Abstract
Context:
In X-linked hypophosphatemia (XLH), elevated fibroblast growth factor 23 (FGF23) decreases the renal tubular maximum reabsorption rate of phosphate/glomerular filtration rate (TmP/GFR) and serum inorganic phosphorus (Pi), resulting in rickets and/or osteomalacia.
Objective:
The objective was to test the hypothesis that monthly KRN23 (anti-FGF23 antibody) would safely improve serum Pi in adults with XLH.
Design:
Two sequential open-label phase 1/2 studies were done.
Setting:
Six academic medical centers were used.
Participants:
Twenty-eight adults with XLH participated in a 4-month dose-escalation study (0.05–0.6 mg/kg); 22 entered a 12-month extension study (0.1–1 mg/kg).
Intervention:
KRN23 was injected sc every 28 days.
Main Outcome Measure:
The main outcome measure was the proportion of subjects attaining normal serum Pi and safety.
Results:
At baseline, mean TmP/GFR, serum Pi, and 1,25-dihydroxyvitamin D [1,25(OH)2D] were 1.6 ± 0.4 mg/dL, 1.9 ± 0.3 mg/dL, and 36.6 ± 14.3 pg/mL, respectively. During dose escalation, TmP/GFR, Pi, and 1,25(OH)2D increased, peaking at 7 days for TmP/GFR and Pi and at 3–7 days for 1,25(OH)2D, remaining above (TmP/GFR, Pi) or near [1,25(OH)2D] pre-dose levels at trough. After each of the four escalating doses, peak Pi was between 2.5 and 4.5 mg/dL in 14.8, 37.0, 74.1, and 88.5% of subjects, respectively. During the 12-month extension, peak Pi was in the normal range for 57.9–85.0% of subjects, and ≥25% maintained trough Pi levels within the normal range. Serum Pi did not exceed 4.5 mg/dL in any subject. Although 1,25(OH)2D levels increased transiently, mean serum and urinary calcium remained normal. KRN23 treatment increased biomarkers of skeletal turnover and had a favorable safety profile.
Conclusions:
Monthly KRN23 significantly increased serum Pi, TmP/GFR, and 1,25(OH)2D in all subjects. KRN23 has potential for effectively treating XLH.
Loss-of-function mutations in PHEX cause X-linked hypophosphatemia (XLH), the most common heritable form of rickets. The estimated prevalence of XLH is 1:20 000 (1). PHEX mutations cause increased bone expression of fibroblast growth factor 23 (FGF23) (2, 3). FGF23 reduces renal tubular phosphate reabsorption and serum inorganic phosphorus (Pi) concentration (4–6). FGF23 also alters vitamin D metabolism, resulting in low or normal serum 1,25-dihydroxyvitamin D [1,25(OH)2D] levels, which are inappropriate relative to the hypophosphatemia in XLH (1, 2, 7).
The mineralization rate is markedly decreased, resulting in osteomalacia and rickets. Skeletal deformities develop, including leg bowing and short stature. Adult manifestations include bone pain and insufficiency fractures. Although therapy with calcitriol and phosphate improves skeletal mineralization (8–11), defective renal phosphate reabsorption and 1,25(OH)2D production persist. Treatment effects on skeletal deformity and growth are variable (12). Therapeutic responses are limited by poor compliance because multiple daily doses are required and adverse gastrointestinal effects occur. Serious long-term complications (hypercalciuria, nephrocalcinosis, and hyperparathyroidism) necessitate frequent monitoring and dose titration (12).
KRN23 is a recombinant human IgG1 monoclonal antibody that binds FGF23 and blocks its activity (13). In a phex-deficient mouse, anti-FGF23 antibody increased serum Pi and 1,25(OH)2D levels, improving rickets, osteomalacia, and skeletal growth (14). In a phase 1 double-blind, placebo-controlled study of adults with XLH, a single iv or sc dose of KRN23 increased phosphate reabsorption (renal tubular maximum reabsorption rate of phosphate/glomerular filtration rate [TmP/GFR]), serum Pi, and 1,25(OH)2D (15). The half-life of KRN23 after sc dosing was 13–19 days, and serum Pi remained higher than baseline beyond 4 weeks, supporting dosing every 4th week (15, 16). We therefore conducted a multicenter phase 1/2 open-label, dose-escalation study in adults with XLH to evaluate the safety and efficacy of sc KRN23 administered every 28 days for four doses, followed by a 12-month extension study.
Subjects and Methods
Study oversight
The study was approved by each participating institution's review board and conducted in accordance with the International Conference on Harmonization E6 Good Clinical Practice guidelines and the Declaration of Helsinki. Data were analyzed according to a prespecified analysis plan by the sponsor, in conjunction with the investigators, and monitored by a data safety monitoring committee. All subjects provided written informed consent before screening.
Role of the funding source
The study funder provided KRN23 and contributed to the study design, data collection and analysis. All authors had full access to all data in the study, determined to submit these data, contributed to writing the manuscript, and agreed upon the final paper content.
Subjects
Six sites enrolled subjects. Inclusion criteria included age ≥18 years, clinical diagnosis of XLH, TmP/GFR <2.0 mg/dL, creatinine clearance ≥60 mL/min (Cockcroft-Gault equation), serum calcium <10.8 mg/dL, and serum intact FGF23 >30 pg/mL, because previous publications suggest that such FGF23 values are physiologically inappropriate during hypophosphatemia (17). Exclusion criteria included pregnancy or lactation, major surgery, or receipt of live vaccine or monoclonal antibody products within 3 months before screening. Subjects discontinued vitamin D (or its analogs), calcium or phosphate supplements, or aluminum hydroxide at least 10 days before the screening visit, and thereafter these were not permitted throughout the study. One subject continued cinacalcet due to pre-existing hyperparathyroidism. Ten subjects had received KRN23 in the previous single-dose study (15); all had sufficient washout of KRN23 before entering the multiple-dose study.
Study design and treatment
Subjects were enrolled in a 4-month open-label, phase 1/2 dose-escalation study with the option to continue in a 12-month extension. The open-label design was selected for safe titration of KRN23 dose based on serum Pi. KRN23 was manufactured, labeled, and packaged in single-use vials by Kyowa Hakko Kirin Co, Ltd.
