Abstract
Bacillus thuringiensis serovar israelensis is a wide-spread soil bacterium affiliated with the B. cereus group (Bcg) and is widely used in biocontrol products applied against mosquito and black fly larvae. For monitoring and quantification of applied B. thuringiensis serovar israelensis and its effect on indigenous B. thuringiensis serovar israelensis and Bcg assemblages, efficient and reliable tools are essential. The abundance and properties of B. thuringiensis serovar israelensis strains in the environment traditionally have been investigated with cultivation-dependent techniques, which are hampered by low sensitivity and the morphological similarity between B. cereus and B. thuringiensis. Currently available PCR-based detection and quantification tools target markers located on plasmids. In this study, a new cultivation-independent PCR-based method for efficient and specific quantification of B. thuringiensis serovar israelensis and Bcg is presented, utilizing two sets of PCR primers targeting the bacterial chromosome. Sequence database searches and empirical tests performed on target and nontarget species, as well as on bulk soil DNA samples, demonstrated that this diagnostic tool is specific for B. thuringiensis serovar israelensis and Bcg. The method will be useful for comparisons of Bcg and B. thuringiensis serovar israelensis abundances in the same samples. Moreover, the effect of B. thuringiensis serovar israelensis-based insecticide application on the total Bcg assemblages, including indigenous populations, can be investigated. This type of information is valuable in risk assessment and policy making for use of B. thuringiensis serovar israelensis in the environment.
INTRODUCTION
The bacterium Bacillus thuringiensis (Bt) is one of the grand successes in microbial pest control (1). After its first discovery in 1901 and description by Berliner in 1911, the first biocontrol product based on Bt, Sporein, for control of the European corn borer (Ostrinia nubilalis), was commercially available already in 1938 (1). The crystalline, proteinaceous δ-endotoxins of Bt are formed during sporulation, and their lethal effect is manifested only after larval ingestion of spores or free crystals (2, 3). Bt is a member of the Bacillus cereus group (Bcg), which consists of the Gram-positive rod-shaped spore-forming bacterial species B. cereus, Bt, B. anthracis, B. mycoides, B. pseudomycoides, and B. weihenstephanensis (4, 5). However, a multidatatype phylogenetic analysis of Bcg strains isolated from various sample types, including environmental, clinical, food, and diary sources, did not reflect current Bcg taxonomy well. This finding indicated that the phylogeny of this bacterial group is more complicated than reflected by the division into the current six species (6).
The bacterium B. thuringiensis serovar israelensis produces toxins active against nematoceran larvae and has been widely used for biocontrol of mosquitoes and black flies (3). B. thuringiensis serovar israelensis was described in 1976 (7) and has been shown to have a worldwide distribution. It is generally found in soil but also has been isolated from insects, plants, and mushroom compost (8, 9). B. thuringiensis serovar israelensis-based biocontrol products are used mainly in inundation biological control, where, due to the quickly reduced activity of the crystal toxins (10) and because they are only formed during sporulation, the product needs to be reapplied in order to keep the abundance of larvae below a critical level.
For a long time, investigations of the abundance of Bt in the environment relied on cultivation on selective media (11, 12), subsequently in combination with identification of B. thuringiensis serovar israelensis with PCR (13), serotype characterization (14, 15), and/or amplified fragment length polymorphism genotyping (14). Cultivation approaches are comparatively insensitive, laborious, and hampered by the morphological and phenotypic similarities between B. cereus and Bt. The cry genes, which together with other plasmid-borne genes encode the δ-endotoxins, have been used as markers differentiating between B. thuringiensis serovar israelensis and B. cereus (16, 17). The genes also have been targets for PCR-based monitoring of B. thuringiensis serovar israelensis populations in wetlands (18). However, the plasmid has a high potential for horizontal transfer and can be lost in the environment (19–21) and can be picked up by strains of B. cereus (22). Therefore, primers differentiating B. cereus and B. thuringiensis serovar israelensis on the chromosomal level are needed for comparing their populations in various habitats and B. thuringiensis serovar israelensis product-treated as well as untreated environments. Furthermore, the cry genes often are associated with transposable elements (5). This makes plasmid-borne genes comparatively unstable and less reliable as genetic markers. Hence, development of more robust methods has a strong potential to improve detection and quantification of Bcg bacteria in the environment.
In this study, a cultivation-independent PCR-based method, using new primers targeting the bacterial chromosome, was developed for quantification of B. thuringiensis serovar israelensis and Bcg in the environment. Phylogenetic analysis based on published genomes of Bcg strains was used to identify useful chromosomal loci. The developed PCR primers were validated for target specificity as well as for PCR amplification efficiency and potential inhibition using soil DNA extracts.
MATERIALS AND METHODS
Soil samples.
Soil samples were collected in May 2013 from five different sites, two forest swamps (FS1, lat 60.296645, long 16.842440; FS2, lat 60.291774, long 16.830497; latitude and longitude coordinates) and three wet meadows (WM1, lat 60.220125, long 16.752357; WM2, lat 60.248941, long 16.784031; WM3, lat 60.249198, long 16.783335), all located in the River Dalälven floodplains in central Sweden. With the exception of WM1, all sites have been subjected to mosquito control with B. thuringiensis serovar israelensis since the year 2000 within the Biological Mosquito Control project (23). At each site, five 10-cm-deep soil cores (diameter, 2.5 cm) were taken at evenly distributed positions within 1 m2 and pooled. Soil samples were transported in cooling boxes to the laboratory, where they were stored at 4°C until further processing and experiments. Samples were homogenized by passing through a 4-mm sieve and their dry weight determined.
Homogenized soil from site WM1 (not treated with B. thuringiensis serovar israelensis) was autoclaved, and the absence of Bcg cells was confirmed by dilution to 1:100, heating to 65°C for 40 min, and plating in triplicates on T3 agar (24) supplemented with 25 mg liter−1 DelvoCid Instant (DSM, Düsseldorf, Germany) (25). After 24 h at 30°C, plates were checked for the absence of Bcg-like colonies (rugose, ice crystal-like appearance and a diameter of >1 mm).
Bacterial strains.
A collection of 68 Bacillus strains was established (Table 1). The collection covers representatives of both Bcg species, including different Bt serovars and B. thuringiensis serovar israelensis strains isolated from commercial biocontrol products (AM65-52 and DSM 5724), and soil in Sweden (strains 06:11, 06:12, 08:36, 08:37, and 08:38). Strains were grown and maintained on T3 agar (24). Spores of B. thuringiensis serovar israelensis strain AM65-52 used in the commercial B. thuringiensis serovar israelensis-based product Vectobac-G (Valent Biosciences, Libertyville, FL) were obtained by inoculating liquid T3 medium followed by incubation at 28°C for 5 days (26). The cultures were checked for spores and crystals by phase-contrast microscopy. The density of spores was determined by cultivating 30 μl of 105 dilutions on T3 agar after vegetative cells in the spore solution had been eliminated by heating to 75°C for 15 min.
TABLE 1.
