Abstract
Because of high diurnal water quality fluctuations in raw municipal wastewater, the use of proportional autosampling over a period of 24 h at municipal wastewater treatment plants (WWTPs) to evaluate carbon, nitrogen, and phosphorus removal has become a standard in many countries. Microbial removal or load estimation at municipal WWTPs, however, is still based on manually recovered grab samples. The goal of this study was to establish basic knowledge regarding the persistence of standard bacterial fecal indicators and Bacteroidetes genetic microbial source tracking markers in municipal wastewater in order to evaluate their suitability for automated sampling, as the potential lack of persistence is the main argument against such procedures. Raw and secondary treated wastewater of municipal origin from representative and well-characterized biological WWTPs without disinfection (organic carbon and nutrient removal) was investigated in microcosm experiments at 5 and 21°C with a total storage time of 32 h (including a 24-h autosampling component and an 8-h postsampling phase). Vegetative Escherichia coli and enterococci, as well as Clostridium perfringens spores, were selected as indicators for cultivation-based standard enumeration. Molecular analysis focused on total (AllBac) and human-associated genetic Bacteroidetes (BacHum-UCD, HF183 TaqMan) markers by using quantitative PCR, as well as 16S rRNA gene-based next-generation sequencing. The microbial parameters showed high persistence in both raw and treated wastewater at 5°C under the storage conditions used. Surprisingly, and in contrast to results obtained with treated wastewater, persistence of the microbial markers in raw wastewater was also high at 21°C. On the basis of our results, 24-h autosampling procedures with 5°C storage conditions can be recommended for the investigation of fecal indicators or Bacteroidetes genetic markers at municipal WWTPs. Such autosampling procedures will contribute to better understanding and monitoring of municipal WWTPs as sources of fecal pollution in water resources.
INTRODUCTION
Microbial fecal contamination of aquatic systems by municipal wastewater represents a significant threat to public health (1). Thus, appropriate wastewater disposal technologies and fecal pollution monitoring programs are critical for safeguarding our water resources. Standard fecal indicators, as well as recently developed genetic microbial source tracking (MST) markers, are used to monitor the microbial fecal loads emitted from wastewater treatment plants (WWTPs) and their impact on receiving waters (2–6). Microbiological sampling of WWTPs is commonly based on manually recovered samples (7). However, the concept behind these methods neglects temporal fluctuations in water quality. Large diurnal variations have been reported for key chemical parameters, such as nutrients, in raw wastewater (8). Determination of the efficacy of carbon, nitrogen, and phosphorus removal at WWTPs is thus frequently based on automated diurnal sampling. For example, in Austria, automated sampling procedures for chemical parameters are required for the official performance testing of WWTPs with more than 1,000 population equivalents (PE), and these procedures use sampling volumes that are proportional to the observed water influx levels over a period of 24 h (9).
Automated sampling is infrequently used for monitoring of microbial fecal pollution. A key argument against the use of automated sampling procedures is the unknown, low, or differential persistence of microbial targets, especially when longer storage periods (i.e., >8 h) are used. This deficiency can potentially lead to false-negative results or underestimation of target concentrations (10–13). Nonetheless, several studies have demonstrated the potential of automated sampling procedures for pollution microbiology (2, 14–17). For example, autosampling was used to elucidate previously unobserved microbial fecal pollution dynamics in alpine water resources, results that had significant implications for water quality management (14, 18). To keep the effects of microbial die-off within a negligible range, batches of collected samples were recovered from an automatic sampling device within 24 h and analyzed immediately (14).
The goal of this study was to establish basic knowledge regarding the persistence of standard bacterial fecal indicators and Bacteroidetes genetic MST markers in municipal wastewater in order to evaluate their suitability for automated sampling procedures. Raw and treated wastewater samples from representative municipal WWTPs were investigated in microcosm experiments at 5 and 21°C for a period of 32 h. This time span reflects the 24-h autosampling period required for WWTP performance testing in the European Community and an 8-h postsampling phase (equivalent to 1 working day) that includes sample transport and processing. Surprisingly, in contrast to natural systems such as rivers and lakes, no information is available for raw and treated wastewater of municipal origin regarding the persistence of fecal indicators and genetic markers (19–23). Here, the fecal indicator bacteria Escherichia coli, enterococci, and Clostridium perfringens spores were selected as representatives for cultivation-based standard determination, while molecular quantification by quantitative PCR (qPCR) was used to elucidate total and human-associated genetic Bacteroidetes markers. Additionally, 16S rRNA gene-based next-generation sequencing (NGS) was used to selected samples to further evaluate the results recovered from the microbial communities investigated on a more general screening level. We hypothesized that only the spores of C. perfringens are appreciably stable in raw and treated wastewater of municipal origin, whereas vegetative cells of E. coli and enterococci, as well as genetic markers of Bacteroidetes, exhibit significant concentration reductions at 5 and 21°C during the storage period selected.
MATERIALS AND METHODS
WWTPs investigated.
Three municipal WWTPs (no. 2, 3, and 4) in the area of Vienna, Austria, with sizes ranging from 23,000 to 140,000 PE, were selected as representative plants for the Austrian/European region (24). For detailed information on the characteristics of the WWTPs, the chemical analysis of the raw and treated wastewater, and the methodology, see Table S1 in the supplemental material. Samples were taken in both summer and winter to account for potential seasonal differences. Industrial influence at the selected plants was moderate, and thus no inhibitory or toxic effects were expected. The annual mean chemical oxygen demand (COD) and total nitrogen (TN) and total phosphorus (TP) concentrations in the raw municipal wastewater investigated were 460 to 560, 45 to 55, and 4 to 10 mg liter−1, respectively. At the time of this study, WWTPs 3 and 4 were using activated sludge treatment with nitrification and denitrification. Phosphorus removal was achieved by chemical precipitation, which is required for sensitive areas in the European Union (25). Overall, elimination rates for COD, TN, and TP were ≥94%, ≥90%, and approximately 80%, respectively. In contrast to WWTPs 3 and 4, WWTP 2 was overloaded without showing denitrification, and it therefore displayed low rates of nitrogen removal. No disinfection was applied at the WWTPs investigated.
Sampling and microcosm experiments.
Grab samples from the influent and effluent sites of the WWTPs investigated were collected in sterile 5-liter plastic bottles (Azlon, Great Britain). Samples were kept cold in the dark and immediately transported to the laboratory. There, samples were thoroughly shaken, subdivided between two 2-liter bottles, carefully temperature equilibrated within 3 to 5 h (the time required depended on the sampling temperature), and incubated at 5 ± 2 or 21 ± 1°C for batch culture microcosm experiments spanning a minimum of 168 h. Although the main focus of the experiments was on persistence during short-term storage (≤32 h), some points of observation were also selected at incubation times of >32 h to achieve a reference to longer-term storage. At defined intervals (Table 1), 70-ml subfractions were recovered from the microcosms, homogenized in an ultrasonic bath (SONOREX; Bandelin, Germany) for 5 min, and subjected to microbiological analyses (analyses were performed with several dilutions and duplicates). Before subfractions were removed from microcosms, they were thoroughly shaken with inversion of the bottles. The remainder of each 5-liter municipal wastewater sample was used for chemical analysis (see Table S1 in the supplemental material). The extent of statistical variation at the experimental trial level of the microcosms was also estimated. This was done during four persistence experiments by using replicate measurements for AllBac, BacHum-UCD, and HF183 TaqMan qPCR determinations. The results did not reveal any detectable systematic effect on the regression coefficients due to the replication effort (Mann-Whitney U test, P > 0.5, n = 4 × 12).