The screening visit occurred within 30 days before initial dosing. Subjects were treated with four sc doses of KRN23 every 28 days using stepwise dose escalation from 0.05 to 0.1 to 0.3 to 0.6 mg/kg. Fasting serum Pi on day 26 post-dosing was used to guide dose changes.
The initial dose of the subsequent extension phase study was administered 53 days (mean; range, 48–65 d) after the last dose of the escalation phase. During the extension, doses ranged from 0.1 to 1 mg/kg. Fasting serum Pi at both peak and day 25 informed dose adjustments to avoid hyperphosphatemia. The final visit of the extension occurred 38 ± 5 days after the final dose. (For visit schedule and dosing algorithms, see Supplemental Data).
Study outcomes
Efficacy
The primary efficacy outcome was the proportion of subjects achieving maximum fasting serum Pi within the normal range (>2.5 to ≤4.5 mg/dL) (21). Pi data are presented in the following ranges: less than normal (≤2.5 mg/dL), lower half of the normal range (>2.5 to ≤3.5 mg/dL), upper half of the normal range (>3.5 to ≤4.5 mg/dL), and above the normal range (>4.5 mg/dL). Secondary outcomes included changes from baseline in calculated TmP/GFR (18), serum Pi, and 1,25(OH)2D.
Safety
Primary safety outcomes included adverse events (AEs), changes in safety laboratory measurements, vital signs, physical findings, electrocardiogram (ECG), renal ultrasound, and cardiac computed tomography (CT) for coronary artery and aortic valve calcium scoring.
Study assessments
Additional measurements included serum 25-hydroxyvitamin D [25(OH)D], creatinine, calcium, and PTH; 2-hour fasting urinary excretion of phosphorus, calcium, and creatinine; and 24-hour urinary calcium, phosphate, and creatinine. Bone turnover markers included serum bone alkaline phosphatase (BALP), procollagen type 1 amino-terminal propeptide, carboxy-terminal telopeptide of type 1 collagen, and osteocalcin. Serum intact FGF23 and anti-KRN23 antibodies were measured as described (15). All other measurements were performed by Quest Diagnostics (Supplemental Data).
Safety assessments included blood chemistry, hematology, urinalysis, physical examination, and ECGs. AEs (graded using the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0) (19) were categorized by relationship to KRN23 treatment. Renal ultrasonography and cardiac CT assessed, respectively, nephrocalcinosis and aortic valve/coronary artery calcification at screening, upon completion of dose escalation, and after completion of the extension study.
Statistical analyses
Descriptive statistics were calculated. Pearson's correlations of baseline FGF23 (log10 transformed) with TmP/GFR, Pi, and 1,25(OH)2D were performed. The proportion of subjects with maximum post-dose Pi levels in each predescribed range was summarized by visit day after each dose. All post-dose values were compared to baseline values (visit 2 before the initial KRN23 dose) using paired t tests. P values remaining <.05 after Bonferroni adjustment for multiple comparisons (ie, P values multiplied by the number of comparisons) were considered significant. Statistical analyses were performed using SAS software (version 9; SAS Institute Inc).
Results
Subjects
KRN23 was administered to 28 subjects during the dose-escalation study; 26 subjects (92.9%) received four doses (Figure 1). Of these, 22 subjects entered the extension study; 19 subjects received all 16 doses. Two subjects withdrew during dose escalation, and three withdrew during the extension. Baseline characteristics and biochemistry data are shown in Table 1. Baseline values for the 22 subjects entering the extension were similar to those for the entire cohort. All subjects reported past use of phosphate and high-dose vitamin D or vitamin D analogs for XLH, mostly in childhood. During the year before enrollment, only 14 subjects received XLH treatment; 13 received phosphate (250–4500 mg elemental phosphorus/d; median, 1000 mg/d), and 14 received vitamin D analogs (0.25–3 μg/d; median, 0.75 μg/d; calcitriol in 13 subjects, paricalcitol in one subject). These 14 had stopped treatment between 11 and 154 days (median, 14) before the screening visit. For the 10 subjects who had participated in the single-dose KRN23 study (15), at least 398 days (or 21 KRN23 elimination half-lives) had elapsed, and KRN23 was not detectable in serum at baseline.
Figure 1.
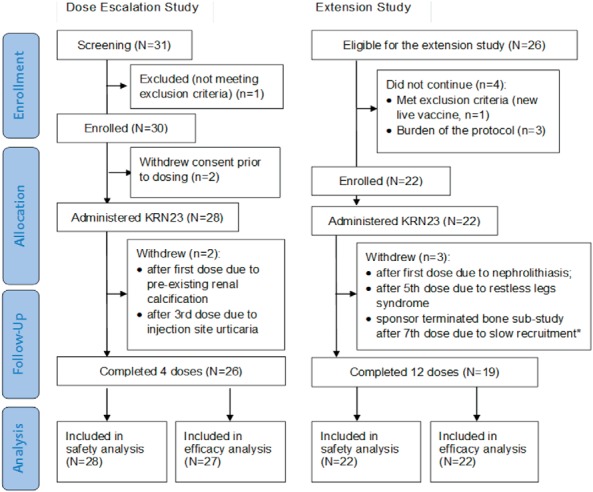
CONSORT diagram for the dose-escalation and extension studies. Twenty-eight adult subjects with XLH entered the 4-month dose-escalation trial, and 22 subjects who had completed the dose-escalation study entered the 12-month extension trial. Two subjects withdrew early during the dose-escalation trial, and three subjects withdrew early during the extension trial. *, One subject in a planned bone substudy (see Supplemental Data).
Table 1.