Bacillus strains used in this studya
| Species | Origin | Bacterial strainb | Presence of: |
||
|---|---|---|---|---|---|
| Cry4 | Bti1 | Bcg1 | |||
| B. cereus group | |||||
| B. cereus | Milk | ATCC 14579T | − | − | + |
| B. cereus | Unknown | AH 184 | − | − | + |
| B. cereus | Human wound | F 837/76 | − | − | + |
| B. cereus | Milk | ATCC 4342 | − | − | + |
| B. cereus | Blood | ATCC 7064 | − | − | + |
| B. cereus | Powdered milk | ATCC 33018 | − | − | + |
| B. cereus | Spodoptera frugiperda | T01 176:C2 | − | − | + |
| B. cereus | Rice | F 3502/73 | − | − | + |
| B. cereus | Soil | ATCC 6464 | − | − | + |
| B. cereus | Cheese spoilage | ATCC 10987 | − | − | + |
| B. mycoides | Soil | DSM 2048T | − | − | + |
| B. mycoides | Soil | ATCC 6462T | − | − | + |
| B. pseudomycoides | Unknown | DSM 12442 | − | − | + |
| B. pseudomycoides | Soil | CECT 7065T | − | − | + |
| B. weihenstephanensis | Pasteurized milk | DSM 11821T | − | − | + |
| B. thuringiensis | Soil | 06:03 | − | − | + |
| B. thuringiensis | Soil | 09:02 | − | − | + |
| B. thuringiensis serovar kenyae | Corcyra cephalonica | HD-136 | − | − | + |
| B. thuringiensis serovar alesti | Bombyx mori | HD-4 | − | − | + |
| B. thuringiensis serovar aizawai | Heliothis assulta | HD-112 | − | − | + |
| B. thuringiensis serovar canadensis | Unknown | HD-224 | − | − | + |
| B. thuringiensis serovar colmeri | Grain dust | HD-847 | − | − | + |
| B. thuringiensis serovar dakota | Unknown | HD-932 | − | − | + |
| B. thuringiensis serovar darmstadiensis | Unknown | HD-146 | − | − | + |
| B. thuringiensis serovar dendrolimus | Dendrolimus sibericus | HD-7 | − | − | + |
| B. thuringiensis serovar entomocidus | Unknown | HD-110 | − | − | + |
| B. thuringiensis serovar finitimus | Malacosoma distria | HD-3 | − | − | + |
| B. thuringiensis serovar galleriae | Dendrolimus sibericus | HD-29 | − | − | + |
| B. thuringiensis serovar indiana | Soya been field | HD-521 | − | − | + |
| B. thuringiensis serovar kuamotoensis | Silkworm litter | HD-867 | − | − | + |
| B. thuringiensis serovar kurstaki | Pectinophora gossypiella | HD-1 | − | − | + |
| B. thuringiensis serovar kurstaki | Ephestia kühniella | HD-73 | − | − | + |
| B. thuringiensis serovar kyushuensis | Bombyx mori | HD-541 | − | − | + |
| B. thuringiensis serovar morrisoni | Unknown | HD-12 | − | − | + |
| B. thuringiensis serovar ostriniae | Unknown | HD-501 | − | − | + |
| B. thuringiensis serovar pakistani | Cydia pomonella | HD-395 | − | − | + |
| B. thuringiensis serovar shandogiensis | Unknown | HD-1012 | − | − | + |
| B. thuringiensis serovar sotto | Unknown | HD-770 | − | − | + |
| B. thuringiensis serovar thompsoni | Unknown | HD-542 | − | − | + |
| B. thuringiensis serovar thuringiensis | Unknown | HD-22 | − | − | + |
| B. thuringiensis serovar thuringiensis | Ephestia kühniella | HD-2 | − | − | + |
| B. thuringiensis serovar tochigiensis | Silkworm litter | HD-868 | − | − | + |
| B. thuringiensis serovar tohokuensis | Silkworm litter | HD-866 | − | − | + |
| B. thuringiensis serovar tolworthi | Unknown | HD-537 | − | − | + |
| B. thuringiensis serovar toumanoffi | Galleria mellonella | HD-201 | − | − | + |
| B. thuringiensis serovar israelensis | Commercial product | DSM 5724CM | + | + | + |
| B. thuringiensis serovar israelensis | Soil | 06:11 | + | − | + |
| B. thuringiensis serovar israelensis | Soil | 06:12 | + | + | + |
| B. thuringiensis serovar israelensis | Soil | 08:36 | + | + | + |
| B. thuringiensis serovar israelensis | Soil | 08:37 | + | + | + |
| B. thuringiensis serovar israelensis | Soil | 08:38 | + | + | + |
| B. thuringiensis serovar israelensis | Culex pipiens | HD-567 CM | − | + | + |
| B. thuringiensis serovar israelensis | Plasmid cured mutant | 4Q2-72 | + | + | + |
| B. thuringiensis serovar israelensis | Unknown | Dbt357 | − | − | + |
| B. thuringiensis serovar israelensis | Unknown | HBt1 | + | + | + |
| B. thuringiensis serovar israelensis | Unknown | HBt17 | − | − | + |
| B. thuringiensis serovar israelensis | Soil | Bta2 | + | + | + |
| B. thuringiensis serovar israelensis | Vectobac-G | AM65-52 CM | + | + | + |
| B. thuringiensis serovar israelensis | Soil | S128 | + | + | + |
| B. thuringiensis serovar israelensis | Unknown | LH-1 | + | + | + |
| B. thuringiensis serovar israelensis | Unknown | NB31 | + | + | + |
| Non-B. cereus group | |||||
| B. amyloliquefaciens | Soil | DSM 7T | − | − | − |
| B. circulans | Soil | DSM 11T | − | − | − |
| B. licheniformis | Unknown | DSM 1320 | − | − | − |
| B. megaterium | Milk | DSM 32T | − | − | − |
| B. polymyxa | Unknown | DSM 36T | − | − | − |
| B. sphaericus | Milk | DSM 2898 | − | − | − |
| B. subtilis | Unknown | DSM 10T | − | − | − |
The Bacillus strains, including 12 species and 27 subspecies, were tested with the novel PCR-based diagnostics of B. cereus group and specifically B. thuringiensis serovar israelensis using chromosome-directed primers Bti1 and Bcg1, respectively, as well as the cry4Aa and cry4Ba genes targeting primers (Cry4) as a control.
A superscript T indicates type strain, and superscript CM indicates a strain used in a commercial product.
DNA extraction.
For DNA preparation from Bacillus strains, bacteria were cultivated on Luria-Bertani agar (LB) and incubated at 30°C overnight. Bacterial cells were suspended in Tris-EDTA and lysed by boiling. DNA-containing extracts were separated from cell debris by centrifugation (27) and stored at −20°C. Soil DNA was extracted from 250-mg subsamples using the PowerLyzer PowerSoil DNA isolation kit (Mo Bio Laboratories, Carlsbad, CA) according to the manufacturer's instructions. A FastPrep-24 instrument (MP Biomedicals, Santa Ana, CA) was used for bead beating the soil samples (6,000 rpm for 45 s). The quality of soil DNA extracts was examined by electrophoresis (1% [wt/vol] agarose gels and ethidium bromide staining were used throughout). The DNA concentrations of extracts were determined with Pico-Green (Invitrogen, Carlsbad, CA) using a Qubit fluorometer (Invitrogen).
Primer design.
In order to locate chromosome regions suitable for designing Bcg- and B. thuringiensis serovar israelensis-targeting primers, comparative analysis of available annotated Bt genomes and one B. cereus genome (Bacillus cereus G9842 [NCBI accession number CP001186], Bt HD-771 [CP003752], B. thuringiensis serovar israelensis HD-789 [CP003763], and B. thuringiensis serovar kurstaki HD-73 [CP004069]) was undertaken using the software Genious 6.0.5 (28). Aligned contigs were visually scanned for regions containing high mutation rates (29), i.e., showing high net nucleotide differences. Within the identified regions, putative primer pairs targeting Bcg (Bcg1_for/rev) and B. thuringiensis serovar israelensis (Bti1_for/rev) were designed. In a first check that the putative primers target Bcg-specific (i.e., B. cereus, B. anthracis, B. mycoides, B. pseudomycoides, B. weihenstephanensis, and different Bt serovars) or B. thuringiensis serovar israelensis-specific signatures, BLAST sequence homology searches using concatenated primer sequences were performed in the whole-genome shotgun contig (WGS) database of NCBI (30) restricted to Bacillus spp. The BLAST sequence homology searches were run with the default settings and the number of hits set to 500 for Bcg- and B. thuringiensis serovar israelensis-specific primers (26 February 2015).
