Abstract
The primate basal ganglia are fundamental to the Ackermann and colleagues’ proposal. However, primates and rodents are models for human cognitive functions involving basal-ganglia circuits and links between striatal function and vocal communication come from songbirds. We suggest that the proposal is better integrated in cognitive and/or motor theories on spoken language origins and with more analogous nonhuman animal models.
Ackermann and colleagues present an interesting twist on the well-weathered hypothesis of a direct cortico-bulbar tract as a key step in the evolution of spoken language in humans, or song in vocal-learning birds. The authors seek to generate a new hypothesis that the basal ganglia, in particular, functionally reorganized during human evolution for spoken language and also change in function during ontogeny with the learning of speech. Curiously, however, the basal ganglia, after supporting a language learning role during child development, are proposed to revert to a seemingly more evolutionarily conserved functional role of supporting “emotive-prosodic” modulation in adult humans. This illustrates how the proposal flexes to encompass most data and risks being empirically untestable. Especially unclear is what similarities or differences are hypothesized to exist between humans and different animal models, where presumably homologous or analogous neurobiological mechanisms can be clarified.
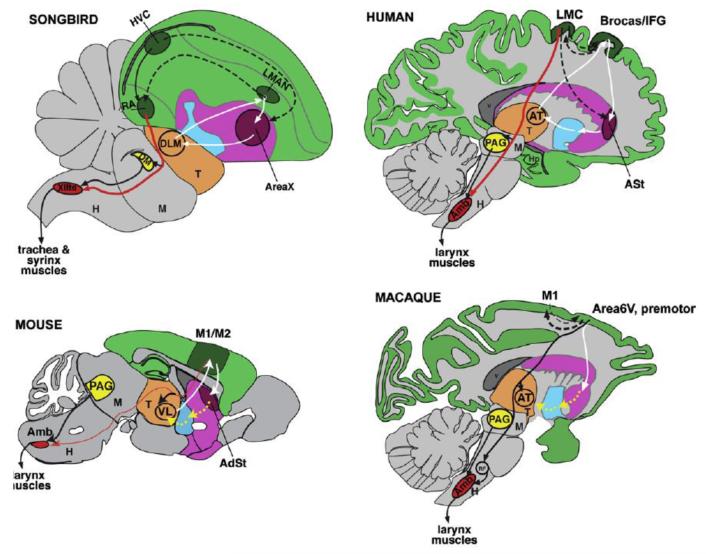
Although we have little doubt that the basal ganglia were an evolutionary substrate for spoken language, one among many others, the current proposal requires considerable strengthening. We make two key suggestions. First, the hypothesis needs to be grounded in, or its key tenets distinguished from, certain cognitive and/or motor theories. Such theories have proposed that specific improvements occurred in vocal learning systems or motor pathways of humans and some birds, including cortico-striatal-thalamic circuits (Arriaga & Jarvis, 2013; Feenders et al., 2008; Fitch, Huber, & Bugnyar, 2010; Fitch & Jarvis, 2012; Petkov & Jarvis, 2012; Wild, 1997). Second, we propose that the key tenets of the proposal, if clarified, can be comparatively tested in studies between, for instance, human and nonhuman primates, and songbirds and vocal non-learning birds, and any of these species and rodents (see Figure 1). Such comparative analyses have already been used in the past to test for the hypothesised differences in the cortico-striatal system between some of these species, and can still be used to comparatively test additional aspects of the current proposal.
Figure 1.
Summary diagrams of vocal systems in songbirds, humans, monkeys and mice. Modified from Arriaga and Jarvis (2013). Cortoco-striatal-thalamic loops are schematized from data in humans and songbirds. Yellow dashed lines in macaque monkeys and mice show proposed cortico-striatal-thalamic connections for vocalization that need to be tested.