Subject Demographics and Disease Characteristics at Baseline
| Characteristicsa | KRN23 Dose- Escalation Study | KRN23 Dose-Extension Study | Reference Range (Ref) |
|---|---|---|---|
| Demographics | n = 28 | n = 22 | NA |
| Age, y | 41.9 ± 13.8 (19, 66) | 42.0 ± 14.6 (19, 66) | NA |
| Sex (male, female), n | 9, 19 | 9, 13 | NA |
| Race (Caucasian, African American), n | 27, 1 | 21, 1 | NA |
| Weight, median (range), kg | 70.1 (46.4, 121.9) | 75.3 (51.3, 124.3) | NA |
| Height, cm | 150.3 ± 12.2 (121.9, 170.2) | 150.9 ± 12.7 (121.9, 170.2) | NA |
| Laboratory measurementsb | |||
| Intact FGF23, median (range), pg/mL | 95 (36, 3520) | 85 (36, 3520) | 8–54 (20) |
| Serum Pi, mg/dL | 1.9 ± 0.3 (1.2, 2.8) | 1.9 ± 0.3 (1.2, 2.8) | 2.5–4.5 (21) |
| TmP/GFR, mg/dL | 1.6 ± 0.4 (0.8, 2.3) | 1.6 ± 0.3 (0.9, 2.0) | 2.5–4.2 (22) |
| Serum 1,25(OH)2D, pg/mLc | 36.6 ± 14.3 (10, 62) | 36.4 ± 12.6 (10, 61) | 15.9–55.6 (23) |
| Serum 25(OH)D, ng/mL | 25.0 ± 9.1 (12, 44) | 23.1 ± 8.68 (12, 37) | 32–100 (23) |
| Creatinine clearance, mL/min | 144.3 ± 63.3 (63.2, 349.6) | 152.1 ± 67.3 (70.6, 349.6) | Male, 97–137; female, 88–128 (24) |
| Serum total calcium, mg/dL | 9.1 ± 0.4 (8.5, 10.2) | 9.1 ± 0.4 (8.5, 10.2) | 8.5–10.3 (21) |
| Serum PTH, median (range), pg/mL | 74 (38, 143) | 68.5 (40, 143) | 10–65 (21) |
| BALP, μg/L | 28.3 ± 12.8 (8.2, 52.4) | 31.1 ± 12.3 (13.2, 52.4) | Male, 3.7–20.9; premenopausal female, 2.9–14.5, and postmenopausal female, 3.8–22.6 (21) |
| 24-h urine calcium, median (range), mg/24 h | 67 (11, 253) | 78.5 (11, 253) | Male, 50–300; female, 50–250 (21) |
| 2-h calcium/creatinine ratio (mg/g creatinine), median (range) | 36 (7, 192) | 41 (7, 192) | Male, 10–240; female, 10–320 (21) |
| 24-h urine phosphate (mg), median (range) | 2444 (1087, 4785) | 2432 (1087, 4785) | Male, 1103–4903; female, 521–3677 (21) |
Abbreviation: NA, not applicable.
This table provides baseline values on all 28 subjects that entered the dose-escalation trial as well as the 22 subjects that later went on to enter the 12-month extension trial. Data are presented from screening or baseline from the dose-escalation study, and are expressed as mean ± SD (range), except for the skewed data, where median (range) is noted.
Conversion factors: to convert the values for phosphorus and TmP/GFR (mg/dL) to millimoles per liter, multiply by 0.32. To convert calcium (mg/dL) to millimoles per liter, multiply by 0.25. To convert 1,25(OH)2D (pg/mL) to picomoles per liter, multiply by 2.4. To convert 25(OH)D (ng/mL) to nanomoles per liter, multiply by 2.496. To convert PTH (pg/mL) to picomoles per liter, multiply by 0.1061. To convert creatinine (g/24 h) to millimoles per 24 hours, multiply by 8.84. To convert urine calcium/creatinine ratio to millimoles per mole, multiply by 2.828.
n = 24 for serum 1,25(OH)2D.
Baseline biochemistry
At the screening visit, all subjects had serum Pi ≤2.5 mg/dL. However, at visit 2, which was counted as the baseline for analysis, one subject had increased to Pi 2.8 mg/dL. Mean ± SD baseline serum Pi (1.9 ± 0.3 mg/dL) and TmP/GFR (1.6 ± 0.4 mg/dL) were below the normal range. Mean serum 1,25(OH)2D (36.6 ± 14.3 pg/mL) was normal, and median serum FGF23 was elevated (95 pg/mL, distribution skewed) (Table 1). Baseline serum FGF23 correlated inversely with baseline serum Pi, TmP/GFR, and 1,25(OH)2D (r = −0.329, −0.469, −0.530, respectively), excluding one subject with extremely high serum FGF23 (3520 pg/mL).
KRN23 dose
Doses were increased during the first four doses and titrated further during the extension based on serum Pi levels. After the eighth dose (dose 4 of the extension), dose levels were relatively stable; over 80% of subjects received 0.6 or 1 mg/kg KRN23. Four subjects (18.2%) required dose reductions based on serum Pi during the extension, although some subsequently resumed the larger dose. Summary dose information is available in Supplemental Table 1.
Primary outcome
Serum Pi
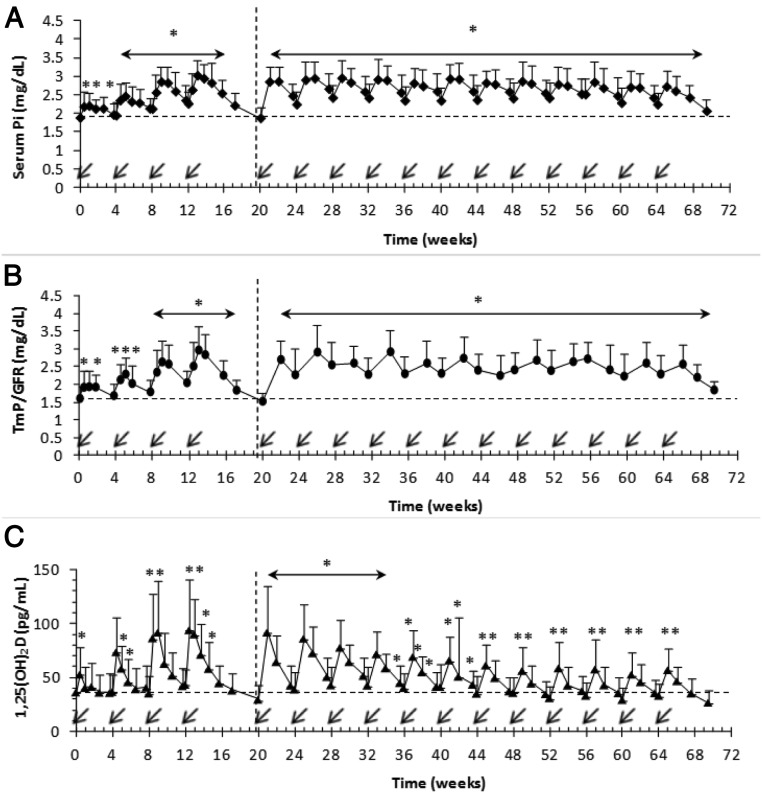
During the dose-escalation study, after each dose of KRN23, mean serum Pi peaked by day 7, decreasing to a trough on day 28 (Figure 2A). The proportion of subjects with serum Pi between >2.5 and ≤4.5 mg/dL was 3.7% at baseline, increasing to 14.8, 37.0, 74.1, and 88.5% on day 7 after each of the four successive doses (Table 2).
