Abstract
Simulation models (SMs) combine information from a variety of sources to provide a useful tool for examining how the effects of obesity unfold over time and impact population health. SMs can aid in the understanding of the complex interaction of the drivers of diet and activity and their relation to health outcomes. As emphasized in a recently released report of the Institute or Medicine, SMs can be especially useful for considering the potential impact of an array of policies that will be required to tackle the obesity problem. The purpose of this paper is to present an overview of existing SMs for obesity. First, a background section introduces the different types of models, explains how models are constructed, shows the utility of SMs, and discusses their strengths and weaknesses. Using these typologies, we then briefly review extant obesity SMs. We categorize these models according to their focus: health and economic outcomes, trends in obesity as a function of past trends, physiologically-based behavioral models, environmental contributors to obesity, and policy interventions. Finally, we suggest directions for future research.
Keywords: Simulation models, obesity, policy
Introduction
Computer simulation is widely used to understand and predict behavior in complex systems [1, 2]. The use of simulation models (SMs) in chronic disease prevention is at a nascent stage but has tremendous potential to bridge the gap between research and practice [3–5]. SMs combine information from different sources to provide a useful tool for examining how the effects of public health policies and risk factors unfold over time in complex systems and impact population health [4, 5]. For example, the effects of tobacco control policies on smoking prevalence and health outcomes have been modeled by Mendez and Warner [6, 7], Tengs et al.[8, 9], Ahmad [10–12] and Levy et al.[4, 13, 14].
Like smoking, the effects of obesity on health extend well into the future, not only because of the implications of current obesity on future health, but also because of the difficulty of reducing an individual’s weight [15–18]. In contrast to smoking, which is not necessary for survival, obesity depends on the influence of two key behaviors that are essential to life: diet and physical activity. Although excess weight gain is the result of energy intake exceeding energy expenditure over an extended period of time, there are complex interactions among multiple biological, psychosocial, cultural, environmental, and economic drivers of behavior [3–5, 19–21]. Furthermore, unlike smoking which, can be fairly easily defined as a discrete behavior, the daily imbalance driving excess weight gain is as little as 100 kcal/day [22, 23], making it more difficult to pinpoint and control specific target behaviors. SMs can help us understand the complex interaction of the drivers of diet and activity and how these behaviors affect current and future health outcomes.
As emphasized in a recently released report of the Institute or Medicine [24], SMs can be especially useful for considering the array of policies that will be required to tackle the obesity problem [25]. Purely empirical evaluations (e.g., randomized control trials or natural experiments) often examine the effect of a limited number of policies and often only for a select population because it is difficult to disentangle the effects of different policies on behaviors, obesity rates and health outcomes [25].
The purpose of this paper is to present an overview of existing SMs for obesity. First, a background section discusses the different types of models, the elements of model building and some strengths and weaknesses of SMs. Using the typologies in the background section, we then review extant obesity SMs. Due to the breadth of the scope of models, we summarize and discuss general limitations of current models but do not critically analyze each individual model. Finally, we suggest directions for future research.
Simulation Models: Background
A. Characteristics of Simulation Models
SMs usually consist of a collection of mathematical equations that quantitatively map the relationships between a number of inputs and one or more outputs. Outputs from one set of equations can be used as inputs to a second set of equations. For example, the effects of public policies can be seen as involving stages: public policies affect environments and knowledge which influence diet and physical activity, which in turn determine weight, which in turn influence health outcomes. Bidirectional relationships (or feedback loops) can also be specified. For example, obesity rates may affect social norms regarding acceptable body size, which in turn may affect dietary behavior or activity levels [26].
SMs can be static or dynamic. Static SMs consider snapshots at two points in time or two different scenarios at a single point in time. Dynamic SMs consider the path of changes in an outcome variable over time. SMs are further classified as macro or micro models. Macro models distinguish groups of individuals. For obesity, groups may be characterized by socio-demographic status (e.g., by gender, age, or income), and further distinguished by weight class (e.g., normal, overweight, or obese). While a macro model tracks the proportions in each category, micro models track individual characteristics (e.g., mapped from a multivariate distribution of body mass index (BMI) by age and gender).
SMs may simulate events of a single cohort of individuals over their lifetime or of the entire population over a specific time period. The transitions to and from categories (such as age, or weight) may be specified as occurring in discrete or continuous time. Discrete time models allow state transitions at fixed time intervals only (e.g., yearly), whereas continuous time models allow transitions at any point in time. Transition rules (e.g., from weight x in year 1 to weight x+y in year 2) may depend on individual characteristics, such as age and/or gender and past weight. However, to be parsimonious, transitions are usually specified as Markov [27, 28], whereby they depend only on the current state (e.g., by carrying all past information forward into the current state, weight in period t+1 depends only on weight in period t).
The transition rules may be deterministic with probability equaling 1, such as changes in food consumption due to price change, or stochastic with multiple possible outcome values each occurring with some degree of probability, such as the chance of dying within a given time period [29]. One frequently used type of probabilistic method is the Monte Carlo Model (MCM), which draws randomly from a fixed distribution of population characteristics. The transitions may be further structured, as with agent-based models [30], which employ rules for individual (or “agent”) behavior and interactions with other agents (e.g., to show the effects on obesity of price through maximizing behavior or of social norms through interdependent behavior).
B. Steps in the Development of a Simulation Model
Development of an SM generally involves the following elements: 1) define the scope of the problem; 2) choose the type of the model and determine its basic structure; 3) estimate parameters; 4) validate the model; and 5) conduct sensitivity analyses.
A basic structure is driven by model type (micro vs. macro, stochastic vs. deterministic, etc.), the heterogeneity of the population and outcomes to be considered, and the causal pathways from upstream factors to downstream outcomes. These choices are dependent on the problem being considered, the data available, and the trade-off between the simplicity and complexity of the model. A more complex model may be better equipped to represent reality, but simpler models require less information and are generally more transparent.
Once the transitions between states are specified, initial model parameters may be statistically estimated or derived from published sources. When solid empirical evidence is not available, expert opinion can be used to estimate parameters of the model[25]. Calibration refers to the selection or refinement of model parameters to reproduce expected or observed results.
Validation is the process of assessing whether the model is consistent with data that were not used for calibration, also called ‘external validation’. By showing that the simulated outcomes of the model conform to historical or other empirical data, the credibility of the model is enhanced.
Sensitivity analysis is used to show how the model results vary under plausible values of parameters (parameter uncertainty) or key assumptions made in the design of the model (model uncertainty, e.g., intervention delivery by a practice nurse rather than a general practitioner). If the conclusions continue to hold under varied conditions, then the model is said to be robust.
C. The Benefits and Limitations of Simulation Models
Traditional epidemiological studies are often ill equipped to simultaneously consider multiple associations and pathways in a complex problem like obesity. The core strength of SMs is to make the complexities tractable by providing a framework for examining nonlinear dynamics, time delayed effects, multiple interactions and feedback loops.[31, 32]. SMs provide a means for integrating the knowledge base from a variety of diverse fields, such as statistics, epidemiology, biology, nutrition, sociology, psychology, and economics to depict the multitude of genetic, environmental and behavioral influences on obesity [20, 21, 32]. Indeed, SMs can be used to synthesize and systematically combine information from many disciplines, thereby helping to understand the “big picture” rather than considering pieces of the system in isolation.
Not only can SMs provide a framework for indentifying research priorities, but they can also support policy-makers to achieve evidence-based decisions [33]. SMs can demonstrate the need for public health policy by quantifying and forecasting the effects of obesity on health and other outcomes. SMs can show the successes and failures of past policies, as well as predicting hypothetical policy proposals before their implementation. By using SMs to examine the effects of different policies individually and in various combinations, an overall strategy to the obesity problem can be more effectively developed.
