Abstract
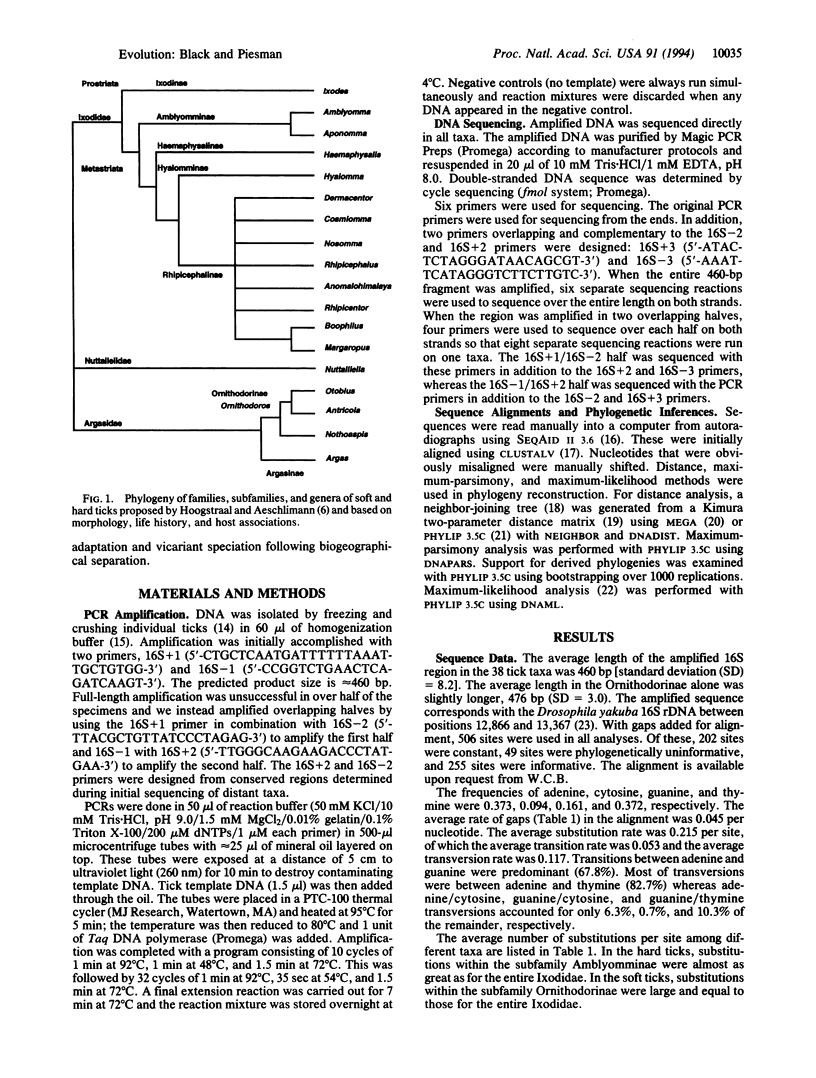
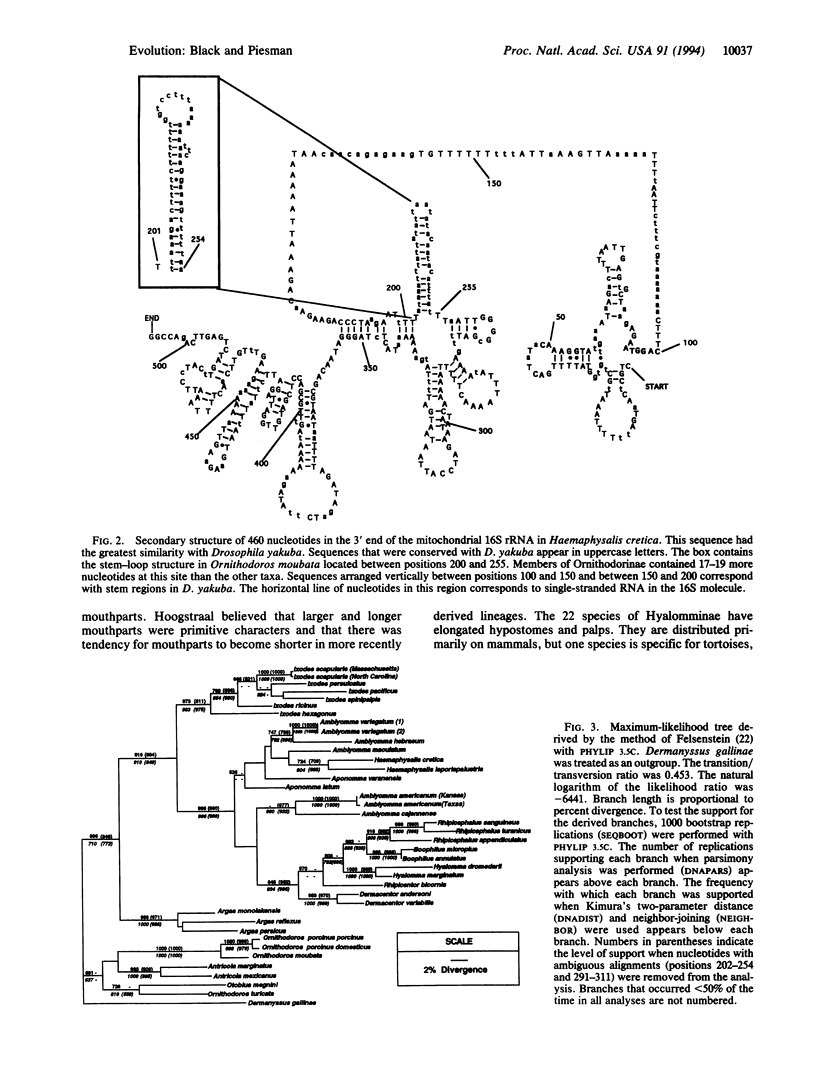
Ticks are parasitiform mites that are obligate hematophagous ectoparasites of amphibians, reptiles, birds, and mammals. A phylogeny for tick families, subfamilies, and genera has been described based on morphological characters, life histories, and host associations. To test the existing phylogeny, we sequenced approximately 460 bp from the 3' end of the mitochondrial 16S rRNA gene (rDNA) in 36 hard- and soft-tick species; a mesostigmatid mite, Dermanyssus gallinae, was used as an outgroup. Phylogenies derived using distance, maximum-parsimony, or maximum-likelihood methods were congruent. The existing phylogeny was largely supported with four exceptions. In hard ticks (Ixodidae), members of Haemaphysalinae were monophyletic with the primitive Amblyomminae and members of Hyalomminae grouped within the Rhipicephalinae. In soft ticks (Argasidae), the derived phylogeny failed to support a monophyletic relationship among members of Ornithodorinae and supported placement of Argasinae as basal to the Ixodidae, suggesting that hard ticks may have originated from an Argas-like ancestor. Because most Argas species are obligate bird octoparasites, this result supports earlier suggestions that hard ticks did not evolve until the late Cretaceous.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clary D. O., Wolstenholme D. R. The mitochondrial DNA molecular of Drosophila yakuba: nucleotide sequence, gene organization, and genetic code. J Mol Evol. 1985;22(3):252–271. doi: 10.1007/BF02099755. [DOI] [PubMed] [Google Scholar]