Figure 2.
Effect of KRN23 on serum Pi (A), TmP/GFR (B), and 1,25(OH)2D (C) in adults with XLH. KRN23 administration (arrow) occurred every 28 days. Data are presented as mean ± SD. *, P values remaining <.05 after Bonferroni adjustment for multiple comparisons were considered significantly different from the baseline value. An asterisk over a horizontal line with arrowheads indicates that all values shown under that line were different from baseline (P < .05). The horizontal broken lines indicate baseline level. The vertical broken lines demarcate the 4-month dose-escalation study from the 12-month extension study; n = 28 for the dose-escalation study; n = 22 for the extension study.
Table 2.
Proportion of Subjects With Serum Pi in the Normal Range on Day 7 Post-Dose: Efficacy Population
| Dose/Day Relative to Each Dose | n | Serum Pi, mg/dL |
||||
|---|---|---|---|---|---|---|
| ≤2.5 | >2.5 to ≤3.5 | >3.5 to ≤4.5 | >4.5 | >2.5 to ≤4.5 (normal range) | ||
| Dose-escalation study (n = 27)a | ||||||
| Day 0 (Pre-KRN23) | 27 | 26 (96.3) | 1 (3.7) | 0 (0) | 0 (0) | 1 (3.7) |
| Dose 1/d 7 | 27 | 23 (85.2) | 4 (14.8) | 0 (0) | 0 (0) | 4 (14.8) |
| Dose 2/d 7 | 27 | 17 (63.0) | 10 (37.0) | 0 (0) | 0 (0) | 10 (37.0) |
| Dose 3/d 7 | 27 | 7 (25.9) | 20 (74.1) | 0 (0) | 0 (0) | 20 (74.1) |
| Dose 4/d 7 | 26 | 3 (11.5) | 19 (73.1) | 4 (15.4) | 0 (0) | 23 (88.5) |
| Dose 4/d 36 (end of dose escalation) | 26 | 22 (84.6) | 4 (15.4) | 0 (0) | 0 (0) | 4 (15.4) |
| Extension study (n = 22) | ||||||
| Day 0 (day 137 from baseline)b | 21c | 21 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0) | 0 (0.0) |
| Dose 5/d 7 | 22 | 4 (18.2) | 17 (77.3) | 1 (4.5) | 0 (0) | 18 (81.8) |
| Dose 6/d 7 | 21 | 6 (28.6) | 13 (61.9) | 2 (9.5) | 0 (0) | 15 (71.4) |
| Dose 7/d 7 | 21 | 5 (23.8) | 15 (71.4) | 1 (4.8) | 0 (0) | 16 (76.2) |
| Dose 8/d 7 | 21 | 7 (33.3) | 11 (52.4) | 3 (14.3) | 0 (0) | 14 (66.7) |
| Dose 9/d 7 | 20 | 5 (25.0) | 15 (75.0) | 0 (0.0) | 0 (0) | 15 (75.0) |
| Dose 10/d 7 | 20 | 3 (15.0) | 15 (75.0) | 2 (10.0) | 0 (0) | 17 (85.0) |
| Dose 11/d 7 | 20 | 4 (20.0) | 16 (80.0) | 0 (0.0) | 0 (0) | 16 (80.0) |
| Dose 12/d 7 | 18 | 7 (38.9) | 8 (44.4) | 3 (16.7) | 0 (0) | 11 (61.1) |
| Dose 13/d 7 | 18 | 5 (27.8) | 12 (66.7) | 1 (5.6) | 0 (0) | 13 (72.2) |
| Dose 14/d 7 | 19 | 7 (36.8) | 10 (52.6) | 2 (10.5) | 0 (0) | 12 (63.2) |
| Dose 15/d 7 | 19 | 5 (26.3) | 13 (68.4) | 1 (5.3) | 0 (0) | 14 (73.7) |
| Dose 16/d 7 | 19 | 8 (42.1) | 9 (47.4) | 2 (10.5) | 0 (0) | 11 (57.9) |
| Dose 16/d 38 (end of extension study) | 19 | 18 (81.8) | 1 (4.5) | 0 (0) | 0 (0) | 0 (0) |
Data are expressed as number (percentage) of subjects treated with KRN23.
Twenty-eight subjects were enrolled, but one withdrew before completing one dosing cycle, so only 27 could be evaluated for efficacy.
Mean interval from the last visit (d 120) in the dose-escalation study to the first day in the extension study was 17 days.
One subject was missing serum Pi value on day 0 of the extension study, so n = 21 at this time point.
Mean ± SD peak (d 7) and trough (d 28) serum Pi levels increased after each successive dose, with the peak level reaching 3.0 ± 0.4 mg/dL after the fourth dose. All post-dose serum Pi values from doses 2 to 16, including troughs, were greater than baseline (P < .05) (Figure 2A), except for the visits 5–6 weeks after the last dose of the studies.
