Abstract
Human norovirus (HuNoV) is the leading cause of foodborne illnesses, with an increasing number of outbreaks associated with leafy greens. Because HuNoV cannot be routinely cultured, culturable feline calicivirus (FCV), murine norovirus (MNV), porcine sapovirus (SaV), and Tulane virus (TV) have been used as surrogates. These viruses are generated in different cell lines as infected cell lysates, which may differentially affect their stability. Our objective was to uniformly compare the survival of these viruses on postharvest lettuce while evaluating the effects of cell lysates on their survival. Viruses were semipurified from cell lysates by ultrafiltration or ultracentrifugation followed by resuspension in sterile water. Virus survival was examined before and after semipurification: in suspension at room temperature (RT) until day 28 and on lettuce leaves stored at RT for 3 days or at 4°C for 7 and 14 days. In suspension, both methods significantly enhanced the survival of all viruses. On lettuce, the survival of MNV in cell lysates was similar to that in water, under all storage conditions. In contrast, the survival of FCV, SaV, and TV was differentially enhanced, under different storage conditions, by removing cell lysates. Following semipurification, viruses showed similar persistence to each other on lettuce stored under all conditions, with the exception of ultracentrifugation-purified FCV, which showed a higher inactivation rate than MNV at 4°C for 14 days. In conclusion, the presence of cell lysates in viral suspensions underestimated the survivability of these surrogate viruses, while viral semipurification revealed similar survivabilities on postharvest lettuce leaves.
INTRODUCTION
Human noroviruses (HuNoVs) are the leading cause of acute viral gastroenteritis in the United States (1). These viruses are 28 to 35 nm in diameter, nonenveloped, single-stranded RNA viruses that are members of the Caliciviridae family. An increasing number of HuNoV outbreaks have been associated with leafy greens (2). For example, food was the primary vehicle of transmission in 1,008 HuNoV outbreaks reported between 2009 and 2012 in the United States, and leafy greens were the primary food associated with these outbreaks (1). Therefore, there is a need to understand the occurrence and survival/persistence of HuNoV on leafy greens. However, assessing the survival of HuNoVs on leafy greens is hampered by the fact that these viruses are still refractory to routine growth in cell culture; hence, their infectivity cannot be readily quantified (3, 4). Therefore, surrogate culturable viruses are often used as proxies to investigate HuNoV transmission routes, disinfection, and survival in the environment.
Among members of the Caliciviridae family, feline calicivirus (FCV) has been the most widely used HuNoV surrogate since the 1990s (5). In 2004, murine norovirus (MNV), a virus genetically more closely related to HuNoV, was propagated in cell culture (6) and, since then, it has been widely used as a HuNoV surrogate (7). In 2004, bile acids in intestinal contents were identified essential for porcine sapovirus (SaV; Cowden strain) replication in vitro (8), and later the use of SaV as a HuNoV surrogate was established (9–11). In 2008, Tulane virus (TV) was propagated in cell culture (12) and is currently being investigated as a surrogate for HuNoV (9, 13). These HuNoV calicivirus surrogates differ in their susceptible hosts, disease symptoms, cell surface receptor binding, and physiochemical properties. For example, while FCV causes respiratory tract disease in domestic cats (14), MNV causes modest intestinal pathology in wild-type mice (15), TV causes fever, diarrhea, and inflammation of the duodenum in rhesus macaques (16), and SaV causes gastroenteritis in gnotobiotic piglets (17, 18). Previous studies were limited to assessing the survival of either one or two of these surrogates on leafy greens (11, 13, 19–23). In addition, different studies have used different viral suspension matrices, including undiluted or diluted virus-infected cell lysates in phosphate-buffered saline (PBS), Hanks' balanced salt solution (HBSS), soil, or fecal solutions (11, 13, 19–23). For example, FCV generated in cell lysates and diluted in cell culture medium was 90% inactivated within 1.5 days when inoculated on lettuce leaves that were stored at 4°C (19), whereas when suspended in 10% fecal solution the virus survived for at least 7 days (21). A 2-log reduction was reported for hepatitis A virus generated in cell lysates and inoculated on lettuce leaves stored at 4°C for 7 days (24), whereas less than a 0.5-log reduction occurred when the virus was suspended in fecal solution (25). Uniform comparisons for all calicivirus HuNoV surrogate survival rates on leafy greens are lacking. This is important because different cell lysate matrices may have different effects on the survival of these surrogate viruses, hampering direct comparisons, an understanding of their comparative survival on leafy greens, and the utility of these viruses as appropriate surrogates in studies concerned with survival on leafy greens.
Viruses are usually generated as infected cell lysates following repeated freeze-thaw cycles implemented during the process of harvesting the virus from infected cells. Although a brief low-speed centrifugation (2,000 to 2,500 × g for 20 to 30 min at 4°C) is usually performed to remove cellular debris, such as nuclei, the cytosolic and membrane contents (with active enzymes) are retained in the virus stocks. For example, LLC-PK1 and LLC-MK2, used for SaV and TV generation, respectively, are known to secrete significant amounts of plasminogen activator, a serine protease (26), while RAW 264.7 cells (used for MNV generation) secrete lysozyme, a glycoside hydrolase (27). Collectively, surrogate viruses propagated in these different cell lines are essentially suspended in different infected cell lysates containing different secretions and medium components, all of which may interact to affect the stability of these viruses. Therefore, the overall objectives of this study were to uniformly compare the survival rates of FCV, SaV, MNV, and TV on postharvest lettuce under the same suspension matrix (water) while simultaneously assessing the effects of infected cell lysates on their survival.
MATERIALS AND METHODS
Propagation of viruses.
