Abstract
Vanilla beans were analyzed using biochemical methods, which revealed that glucovanillin disperses from the inner part to the outer part of the vanilla bean during the curing process and is simultaneously hydrolyzed by β-d-glucosidase. Enzymatic hydrolysis was found to occur on the surface of the vanilla beans. Transcripts of the β-d-glucosidase gene (bgl) of colonizing microorganisms were detected. The results directly indicate that colonizing microorganisms are involved in glucovanillin hydrolysis. Phylogenetic analysis based on 16S rRNA gene sequences showed that the colonizing microorganisms mainly belonged to the Bacillus genus. bgl was detected in all the isolates and presented clustering similar to that of the isolate taxonomy. Furthermore, inoculation of green fluorescent protein-tagged isolates showed that the Bacillus isolates can colonize vanilla beans. Glucovanillin was metabolized as the sole source of carbon in a culture of the isolates within 24 h. These isolates presented unique glucovanillin degradation capabilities. Vanillin was the major volatile compound in the culture. Other compounds, such as α-cubebene, β-pinene, and guaiacol, were detected in some isolate cultures. Colonizing Bacillus isolates were found to hydrolyze glucovanillin in culture, indirectly demonstrating the involvement of colonizing Bacillus isolates in glucovanillin hydrolysis during the vanilla curing process. Based on these results, we conclude that colonizing Bacillus isolates produce β-d-glucosidase, which mediates glucovanillin hydrolysis and influences flavor formation.
INTRODUCTION
Vanilla flavoring obtained from cured Vanilla planifolia beans is widely used in food, beverages, and cosmetics, such that the total worldwide consumption is markedly increasing (1, 2). The characteristics of the vanilla flavor are formed only during a careful curing process that yields the main aromatic constituent, vanillin, and over 200 other volatile compounds with delicate sweet fragrances (3).
The conventional curing process starts with a blanching step in which the mature green vanilla bean is immersed in hot water for 3 to 5 min. The vanilla beans are then subjected to a process that involves periodic sweating and drying. During the remaining part of the day, the vanilla beans are allowed to acclimate on wooden racks in a well-ventilated room and are then stored in small bundles in plastic vacuum bags at room temperature (4, 5).
In fresh vanilla beans, vanillin is exclusively present in a conjugated form, principally as glucovanillin. At this stage, the beans display no trace of vanilla flavor (6, 7). One of the most important aspects of curing is when glucovanillin comes into contact with β-d-glucosidase, thus releasing free vanillin (8). Thermal treatment, plant enzyme reactions, and microbial activity are all important in vanillin flavor generation (9). Röling et al. (10) reported that differences in microbial abundance, communities, and strain characteristics result in variations in vanilla flavor. These results show that colonizing microorganisms can contribute to flavor formation. Recently, numerous studies have revealed that endogenous plant β-d-glucosidase hydrolyzes glucovanillin; however, the microbial contribution to vanillin formation has never been fully investigated (11).
To characterize microorganisms that colonize vanilla beans on the basis of whether they contribute to flavor formation or not, in this study, we investigated the localization, concentration, and hydrolysis of glucovanillin. Furthermore, the colonizing microorganisms were identified to be β-d-glucosidase-producing Bacillus isolates, and the β-d-glucosidase gene (bgl) was cloned, sequenced, and phylogenetically analyzed. Glucovanillin hydrolysis activity was evaluated, and volatile compound formation in the culture was analyzed.
MATERIALS AND METHODS
Determination of glucovanillin and vanillin.
Vanilla beans were collected in Hainan, China, and cured by the hot air processing method (12). The fleshy area (from the surface to the mesocarp, approximately 50% of the bean) and the placental area (from the mesocarp to the seeds, approximately 50% of the bean) were carefully dissected from the vanilla beans. Seeds were separated from the placental tissue by gentle brushing. Extractions of glucovanillin and vanillin were performed according to the methods of Kumar et al. (13) and Dong et al. (12), respectively. Glucovanillin hydrolysis in the cultures was studied in 10 ml of sterilized mineral salts medium inoculated with 0.1 ml of an initial culture containing each isolate. All cultures were incubated at 28°C. Sample aliquots were collected at regular time intervals. Glucovanillin and vanillin concentrations were analyzed in triplicate using high-pressure liquid chromatography (HPLC; 1260 series HPLC; Agilent, Santa Clara, CA, USA) according to the method of Dong et al. (12), with slight modification. The HPLC was equipped with a Zorbax Eclipse Plus C18 column (4.6 mm by 100 mm; particle size, 3.5 μm; Agilent). Isocratic elution at a flow rate of 1.0 ml/min was performed using a mixture of 20% methanol and 80% acidified water. A UV detector at 280 nm was used, and the column temperature was maintained at 26°C.
Isolation of β-d-glucosidase-producing bacteria.
Cured vanilla beans were chopped into 0.2- to 0.5-cm pieces and placed in a bottle containing sterile distilled water. The bottle was shaken, and then sterile distilled water was used for plating on LB medium. Colonies that developed on the plates were purified until a uniform morphology was obtained. The colonies were transferred to LB medium containing esculin and ferric ammonium citrate. The plates were incubated for 24 h, and colonies surrounded by black halos were selected. Twenty flourishing bacterial isolates (isolates XY1 to XY20) were finally selected for further study.
DNA and RNA manipulation.
Vanilla beans were immersed in sterile normal saline buffer, and the solution was shaken for 1 h before the colonizing microorganisms were eluted. After centrifugation, RNA was extracted from the microorganisms. The procedure was conducted using the RNAiso Plus extraction reagent (TaKaRa) according to the manufacturer's instructions. On the basis of bacterial homology analysis by use of the blastn program, primers were designed for reverse transcription-PCR (RT-PCR) analysis of RNA that codes for β-d-glucosidase. Genomic DNA was isolated using a TIANamp bacteria DNA kit (Tiangen) according to the manufacturer's instructions. The 16S rRNA gene and bgl were amplified from the isolates (14). All the primers used in this study are provided in Table 1.