TABLE 1.
Full data set for the persistence of standard fecal indicators and MST markers in raw and treated municipal wastewater at 5°C recovered from microcosm experiments
| Method, sample, and SE (WWTP)a | Timeb | Microcosm experiment descriptive statisticsc |
Regression analysis of microcosm data |
||||
|---|---|---|---|---|---|---|---|
| Meand | Mine | Maxf | dg | kg | Log/% reductionh | ||
| AllBac qPCR | |||||||
| Influent | |||||||
| 1 (2) | a | 10.0 | 9.3 | 10.3 | 10.0 | 0.003 | |
| 3 (4) | b | 10.3 | 10.1 | 10.4 | 10.2 | 0.004 | |
| 7 (3) | c | 10.6 | 10.0 | 11.2 | 10.2 | 0.034 | |
| 9 (3) | d | 10.7 | 10.6 | 10.8 | 10.8 | −0.005 | |
| 11 (4) | i | 10.1 | 9.7 | 10.2 | 10.0 | 0.001 | |
| 13 (2) | i | 10.5 | 10.3 | 10.6 | 10.5 | −0.003 | |
| Effluent | |||||||
| 2 (2) | a | 7.7 | 7.2 | 7.9 | 7.8 | −0.003 | |
| 4 (4) | b | 7.9 | 7.7 | 8.2 | 8.1 | −0.007 | |
| 8 (3) | c | 7.8 | 7.1 | 9.8 | 7.2 | 0.057 | |
| 10 (3) | d | 7.7 | 7.5 | 8.0 | 7.7 | 0.001 | |
| 12 (4) | i | 8.8 | 8.7 | 8.8 | 8.8 | 0.001 | |
| 14 (2) | i | 8.2 | 8.0 | 8.5 | 8.3 | −0.008 | |
| BacHum-UCD qPCR | |||||||
| Influent | |||||||
| 1 (2) | a | 8.9 | 8.4 | 9.2 | 8.9 | −0.001 | |
| 3 (4) | b | 8.7 | 8.5 | 8.9 | 8.6 | 0.002 | |
| 7 (3) | c | 9.1 | 8.9 | 9.5 | 9.2 | −0.011 | |
| 9 (3) | d | 9.0 | 8.8 | 9.0 | 9.0 | −0.004 | |
| 11 (4) | i | 8.7 | 8.4 | 9.2 | 8.6 | 0.015 | |
| 13 (2) | i | 9.5 | 9.0 | 9.7 | 9.3 | 0.010 | |
| Effluent | |||||||
| 2 (2) | a | 6.7 | 6.1 | 6.9 | 6.7 | −0.002 | |
| 4 (4) | b | 6.1 | 5.8 | 6.6 | 6.4 | −0.019 | |
| 8 (3) | c | 6.6 | 6.1 | 8.1 | 6.1 | 0.042 | |
| 10 (3) | d | 5.6 | 5.4 | 5.9 | 5.6 | 0.000 | |
| 12 (4) | i | 8.0 | 7.7 | 8.2 | 7.7 | 0.018 | |
| 14 (2) | i | 7.0 | 6.7 | 7.1 | 6.9 | 0.004 | |
| HF183 TaqMan qPCR | |||||||
| Influent | |||||||
| 1 (2) | a | 8.5 | 7.5 | 9.1 | 8.4 | 0.006 | |
| 3 (4) | b | 8.4 | 8.2 | 8.6 | 8.3 | 0.003 | |
| 7 (3) | c | 8.0 | 7.6 | 8.8 | 7.8 | 0.019 | |
| 9 (3) | d | 9.4 | 9.2 | 9.4 | 9.4 | −0.002 | |
| 11 (4) | i | 8.5 | 8.2 | 8.7 | 8.3 | 0.010 | |
| 13 (2) | i | 9.0 | 8.7 | 9.3 | 8.9 | 0.007 | |
| Effluent | |||||||
| 2 (2) | a | 6.5 | 6.1 | 6.8 | 6.6 | −0.008 | |
| 4 (4) | b | 5.7 | 5.5 | 6.1 | 6.0 | −0.015i | 0.48/66 |
| 8 (3) | c | 5.6 | 4.9 | 7.1 | 4.7 | 0.074 | |
| 10 (3) | d | 6.1 | 5.8 | 6.9 | 6.1 | 0.003 | |
| 12 (4) | i | 7.5 | 7.3 | 7.7 | 7.3 | 0.013 | |
| 14 (2) | i | 6.5 | 6.4 | 6.6 | 6.5 | 0.000 | |
| E. coli (cultivation based) | |||||||
| Influent | |||||||
| 1 (2) | a | 6.8 | 6.8 | 6.8 | 6.8 | 0.000 | |
| 3 (4) | b | 6.4 | 6.2 | 6.6 | 6.5 | −0.004 | |
| 5 (4) | e | 6.9 | 6.9 | 7.0 | 6.9 | 0.000 | |
| 7 (3) | c | 6.2 | 6.2 | 6.3 | 6.3 | −0.003 | |
| 9 (3) | d | 6.7 | 6.6 | 6.9 | 6.7 | −0.002 | |
| 11 (4) | i | 7.8 | 7.7 | 8.1 | 8.1 | −0.015 | |
| 13 (2) | i | 6.1 | 6.1 | 6.2 | 6.2 | −0.003 | |
| Effluent | |||||||
| 2 (2) | a | 4.4 | 4.3 | 4.6 | 4.5 | −0.005 | |
| 4 (4) | b | 4.7 | 4.6 | 5.0 | 4.9 | −0.010 | |
| 6 (4) | e | 4.6 | 4.5 | 4.6 | 4.6 | 0.000 | |
| 8 (3) | c | 3.7 | 3.6 | 3.8 | 3.6 | 0.006 | |
| 10 (3) | d | 3.6 | 3.5 | 3.8 | 3.7 | −0.002 | |
| 12 (4) | i | 5.2 | 5.2 | 5.3 | 5.3 | −0.004 | |
| 14 (2) | i | 4.0 | 3.9 | 4.1 | 4.1 | −0.005 | |
| C. perfringens spores (cultivation based) | |||||||
| Influent | |||||||
| 1 (2) | a | 5.0 | 4.9 | 5.1 | 5.0 | 0.005 | |
| 3 (4) | b | 4.9 | 4.7 | 4.9 | 4.8 | 0.003 | |
| 7 (3) | c | 4.6 | 4.5 | 4.7 | 4.6 | −0.003 | |
| 11 (4) | i | 4.5 | 4.4 | 4.6 | 4.5 | 0.000 | |
| 13 (2) | i | 4.7 | 4.6 | 4.8 | 4.7 | 0.003 | |
| Effluent | |||||||
| 2 (2) | a | 3.9 | 3.8 | 4.0 | 3.9 | 0.006 | |
| 4 (4) | b | 3.1 | 3.1 | 3.2 | 3.2 | −0.002 | |
| 8 (3) | c | 3.0 | 2.9 | 3.0 | 3.0 | 0.003 | |
| 12 (4) | i | 3.9 | 3.7 | 4.0 | 3.8 | 0.004 | |
| 14 (2) | i | 3.9 | 3.7 | 4.0 | 3.9 | −0.002 | |
| Enterococci (cultivation based) | |||||||
| Influent | |||||||
| 11 (4) | i | 5.4 | 5.3 | 5.6 | 5.3 | 0.006 | |
| 13 (2) | i | 5.7 | 5.6 | 5.9 | 5.6 | 0.006 | |
| Effluent | |||||||
| 12 (4) | i | 4.3 | 4.3 | 4.3 | 4.3 | −0.001 | |
| 14 (2) | i | 4.1 | 4.0 | 4.2 | 4.0 | −0.001 | |
SE, sampling event number. In parentheses is the number of the WWTP investigated.