SMs are useful for synthesizing existing data; but their forecasts are dependent on the quality of the data that serve as inputs (e.g. measured versus self-reported weights and heights) and dependent on the quality of the evidence for effects of different risk factors, intervening variables and policies. In addition, a natural consequence of representing problems as models is that simplifying assumptions must be made. For example, an SM may treat all people of a specific age (or gender, race, weight range, etc) as a single category, such as age 20–45, even though there may be incremental differences among people within this age range. The impact of an assumption on outcomes will depend on the problem analyzed and can be addressed through sensitivity analysis.
Review of Current Obesity Models
Table 1 summarizes the different obesity models that have been published or presented at international conferences. We limit the table to models that incorporate trends in obesity over time with transitions by age group.
Table 1.
Dynamic Simulation Models of Obesity*
| Health Outcome Models | |||||||
|---|---|---|---|---|---|---|---|
| Reference | Modeling Method | Focus Population Covered | Main data source (calibration/validation) | Obesity Outcomes | Health & Economic Outcomes | Study Description | Results |
| van Baal, Polder, et al., (2008) | Discrete-time Markov micro model | Holland: Cohorts of 500 men & 500 women aged 20 y at baseline, | Longitudin al Cost & Use files of the Medicare Current Beneficiary Survey from 1992 to 2001 | Never-smokers with BMI> 30; NW never-smoker (18.5<BMI< 25) & NW “smoking” cohort | Health care costs, Disease incidence | Estimated lifetime health-care costs for obese adults compared to similar cohorts of smokers & “healthy-living” persons | Annual health expenditure < 56 y was highest for obese, but smokers > 55 y incurred higher costs. Because of differences in life expectancy, lifetime health expenditure of obese individuals was less than for low-risk individuals, but more than for smokers. |
| Light-wood, Bibbins-Domingo, Coxson 2009 | Discrete-time Markov cohort macro model | US: Adults aged 35–64 years in the US population in 2020 to 2050. | NHANES for 1971 to 1974,1976 to 1980, 1988 to 1994, & 1999 to 2000 | Adolescent OW as BMI above the 95th percentile on the CDC growth charts & adult obesity as BMI≥ 30 kg/m2 | Medical costs & lost productivity due to earlier mortality & morbidity from obesity & associated CHD & diabetes | Historical data on OW adolescents used to predict obesity in adults. MEPS data used to estimate costs due to CHD. Census & Bureau of Labor & Statistics data used to measure productivity loss. | Today’s OW youth will result in 161 million life-years complicated by obesity, diabetes, or CHD & 1.5 million life-years lost. The cumulative excess attributable total costs are estimated at $208 billion because of lost productivity from earlier death or morbidity & $46 billion from direct medical costs. |
| Trend in Obesity Models | |||||||
| Basu (2009) | Discrete-time Markov macro model | US: Children (6–17 y) & adults (>17 y) by age group, sex, & race from 2004–2014 | MEPS for 2001–2002 & 2004–2005 to estimate 1-year transitions across BMI categories. Validation with 2005–2006 NHANES | Adult: NW (BMI< 25), OW (25≤BMI < 30), obese 1 (30≤BMI) & <35), 2 (35≤BMI<40), & 3 (BMI≥40). Youth: NW (BMI < 85th %ile), at risk for OW (85th ≤ BMI < 95th %ile), OW 1 (95th ≤ BMI < 99th %ile), & OW 2 (BMI ≥ 99th %ile) |
None | A probabilistic, statistically-based population-level model used to forecast distributions of obesity from 2004–2014. | Projects: 1) Most adults in 2004–5 remained in their initial BMI category; 2) Children showed more movement from other BMI categories into OW class 2 relative to 2001–2002. Significant increases (5%–14% over 5 y) in the risk of OW among children 6 to 9 y, & 3) increases in adult OW for future, with obesity remaining at current levels. |
| Brown, Byatt, Marsh, et al. (2010) | Discrete time, Markov, MCM | UK & US: All Ages | Health Survey for England, 1993–2007, also applied to US using NHANES data | Adult: BMI groups (<20, 20–25, 25–30, 30–40, >40). Youth: 85th, 95th %ile (IOTF), by age, gender & social classes |
Disease incidence by type for major BVMI-related diseases | A statistical model (non-linear) used to estimate weight gain trajectories by age, gender & SES, the parameters of which were used in a microsimulation model. | Projects flattening in the upward trend in obesity & the obesity rate is growing faster among low SES than other SES groups. US projections were similar; with UK lagging in time relative the US. |
| Flanagan, Finès, Nadeau, et. al. (2009) | Discrete-event, Continuous time, MCM | Canada: All ages (initially adults, later extended to youth) | Canadian Community Health Survey 2001 for levels, National Population Health Survey for transitions | BMI value as categories: Underweight (BMI < 18.5), NW (BMI 18.5–25), OW (BMI 25–30), Obese (BMI>=30) | Impact on disease onset (osteoarthri tis, acute myocardial infarction, diabetes) & health-related quality of life | BMI projected from past BMI & changes in income, region & education. Obesity affects health through longitudinal risk factor & disease sub-modules. Rich on population characteristics, links to specific diseases, & other lifestyle factors (e.g., smoking). | Projects slow upward trends in obesity; a 10% increase over the next 20 years, with steeper slopes in males. Projections are partially driven by aging of the population & by recent obesity trends. |
| Homer, Milstein, Dietz, (2006) | System dynamics, discrete time, Markov, macro model | US: All ages | NHANES (1971–4, 76–80, 88–94, 99–02); NHES (1960–2, 63–5, 66–70) | Normal weight (BMI<25), moderately overweight (25–30 or 85th %ile), moderately obese (30–5 or 95th %ile), severely obese (BMI>35 or 99th %ile) | Disease incidence (especially diabetes & CHD), Obesity-attributed Illness cost & unhealthy days | Trend analysis used to consider obesity transitions by age. Obesity was affected by policies through energy balance equation. The health model incorporates other life style factors (smoking), | Finds: 1) Extrapolations assuming continued linear growth exaggerate future obesity. 2) The daily caloric imbalance relative to 1970 accounting for obesity growth is 1–3% of daily caloric intake. 3) Interventions focused on youth alone have small impact on adult obesity. 4) With caloric balance by 2015, full adverse health impact of obesity will still continue far into future. |
| Wang, Colditz, & Kuntz, (2007) | Statistically -based model estimated with individual level data | US: Children (6–17 y) and adults (>17 y) by age group, sex, & race from 2004–2014. | 4 NHANES surveys; 1971 to 2004. Validated using cohorts in the Nurses’ Health Study and Health Profession als Follow-up Study. | BMI distributions | None | Within race & sex, regression models fitted to create smoothed mean BMI curves by age for 1970 to 2010. Linking corresponding birth cohorts across age- & year-specific mean BMI projections, the trajectory of relative BMI throughout each cohort’s lifetime was projected. | BMI secular trends in the past 3 decades differ by birth cohort, sex, & race. If trends continue, obesity prevalence in 2010 will reach 35%, 36%, 33%, & 55% among white men, white women, black men, & black women, resp., translating into 9.3 million more obese adults 20 to 74 y than in 2000. |
| Sassi, Devaux, Cecchini et al. (2009) | Statistically -based model using individual level data | OECD nations: all ages | National Health Surveys | BMI (overweight and obese) | None | Using data by age, gender and SES from repeated cross-sectional national surveys, statistical models estimated with age, period & cohort effects, and the clustering of individuals in households | Future trends show a progressive stabilisation or slight shrinkage of pre-obesity rates, but continued increase in obesity rates. Pronounced disparities by SES in women, with mixed patterns in men. |