TABLE 1.
PCR primers used for 16S rRNA gene and bgl sequence analyses
| Primer | Sequence (5′-3′) | Approximate length of product (bp) | Main applicationa |
|---|---|---|---|
| A 8-27fb | AGAGTTTGATCCTGGCTCAG | 1,476 | A |
| B 1523-1504rb | AAGGAGGTGATCCAGCCGCA | ||
| GRTF2 | CACCACCAATTTGTGGC | 47 | B |
| GRTR3 | GCTATCATACAGCCGATTT | ||
| GF4 | GCNYTNTTYGCNGARATGGG | 1,200 | C |
| GR4 | YTCNCCRTTNGTNGCDATNCA | ||
| GF5 | TGATCTGTTTGATGAGCTG | 801 | C |
| GR5 | AATGGGGTAGTCGTCCTG | ||
| GF6 | GCGCTGTTTGCTGAGATG | 799 | C |
| GR6 | ATGGGGTAGTCGTCCTGAA | ||
| GF7 | TTTGAAGAAGGAGAAAACAA | 427 | C |
| GR7 | ATCAATTTGCCAGCCCCA |
A, 16S rRNA gene PCR amplification; B, RNA-encoded β-d-glucosidase RT-PCR amplification; C, bgl PCR amplification.
Cited from Zhou et al. (14).
RNA reverse transcription, PCR amplification, product purification, and cloning into Escherichia coli DH5α were performed as described in a previous report (15). PCRs were performed in 25-ml volumes containing 2.5 ml 10× PCR buffer, 200 mM deoxynucleoside triphosphates, 80 nM each primer, 1.25 U Taq DNA polymerase (TaKaRa, Dalian, China), and 1 μl genomic DNA extract (approximately 10 ng). The reactions were programmed for an initial incubation at 94°C for 3 min, followed by 35 cycles at 94°C for 45 s, 51 to 56°C for 45 s, and 72°C for 30 to 120 s and then a final extension reaction at 72°C for 10 min. PCR products and clones were sequenced by the Beijing Genomics Institute, Beijing, China.
Phylogenetic analysis.
DNA sequences were analyzed using DNAssist (version 2.2) (16). Sequence comparisons with close relatives available from GenBank were performed using the blastn program to approximate the phylogenetic affiliations of the strains. The type strain sequences were obtained from NCBI. The ClustalX program (version 1.81) was then used to adjust the sequences (17). Alignment gaps and ambiguous characters were treated as missing information. Neighbor-joining analyses were performed in MEGA software (version 5) (18), in which all characters were unordered and reversible, and 1,000 bootstrap replicates were run.
Inoculation of vanilla beans with GFP-tagged Bacillus subtilis XY11.
Plasmid pHAPII (GenBank accession number HM151400), an E. coli-B. subtilis shuttle vector that contains the green fluorescent protein (GFP) gene, was provided by Zhenhua Zhang. Representative isolates of B. subtilis XY11 were selected for colonization analysis. The protocol for electroporation of the isolate was carried out as described previously by Zhang et al. (19). GFP-tagged XY11 was inoculated into LB medium containing 20 μg/ml kanamycin and grown to stationary phase. The bacterial cells were washed twice in M8 buffer (22 mM Na2HPO4, 22 mM KH2PO4, 100 mM NaCl) and finally suspended in M8 buffer. Vanilla beans were soaked in the suspension for 30 min at 30°C. Then, the samples were subjected to incubation for 3 days at 30°C prior to microscopic observation. Uninoculated vanilla beans were prepared as controls.
Microscopy.
Vanilla beans were monitored at 3 days after inoculation and washed. Fluorescent strain XY11 cells on the samples were visualized by a confocal laser scanning microscope (CLSM; Ultra View VoX; PerkinElmer, USA). The vanilla beans were cut into pieces 1 to 2 cm long, and the segments were placed on a slide and visualized at an excitation wavelength of 488 nm. The emitted light of the GFP was detected in the range of 500 to 600 nm, and the images were obtained using Leica confocal software (version 2.61).
GC-MS analysis.
The aromatic constituents of cultures that had been incubated for 24 h were extracted with equal volumes of acetic ether and dichloromethane. Gas chromatography (GC)-mass spectrometry (MS) analysis was performed using an Agilent 7890A gas chromatograph coupled to an Agilent 5975C quadrupole mass spectrometer. Volatile compounds were separated on a DB-5 fused-silica capillary column (length, 30 m; inside diameter, 0.25 mm; film thickness, 0.25 μm; J&W Scientific, Folsom, CA, USA). The column temperature was programmed to increase from 40°C to 65°C at a rate of 1.5°C/min; the column was held at the initial temperature and at 65°C for 2 min; the temperature was raised further to 70°C at a rate of 0.5°C/min, to 90°C at a rate of 5°C/min, to 170°C at a rate of 3°C/min, and to 290°C at a rate of 4°C/min; and the column was maintained at a final temperature of 290°C for 2 min. Samples were injected in the splitless mode. The electron impact energy was 70 eV, and the ion source and quadrupole temperatures were set at 230°C and 150°C, respectively. Electron impact mass spectra were recorded in the 30- to 300-atomic-mass-unit range at 1-s intervals. Compounds were identified on the basis of the linear retention index, the interpretation of their mass spectra, and the data available in the spectral library (Wiley/NIST Libraries) of the instrument. The linear retention index was calculated using n-alkanes (C-8 to C-40) as a reference.