Analysis times during microcosm experiments: a (n = 5), 0, 4, 8, 20, and 24 h; b (n = 6), 0, 7, 19, 24, 27, and 43 h; c (n = 6), 0, 4, 8, 12, 22, and 24 h; d (n = 5), 0, 5, 18, 27, and 35 h; e (n = 5), 0, 5, 11, 17, and 25 h; i (n = 5), 0, 9,5, 20, 24, and 29 h.
Values obtained by qPCR are in log10 ([ME + 1] 100 ml−1) (where ME is marker equivalents), and those obtained by cultivation are in log10 ([CFU + 1] 100 ml−1).
Mean, arithmetic mean.
Min, minimum value.
Max, maximum value.
d and k are linear regression coefficients. d is the intercept with the y axis log10 ([ME + 1] 100 ml−1) or log10 ([CFU + 1] 100 ml−1). k is the slope {log10 ([ME + 1] 100 ml−1) or log10 [CFU 100 ml−1]} per hour.
Log10 reduction calculated from regression model for a sample storage time of 32 h at 5°C (calculated for significant regression coefficients only). The value after the slash is the percent reduction, relating to the delogarithmized absolute values.
Statistically significant coefficient (P ≤ 0.05, Bonferroni corrected).
Microbiological and molecular analyses.
Cultivation-based enumeration of E. coli bacteria, enterococci, and C. perfringens spores was performed by membrane filtration using appropriate dilutions as previously described (26, 27). For quantification of C. perfringens spores, 5-ml (influent) and 15-ml (effluent) aliquots from the batch sample were pasteurized at 60 ± 2°C for 15 min. C. perfringens was analyzed according to ISO standard 14189 (28), on the basis of selective growth on tryptose sulfite cycloserine agar (Scharlau, Spain) at 44°C and subsequent colony identification by acid phosphatase reaction (29). Enumeration of presumptive E. coli bacteria on the basis of ISO standard 16649-1 (30) was done with chromogenic tryptone bile agar with X-glucuronide (Oxoid, Thermo Fisher Scientific Inc., Cheshire, United Kingdom) at 44°C. Enumeration of enterococci on the basis of ISO standard 7899-2 (31) was done with Slanetz-Bartley medium (Oxoid) and dry-heat incubation at 44 ± 0.5°C for 44 ± 4 h. Appropriate control strains were used to ensure the quality of the medium.
Detection of genetic MST markers was based on total and human-associated Bacteroidetes assays. Respective 16S rRNA gene markers for AllBac (32), BacHum-UCD (33), and HF183 TaqMan (34) were quantified by qPCR. For DNA extraction, we used polycarbonate membrane filtration (0.2-μm Isopore membrane filter GTTP; Millipore, Cork, Ireland) of 10-ml (influent) and 50-ml (effluent) batch sample aliquots, as previously described (35, 36), followed by phenol-chloroform DNA extraction. Cell lysis was carried out with a FastPrepR-24 Instrument (MP Biomedicals Inc., Irvine, CA) at a speed setting of 6 m/s for 30 s each. The extracted DNA was stored at −20°C prior to analysis of two dilutions (10- and 100-fold) to test for PCR inhibition. The rotor discs were loaded with Master Mix and sample by a Qiagility Robot (Qiagen, Hilden, Germany), and measurements were subsequently performed on a Rotorgene Q Cycler (Qiagen). For the AllBac qPCR assay, we used 2.5 μl of the appropriate DNA sample dilution, 600 nM primer AllBac296f, 600 nM primer AllBac412r, 25 nM TaqMan MGB probe AllBac375Bhqr (32), 0.4 g liter−1 bovine serum albumin (Roche Diagnostics, Mannheim, Germany), and 7.5 μl of iQ Supermix (Bio-Rad, Hercules, CA) in a total reaction volume of 15 μl. We also added 5 mM MgCl2 to obtain a total Mg2+ concentration of 8 mM (32). For the BacHum-UCD assay, we used 2.5 μl of the respective DNA sample dilution, 400 nM primer BacHum-160f, 400 nM primer BacHum-241r, 80 nM TaqMan MGB probe BacHum-193p (33), 0.4 g liter−1 bovine serum albumin, and 7.5 μl of iQ Supermix in a total reaction volume of 15 μl. For the HF183 TaqMan assay, we used 2.5 μl of the respective DNA sample dilution, 100 nmol liter−1 primer HF183, 100 nmol liter−1 primer BFD REV, 80 nmol liter−1 TaqMan MGB probe BFDFAM (34), 0.4 g liter−1 bovine serum albumin, and 7.5 μl of iQ Supermix in a total reaction volume of 15 μl. The PCR program for AllBac was 95°C for 3 min and 45 cycles of 95°C for 30 s and 60°C for 45 s. For BacHum-UCD, the PCR program was 95°C for 3 min and 45 cycles of 95°C for 15 s and 60°C for 1 min. For the HF183 TaqMan assay, the PCR program was 95°C for 3 min and 45 cycles of 95°C for 15 s and 60°C for 30 s. Real-time data were collected during the 60°C primer-annealing step. Quantification was based on appropriate standard dilutions of plasmid DNA (37) and presented as marker equivalents per volume (ME/vol) according to Reischer et al. (36). For a detailed description of the NGS methodology used here, which was based on the V1-V2 region of the 16S rRNA gene, see the supplemental material.