Primer specificity tests.
Primer specificity was evaluated experimentally by applying two different approaches. In the first, PCR amplification of genomic DNA from the Bacillus strain collection was assessed with the primer pairs Bcg1_for/rev and Bti1_for/rev (Tables 1 and 2). As a control, cry4Aa and cry4Ba genes targeting primers Un4(d)/Cry4R (Cry4) (17) were assessed as well. The reaction volume was 20 μl, containing 1 μl bacterial DNA extract, 1× Fermentas DreamTaq green PCR master mix (Thermo Fisher Scientific, Waltham, MA), and 0.3 μM each primer (Life Technologies, Carlsbad, CA). PCR was performed with a C1000 thermal cycler (Bio-Rad, Hercules, CA) with a 3-min initial activation at 95°C, followed by 35 cycles of 30 s of denaturation at 95°C; 30 s of annealing at 60°C for the Bti1 primer, 65°C for the Bcg1 primer, and 67°C for the Cry4 primer; 1 min of extension at 72°C; and finally an extension step for 10 min at 72°C. PCR products were visualized by electrophoresis.
TABLE 2.
Nucleotide sequences of the novel chromosome-directed primersa
| Primer | Sequence (5′–3′) | Oligonucleotide positionb (5′–3′) | Amplified fragment length (bp) |
|---|---|---|---|
| Bti1_for | CAAACATTTCATTCCAATAACA | 3152129–3152150 | 190 |
| Bti1_rev | ATACTGTGTGGGATGCTTATTA | 3152310–3152331 | |
| Bcg1_for | AACAGGCTCCATACAATGGTAT | 5274038–5274059 | 250 |
| Bcg1_rev | TGGTAGCGTTTCTTCGTCTTAT | 5274262–5274283 |
Novel chromosome-directed primers for diagnostics of the Bacillus cereus group, and specifically the insecticidal Bacillus thuringiensis serovar israelensis, were used in quantitative PCR.
Positions are given relative to the reference genome Bacillus thuringiensis serovar israelensis HD-789, GenBank accession number CP003763.
The quality of Bacillus DNA was checked by PCR using 0.5 μM universal bacterial primers SD-arch-0519-aS-15 and SD-Bact-0785-bA-18 (31) by following the PCR conditions described above with the addition of 0.1 mg ml−1 bovine serum albumin (BSA) and annealing at 52°C with 32 cycles.
In the second approach, the specificity of primers was checked by determining the identity of PCR products amplified with the Bti1 and Bcg1 primer pairs from soil DNA. The same PCR conditions were used as those described above, and 0.6 mg ml−1 BSA (GE Healthcare, Piscataway, NJ) was added. The annealing temperature was lowered to 56 and 63°C for the B. thuringiensis serovar israelensis- and Bcg-specific PCR, respectively, and the number of cycles was increased to 40. Amplification products were cloned using the TOPO TA cloning kit and One Shot TOP10 chemically competent Escherichia coli cells (Life Technologies) according to the manufacturer's recommendations. Colony PCR with 0.5 μM M13 forward (−20) and M13 reverse primers was performed on 12 randomly selected clones for each soil sample, using the conditions described above but with an annealing temperature of 55°C and 25 cycles. PCR products were checked for quality by electrophoresis, followed by sequencing (Macrogen, Republic of South Korea) using M13 forward (−20) and M13 reverse primers. The clones were divided into sequence homology groups using the software Bioedit, version 7.1.3.0 (32). BLAST sequence similarity searches with representative sequences from each homology group were performed in the WGS database of NCBI (30), restricted to Bacillus spp. The default settings were used with the number of hits set to 500 for B. thuringiensis serovar israelensis and Bcg primer fragments, and sequences with 100% compliance were retrieved (26 February 2015).
A phylogenetic analysis of the Bcg sequences retrieved from soil was performed on an alignment consisting of one representative sequence from each homology group of clones as well as one representative sequence from the 100% sequence similarity hits. In order to cover the different accession numbers assigned to the 100% sequence similarity hits, one representative for each individual accession number identity at subspecies, species, and genus levels was selected for each homology group. Maximum likelihood as well as neighbor-joining tree analyses were performed based on the Jukes-Cantor model, applying 1,000 bootstrap resamplings and the default settings in the software MEGA 5 (33).
Cloning and purification of plasmid DNA standards.
For use as a standard in the quantitative real-time PCR (qPCR), a plasmid was constructed containing a fragment amplified by the Bti1 primers from DNA of B. thuringiensis serovar israelensis strain AM65-52 and cloned into a pCR4-TOPO vector (Life Technologies) as described above. Positive clones were grown in liquid LB overnight, and plasmids were harvested using the QIAprep spin miniprep kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. Plasmid DNA was quantified with Pico-Green (Invitrogen, Carlsbad, CA) using a Qubit fluorometer (Invitrogen). The plasmid concentration was adjusted to 106 copies μl−1, and 10-fold serial dilutions were prepared.
Quantification efficiency.
For checking the efficiency of the qPCR with soil extracts, reactions were performed using 10-fold serial dilutions of plasmid DNA with or without addition of 5 ng of soil DNA (extracted from the autoclaved soil from site WM1).
qPCR was performed in 20-μl reaction volumes containing 10 μl of IQ SYBR green supermix (Bio-Rad), 0.5 μM each B. thuringiensis serovar israelensis- and Bcg-specific primer pair, 0.1 mg ml−1 BSA (GE Healthcare), and 5 μl template, using a CFX 96 real-time system (Bio-Rad). Cycling conditions were 3 min of initial activation at 95°C, followed by 35 cycles of 15 s of denaturation at 95°C and 1 min of annealing and extension at 60 or 65°C for B. thuringiensis serovar israelensis- and Bcg-specific amplification, respectively. Following amplification, a melting curve analysis was performed, ranging from 55°C to 95°C with 0.5°C increments for 5 s. For each template dilution, the reactions were performed in triplicates. The threshold line and the sample specific threshold cycle numbers (CT) were determined with the default parameters of the qPCR instrument software (Bio-Rad CFX Manager, version 3.1). Efficiency values (E) and correlation coefficients (R2) were calculated by the software for each standard quantification curve of CT against the number of input copies.
Potential PCR inhibition.
From all five soil samples, 5 μl of soil DNA extracts (containing 0, 0.2, 1, 2, 3, or 4 ng DNA μl−1) was added to qPCR vials and amended with 106 copies of the B. thuringiensis serovar israelensis-specific qPCR plasmid standard, resulting in a total reaction volume of 20 μl (34). qPCR was performed in triplicates using the instrumentation and reaction composition described above, except for adding 0.3 μM each M13 forward (−20) and M13 reverse primer with an annealing temperature of 61°C. Cycling conditions were set at 5 min of initial activation at 95°C, followed by 35 cycles of 40 s of denaturation at 95°C and 40 s of annealing at 61°C, and finally 30 s of extension at 72°C. Following amplification, a melting curve analysis was performed, ranging from 55°C to 95°C with a 0.5°C increment every 5 s.
Statistical analyses.
For statistical evaluation of inhibitory effects of soil DNA extracts on PCR, pairwise t testing with Welch approximation to the degrees of freedom was applied, using the “stats” package of the R software (version 2.15.1; R Foundation for Statistical Computing, Vienna, Austria). P values were manually Bonferroni corrected due to multiple testing.
CT values from different dilutions of plasmid-based standard curves for Bcg and B. thuringiensis serovar israelensis with and without soil DNA extracts were tested for statistical differences by applying parametrical tests using the function “pairwiset.test” (version 2.15.1; R Foundation for Statistical Computing, Vienna, Austria). The residuals were checked for normal distribution.
Nucleotide sequence accession numbers.
