Abstract
Background
Fluoroquinolone resistance in Pseudomonas aeruginosa may be due to efflux pump overexpression and/or target mutations. We designed this study to investigate the efflux pump mediated fluoroquinolone resistance and check the increasing effectiveness of fluoroquinolones in combination with an efflux pumps inhibitor among P. aeruginosa isolates from burn wounds infections.
Materials and Methods
A total of 154 consecutive strains of P. aeruginosa were recovered from separate patients hospitalized in a burn hospital, Tehran, Iran. The isolates first were studied by disk diffusion antibiogram for 11 antibiotics and then minimum inhibitory concentration (MIC) experiments were performed to detect synergy between ciprofloxacin and the efflux pump inhibitor, carbonyl cyanide-m-chlorophenyl hydrazone (CCCP). Then to elucidate the inducing of multi drug resistance due to different efflux pumps activation in Fluoroquinolone resistant isolates, synergy experiments were also performed in random ciprofloxacin resistant isolates which have overexpressed efflux pumps phenotypically, using CCCP and selected antibiotics as markers for Beta-lactams and Aminoglycosides. The isolates were also tested by polymerase chain reaction (PCR) for the presence of the MexA, MexC and MexE, which encode the efflux pumps MexAB-OprM, MexCD-OprJ and MexEF-OprN.
Results
Most of the isolates were resistant to 3 or more antibiotics tested. More than half of the ciprofloxacin resistant isolates exhibited synergy between ciprofloxacin and CCCP, indicating the efflux pump activity contributed to the ciprofloxacin resistance. Also increased susceptibility of random ciprofloxacin resistant isolates of P. aeruginosa to other selected antibiotics, in presence of CCCP, implied multidrug extrusion by different active efflux pump in fluoroquinolones resistant strains. All of Ciprofloxacin resistant isolates were positive for MexA, MexC and MexE genes simultaneously.
Conclusion
In this burn hospital, where multidrug resistant P. aeruginosa isolates were prevalent, ciprofloxacin resistance and multidrug resistance due to the overexpression of fluoroquinolones mediated efflux pumps has also now emerged. Early recognition of this resistance mechanism should allow the use of alternative antibiotics and use an efflux pumps inhibitor in combination with antibiotic therapy.
Keywords: Pseudomonas aeruginosa; Antibiotic therapy; Efflux pump; Ciprofloxacin, Carbonyl cyanide-m-chlorophenyl hydrazone; Burned patients
Introduction
Pseudomonas aeruginosa is an opportunistic human pathogen and one of the leading causes of nosocomial infections worldwide [1] and causes a variety of infections especially in immunocompromised patients such as burned patients. Infections caused by P. aeruginosa are associated with significant morbidity and mortality [2,3]. The organism exhibits a high level of intrinsic resistance and only a limited number of antimicrobial agents are active against it [4]. In addition, P. aeruginosa has acquired multiple mechanisms of resistance against all available anti pseudomonal agents [4,5]. Fluoroquinolones(FQ) are one of the major classes of antibiotics used in the treatment of infections caused by P. aeruginosa [1] and Large-scale surveillance studies have reported an increasing rate of FQ resistance among clinical isolates of P. aeruginosa [6]. Overexpression of efflux pumps (EPs) and Target based mutation in gyrase and/or topoisomerase contribute to FQ resistance [4]. Of note, in vitro data indicate that at FQ concentrations near the MIC, efflux mutants are preferentially selected before target mutations [7]. Analysis of the genome sequence of P. aeruginosa revealed the existence of 12 potential resistance nodulation division (RND) efflux pump systems [1]. Of these, Six EPs have been well characterized thus far: Mex-AB-OprM, MexCD-OprJ, MexEF-OprN, MexXY-OprM, Mex-JK-OprM, and MexVW-OprM [8,9,10]. The first three have an important role in FQ resistances. All of the pumps can expel a variety of compounds ranging from detergents to structurally unrelated antimicrobial agents from the cytoplasm or periplasmic space [10,11]. While each pump has a preferential set of antimicrobial agent substrates, the FQs are universal substrates for all known Mex pumps [12]. Phenotypic and genetic tests may be used in laboratories practice in order to identify the presence of acquired resistance due to efflux pump overexpression among P. aeruginosa isolates. Increased expression of efflux pumps could increases the MICs of many antimicrobials. A series of different compounds have been identified as efflux pump inhibitors (EPIs) with the ability to broadly inhibit several known multidrug EPs in P. aeruginosa. In this study the resistance due to overexpression of the efflux system was evaluated by phenotypic test using efflux pump inhibitor carbonyl cyanide 3-chlorophenylhydrazone (CCCP). CCCP is a known proton motive force and RND efflux pump inhibitor [13,14], that can be added in Mueller-Hinton agar during its preparation. This phenotypic test is useful to detect efflux pump overexpression that contributes to or determines Fluoroquinolone and multi drug resistance in the study isolates. The minimum inhibitory concentrations (MICs) of antibiotics for strains overexpressing an efflux pump are usually 2 or more folds higher than those strains of that species which didn't have overexpressing an efflux pump.In this study, we hypothesized that the FQ resistant among P. aeruginosa isolated from burn wound infections (may be due to widespread use of FQ agents to treat burn wounds) could be in corporate with resistance to other existing antipseudomonal agents such as Beta-lactams and Aminoglycosides through FQ selected overexpression of multidrug efflux pumps. The specific aims of our study were to use CCCP as a screening agent to evaluate (i) the prevalence of efflux pump-mediated resistance among clinical isolates of P. aeruginosa (ii) the contribution of efflux pumps overexpression as the supposed mechanism for the multi drug resistance (MDR) phenotype in P. aeruginosa (iii) effectiveness increase of FQs in combined with an efflux pump inhibitor.
Materials and Methods
1. Bacterial isolates
The study included a total of 154 P. aeruginosa non repetitive isolates recovered consecutively from clinical burn wound infections of separate patients at a burn hospital of Tehran, Iran. Bacterial isolates first were identified based on the standard biochemical tests [15]. Then phenotypic identification was confirmed at the species level by using PCR amplification of OprI and OprL gene [16] (Table 1). Bacterial genomic DNA was extracted from the all isolates, as well as from the reference strains of P.aeruginosa, by employment boiling method. For this purpose, all isolates were inoculated aerobically on nutrient agar for 18-24 hour at 37℃. Depending on colony size, three to six colonies were picked from plates and mixed in to 0.1 mL DNase/RNase-free water in sterile 1.5-mL tubes to obtain a turbid suspension of bacteria (~ 1-2 × 109 cells/mL). The cell suspensions were held in a boiling water-bath for 10 min to lyse the cells and then centrifuged at 10,000 g at 4℃ for 10 minutes. Finally the supernatant transmitted in sterile conditions into another tube and used as DNA template. Extracted DNA was stored at -20℃ prior to PCR amplification. Also all the confirmed P. aeruginosa isolates were stored at -70℃ in Trypticase soy broth supplemented with 10% glycerol until ready for testing. Control strains included PAO1 (wild type) [17] and P. aeruginosa ATCC 27853.
Table 1. Primers used in this study.
| Primer | 5'-sequence-3' | Product length (bp) | Reference |
|---|---|---|---|
| OprI-F | ATGAACAACGTTCTGAAATTCTCTGCT | 249 | 16 |
| OprI-R | CTTGCGGCTGGCTTTTTCCAG | ||
| OprL-F | ATGGAAATGCTGAAATTCGGC | 504 | 16 |
| OprL-R | CTTCTTCAGCTCGACGCGACG | ||
| mexA1 | CGACCAGGCCGTGAGCAAGCAGC | 316 | 24 |
| mexA2 | GGAGACCTTCGCCGCGTTGTCGC | ||
| mexC3 | GTACCGGCGTCATGCAGGGTTC | 164 | 24 |
| mexC4 | TTACTGTTGCGGCGCAGGTGACT | ||
| mexE4 | CCAGGACCAGCACGAACTTCTTGC | 114 | 24 |
| mexE5 | CGACAACGCCAAGGGCGAGTTCACC |
2. In vitro susceptibility testing