| Behavioral Models | |||||||
| Bahr, Browning, & Wyatt, et al (2009) | Social network micro model of prediction | US: all ages | Framing-ham Study | Under, normal and over-weight and obese | None | Rule of social interaction (majority) were applied to individuals based on interaction with neighbors & dependent on social volatility. | Individuals with similar BMI cluster into groups driven by social forces toward increasing obesity. Traditional weight management inter-ventions fail because they do not consider surrounding clusters & wider social network. Interventions should targeting well-connected &/or NW individuals. |
| Burke & Heil (2007) | Agent-based, discrete time model | US: women ages 30–60 | Calibrated to American women (30 to 60 y) & weight distribution validated to NHANES surveys from 1976 through 2000. | Average weight & rate of obesity | None | Model of food & non-food consumption, focusing on falling food prices in an economic model involving endogenous body weight norms (aspiration to weigh less than average, which becomes relaxed as weight increases) & an explicit description of human basal metabolism (heterogeneous across population, increasing & concave in body weight). | Accurately predicts increases in average weight & obesity rates, especially the relative growth in upper quantiles. Food prices affect weight of heavier individuals more. The lagged adjustment of weight norms explains recent trends of rising obesity rates since the mid 1990s, despite apparent leveling off of price declines. Policies should focus on the upper 5% of the weight distribution. |
| Hammond, & Epstein (2007) | Agent-based discrete time model | General model, not a specific population | Hypothetical population | Change in BMI & weight distributions | None | Individual behavior in the context of the physiology of dieting & exercise, & socially influenced weight changes. Focus on the role of social norms with the interaction of physiological realities, & the impact of media & public health messages. | Shows how the core equations governing the physiology of weight change can generate many known observations about diet & weight gain, including: the difficulty of maintaining a diet, high rates of recidivism after dieting, & substantial individual heterogeneity in the success of diets. |
| Policy Models | |||||||
| Carter, Vos, & Moodie (2008) | Macro model used to determine C-E of interventions. | Australia (also being applied in New Zealand & USA): various ages | Various nations: various published sources | BMI | DALYs (aggregated over diseases), $/DALY saved | Type of modeling depends on disease. Starts with single cohort, discrete time, macro models, moves to multiple cohort/dynamic population/or micro model dependent on the disease & research question. C-E analysis includes the link from interventions to behavioural change to caloric intake/expenditure to change in BMI to health/cost outcomes (primarily DALYs) over lifetime of the cohort. Comprehensive intervention evaluation strategy used to set priorities. | Model has been used to evaluate 13 interventions targeting youth obesity & 150 interventions across a range of chronic diseases as part of the Australian ACE Prevention project, & to determine most cost-effective package of interventions. Ultimate goal – help decision-makers move resources towards more efficient options by new investments in proven C-E packages of interventions, or shifting funds from less efficient current practices to more efficient preventive action |
| Sassi, Cecchin, Lauer (2009) | Discrete-time, Markov micro model following current cohorts to determine C-E | OECD nations: by age, gender and SES | National Health Surveys | Intake of fats, fruits and vegetables, level of physical activity, and BMI | DALYs (aggregated over diseases), $/DALY saved | Based on the WHO-CHOICE (CHOosing Interventions that are Cost-Effective), model assesses the efficiency of prevention-oriented policy options affecting chronic diseases. Considers the links from the effect of policies (effectiveness based on best studies) to BMI to health outcomes using a dynamic model | Most interventions have favourable C-E ratios, but those tackling individual determinants or targeted to narrow group have a limited population impact. Although the most efficient interventions are outside the health sector, health care systems can have important impact by focusing on those at high risk. Interventions targeting younger ages affect health mostly in the future. |
| Schroeter, Jayson Lusk &, Tyner 2008. | Economic macro model | US: all ages | Various published surveys | Weight gain in terms of BMI | None | The model identifies conditions under which price & income changes are most likely to change weight. An economic model considers the effect of prices of high caloric & low caloric foods & of income (using price & income elasticities) & applies a caloric intake equation to examine the relationship of consumption to weight. | Although raising the price of high-calorie food will decrease their consumption, the effect on weight can be ambiguous. e.g., a tax on food away from home could lead to an increase in body weight. The analysis shows the need to employ economic modeling when developing public policy to reduce obesity. |
The table is limited to dynamic models that track a population by age group over time
Abbreviations
MEPS = Medical Expenditure Panel Survey
NHANES = National Health & Nutrition Examination Surveys
NW= normal weight
OW = overweight y = years old
MCM = Monte Carlo Method
C-E = cost effectiveness
We begin by reviewing SMs focusing on the health and economic consequences of obesity, since that is the ultimate public health concern and often defines the problem. We then consider SMs that project future rates of obesity based on historical trends, followed by models which relate dietary and physical activity to obesity, SMs that relate diet and physical activity to environmental conditions and, finally, SMs of specific interventions and policies.
A. Health and Economic Consequences of Obesity
Obesity has been associated with a higher risk of heart disease, diabetes, cancers and other disease [34]. Each of these outcomes is associated with different burdens of morbidity and mortality. The total mortality risk attributable to obesity is usually estimated in terms of the average years of life lost at each age [35–37]. Morbidity varies by type and severity of disease, the effect of which can be aggregated in terms of medical costs [37], years of life lost [38, 39], quality-adjusted or disability-adjusted life years lost (QALYs or DALYs) [33, 40–42].
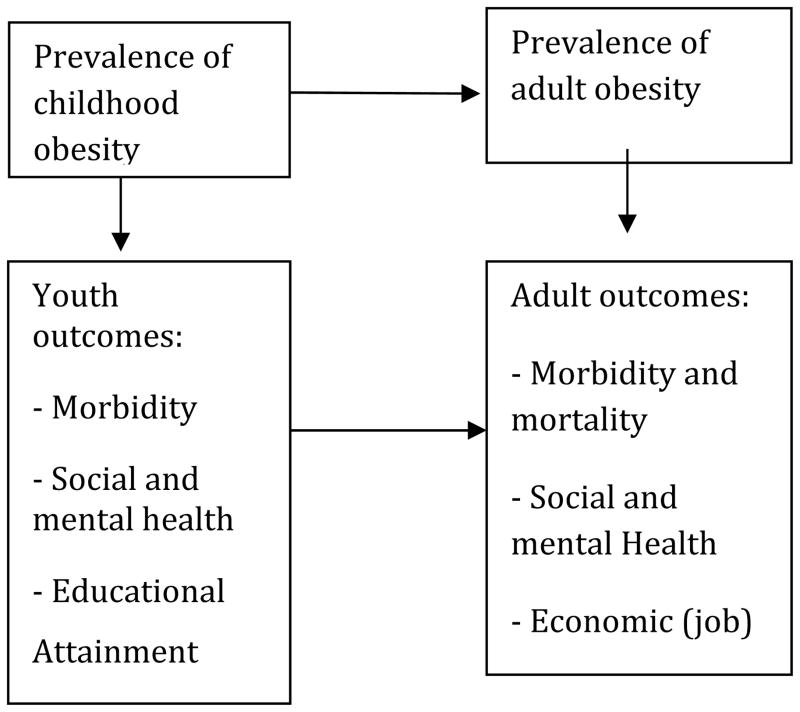
The excess risks of morbidity and mortality due to obesity may be pronounced at later ages, but health effects arise as early as childhood. As shown in Figure 1, obesity has been associated with other adverse outcomes, including emotional distress (including social health issues, such as discrimination) and lost productivity [43–45], and, in the case of youth, reduced educational achievement and poor psycho-social outcomes [46, 47]. A dynamic micro-SM developed by van Ball et al.[48] showed how obesity-related medical costs vary over the life cycle for a typical cohort. They found that increased medical costs attributable to obesity at younger ages may be offset by reduced costs at later ages when the obese individual is less likely to be alive. In a macro-SM, Lightwood et al.[49] simulated obesity transitions by age. Incorporating a broad array of risk factors and disease outcomes, they predicted the future effects of current adolescent obesity on medical costs and productivity loss. Finkelstein et al.[50–52] also considered employment effects but used distributions of obesity at each age rather than modeling transitions by age.