Nucleotide sequence accession numbers.
The nucleotide sequences of the rRNA genes and bgl of isolates XY1 to XY20 identified in this study have been placed in GenBank under accession numbers KF986303 to KF986322 and KJ572540 to KJ572559, respectively.
RESULTS
Distribution of glucovanillin and vanillin in vanilla beans.
To examine the distribution of glucovanillin in vanilla beans collected during the curing process, the fleshy and placental areas were separated. The content of glucovanillin is shown in Fig. 1. The glucovanillin content in the fleshy area of green, freshly blanched, sweating, drying, and cured beans was 0.037 ± 0.0003 mmol/g, 0.48 ± 0.0136 mmol/g, 0.259 ± 0.0128 mmol/g, 0.149 ± 0.0114 mmol/g, and 0 mmol/g, respectively. For the placental areas, the glucovanillin content decreased gradually. The highest glucovanillin content was observed in green beans, whereas the lowest was observed in cured beans. As can be seen, the glucovanillin content in the placental area of green, freshly blanched, sweating, drying, and cured beans was 0.883 ± 0.0033 mmol/g, 0.587 ± 0.0068 mmol/g, 0.289 ± 0.0182 mmol/g, 0.133 ± 0.0119 mmol/g, and 0 mmol/g, respectively.
FIG 1.
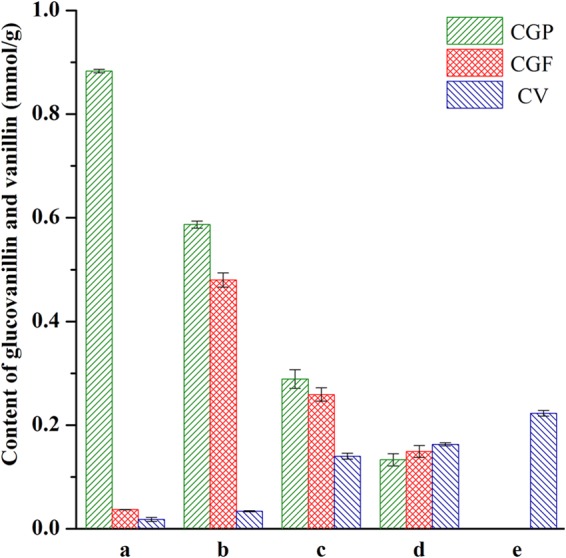
Glucovanillin and vanillin contents of vanilla beans at different stages of the curing process. a, green vanilla beans; b, freshly blanched vanilla beans; c, sweating vanilla beans; d, drying vanilla beans; e, cured vanilla beans; CGP, content of glucovanillin in the placental area as the dry weight of the placental area; CGF, content of glucovanillin in the fleshy area as the dry weight of the fleshy area; CV, content of vanillin as the dry weight of the total vanilla bean.
Among the various flavor compounds reported in vanilla extract, vanillin is the most important component. The vanillin contents of beans collected during different curing periods increased progressively and are shown in Fig. 1. The highest vanillin content was observed in cured beans (0.223 ± 0.0055 mmol/g), whereas the vanillin content in green beans approached 0.018 ± 0.0039 mmol/g. Vanilla beans collected during the blanching, sweating, and drying periods produced only 0.034 ± 0.0011 mmol/g, 0.14 ± 0.0056 mmol/g, and 0.163 ± 0.0028 mmol/g vanillin, respectively.
Detection of bgl transcription.
To evaluate whether colonizing microorganisms produce exogenous β-d-glucosidase, involved in glucovanillin hydrolysis, bgl transcription was monitored. The transcription of bgl was detected in the samples obtained during the blanching, sweating, and drying periods, but the gene was not detected in the sample obtained during the conditioning process (Fig. 2). Failure to detect the bgl transcript in the sample obtained during the conditioning process may be due to the lack of transcription, or the gene could not be detected in the sample used in this study. PCR products were cloned and sequenced, and blastn analysis showed that the bgl fragment has 100% sequence identity to bgl from Bacillus strains (DDBJ accession numbers AB976521 to AB976523).
FIG 2.

RT-PCR amplification of bgl from microorganisms extracted from vanilla beans at different curing process stages. Lane a, a freshly blanched vanilla bean; lane b, a sweating vanilla bean; lane c, a drying vanilla bean; lane d, a conditioning vanilla bean; lane P, positive control (genomic DNA); lane N, negative control (double-distilled water); lanes M, size markers (D2000). The gels were run under the same experimental conditions.
Isolation and identification of strains.