One issue is whether and which basal-ganglia dependent differences exist between humans and other nonhuman primates or mammals. There is little direct comparative evidence in the primate literature to suggest that the cortico-striatal-thalamic system is strikingly different in humans relative to nonhuman primates. In fact, as the authors note, nonhuman primates and rodents are used as cellular model systems for human basal-ganglia related cognitive function on motor and procedural learning, habit forming, reward and decision-making, and sensory-motor timing relationships (Matell & Meck, 2004; Schultz, Tremblay, & Hollerman, 2000). Presumably, the proposal is that the basal ganglia, as part of a cognitive system, increased in capacity in humans to support language learning (Friederici, 2011; Petkov & Jarvis, 2012; Petkov & Wilson, 2012). In this regard, it is possibly interesting that Artificial Grammar learning tasks, which were developed in the infant learning literature and that tap into rule-based procedural learning, appear to show differences between different species of monkeys (Wilson et al., in press) and between monkeys and humans (Fitch & Hauser, 2004). These observations were predicted by cognitive theories on spoken language origins (Arriaga & Jarvis, 2013; Petkov & Jarvis, 2012).
Thus, the proposal lacks the strength of the specificity of the direct cortico-bulbar hypothesis, and at the same time suffers from the limitation of overemphasis on a region vital for cognition, whose function is lost without the context of the cortico-striatal-thalamic circuits that are formed in the brains of birds and mammals. As a historical example, the direct cortico-bulbar hypothesis is now seen to be grounded in motor theories of spoken language origins (Petkov & Jarvis, 2012). It is very specific that a monosynaptic change allowed learned sensory patterns to be vocally produced. But its strength in specificity was also its Achilles heel, leaving unanswered how humans and other mammals differ in their neurobiological substrates for learned auditory patterns, and which are linked to vocal motor output (via the nucleus ambiguus). Cognitive theories and the current proposal aim to address this shortcoming. Moreover, even the tenet of a presence vs. absence of a direct cortico-bulbar tract is being challenged by recent data: Mice appear to have a sparse but still present direct cortico-bulbar projection to the nucleus ambiguus and greater vocal-production-plasticity capabilities than has been thought (Arriaga & Jarvis, 2013; Arriaga, Zhou, & Jarvis, 2012), features that had been thought to be unique to humans and vocal learning birds.
Notably, the more precise link that the authors are pursuing with regards to the origins of spoken language and basal ganglia function, already has an evolutionary counterpart in vocal-learning and vocal-non learning birds. The avian striatal vocal nucleus (called Area X in songbirds) sits within a cortico-striatal-thalamic loop, which is important for song learning (Jarvis, 2004, 2006; Jarvis et al., 2000), including covert-skill song learning (Charlesworth, Warren, & Brainard, 2012). Moreover, Feenders et al. (2008), by compared the anterior-forebrain pathway in vocal learning birds to this pathway in vocal non-learning birds, found evidence to develop a motor theory of vocal learning origin. This theory proposes that the anterior-forebrain song pathway (including Area X) independently arose multiple times in vocal learning birds from a set of regions that in vocal non-learning birds control non-vocal motor actions. The discrete striatal Area X that sits within the cortico-striatal-thalamic vocal-learning loop (Fig. 1) is not present in vocal non-learning birds. Motor striatal regions outside of Area X, or the comparable forebrain regions in vocal non-learning birds, are more diffuse and relate to these animals’ non-vocal motor learning abilities. Thus, considerable insights on the cortico-striatal-thalamic system have already been provided by avian models. These are only briefly alluded to but not meaningfully used to inform the current proposal.
In summary, the proposal is an interesting review of the literature with an emphasis on the basal-ganglia as an evolutionary substrate for spoken language. However, we found it heavy on conjecture and light on empirical hypotheses, which as we have suggested can be strengthened by, (1) taking a broader evolutionary perspective that allows integrating data from birds and mammals, and (2) delineating more carefully how the current proposal can be integrated within or distinguished from other theories on spoken language origins.