Cell culture propagation of SaV (Cowden strain), FCV (F9 strain), MNV (S7 strain), and TV in an LLC porcine kidney cell line (LLC-PK1; ATCC CL-101), the Crandell-Rees feline kidney cell line (CRFK; ATCC CCL-94), a mouse leukemic macrophage cell line (RAW 264.7; ATCC TIB-71), and an LLC monkey kidney cell line (LLC-MK2; ATCC CCL-7) was done as described previously (11, 12, 28). The MNV S7 strain was generously provided by Yukinobu Tohya, Department of Veterinary Medicine, Nihon University, Japan (29). As reported previously, SaV and FCV were cultured in minimum essential medium (MEM; Life Technologies, Grand Island, NY) supplemented with nonessential amino acids (1%) without or with 2% fetal bovine serum (FBS), respectively (11). TV and MNV were cultured in M199 and Dulbecco's modified Eagle's medium (DMEM) with high glucose (Life Technologies) supplemented with 5% and 10% FBS, respectively (12, 28). In addition, all culture media used for cell maintenance and virus generation were supplemented with 1% HEPES and 1% antibiotic-antimycotic cocktail (Invitrogen, Carlsbad, CA, USA). FBS (HyClone FBS characterized; Thermo Scientific, Rockford, IL) used in culturing the cells and the viruses (except for SaV) either received no heat inactivation (LLC-PK1) or was heat inactivated at 56°C for 30 min (CRFK/FCV and LLC-MK2/TV) or at 60°C for 60 min (RAW 264.7/MNV) as previously described (11). Briefly, confluent monolayers of cells (except for RAW 264.7 cells, which were used at 90% confluence) were inoculated with each virus at a multiplicity of infection (MOI) of 0.05. Cultures were harvested when 90% of the cells showed cytopathic effects (CPE) at days 1.5, 2, 2.5, and 3 postinfection for FCV, MNV, TV, and SaV, respectively. Viruses were released by applying three cycles of freezing-thawing, and the cell debris was removed by centrifugation at 2,500 × g for 30 min at 4°C. The supernatants containing the viruses were aliquoted, stored at −80°C, and used in all subsequent experiments. Virus titers were determined in CPE-based assays and expressed as the tissue culture infectious dose affecting 50% of the cultures (TCID50), as described below. Viral yields for SaV, TV, and MNV (∼6 log10 TCID50/ml) were not significantly different from each other. However, FCV was generated at a significantly higher yield (∼8 log10 TCID50/ml). Therefore, the FCV stocks were diluted 100-fold with cell lysates (centrifuged as described above) obtained from control noninfected CRFK cells that were incubated under similar conditions as the infected cells.
Semipurification of viruses.
To accurately compare the survival of the four surrogate viruses, two approaches were followed to semipurify the viruses from cell lysates: (i) ultrafiltration with Amicon 100K Ultra-15 centrifugal devices (Millipore, Billerica, MA) at 4,000 × g for 0.5 h at 4°C (as recommended by the manufacturer); (ii) ultracentrifugation in Beckman tubes (Beckman Coulter, Brea, CA) at 112,700 × g for 1.5 h at 4°C. Then, viruses were resuspended to their original volumes using distilled water which was filtered through a 0.22-μm filter in a Milli-Q water system (Millipore) and autoclaved. The pHs of cell lysate suspensions of FCV, SaV, MNV, and TV were 7.90, 7.73, 7.23, and 8.42, respectively. The pH of the reconstituted viral solutions in sterile water was similar to that of sterile water (pH 7.86) used for the resuspension. There were no significant differences in viral titers before or after ultracentrifugation or ultrafiltration for FCV, SaV, MNV, and TV.
Survival experiments.
The survival of viruses before and after semipurification was examined in suspension by incubating 1-ml aliquots of the different surrogates in clear 1.5-ml microcentrifuge tubes at room temperature (RT; ∼20°C) inside an opaque Tupperware box (to generate dark conditions) for 0, 7, 14, 21, and 28 days. One-milliliter aliquots of sterile water served as negative controls. The survival of FCV, SaV, MNV, and TV on postharvest lettuce leaves was assessed using romaine lettuce heads (Lactuca sativa) purchased from a local grocery store. Healthy outer leaves were hand washed thoroughly with sterile water and then allowed to dry before inoculation with viruses. To determine the detection limit for each virus, 10-fold serially diluted viral suspensions were spot inoculated onto lettuce leaves (1 ml per leaf at ∼30 cm2/leaf), allowed to dry for 2 h in a biological safety level II (BSL2) cabinet, and thereafter immediately processed as described below to determine viral titers at day 0 (defined as following the 2-h drying period). Semipurified and unpurified viruses were spot inoculated onto lettuce leaves (1 ml per leaf at ∼30 cm2/leaf), allowed to dry, and then bagged in sterile Whirl-Pak bags (Nasco, Salida, CA, USA), preserving the relative humidity at 90 to 95% as measured by a relative humidity meter (Fluke, Everett, WA). Samples were stored under dark conditions (inside an opaque Tupperware box) either at RT for 3 days or at 4°C for 7 and 14 days. Lettuce leaves spot inoculated with 1 ml of sterile water served as negative controls. Viruses were recovered from the lettuce samples as described previously (10). Briefly, each lettuce sample was weighed, cut into small pieces, and transferred into a 50-ml Falcon tube containing 30 ml of MEM supplemented with 1% antibiotic-antimycotic cocktail (Invitrogen, Carlsbad, CA, USA) and 2% heat-inactivated (60°C for 1 h) FBS. Samples were shaken vigorously (1 min vortexing and then 10 min shaking at 250 rpm at 4°C), and the resulting solutions were centrifuged at 2,095 × g for 10 min to eliminate plant debris and bacterial cells. The supernatants were ultracentrifuged at 112,700 × g for 1.5 h to concentrate the viruses. The resulting pellets were suspended in 1 ml of sterile PBS (0.01 M, pH 7.4). The infectivity titers (TCID50/g) were determined on day 0 and on postinoculation days 3, 7, and 14, as described below.
Infectivity assays.