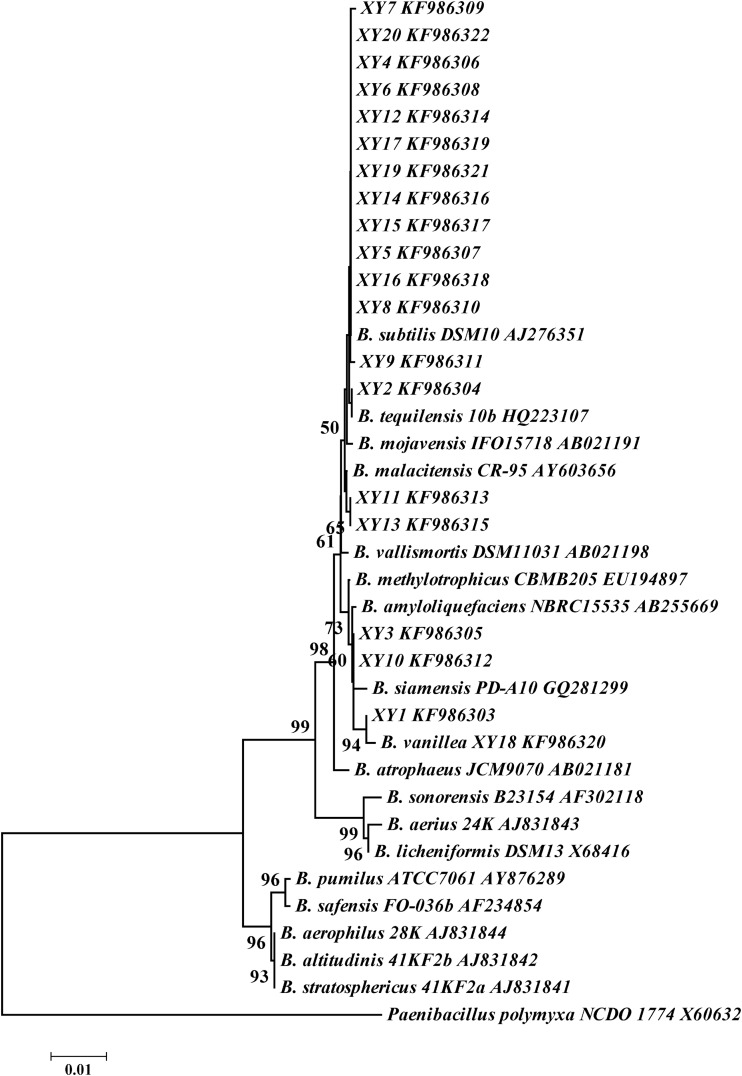
For the purpose of characterizing the microorganisms hydrolyzing glucovanillin, strains colonizing the vanilla beans were isolated and identified. The liquid from washed vanilla beans could be used for strain isolation. Colonies were developed on plates and were then purified. Thirty-two isolates were obtained, and 20 isolates were selected for analysis. The 16S rRNA gene sequences of the isolates showed high levels of similarity (more than 99%) to those of the type strains of B. vanillea and B. subtilis. Neighbor-joining analysis showed that isolates XY1, XY3, and XY10 were the most closely related to B. vanillea XY18. XY18 was deposited in the China General Microbiological Culture Collection Center and The Netherlands Culture Collection of Bacteria (CGMCC 8629 = NCCB 100507). The other 16 isolates were placed in the B. subtilis clade (Fig. 3). Therefore, the isolates that formed halos were identified as Bacillus species (20–23).
FIG 3.
Bacillus 16S rRNA gene phylogram based on neighbor joining. Numbers at the branches are the percentages of trees containing the corresponding clade on the basis of 1,000 bootstrap replications. The strains selected for analysis were type strains.
Detection of β-d-glucosidase-producing isolates.
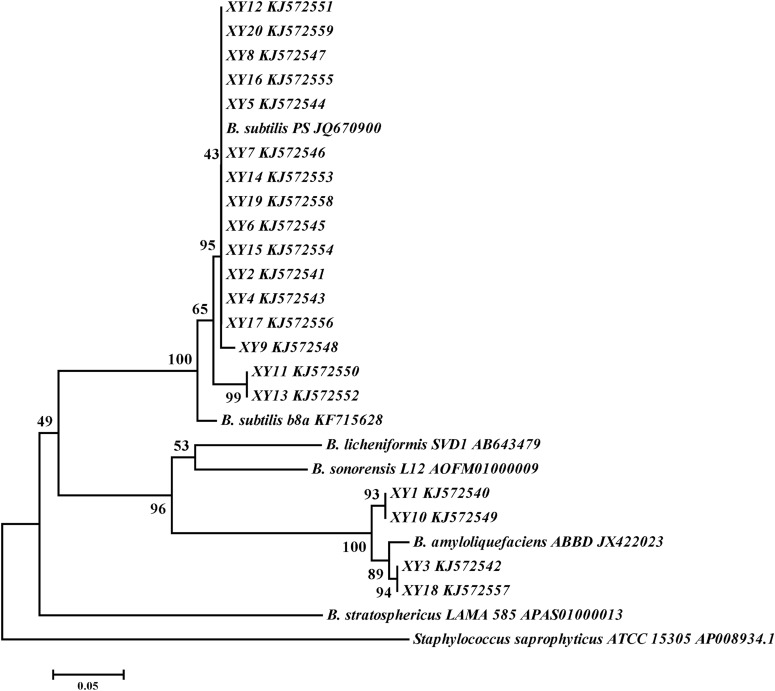
After several rounds of transfers, colonies that could degrade esculin were isolated from the culture medium. Colonies isolated from the vanilla beans formed black halos, indicating that β-d-glucosidase-producing bacteria colonized the cured vanilla beans. bgl fragments were amplified from 20 bacterial isolates and were finally selected for further study. Sequence alignment revealed that all of the amplified fragments shared the most identity with bgl from Bacillus isolates. Phylogenetic trees were constructed on the basis of the sequences of the bgl fragments from 20 isolates along with the sequences of those from representative Bacillus isolates. The topology of the tree was similar to that of the 16S rRNA gene tree, and the bgl cluster exhibited a close relationship with the isolate taxonomy (Fig. 4).
FIG 4.
Bacillus bgl phylogram based on neighbor joining. Numbers at the branches are the percentages of trees containing the corresponding clade on the basis of 1,000 bootstrap replications.
Colonization of vanilla beans by GFP-tagged B. subtilis XY11.
Three days after inoculation, vanilla beans were examined with a CLSM. No fluorescent cells could be observed for the controls (Fig. 5a). However, the GFP-tagged XY11 cells were easily distinguished from the background fluorescence of vanilla beans inoculated with the isolates. The images showed that the green fluorescent B. subtilis XY11 cells colonized the vanilla beans. The location of the cells was found to be discontinuous and dispersive (Fig. 5b).
FIG 5.