Data analysis and statistics.
All microbial data were expressed as log10 (x + 1). Regression analysis and descriptive statistics were calculated with IBM SPSS Statistics version 20.0.0 (IBM, Germany). To account for the multiple tests that were carried out, statistical significance levels were Bonferroni corrected. All graphs were prepared with SigmaPlot 11.0 (SPSS Inc., Chicago, IL) and CorelDraw X5 (Corel, Canada).
RESULTS
All experiments with raw municipal wastewater samples, including influents from WWTPs 2, 3, and 4, revealed high stability of the microbiological parameters investigated at 5 and 21°C during the 32-h storage period (Tables 1 and 2; Fig. 1). Only 2 of 64 regression coefficients of microcosm experiments using raw wastewater displayed a negative value that deviated significantly from zero (P ≤ 0.05, Bonferroni corrected). These statistically significant regression coefficients were from the human-associated Bacteroidetes marker BacHum-UCD and HF183 TaqMan, accounting for a maximum 0.5-log10 concentration decrease in the regression model during storage for 32 h at 21°C (Table 2). All measurements of vegetative E. coli and enterococci and the genetic Bacteroidetes markers resulted in more pronounced concentration decreases at the 96- and 264-h time points. C. perfringens spores did not show any relevant concentration decrease during the whole observation period (Fig. 1; Tables 1 and 2).
TABLE 2.
Full data set for the persistence of standard fecal indicators and MST markers in raw and treated municipal wastewater at 21°C recovered from microcosm experiments
| Method, sample, and SE (WWTP)a | Timeb | Microcosm experiment descriptive statisticsc |
Regression analysis of microcosm data |
||||
|---|---|---|---|---|---|---|---|
| Meand | Mine | Maxf | dg | kg | Log/% reductionh | ||
| AllBac qPCR | |||||||
| Influent | |||||||
| 1 (2) | a | 10.0 | 9.5 | 10.4 | 9.9 | 0.007 | |
| 3 (4) | b | 10.2 | 10.1 | 10.4 | 10.1 | 0.004 | |
| 7 (3) | c | 10.3 | 10.1 | 10.6 | 10.3 | 0.004 | |
| 9 (3) | d | 10.9 | 10.7 | 11.1 | 10.8 | 0.003 | |
| 11 (4) | i | 10.0 | 9.5 | 10.3 | 9.7 | 0.017 | |
| 13 (2) | i | 10.7 | 10.4 | 10.9 | 10.5 | 0.006 | |
| Effluent | |||||||
| 2 (2) | a | 7.4 | 7.0 | 7.9 | 7.8 | −0.030i | 0.96/89 |
| 4 (4) | b | 7.8 | 7.3 | 8.2 | 7.9 | −0.002 | |
| 8 (3) | c | 7.22 | 6.95 | 7.52 | 7.3 | −0.011 | |
| 10 (3) | d | 7.40 | 7.2 | 7.6 | 7.5 | −0.006 | |
| 12 (4) | i | 8.75 | 8.62 | 8.98 | 8.9 | −0.011 | |
| 14 (2) | i | 8.02 | 7.65 | 8.30 | 8.3 | −0.014 | |
| BacHum-UCD qPCR | |||||||
| Influent | |||||||
| 1 (2) | a | 8.7 | 8.4 | 9.1 | 8.8 | −0.009 | |
| 3 (4) | b | 8.3 | 8.1 | 8.6 | 8.5 | −0.010i | 0.30/50 |
| 7 (3) | c | 9.2 | 8.9 | 9.3 | 9.1 | 0.008 | |
| 9 (3) | d | 8.9 | 8.7 | 8.9 | 8.9 | −0.002 | |
| 11 (4) | i | 8.4 | 8.1 | 8.6 | 8.5 | −0.002 | |
| 13 (2) | i | 9.5 | 9.1 | 9.7 | 9.3 | 0.011 | |
| Effluent | |||||||
| 2 (2) | a | 6.1 | 5.5 | 6.9 | 6.8 | −0.045i | 1.89/99 |
| 4 (4) | b | 5.2 | 4.2 | 6.3 | 6.2 | −0.049i | 1.57/97 |
| 8 (3) | c | 6.1 | 5.7 | 6.4 | 6.3 | −0.023 | |
| 10 (3) | d | 5.0 | 4.0 | 5.5 | 5.2 | −0.013 | |
| 12 (4) | i | 7.9 | 7.8 | 7.9 | 7.9 | 0.001 | |
| 14 (2) | i | 6.7 | 6.5 | 7.1 | 7.0 | −0.014 | |
| HF183 TaqMan qPCR | |||||||
| Influent | |||||||
| 1 (2) | a | 8.4 | 7.5 | 8.9 | 8.3 | 0.009 | |
| 3 (4) | b | 8.0 | 7.7 | 8.4 | 8.3 | −0.015i | 0.48/66 |
| 7 (3) | c | 8.1 | 7.8 | 8.8 | 7.7 | 0.032 | |
| 9 (3) | d | 9.2 | 9.1 | 9.4 | 9.4 | −0.007 | |
| 11 (4) | i | 8.2 | 7.9 | 8.5 | 8.3 | −0.005 | |
| 13 (2) | i | 9.0 | 8.5 | 9.3 | 8.8 | 0.009 | |
| Effluent | |||||||
| 2 (2) | a | 6.1 | 5.3 | 7.0 | 6.7 | −0.046i | 1.42/96 |
| 4 (4) | b | 6.0 | 4.7 | 3.9 | 5.8 | −0.054 | |
| 8 (3) | c | 5.0 | 4.5 | 5.6 | 5.0 | 0.002 | |
| 10 (3) | d | 5.4 | 4.0 | 6.0 | 5.7 | −0.016 | |
| 12 (4) | i | 7.4 | 7.3 | 7.5 | 7.4 | 0.000 | |
| 14 (2) | i | 6.3 | 6.0 | 6.6 | 6.5 | −0.015 | |
| E. coli (cultivation based) | |||||||
| Influent | |||||||
| 1 (2) | a | 6.8 | 6.7 | 6.9 | 6.8 | −0.001 | |
| 3 (4) | b | 6.3 | 6.2 | 6.5 | 6.4 | −0.006 | |
| 5 (4) | e | 6.8 | 6.6 | 6.9 | 6.8 | −0.006 | |
| 7 (3) | c | 6.4 | 6.3 | 6.4 | 6.4 | 0.000 | |
| 9 (3) | d | 6.7 | 6.6 | 6.9 | 6.8 | −0.004 | |