Viruses were titrated for TCID50 by using their respective cell lines cultured in 96-well plates. Briefly, 1- to 2-day-old confluent cell monolayers (except for RAW 264.7 cells, which were used at ∼50% confluence) in 96-well plates were infected in quadruplet with serially diluted samples (1:10 in the respective cell culture medium supplemented with 1% antibiotic-antimycotic cocktail) and incubated at 37°C. The plates were inspected daily for CPE, whereby final observations were performed on day 5 for FCV, MNV, and TV. For SaV, on day 5 virus-infected cells in the highest dilution wells showing isolated CPE and negative-control wells were indistinguishable. Therefore, an immunohistochemistry protocol described previously (10) was used to stain virus-infected cells to determine the TCID50 of SaV on day 5. The CPE for SaV, TV, and FCV manifested as rounding of the cells followed by their detachment from the cell monolayer, while that for MNV was observed as shrinking of the cells, detachment, and loss of translucent appearance. The wells with infected cells were scored positive, and the viral titers were estimated following the Reed-Muench equation for the calculation of the TCID50 (30). Virus negative-control processed leaf samples were used to assess cytotoxic effects of plant debris on cells. No cytotoxic effects or bacterial contamination was observed for any of the cell lines.
Statistical analysis.
Prism version 5 (GraphPad Software, USA) was used for statistical analyses. The entire data set was log10 transformed, and the reduction in infectivity titers at each incubation day was reported. The reductions in infectivity were calculated by subtracting titers at each incubation day from their respective titers at day 0. Significant differences in mean reductions in infectivity titers of the different surrogate viruses were determined by using either one-way (followed by Tukey post hoc) or two-way (followed by Bonferroni posttest) analysis of variance (ANOVA), depending on whether one or two factors were compared. Each experiment was repeated twice with triplicate samples per virus per time point per treatment. Differences in means were considered significant when the P value was <0.05. Data are expressed as means ± standard errors (SE).
RESULTS
Semipurification enhanced the survival of all viruses in suspension at RT.
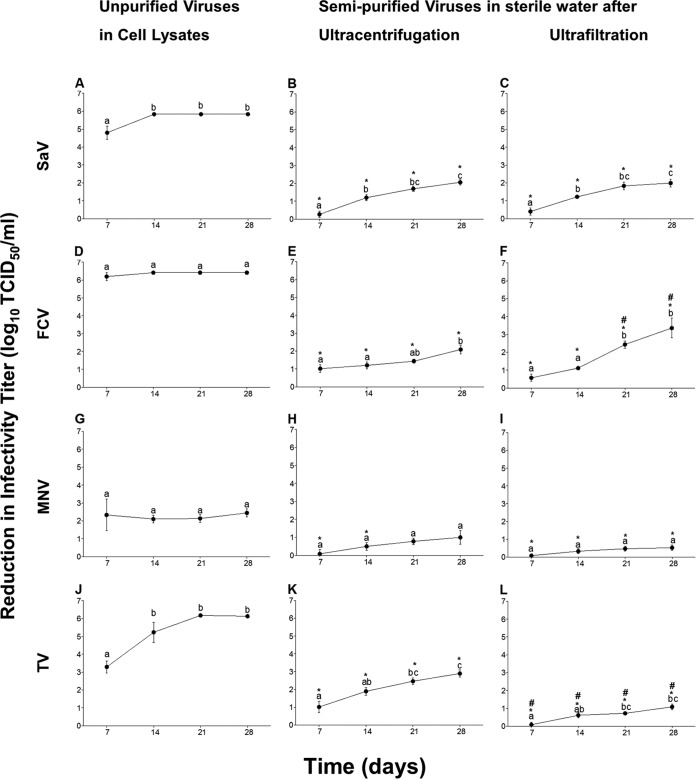
On day 0, there were no significant differences in the virus titers for FCV, SaV, MNV, and TV (6.41 ± 0.84, 5.83 ± 0.07, 6.63 ± 0.01, and 6.17 ± 0.31 log10 TCID50/ml, respectively). FCV, SaV, and TV generated in cell lysates were completely inactivated on day 7, 14, and 21, respectively (Fig. 1A, D, and J). In contrast, MNV showed an ∼2-log reduction in infectivity titer by day 7, which remained stable through day 28 (Fig. 1G). All viruses purified by either ultracentrifugation or ultrafiltration exhibited significantly lower reduction in infectivity titers compared to corresponding viruses in cell lysates (Fig. 1). In addition, ultrafiltration had a more positive effect on TV survival than ultracentrifugation (Fig. 1K and L), while there was the opposite effect in the case of FCV at days 21 and 28 (Fig. 1E and F). In contrast, there were no significant differences between the effect of ultrafiltration in comparison to ultracentrifugation on either SaV (Fig. 1B and C) or MNV survival (Fig. 1H and I). Ultrafiltered and ultracentrifuged MNV was stable over time, showing no significant differences in infectivity reduction titers on days 7, 14, 21, and 28 (Fig. 1H and I). In contrast, ultracentrifuged and ultrafiltered SaV, FCV, and TV showed significantly increased reductions in infectious titers on day 28 compared to day 7 (Fig. 1). Overall, the semipurified viruses were still infectious on day 28. Purification by ultracentrifugation showed that MNV had the lowest significant reduction in infectivity titers, followed by FCV, SaV, and TV, which showed similar reductions in titers (∼1, 2, 2, and 2 log10 TCID50/ml, respectively). While purification by ultrafiltration showed that MNV had a similar reduction rate as TV, followed by SaV and then by FCV (∼0.5, 1, 2, and 3 log10 TCID50/ml, respectively).
FIG 1.
Reductions in viral infectivity titers (log10 TCID50 per milliliter) for SaV (A, B, and C), FCV (D, E, and F), MNV (G, H, and I), and TV (J, K, and L) incubated at RT for 7, 14, 21, and 28 days. Viruses were left suspended in their virus-infected cell lysates or were semipurified by ultracentrifugation and ultrafiltration followed by resuspension in sterile water. Significant differences between time points within each virus are indicated by at least one different alphabet letter. Significant differences between semipurified viruses with respect to unpurified viruses are indicated by *, while those between the two purification methods are indicated by #.
