride (CCl4). Mice received vehicle (saline/olive oil) by subcutaneous injection, CCl4 by subcutaneous injection or CCl4 (subcutaneous) plus CCN4mAb by subcutaneous injection. The pro-inflammatory and pro-fibrotic factors were determined by Western blot. The biochemistry and histopathology, collagen deposition and nuclear factor (NF)-κB activity were also assessed.
Results
Chronic CCl4 treatment caused liver injury and collagen accumulation. The expression levels of CCN4, pro-inflammatory and pro-fibrotic mediators as well as the activity of NF-κB were markedly increased. Treatment with CCN4mAb significantly inhibited CCl4-induced CCN4 expression, leading to attenuated CCl4-induced liver injury and the inflammatory response. CCN4 blockade also significantly reduced the formation of collagen in the liver and the expression of α-smooth muscle actin and transforming growth factor β1.
Conclusions
CCN4 inhibition by CCN4mAb in vivo significantly attenuated the CCl4-induced liver injury and the progression of liver fibrosis. CCN4 may represent a novel therapeutic target for liver injury and fibrosis.
Keywords: CCN4, inflammation, liver fibrosis, carbon tetrachloride (CCl4)
Introduction
In the liver, the progressive accumulation of connective tissue, termed fibrosis, represents a very frequent event in hepatic disorders [1]. The mortality of patients with liver fibrosis is gradually increasing [2] and long-term liver fibrosis can lead to hepatocellular carcinoma, which is a challenging malignancy worldwide [3]. Although many agents have been used to treat hepatic fibrosis, there is hardly any effective treatment [4]. An effective therapy for treatment of hepatic fibrosis is thus urgently needed.
The CCN family of proteins, including 6 members, shows a high degree of structural similarity [5]. CCN2, a down-stream mediator of transforming growth factor (TGF)-β, is overexpressed in fibrotic disorders found in a variety of tissues and organs and has been shown to display distinct pro-fibrotic function [6]. However, deletion of CCN2 does not suffice to prevent fibrosis of the severely injured kidney [7]. CCN3, another member of the CCN family, is a negative regulator of CCN2 and an inhibitor of renal fibrosis [8]. CCN3 was also implicated in cirrhosis [9]. These findings indicate that fibrogenic induction is complex and regulated by CCN family members functioning together [10].
CCN4, also termed WNT-inducible signaling pathway protein-1 (WISP-1), has been implicated in fibrosis [11, 12]. CCN4 can stimulate cardiomyocyte hypertrophy, fibroblast proliferation, and extracellular matrix (ECM) expression [11]. Further data show that CCN4 mediates pulmonary fibrosis, and neutralizing monoclonal antibodies (mAbs) specific for CCN4 reduced the pulmonary fibrosis in a mouse model [12]. CCN4 is also upregulated in patients with idiopathic pulmonary fibrosis [12]. However, it remains elusive whether CCN4 contributes to hepatic fibrosis. Here, we investigated whether specific blockade of CCN4 could attenuate chronic liver injury and fibrosis in a mouse model induced by carbon tetrachloride (CCl4).
Material and methods
Mouse hepatic fibrosis induction and treatments
All procedures were approved by the Ethics Committee of our hospital. Eight-week-old male C57BL/6 mice were used and maintained under standard conditions. Hepatic fibrosis was induced by subcutaneously injecting a 1 : 1 solution of CCl4 in olive oil (Chemical Agent Company of Shanghai, China). The solution was administered three times a week for 4 weeks. At the end of the 1st week, CCl4-injected mice were divided into two groups: CCl4 injection alone and CCl4 injection plus CCN4mAb by subcutaneous infusion twice a week. The usage of CCN4mAb was based on previous literature [13].
The animals were divided into three groups (n = 12 per group): (1) group 1, normal control with vehicle (saline/olive oil) alone by subcutaneous injection administration; (2) group 2, CCl4 (dissolved in olive oil) alone by subcutaneous injection; (3) group 3, CCl4 by subcutaneous injection + CCN4mAb by subcutaneous injection.