- Clary D. O., Wolstenholme D. R. The ribosomal RNA genes of Drosophila mitochondrial DNA. Nucleic Acids Res. 1985 Jun 11;13(11):4029–4045. doi: 10.1093/nar/13.11.4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein J. Evolutionary trees from DNA sequences: a maximum likelihood approach. J Mol Evol. 1981;17(6):368–376. doi: 10.1007/BF01734359. [DOI] [PubMed] [Google Scholar]
- Higgins D. G., Sharp P. M. Fast and sensitive multiple sequence alignments on a microcomputer. Comput Appl Biosci. 1989 Apr;5(2):151–153. doi: 10.1093/bioinformatics/5.2.151. [DOI] [PubMed] [Google Scholar]
- Hoogstraal H. Argasid and nuttalliellid ticks as parasites and vectors. Adv Parasitol. 1985;24:135–238. doi: 10.1016/s0065-308x(08)60563-1. [DOI] [PubMed] [Google Scholar]
- Johnson B. J., Happ C. M., Mayer L. W., Piesman J. Detection of Borrelia burgdorferi in ticks by species-specific amplification of the flagellin gene. Am J Trop Med Hyg. 1992 Dec;47(6):730–741. doi: 10.4269/ajtmh.1992.47.730. [DOI] [PubMed] [Google Scholar]
- Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 1980 Dec;16(2):111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- Oliver J. H., Jr, Owsley M. R., Hutcheson H. J., James A. M., Chen C., Irby W. S., Dotson E. M., McLain D. K. Conspecificity of the ticks Ixodes scapularis and I. dammini (Acari: Ixodidae). J Med Entomol. 1993 Jan;30(1):54–63. doi: 10.1093/jmedent/30.1.54. [DOI] [PubMed] [Google Scholar]
- Saitou N., Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987 Jul;4(4):406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Wesson D. M., McLain D. K., Oliver J. H., Piesman J., Collins F. H. Investigation of the validity of species status of Ixodes dammini (Acari: Ixodidae) using rDNA. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):10221–10225. doi: 10.1073/pnas.90.21.10221. [DOI] [PMC free article] [PubMed] [Google Scholar]