Two-way ANOVA performed for each virus separately (with suspension matrix and time as factors) revealed that the virus suspension matrix exerted a significant effect (P < 0.05) on the infectivity reductions for FCV, SaV, MNV, and TV, accounting for ∼87, 91, 48, and 79% of the total variance in the data set. Time had a less significant effect, accounting for 5, 7, and 12% of the total variance for FCV, SaV, and TV, respectively. For MNV, time had no significant impact on infectivity reductions, accounting for 2% of total variance. The interaction between the two factors was significant only for FCV and TV (accounting for ∼3% of total variance).
Semipurification enhanced the survival of FCV, SaV, and TV on postharvest lettuce leaves.
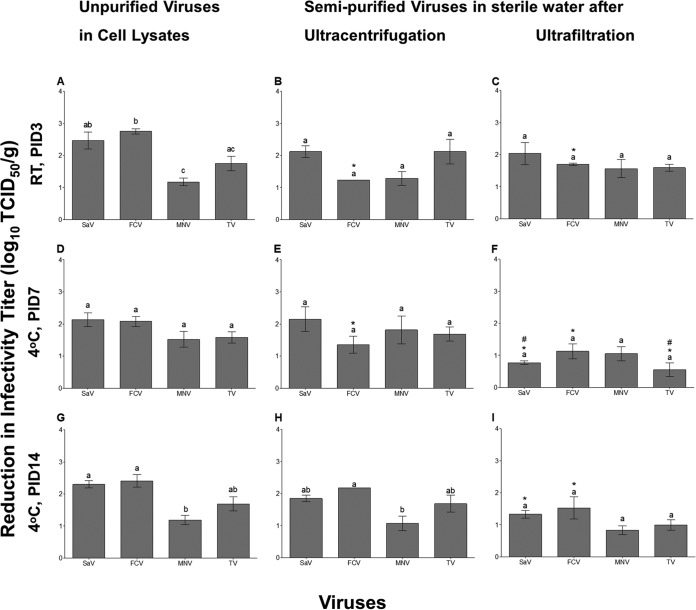
The minimum viral concentration that could be detected per gram of lettuce leaves was not significantly different among FCV, SaV, MNV, and TV (1.42 ± 0.7, 1.7 ± 0.30, 0.84 ± 0.11, and 0.75 ± 0.1 log10 TCID50/g, respectively). By subtracting the titers of the recovered viruses obtained on day 0 (following the 2-h drying period) from the viral titers of the inocula, it was found that an average of 1.3 ± 0.21, 1.18 ± 0.22, 0.61 ± 0.14, and 0.86 ± 0.15 log10 TCID50/ml was lost during the recovery process for FCV, SaV, MNV, and TV, respectively. This recovery loss was not significantly different between the four viruses. On day 0, there were no significant differences in the recovered virus infectivity titers from lettuce leaves among FCV, SaV, MNV, and TV (4.44 ± 0.17, 3.44 ± 0.48, 3.97 ± 0.5, and 3.99 ± 0.12 log10 TCID50/g of lettuce, respectively).
The four viruses incubated at RT for 3 days on lettuce leaves exhibited significant differences in infectivity reductions when suspended in cell lysates (Fig. 2A). In contrast, there were no significant differences in the infectivity reductions among the semipurified surrogate viruses (Fig. 2B and C). Both ultrafiltration and ultracentrifugation significantly improved the persistence of FCV in comparison to FCV in cell lysates (Fig. 2B and C). However, purification had no effect on the persistence of SaV, MNV, and TV. When the four viruses were incubated on lettuce leaves at 4°C for 7 days, a similar reduction in infectivity titers was detected among SaV, FCV, MNV, and TV in cell lysates (Fig. 2D) and following ultracentrifugation (Fig. 2E) or ultrafiltration purification (Fig. 2F). Ultracentrifugation only enhanced the persistence of FCV (Fig. 2E). While ultrafiltration enhanced the persistence of SaV, FCV, and TV compared to viruses in cell lysates (Fig. 2F). On day 14 at 4°C, only ultrafiltration produced significant enhancement in the persistence of SaV and FCV compared to unpurified viruses (Fig. 2I). The survival of MNV was not significantly different between semipurified and unpurified viruses on days 7 (Fig. 2D, E, and F) or 14 (Fig. 2G, H, and I). Overall, all purified viruses showed similar persistence to each other on lettuce stored under all conditions, with the exception of ultracentrifugation-purified FCV, which showed a significantly greater infectivity reduction than MNV at 4°C at 14 days (Fig. 2H).
FIG 2.
Reduction in infectivity titers (log10 TCID50 per gram) for SaV, FCV, MNV, and TV on lettuce leaves incubated at RT for 3 days (A, B, and C), at 4°C for 7 days (D, E, and F), or at 4°C for 14 days (G, H, and I). Significant differences among the viruses are indicated by at least one different alphabet letter. Significant differences between semipurified viruses with respect to unpurified viruses are indicated by *, while those between the two purification methods are indicated by #.
Comparing the survival rates of viruses in cell lysates incubated on lettuce leaves at 4°C to those lysates incubated at RT revealed that a period of 3 days of incubation at RT exerted a similar reduction effect on infectivity titers for all viruses as did a period of 7 days of incubation at 4°C (Fig. 2A and D). In addition, no further significant reduction in infectivity titers was observed on day 14 compared to day 7 for any viruses (Fig. 2D and G). A similar trend was observed for viruses that were ultracentrifuged and ultrafiltered, with the exception of ultracentrifuged FCV, which showed a greater reduction in infectivity on day 14 (4°C) than on day 7 (4°C) or day 3 (RT) (Fig. 2H, E, and B), and ultrafiltered SaV and TV, which showed a significantly greater reduction when incubated at RT for 3 days versus at 4°C for 7 days (Fig. 2C and F).
Two-way ANOVA performed for the results with each virus separately (with suspension matrix and storage conditions as factors) revealed that the virus suspension matrix exerted significant effects on the infectivity reductions for FCV, SaV, and TV, accounting for ∼43, 51, and 38% of total variance. Storage conditions (3 days at RT or 7 and 14 days at 4°C) had fewer but significant effects for FCV, SaV, and TV, accounting for 14, 14, and 18% of total variance, respectively. In contrast, neither suspension matrix nor storage condition accounted for any significant effects on MNV infectivity reductions. The interaction between the two factors was significant only for SaV (accounting for 15% of total variance).