Sample collection and histopathologic analysis
Mice were sacrificed at the end of the 4th week after initial injection (24 h after the last injection). Serum was obtained and stored at –20°C. Liver tissues were fixed in 10% phosphate-buffered formalin and embedded in paraffin. The paraffin blocks were cut into tissue sections (4 µm). Sections were stained with hematoxylin and eosin. The collagen accumulated in the liver sections was stained with 0.1% Picro-Sirius Red (Polysciences Inc.) and quantified by Image Analyzer (Leica Microsystems Ltd.). The percentage area of the total amount of collagen was determined by the sum areas of Sirius Red positive stain divided by the reference field multiplied by 100. The percentage positive area of the central veins was determined by the sum areas of Sirius Red positive stain in the central veins divided by the reference field multiplied by 100. The percentage positive of the perihepatic region was calculated by subtracting the percentage positive of total area and the percentage of central vein.
Determination of serum alanine aminotransferase (ALT) activity
The ALT assay was performed using a commercially available kit according to the manufacturer's protocol (Wako Pure Chemical).
RNA extraction and reverse transcriptase polymerase chain reaction (RT-PCR)
Total RNA was extracted from liver tissue using Trizol (Life Technology). The preparation of the first-strand cDNA was performed using First-Strand Synthesis System (Invitrogen). The CCN4 mRNA was measured by RT-PCR. The primers for CCN4 were: forward: 5’-AGAGCCGCCTCTGCAACTT-3’, reverse: 5’-GGAGAAGCCAA GCCCATCA-3’. GAPDH was amplified as an internal control.
Measurement of TNF-α, IL-6 and CCL2
Sera were collected for the measurement of tumor necrosis factor (TNF)-α, interleukin (IL)-6 and CCL2 by ELISA (R&D Systems) according to the manufacturer's instructions.
Western blot
The liver tissues were lysed at 4°C in RIPA buffer (Qiagen) and extracts clarified at 12,000×g for 25 min. Aliquots (60–90 µg protein) were electrophoretically separated, transferred to nitrocellulose membranes, incubated overnight with specific primary antibodies (anti-TGF-β1, 1 : 2000, anti-α smooth muscle actin (anti-α-SMA) antibody, 1 : 1000; anti-CCN4 antibody, 1 : 500; 1 : 1000; anti-β-tubulin antibody, 1 : 5000; all from Abcam). After washing three times, primary antibodies were detected with secondary antibody (Abcam, 1 : 5000). Immunoreactive proteins were visualized with ECL reagent and quantitated by densitometry. Activity of nuclear factor-κB (NF-κB) was determined by active NF-κBp65 (Abcam, 1 : 2000) using Western blot. Histone H3 was used as a nuclear internal control.
Statistical analysis
Data were expressed as mean ± SD. Statistical comparison between groups was done using the Kruskal-Wallis test. Value of p < 0.05 was considered statistically significant.
Results
Enhanced CCN4 expression in CCl4-induced liver fibrosis
As expected, livers from group 2 exhibited increased mRNA (0.51 ±0.17 vs. 1.51 ±0.36, p < 0.01) and protein levels (0.5 ±0.13 vs. 1.7 ±0.30, p < 0.01) of CCN4, when compared with group 1 (Figure 1).
Figure 1.
CCN4 expression in livers after CCl4 induction. The relative mRNA (A and B) and protein expression (C and D) was expressed as the ratio of band intensity for CCN4 relative to that for the internal control (GAPDH or β-tubulin)
N = 12 mice per group. *P < 0.01, compared with vehicle-treated normal control. Lane 1–2: vehicle (saline/olive oil)-treated normal mice; Lane 3–4: CCl4 by subcutaneous injection.