Figure I.
Future Health and Economic Outcomes
Current models generally employ a Markov assumption, whereby health outcomes depend only on current levels of BMI. However, health outcomes over the life cycle of an individual may depend on how BMI persists over time as well as the relative health risks associated with different levels of BMI at each age [39, 53]. Obesity may create risks that are cumulative over time (e.g. atherosclerosis) or cause a disease with future ramifications (e.g., diabetes which is itself a risk factor for heart disease). Data on diseases that can be linked to obesity are often prevalence-based, since data on the incidence and duration of disease are often not available. In addition, the nature and extent of adverse effects may depend on the level of BMI in a non-linear fashion [54–56], and vary by age, gender or socio-economic status in a way not captured by simplified models.
The effects of obesity on health have been considered in models devoted to obesity or in broader models that simultaneously consider other risk factors [57, 58] along with a broad array of diseases [59–61]. Other risk factors, such as smoking, may reinforce the risks associated with obesity or may lead to higher background risk relative to obesity [62–65]. The inter-related effect of life-style factors is potentially quite complex, especially since health outcomes may depend on past as well as current BMI. Because health outcomes associated with obesity, especially heart disease and cancers, are dependent on the complex relationship of multiple risk factors, the ability to validate the role of obesity in health outcomes is limited.
Another limitation is that current models do not distinguish the effects of diet and physical activity on health outcomes. The magnitude of their effects may depend on how obesity is measured [56]. Furthermore, physical activity and certain types of foods may affect health outcomes above and beyond their effects through BMI, such as the effect of sugar-sweetened beverages on diabetes [66] or of trans fats on heart disease [67]. Dall et al.[68, 69] considered how higher daily intake of calories and sodium can increase the prevalence of excess weight and hypertension.
B. Trends in BMI as a Function of Past Trends
SMs projecting future trends in obesity prevalence may inform future health care needs and identifying underserved populations. Statistical models employing simple secular trends have been applied to 106 countries by Kelly et al.[70]. Dynamic SMs enable more sophisticated analyses that incorporate changing socio-demographic characteristics of the population. They generally begin with the population differentiated by BMI or obesity rates, age and other relevant population characteristics [71–73]. Over time, they simulate how obesity outcomes change in parallel to demographic changes through births and deaths.
Dynamic SMs explicitly consider transition probabilities between BMI categories by age, where the transitions may be estimated statistically by following age groups over time. For the United States, Basu [74] used Medical Expenditure Panel Survey data (2001–2002, 2004–2005) to estimate and compare the 1-year transitions across BMI categories, and validated the model using the 2005–2006 National Health and Nutrition Examination Survey. Although the absolute levels of obesity remained high among US adults, the author projected that the growth in obesity would plateau while rates for children would continue to grow.
Brown et al.[32, 75] developed a MCM for the United Kingdom based on a statistical model which estimated weight gain trajectories by age, gender and socio-economic status (SES) from the year 1993. For Canada, the Population-Health Model (POHEM) [76–78] has been expanded to consider the role of obesity along with other lifestyle factors (e.g., smoking). BMI trends were projected as a function of past levels and changes in BMI, education, income and region. Both the UK and Canadian model have been validated and used to predict the impact on health through longitudinal risk factor and disease sub-modules. Both have found that obesity rates among some of the younger age groups have flattened, but obesity rates in adults and obesity-related diseases are predicted to continue climbing in the foreseeable future [74–77].
In all the above models, transitions in obesity vary by age, but others have taken into account how trends may vary by cohort [79]. For example, those exposed to lifestyles of the 1940s and 1950s while growing up may have different lifestyle risks than those born in later decades. Wang et al.[73] forecasted the BMI distribution in the U.S. population, incorporating demographic shifts in sex, age, and race over time. Statistical analysis showed BMI secular trends in the past 3 decades differ significantly by birth cohort, and is likely to have different impact on the aging population by race. For 13 OECD nations, Sassi et al.[80] have also incorporated age, period and cohort effects, as well as the clustering of individuals into households. They predicted that pre-obesity rates would stabilize whereas obesity rates and SES disparities, particularly among women would continue to grow.
Since sufficient longitudinal data are generally not available, synthetic cohorts, (i.e., linking age groups across cross-sectional data at different time points) are often used, so that data may not be comparable over time. Specifically, since weight may fluctuate over time, synthetic cohort data may not adequately capture the duration of obesity. which may limit the ability to link trends in obesity with health outcomes. Longitudinal data will be needed to surmount these obstacles. In addition, the models above have been validated over a limited number of years. It will be important to consider how well their models predict overall obesity rates, as well as by age gender and socio-economic status, as new data become available.
To fully incorporate age, period and cohort effects, dynamic SMs will require data with consistent measures of obesity over time, and which links adult to youth obesity [81–83]. The age-period-cohort models may predict accurately over short-term horizons but may be unstable over longer periods (i.e., the predicted trajectories may change) if underlying patterns in diet and physical activity change. Homer et al.[84] incorporated an energy balance equation into a macro-SM to project future trends in obesity rates. They found that: 1) extrapolations assuming continued linear growth may exaggerate future prevalence; 2) the daily caloric imbalance that accounts for obesity growth was 1–3% of daily caloric intake; and 3) current policy efforts focusing on school-age children alone will not have much impact on obesity-related diseases at the population level for several decades. However, use of the energy balance equation in macro models may be subject to limitations as discussed in the next section.
C. Physiologically-Based Behavioral Models
Predicting the change of body weight that results from a specific change in diet or physical activity generally involves a “below the skin” mathematical model of energy balance. These models aim to link changes in diet or physical activity to changes in body weight, either at the individual or at the population level. Because the literature is large, this section provides an overview focusing on their implication for SMs that consider upstream and downstream effects.
The simplest model proposed by Hill et al.[22] provides a measure of the average daily energy imbalance underlying body weight change by assuming that a pound of weight represented 3500 kcal excess storage and that excess energy was converted to storage at a 50% efficiency. Based on historical trends, they estimated that the median daily energy imbalance gap between intake and expenditure needed to explain a population weight gain of 1.8–2.0 lbs per year was about 30 kcal/d. Another simple model developed by Swinburn et al.[85] used studies of total energy expenditure (TEE) measured by the doubly labeled water technique. Under the assumption that TEE equals total energy intake (TEI) when weight is stable, they developed equations to estimate the difference in energy flux between two steady states of weight balance with the higher weight. Hall and Jordan developed a spreadsheet-based model of the change in steady-state body weight that explicitly included the effect of both TEI along with physical activity changes and metabolic adaptation impacting TEE [86]. Using data from 8 longitudinal weight-loss studies, their model calculations closely matched the weight change, and also accurately predicted the proportion of weight change resulting from the loss of body fat. In applying their respective equations, both Swinburn et al.[87] and Hall and Chow [88] concluded that the increase in US food supply over the last 30 years was more than sufficient to explain the concurrent increase in weight in the US population. Although the two models came up with different absolute values for the predicted weight gain with further methodological adjustment [89, 90], both models concurred that an increased TEI of about 94kJ/day would result in a weight increase of about 1kg for adults. Most studies examining behavioural predictors of weight gain have been inconclusive [91], probably because the size of the energy gap which distinguishes weight gainers from non-gainers is below the threshold of current measurements. However, most predictive models make the assumption that weight change is due to behavioural changes in energy intake or physical activity rather than some primary readjustment of body composition [92]
To calculate the time course of body weight change, differential equation models have been developed that use food intake as a model input and calculate changes in energy expenditure and body weight over time. Flatt [93] was the first to attempt to model how food intake is regulated in humans using a two-compartment model considering body glycogen and fat. Chow and Hall [94] have shown that three-dimensional macronutrient (protein, carbohydrate and fat) balance models [95, 96] can be reduced to two dimensional models of body fat and fat free mass, which only under a specific set of conditions can be simplified to a one dimensional equation for body weight change [97]. To consider the independent role of exercise, Christiansen et al.[98] hypothesized how weight gain may result in decreased physical activity, thereby forming a positive feedback loop that generates self-promoting increases in weight over time. Abdel-Hamid [99, 100] has proposed a system dynamics model of body weight change in terms of physical activity and food intake, but the equations have not been published. The estimates from physiologically-based behavioral models can be useful in showing the effects at a population level of an intervention that reduces caloric intake, by assuming that the intervention yields a similar shift in caloric intake across the distribution of individuals [40–42, 101].