DISCUSSION
Our study demonstrated that the presence of cell lysates in viral suspensions resulted in the complete inactivation of FCV, SaV, and TV by day 7, 14, and 21, whereas it had lesser effects on MNV during 28 days. The replacement of a cell lysate suspension matrix by sterile water slightly changed the pH values for TV (8.4 to 7.8) and MNV (7.2 to 7.8) but not for SaV and FCV suspension solutions. However, based on previous studies, there were no significant differences in infectivity titers at pH 7 or 8 for MNV and TV incubated at RT for 30 min (13). The lower survival of these surrogate viruses in cell lysates may have been due to the fact that as infected cells die, they release proteolytic enzymes, reactive oxygen species, and other metabolic by-products which may exert damaging effects on viral capsid proteins (31, 32). One major difference in the virus-infected cell lysates of FCV, SaV, MNV, and TV was the concentration of FBS used in their propagation. We propagated these viruses as reported previously for MNV in 10% FBS, TV in 5%, FCV in 2%, and SaV in 0% FBS (11, 12, 28). However, other studies have used various concentrations of FBS for propagation of these viruses. For example, MNV was propagated in 2% (7, 33) or 5% FBS (34), TV in 2% (9, 33) or 10% (13, 35), and FCV in 0 to 2% (19, 36, 37) or 10% FBS (7, 21, 38). Serum contains lipids, attachment factors, and stabilizing and detoxifying factors needed to inhibit proteases (39). When added directly to the viral suspensions, FBS had protective effects on viruses incubated at RT (40–42) or on viruses exposed to UV or gamma irradiation (43, 44). Although the exact mechanism for such effects is unknown, it may be that the high protein load of FBS (32) associates with the viral particles, stabilizing them, or deactivates the action of proteases present in the cell lysate (39). However, it is not known whether FBS added to culture media used for viral propagation has any effect on the stability of the generated viruses. In our study, MNV propagated in 10% FBS showed stable infectivity reductions until day 28, while FCV and SaV propagated in a solution with a lower percent FBS showed complete inactivation. The latter suggested that a higher percentage of FBS may enhance virus stability at RT. However, when we recultured all viruses under 10% or 0% FBS and incubated them for 7 days at RT, we observed a significantly lower log reduction in infectivity titers with 10% FBS for FCV and TV versus 0% FBS (3.01 ± 0.29 and 2.34 ± 0.23 versus 5.35 ± 0.85 and 4.08 ± 0.19 log10 TCID50/ml, respectively) but not for MNV (0.82 ± 0.12 versus 1.12 ± 0.1) and SaV (4.4 ± 0.22 versus 3.5 ± 0.12). Therefore, FBS in cell lysates may be one of the factors that differentially impact the stability of certain viruses. Enteric calicivirus capsids are considered to be more stable than respiratory calicivirus capsids (45), which may explain the faster inactivation of FCV in suspension compared to inactivation of SaV, MNV, and TV. Consistent with our results, it was previously reported that MNV was more stable than FCV when both were cultured under 5% FBS (34) or even when FCV was cultured at a higher FBS concentration than was MNV (10 versus 3%, respectively) (7). While it was beyond the scope of our study to identify the components of the cell lysates mediating the observed effects on the four viruses, it may be that multiple factors in the cell lysates interact to affect the stability of these viruses. Therefore, it is important to purify viral suspensions from cell lysates before choosing to reconstitute them in a given matrix, such as water, a fecal suspension, a soil solution, etc. This will minimize the negative impact of the cell lysates and allow uniform comparisons of HuNoV surrogate survival on leafy greens.
Semipurification of viruses from their cell lysates resulted in a less-degrading and/or more protective effect on the stability of FCV, SaV, MNV, and TV viruses in suspension. The proteolytic enzymes require a higher centrifugal speed to be pelleted (∼500,000 × g) (46), and in general the average molecular mass of eukaryotic proteins is less than 100 kDa (47). Therefore, both ultracentrifugation and ultrafiltration, under the conditions used in our study, are expected to remove many damaging cellular by-products. Also, we performed the survival experiments of viruses in suspension at RT and not at 4°C as a proof of concept that cell lysates can exert a negative effect on virus stability. It is expected that such a negative effect would be more pronounced at RT than 4°C, as the activities of enzymes are slower at 4°C. At a similar initial titer, a previous study reported a lower reduction in infectivity for FCV (3-log10 reduction by day 7 versus ∼6-log10 reduction in the TCID50/ml by day 7 in our study) when generated in cell lysates that were diluted 10-fold in culture media (36). In contrast, our study showed that semipurified FCV exhibited 1-log reduction on day 7 at RT. While dilution of cell lysates lessens the negative effects on the survival of FCV, it decreases the initial viral titers (which may not be desirable) and still leaves damaging cellular by-products. Ultrafiltration and ultracentrifugation treatments showed different effects on reductions in infectivity for the different surrogate viruses. This may have been due to the fact both treatments can retain disparate cytosolic contents that may have differential effects on different viruses. For example, ultracentrifugation at ∼100,000 × g for 1 h retains, in addition to the viruses, the membrane fraction of the cells while the cytosolic contents are removed with the supernatants (46, 48), whereas ultrafiltration retains the lysate contents that are greater than 100 kDa. Therefore, it is equally important when removing cell lysates from the viruses to assess the effect of the viral purification method on the stability of the virus before further applications.