CCN4mAb reduced CCl4-induced necrosis, inflammatory infiltration and cellular injury
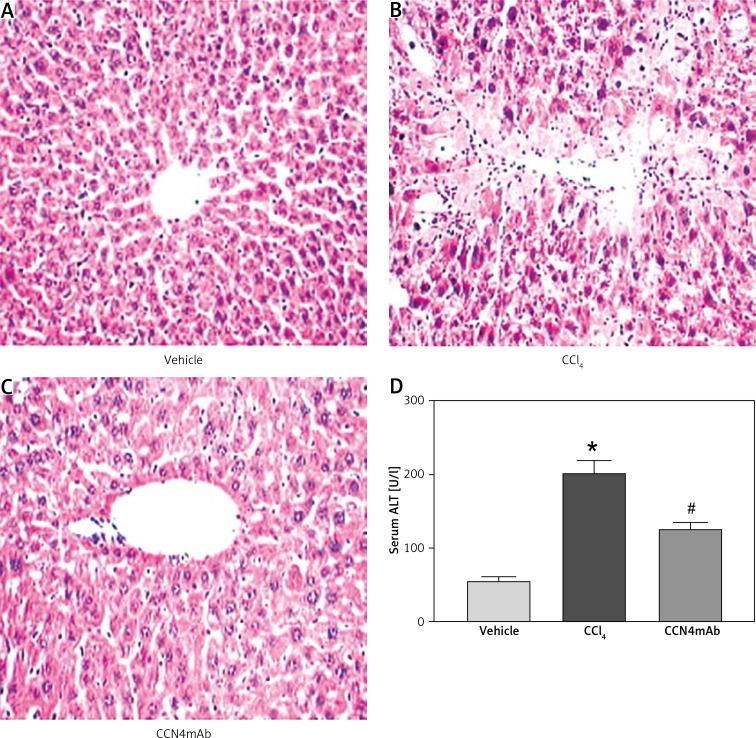
We found that CCl4 treatment induced the formation of necrosis in the liver with large amounts of inflammatory infiltration surrounding the centrilobular veins (Figure 2 B). The CCN4mAb treatment significantly attenuated the necrosis (Figure 2 C). CCl4 also increased serum ALT in comparison with group 1 (56.7 ±11.6 vs. 208.2 ±37.4 vs. 116 ±29.4 U/l, p < 0.01) (Figure 2 D). Group 3 showed significantly reduced serum ALT and histopathological damage compared with the CCl4-treated group (p < 0.01), although the CCN4mAb treatment did not normalize the serum ALT (vehicle vs. CCN4mAb, p < 0.05).
Figure 2.
Representative photographs of HE staining: A – vehicle-treated normal control, B – CCl4-treated and C – CCl4 + CCN4mAb-treated groups. Liver necrotic area was present in CCl4-treated mice with significantly large amounts of inflammatory cell infiltration surrounding the centrilobular veins. CCl4 treatment resulted in obviously increased (D) serum ALT level
*p < 0.01, vs. vehicle-treated normal control; #p < 0.01 vs. CCl4-treated group (n = 10–12/group). Magnification: 200×.
CCN4mAb reduced CCl4-induced collagen accumulation in the liver
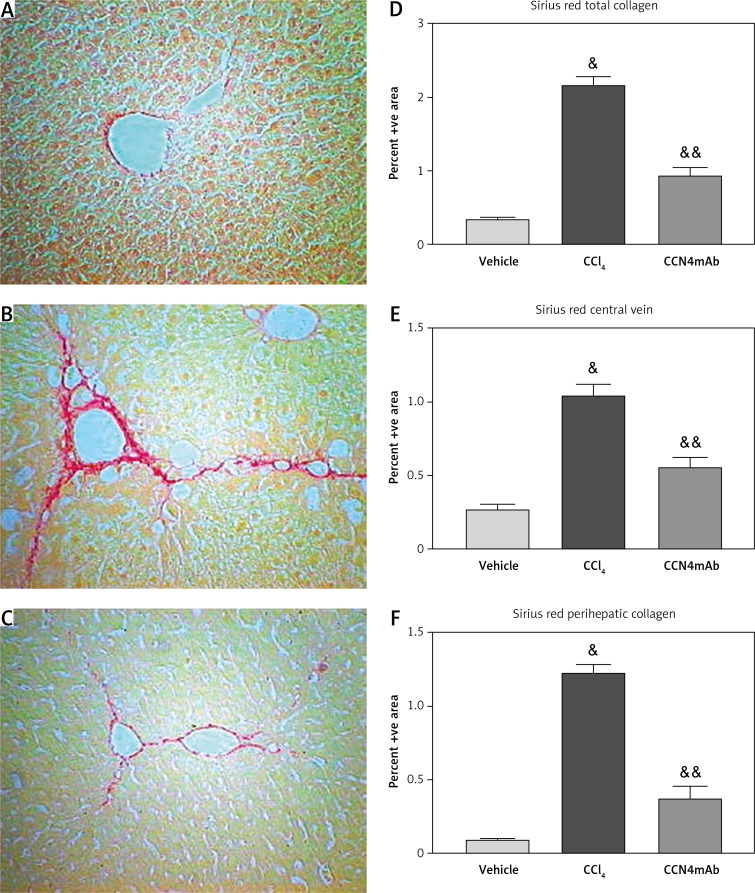
Chronic CCl4 treatment showed a significant increase in collagen accumulation in the liver (0.37 ±0.04 vs. 2.14 ±0.22 vs. 0.91 ±0.24, p < 0.01, Figure 3 D). The deposition of collagen in the pericellular area and along the central vein was profoundly elevated (p < 0.01) (respectively 0.26 ±0.07 vs. 1.08 ±0.18 vs. 0.54 ±0.14, p < 0.01, Figure 3 E and 0.13 ±0.02 vs. 1.26 ±0.15 vs. 0.42 ±0.19, p < 0.01, Figure 3 F). Overall, the total amount of collagen was markedly increased in group 2. Treatment with CCN4mAb resulted in an obvious reduction of collagen accumulation within the liver compared with group 2 (p < 0.01).