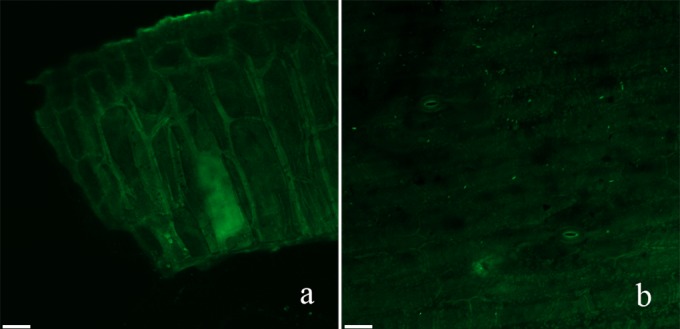
Confocal laser scanning micrographs of the colonization of GFP-tagged XY11 on vanilla beans. (a) Control; (b) GFP-tagged B. subtilis XY11-colonized vanilla beans. Bars = 49 μm.
Hydrolysis of glucovanillin.
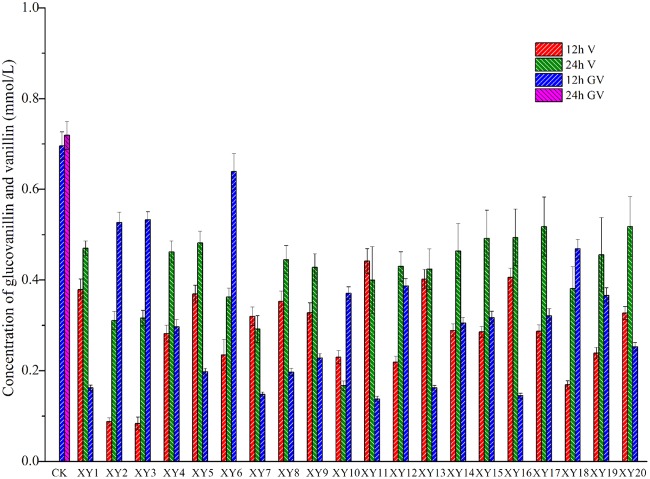
Glucovanillin was metabolized in cultures of 20 isolates with glucovanillin as the sole carbon source. Partial conversion occurred at 12 h, and complete conversion was achieved within 24 h. This result is similar to that obtained previously by direct evaluations of biochemistry, in which glucovanillin could be hydrolyzed by colonizing Bacillus isolates. The ability to hydrolyze glucovanillin to vanillin was unique among the isolates. As shown in Fig. 6, the HPLC profiles indicated the concentrations of glucovanillin and vanillin. According to the ability to convert glucovanillin to vanillin (Tukey test), the group that was the most able to convert glucovanillin to vanillin included XY1, XY4, XY5, XY8, XY9, XY11, XY12, XY13, XY14, XY15, XY16, XY17, XY18, XY19, and XY20, and the group that was the least able to convert glucovanillin to vanillin included XY7 and XY10. This result suggests that isolates of the group that was the most able to convert glucovanillin to vanillin are suitable for assisting in vanillin production. Compared with the data points for the theoretical amount of vanillin that could be converted, the quantity of glucovanillin actually converted was reduced. This result indicates that the isolates converted glucovanillin to vanillin and other compounds (1). Alternatively, the isolates converted glucovanillin solely to vanillin and transformed vanillin to other compounds.
FIG 6.
Hydrolysis of glucovanillin by 20 isolates. GV, glucovanillin; V, vanillin. All the isolates belonged to the genus Bacillus, and the species were as follows: B. subtilis for isolates XY2, XY4, XY5, XY6, XY7, XY8, XY9, XY11, XY12, XY13, XY14, XY15, XY16, XY17, XY19, and XY20 and B. vanillea for isolates XY1, XY3, XY10, and XY18. CK, without inoculating any isolates.
Odor compounds produced by Bacillus strains.
GC-MS analysis was performed to evaluate the volatile compound composition of metabolized glucovanillin (23). Organic solvents that differ in polarity were tested because volatile compounds related to vanilla present a wide range of functional groups (Table 2). The GC-MS profiles of the culture extracts showed the presence of compounds A, B, C, and D. Compound A, with a retention time (tR) of 47.065 min, showed a prominent protonated molecular ion that matched that of the α-cubebene standard. Compound B, with a tR of 16.305 min, showed a molecular ion that matched that of the β-pinene standard. Compound C, with a tR of 26.329 min, showed a molecular ion that matched that of the guaiacol standard. Compound D, with a tR of 47.965 min, showed a molecular ion that matched that of the vanillin standard.
TABLE 2.
Volatile compounds detected in aroma extracts obtained from isolate cultures using acetic ether and dichloromethane
| Compound | Isolates |
|---|---|
| α-Cubebene | XY9,a XY16a |
| β-Pinene | XY5,a XY10a |
| Guaiacol | XY1,a XY2,a XY3,a,b XY5,a XY6,a XY7,a,b XY8,a XY9,a XY10,a,b XY11,a XY12,b XY16,b XY18,a,b XY19a |
| Vanillin | XY1,a,b XY2,a,b XY3,a,b XY4,a,b XY5,a,b XY6,a,b XY7,a,b XY8,a,b XY9,a,b XY10,a,b XY11,a,b XY12,a,b XY13,a,b XY14,a,b XY15,a,b XY16,a,b XY17,a,b XY18,a,b XY19,a,b XY20a,b |
Acetic ether extract.
Dichloromethane extract.