Throughout the extension, serum Pi increased in all subjects, with most attaining a normal peak serum Pi (>2.5 to ≤ 4.5 mg/dL), ranging from 57.9–85.0% of subjects for each dosing cycle. In addition, 25–42.9% of subjects maintained a normal serum Pi even at the trough levels during the extension study. Serum Pi never exceeded 4.5 mg/dL in any subject (Table 2). Fluctuations of serum Pi during each dosing interval were small. During the extension, mean ± SD peak serum Pi ranged from 2.6 ± 0.4 mg/dL to 3.0 ± 0.5 mg/dL, and mean trough values ranged from 2.3 ± 0.4 mg/dL to 2.5 ± 0.4 mg/dL, compared to serum Pi of 1.9 ± 0.3 mg/dL at baseline.
Secondary outcomes
TmP/GFR
TmP/GFR increased in an identical pattern to serum Pi, peaking at day 7 or 14 (Figure 2B) after each KRN23 dose. Mean ± SD peak TmP/GFR increased to 3.0 ± 0.7 mg/dL after the fourth dose. TmP/GFR was greater than baseline (P < .05) for most samples including troughs, except for the visits at the end of the dose-escalation and extension studies (which were more than 4 wk after the previous doses; Figure 2B). The peak TmP/GFR ranged from 2.2 ± 0.4 to 2.9 ± 0.6 mg/dL for doses 5–16.
Serum 1,25(OH)2D
Mean serum 1,25(OH)2D concentration peaked 3–7 days after dosing during dose escalation and 7 days after dosing during the extension, returning to near baseline values before the next dose (Figure 2C). Peak 1,25(OH)2D was greater than baseline for all doses (P < .01); however, the peak value gradually decreased during later cycles of the extension.
Bone markers
Serum procollagen type 1 amino-terminal propeptide was significantly increased above baseline after each dose (P < .01) during escalation and remained elevated during the extension. Serum osteocalcin increased significantly after dose 4 (P < .01) and remained elevated throughout the extension. BALP was significantly increased by the beginning of the extension period (P < .01) and declined by the last study visit. Carboxy-terminal telopeptide of type 1 collagen also increased transiently during the extension study (Supplemental Figure 1).
Safety
Biochemical parameters
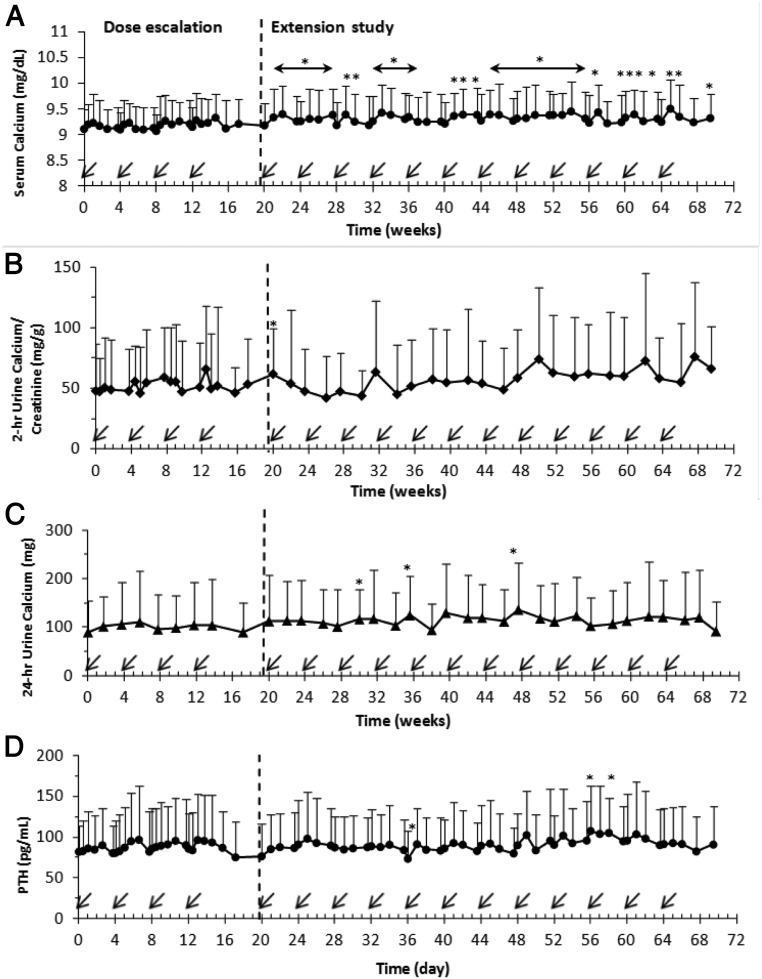
Mean serum calcium increased minimally above baseline at multiple time points (P < .05) but remained within the normal range throughout the study (Figure 3A). There were no consistent changes in the mean 2-hour urine calcium/creatinine ratio (Figure 3B) or 24-hour urinary calcium excretion (Figure 3C), although mild increases in mean 24-hour urine calcium (of <44 mg/d) occurred only three times during the extension. Transient hypercalcemia (calcium > 10.5 mg/dL) occurred in two subjects (range, 10.8 to 11.1 mg/dL), and hypercalciuria occurred in five subjects (persistent in one subject, only once in three subjects). The subjects with transient hypercalcemia had baseline mild hyperparathyroidism (PTH, 141 and 138 pg/mL with simultaneous serum calcium of 10.2 and 10.0 mg/dL, respectively). PTH (Figure 3D) was relatively stable, with few minor fluctuations throughout the study. No consistent changes in serum creatinine or 25(OH)D (Supplemental Figure 2, A and B) occurred. There was considerable interindividual variability in 24-hour urinary phosphate (Supplemental Figure 2C) and calcium excretion throughout the study (Figure 3, B and C).
Figure 3.
Effect of multiple-dose administration of KRN23 in adults with XLH on serum calcium (A), 2-hour urine calcium/creatinine ratio (B), 24-hour urinary calcium excretion (C), and PTH (D). Arrows indicate day for KRN23 administration (every 28 days). Data are presented as mean ± SD. *, P values remaining <.05 after Bonferroni adjustment for multiple comparisons were considered significantly different from the baseline value. An asterisk over a horizontal line with arrowheads indicates that all values shown under that line were different from baseline (P < .05). The vertical broken lines demarcate the 4-month dose-escalation study from the 12-month extension study; n = 28 for the dose-escalation study; n = 22 for the extension study.