Figure 3.
Sirius Red staining on the sections of (A) vehicle-treated normal control, (B) CCl4-treated and (C) CCl4 + CCN4mAb-treated groups. Histograms show the percentage area of Sirius Red staining of collagen of (D) whole liver section, (E) along central vein and (F) pericellular area. CCl4 treatment increased collagen deposition in central vein and pericellular area
&p < 0.01, versus vehicle-treated normal control; &&p < 0.01 compared with CCl4-treated group (n = 10–12/group).
CCN4mAb treatment reduced the production of proinflammatory cytokines
Serum levels of TNF-α (90.8 ±17.4 vs. 241.4 ±18.1 vs. 112 ±13.0 pg/ml, p < 0.01), IL-6 (189 ±20.1 vs. 452.4 ±28.3 vs. 206.6 ±21.0, p < 0.01) and CCL2 (2097 ±149.5 vs. 9812.3 ±896.8 vs. 2301.4 ±202.5, p < 0.01) were significantly elevated by more than 3-fold respectively compared with the vehicle-treated control group) (Figure 4). Of note, group 3 showed markedly reduced serum levels of TNF-α, IL-6 and CCL2 (all p < 0.01), as indicated by ELISA. These data indicate that CCN4mAb treatment also significantly reduced the CCl4-induced inflammatory response.
Figure 4.
CCN4mAb treatment reduced the production of serum TNF-α, IL-6 and CCL2
**P < 0.01, compared with vehicle-treated normal control; ##p < 0.01, compared with CCl4-treated group (n = 10–12/ group). 1: vehicle (saline/olive oil)-treated normal mice; 2: CCl4 by subcutaneous injection. 3: CCl4 plus CCN4mAb treatment. N = 12 mice per group.