Although fresh produce should be kept cool during its shelf life (∼2 weeks), it can be accidently exposed to RT for short periods of time between harvest and consumption. Therefore, we assessed the survival of FCV, SaV, MNV, and TV on lettuce at both 4°C and RT. Because our “in suspension” experiments provided evidence for the negative impact of cell lysates on the survival of these viruses, we exchanged the different cell lysate matrices to a uniform matrix (water) prior to assessing their survival on lettuce leaves. Irrigation water (preharvest contamination source) and wash water (postharvest contamination source) are potential vehicles for HuNoV contamination of lettuce, justifying the use of water as a suspension matrix in our study. Whether semipurified viruses suspended in water persist longer than viruses mixed with different matrices is largely dependent on the matrix. For example, certain food matrices may inactivate the viruses faster than others (49), while other matrices, such as soil or sediments, to which viruses can adsorb may help the viruses persist longer (as in the case of fecal contamination in rivers and on beaches) (50). In addition, our results indicated that the survival of semipurified MNV on lettuce was not significantly different than that of MNV in cell lysates. Therefore, the survival of semipurified viruses may vary depending on the resuspension matrix. Overall, viruses semipurified by ultrafiltration from cell lysates and suspended in water showed similar persistence to each other under all storage conditions. Under the experimental conditions applied, FCV, SaV, MNV, and TV will be negatively charged, because the calculated isoelectric points (pI; 4.9, 5.4, 4.7, and 4.8, respectively) (11, 51, 52) of their major capsid proteins are lower than the pH of the suspension matrix (water or cell lysates). Therefore, the viral pI is not the primary factor that explains the similar survival characteristics of the semipurified viruses on lettuce, which is consistent with results of a previous study (51). In addition, our results showed that cell lysates and storage conditions exert a significant effect on the survival of FCV, SaV, and TV but not MNV, which may reflect an inherently more stable capsid structure for MNV. In contrast to a previous study that reported an ∼4-log10 reduction in FCV infectivity titer within 7 days at 4°C when the virus was inoculated in cell lysates on iceberg lettuce (19), our study showed an ∼2-log10 reduction at 4°C for 7 days. The latter was cultured in 2% FBS, while the former was cultured in 0% FBS, suggesting that cell lysate contents such as FBS may affect virus survival on lettuce. It was reported, however, that FCV reconstituted in a 10% fecal suspension was reduced by ∼2 log10 on day 7 at 4°C on lettuce (21), indicating again the importance of considering the effect of the suspension matrix in determining virus survival. A 1.5-log reduction in infectivity for 3 days at RT was estimated for MNV suspended in a soil solution and inoculated on lettuce (20). That level is similar to the reduction levels obtained for semipurified and unpurified MNV in our study on day 3 at RT (∼1 to 1.5 log10 TCID50/g), supporting our finding that the suspension matrix for MNV has a limited effect on the virus survival on lettuce. Collectively, virus-infected cell lysates may lessen the survival potential of FCV, SaV, and TV surrogate viruses on lettuce, warranting careful examination of the survival of these HuNoV surrogates without such influences.
In conclusion, the presence of cell lysates can underestimate the survival potential of these viruses, limiting their utility as HuNoV surrogates. Since HuNoV is widely known for its high stability in the environment, enhancing the stability of FCV, SaV, MNV, and TV should benefit efforts focused on using these viruses as HuNoV surrogates. Uniform comparisons of FCV, SaV, MNV, and TV in a similar suspension matrix (water) revealed that these viruses have similar stabilities on postharvest lettuce leaves. The observation that all surrogate viruses remained infectious throughout the lettuce shelf life highlights an increased risk to consumers from accidental HuNoV contamination events and calls for effective countermeasures for fresh produce decontamination.
ACKNOWLEDGMENTS
We thank Issmat Kassem and Michele Williams for their critical review of the manuscript.
This project was supported by Agriculture and Food Research Initiative grant 2011-67017-30067 and grant 2011-68003-30395 from the USDA National Institute of Food and Agriculture. Salaries and research support were provided by state and federal funds provided to the Ohio Agricultural Research and Development Center (OARDC) of The Ohio State University.
REFERENCES
- 1.Hall AJ, Wikswo ME, Pringle K, Gould LH, Parashar UD. 2014. Vital signs: foodborne norovirus outbreaks—United States, 2009–2012. MMWR Morb Mortal Wkly Rep 63:491–495. [PMC free article] [PubMed] [Google Scholar]
- 2.Gould LH, Walsh KA, Vieira AR, Herman K, Williams IT, Hall AJ, Cole D. 2013. Surveillance for foodborne disease outbreaks—United States, 1998–2008. MMWR Surveill Summ 62(SS–02): 1–34. [PubMed] [Google Scholar]