Compared to adults, Butte and Ellis [102] showed that the daily energy gap was higher for children who were rapidly gaining weight. Jordan and Hall [103] considered the dynamic relations between diet, macronutrient oxidation, and energy expenditure during normal infant growth to show the complex metabolic adaptations occurring during normal growth. Simplified methods provide useful metrics for modeling youth weight gain. A number of studies [23, 104, 105] have modeled different aspects of unhealthy weight gain in children but there is little consensus because of the different populations considered (general population versus the obese) and different conceptualization of the ‘energy gaps’ being measured. The energy requirements of growth make modeling weight gain in children more complex than for adults
D. Environmental Contributors to Obesity
While diet and physical activity are the underlying behaviors that explain weight changes, the environment in which people work, live, and go to school can enable or constrain healthy behaviors and help explain changes in obesity prevalence. In contrast to physiological models, models in this section explore “above the skin” factors associated with obesity in a psycho-social or economic framework.
Edwards et al.[106, 107] developed a SimObesity model to consider childhood social networks within neighborhoods. Their results show that social capital and poverty are strongly associated with childhood obesity. Network analysis [26, 108–112] examines how characteristics of social networks might explain obesity rates. Christakis and Fowler [26] showed that adult obesity spreads over time through social ties at home, work and in their geographic neighborhood using a statistical model. Trogden and Nonnemaker [109] and Halliday and Kwak [111] confirmed these findings in adolescent social networks, but Cohen-Cole and Fletcher [110] suggested that environmental influences, rather than social ties, explain peer influences on weight status. In response, Fowler and Christakis [112] confirmed their results and highlighted the importance of their findings for policy, noting the potential bias in effect sizes from failing to account for ongoing network effects.
In contrast to these statistically-based, static network models, Bahr et al.[108] developed a dynamic, micro-SM of the spread of obesity, which employed a majority rule dependent on the interaction with neighbors and social volatility. They found that for a wide variety of conditions, individuals with similar BMIs were found to cluster into groups, and social forces drove these groups toward increasing obesity. Their results suggest that interventions should target well-connected or normal weight individuals in high risk demographic groups, and that conventional, individual focused weight management interventions should consider the wider social network. The network models appear promising but await validation.
Burke et al.[113] developed an agent-based model to explore a variety of factors that could explain observed increases in obesity prevalence. Their model featured falling food prices, body weight norms, and human metabolism (with heterogeneity). The model accurately predicted increases in average weight and obesity prevalence, as well as increases in the upper quintiles. Food prices had greater effects on heavier individuals due to the shape of the metabolic rate curve. Continuing increases in obesity rates, despite a leveling off of price declines, were explained by the lagged adjustment of weight norms.
An agent-based model by Hammond and Epstein [114] incorporated the role of physical activity, the interaction between physiological states and dietary decisions, social influence and norms, and the impact of media and public health messages. The authors showed how core equations reflecting the physiology of weight change could reproduce many observed phenomena. Specifically, their model helps explain the difficulty of maintaining a diet, high rates of recidivism after dieting, and substantial individual heterogeneity in the success of different types of diets. However, the model will need to be validated.
The models described in this section are useful for understanding a wide variety of environmental determinants of obesity. As argued by Hammond and Epstein [114], models that integrate social, physiological, and economic aspects can provide deeper explanations of the observed dynamics of obesity and suggest policies tailored to specific communities. However, issues of causality, such as feedback effects of obesity rates on norms, will need to be addressed. As more is learned, SMs may also elucidate the implications of the interplay between genes and environment for BMI and health policy. Research already shows that genetic traits can help set bounds on the effects that changes in the environment may have [115].
E. Policy Intervention Models
Policy intervention models consider potential levers to influence the environment, e.g., price and advertising, or to directly influence individual diet and exercise through education. The Assessing Cost-Effectiveness in Obesity (ACE) project provides a useful framework for considering the effect of policy interventions. The investigators modeled the average effect of a policy intervention on TEE or TEI, which in turn affects average BMI and, subsequently, health/cost outcomes (primarily DALYs) [33, 40–42, 101]. The ACE framework [33] has been applied to 150 interventions across a range of chronic diseases (ACE Prevention), including obesity (ACE Obesity), in order to determine the most cost-effective package of interventions. Separately, they consider factors important to policy making, but difficult to model, such as equity, implementation feasibility, and acceptability to stakeholders. The goal is to help decision-makers determine the best way to efficiently allocate resources.
As part of the ACE studies, Vreeman et al.[116] constructed a static, snapshot model to evaluate a policy reducing children’s exposure to TV food advertising. Parameter estimates relating advertising to consumption levels were derived from the empirical literature and from expert opinion. Using evidence from a randomized controlled trial and from ecological studies of TV advertising of marketing bans, Magnus et al.[117] also found that banning television ads for energy-dense, nutrient-poor foods during children’s peak viewing times was cost-effective. These studies highlight different methods for obtaining effect sizes when empirical results are subject to high uncertainty.
Using the WHO-CHOICE (Choosing Interventions that are Cost-Effective), Sassi et al. [118] considered the effect of a broad array of interventions on the consumption of fats, fruits and vegetables, and BMI, which in turn affected health outcomes. They conclude that the obesity problem requires a broad array of policies targeting different demographic groups.
Schroeter et al.[119] developed an economic macro-model to identify conditions under which price and income changes are most likely to affect weight. They modeled the effect of income and prices of high and low caloric foods on consumption, and subsequently body weight. They found that, although raising the price of high-calorie food could decrease consumption, the resulting impact on body weight was unclear. For example, a tax on food eaten away from home could lead to an increase in body weight due to substitution of other high caloric foods. The study shows how SMs may be used to detect unintended consequences, and the importance of the scope of foods covered by a policy aimed at diet.
SMs addressing policy interventions are at early stages of development. To develop a comprehensive approach, SMs will ultimately need to simultaneously consider multiple policies, how the effect of a policy depends on the manner in which it is implemented and the other policies in effect, how the effects vary by socio-demographic group, and how the effects vary over time. Results from the tobacco policy simulation literature demonstrate the potential importance of each of these factors [4, 10, 11, 120].
In a model examining the effectiveness of policies designed to limit youth access, Levy and Friend [121] show that in order to effectively restrict the sales of cigarettes to minors, compliance checks, penalties and publicity must all be at sufficient levels to halt most sales. When the policies are combined, increasing marginal reductions in youth smoking are predicted to some threshold, followed by diminishing returns. This non-linear relationship, as typified by an S-shaped curve, is also seen in the dissemination of information policies [122].
Even when a policy effectively reduces youth access to tobacco from retail sources, youth may turn to non-retail sources for their cigarettes, such as older peers, parents or theft, suggesting the need for additional policies [121]. Figure 2 shows the different types of youth-oriented obesity policies and how the effect of different policies may interact. For example, an effective school educational curriculum might reinforce in-school restrictions on sugar-sweetened beverages by reducing their consumption outside of school as well. While in some cases policies are mutually reinforcing, in other cases the effect of a policy may be less when implemented with another policy in place than when implemented alone.