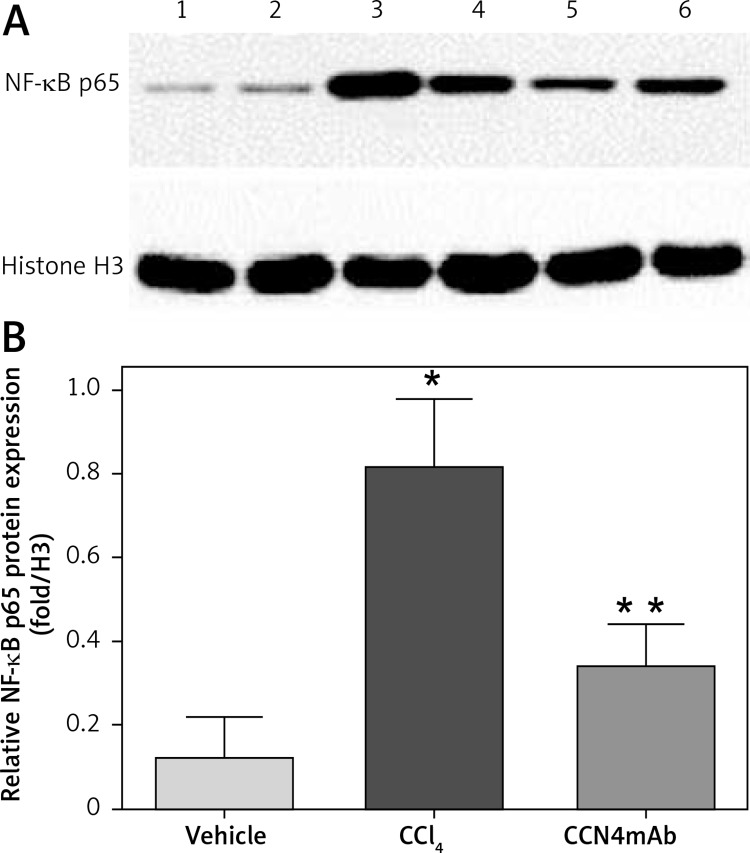
CCN4mAb inhibited activity of NF-κB induced by CCl4
Chronic CCl4 administration increased the activity of NF-κB to 4-fold relative to group 1 (p < 0.01) (0.12 ±0.07 vs. 0.81 ±0.11 vs. 0.37 ±0.08, p < 0.05, Figure 5). The activity level of NF-κB was significantly reduced via treatment with CCN4mAb, in comparison with that in group 2 (p < 0.01).
Figure 5.
NF-κB activity shown by Western blot. A – NF-κB p65 protein expression in vehicle-treated (lanes 1–2), CCl4-treated (lanes 3–4) and CCN4mAb-treated (lanes 5–6) mice. B – Relative NF-κB p65 protein expression relative to internal control histone H3
*p < 0.01 versus vehicle-treated group; **p < 0.01 versus CCl4-treated group, n = 10 per group, bars represent mean ± SD.
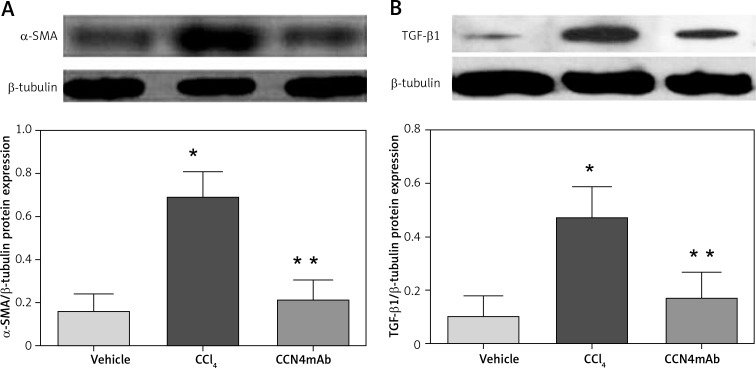
CCN4mAb inhibited protein expression of pro-fibrotic factors
Chronic treatment with CCl4 enhanced the protein expression levels of α-SMA (0.17 ±0.08 vs. 0.68 ±0.12 vs. 0.21 ±0.09, p < 0.01, Figure 6 A) and TGF-β1 (0.09 ±0.05 vs. 0.45 ±0.11 vs. 0.18 ±0.09, p < 0.01, p < 0.01, Figure 6 B). CCN4mAb treatment significantly down-regulated the expression levels of these pro-fibrotic markers (p < 0.01).
Figure 6.
Protein expression levels of pro-fibrotic factors (A) α-SMA and (B) TGF-β1. Chronic CCl4 treatment up-regulated the protein expression of α-SMA and TGF-β1 (*p < 0.001). The protein expression levels were expressed as the ratio of band intensity for target protein relative to that for internal control (β-tubulin), n = 12 mice per group.
*P < 0.01, compared with vehicle-treated normal control; **p < 0.01 compared with CCl4-treated group
Discussion
In this study, we tested the effects of CCN4 inhibition on liver injury and fibrosis. Due to the absence of specific inhibitors of CCN4, we used CCl4mAb in this study. As a result, CCN4mAb administration effectively attenuated the CCl4-induced liver injury and fibrosis.