| 11 (4) | i | 8.0 | 7.9 | 8.2 | 8.1 | −0.009 | |
| 13 (2) | i | 6.1 | 6.1 | 6.1 | 6.1 | 0.001 | |
| Effluent | |||||||
| 2 (2) | a | 3.9 | 3.4 | 4.4 | 4.5 | −0.042i | 1.35/96 |
| 4 (4) | b | 4.1 | 3.3 | 5.1 | 5.0 | −0.041i | 1.31/95 |
| 6 (4) | e | 4.2 | 3.9 | 4.6 | 4.5 | −0.024 | |
| 8 (3) | c | 3.6 | 3.5 | 3.8 | 3.8 | −0.009i | 0.29/49 |
| 10 (3) | d | 3.7 | 3.6 | 3.8 | 3.7 | −0.002 | |
| 12 (4) | i | 5.2 | 5.2 | 5.3 | 5.3 | −0.005 | |
| 14 (2) | i | 3.8 | 3.5 | 4.0 | 4.0 | −0.017 | |
| C. perfringens spores (cultivation based) | |||||||
| Influent | |||||||
| 1 (2) | a | 5.1 | 4.9 | 5.2 | 5.0 | 0.006 | |
| 3 (4) | b | 4.9 | 4.8 | 5.0 | 4.8 | 0.001 | |
| 7 (3) | c | 4.5 | 4.4 | 4.6 | 4.5 | −0.002 | |
| 11 (4) | i | 4.5 | 4.3 | 4.6 | 4.4 | 0.005 | |
| 13 (2) | i | 4.7 | 4.6 | 4.8 | 4.6 | 0.003 | |
| Effluent | |||||||
| 2 (2) | a | 4.0 | 3.9 | 4.1 | 3.9 | 0.004 | |
| 4 (4) | b | 3.1 | 3.1 | 3.2 | 3.2 | −0.002 | |
| 8 (3) | c | 3.0 | 2.9 | 3.1 | 3.0 | 0.004 | |
| 12 (4) | i | 3.8 | 3.7 | 3.9 | 3.9 | −0.004 | |
| 14 (2) | i | 3.8 | 3.7 | 3.9 | 3.8 | 0.001 | |
| Enterococci (cultivation based) | |||||||
| Influent | |||||||
| 11 (4) | i | 5.4 | 5.3 | 5.5 | 5.3 | 0.003 | |
| 13 (2) | i | 5.68 | 5.46 | 5.81 | 5.6 | 0.005 | |
| Effluent | |||||||
| 12 (4) | i | 4.2 | 4.1 | 4.3 | 4.3 | −0.005 | |
| 14 (2) | i | 3.96 | 3.87 | 4.03 | 4.2 | −0.007 | |
SE, sampling event number. In parentheses is the number of the WWTP investigated.
Analysis times during microcosm experiments: a (n = 5), 0, 4, 8, 20, and 24 h; b (n = 6), 0, 7, 19, 24, 27, and 43 h; c (n = 6), 0, 4, 8, 12, 22, and 24 h; d (n = 5), 0, 5, 18, 27, and 35 h; e (n = 5), 0, 5, 11, 17, and 25 h; i (n = 5), 0, 9,5, 20, 24, and 29 h.
Values obtained by qPCR are in log10 ([ME + 1] 100 ml−1) (where ME is marker equivalents), and those obtained by cultivation are in log10 ([CFU + 1] 100 ml−1).
Mean, arithmetic mean.
Min, minimum value.
Max, maximum value.
d and k are linear regression coefficients. d is log10 ([ME + 1] 100 ml−1) or log10 (CFU 100 ml−1). k is the difference in log10 [(ME + 1) 100 ml−1] or log10 ( CFU 100 ml−1) values per hour between data points.
Log10 reduction calculated from regression model for a sample storage time of 32 h at 21°C (calculated for significant regression coefficients only). The value after the slash is the percent reduction, relating to the delogarithmized absolute values.
Statistically significant coefficient (P ≤ 0.05, Bonferroni corrected).
FIG 1.
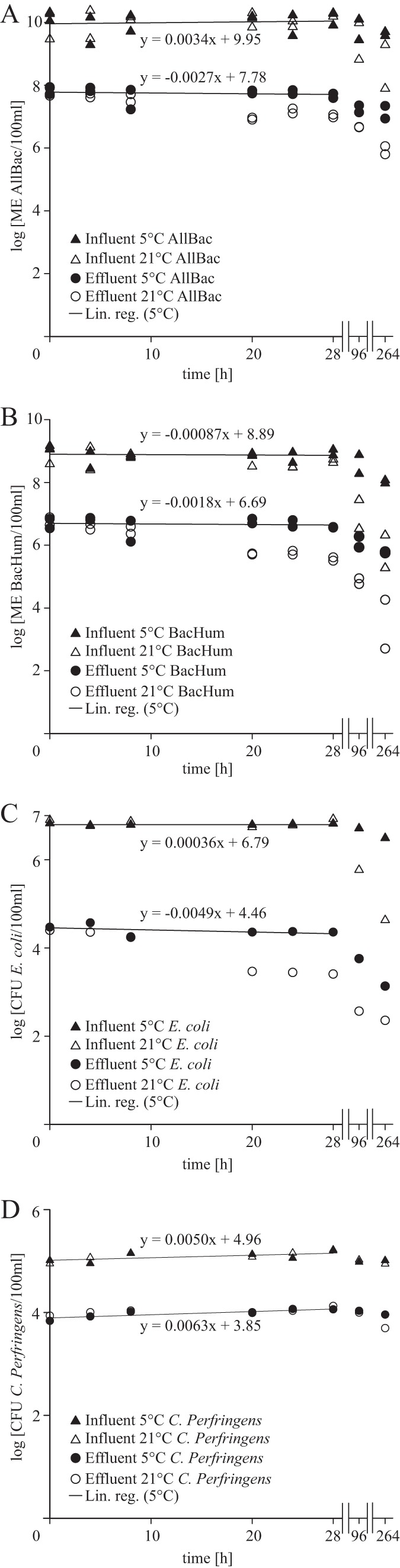
Persistence of standard fecal indicators and genetic MST markers in raw (influent) and treated (effluent) municipal wastewater at 5 and 21°C. The data shown are a representative set; Tables 1 and 2 contain the complete data. Linear regression analysis was performed for 28 h and is shown only for the 5°C storage conditions (values for samples taken at 96 and 264 h are given as control measurements). Panels: A, AllBac analysis of genetic fecal markers for the total Bacteroidetes populations; B, BacHum-UCD analysis of genetic fecal markers for human-associated Bacteroidetes populations; C, cultivation-based enumeration of E. coli bacteria; D, cultivation-based enumeration of C. perfringens spores. Lin. reg., linear regression.
The persistence of the microbial parameters investigated in treated wastewater samples at 5°C was also high (Fig. 1). With the exception of one experiment, regression analysis did not detect any statistically significant changes in the time frame investigated (Table 1). In contrast, nine of the microcosm experiments carried out with treated wastewater at 21°C revealed significant negative regression coefficients for E. coli and the genetic Bacteroidetes markers (P ≤ 0.05, Bonferroni corrected, Table 2). Concentration decreases of up to 1.9 log10 for a 32-h storage period were apparent when the regression model was used (Table 2). Additionally, all measurements taken at 96 and 264 h yielded large and significant reductions for E. coli, enterococci, and the genetic Bacteroidetes markers; again, no notable decrease in C. perfringens spores was found in any of these storage experiments (Table 1; Fig. 1).