Adverse events
AEs were reported in 27 (96%) of the 28 subjects during the studies, and 18 subjects (64%) had AEs considered drug-related by investigators. Common AEs included arthralgia (n = 12); nasopharyngitis (n = 11); back pain (n = 10); extremity pain (n = 7); diarrhea, sinusitis, upper respiratory infection, dizziness, and headache (n = 6 each); and injection site reaction and restless leg syndrome (n = 5 each). The most common drug-related AEs were diarrhea and arthralgia (n = 2 each) and injection site reactions (n = 5). Six grade 3 AEs occurred once each among five subjects: myalgia, extremity pain, cervical spine stenosis, breast cancer, hypertensive crisis (all unrelated to study drug), and restless leg syndrome (related to study drug). Two subjects discontinued due to drug-related AEs: one with moderate urticarial injection site reaction, and one with severe restless leg syndrome. There were no deaths.
Other safety monitoring
There were no significant or consistent changes in vital signs, ECGs, physical examination findings, or for laboratory safety parameters. Anti-KRN23 antibodies were not detected in any subject.
Cardiac CT scans were conducted for 28 subjects at baseline, 11 subjects upon completing dose escalation, 15 subjects after the sixth dose, and 19 subjects upon completing the extension study. Only three subjects had minor baseline coronary artery or aortic valve calcification (Agatston scores, 1, 6, and 9). One subject had an increase from 0 to 3 in coronary artery Agatston score, upon completion of dose escalation. During the extension study, one subject had increases in coronary artery and aortic valve calcification (Agatston scores increasing from 6 to 47 and from 0 to 5, respectively). Among these two subjects, one had similar biochemical values to other subjects, whereas the other did have parathyroid autonomy at baseline, with persistent hyperparathyroidism during the study and intermittent mild hypercalcemia. Twenty-four-hour urine calcium was not elevated in either subject, and the serum 1,25(OH)2D and Pi concentrations during the study were similar to other subjects.
Renal ultrasonograms were performed in 28 subjects at screening and upon completion of dose escalation, as well as in 22 at the end of the extension study or at early withdrawal. Ten subjects had baseline nephrocalcinosis (n = 7) and/or nephrolithiasis (n = 4); one had both. There was no evidence of progressive nephrocalcinosis during the dose-escalation or extension studies. One subject with baseline nephrolithiasis withdrew after dose 5 due to nephrolithiasis symptoms.
Discussion
Subcutaneous administration of KRN23 every 4 weeks for 16 months resulted in increased TmP/GFR, serum Pi, and 1,25(OH)2D from baseline in all subjects, consistent with inhibition of FGF23 activity. Serum Pi and TmP/GFR increased after each dose, peaked near day 7, and then declined, remaining above pre-dose levels before subsequent dosing. Serum Pi never exceeded the normal range in any subject. Effects on TmP/GFR, serum Pi, and 1,25(OH)2D were related to the area under the serum KRN23 concentration-time curve (25, 26). Throughout the extension study, the serum Pi response was similar among subjects and suggested that KRN23 can sustain stable serum Pi levels within a safe and therapeutic range throughout this dosing interval.
Changes in TmP/GFR followed a similar temporal pattern after KRN23 administration, providing evidence for a KRN23 effect upon renal phosphate reabsorption. Although dietary phosphate content chronically leads to regulation of TmP/GFR (1), to limit the effect of the food, subjects were not permitted to change current diet and activity/exercise regimen from their baseline during the study. For all assessments of TmP/GFR, samples were collected in a fasted state; however, food intake would influence the 24-hour urine phosphate. Mean 24-hour urine phosphate excretion did not change despite the increase in TmP/GFR, possibly due to the wide variance of this measure, chronic high dietary phosphorus intake, and/or increased intestinal absorption due to the increased 1,25(OH)2D level.
The combined changes in TmP/GFR, serum Pi, and 1,25(OH)2D are predicted to have beneficial effects on osteomalacia in subjects with XLH. The changes in the bone formation markers during KRN23 treatment suggest potential improvement in mineralization. Increases in osteocalcin occur in XLH subjects after 1,25(OH)2D therapy (27), and repeated injection of anti-FGF-23 antibodies enhanced mineralization of osteoid in adult hyp mice (14). Further studies are necessary to determine whether KRN23 definitively alters skeletal outcomes.
KRN23 had a favorable safety profile. Most AEs were mild or unrelated to study drug. Mean serum calcium remained normal; however, two subjects had mild intermittent hypercalcemia (which can also occur with calcitriol therapy). Mean urinary calcium excretion remained within the normal range, although one subject had persistently elevated urinary calcium excretion, and four subjects had solitary (n = 3) or intermittent (n = 1) hypercalciuria. Long-term studies will be necessary to determine whether increased skeletal mineralization accounts for stable serum and urinary calcium.
Patients with XLH develop hyperparathyroidism in response to long-term treatment with phosphate. The median baseline PTH level of this cohort was increased, and hyperparathyroidism was present in 18 of 28 subjects. Mean PTH did not decrease in response to elevations in 1,25(OH)2D, perhaps due to the transient nature of 1,25(OH)2D changes, or possibly indicative of underlying parathyroid autonomy.
Subjects with XLH may develop nephrocalcinosis (12) during treatment with oral phosphate and calcitriol. In the present study, there was no evidence of worsening nephrocalcinosis being found in any subject. Rarely, patients have developed cardiac calcifications with phosphate and calcitriol regimens; two subjects in our study had small increases in coronary artery or aortic valve calcification score, consistent with minimal-mild calcification (28, 29) and not at values commonly associated with significant risk for cardiac disease (15). One of these two patients had evidence of baseline parathyroid autonomy and intermittent hypercalcemia. Although the data are generally reassuring, monitoring for calcifications will continue to be part of the safety assessments of future trials.