First the antibacterial susceptibility testing of selected P. aeruginosa species was done by disk diffusion method on Mueller-Hinton Agar (Control S, Madrid, Spain). All isolates were tested for susceptibility to imipenem (10 µg), cefepime (30 µg), Ticarcillin (75 µg), Aztreonam(30 µg), Tobramycin (10 µg), Gentamicin (10 µg), Colistin (25 µg), Amikacin (30 µg), Ciprofloxacin (5 µg), Piperacillin (100 µg), Piperacillin-tazobactam (110 µg) (all from Mast, Merseyside. UK). Then, to investigate the prevalence of Efflux pump mediated FQ resistance, synergy experiments were performed for all 154 strains of P. aeruginosa using ciprofloxacin, as a representative of FQ agents which are typically exported by all of 3 most important efflux pumps, and the efflux pump inhibitor. CCCP (Sigma-Aldrich, ST. Louis, MO, USA) was incorporated in Mueller-Hinton agar (Control S, Spain) at concentrations of 12.5 µm and ciprofloxacin susceptibility testing was performed by the two fold serial dilution method using a final inoculum of 106 cells/mL in agar plates with and without CCCP [18]. Then to elucidate the inducing of multi drug resistance due to different efflux pumps activation in Fluoroquinolone resistant isolates, synergy experiments were also performed in random ciprofloxacin resistant isolates which have overexpressed efflux pumps phenotypically, using CCCP and antibiotics selected as markers for beta-lactams [imipenem (IMI) & cefepime (CEF)] and Aminoglycosides [gentamicin (GEN)]. P. aeruginosa ATCC 27853 and PAO1 was used as reference strain [19]. Both of these methods were carried out according to the guidelines established by the clinical and laboratory standards institute (CLSI) [20]. The latter synergy tests were performed in order to check the contribution of efflux pumps to the resistance to FQs, Beta-lactams and Aminoglycosides that are selectively extracted by FQ mediated efflux pumps commonly found in pseudomonads [21].
3. PCR for Mex-Opr efflux systems
To investigate the ratio between existing the different EPs genes and overexpressed efflux pumps ranges, all the isolates were tested by PCR for the presence of MexA, MexC and MexE genes, which are representative of MexAB-OprM, Mex-CD-OprJ and MexEF-OprN efflux systems respectively, using previously described primers [18,22]. Primer designation and their sequences are shown in Table 1.
Results
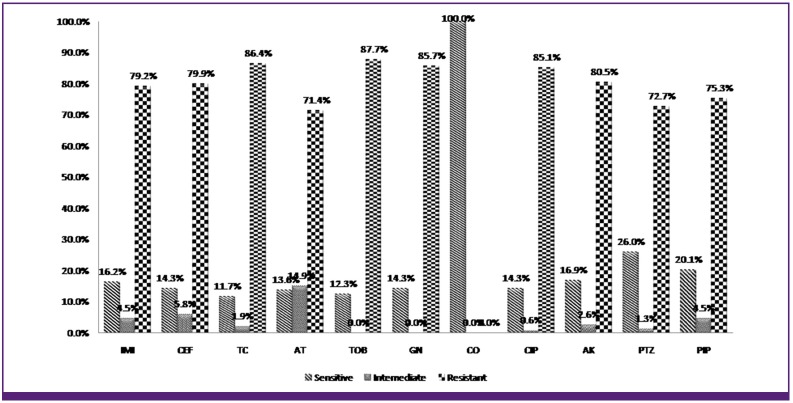
154 strains of P. aeruginosa were isolated from hospitalized burned patients and confirmed using biochemical tests and confirmatory PCR assays. The antibiogram results representing 143 (92.8%) of the P. aeruginosa isolates exhibiting resistance to two or more antibiotics. According to our antibiogram results, all of the isolates only were susceptible to colistin. The most resistance was seen against tobramycin (87.7%) and the least resistance was seen against aztreonam (71.4%).The preliminary results of ciprofloxacin susceptibility test using the disk agar diffusion method showed that 85.1% of the P.aeruginosa strains were resistant to this antibiotic. Figure 1 summarizes the susceptibility status of the isolates against 11 studied antibiotics according to disk diffusion method. MIC for ciprofloxacin ranged from 0.5 to 128 mg/L, According to the established breakpoint values recommended by CLSI [20]. The P.aeruginosa isolates with MIC ≥ 4 mg/L are considered as ciprofloxacin resistant. The preliminary results of ciprofloxacin susceptibility test using the MIC method showed that 127 (82.5%) of the P. aeruginosa strains were resistant to this antibiotic. Results of Synergy tests indicated that by using CCCP as EPI, the MICs of ciprofloxacin for 51 (61%) of the ciprofloxacin resistant isolates decreased (2 to 256 folds) on the CCCP-supplemented plate. Table 2 shows the Effects of CCCP on the Ciprofloxacin MIC reduction in ciprofloxacin resistant isolates of P. aeruginosa. Also in other synergy tests by using CCCP and other antibiotics, we observed increased susceptibility of random selected ciprofloxacin resistant isolates to selected antibiotics as markers for beta-lactams [imipenem (IMI) & cefepime (CEF)] and Aminoglycosides [gentamicin (GEN)]. Table 3 shows the MIC values for ciprofloxacin measured in the absence and in the presence of 12.5 µm CCCP as efflux pump inhibitor in clinical strains of P. aeruginosa. The MexA, MexC and MexE genes were amplified by PCR in 147 (95.4%) isolates simultaneously, and 5 (3.2%) isolates revealed the presence of MexA and MexE simultaneously. 1 (0.6%) isolate had only the MexE gen and 1 (0.6%) had none of the Mex genes. All of the ciprofloxacin resistant isolate had MexA, MexC and MexE genes simultaneously.