- 3.Duizer E, van Duynhoven Y, Vennema H, Koopmans M. 2004. Failure to detect norovirus in a large group of asymptomatic individuals by Marshall et al. Public Health 118:455–456. http://dx.doi.org/10.1016/j.puhe.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Takanashi S, Saif LJ, Hughes JH, Meulia T, Jung K, Scheuer KA, Wang Q. 2014. Failure of propagation of human norovirus in intestinal epithelial cells with microvilli grown in three-dimensional cultures. Arch Virol 159:257–266. doi: 10.1007/s00705-013-1806-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richards GP. 2012. Critical review of norovirus surrogates in food safety research: rationale for considering volunteer studies. Food Environ Virol 4:6–13. doi: 10.1007/s12560-011-9072-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wobus CE, Karst SM, Thackray LB, Chang KO, Sosnovtsev SV, Belliot G, Krug A, Mackenzie JM, Green KY, Virgin HW. 2004. Replication of norovirus in cell culture reveals a tropism for dendritic cells and macrophages. PLoS Biol 2:e432. doi: 10.1371/journal.pbio.0020432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cannon JL, Papafragkou E, Park GW, Osborne J, Jaykus LA, Vinje J. 2006. Surrogates for the study of norovirus stability and inactivation in the environment: a comparison of murine norovirus and feline calicivirus. J Food Prot 69:2761–2765. [DOI] [PubMed] [Google Scholar]
- 8.Chang KO, Sosnovtsev SV, Belliot G, Kim Y, Saif LJ, Green KY. 2004. Bile acids are essential for porcine enteric calicivirus replication in association with down-regulation of signal transducer and activator of transcription 1. Proc Natl Acad Sci U S A 101:8733–8738. doi: 10.1073/pnas.0401126101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cromeans T, Park GW, Costantini V, Lee D, Wang Q, Farkas T, Lee A, Vinje J. 2014. Comprehensive comparison of cultivable norovirus surrogates in response to different inactivation and disinfection treatments. Appl Environ Microbiol 80:5743–5751. doi: 10.1128/AEM.01532-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Esseili MA, Wang Q, Zhang Z, Saif LJ. 2012. Internalization of sapovirus, a surrogate for norovirus, in romaine lettuce and the effect of lettuce latex on virus infectivity. Appl Environ Microbiol 78:6271–6279. doi: 10.1128/AEM.01295-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Q, Zhang Z, Saif LJ. 2012. Stability of and attachment to lettuce by a culturable porcine sapovirus surrogate for human caliciviruses. Appl Environ Microbiol 78:3932–3940. doi: 10.1128/AEM.06600-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farkas T, Sestak K, Wei C, Jiang X. 2008. Characterization of a rhesus monkey calicivirus representing a new genus of Caliciviridae. J Virol 82:5408–5416. doi: 10.1128/JVI.00070-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirneisen KA, Kniel KE. 2013. Comparing human norovirus surrogates: murine norovirus and Tulane virus. J Food Prot 76:139–143. doi: 10.4315/0362-028X.JFP-12-216. [DOI] [PubMed] [Google Scholar]
- 14.Radford AD, Turner PC, Bennett M, McArdle F, Dawson S, Glenn MA, Williams RA, Gaskell RM. 1998. Quasispecies evolution of a hypervariable region of the feline calicivirus capsid gene in cell culture and in persistently infected cats. J Gen Virol 79:1–10. [DOI] [PubMed] [Google Scholar]
- 15.Kahan SM, Liu G, Reinhard MK, Hsu CC, Livingston RS, Karst SM. 2011. Comparative murine norovirus studies reveal a lack of correlation between intestinal virus titers and enteric pathology. Virology 421:202–210. doi: 10.1016/j.virol.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sestak K, Feely S, Fey B, Dufour J, Hargitt E, Alvarez X, Pahar B, Gregoricus N, Vinje J, Farkas T. 2012. Experimental inoculation of juvenile rhesus macaques with primate enteric caliciviruses. PLoS One 7:e37973. doi: 10.1371/journal.pone.0037973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang QH, Costantini V, Saif LJ. 2007. Porcine enteric caliciviruses: genetic and antigenic relatedness to human caliciviruses, diagnosis and epidemiology. Vaccine 25:5453–5466. doi: 10.1016/j.vaccine.2006.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flynn WT, Saif LJ, Moorhead PD. 1988. Pathogenesis of porcine enteric calicivirus-like virus in four-day-old gnotobiotic pigs. Am J Vet Res 49:819–825. [PubMed] [Google Scholar]
- 19.Allwood PB, Malik YS, Hedberg CW, Goyal SM. 2004. Effect of temperature and sanitizers on the survival of feline calicivirus, Escherichia coli, and F-specific coliphage MS2 on leafy salad vegetables. J Food Prot 67:1451–1456. [DOI] [PubMed] [Google Scholar]
- 20.Fallahi S, Mattison K. 2011. Evaluation of murine norovirus persistence in environments relevant to food production and processing. J Food Prot 74:1847–1851. doi: 10.4315/0362-028X.JFP-11-081. [DOI] [PubMed] [Google Scholar]
- 21.Mattison K, Karthikeyan K, Abebe M, Malik N, Sattar SA, Farber JM, Bidawid S. 2007. Survival of calicivirus in foods and on surfaces: experiments with feline calicivirus as a surrogate for norovirus. J Food Prot 70:500–503. [DOI] [PubMed] [Google Scholar]
- 22.Baert L, Uyttendaele M, Vermeersch M, Van Coillie E, Debevere J. 2008. Survival and transfer of murine norovirus 1, a surrogate for human noroviruses, during the production process of deep-frozen onions and spinach. J Food Prot 71:1590–1597. [DOI] [PubMed] [Google Scholar]
- 23.Escudero BI, Rawsthorne H, Gensel C, Jaykus LA. 2012. Persistence and transferability of noroviruses on and between common surfaces and foods. J Food Prot 75:927–935. doi: 10.4315/0362-028X.JFP-11-460. [DOI] [PubMed] [Google Scholar]
- 24.Croci L, De Medici D, Scalfaro C, Fiore A, Toti L. 2002. The survival of hepatitis A virus in fresh produce. Int J Food Microbiol 73:29–34. doi: 10.1016/S0168-1605(01)00689-4. [DOI] [PubMed] [Google Scholar]
- 25.Bidawid S, Farber JM, Sattar SA. 2001. Survival of hepatitis A virus on modified atmosphere-packaged (MAP) lettuce. Food Microbiol 18:95–102. doi: 10.1006/fmic.2000.0380. [DOI] [Google Scholar]
- 26.Hull RN, Cherry WR, Weaver GW. 1976. The origin and characteristics of a pig kidney cell strain, LLC-PK. In Vitro 12:670–677. doi: 10.1007/BF02797469. [DOI] [PubMed] [Google Scholar]
- 27.ATCC. 2014. RAW 264.7 (ATCC TIB-71); general information. http://www.atcc.org/products/all/TIB-71.aspx#characteristics. Accessed 9 December 2014. [Google Scholar]