The current multidose KRN23 studies advance the translation of earlier murine studies (14) to treatment of human subjects with XLH. With the current cumbersome regimen of multiple daily oral doses of calcitriol and phosphate, serum Pi fluctuates considerably through the day (8). In contrast, KRN23 administered every 4 weeks produced sustained increases in serum Pi throughout the dosing interval. Although serum Pi was not normal in all subjects through the entire dosing cycle, 25–42.9% of subjects sustained normal serum Pi throughout an entire dosing cycle, a more physiological pattern as compared to the multiple daily fluctuations seen with current standard therapy. Modifications of the dosing algorithm may allow for sustained normophosphatemia in a greater number of subjects, potentially enhancing skeletal mineralization and improving muscle weakness. In this regard, KRN23 significantly improved patient self-perception of physical functioning using instruments for health-related quality of life (30).
Our studies indicate sustained KRN23 dose-dependent improvement in TmP/GFR, serum Pi, and 1,25(OH)2D in adults with XLH. Thus, KRN23 has the potential to improve biochemical and skeletal outcomes in adults and children with XLH, with greater convenience and compliance than multiple daily doses of calcitriol and phosphate.
Acknowledgments
We are particularly grateful to the dedicated subjects who participated in the study. We thank clinical study coordinators and subinvestigators, Elizabeth Olear and Rebecca Sullivan at Yale University School of Medicine, New Haven, Connecticut; Connie Sullivan and Marian Hart at Indiana University, Indianapolis, Indiana; Margaret Stewart at Duke University Medical Center, Durham, North Carolina; Nathaniel Jacob Harrison and Monika Ruscheinsky at the University of Texas Health Science Center at Houston, Houston, Texas; Michaela Durigova at Shriners Hospital for Children, Montreal, Canada; and Dr Farzana Perwad, Stephanie Lemp, and Vinodhini Lakshman at University of California, San Francisco, California. We thank Val Barra and Yamamoto Katsuhiko (Kyowa Hakko Kirin California Inc, La Jolla, California) for analyses of KRN23 and anti-KRN23 antibody. We also thank Shirley McKernan (Kyowa Hakko Kirin Pharma Inc) who contributed to the editing and formatting.
The study was funded by Kyowa Hakko Kirin Pharma Inc.
Trial Registration: ClinicalTrials.gov NCT01340482 for KRN23 INT-001 and NCT01571596 for KRN23-INT-002.
Author Contributions: E.A.I., M.D.R., T.J.W., M.A.K., M.P., and T.O.C. participated in the design of the study. E.A.I., X.Z., M.D.R., T.J.W., M.A.K., M.V., F.H.G., A.A.P., K.I., M.P., and T.O.C. participated in patient accrual, study monitoring, and data collection. All authors analyzed and interpreted the data. T.I. was the study biostatistician responsible for the statistical analyses. All authors were members of the writing group and participated in development of the report, agreed on the content, reviewed drafts, and approved the final version.
Disclosure Summary: T.O.C., M.D.R., E.A.I., T.J.W., K.I., A.A.P., F.H.G., and M.P. had received research grants during study conduct from Kyowa Hakko Kirin Pharma Inc. X.Z., M.A.K., T.I., M.V., and J.S.H. are employed by Kyowa Hakko Kirin Pharma Inc. Additionally, E.A.I., T.O.C., M.P., F.H.G., T.J.W., A.A.P., and K.I. have received research grants and/or consulting fees (other than Advisory Board or Board of Directors) from Ultragenyx Pharmaceuticals Inc for subsequent work related to KRN23.
Footnotes
- AE
- adverse event
- BALP
- bone alkaline phosphatase
- CT
- computed tomography
- ECG
- electrocardiogram
- FGF23
- fibroblast growth factor 23
- 1,25(OH)2D
- 1,25-dihydroxyvitamin D
- 25(OH)D
- 25-hydroxyvitamin D
- Pi
- inorganic phosphorus
- TmP/GFR
- renal tubular maximum reabsorption rate of phosphate/glomerular filtration rate
- XLH
- X-linked hypophosphatemia.
References
- 1. Burnett CH, Dent CE, Harper C, Warland BJ. Vitamin D-resistant rickets. Analysis of twenty-four pedigrees with hereditary and sporadic cases. Am J Med. 1964;36:222–232. [DOI] [PubMed] [Google Scholar]
- 2. Imel EA, Econs MJ. Fibroblast growth factor 23: roles in health and disease. J Am Soc Nephrol. 2005;16:2565–2575. [DOI] [PubMed] [Google Scholar]
- 3. Liu S, Quarles LD. How fibroblast growth factor 23 works. J Am Soc Nephrol. 2007;18(6):1637–1647. [DOI] [PubMed] [Google Scholar]
- 4. Larsson T, Marsell R, Schipani E, et al. Transgenic mice expressing fibroblast growth factor 23 under the control of the α1(I) collagen promoter exhibit growth retardation, osteomalacia, and disturbed phosphate homeostasis. Endocrinology. 2004;145:3087–3094. [DOI] [PubMed] [Google Scholar]
- 5. Shimada T, Urakawa I, Yamazaki Y, et al. FGF-23 transgenic mice demonstrate hypophosphatemic rickets with reduced expression of sodium phosphate cotransporter type IIa. Biochem Biophys Res Commun. 2004;314:409–414. [DOI] [PubMed] [Google Scholar]
- 6. Gattineni J, Bates C, Twombley K, et al. FGF23 decreases renal NaPi-2a and NaPi-2c expression and induces hypophosphatemia in vivo predominantly via FGF receptor 1. Am J Physiol Renal Physiol. 2009;297:F282–F291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Haussler M, Hughes M, Baylink D, Littledike ET, Cork D, Pitt M. Influence of phosphate depletion on the biosynthesis and circulating level of 1α,25-dihydroxyvitamin D. Adv Exp Med Biol. 1977;81:233–250. [DOI] [PubMed] [Google Scholar]