Figure 2. Effects of Multiple Youth-Oriented Policies on Overweight and Obesity.
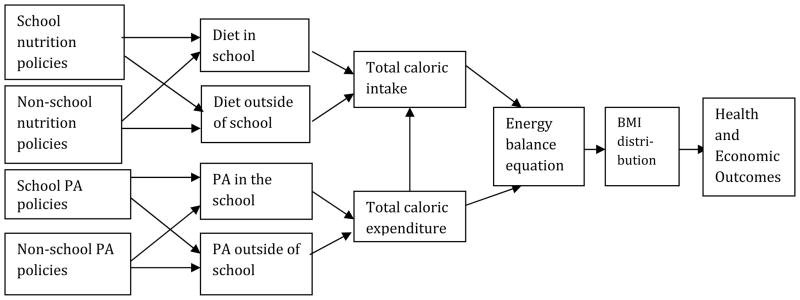
PA = physical activity
While Figure 2 shows uni-directional effects, SMs can also be useful in understanding feedback effects. For example, as individuals lose weight in response to a policy, social norms may change. The change in social norms may create greater political pressure to implement new, stronger policies, thereby enhancing the effect of past policies. On the other hand, the effects of a policy could diminish over time if, for example, food manufacturers adapt their marketing practices to maintain sales in response to a policy restricting a particular type of marketing.
SMs can also explore the effects of policy on different subpopulations, so that policies can target high risk groups. Higher tobacco taxes have been found to have more prominent effects on those at lower ages (who are less addicted) and in families with lower income (who can less afford the higher prices) [10, 123, 124]. Similarly, youth at risk of becoming overweight and from low SES families have been found to be particularly sensitive to fruit and vegetable prices [125, 126], and high BMI youth [127, 128] were more sensitive to food prices.
For cigarettes, it has been shown that policies directed at youth only affect a small portion of the overall population and thus will have a limited impact on overall smoking prevalence in the near term [13]. Similarly, obesity policies aimed at preventing normal weight youth from gaining excess weight will also have delayed effects on adult obesity rates [118], while those policies aimed at all currently overweight individuals, such as a tax on sugar-sweetened beverages, may have more immediate effects on the overall population.
SMs can also be used to show how the effects of a policy vary over time. Alternatively, the benefits of a policy may grow over time as its effects disseminate through social networks and social norms change. In some cases, however, a policy’s impact may be greatest when first implemented, but the incremental effects may diminish over time. Just as smokers may later relapse [129], some individuals may initially be affected by diet or exercise interventions, but may revert back to a poorer diet or sedentary lifestyle.
Policy models are at an early stage, without a strong evidence base to underpin the evaluations of interventions and the assumptions around the maintenance of effect over time. Studies will be needed that consider the dynamic and interactive effects of policies, feedback effects and how policies affect different socio-demographic and weight groups. In developing these models, longitudinal data on weight changes over periods of policy change will be needed in order to accurately assess these effects. Consistent measures of policy implementation and outcomes will help facilitate comparisons across studies.
Conclusions
Obesity is complex, not just complicated [31]. Many factors contribute to the problem, and they relate to each other in nonlinear fashions, are subject to time delays, and change over time. Solutions will be needed that are appropriate for complex problems. Information on solutions is limited to date and resides in many different sources. Simulation models can be used to combine multiple sources of information to elucidate and test potential solutions.
Simulation models of obesity issues, particularly policies directed at obesity, are at a nascent stage of development. Most of the models focus on one or two links in the process from changes in public policy to the health implications of obesity, and often utilize different modeling approaches (e.g. static vs. dynamic, micro vs. macro, etc). While the models are distinct and suited to different issues, they may complement each other in shedding light on the nature of the problem and potential solutions. As models are developed for different countries with different menus of policies and these models are validated, the effects of policies in various combinations can be more fully understood. Nevertheless, no one model can address all questions well. Models are best designed with a specific set of questions in mind. While it is tempting to try and make models comprehensive, the model can become so unwieldy in all its complexity that the results are no longer transparent and validation becomes nearly impossible.
For the insights of the different models to be most useful, it will be important for models to be transparent. Although there are divergent views on what constitutes the optimal level of transparency [130, 131], it often refers to a clear statement of model structure (assumptions, equations and algorithms), the data used to calibrate or estimate model parameters, goodness of fit to calibration data, and validation results. Technical appendices, including supplemental materials published online, can be critical. For example, the Cancer Intervention and Surveillance Modeling Network provides online model profiles (http://cisnet.cancer.gov) with these elements.
Comparative modeling involves comparing the results of different models used to model the same problem. This allows for a more systematic examination and understanding of the specific ways that model structure, assumptions, and parameters influence model outcomes. Examples of successful comparative modeling in other health areas include the 7 CISNET models for breast cancer [132, 133] used to estimate the combined effects of screening and treatment and the Mt. Hood Challenge comparing diabetes models [134].” When the results from models differ, sensitivity analysis can help to consider how their results depend on underlying assumptions and parameters. Examples of this comparative modeling approach include the 7 CISNET models for breast cancer used to estimate the combined effects of screening and treatment and the Mt. Hood Challenge comparing diabetes models. For obesity, the Robert Wood Johnson Foundation (RWJF) and the Office of Behavioral and Social Sciences Research at the National Institutes of Health (OBSSR) jointly sponsored the Collaborative Obesity Modeling Network (COMNet; http://obesitymodeling.net/), which provided support for modeling teams in the U.S., the U.K, Canada and Australia to meet and compare their models. In 2009, the Comparative Modeling Network for Obesity Policy (CompMod), (was formed, and consists of six modeling teams utilizing different modeling modalities to address a common group of policy questions. CompMod is funded by the Eunice Kennedy Shriver National Institute on Child Health and Human Development (NICHD), OBSSR, and RWJF and is part of the National Collaborative on Childhood Obesity Research (NCCOR www.nccor.org).
Because of the complexity of the obesity problem, it is likely that different models will focus on different policies and a limited number of links from policy implementation to behaviors to BMI to disease outcomes. Nevertheless, the different models can inform each other. The development of simulation models is itself a dynamic (iterative) process. Simulation models can provide guidance on the set of hypotheses meriting further empirical study and even point to the types of data that need to be collected. As better information becomes available, simulation models can be adapted and improved. Thereby, simulation models serve as a summary of our knowledge and a structure for improving our knowledge of the obesity problem and potential solutions.
Acknowledgments
The authors gratefully acknowledge support from the Robert Wood Johnson Foundation (RWJF; contract #63048) which supported David Levy to devote his time to this review. In addition the authors gratefully acknowledge support from the NIH Office of Behavioral and Social Sciences Research (OBSSR; contract # HHSN200700356P) and RWJF (contract #) which together supported a series of meetings of the Collaborative Obesity Modeling Network (COMNet) where this work was conceived and developed. While not all COMNet members are authors on this article, the authors acknowledge the contributions of the entire COMNet group in stimulating and refining this article. In particular, the authors would like to thank Diane Finegood, Bill Flanagan and Kevin Hall for their very useful comments on earlier drafts of this work.
Footnotes
The findings and conclusions in this article are those of the authors and do not necessarily represent any official position of the Office of Behavioral and Social Sciences Research, or the National Institutes of Health.
Potential Conflicts of Interest: None
Contributor Information
David T. Levy, Pacific Institute for Research and Evaluation and Department of Economics, University of Baltimore, Baltimore, MD.
Patricia L. Mabry, Office of Behavioral and Social Sciences Research, National Institutes of Health, Bethesda, MD.