Figure 1. The susceptibility status of the isolates against 11 studied antibiotics according to disk diffusion. IMI, imipenem; CEF, cefepime; TC, ticarcillin; AT, aztreonam; TOB, tobramycin; GN, gentamicin; CO, colistin; CIP, ciprofloxacin; AK, amikacin; PTZ, piperacillin-tazobactam; PIP, piperacillin.
Table 2. Effects of CCCP on the Ciprofloxacin MIC in resistant isolates of P. aeruginosa.
| Ciprofloxacin Resistant isolates, No. (%) | Fold reduction in MIC + CCCP |
|---|---|
| 49 (39%) | 0 |
| 42 (33%) | 2 |
| 10 (8%) | 4 |
| 4 (3%) | 8 |
| 5 (4%) | 16 |
| 8 (6%) | 32 |
| 1 (1%) | 64 |
| 0 (0%) | 128 |
| 8 (6%) | 256 |
CCCP, carbonyl cyanide-m-chlorophenylhydrazone; MIC, minimal inhibitory concentration.
Table 3. Ciprofloxacin resistant strains of P. aeruginosa used in this study and MICs of the four antibiotics selected as markers for Beta-lactams [Imipenem (IMI) & Cefepime (CEF)], Fluoroquinolones [Ciprofloxacin (CIP)] and Aminoglycosides [Gentamicin (GEN)] tested in the absence or in the presence of the efflux inhibitor CCCP.
| Strains | MIC (mg/L) | |||||||
|---|---|---|---|---|---|---|---|---|
| IMI | CEF | CIP | GEN | |||||
| -CCCP | +CCCP | -CCCP | +CCCP | -CCCP | +CCCP | -CCCP | +CCCP | |
| 208 | 256 | 4 | 1,024 | 0.5 | 128 | 0.5 | 4 | 2 |
| 209 | 128 | 4 | 1,024 | 0.5 | 128 | 0.5 | 1,024 | 1 |
| 211 | 256 | 4 | 1,024 | 0.5 | 128 | 0.5 | 1,024 | 1 |
| 212 | 256 | 2 | 1,024 | 0.5 | 128 | 0.5 | 1,024 | 1 |
| 215 | 256 | 8 | 1,024 | 8 | 128 | 0.5 | 1,024 | 1 |
| 322 | 128 | 128 | 1,024 | 0 | 128 | 0.5 | 32 | 1 |
| 477 | 256 | 4 | 512 | 512 | 8 | 0.5 | 256 | 128 |
| 481 | 32 | 8 | 64 | 32 | 16 | 2 | 1,024 | 512 |
| 483 | 32 | 8 | 64 | 64 | 16 | 1 | 1,024 | 512 |
| 488 | 128 | 8 | 64 | 32 | 16 | 0.5 | 128 | 64 |
| 517 | 256 | 128 | 1,024 | 512 | 8 | 0.5 | 256 | 256 |
| 520 | 256 | 128 | 1,024 | 1,024 | 16 | 0.5 | 256 | 256 |
| 529 | 128 | 64 | 512 | 256 | 16 | 0.5 | 128 | 16 |
| 538 | 256 | 256 | 1,024 | 512 | 16 | 0.5 | 256 | 128 |
| 539 | 64 | 4 | 512 | 256 | 16 | 0.5 | 128 | 128 |
| 599 | 128 | 64 | 1,024 | 64 | 16 | 0.5 | 128 | 64 |
| PAO1 | 2 | 2 | 0.5 | 0.5 | 1 | 1 | 2 | 2 |
-CCCP, in the absence of carbonyl cyanide-m-chlorophenylhydrazone; +CCCP, in the presence of carbonyl cyanide-m-chlorophenylhydrazone.