The sequences from sequence homology groups (SHG) representing Bcg strains detected in soil samples here have been deposited in GenBank under accession numbers KP863940 to KP863943 for SHG1 Bcg to SHG4 Bcg and KP863944 and KP863945 for SHG1 B. thuringiensis serovar israelensis and SHG2 B. thuringiensis serovar israelensis, respectively.
RESULTS
Primer design.
Based on whole-genome comparisons, two intergenic regions were selected for designing primer pairs targeting the Bcg and B. thuringiensis serovar israelensis, respectively (Table 2). The region flanked by the primers Bcg1_for/rev (Table 2) is located across an intergenic region and a gene predicted as a transcriptional regulator (region 5274038 to 5274283 of reference genome Bacillus thuringiensis serovar israelensis HD-789; NCBI accession number CP003763) (35). For the primers Bti1_for/rev, the target is located in an intergenic region (positions 3152129 to 3152331) (35).
The BLAST similarity searches with concatenated Bcg-targeting primer sequences on the WGS database of NCBI recovered 253 hits with 100% sequence similarity for the full query length (Table 3) (26 February 2015). Among them, 237 accession numbers were those of target species, i.e., the Bcg members B. cereus, B. anthracis, B. mycoides, B. pseudomycoides, B. weihenstephanensis, and Bt. A further 13 of the hits were identified as Bacillus species, while one hit was with each of the nontarget species B. subtilis (strain B7-S; AZNI01000038), B. gaemokensis (BL3-6; JOTM01000032), and B. manliponensis (BL4-6; JOTN01000018). Among the hits with less than 100% sequence similarity, there were accession numbers identified as Bacillus species but none as Bcg members independent of the covered query length. When restricting the BLAST search to the nucleotide database of NCBI, among the 90 retrieved 100% match hits, 87 were assigned to the Bcg (26 February 2015) (data not shown). The remaining three were assigned to B. bombyseptieus, B. toyonensis, and B. cytotoxicus (data not shown).
TABLE 3.
Taxonomic identification of the 100% match hits of the concatenated Bacillus cereus group and B. thuringiensis serovar israelensis-targeting primer sequences to accession numbers in the WGS database of NCBI
| Bacterial speciesa | No. of primer sequences targeting: |
|
|---|---|---|
| B. cereus group | B. thuringiensis serovar israelensis | |
| B. anthracis | 58 | |
| B. cereus | 139 | 2 |
| B. mycoides | 5 | |
| B. pseudomycoides | 1 | |
| B. weihenstephanensis | 4 | |
| B. thuringiensis | 12 | 1 |
| B. thuringiensis serovar aizawai | 2 | |
| B. thuringiensis serovar andalousinesis | 1 | |
| B. thuringiensis serovar berliner | 1 | |
| B. thuringiensis serovar huazhongensis | 1 | |
| B. thuringiensis serovar israelensis | 2 | 2 |
| B. thuringiensis serovar kurstaki | 2 | |
| B. thuringiensis serovar monterrey | 1 | |
| B. thuringiensis serovar morrisoni | 1 | |
| B. thuringiensis serovar pakistani | 1 | |
| B. thuringiensis serovar pondicheriensis | 1 | |
| B. thuringiensis serovar pulsiensis | 1 | |
| B. thuringiensis serovar sotto | 1 | |
| B. thuringiensis serovar thuringiensis | 1 | |
| B. thuringiensis serovar tochigiensis | 1 | |
| B. thuringiensis serovar tolworthi | 1 | |
| B. gaemokensis | 1 | |
| B. manliponensis | 1 | |
| B. subtilis | 1 | |
| Bacillus sp. | 13 | 3 |
| Total no. of hits | 253 | 8 |
Species were assigned to accession numbers with 100% sequence similarity to concatenated primer sequences according to the nomenclature adopted from NCBI.
The corresponding BLAST sequence similarity searches with the B. thuringiensis serovar israelensis-targeting primers revealed 100% similarity with accession numbers identified as Bacillus thuringiensis serovar israelensis ATCC 35646 (AAJM01000297), Bacillus thuringiensis serovar israelensis 4Q7 (JEOC01000010), Bacillus thuringiensis IBL 4222 (ACNL01000121), and Bacillus sp. strains WBUNB009 (ANFK01000002), L_1B0_8 (JXIT01000096), and L_1B0_5 (JXIS01000041) (Table 3) (25 February 2015). Additionally, there was a 100% match with two accession numbers identified as Bacillus cereus VD045 (AHET01000010) and AH676 (ACMQ01000156).
Primer specificity.
Among the 68 bacterial strains tested with the primer pairs, the Bti1 and Cry4 primers gave amplification products only with B. thuringiensis serovar israelensis strains, although four B. thuringiensis serovar israelensis strains (Dbt357, HBt17, HD567, and 06:11) gave no PCR product for either one or both B. thuringiensis serovar israelensis primer pairs (Table 1). The Bcg1 primers were positive for all Bcg bacteria but for none of the strains of non-Bcg Bacillus species (Table 1). The PCR with universal bacterial primers produced amplicons of the expected sizes for all strains of the collection (data not shown), confirming acceptable quantity and quality of DNA extracts for all of the tested strains.
PCR amplification with the B. thuringiensis serovar israelensis- and Bcg-targeting primers from soil DNA extracts produced PCR amplicons of the expected sizes (data not shown). PCR fragments were cloned, and 12 clones per soil sample were arbitrarily selected and sequenced. All 48 clones retrieved for B. thuringiensis serovar israelensis contained a fragment with the expected length. Sequences formed two sequence homology groups (SHG), SHG1 and SHG2 B. thuringiensis serovar israelensis, which varied in one deleted base. Thirty-five identical clones were designated SHG1 B. thuringiensis serovar israelensis (KP863944) (Table 4). These clones are identical to five accession numbers in the NCBI database, identified as Bacillus thuringiensis IBL 4222 (ACNL01000121), Bacillus thuringiensis serovar israelensis 4Q7 (JEOC01000010), Bacillus thuringiensis serovar israelensis ATCC 35646 (AAJM01000297), and Bacillus sp. strains L_1B0_8 and L_1B05 (JXIT01000096 and JXIS01000041) (Table 5) (26 February 2015). Two clones (coming from two different soil samples) were designated SHG2 B. thuringiensis serovar israelensis (KP863945) (Table 4). The remaining 11 clones had single-base-pair differences at individual positions and were not further analyzed. There were no database accession numbers having 100% similarity with SHG2 B. thuringiensis serovar israelensis or any of the clone sequences containing single-base-pair differences.
TABLE 4.
Distribution of Bacillus clones retrieved from soil samplesa
| Soil sample | No. of clones of: |
|||||
|---|---|---|---|---|---|---|
| SHG1 Bcg (KP863940) | SHG2 Bcg (KP863941) | SHG3 Bcg (KP863942) | SHG4 Bcg (KP863943) | SHG1 B. thuringiensis serovar israelensis (KP863944) | SHG2 B. thuringiensis serovar israelensis (KP863945) | |
| FS1 | 9 | 0 | 0 | 0 | 11 | 1 |
| FS2 | 2 | 3 | 2 | 1 | 8 | 0 |
| WM2 | 0 | 0 | 1 | 6 | 9 | 0 |
| WM3 | 3 | 0 | 2 | 4 | 7 | 1 |
| Total no. of clones | 14 | 3 | 5 | 11 | 35 | 2 |
Soil clones were determined using DNA extracts from soil samples from the River Dalälven floodplains, Sweden. The number of clones retrieved from forested swamps (FS) 1 and 2 and wet meadows (WM) 2 and 3 are listed, as well as their distribution on the sequence homology groups (SHG) of B. cereus group (Bcg) and B. thuringiensis serovar israelensis.
TABLE 5.