Y. Claire Wang, Dept of Health Policy and Management, Columbia Mailman School of Public Health, NY, NY.
Steve Gortmaker, Department of Society, Human Development, and Health, Harvard School of Public Health, Boston, Massachusetts.
Terry T-K Huang, Dept of Health Promotion and Social and Behavioral Health, University of Nebraska Medical Center, Omaha, Nebraska.
Tim Marsh, National Heart Forum, London, England.
Marj Moodie, Health Economics Unit, Deakin Population Health, Deakin University, Australia.
Boyd Swinburn, Alfred Deakin Professor, and Director, WHO Collaborating Center for Obesity Prevention Deakin University, Australia.
References
- 1.Citro CF, Hanushek EA, editors. Review and Recommendations. I. National Research Council; 1991. Improving Information for Social Policy Decisions: The Uses of Microsimulation Modeling. [Google Scholar]
- 2.Orcutt G. A new type of socio-economic system. Review of Economics and Statistics. 1957;80:1081–1100. [Google Scholar]
- 3.Sterman JD. Learning from evidence in a complex world. Am J Public Health. 2006;96(3):505–14. doi: 10.2105/AJPH.2005.066043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levy DT, Bauer JE, Lee HR. Simulation modeling and tobacco control: creating more robust public health policies. Am J Public Health. 2006;96(3):494–8. doi: 10.2105/AJPH.2005.063974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Homer JB, Hirsch GB. System dynamics modeling for public health: background and opportunities. Am J Public Health. 2006;96(3):452–8. doi: 10.2105/AJPH.2005.062059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mendez D, Warner KE, Courant PN. Has smoking cessation ceased? Expected trends in the prevalence of smoking in the United States. Am J Epidemiol. 1998;148(3):249–58. doi: 10.1093/oxfordjournals.aje.a009632. [DOI] [PubMed] [Google Scholar]
- 7.Mendez D, Warner KE. Adult cigarette smoking prevalence: declining as expected (not as desired) Am J Public Health. 2004;94(2):251–2. doi: 10.2105/ajph.94.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tengs TO, Osgood ND, Lin TH. Public health impact of changes in smoking behavior: results from the Tobacco Policy Model. Med Care. 2001;39(10):1131–41. doi: 10.1097/00005650-200110000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Tengs TO, et al. Federal policy mandating safer cigarettes: a hypothetical simulation of the anticipated population health gains or losses. J Policy Anal Manage. 2004;23(4):857–72. doi: 10.1002/pam.20051. [DOI] [PubMed] [Google Scholar]
- 10.Ahmad S. Increasing excise taxes on cigarettes in California: a dynamic simulation of health and economic impacts. Prev Med. 2005;41(1):276–83. doi: 10.1016/j.ypmed.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 11.Ahmad S, Billimek J. Estimating the health impacts of tobacco harm reduction policies: a simulation modeling approach. Risk Anal. 2005;25(4):801–12. doi: 10.1111/j.1539-6924.2005.00647.x. [DOI] [PubMed] [Google Scholar]
- 12.Ahmad S, Billimek J. Limiting youth access to tobacco: Comparing the long-term health impacts of increasing cigarette excise taxes and raising the legal smoking age to 21 in the United States. Health Policy. 2006 doi: 10.1016/j.healthpol.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 13.Levy DT, Cummings KM, Hyland A. A simulation of the effects of youth initiation policies on overall cigarette use. Am J Public Health. 2000;90(8):1311–4. doi: 10.2105/ajph.90.8.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levy DT, Nikolayev N, Mumford EA. Recent Trends in Smoking and the Role of Public Policies: Results from the SimSmoke Tobacco Control Policy Simulation Model. Addiction. 2005;10(10):1526–37. doi: 10.1111/j.1360-0443.2005.01205.x. [DOI] [PubMed] [Google Scholar]
- 15.Whitaker RC, et al. Predicting obesity in young adulthood from childhood and parental obesity. N Engl J Med. 1997;337(13):869–73. doi: 10.1056/NEJM199709253371301. [DOI] [PubMed] [Google Scholar]
- 16.Must A, Strauss RS. Risks and consequences of childhood and adolescent obesity. Int J Obes Relat Metab Disord. 1999;23(Suppl 2):S2–11. doi: 10.1038/sj.ijo.0800852. [DOI] [PubMed] [Google Scholar]
- 17.Freedman DS, et al. Racial differences in the tracking of childhood BMI to adulthood. Obes Res. 2005;13(5):928–35. doi: 10.1038/oby.2005.107. [DOI] [PubMed] [Google Scholar]
- 18.Freedman DS, et al. The relation of childhood BMI to adult adiposity: the Bogalusa Heart Study. Pediatrics. 2005;115(1):22–7. doi: 10.1542/peds.2004-0220. [DOI] [PubMed] [Google Scholar]
- 19.Hammond RA. Complex systems modeling for obesity research. Prev Chronic Dis. 2009;6(3):A97. [PMC free article] [PubMed] [Google Scholar]
- 20.Huang TT, et al. A systems-oriented multilevel framework for addressing obesity in the 21st century. Prev Chronic Dis. 2009;6(3):A82. [PMC free article] [PubMed] [Google Scholar]
- 21.Huang TT, Glass TA. Transforming research strategies for understanding and preventing obesity. Jama. 2008;300(15):1811–3. doi: 10.1001/jama.300.15.1811. [DOI] [PubMed] [Google Scholar]
- 22.Hill JO, et al. Obesity and the environment: where do we go from here? Science. 2003;299(5608):853–5. doi: 10.1126/science.1079857. [DOI] [PubMed] [Google Scholar]
- 23.Wang YC, et al. Estimating the energy gap among US children: a counterfactual approach. Pediatrics. 2006;118(6):e1721–33. doi: 10.1542/peds.2006-0682. [DOI] [PubMed] [Google Scholar]
- 24.Institute of Medicine. Bridging the Evidence Gap in Obesity Prevention: A Framework to Inform Decision Making. The National Academy Press; Washington, DC: 2010. [PubMed] [Google Scholar]
- 25.Swinburn B, Gill T, Kumanyika S. Obesity prevention: a proposed framework for translating evidence into action. Obesity Reviews. 2005;6(1):23–33. doi: 10.1111/j.1467-789X.2005.00184.x. [DOI] [PubMed] [Google Scholar]
- 26.Christakis NA, Fowler JH. The spread of obesity in a large social network over 32 years. N Engl J Med. 2007;357(4):370–9. doi: 10.1056/NEJMsa066082. [DOI] [PubMed] [Google Scholar]
- 27.Sonnenberg FA, Beck JR. Markov models in medical decision making: a practical guide. Med Decis Making. 1993;13(4):322–38. doi: 10.1177/0272989X9301300409. [DOI] [PubMed] [Google Scholar]
- 28.Cox DR, MHD . The theory of stochastic processes. London: Chapman and Hall Ltd; 1965. [Google Scholar]
- 29.Tesfatsion L, JKL . Handbook of Computational Economics. Vol. 2. North-Holland, Amsterdam: Elseiver; 2006. [Google Scholar]
- 30.Bonabeau E. Agent-based modeling: methods and techniques for simulating human systems. Proc Natl Acad Sci. 2002;99(Suppl 3):7280–7287. doi: 10.1073/pnas.082080899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Finegood D, Merth T, Rutter H. Implications of the foresight obesity system map for solutions to childhood obesity. Obesity (Silver Spring) 2009;18(Suppl 1):S13–6. doi: 10.1038/oby.2009.426. [DOI] [PubMed] [Google Scholar]
- 32.Jones A, et al. Tackling obesities: future choices — obesogenic environments — evidence review. London (GB): Government Office for Science, Foresight Programme; 2007. [Google Scholar]