Discussion
The findings of the present study clearly show the high incidence of resistant P. aeruginosa strains in burned patients for most of the antibiotics tested. It seems that resistance of P. aeruginosa to most therapeutically antibiotics is common in burned patients of Iran. Almost of the isolates were resistant to two or more antibiotics tested, except colistin showing the highest (100%) antibacterial activity. Only 8 (5.2%) P. aeruginosa isolates were fully susceptible. In case of ciprofloxacin as an important therapeutically choice for P. aeruginosa we observed resistance among 85% of isolates and all of ciprofloxacin-resistant P. aeruginosa were resistant to one or more other tested antibiotics except colistin with no detected resistance. It could be a reason to approve our hypothesis that the FQ resistant among P. aeruginosa isolated from burn wound infections could be in coordinate with resistance to other existing antipseudomonal agents through overexpression of FQ mediated multidrug efflux pumps, because these efflux pumps have broad substrate specificity, and extrude many antibiotic classes including B-lactams, quinolones and aminoglycosides[18]. Widespread use of FQ agents could be one of the main reasons to inducing FQ resistance among P. aeruginosa isolated from burn wound infections, so it has an adverse collateral effect on the susceptibility of P. aeruginosa to other existing antipseudomonal agents through FQ selected overexpression of multidrug efflux pumps. To more examination of this assumption, we employed the EPI compound, CCCP, to phenotypic investigation of efflux pump-overexpressed in clinical isolates of P. aeruginosa. Its why that the susceptibility of P. aeruginosa strains to antimicrobial agents can be significantly enhanced by pump inactivation [21]. According to our observations, many of clinical isolates of P. aeruginosa with a wide range of resistance phenotypes showed increased susceptibilities to ciprofloxacin in the presence CCCP and the MIC of more than half of ciprofloxacin resistant strains decreased two or more fold and the ratio of sensitive strains increased from 25% to 51%. The result of synergy tests observed between CCCP and ciprofloxacin approved its selective extrusion by the above efflux systems contribute to the ciprofloxacin resistance. The genetically investigation of existence of MexA, MexC and MexE genes, which are representative of MexAB-OprM, MexCD-OprJ and MexEF-OprN efflux systems respectively, showed that all of ciprofloxacin resistant strains have these 3 Mex genes simultaneously, but according to phenotypic investigation of efflux pump, half of them showed overexpression of multidrug efflux pumps, so fortunately some of Eps are yet inactive. Also Increased susceptibility of ciprofloxacin resistant P. aeruginosa isolates to selected antibiotics as markers for Beta-lactams [imipenem (IMI) & cefepime (CEF)] and Aminoglycosides [gentamicin (GEN)], in the presence of CCCP implied multidrug extrusion by different active FQ mediated efflux pump in FQ resistant strains. After the addition of CCCP, the accumulation of antibiotics could have increased, and thus led to lower MICs for P. aeruginosa isolates. Inhibition of efflux by CCCP in some isolates brought MICs down even to a level similar to those sensitive strains of P. aeruginosa. So efflux pumps have an important role in FQ resistant isolates of P. aeruginosa as an important and clinically refractory organism. A major beneficial consequence of inhibition of multiple efflux pumps demonstrated in this study is a notable decrease in the frequency of emergence of P. aeruginosa strains with clinically relevant levels of resistance to fluoroquinolones, also the efflux pump inhibitors appears to be an attractive approach to improvement of the clinical efficacies of other antibiotics that are substrates of such pumps [23]. Because the FQs are universal substrates for all known Mex pumps [10,12]. Thus, FQ exposure as one of the main reasons to inducing FQ resistance among P. aeruginosa isolated from burn wound infections, could has the possibility to select for mutants with the multidrug resistant phenotype via efflux pump overexpression. By inhibiting Efflux pumps in combination with antibiotic therapy we could overcome some resistance among P. aeruginosa isolates. However we should consider the prevalence of efflux mediated resistance, besides other mechanisms that contribute to resistance to a particular antibiotic and interactions between different mechanisms of resistance. From our experience with clinical isolates of P. aeruginosa recovered from burned patients, it is evident that the utility of Efflux pump inhibitors should be in combination with antibacterial agents for antibacterial therapy; these compounds may prove to be very useful for study of the contribution and prevalence of efflux in acquired and intrinsic resistance to multiple antibiotics in other gram-negative bacteria.