CCN4 is overexpressed in normal skin fibroblasts and it can stimulate cell proliferation and induces morphological transformation [14]. CCN4 exerts prohypertrophic and mitogenic effects stimulating cardiac fibroblast proliferation, and collagen expression [11]. The pro-fibrogenic role of CCN2 has been widely accepted, and the CCN2/TGF-β1 axis plays an important role in the development and progression of fibrosis in many organ systems, including the liver [15]. In contrast, CCN3/NOV small interfering RNA enhances fibrogenic gene expression in primary hepatic stellate cells and a cirrhotic fat storing cell line [9]. Our results suggest that increased CCN4 activity is present in CCl4-induced liver fibrosis and contributes to fibrogenesis.
Inflammation was closely associated with liver fibrosis [16, 17]. The NF-κB is a nuclear transcriptional activator that plays a central role in the stress response and inflammation. Activation of NF-κB can promote the production of collagen and inflammatory chemokines in the process of liver fibrosis [18]. Accumulating evidence has indicated that monocyte infiltration into the liver is a major pathogenic factor for chronic hepatic inflammation and fibrosis [19]. In the present study, administration of CCN4mAb reduced CCl4-induced expression of TNF-α, IL-6 and CCL2. Also, CCN4mAb reduces the activity of NF-κB. All these data lend support to the pro-inflammatory property of CCN4. We propose that the anti-inflammatory effect of CCN4mAb may in part be regulated by NF-κB, TNF-α, IL-6 and CCL2, which could attenuate the downstream inflammatory process.
The TGF-β1 is a well-established major fibrogenic cytokine mainly produced by α-SMA-positive myofibroblasts [20, 21]. α-SMA may be a valuable marker in the evaluation of fibrosis progression [22]. We found that CCl4-induced increased expression levels of TGF-β1 and α-SMA were reduced by CCN4mAb. Thus, the protective effect of CCN4mAb may be partly due to decreased activation of pro-fibrotic factors. Consistent with our study, CCN3 overexpression prominently repressed type I collagen and α-SMA gene expression and also showed a decrease in CCN4 gene expression [10]. In contrast, CCN3 small interfering RNA enhances fibrogenic gene expression in primary hepatic stellate cells and the cirrhotic fat storing cell line CFSC [9]. These findings indicate that fibrotic gene induction is under the control of a complex network involving different CCN family members.
Our results offer new insights into the potentially important role of CCN4 in hepatic fibrosis. CCN4 blockade via CCN4mAb has protective effects on attenuating CCl4-induced liver injury and fibrosis. The precise mechanism of CCN4 in liver fibrosis remains unclear and it requires further investigations into the role of this potentially important factor in liver fibrosis.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Poli G. Pathogenesis of liver fibrosis: role of oxidative stress. Mol Aspects Med. 2000;21:49–98. doi: 10.1016/s0098-2997(00)00004-2. [DOI] [PubMed] [Google Scholar]
- 2.Brenner DA. Molecular pathogenesis of liver fibrosis. Trans Am Clin Climatol Assoc. 2009;120:361–8. [PMC free article] [PubMed] [Google Scholar]
- 3.Cabibbo G, Maida M, Genco C, Antonucci M, Cammà C. Causes of and prevention strategies for hepatocellular carcinoma. Semin Oncol. 2012;39:374–83. doi: 10.1053/j.seminoncol.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 4.Rosenbloom J, Castro SV, Jimenez SA. Narrative review: fibrotic diseases: cellular and molecular mechanisms and novel therapies. Ann Intern Med. 2010;152:159–66. doi: 10.7326/0003-4819-152-3-201002020-00007. [DOI] [PubMed] [Google Scholar]
- 5.Brigstock DR. The CCN family: a new stimulus package. J Endocrinol. 2003;178:169–75. doi: 10.1677/joe.0.1780169. [DOI] [PubMed] [Google Scholar]