References (APA Standard)
- Arriaga G, Jarvis ED. Mouse vocal communication system: are ultrasounds learned or innate? Brain Lang. 2013;124(1):96–116. doi: 10.1016/j.bandl.2012.10.002. doi: 10.1016/j.bandl.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arriaga G, Zhou EP, Jarvis ED. Of mice, birds, and men: the mouse ultrasonic song system has some features similar to humans and song-learning birds. PLoS ONE. 2012;7(10):e46610. doi: 10.1371/journal.pone.0046610. doi: 10.1371/journal.pone.00466102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlesworth JD, Warren TL, Brainard MS. Covert skill learning in a cortical-basal ganglia circuit. Nature. 2012;486(7402):251–255. doi: 10.1038/nature11078. doi: 10.1038/nature11078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feenders G, Liedvogel M, Rivas M, Zapka M, Horita H, Hara E, Jarvis ED. Molecular mapping of movement-associated areas in the avian brain: a motor theory for vocal learning origin. PLoS ONE. 2008;3(3):e1768. doi: 10.1371/journal.pone.0001768. doi: 10.1371/journal.pone.0001768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch WT, Hauser MD. Computational constraints on syntactic processing in a nonhuman primate. Science. 2004;303(5656):377–380. doi: 10.1126/science.1089401. doi: 10.1126/science.1089401303/5656/377 [pii] [DOI] [PubMed] [Google Scholar]
- Fitch WT, Huber L, Bugnyar T. Social cognition and the evolution of language: constructing cognitive phylogenies. Neuron. 2010;65(6):795–814. doi: 10.1016/j.neuron.2010.03.011. doi: 10.1016/j.neuron.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch WT, Jarvis ED. Birdsong and other animal models for human speech, song, and vocal learning. In: Arbib M, editor. Language, Music and the Brain. MIT Press; Cambridge MA: 2012. [Google Scholar]
- Friederici AD. The brain basis of language processing: from structure to function. Physiol Rev. 2011;91(4):1357–1392. doi: 10.1152/physrev.00006.2011. doi: 91/4/1357 [pii]10.1152/physrev.00006.2011. [DOI] [PubMed] [Google Scholar]
- Jarvis ED. Learned birdsong and the neurobiology of human language. Ann N Y Acad Sci. 2004;1016:749–777. doi: 10.1196/annals.1298.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis ED. Selection for and against vocal learning in birds and mammals. Ornithological Science. 2006;5:5–14. [Google Scholar]
- Jarvis ED, Ribeiro S, da Silva ML, Ventura D, Vielliard J, Mello CV. Behaviourally driven gene expression reveals song nuclei in hummingbird brain. Nature. 2000;406(6796):628–632. doi: 10.1038/35020570. doi: 10.1038/35020570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matell MS, Meck WH. Cortico-striatal circuits and interval timing: coincidence detection of oscillatory processes. Brain Res Cogn Brain Res. 2004;21(2):139–170. doi: 10.1016/j.cogbrainres.2004.06.012. doi: 10.1016/j.cogbrainres.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Petkov CI, Jarvis ED. Birds, primates, and spoken language origins: behavioral phenotypes and neurobiological substrates. Front Evol Neurosci. 2012;4:12. doi: 10.3389/fnevo.2012.00012. doi: 10.3389/fnevo.2012.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkov CI, Wilson B. On the pursuit of the brain network for proto-syntactic learning in non-human primates: conceptual issues and neurobiological hypotheses. Philos Trans R Soc Lond B Biol Sci. 2012;367(1598):2077–2088. doi: 10.1098/rstb.2012.0073. doi: 10.1098/rstb.2012.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Tremblay L, Hollerman JR. Reward processing in primate orbitofrontal cortex and basal ganglia. Cereb Cortex. 2000;10(3):272–284. doi: 10.1093/cercor/10.3.272. [DOI] [PubMed] [Google Scholar]
- Wild JM. Neural pathways for the control of birdsong production. J Neurobiol. 1997;33(5):653–670. doi: 10.1002/(sici)1097-4695(19971105)33:5<653::aid-neu11>3.0.co;2-a. doi: 10.1002/(SICI)1097-4695(19971105)33:5. [DOI] [PubMed] [Google Scholar]
- Wilson B, Slater H, Kikuchi Y, Milne A, Marslen-Wilson W, Smith K, Petkov CI. Auditory artificial-grammar learning in macaque and marmoset monkeys. J Neurosci. doi: 10.1523/JNEUROSCI.2414-13.2013. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]