Most of resistance to FQs among pseudomonades contribute to constitutively expressed efflux pumps. Therefore, efflux pump inhibitors are expected to enhance the activities of FQs. FQ mediated efflux pumps from other gram negative bacteria also belong to the same family of transporters. This increases the possibility that a single inhibitor might be active against multiple efflux pumps [23]. While initial reports have demonstrated the multifactorial benefits of the Efflux pump inhibitor approach in combating drug resistance; substantial efforts are needed before inhibitors can be used clinically. Our efforts are focused on the evaluation of the activities of the CCCP agent. Should our preliminary observations be confirmed in larger studies, it could be of importance to the ongoing efforts to elucidate the mechanisms of Efflux pumps mediated resistance development and identify clinically applicable inhibitors.
Acknowledgement
The authors would like to acknowledge to Iran University of Medical Sciences, for supporting this research. Also, we gratefully acknowledge to Antimicrobial Resistant Research Centre staffs for their co operations.
References
- 1.Askoura M, Mottawea W, Abujamel T, Taher I. Efflux pump inhibitors (EPIs) as new antimicrobial agents against Pseudomonas aeruginosa. Libyan J Med. 2011;6:5870. doi: 10.3402/ljm.v6i0.5870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fagon JY, Chastre J, Wolff M, Gervais C, Parer-Aubas S, Stéphan F, Similowski T, Mercat A, Diehl JL, Sollet JP, Tenaillon A. Invasive and non-invasive strategies for management of suspected ventilator associated pneumonia. Ann Intern Med. 2000;132:621–630. doi: 10.7326/0003-4819-132-8-200004180-00004. [DOI] [PubMed] [Google Scholar]
- 3.Harris A, Torres-Viera L, Vekataraman L, DeGirolami P, Samore M, Carmeli Y. Epidemiology and clinical outcomes of patients with multiresistant Pseudomonas aeruginosa. Clin Infect Dis. 1999;28:1128–1133. doi: 10.1086/514760. [DOI] [PubMed] [Google Scholar]
- 4.Hancock RE. Resistance mechanisms in Pseudomonas aeruginosa and other non fermentative gram-negative bacteria. Clin Infect Dis. 1998;27(Suppl 1):S93–S99. doi: 10.1086/514909. [DOI] [PubMed] [Google Scholar]
- 5.Sefton AM. Mechanisms of antimicrobial resistance: their clinical relevance in the new millennium. Drugs. 2002;62:557–566. doi: 10.2165/00003495-200262040-00001. [DOI] [PubMed] [Google Scholar]
- 6.Kriengkauykiat J, Porter E, Lomovskaya O, Wong-Beringer A. Use of an efflux pump inhibitor to determine the prevalence of efflux pump-mediated fluoroquinolone resistance and multidrug resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2005;49:565–570. doi: 10.1128/AAC.49.2.565-570.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Köhler T, Michea-Hamzehpour M, Plesiat P, Kahr AL, Pechere JC. Differential selection of multidrug efflux systems by quinolones in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1997;41:2540–2543. doi: 10.1128/aac.41.11.2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chuanchuen R, Narasaki CT, Schweizer HP. The MexJK efflux pump of Pseudomonas aeruginosa requires OprM for antibiotic efflux but not for efflux of triclosan. J Bacteriol. 2002;184:5036–5044. doi: 10.1128/JB.184.18.5036-5044.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Y, Mima T, Komori Y, Morita Y, Kuroda T, Mizushima T, Tsuchiya T. A new member of the tripartite multidrug efflux pumps, MexVW-OprM, in Pseudomonas aeruginosa. J Antimicrob Chemother. 2003;52:572–575. doi: 10.1093/jac/dkg390. [DOI] [PubMed] [Google Scholar]