Numbers and taxonomic identification of the 100% match hits with a representative sequence from each of the Bacillus sequence homology groupsa
| Bacterial species | No. of PCR hits for representative sequence of: |
|||||
|---|---|---|---|---|---|---|
|
Bacillus cereus group |
B. thuringiensis serovar israelensis |
|||||
| SHG1 (KP863940) | SHG2 (KP863941) | SHG3 (KP863942) | SHG4 (KP863943) | SHG1 (KP863944) | SHG2 (KP863945) | |
| B. anthracis | 58 | |||||
| B. cereus | 70 | 9 | 42 | 5 | 5 | |
| B. mycoides | 1 | |||||
| B. weihenstephanensis | 3 | 1 | ||||
| B. thuringiensis | 10 | 1 | 1 | |||
| B. thuringiensis serovar aizawai | 2 | |||||
| B. thuringiensis serovar andalousinensis | 1 | |||||
| B. thuringiensis serovar berliner | 1 | |||||
| B. thuringiensis serovar huazhongensis | 1 | |||||
| B. thuringiensis serovar israelensis | 2 | 2 | ||||
| B. thuringiensis serovar kurstaki | 2 | |||||
| B. thuringiensis serovar monterrey | 1 | |||||
| B. thuringiensis serovar morrisoni | 1 | |||||
| B. thuringiensis serovar pakistani | 1 | |||||
| B. thuringiensis serovar pondicheriensis | 1 | |||||
| B. thuringiensis serovar pulsiensis | 1 | |||||
| B. thuringiensis serovar thuringiensis | 1 | |||||
| B. thuringiensis serovar tolworthi | 1 | |||||
| B. subtilis | 1 | |||||
| Bacillus spp. | 2 | 7 | 2 | |||
| Total no. of hits | 138 | 9 | 73 | 6 | 10 | 0 |
Soil clones were determined using DNA extracts from soil samples from the River Dalälven floodplains, Sweden. The numbers and taxonomic identification of the 100% match hits (BLAST searches in the whole genome shot-gun contigs database of NCBI) with a representative sequence (corresponding NCBI accession number in parentheses) from each of the sequence homology groups are listed. Species were assigned to accession numbers with 100% sequence similarity to SHGs according to the nomenclature adopted from NCBI.
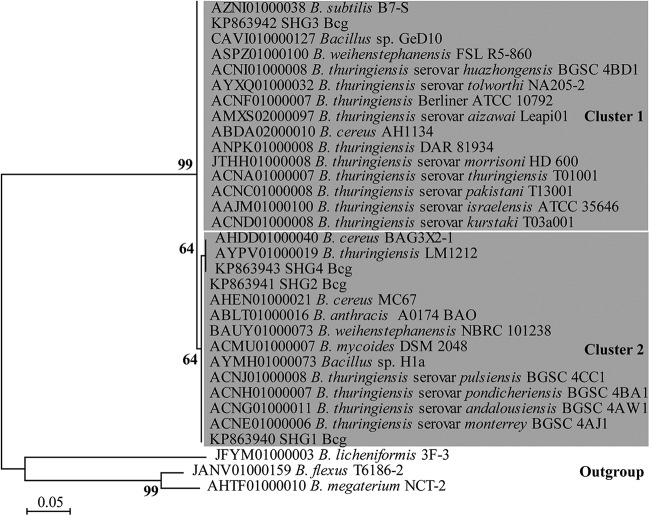
Similar to the B. thuringiensis serovar israelensis clones, all 48 retrieved Bcg clones contained a PCR fragment of the expected size. Among these, 46 had similarities between 0.97 and 1 with base pair differences in 17 positions; however, for two clones the sequences were of insufficient quality (data not shown). Thirty-three clones were divided into four SHG (SHG1 Bcg to SHG4 Bcg, KP863940 to KP863943) (Table 4), while 13 clones revealed single-base-pair differences at individual positions and were not analyzed further. The matches in the searches with representatives of each of the B. cereus homology groups were dominated by B. cereus, with lesser contributions from other Bcg species, 1 B. subtilis strain, and 11 unidentified Bacillus strains (Table 5) (26 February 2015). Perfect matches to sequences assigned to B. anthracis were found only for SHG1 Bcg, while Bt (including B. thuringiensis serovar israelensis) accession numbers were found almost exclusively in SHG3 Bcg. The phylogenetic analyses of the alignment containing sequences with 100% similarities to representatives of the different Bcg homology groups separated them into two main clusters with a bootstrap value of 99 (Fig. 1). Cluster 1 contains all sequences of SHG3 Bcg, including representatives of the accession numbers identified as B. thuringiensis serovar israelensis. In cluster 2, all sequences identical to the other sequence homology groups clustered together, including the sequences assigned to B. anthracis.
FIG 1.
Phylogenetic tree built on NCBI accession numbers with 100% similarity to sequence homology groups formed of soil clones obtained with Bcg1_for/rev primers from targets amplified from soil DNA. Representative sequences from each accession number at subspecies, species, and genus level for each of the sequence homology groups, as well as from different homology groups among clone sequences (SHG1 Bcg to SHG4 Bcg; KP863940 to KP863943), were included. The tree was constructed using maximum-likelihood analysis based on the Jukes-Cantor model of Bacillus cereus group 1 (Bcg) sequences from Bcg bacteria with 1,000 iterations of bootstrapping.
Sensitivity of real-time PCR quantification.
The standard curve containing B. thuringiensis serovar israelensis plasmid dilutions was linear (R2 = 0.997) over seven orders of magnitude with an amplification efficiency of 91.8% using B. thuringiensis serovar israelensis-targeting primers. The melting curve analysis confirmed that amplification was specific (data not shown). With addition of soil DNA extract, the slope of the standard curve changed from −3.536 to −3.318, and small but significant differences were found for 5 × 104, 5 × 103, and 50 copies (Table 6). However, the correlation still was linear over seven orders of magnitude (R2 = 0.986), with an amplification efficiency of 100.2%. The detection sensitivity and amplification efficiency for the Bcg-targeting primers was similar to that for the B. thuringiensis serovar israelensis primers, and slopes were almost parallel for pure plasmid (−3.322) and plasmid plus soil DNA (−3.363) (Table 6).
TABLE 6.
Validation of quantitative PCR targeting the Bcg1 and Bti1 regions of the Bacillus cereus group and B. thuringiensis serovar israelensis, respectively, with cloned DNA of B. thuringiensis serovar israelensis AM65-52a
| Sample type | E [%] | R2 |
CT at copy no. of: |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 5 × 107 | 5 × 106 | 5 × 105 | 5 × 104 | 5 × 103 | 5 × 102 | 50 | |||
| Bcg1 plasmid | 98.3 | 0.996 | 11.48 ± 0.25 | 16.11 ± 0.08 | 18.97 ± 0.14 | 22.03 ± 0.07 | 25.39 ± 0.13 | 28.93 ± 0.25 | 32.23 ± 0.78 |
| Bcg1 plasmid plus soil | 100.0 | 0.994 | 11.88 ± 0.06 | 16.21 ± 0.07 | 19.30 ± 0.64 | 22.03 ± 0.14 | 25.33 ± 0.21 | 28.94 ± 0.03 | 32.39 ± 1.05 |
| Bti1 plasmid | 91.8 | 0.997 | 12.74 ± 0.16 | 16.23 ± 0.04 | 20.69 ± 0.14 | 24.03 ± 0.05* | 27.20 ± 0.17* | 30.82 ± 0.51 | 33.89 ± 0.48* |
| Bti1 plasmid plus soil | 100.2 | 0.987 | 12.85 ± 0.19 | 16.23 ± 0.07 | 21.73 ± 0.56 | 23.70 ± 0.17* | 27.07± 0.37* | 30.24 ± 0.15 | 32.67 ± 0.52* |
Serial 10-fold dilutions (107 to 10) of plasmid DNA or plasmid DNA spiked with 5 ng soil DNA were analyzed. The average efficiency value (E) and correlation coefficient (R2) for the four different dilution series are indicated. CT values are the averages from three repetitions ± standard deviations. An asterisk indicates significantly (P < 0.05) different copy numbers between dilutions of Bti1 plasmid DNA with and without soil extract addition.