- 8. Glorieux FH, Marie PJ, Pettifor JM, Delvin EE. Bone response to phosphate salts, ergocalciferol, and calcitriol in hypophosphatemic vitamin D-resistant rickets. N Engl J Med. 1980;303:1023–1031. [DOI] [PubMed] [Google Scholar]
- 9. Harrell RM, Lyles KW, Harrelson JM, Friedman NE, Drezner MK. Healing of bone disease in X-linked hypophosphatemic rickets/osteomalacia. Induction and maintenance with phosphorus and calcitriol. J Clin Invest. 1985;75:1858–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Petersen DJ, Boniface AM, Schranck FW, Rupich RC, Whyte MP. X-linked hypophosphatemic rickets: a study (with literature review) of linear growth response to calcitriol and phosphate therapy. J Bone Miner Res. 1992;7:583–597. [DOI] [PubMed] [Google Scholar]
- 11. Costa T, Marie PJ, Scriver CR, et al. X-linked hypophosphatemia: effect of calcitriol on renal handling of phosphate, serum phosphate, and bone mineralization. J Clin Endocrinol Metab. 1981;52:463–472. [DOI] [PubMed] [Google Scholar]
- 12. Carpenter TO, Imel EA, Holm IA, Jan de Beur SM, Insogna KL. A clinician's guide to X-linked hypophosphatemia. J Bone Miner Res. 2011;26(7):1381–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yamazaki Y, Tamada T, Kasai N, et al. Anti-FGF23 neutralizing antibodies show the physiological role and structural features of FGF23. J Bone Miner Res. 2008;23(9):1509–1518. [DOI] [PubMed] [Google Scholar]
- 14. Aono Y, Yamazaki Y, Yasutake J, et al. Therapeutic effects of anti-FGF23 antibodies in hypophosphatemic rickets/osteomalacia. J Bone Miner Res. 2009;24:1879–1888. [DOI] [PubMed] [Google Scholar]
- 15. Carpenter TO, Imel EA, Ruppe MD, et al. Randomized trial of the anti-FGF23 antibody KRN23 in X-linked hypophosphatemia. J Clin Invest. 2014;124:1587–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang X, Carpenter T, Imel E, et al. Pharmacokinetics and pharmacodynamics of a human monoclonal anti-FGF23 antibody (KRN23) after single-dose administration to patients with X-linked hypophosphatemia. J Bone Miner Res. 2013;28(suppl 1)SU0169. [Google Scholar]
- 17. Endo I, Fukumoto S, Ozono K, et al. Clinical usefulness of measurement of fibroblast growth factor 23 (FGF23) in hypophosphatemic patients: proposal of diagnostic criteria using FGF23 measurement. Bone. 2008;42(6):1235–1239. [DOI] [PubMed] [Google Scholar]
- 18. Payne RB. Renal tubular reabsorption of phosphate (TmP/GFR): indications and interpretation. Ann Clin Biochem. 1998;35:201–206. [DOI] [PubMed] [Google Scholar]
- 19. National Cancer Institute. Common Terminology Criteria for Adverse Events version 4.03 (CTCAE). http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010–06–14_QuickReference_5x7.pdf. Published June 14, 2010.
- 20. Yamazaki Y, Okazaki R, Shibata M, et al. Increased circulatory level of biologically active full-length FGF-23 in patients with hypophosphatemic rickets/osteomalacia. J Clin Endocrinol Metab. 2002;87(11):4957–4960. [DOI] [PubMed] [Google Scholar]
- 21. Quest Investigator Manual for the Kyowa Hakko Kirin Pharma, Inc. Trial: A Phase I/II, Open-Label, Repeat-Dose, Dose-Escalation Study of KRN23 in Adult Subjects With X-Linked Hypophosphatemia. Revision 1.4, Valencia, CA: Quest Diagnostics; August 16, 2011. [Google Scholar]
- 22. Walton RJ, Bijvoet OL. Nomogram for derivation of renal threshold phosphate concentration. Lancet. 1975;2(7929):309–310. [DOI] [PubMed] [Google Scholar]
- 23. Investigator Laboratory Instruction Manual for Kirin Pharma. Esoterix Clinical Trials Services. Cranford, NJ: Esoterix; October 28, 2008. [Google Scholar]
- 24. National Institutes of Health. Creatinine clearance test. http://www.nlm.nih.gov/medlineplus/ency/article/003611.htm Accessed October 21, 2014.
- 25. Zhang X, Imel EA, Ruppe MD, et al. Pharmacokinetics (PK) and pharmacodynamics (PD) following four monthly doses of a human monoclonal anti-FGF23 (Fibroblast Growth Factor 23) antibody (KRN23) in adults with X-linked hypophosphatemia (XLH). In: Proceedings from The Endocrine Society's 96th Annual Meeting and Expo; June 21–24, 2014; Chicago, IL Abstract MON-0206. [Google Scholar]
- 26. Zhang X, Imel EA, Ruppe MD, et al. Pharmacokinetics (PK) and pharmacodynamics (PD) of a human anti-FGF23 antibody (KRN23) in a long-term extension study of adults with X-linked hypophosphatemia (XLH). J Bone Miner Res. 2014;29(suppl 1):S483. [Google Scholar]
- 27. Gundberg CM, Cole DE, Lian JB, Reade TM, Gallop PM. Serum osteocalcin in the treatment of inherited rickets with 1,25-dihydroxyvitamin D3. J Clin Endocrinol Metab. 1983;56:1063–1067. [DOI] [PubMed] [Google Scholar]
- 28. Halliburton SS, Stillman AE, White RD. Noninvasive quantification of coronary artery calcification: methods and prognostic value. Cleve Clin J Med. 2002;69(suppl 3):S6–S11. [DOI] [PubMed] [Google Scholar]
- 29. Moltz KC, Friedman AH, Nehgme RA, Kleinman CS, Carpenter TO. Ectopic cardiac calcification associated with hyperparathyroidism in a boy with hypophosphatemic rickets. Curr Opin Pediatr. 2001;13(4):373–375. [DOI] [PubMed] [Google Scholar]
- 30. Ruppe MD, Zhang X, Imel EA, et al. Effect of four monthly doses of a human monoclonal anti-FGF23 (Fibroblast Growth Factor 23) antibody (KRN23) on quality of life in X-linked hypophosphatemia (XLH). In: Proceedings from The Endocrine Society's 96th Annual Meeting and Expo; June 21–24, 2014; Chicago, IL Abstract MON-2010. [Google Scholar]