DISCUSSION
Biochemical data revealed that the amount of glucovanillin in the fleshy areas increased after the initial blanching step and decreased progressively during the sweating, drying, and conditioning processes (Fig. 1). Interestingly, total glucovanillin increased after the blanching treatment, possibly due to the heat treatment leading to cellular decompartmentalization and improving the extraction of glucovanillin (24). The present study proved that glucovanillin dispersed from the placental area to the fleshy area and was completely hydrolyzed by β-d-glucosidase. This may reflect the fact that glucovanillin comes into contact with microorganisms on the surface of vanilla beans, resulting in hydrolysis by these microorganisms.
Previous studies have reported nearly total losses of endogenous β-d-glucosidase activity in the first 24 h after heat treatment during traditional and laboratory curing; nonetheless, glucovanillin hydrolysis and vanillin formation continued during the later stages (25, 26). Similarly, a discrepancy between the loss of endogenous β-d-glucosidase activity and the conversion of glucovanillin to vanillin was also observed in this study (data not shown). This could indicate that exogenous β-d-glucosidase is involved in glucovanillin hydrolysis because it could not be detected using the endogenous enzymatic test.
The inoculation of GFP-tagged isolates showed that the Bacillus isolates can colonize vanilla beans (Fig. 5). RT-PCR analysis revealed that the colonizing Bacillus isolates transcribed bgl after the loss of endogenous β-d-glucosidase activity (Fig. 2). This could explain the total hydrolysis of glucovanillin, despite the huge loss in endogenous enzymatic activity after the 24-h heat treatment (6). Odoux et al. (8) observed a small amount of residual β-d-glucosidase activity that could not be quantified under the conditions of their test; however, β-d-glucosidase continued hydrolyzing glucovanillin with extremely low kinetics to achieve total glucovanillin hydrolysis. This finding agrees with the low kinetics of the hydrolysis reactions. The colonizing Bacillus isolates produced exogenous β-d-glucosidase to hydrolyze glucovanillin.
Most previous studies showed that the hydrolytic release of vanillin is catalyzed by the endogenous β-d-glucosidase of vanilla beans (25–27). Only a few studies have demonstrated that β-d-glucosidase might also originate from microorganisms. The vanillin content obtained with the conventional method of curing of vanilla beans was significantly greater than that obtained without microorganism assistance with curing. Furthermore, a control experiment showed that the vanillin content in the sample cured with the assistance of the Bacillus isolates was greater than that in the sterile sample (see Fig. S1 in the supplemental material). Large differences in the number, species composition, and enzymatic abilities of vanilla-colonizing microorganisms have been observed (10, 28, 29). This indicates that the development of vanilla flavor could be affected by microbial activity. However, bgl was not monitored in the colonizing isolates. Evidence for the involvement of microorganisms in glucovanillin hydrolysis and their role in flavor formation has not been reported. In this study, an abundance of Bacillus isolates with bgl was isolated from cured vanilla beans (Fig. 3 and 4). Bacillus isolates were able to metabolize glucovanillin in culture (Fig. 6). This indirectly reveals that Bacillus isolates colonizing vanilla beans have the ability to produce β-d-glucosidase and hydrolyze glucovanillin.
Strains isolated from soil identified as B. subtilis, B. fusiformis, and B. pumilus are capable of transforming isoeugenol to vanillin (30–32). Additionally, another B. subtilis isolate showed the ability to produce tetramethylpyrazine (a commonly occurring alkylpyrazine that is responsible for the odor of oriental foods, such as Chinese liquors) via the precursor 3-hydroxy-2-butanone (33). Combined with the findings of the present study, this suggests that Bacillus isolates can convert aromatic precursors and influence flavor formation (34, 35).
GC-MS analysis was performed to identify aromatic compounds produced by colonizing Bacillus isolates that hydrolyze glucovanillin. Guaiacol, a volatile compound abundant in vanilla beans, was detected in 14 isolate culture extracts. Phenolic compounds are responsible for the characteristic notes of the V. planifolia flavor, giving sweet, smoky, woody, and balsamic notes (23). Brunschwig et al. (36) reported that guaiacol was rather intense in the V. tahitensis flavor, with odor-specific magnitude estimation values being 6.5% to 8.1%. Phenolic compounds were also identified to be the key components of Tahitian vanilla flavor, yielding strong smoky notes. In this study, Bacillus isolates recovered from cured vanilla beans converted glucovanillin to guaiacol, indicating that the mechanism of guaiacol generation may involve colonizing microorganisms instead of Vanilla species. Clear differences between the aromatic compounds identified in organic aroma extracts from cultures of each isolate were observed (Table 2). The major volatile compound, vanillin, was detected in all cultures. However, α-cubebene, β-pinene, and guaiacol were observed in only a few cultures. The characteristic notes of vanilla beans might depend on not only the Vanilla species but also the colonizing isolates.
A previous study has shown that Alicyclobacillus acidoterrestris in apple juice started to form guaiacol from vanillin when the spore count exceeded the critical level of 104 CFU/ml (37). Similarly, the guaiacol detected in the extracts of Bacillus isolates was possibly formed from vanillin. To our knowledge, there is still a lack of any evidence that α-cubebene and β-pinene could be transformed from vanillin and glucovanillin by bacterial metabolism. The pathway of formation of these two compounds remains unclear. Furthermore, the isolates persist to produce α-cubebene, β-pinene, and guaiacol after preservation; alternatively, the deterioration of this ability occurs. Further research of this is needed.
Reconstitution of the vanilla flavor profile with β-d-glucosidase-producing isolates would be the scope of future studies in this area. Moreover, the whole sequence of bgl needs to be cloned, and the enzymatic activity of exogenous β-d-glucosidase needs to be evaluated. In summary, this study presents direct and indirect evidence that β-d-glucosidase-producing Bacillus isolates recovered from vanilla beans are involved in glucovanillin hydrolysis and influence flavor formation during the curing of vanilla.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by funds from the National Science and Technology Support Program (2012BAD36B03) and the Chinese Central Public-Interest Scientific Institution Basal Research Fund (1630052012017).