Potential PCR inhibition was tested with the addition of five different soil DNA extracts in increasing concentrations. The total amount of soil DNA was lowest in the extract from soil WM1 (20 ng μl−1) and highest from soil FS1 (73 ng μl−1). Consequently, the volume of raw soil DNA extract added to the PCR was 3.65 times higher from soil WM1 than from soil FS1. Extracts from soils FS2 and WM3 gave low, though significant, inhibition already with addition of 5 ng of soil DNA (Fig. 2). PCR inhibition increased with 10 ng and was significant for all samples except WM1. When we added more than 10 ng soil DNA, all extracts caused inhibition.
FIG 2.
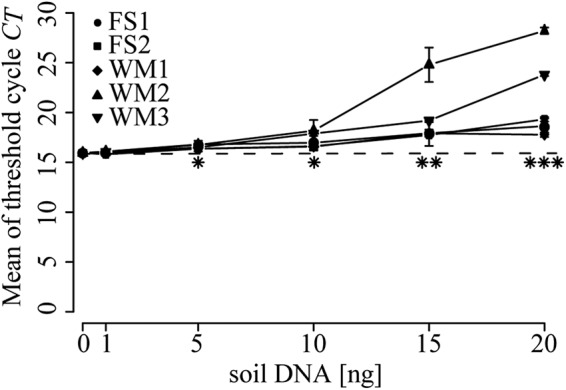
Quantitative real-time PCR analyses for determining potential PCR inhibition by soil DNA extracts. Extracts corresponding to 1, 5, 10, 15, or 20 ng of soil DNA were added to PCR mixtures containing 106 copies of the recombinant plasmid pCR4-Topo (Invitrogen). The dashed line indicates the mean CT of the control without addition of soil DNA. CT values are the averages from three replicates, and error bars represent standard deviations. Asterisks indicate significant deviations from the control (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
DISCUSSION
Whole-genome comparisons among four strains from Bcg allowed the identification of Bcg- and B. thuringiensis serovar israelensis-specific marker sequences located on the bacterial chromosome. B. cereus and Bt are genetically closely related, although they differ in some physiological properties and, notably, insecticidal activity (5). So far, the only way to differentiate between B. cereus and Bt was based on the presence of a plasmid carrying the genes responsible for the formation of the insecticidal crystals (5). In this study, we present new Bcg- and B. thuringiensis serovar israelensis-specific primers targeting markers located on the chromosome. This method provides comparatively stable and reliable PCR-based differentiation, detection, and quantification of the Bcg and B. thuringiensis serovar israelensis bacteria and is suitable for environmental studies. Since the markers are located on the chromosome, the drawbacks of using targets located on exchangeable plasmids (19–21) are avoided. This method will be useful, for example, in studies of effects on indigenous Bcg assemblages of application of B. thuringiensis serovar israelensis-based insecticides.
Primer design and evaluation of specificity.
BLAST similarity searches on NCBI databases for the Bcg primers gave one match to an accession number identified as a non-Bcg strain, B. subtilis B7-S (Fig. 1 and Table 3). However, several lines of evidence suggest that B7-S is not correctly identified. To begin with, the size and G+C content of the B. subtilis B7-S genome are 5.3 Mb and 35.1%, respectively (36), while for other B. subtilis strains deposited in NCBI, genome sizes varied between 4.01 and 4.22 Mb (except for BEST7613, with 7.59 Mb), and G+C content ranged from 43.5 to 45.7%. The B. cereus genomes, on the other hand, are very similar to strain B7-S, with sizes ranging from 5.29 to 5.84 Mb and G+C contents from 35.2 to 35.5%. In silico DNA-DNA hybridization of B. cereus type strain ATCC 14579 (AZNI01000038) with B. subtilis B7-S indicates that B7-S is actually a B. cereus strain (http://ggdc.dsmz.de) (data not shown). Moreover, no PCR product was obtained from the type strain B. subtilis DSM 10 (Table 1). Together, these findings indicate that B. subtilis B7-S represents a misclassified entry in the databases. Perfect hits also were found in NCBI databases with B. toyonensis, B. cytotoxicus, B. gaemokensis, and B. manliponensis (37–40). Although these novel Bacillus species have been proposed to belong to the Bcg, valid species descriptions still are missing. Hence, these strains were not included in our collection for primer specificity tests. Additionally, B. bombyseptieus also was a perfect hit for the Bcg primers. This species has been reported to form insecticidal crystals during sporulation, and the released toxins induce host responses when entering the larval midgut of a silkworm (41). The high similarity of B. bombyseptieus to B. thuringiensis serovar chinensis CT-43 (>96.46%) (42) supports its affiliation with the Bcg.
Other primers and probes targeting the chromosome have been designed for specific detection and quantification of the Bcg as well as differentiation among Bcg species (43, 44). However, our BLAST similarity searches on WGS databases in NCBI revealed that these Bcg primers missed a certain proportion of Bcg strains whose genome sequences have been published recently (22 April 2015) (data not shown). Thus, the specificity tests performed in this study indicate that our primers are the first that pick up the total Bcg as it is currently known using a single primer pair.
The target fragment for the B. thuringiensis serovar israelensis primers matched accession numbers from WGS databases in NCBI assigned to the nontarget B. cereus strains AH676 and VD045. For strain VD045, potential plasmid transfer through conjugation has been reported, and the identification at the species level has been questioned (45). Thus, at least VD045 may actually represent a B. cereus strain which is able to take up the plasmid carrying the genes responsible for insect toxicity. In addition, 100% matches were found with accession numbers identified only at the species or genus level, such as Bt IBL 4222 and Bacillus sp. strains WBUNB009, L_1B0_8, and L_1B0_5. Interestingly, for three of these strains (IBL 4222, L_1B0_8, and L_1B0_5), contigs were found in the database having annotated genes encoding Cry4Aa and Cry4Ba, which is specific for B. thuringiensis serovar israelensis. This finding indicates the affiliation of these strains is with serovar israelensis.
Two of the B. thuringiensis serovar israelensis strains in the collection were not amplified with the B. thuringiensis serovar israelensis-targeting primers. However, these two strains were not amplified when using B. thuringiensis serovar israelensis primers targeting the B. thuringiensis serovar israelensis-specific cry4Aa and cry4Ba genes (17) located on the plasmid either (Table 1), implying that their affiliation with serovar israelensis is uncertain. Thus, this result does not really put the B. thuringiensis serovar israelensis specificity of the new primers into question.
In PCR amplifications using the soil DNA extracts, the Bcg and B. thuringiensis serovar israelensis primers generated amplicons of the expected sizes and sequences. The unequivocal phylogenetic placement of the Bcg1 soil clones into two different clusters containing exclusively Bcg members supports the specificity of the new PCR-based detection tool (Fig. 1). The similarity searches with a group-representative sequence revealed 11 times more perfect matches for SHG3 Bcg than for SHG4 Bcg (Table 5), indicating a stronger representation of SHG3 Bcg in the database. Most clones in SHG1 Bcg were recovered from forested swamps, while most in SHG4 Bcg originated in wet meadows. This indicates that the abundances of the groups is associated with a particular habitat type. Both the Bcg and B. thuringiensis serovar israelensis primers generated sequences with single-nucleotide mutations at individual positions and no 100% match to any sequence in the database. It is likely that this finding can partly be explained by random sequencing errors, which cannot be avoided and need to be taken into account.
Extraction, PCR efficiencies, and potential PCR inhibition.