- 10.Poole K. Efflux-mediated resistance to fluoroquinolones in gram negative bacteria. Antimicrob Agents Chemother. 2000;44:2233–2241. doi: 10.1128/aac.44.9.2233-2241.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gotoh N, Tsujimoto H, Tsuda M, Okamoto K, Nomura A, Wada T, Nakahashi M, Nishino T. Characterization of the MexC-MexD-OprJ multidrug efflux system in Delta mexA-mexB-oprM mutants of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1998;42:1938–1943. doi: 10.1128/aac.42.8.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schweizer H.P. Efflux as a mechanism of resistance to antimicrobials in Pseudomonas aeruginosa and related bacteria: unanswered question. Genet Mol Res. 2003;2:48–62. [PubMed] [Google Scholar]
- 13.Ikonomidis A, Tsakris A, Kanellopoulou M, Maniatis AN, Pournaras S. Effect of the proton motive force inhibitor carbonyl cyanide-m-chlorophenylhydrazone (CCCP) on Pseudomonas aeruginosa biofilm development. Lett Appl Microbiol. 2008;47:298–302. doi: 10.1111/j.1472-765x.2008.02430.x. [DOI] [PubMed] [Google Scholar]
- 14.Pagès JM, Masi M, Barbe J. Inhibitors of efflux pumps in Gram-negative bacteria. Trends Mol Med. 2005;11:382–389. doi: 10.1016/j.molmed.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 15.Barrow G, Feltham R. Characters of gram-negative bacteria in Cowan & steel manual for identification of medical bacteria. 3rd ed. Cambridge, UK: 2003. pp. 130–131. [Google Scholar]
- 16.De Vos D, Lim A, Jr, Pirnay JP, Struelens M, Vandenvelde C, Duinslaeger L, Vanderkelen A, Cornelis P. Direct detection and identification of Pseudomonas aeruginosa in clinical samples such as skin biopsy specimens and expectorations by multiplex PCR based on two outer membrane lipoprotein genes, oprI and oprL. J Clin Microbiol. 1997;35:1295–1299. doi: 10.1128/jcm.35.6.1295-1299.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hancock RE, Nikaido H. Outer membranes of gram-negative bacteria. XIX. Isolation from Pseudomonas aeruginosa PAO1 and use in reconstitution and definition of the permeability barrier. J Bacteriol. 1978;136:381–390. doi: 10.1128/jb.136.1.381-390.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pournaras S, Maniati M, Spanakis N, Ikonomidis A, Tassios PT, Tsakris A, Legakis NJ, Maniatis AN. Spread of efflux pump-overexpressing, non-metallo-beta-lactamase-producing, meropenem-resistant but ceftazidime-susceptible Pseudomonas aeruginosa in a region with blaVIM endemicity. J Antimicrob Chemother. 2005;56:761–764. doi: 10.1093/jac/dki296. [DOI] [PubMed] [Google Scholar]
- 19.Bonfiglio G, Carciotto V, Russo G, Stefani S, Schito GC, Debbia E, Nicoletti G. Antibiotic resistance in Pseudomonas aeruginosa: an Italian survey. J Antimicrob Chemother. 1998;41:307–310. doi: 10.1093/jac/41.2.307. [DOI] [PubMed] [Google Scholar]
- 20.Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; twenty-second informational supplement. Wayne, PA: CLSI; 2010. [Google Scholar]
- 21.Masuda N, Sakagawa E, Ohya S, Gotho N, Tsujimoto H, Nishino T. Substrate specificities of MexAB-OprM, Mex-CD-OprJ, and MexXY-OprM efflux pumps in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2000;44:3322–3327. doi: 10.1128/aac.44.12.3322-3327.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quale J, Bratu S, Landman D, Heddurshetti R. Molecular epidemiology and mechanisms of carbapenem resistance in Acinetobacter baumannii endemic in New York City. Clin Infect Dis. 2003;37:214–220. doi: 10.1086/375821. [DOI] [PubMed] [Google Scholar]
- 23.Lomovskaya O, Warren MS, Lee A, Galazzo J, Fronko R, Lee M, Blais J, Cho D, Chamberland S, Renau T, Leger R, Hecker S, Watkins W, Hoshino K, Ishida H, Lee VJ. Identification and characterization of inhibitors of multidrug resistance efflux pumps in Pseudomonas aeruginosa: novel agents for combination therapy. Antimicrob Agents Chemother. 2001;45:105–116. doi: 10.1128/AAC.45.1.105-116.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]