To further evaluate our results regarding the 16S rRNA gene bacterial community composition and the persistence of Bacteroidetes populations at the phylum scale, one representative microcosm series from the WWTP 2 effluent was chosen for additional 454 amplicon pyrosequencing analysis. Taxonomic pyrosequencing analysis of the 16S rRNA gene microbial community composition revealed a clear predominance of the phyla Proteobacteria and Bacteroidetes, with average relative abundances of 60% ± 5% and 27% ± 6%, respectively. The next most predominant phyla were Actinobacteria and Firmicutes, with average abundances of 2% ± 0.6% and 2% ± 0.7%, respectively. Microbial community structure analysis with a unweighted UniFrac algorithm combined with principal-coordinate analysis did not detect any notable changes in the Bacteroidetes community composition during the short-term period of storage at 5°C investigated (Fig. 2). In contrast, major changes in the total Bacteroidetes community structure became apparent under 21°C incubation conditions and also at the later time points (96 and 264 h) of the 5°C microcosms experiments (Fig. 2).
FIG 2.
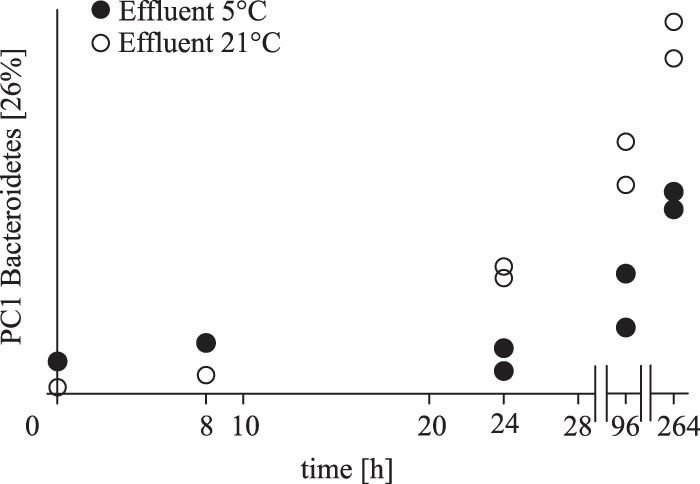
16S rRNA gene-based qualitative UniFrac community structure dynamics in the microcosm experiments with WWTP 2 effluent. The first principal coordinate (PC1) versus time is shown for the phylum Bacteroidetes (26% of the total variance is explained by PC1) on the x and y axes, respectively. Black and open dots represent microcosm experiments at 5 and 21°C, respectively. Analyses at the 0- and 8-h time points are shown as a single analysis, whereas analyses at the 24-, 96-, and 264-h time points are shown as duplicate analyses.
DISCUSSION
The data obtained from the microcosm experiments clearly contradicted the initial hypothesis regarding the low persistence of the microbial indicators investigated in municipal wastewater during short-term storage (32 h) at 5°C. In addition to the highly resistant C. perfringens spores (26, 38, 39), the vegetative E. coli cells and the genetic Bacteroidetes markers displayed remarkable stability at 5°C in the defined time frame. Although qPCR-based detection of a genetic DNA marker does not indicate cell viability (40), a significantly increasing or decreasing trend in the DNA target concentration due to cell growth, degradation, or grazing effects would have been detected by the molecular quantification methods used here (6, 41). Furthermore, the stability of the molecular signatures of Bacteroidetes cells was supported by data on the differing taxonomic levels investigated, which were quantified by the BacHum-UCD, HF183 TaqMan, and AllBac qPCR assays (32–34) and qualitatively screened by 16S rRNA gene NGS community structure analysis (42).
Strong decreases in the representative bacteria were observed only in the microcosm experiments at 21°C using untreated wastewater samples, with E. coli and genetic Bacteroidetes markers displaying losses of up to 99% of their original populations (Table 2). However, not all of these experiments yielded such a marked decrease, most likely because storage periods longer than 32 h would have been needed to reach these levels. No signs of toxicological inhibition of the microbial community in the activated sludge, which generally manifests itself as inhibition of aerobe/anaerobe heterotrophy or specific inhibition of nitrification, were discernible at the WWTPs (see the WWTP data in the supplemental material). Measurements at 96 and 264 h also revealed a clearly decreasing response, further supporting the absence of inhibiting substances. Very surprisingly, no decreasing effect was detectable in the microcosm experiments with raw municipal wastewater samples at 21°C. Extremely high levels of organic substrates (CODs of up to 680 mg liter−1 were measured in raw municipal wastewater), and the absence of oxygen may have contributed to this short-term stability effect. This is only a preliminary speculation, and further investigations beyond the scope of this study are needed to clarify the actual reason for our observation.
The effluent and influent characteristics selected represent a typical range of municipal wastewaters occurring at WWTPs in Austria (see Table S1 in the supplemental material) with respect to catchment type, wastewater channels, and treatment plant performance (24). Our results can be taken as a strong indication that microbial persistence is not a limiting factor in short-term storage at 5°C of raw and treated municipal wastewater samples. It is important to emphasize that disinfection was not applied at the WWTPs investigated. Disinfection is not required for biological treated wastewater according to Austrian and European regulations. Disinfection is considered only in sensitive areas used for bathing or drinking water production and not for receiving waters without a particular use. Furthermore, the proportion of industrial wastewater input was low to moderate at the WWTPs investigated. No specific inhibitory effects or toxic substances have been reported for these WWTPs (e.g., for respiratory or nitrification measurements). The results obtained thus relate to nondisinfected raw and biological treated wastewater of municipal origin, without the occurrence of microbicidal substances from industrial effluents. Pyrosequencing-based 16S rRNA gene community analysis also demonstrated the typical bacterial community composition expected of wastewater of municipal origin (43, 44). The investigation of effects of disinfection or toxic compounds on the persistence of indicators or fecal markers was not the aim of this study. However, in future, it might also be interesting to elucidate the effect of microbicidal conditions on microbiological parameters with different endpoints during short-term storage (e.g., cultivation-based enumeration versus direct detection of nucleic acids). Further studies may also focus on analysis of the activity of the bacterial community considered at 5°C.
In conclusion, we can recommend 24-h autosampling procedures under 5°C storage conditions not only for chemical analysis but also for representative microbiological investigations of raw and biological treated wastewater of municipal origin when using bacterial standard fecal indicators or Bacteroidetes genetic MST markers. Such autosampling procedures will contribute significantly to a better understanding and monitoring of municipal WWTPs as sources of fecal contamination of water resources (1, 45).