The moderate but significant effects of soil extract addition on the plasmid standard curves confirm that coextracted compounds such as humic acids can inhibit PCR amplification (34). This effect also was evident when mixing a fixed amount of plasmid with different amounts of soil DNA extracts (Fig. 2). Potential inhibition varied among the soils, but it also may depend on the DNA extraction protocol used (34). Generally, for soil extracts containing low target concentrations, it is often an advantage to increase the amount of DNA in order to increase the target concentration and lower the detection limit. In the present study, however, if we increase the amount of extract, the risk for PCR inhibition due to coextracted substances also will increase.
Efficient DNA recovery from bacterial material such as spores in soil also is essential for reliable PCR-based quantification. In one study, the recovery of B. cereus DNA from soil spiked with B. cereus spores varied between 0 and 35%, depending on the number of spores added to the soil, soil type, and DNA extraction kit used (46). Guidi et al. (17), on the other hand, reported 36 and 46% total DNA recovery from added B. thuringiensis serovar israelensis spores. Thus, DNA extraction efficiency is highly dependent on several factors, and additional tests of recovery with appropriate soil types and spore densities are required before using the new primers for quantifying the abundance of the Bcg and B. thuringiensis serovar israelensis in the field.
In conclusion, the tests of specificity, sensitivity, as well as DNA recovery of this study reveal that the new cultivation-independent method gives specific and efficient determination of the abundances of Bcg and B. thuringiensis serovar israelensis in soil samples. This method will be useful in studies of the biogeography and ecology of the Bcg bacteria in general and specifically for assessing effects on resident Bcg and B. thuringiensis serovar israelensis populations in localities treated with B. thuringiensis serovar israelensis-based biological control products.
ACKNOWLEDGMENTS
We thank three anonymous reviewers for their valuable comments on the manuscript.
The project was supported by the Carl Trygger Foundation and the Centre for Biological Control (CBC; http://www.slu.se/cbc) at the Swedish University of Agricultural Sciences (SLU).
We are grateful to Christopher Jones for support in sequence database screening.
REFERENCES
- 1.Lord JC. 2005. From Metchnikoff to Monsanto and beyond: the path of microbial control. J Invertebr Pathol 89:19–29. doi: 10.1016/j.jip.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 2.Bravo A, Likitvivatanavong S, Gill SS, Soberón M. 2011. Bacillus thuringiensis: a story of a successful bioinsecticide. Insect Biochem Mol Biol 41:423–431. doi: 10.1016/j.ibmb.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lacey LA. 2007. Bacillus thuringiensis serovariety israelensis and Bacillus sphaericus for mosquito control. J Am Mosq Control Assoc 23:133–163. doi: 10.2987/8756-971X(2007)23[133:BTSIAB]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 4.Guinebretière M-H, Thompson FL, Sorokin A, Normand P, Dawyndt P, Ehling-Schulz M, Svensson B, Sanchis V, Nguyen-The C, Heyndrickx M, De Vos P. 2008. Ecological diversification in the Bacillus cereus group. Environ Microbiol 10:851–865. doi: 10.1111/j.1462-2920.2007.01495.x. [DOI] [PubMed] [Google Scholar]
- 5.Vilas-Bôas GT, Peruca APS, Arantes OMN. 2007. Biology and taxonomy of Bacillus cereus, Bacillus anthracis, and Bacillus thuringiensis. Can J Microbiol 53:673–687. doi: 10.1139/W07-029. [DOI] [PubMed] [Google Scholar]
- 6.Tourasse NJ, Helgason E, Klevan A, Sylvestre P, Moya M, Haustant M, Økstad OA, Fouet A, Mock M, Kolstø A-B. 2011. Extended and global phylogenetic view of the Bacillus cereus group population by combination of MLST, AFLP, and MLEE genotyping data. Food Microbiol 28:236–244. doi: 10.1016/j.fm.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 7.Goldberg LJ, Margalit J. 1977. A bacterial spore demonstrating rapid larvicidal activity against Anopheles sergentii, Uranotaenia unguiculata, Culex univitattus, Aedes aegypti and Culex pipiens. Mosq News 37:355–358. [Google Scholar]
- 8.Bernhard K, Jarrett P, Meadows M, Butt J, Ellis DJ, Roberts GM, Pauli S, Rodgers P, Burges HD. 1997. Natural isolates of Bacillus thuringiensis: worldwide distribution, characterization, and activity against insect pests. J Invertebr Pathol 70:59–68. doi: 10.1006/jipa.1997.4669. [DOI] [Google Scholar]
- 9.Martin PAW, Travers RS. 1989. Worldwide abundance and distribution of Bacillus thuringiensis isolates. Appl Environ Microbiol 55:2437–2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohana B, Margalit J, Barak ZE. 1987. Fate of Bacillus thuringiensis subsp. israelensis under simulated field conditions. Appl Environ Microbiol 53:828–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hendriksen NB, Hansen BM. 2002. Long-term survival and germination of Bacillus thuringiensis var. kurstaki in a field trial. Can J Microbiol 48:256–261. doi: 10.1139/w02-009. [DOI] [PubMed] [Google Scholar]
- 12.de Melo-Santos MAV, Araújo APD, Rios EMM, Regis L. 2009. Long lasting persistence of Bacillus thuringiensis serovar israelensis larvicidal activity in Aedes aegypti (Diptera: Culicidae) breeding places is associated to bacteria recycling. Biol Control 49:186–191. doi: 10.1016/j.biocontrol.2009.01.011. [DOI] [Google Scholar]
- 13.De Respinis S, Demarta A, Patocchi N, Lüthy P, Peduzzi R, Tonolla M. 2006. Molecular identification of Bacillus thuringiensis var. israelensis to trace its fate after application as a biological insecticide in wetland ecosystems. Lett Appl Microbiol 43:495–501. doi: 10.1111/j.1472-765X.2006.01999.x. [DOI] [PubMed] [Google Scholar]
- 14.Tilquin M, Paris M, Reynaud S, Despres L, Ravanel P, Geremia RA, Gury J. 2008. Long lasting persistence of Bacillus thuringiensis subsp. israelensis (Bti) in mosquito natural habitats. PLoS One 3:e3432. doi: 10.1371/journal.pone.0003432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hajaij M, Carron A, Deleuze J, Gaven B, Setier-Rio M-L, Vigo G, Thiéry I, Nielsen-LeRoux C, Lagneau C. 2005. Low persistence of Bacillus thuringiensis serovar israelensis spores in four mosquito biotopes of a salt marsh in southern France. Microb Ecol 50:475–487. doi: 10.1007/s00248-005-0247-3. [DOI] [PubMed] [Google Scholar]
- 16.Ben-Dov E, Zaritsky A, Dahan E, Barak Z, Sinai R, Manasherob R, Khamraev A, Troitskaya E, Dubitsky A, Berezina N, Margalith Y. 1997. Extended screening by PCR for seven cry-group genes from field-collected strains of Bacillus thuringiensis. Appl Environ Microbiol 63:4883–4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guidi V, De Respinis S, Benagli C, Luthy P, Tonolla M. 2010. A real-time PCR method to quantify spores carrying the Bacillus thuringiensis var. israelensis cry4Aa and cry4Ba genes in soil. J Appl Microbiol 109:1209–1217. doi: 10.1111/j.1365-2672.2010.04741.x. [DOI] [PubMed] [Google Scholar]