- 6.Leask A, Parapuram SK, Shi-Wen X, Abraham DJ. Connective tissue growth factor (CTGF, CCN2) gene regulation: a potent clinical bio-marker of fibroproliferative disease? J Cell Commun Signal. 2009;3:89–94. doi: 10.1007/s12079-009-0037-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Falke LL, Dendooven A, Leeuwis JW, et al. Hemizygous deletion of CTGF/CCN2 does not suffice to prevent fibrosis of the severely injured kidney. Matrix Biol. 2012;31:421–31. doi: 10.1016/j.matbio.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Riser BL, Najmabadi F, Perbal B, et al. CCN3 (NOV) is a negative regulator of CCN2 (CTGF) and a novel endogenous inhibitor of the fibrotic pathway in an in vitro model of renal disease. Am J Pathol. 2009;174:1725–34. doi: 10.2353/ajpath.2009.080241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borkham-Kamphorst E, van Roeyen CR, Van de Leur E, Floege J, Weiskirchen R. CCN3/NOV small interfering RNA enhances fibrogenic gene expression in primary hepatic stellate cells and cirrhotic fat storing cell line CFSC. J Cell Commun Signal. 2012;6:11–25. doi: 10.1007/s12079-011-0141-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abd El Kader T, Kubota S, Janune D, et al. Anti-fibrotic effect of CCN3 accompanied by altered gene expression profile of the CCN family. J Cell Commun Signal. 2013;7:11–8. doi: 10.1007/s12079-012-0180-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colston JT, de la Rosa SD, Koehler M, et al. Wnt-induced secreted protein-1 is a prohypertrophic and profibrotic growth factor. Am J Physiol Heart Circ Physiol. 2007;293:H1839–46. doi: 10.1152/ajpheart.00428.2007. [DOI] [PubMed] [Google Scholar]
- 12.Königshoff M, Kramer M, Balsara N, et al. WNT1-inducible signaling protein-1 mediates pulmonary fibrosis in mice and is upregulated in humans with idiopathic pulmonary fibrosis. J Clin Invest. 2009;119:772–87. doi: 10.1172/JCI33950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Charrier A, Brigstock DR. Regulation of pancreatic function by connective tissue growth factor (CTGF, CCN2) Cytokine Growth Factor Rev. 2013;24:59–68. doi: 10.1016/j.cytogfr.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Desnoyers L, Arnott D, Pennica D. WISP-1 binds to decorin and biglycan. J Biol Chem. 2001;276:47599–607. doi: 10.1074/jbc.M108339200. [DOI] [PubMed] [Google Scholar]
- 15.Nagaraja T, Chen L, Balasubramanian A, et al. Activation of the connective tissue growth factor (CTGF)-transforming growth factor beta1 (TGF-beta1) axis in hepatitis C virus-expressing hepatocytes. PLoS One. 2012;7:e46526. doi: 10.1371/journal.pone.0046526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zimmermann HW, Seidler S, Nattermann J, et al. Functional contribution of elevated circulating and hepatic non-classical CD14CD16 monocytes to inflammation and human liver fibrosis. PLoS One. 2010;5:e11049. doi: 10.1371/journal.pone.0011049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun W, Pei L. Ozone preconditioning and exposure to ketamine attenuates hepatic inflammation in septic rats. Arch Med Sci. 2012;8:918–23. doi: 10.5114/aoms.2012.29278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang H, Zhao LF, Zhao ZF, et al. Heme oxygenase-1 prevents liver fibrosis in rats by regulating the expression of PPARgamma and NF-kappaB. World J Gastroenterol. 2012;18:1680–8. doi: 10.3748/wjg.v18.i14.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wasmuth HE, Tacke F, Trautwein C. Chemokines in liver inflammation and fibrosis. Semin Liver Dis. 2010;30:215–25. doi: 10.1055/s-0030-1255351. [DOI] [PubMed] [Google Scholar]
- 20.Ueberham E, Löw R, Ueberham U, Schönig K, Bujard H, Gebhardt R. Conditional tetracycline-regulated expression of TGF-beta1 in liver of transgenic mice leads to reversible intermediary fibrosis. Hepatology. 2003;37:1067–78. doi: 10.1053/jhep.2003.50196. [DOI] [PubMed] [Google Scholar]
- 21.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–84. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 22.Akpolat N, Yahsi S, Godekmerdan A, Yalniz M, Demirbag K. The value of alpha-SMA in the evaluation of hepatic fibrosis severity in hepatitis B infection and cirrhosis development: a histopathological and immunohistochemical study. Histopathology. 2005;47:276–80. doi: 10.1111/j.1365-2559.2005.02226.x. [DOI] [PubMed] [Google Scholar]