We thank Qinghuang Wang for critical reading of the manuscript and Zhenhua Zhang for the contribution of the plasmid.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.00458-15.
REFERENCES
- 1.Kaur B, Chakraborty D. 2013. Biotechnological and molecular approaches for vanillin production: a review. Appl Biochem Biotechnol 169:1353–1372. doi: 10.1007/s12010-012-0066-1. [DOI] [PubMed] [Google Scholar]
- 2.Korthou H, Verpoorte R. 2007. Vanilla, p 203–217. In Berger RG. (ed), Flavours and fragrances, vol 1 Springer-Verlag, Berlin, Germany. [Google Scholar]
- 3.Sharp MD, Kocaoglu-Vurma NA, Langford V, Rodriguez-Saona LE, Harper JW. 2012. Rapid discrimination and characterization of vanilla bean extracts by attenuated total reflection infrared spectroscopy and selected ion flow tube mass spectrometry. J Food Sci 77:284–292. doi: 10.1111/j.1750-3841.2011.02544.x. [DOI] [PubMed] [Google Scholar]
- 4.Mariezcurrena MD, Zavaleta HA, Waliszewski KN, Sánchez V. 2008. The effect of killing conditions on the structural changes in vanilla (Vanilla planifolia, Andrews) pods during the curing process. Int J Food Sci Technol 43:1452–1457. doi: 10.1111/j.1365-2621.2007.01691.x. [DOI] [Google Scholar]
- 5.Sreedhar RV, Roohie K, Maya P, Venkatachalam L, Bhagyalakshmi N. 2009. Biotic elicitors enhance flavor compounds during accelerated curing of vanilla beans. Food Chem 112:461–468. doi: 10.1016/j.foodchem.2008.05.108. [DOI] [Google Scholar]
- 6.Odoux E. 2006. Glucosylated aroma precursors and glucosidase(s) in vanilla bean (Vanilla planifolia G. Jackson). Fruits 61:171–184. doi: 10.1051/fruits:2006015. [DOI] [Google Scholar]
- 7.Walton NJ, Mayer MJ, Narbad A. 2003. Vanillin. Phytochemistry 63:505–515. doi: 10.1016/S0031-9422(03)00149-3. [DOI] [PubMed] [Google Scholar]
- 8.Odoux E, Escoute J, Verdeil JL. 2006. The relation between glucovanillin, β-d-glucosidase activity and cellular compartmentation during the senescence, freezing and traditional curing of vanilla beans. Ann Appl Biol 149:43–52. doi: 10.1111/j.1744-7348.2006.00071.x. [DOI] [Google Scholar]
- 9.Ranadive AS. 1994. Vanilla—cultivation, curing, chemistry, technology and commercial products, p 517–577. In Charalambrous G. (ed), Spices, herbs and edible fungi. Developments in food science, vol 34 Elsevier, Amsterdam, The Netherlands. [Google Scholar]
- 10.Röling WFM, Josef K, Martin B, Anton A, Stam H, van Verseveld HW. 2001. Microorganisms with a taste for vanilla: microbial ecology of traditional Indonesian vanilla curing. Appl Environ Microbiol 67:1995–2003. doi: 10.1128/AEM.67.5.1995-2003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frenkel C, Ranadive AS, Vázquez JT, Havkin-Frenkel D. 2011. Curing of vanilla, p 84–96. In Havkin-Frenkel D, Belanger FC (ed), Handbook of vanilla science and technology, vol 1 Wiley-Blackwell, Oxford, United Kingdom. [Google Scholar]
- 12.Dong ZZ, Gu FL, Xu F, Wang QH. 2014. Comparison of four kinds of extraction techniques and kinetics of microwave-assisted extraction of vanillin from Vanilla planifolia Andrews. Food Chem 149:54–61. doi: 10.1016/j.foodchem.2013.10.052. [DOI] [PubMed] [Google Scholar]
- 13.Kumar KK, AnanthaKumar AA, Ahmad R, Adhikari S, Variyar PS, Sharma A. 2010. Effect of gamma-radiation on major aroma compounds and vanillin glucoside of cured vanilla beans (Vanilla planifolia). Food Chem 122:841–845. doi: 10.1016/j.foodchem.2010.03.006. [DOI] [Google Scholar]
- 14.Zhou Y, Wei W, Che QL, Xu YX, Wang X, Huang X, Lai R. 2008. Bacillus pallidus sp. nov., isolated from forest soil. Int J Syst Evol Microbiol 58:2850–2854. doi: 10.1099/ijs.0.2008/000075-0. [DOI] [PubMed] [Google Scholar]
- 15.Yong XY, Zhang RF, Zhang N, Chen YL, Huang XQ, Zhao J, Shen QR. 2013. Development of a specific real-time PCR assay targeting the poly-γ-glutamic acid synthesis gene, pgsB, for the quantification of Bacillus amyloliquefaciens in solid-state fermentation. Bioresour Technol 129:477–484. doi: 10.1016/j.biortech.2012.11.092. [DOI] [PubMed] [Google Scholar]
- 16.Patterton HG, Graves S. 2000. DNAssist: the integrated editing and analysis of molecular biology sequences in Windows. Bioinformatics 16:652–653. doi: 10.1093/bioinformatics/16.7.652. [DOI] [PubMed] [Google Scholar]
- 17.Thompson JD, Higgins T, Gibson J. 1994. Clustal W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang N, Wu K, He X, Li SQ, Zhang ZH, Shen B, Yang XM, Zhang RF, Huang QW, Shen QR. 2011. A new bioorganic fertilizer can effectively control banana wilt by strong colonization with Bacillus subtilis N11. Plant Soil 344:87–97. doi: 10.1007/s11104-011-0729-7. [DOI] [Google Scholar]