- 28.Hwang S, Alhatlani B, Arias A, Caddy SL, Christodoulou C, Cunha JB, Emmott E, Gonzalez-Hernandez M, Kolawole A, Lu J, Rippinger C, Sorgeloos F, Thorne L, Vashist S, Goodfellow I, Wobus CE. 2014. Murine norovirus: propagation, quantification, and genetic manipulation. Curr Protoc Microbiol 33:15K.2.1–15K.2.61. doi: 10.1002/9780471729259.mc15k02s33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kitajima M, Oka T, Tohya Y, Katayama H, Takeda N, Katayama K. 2009. Development of a broadly reactive nested reverse transcription-PCR assay to detect murine noroviruses, and investigation of the prevalence of murine noroviruses in laboratory mice in Japan. Microbiol Immunol 53:531–534. doi: 10.1111/j.1348-0421.2009.00152.x. [DOI] [PubMed] [Google Scholar]
- 30.Reed LJ, Muench H. 1938. A simple method of estimating fifty percent endpoints. Am J Hygiene 27:493–497. [Google Scholar]
- 31.Coroadinha AS, Ribeiro J, Roldao A, Cruz PE, Alves PM, Merten OW, Carrondo MJ. 2006. Effect of medium sugar source on the production of retroviral vectors for gene therapy. Biotechnol Bioeng 94:24–36. doi: 10.1002/bit.20778. [DOI] [PubMed] [Google Scholar]
- 32.Genzel Y, Fischer M, Reichl U. 2006. Serum-free influenza virus production avoiding washing steps and medium exchange in large-scale microcarrier culture. Vaccine 24:3261–3272. doi: 10.1016/j.vaccine.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 33.Dicaprio E, Ma Y, Purgianto A, Hughes J, Li J. 2012. Internalization and dissemination of human norovirus and animal caliciviruses in hydroponically grown romaine lettuce. Appl Environ Microbiol 78:6143–6152. doi: 10.1128/AEM.01081-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bae J, Schwab KJ. 2008. Evaluation of murine norovirus, feline calicivirus, poliovirus, and MS2 as surrogates for human norovirus in a model of viral persistence in surface water and groundwater. Appl Environ Microbiol 74:477–484. doi: 10.1128/AEM.02095-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hirneisen KA, Kniel KE. 2013. Norovirus surrogate survival on spinach during preharvest growth. Phytopathology 103:389–394. doi: 10.1094/PHYTO-09-12-0231-FI. [DOI] [PubMed] [Google Scholar]
- 36.Duizer E, Bijkerk P, Rockx B, De Groot A, Twisk F, Koopmans M. 2004. Inactivation of caliciviruses. Appl Environ Microbiol 70:4538–4543. doi: 10.1128/AEM.70.8.4538-4543.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sattar SA, Ali M, Tetro JA. 2011. In vivo comparison of two human norovirus surrogates for testing ethanol-based handrubs: the mouse chasing the cat! PLoS One 6:e17340. doi: 10.1371/journal.pone.0017340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Doultree JC, Druce JD, Birch CJ, Bowden DS, Marshall JA. 1999. Inactivation of feline calicivirus, a Norwalk virus surrogate. J Hosp Infect 41:51–57. doi: 10.1016/S0195-6701(99)90037-3. [DOI] [PubMed] [Google Scholar]
- 39.Gstraunthaler G. 2003. Alternatives to the use of fetal bovine serum: serum-free cell culture. ALTEX 20:275–281. [PubMed] [Google Scholar]
- 40.Bhatt PN, Jacoby RO. 1987. Stability of ectromelia virus strain NIH-79 under various laboratory conditions. Lab Anim Sci 37:33–35. [PubMed] [Google Scholar]
- 41.Kocan RM, Hershberger PK, Elder NE. 2001. Survival of the North American strain of viral hemorrhagic septicemia virus (VHSV) in filtered seawater and seawater containing ovarian fluid, crude oil and serum-enriched culture medium. Dis Aquat Organ 44:75–78. doi: 10.3354/dao044075. [DOI] [PubMed] [Google Scholar]
- 42.Lotlikar MS, Lipson SM. 2002. Survival of spumavirus, a primate retrovirus, in laboratory media and water. FEMS Microbiol Lett 211:207–211. doi: 10.1111/j.1574-6968.2002.tb11226.x. [DOI] [PubMed] [Google Scholar]
- 43.Jean J, Morales-Rayas R, Anoman MN, Lamhoujeb S. 2011. Inactivation of hepatitis A virus and norovirus surrogate in suspension and on food-contact surfaces using pulsed UV light (pulsed light inactivation of food-borne viruses). Food Microbiol 28:568–572. doi: 10.1016/j.fm.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 44.Feng K, Divers E, Ma Y, Li J. 2011. Inactivation of a human norovirus surrogate, human norovirus virus-like particles, and vesicular stomatitis virus by gamma irradiation. Appl Environ Microbiol 77:3507–3517. doi: 10.1128/AEM.00081-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Roda Husman AM, Bijkerk P, Lodder W, Van Den Berg H, Pribil W, Cabaj A, Gehringer P, Sommer R, Duizer E. 2004. Calicivirus inactivation by nonionizing (253.7-nanometer-wavelength [UV]) and ionizing (gamma) radiation. Appl Environ Microbiol 70:5089–5093. doi: 10.1128/AEM.70.9.5089-5093.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P (ed). 2002. Molecular biology of the cell, 4th ed Garland Science, New York, NY. [Google Scholar]
- 47.Milo R, Phillips R. 2014. Cell biology by the numbers; work in progress. Garland Science, New York, NY. [Google Scholar]
- 48.Ichim CV, Wells RA. 2011. Generation of high-titer viral preparations by concentration using successive rounds of ultracentrifugation. J Transl Med 9:137. doi: 10.1186/1479-5876-9-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee SJ, Si J, Yun HS, Ko G. 2015. Effect of temperature and relative humidity on the survival of foodborne viruses during food storage. Appl Environ Microbiol 81:2075–2081. doi: 10.1128/AEM.04093-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bosch A, Pintó RM, Abad FX. 2006. Survival and transport of enteric viruses in the environment, p 151-187 In Goyal S. (ed), Viruses in foods. Springer, New York, NY. [Google Scholar]
- 51.Vega E, Smith J, Garland J, Matos A, Pillaii SD. 2005. Variability of virus attachment patterns to butterhead lettuce. J Food Prot 68:2112–2117. [DOI] [PubMed] [Google Scholar]
- 52.Bolton SL, Kotwal G, Harrison MA, Law SE, Harrison JA, Cannon JL. 2013. Sanitizer efficacy against murine norovirus, a surrogate for human norovirus, on stainless steel surfaces when using three application methods. Appl Environ Microbiol 79:1368–1377. doi: 10.1128/AEM.02843-12. [DOI] [PMC free article] [PubMed] [Google Scholar]