- 18.Guidi V, Patocchi N, Lüthy P, Tonolla M. 2011. Distribution of Bacillus thuringiensis subsp. israelensis in soil of a Swiss wetland reserve after 22 years of mosquito control. Appl Environ Microbiol 77:3663–3668. doi: 10.1128/AEM.00132-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonzales J, Brown B, Carlton B. 1982. Transfer of Bacillus thuringiensis plasmids coding for δ-endotoxin among strains of Bacillus thuringiensis and Bacillus cereus. Proc Natl Acad Sci U S A 79:6951–6955. doi: 10.1073/pnas.79.22.6951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.te Giffel MC, Beumer RR, Klijn N, Wagendorp A, Rombouts FM. 1997. Discrimination between Bacillus cereus and Bacillus thuringiensis using specific DNA probes based on variable regions of 16S rRNA. FEMS Microbiol Lett 146:47–51. doi: 10.1016/S0378-1097(96)00439-9. [DOI] [PubMed] [Google Scholar]
- 21.Ceuppens S, Boon N, Uyttendaele M. 2013. Diversity of Bacillus cereus group strains is reflected in their broad range of pathogenicity and diverse ecological lifestyles. FEMS Microbiol Ecol 84:433–450. doi: 10.1111/1574-6941.12110. [DOI] [PubMed] [Google Scholar]
- 22.Hu XM, Hansen BM, Yuan ZM, Johansen JE, Eilenberg J, Hendriksen NB, Smidt L, Jensen GB. 2005. Transfer and expression of the mosquitocidal plasmid pBtoxis in Bacillus cereus group strains. FEMS Microbiol Lett 245:239–247. doi: 10.1016/j.femsle.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 23.Schäfer ML, Lundström JO. 2014. Efficiency of Bti-based floodwater mosquito control in Sweden–four examples. J Eur Mosq Control Assoc 32:1–8. [Google Scholar]
- 24.Travers RS, Martin PAW, Reichelderfer CF. 1987. Selective process for efficient isolation of soil Bacillus spp. Appl Environ Microbiol 53:1263–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hendriksen NB, Carstensen J. 2013. Long-term survival of Bacillus thuringiensis subsp. kurstaki in a field trial. Can J Microbiol 59:34–38. doi: 10.1139/cjm-2012-0380. [DOI] [PubMed] [Google Scholar]
- 26.Donnarumma F, Paffetti D, Stotzky G, Giannini R, Vettori C. 2010. Potential gene exchange between Bacillus thuringiensis subsp. kurstaki and Bacillus spp. in soil in situ. Soil Biol Biochem 42:1329–1337. doi: 10.1016/j.soilbio.2010.03.014. [DOI] [Google Scholar]
- 27.Hendriksen N, Hansen B. 2011. Diagnostic properties of three conventional selective plating media for selection of Bacillus cereus, B. thuringiensis, and B. weihenstephanensis. Folia Microbiol (Praha) 56:535–539. doi: 10.1007/s12223-011-0079-0. [DOI] [PubMed] [Google Scholar]
- 28.Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, Thierer T, Ashton B, Meintjes P, Drummond A. 2012. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28:1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kepler RM, Rehner SA. 2013. Genome-assisted development of nuclear intergenic sequence markers for entomopathogenic fungi of the Metarhizium anisopliae species complex. Mol Ecol Resources 13:210–217. doi: 10.1111/1755-0998.12058. [DOI] [PubMed] [Google Scholar]
- 30.Altschul SF, Madden TL, Schaffer AA, Zhang JH, Zhang Z, Miller W, Lipman DJ. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klindworth A, Pruesse E, Schweer T, Peplies J, Quast C, Horn M, Glockner FO. 2013. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res 41:e1. doi: 10.1093/nar/gks808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hall TA. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98. [Google Scholar]
- 33.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: Molecular Evolutionary Genetics Analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schneider S, Enkerli J, Widmer F. 2009. A generally applicable assay for the quantification of inhibitory effects on PCR. J Microbiol Methods 78:351–353. doi: 10.1016/j.mimet.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 35.Doggett NA, Stubben CJ, Chertkov O, Bruce DC, Detter JC, Johnson SL, Han CS. 2013. Complete genome sequence of Bacillus thuringiensis serovar israelensis strain HD-789. Genome Announc 1:e01023-13. doi: 10.1128/genomeA.01023-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen P, Li S, Yan L, Wang N, Yan X, Li H. 2014. Draft genome sequence of Bacillus subtilis type strain B7-S, which converts ferulic acid to vanillin. Genome Announc 2:e00025-14. doi: 10.1128/genomeA.00025-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jimenez G, Urdiain M, Cifuentes A, Lopez-Lopez A, Blanch AR, Tamames J, Kampfer P, Kolsto AB, Ramon D, Martinez JF, Codoner FM, Rossello-Mora R. 2013. Description of Bacillus toyonensis sp nov., a novel species of the Bacillus cereus group, and pairwise genome comparisons of the species of the group by means of ANI calculations. Syst Appl Microbiol 36:383–391. doi: 10.1016/j.syapm.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 38.Jung MY, Kim J-S, Paek WK, Lim J, Lee H, Kim PI, Ma JY, Kim W, Chang Y-H. 2011. Bacillus manliponensis sp. nov., a new member of the Bacillus cereus group isolated from foreshore tidal flat sediment. J Microbiol 49:1027–1032. doi: 10.1007/s12275-011-1049-6. [DOI] [PubMed] [Google Scholar]
- 39.Guinebretière M-H, Auger S, Galleron N, Contzen M, De Sarrau B, De Buyser M-L, Lamberet G, Fagerlund A, Granum PE, Lereclus D, De Vos P, Nguyen-The C, Sorokin A. 2013. Bacillus cytotoxicus sp. nov. is a novel thermotolerant species of the Bacillus cereus group occasionally associated with food poisoning. Int J Syst Evol Microbiol 63:31–40. doi: 10.1099/ijs.0.030627-0. [DOI] [PubMed] [Google Scholar]
- 40.Jung M-Y, Paek WK, Park I-S, Han J-R, Sin Y, Paek J, Rhee M-S, Kim H, Song HS, Chang Y-H. 2010. Bacillus gaemokensis sp. nov., isolated from foreshore tidal flat sediment from the Yellow Sea. J Microbiol 48:867–871. doi: 10.1007/s12275-010-0148-0. [DOI] [PubMed] [Google Scholar]
- 41.Huang L, Cheng T, Xu P, Cheng D, Fang T, Xia Q. 2009. A genome-wide survey for host response of silkworm, Bombyx mori during pathogen Bacillus bombyseptieus infection. PLoS One 4:e8098. doi: 10.1371/journal.pone.0008098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng T, Lin P, Jin S, Wu Y, Fu B, Long R, Liu D, Guo Y, Peng L, Xia Q. 2014. Complete genome sequence of Bacillus bombysepticus, a pathogen leading to Bombyx mori black chest septicemia. Genome Announc 2:e00312-14. doi: 10.1128/genomeA.00312-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oliwa-Stasiak K, Kolaj-Robin O, Adley CC. 2011. Development of real-time PCR assays for detection and quantification of Bacillus cereus group species: differentiation of B. weihenstephanensis and rhizoid B. pseudomycoides isolates from milk. Appl Environ Microbiol 77:80–88. doi: 10.1128/AEM.01581-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oliwa-Stasiak K, Molnar CI, Arshak K, Bartoszcze M, Adley CC. 2010. Development of a PCR assay for identification of the Bacillus cereus group species. J Appl Microbiol 108:266–273. doi: 10.1111/j.1365-2672.2009.04419.x. [DOI] [PubMed] [Google Scholar]
- 45.Hu XM, Van der Auwera G, Timmery S, Zhu L, Mahillon J. 2009. Distribution, diversity, and potential mobility of extrachromosomal elements related to the Bacillus anthracis pXO1 and pXO2 virulence plasmids. Appl Environ Microbiol 75:3016–3028. doi: 10.1128/AEM.02709-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dineen SM, Aranda R, Anders DL, Robertson JM. 2010. An evaluation of commercial DNA extraction kits for the isolation of bacterial spore DNA from soil. J Appl Microbiol 109:1886–1896. doi: 10.1111/j.1365-2672.2010.04816.x. [DOI] [PubMed] [Google Scholar]