- 20.Koeppel A, Perry EB, Sikorski J, Krizanc D, Warner A, Ward DM, Rooney AP, Brambilla E, Connor N, Ratcliff RM, Nevo E, Cohan FM. 2008. Identifying the fundamental units of bacterial diversity: a paradigm shift to incorporate ecology into bacterial systematics. Proc Natl Acad Sci U S A 105:2504–2509. doi: 10.1073/pnas.0712205105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakamura LK, Roberts MS, Cohan FM. 1999. Relationship of Bacillus subtilis clades associated with strains 168 and W23: a proposal for Bacillus subtilis subsp. subtilis subsp. nov. and Bacillus subtilis subsp. spizizenii subsp. nov. Int J Syst Evol Microbiol 49:1211–1215. [DOI] [PubMed] [Google Scholar]
- 22.Chen YG, Gu FL, Li JH, Xu F, He SZ, Fang YM. 2015. Bacillus vanillea sp. nov., isolated from the cured vanilla bean. Curr Microbiol 70:235–239. doi: 10.1007/s00284-014-0707-4. [DOI] [PubMed] [Google Scholar]
- 23.Pérez-Silva A, Odoux E, Brat P, Ribeyre F, Rodriguez-Jimenes G, Robles-Olvera V, Garcia-Alvarado MA, Gunata Z. 2006. GC-MS and GC-olfactometry analysis of aroma compounds in a representative organic aroma extract from cured vanilla (Vanilla planifolia G. Jackson) beans. Food Chem 99:728–735. doi: 10.1016/j.foodchem.2005.08.050. [DOI] [Google Scholar]
- 24.Puri M, Sharma D, Barrow CJ. 2012. Enzyme-assisted extraction of bioactives from plants. Trends Biotechnol 30:37–44. doi: 10.1016/j.tibtech.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 25.Dignum MJW, Kerler J, Verpoorte R. 2002. Vanilla curing under laboratory conditions. Food Chem 79:165–171. doi: 10.1016/S0308-8146(02)00125-5. [DOI] [Google Scholar]
- 26.Havkin-Frenkel D, French JC, Pak F, Frenkel C. 2005. Inside vanilla: Vanilla planifolia's botany, curing options and future market prospects. Perfum Flav 30:36–55. [Google Scholar]
- 27.Madhava Naidu M, Kumar PVS, Shyamala BN, Sulochanamma G, Prakash M, Thakur MS. 2012. Enzyme-assisted process for production of superior quality vanilla extracts from green vanilla pods using tea leaf enzymes. Food Bioprocess Technol 5:527–532. doi: 10.1007/s11947-009-0291-y. [DOI] [Google Scholar]
- 28.Odoux E, Escoute J, Verdeil JL, Brillouet JM. 2003. Localization of β-d-glucosidase activity and glucovanillin in vanilla bean (Vanilla planifolia Andrews). Ann Bot 92:437–444. doi: 10.1093/aob/mcg150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.General T, Mamatha V, Divya KA, Appaiah KAA. 2009. Diversity of yeast with β-glycosidase activity in vanilla (Vanilla planifolia) plant. Curr Sci 96:1501–1505. [Google Scholar]
- 30.Hua DL, Ma CQ, Shan L, Song LF, Deng ZX, Maomy ZR, Zhang ZB, Yu B, Xu P. 2007. Biotransformation of isoeugenol to vanillin by a newly isolated Bacillus pumilus strain: identification of major metabolites. J Biotechnol 130:463–470. doi: 10.1016/j.jbiotec.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 31.Shimoni E, Ravid U, Shoham Y. 2000. Isolation of a Bacillus sp. capable of transforming isoeugenol to vanillin. J Biotechnol 78:1–9. doi: 10.1016/S0168-1656(99)00199-6. [DOI] [PubMed] [Google Scholar]
- 32.Zhao LQ, Sun ZH, Zheng P, Zhu LL. 2005. Biotransformation of isoeugenol to vanillin by a novel strain of Bacillus fusiformis. J Biotechnol 27:1505–1509. doi: 10.1007/s10529-005-1466-x. [DOI] [PubMed] [Google Scholar]
- 33.Zhu BF, Xu Y, Fan WL. 2010. High-yield fermentative preparation of tetramethylpyrazine by Bacillus sp. using an endogenous precursor approach. J Ind Microbiol Biotechnol 37:179–186. doi: 10.1007/s10295-009-0661-5. [DOI] [PubMed] [Google Scholar]
- 34.Gilberta JA, van der Lelie D, Zarraonaindia I. 2014. Microbial terroir for wine grapes. Proc Natl Acad Sci U S A 111:5–6. doi: 10.1073/pnas.1320471110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akasaka N, Sakoda H, Hidese R, Ishii Y, Fujiwara S. 2013. An efficient method using Gluconacetobacter europaeus to reduce an unfavorable flavor compound, acetoin, in rice vinegar production. Appl Environ Microbiol 79:7334–7342. doi: 10.1128/AEM.02397-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brunschwig C, Senger-Emonnot P, Aubanel ML, Pierrat A, George G, Rochard S, Raharivelomanana P. 2012. Odor-active compounds of Tahitian vanilla flavor. Food Res Int 46:148–157. doi: 10.1016/j.foodres.2011.12.006. [DOI] [Google Scholar]
- 37.Bahçeci KS, Gökmen V, Acar J. 2005. Formation of guaiacol from vanillin by Alicyclobacillus acidoterrestris in apple juice: a model study. Eur Food Res Technol 220:196–199. doi: 10.1007/s00217-004-1018-y. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.