Supplementary Material
ACKNOWLEDGMENTS
This paper was supported by the Austrian Science Fund (FWF) as part of research projects P22309-B20, P23900-B22, the Vienna Doctoral Programme on Water Resource Systems (W1219-N22), and GWRS-Vienna in cooperation with Vienna Water as part of the (New) Danube-Untere Lobau Network Project (Gewässervernetzung (Neue) Donau-Untere Lobau (Nationalpark Donau-Auen) funded by the Government of Austria (Federal Ministry of Agriculture, Forestry, Environment & Water Management), the Government of Vienna, and the European Agricultural Fund for Rural Development (project LE 07-13). G. H. Reischer was supported by Austrian Science Fund (FWF) project P22032.
We gratefully acknowledge the laboratory assistance provided by Sonja Knetsch and Andrea Lettl. This work represents a joint investigation of the Interuniversity Cooperation Centre for Water & Health (http://www.waterandhealth.at).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.00998-15.
REFERENCES
- 1.Stevens G, Mascarenhas M, Mathers C. 2009. Global health risks: progress and challenges. Bull World Health Organ 87:646–646. doi: 10.2471/BLT.09.070565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Passerat J, Ouattara NK, Mouchel J-M, Rocher V, Servais P. 2011. Impact of an intense combined sewer overflow event on the microbiological water quality of the Seine River. Water Res 45:893–903. doi: 10.1016/j.watres.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 3.Schoen ME, Soller JA, Ashbolt NJ. 2011. Evaluating the importance of faecal sources in human-impacted waters. Water Res 45:2670–2680. doi: 10.1016/j.watres.2011.02.025. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed W, Sritharan T, Palmer A, Sidhu JPS, Toze S. 2013. Evaluation of bovine feces-associated microbial source tracking markers and their correlations with fecal indicators and zoonotic pathogens in a Brisbane, Australia, reservoir. Appl Environ Microbiol 79:2682–2691. doi: 10.1128/AEM.03234-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tambalo DD, Fremaux B, Boa T, Yost CK. 2012. Persistence of host-associated Bacteroidales gene markers and their quantitative detection in an urban and agricultural mixed prairie watershed. Water Res 46:2891–2904. doi: 10.1016/j.watres.2012.02.048. [DOI] [PubMed] [Google Scholar]
- 6.Harwood VJ, Staley C, Badgley BD, Borges K, Korajkic A. 2014. Microbial source tracking markers for detection of fecal contamination in environmental waters: relationships between pathogens and human health outcomes. FEMS Microbiol Rev 38:1–40. doi: 10.1111/1574-6976.12031. [DOI] [PubMed] [Google Scholar]
- 7.ISO. 2006. Water quality—sampling for microbiological analysis (ISO 19458:2006). International Organization for Standardization, Geneva, Switzerland. [Google Scholar]
- 8.Henze MVLM, Ekama G, Brdjanovic D. 2008. Biological wastewater treatment. Principles, modelling and design. IWA Publishing, London, United Kingdom. [Google Scholar]
- 9.ÖWAV. 2010. ÖWAV Arbeitsbehelf 14. Eigen- und Betriebsüberwachung von biologischen Abwasserreinigungsanlagen (>50 EW). 3, vollständig überarbeitete Auflage. Österreicher Wasser und Abfallwirtschaftsverband (ÖWAV), Vienna, Austria. [Google Scholar]
- 10.Green HC, Shanks OC, Sivaganesan M, Haugland RA, Field KG. 2011. Differential decay of human faecal Bacteroides in marine and freshwater. Environ Microbiol 13:3235–3249. doi: 10.1111/j.1462-2920.2011.02549.x. [DOI] [PubMed] [Google Scholar]
- 11.Hoglund C, Stenstrom TA, Jonsson H, Sundin A. 1998. Evaluation of faecal contamination and microbial die-off in urine separating sewage systems. Water Sci Technol 38:17–25. doi: 10.1016/S0273-1223(98)00563-0. [DOI] [Google Scholar]
- 12.Liang ZB, He ZL, Zhou XX, Powell CA, Yang YE, Roberts MG, Stoffella PJ. 2012. High diversity and differential persistence of fecal Bacteroidales population spiked into freshwater microcosm. Water Res 46:247–257. doi: 10.1016/j.watres.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Sokolova E, Astrom J, Pettersson TJ, Bergstedt O, Hermansson M. 2012. Decay of Bacteroidales genetic markers in relation to traditional fecal indicators for water quality modeling of drinking water sources. Environ Sci Technol 46:892–900. doi: 10.1021/es2024498. [DOI] [PubMed] [Google Scholar]
- 14.Stadler H, Skritek P, Sommer R, Mach RL, Zerobin W, Farnleitner AH. 2008. Microbiological monitoring and automated event sampling at karst springs using LEO-satellites. Water Sci Technol 58:899–909. doi: 10.2166/wst.2008.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferguson CM. 1994. Refrigerated autosampling for the assessment of bacteriological water quality. Water Res 28:841–847. doi: 10.1016/0043-1354(94)90090-6. [DOI] [Google Scholar]
- 16.Roser D, Skinner J, LeMaitre C, Marshall L, Baldwin J, Billington K, Kotz S, Clarkson K, Ashbolt N. 2002. Automated event sampling for microbiological and related analytes in remote sites: a comprehensive system, p 123–130. 2nd World Water Congress: Water and Health, Microbiology, Monitoring and Disinfection. IWA Publishing, London, United Kingdom. [Google Scholar]
- 17.Converse RR, Piehler MF, Noble RT. 2011. Contrasts in concentrations and loads of conventional and alternative indicators of fecal contamination in coastal stormwater. Water Res 45:5229–5240. doi: 10.1016/j.watres.2011.07.029. [DOI] [PubMed] [Google Scholar]
- 18.Stadler H, Klock E, Skritek P, Mach RL, Zerobin W, Farnleitner AH. 2010. The spectral absorption coefficient at 254 nm as a real-time early warning proxy for detecting faecal pollution events at alpine karst water resources. Water Sci Technol 62:1898–1906. doi: 10.2166/wst.2010.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Darakas E, Koumoulidou T, Lazaridou D. 2009. Fecal indicator bacteria declines via a dilution of wastewater in seawater. Desalination 248:1008–1015. doi: 10.1016/j.desal.2008.10.017. [DOI] [Google Scholar]
- 20.Lessard EJ, Sieburth JM. 1983. Survival of natural sewage populations of enteric bacteria in diffusion and batch chambers in the marine environment. Appl Environ Microbiol 45:950–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aulenbach BT. 2010. Bacteria holding times for fecal coliform by mFC agar method and total coliform and Escherichia coli by Colilert-18 Quanti-Tray method. Environ Monit Assess 161:147–159. doi: 10.1007/s10661-008-0734-3. [DOI] [PubMed] [Google Scholar]
- 22.Dick LK, Stelzer EA, Bertke EE, Fong DL, Stoeckel DM. 2010. Relative decay of Bacteroidales microbial source tracking markers and cultivated Escherichia coli in freshwater microcosms. Appl Environ Microbiol 76:3255–3262. doi: 10.1128/AEM.02636-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schulz CJ, Childers GW. 2011. Fecal Bacteroidales diversity and decay in response to variations in temperature and salinity. Appl Environ Microbiol 77:2563–2572. doi: 10.1128/AEM.01473-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.BMLFUW. 2012. Kommunale Abwasserrichtlinie der EU-91/271/EWG, Österreichischer Bericht 2012. Bundesministerium für Land- und Forstwirtschaft, Umwelt und Wasserwirtschaft, Vienna, Austria. [Google Scholar]
- 25.Bjerregaard R. 1998. Commission Directive 98/15/EC of 27 February 1998 amending Council Directive 91/271/EEC with respect to certain requirements established in Annex I thereof. Off J Eur Communities 1998:L67/29–L67/30. http://faolex.fao.org/docs/pdf/eur18544.pdf. [Google Scholar]
- 26.Vierheilig J, Frick C, Mayer RE, Kirschner AKT, Reischer GH, Derx J, Mach RL, Sommer R, Farnleitner AH. 2013. Clostridium perfringens is not suitable for the indication of fecal pollution from ruminant wildlife but is associated with excreta from nonherbivorous animals and human sewage. Appl Environ Microbiol 79:5089–5092. doi: 10.1128/AEM.01396-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farnleitner AH, Ryzinska-Paier G, Reischer GH, Burtscher MM, Knetsch S, Kirschner AKT, Dirnboeck T, Kuschnig G, Mach RL, Sommer R. 2010. Escherichia coli and enterococci are sensitive and reliable indicators for human, livestock and wildlife faecal pollution in alpine mountainous water resources. J Appl Microbiol 109:1599–1608. doi: 10.1111/j.1365-2672.2010.04788.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.ISO. 2013. Water quality—enumeration of Clostridium perfringens—method using membrane filtration (ISO 14189). International Organization for Standardization, Geneva, Switzerland. [Google Scholar]
- 29.Ryzinska-Paier G, Sommer R, Haider JM, Knetsch S, Frick C, Kirschner AK, Farnleitner AH. 2011. Acid phosphatase test proves superior to standard phenotypic identification procedure for Clostridium perfringens strains isolated from water. J Microbiol Methods 87:189–194. doi: 10.1016/j.mimet.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.ISO. 2001. Microbiology of food and animal feeding stuffs—horizontal method for the enumeration of beta-glucuronidase-positive Escherichia coli—part 1: colony-count technique at 44 degrees C using membranes and 5-bromo-4-chloro-3-indolyl beta-d-glucoronide (ISO 16649-1:2001 04 15). International Organization for Standardization, Geneva, Switzerland. [Google Scholar]
- 31.ISO. 2000. Water quality—detection and enumeration of intestinal enterococci—part 2: membrane filtration method (ISO 7899-2: 2000). International Organization for Standardization, Geneva, Switzerland. [Google Scholar]
- 32.Layton A, McKay L, Williams D, Garrett V, Gentry R, Sayler G. 2006. Development of Bacteroides 16S rRNA gene TaqMan-based real-time PCR assays for estimation of total, human, and bovine fecal pollution in water. Appl Environ Microbiol 72:4214–4224. doi: 10.1128/AEM.01036-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kildare BJ, Leutenegger CM, McSwain BS, Bambic DG, Rajal VB, Wuertz S. 2007. 16S rRNA-based assays for quantitative detection of universal, human-, cow-, and dog-specific fecal Bacteroidales: a Bayesian approach. Water Res 41:3701–3715. doi: 10.1016/j.watres.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 34.Haugland RA, Varma M, Sivaganesan M, Kelty C, Peed L, Shanks OC. 2010. Evaluation of genetic markers from the 16S rRNA gene V2 region for use in quantitative detection of selected Bacteroidales species and human fecal waste by qPCR. Syst Appl Microbiol 33:348–357. doi: 10.1016/j.syapm.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 35.Griffiths RI, Whiteley AS, O'Donnell AG, Bailey MJ. 2000. Rapid method for coextraction of DNA and RNA from natural environments for analysis of ribosomal DNA- and rRNA-based microbial community composition. Appl Environ Microbiol 66:5488–5491. doi: 10.1128/AEM.66.12.5488-5491.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reischer GH, Kasper DC, Steinborn R, Mach RL, Farnleitner AH. 2006. Quantitative PCR method for sensitive detection of ruminant fecal pollution in freshwater and evaluation of this method in alpine karstic regions. Appl Environ Microbiol 72:5610–5614. doi: 10.1128/AEM.00364-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reischer GH, Kasper DC, Steinborn R, Farnleitner AH, Mach RL. 2007. A quantitative real-time PCR assay for the highly sensitive and specific detection of human faecal influence in spring water from a large alpine catchment area. Lett Appl Microbiol 44:351–356. doi: 10.1111/j.1472-765X.2006.02094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.John DE, Rose JB. 2005. Review of factors affecting microbial survival in groundwater. Environ Sci Technol 39:7345–7356. doi: 10.1021/es047995w. [DOI] [PubMed] [Google Scholar]
- 39.Davies CM, Long JAH, Donald M, Ashbolt NJ. 1995. Survival of fecal microorganisms in marine and freshwater sediments. Appl Environ Microbiol 61:1888–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wuertz S, Wang D, Reischer GH, Farnleitner AH. 2011. Library-independent source tracking methods, p 61–113. In Hagedorn C, Blanch AR, Harwood VJ (ed), Microbial source tracking: methods, applications, and case studies. Springer, New York, NY. [Google Scholar]
- 41.Bae S, Wuertz S. 2009. Rapid decay of host-specific fecal Bacteroidales cells in seawater as measured by quantitative PCR with propidium monoazide. Water Res 43:4850–4859. doi: 10.1016/j.watres.2009.06.053. [DOI] [PubMed] [Google Scholar]
- 42.Shanks OC, Newton RJ, Kelty CA, Huse SM, Sogin ML, McLellan SL. 2013. Comparison of the microbial community structures of untreated wastewaters from different geographic locales. Appl Environ Microbiol 79:2906–2913. doi: 10.1128/AEM.03448-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ranasinghe PD, Satoh H, Oshiki M, Oshima K, Suda W, Hattori M, Mino T. 2012. Revealing microbial community structures in large- and small-scale activated sludge systems by barcoded pyrosequencing of 16S rRNA gene. Water Sci Technol 66:2155–2161. doi: 10.2166/wst.2012.428. [DOI] [PubMed] [Google Scholar]
- 44.Wang X, Hu M, Xia Y, Wen X, Ding K. 2012. Pyrosequencing analysis of bacterial diversity in 14 wastewater treatment systems in China. Appl Environ Microbiol 78:7042–7047. doi: 10.1128/AEM.01617-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.World Health Organization. 2013. Water quality and health strategy 2013–2020. World Health Organization, Geneva, Switzerland. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