- 33.Carter R, et al. Priority setting in health: Origins, description and application of the Assessing Cost Effectiveness (ACE) Initiative. Expert Rev Pharmacoeconomics Outcomes Research. 2008;8(6):593–617. doi: 10.1586/14737167.8.6.593. [DOI] [PubMed] [Google Scholar]
- 34.Popkin BM, et al. Measuring the full economic costs of diet, physical activity and obesity-related chronic diseases. Obes Rev. 2006;7(3):271–93. doi: 10.1111/j.1467-789X.2006.00230.x. [DOI] [PubMed] [Google Scholar]
- 35.Fontaine KR, et al. Years of life lost due to obesity. Jama. 2003;289(2):187–93. doi: 10.1001/jama.289.2.187. [DOI] [PubMed] [Google Scholar]
- 36.Peeters A, et al. Obesity in adulthood and its consequences for life expectancy: a life-table analysis. Ann Intern Med. 2003;138(1):24–32. doi: 10.7326/0003-4819-138-1-200301070-00008. [DOI] [PubMed] [Google Scholar]
- 37.Finkelstein EA, et al. Individual and Aggregate Years-of-life-lost Associated With Overweight and Obesity. Obesity (Silver Spring) 2009 doi: 10.1038/oby.2009.253. [DOI] [PubMed] [Google Scholar]
- 38.Finkelstein EA, et al. Annual medical spending attributable to obesity: payer-and service-specific estimates. Health Aff (Millwood) 2009;28(5):w822–31. doi: 10.1377/hlthaff.28.5.w822. [DOI] [PubMed] [Google Scholar]
- 39.Finkelstein EA, et al. The lifetime medical cost burden of overweight and obesity: implications for obesity prevention. Obesity (Silver Spring) 2008;16(8):1843–8. doi: 10.1038/oby.2008.290. [DOI] [PubMed] [Google Scholar]
- 40.Moodie ML, et al. The Cost-effectiveness of Australia’s Active After-school Communities Program. Obesity (Silver Spring) 2009 doi: 10.1038/oby.2009.401. [DOI] [PubMed] [Google Scholar]
- 41.Moodie M, et al. Cost-effectiveness of active transport for primary school children - Walking School Bus program. Int J Behav Nutr Phys Act. 2009;6:63. doi: 10.1186/1479-5868-6-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haby MM, et al. A new approach to assessing the health benefit from obesity interventions in children and adolescents: the assessing cost-effectiveness in obesity project. Int J Obes (Lond) 2006;30(10):1463–75. doi: 10.1038/sj.ijo.0803469. [DOI] [PubMed] [Google Scholar]
- 43.Finkelstein EA, Ruhm CJ, Kosa KM. Economic causes and consequences of obesity. Annu Rev Public Health. 2005;26:239–57. doi: 10.1146/annurev.publhealth.26.021304.144628. [DOI] [PubMed] [Google Scholar]
- 44.Trogdon JG, et al. Indirect costs of obesity: a review of the current literature. Obes Rev. 2008;9(5):489–500. doi: 10.1111/j.1467-789X.2008.00472.x. [DOI] [PubMed] [Google Scholar]
- 45.Vos T, et al. Assessing cost-effectiveness in mental health: helping policy-makers prioritize and plan health services. Aust N Z J Psychiatry. 2005;39(8):701–12. doi: 10.1080/j.1440-1614.2005.01654.x. [DOI] [PubMed] [Google Scholar]
- 46.Janicke DM, et al. The Relationship Among Child Weight Status, Psychosocial Functioning, and Pediatric Health Care Expenditures in a Medicaid Population. J Pediatr Psychol. 2009 doi: 10.1093/jpepsy/jsp122. [DOI] [PubMed] [Google Scholar]
- 47.Janicke DM, et al. Psychiatric diagnosis in children and adolescents with obesity-related health conditions. J Dev Behav Pediatr. 2008;29(4):276–84. doi: 10.1097/DBP.0b013e31817102f8. [DOI] [PubMed] [Google Scholar]
- 48.van Baal PH, et al. Lifetime medical costs of obesity: prevention no cure for increasing health expenditure. PLoS Med. 2008;5(2):e29. doi: 10.1371/journal.pmed.0050029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lightwood J, et al. Forecasting the future economic burden of current adolescent overweight: an estimate of the coronary heart disease policy model. Am J Public Health. 2009;99(12):2230–7. doi: 10.2105/AJPH.2008.152595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Finkelstein E, Fiebelkorn C, Wang G. The costs of obesity among full-time employees. Am J Health Promot. 2005;20(1):45–51. doi: 10.4278/0890-1171-20.1.45. [DOI] [PubMed] [Google Scholar]
- 51.Finkelstein EA, Brown DS. A cost-benefit simulation model of coverage for bariatric surgery among full-time employees. Am J Manag Care. 2005;11(10):641–6. [PubMed] [Google Scholar]
- 52.Trogdon J, et al. A return-on-investment simulation model of workplace obesity interventions. J Occup Environ Med. 2009;51(7):751–8. doi: 10.1097/JOM.0b013e3181a86656. [DOI] [PubMed] [Google Scholar]
- 53.Bibbins-Domingo K, et al. Adolescent overweight and future adult coronary heart disease. N Engl J Med. 2007;357(23):2371–9. doi: 10.1056/NEJMsa073166. [DOI] [PubMed] [Google Scholar]
- 54.Flegal KM, et al. Estimating deaths attributable to obesity in the United States. Am J Public Health. 2004;94(9):1486–9. doi: 10.2105/ajph.94.9.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Flegal KM, et al. Cause-specific excess deaths associated with underweight, overweight, and obesity. Jama. 2007;298(17):2028–37. doi: 10.1001/jama.298.17.2028. [DOI] [PubMed] [Google Scholar]
- 56.Flegal KM, Graubard BI. Estimates of excess deaths associated with body mass index and other anthropometric variables. Am J Clin Nutr. 2009;89(4):1213–9. doi: 10.3945/ajcn.2008.26698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Milstein B, et al. Charting plausible futures for diabetes prevalence in the United States: a role for system dynamics simulation modeling. Prev Chronic Dis. 2007;4(3):A52. [PMC free article] [PubMed] [Google Scholar]
- 58.Jones AP, et al. Understanding diabetes population dynamics through simulation modeling and experimentation. Am J Public Health. 2006;96(3):488–94. doi: 10.2105/AJPH.2005.063529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Van Meijgaard J, Fielding JE, Kominski GF. Assessing and forecasting population health: integrating knowledge and beliefs in a comprehensive framework. Public Health Rep. 2009;124(6):778–89. doi: 10.1177/003335490912400604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rodgers A, et al. Distribution of major health risks: findings from the Global Burden of Disease study. PLoS Med. 2004;1(1):e27. doi: 10.1371/journal.pmed.0010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Goldman DP, et al. The benefits of risk factor prevention in Americans aged 51 years and older. Am J Public Health. 2009;99(11):2096–101. doi: 10.2105/AJPH.2009.172627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Flegal KM. The effects of changes in smoking prevalence on obesity prevalence in the United States. Am J Public Health. 2007;97(8):1510–4. doi: 10.2105/AJPH.2005.084343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Flegal KM, et al. Impact of smoking and preexisting illness on estimates of the fractions of deaths associated with underweight, overweight, and obesity in the US population. Am J Epidemiol. 2007;166(8):975–82. doi: 10.1093/aje/kwm152. [DOI] [PubMed] [Google Scholar]
- 64.Stewart ST, Cutler DM, Rosen AB. Forecasting the effects of obesity and smoking on U.S. life expectancy. N Engl J Med. 2009;361(23):2252–60. doi: 10.1056/NEJMsa0900459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jia H, Lubetkin EI. The statewide burden of obesity, smoking, low income and chronic diseases in the United States. J Public Health (Oxf) 2009;31(4):496–505. doi: 10.1093/pubmed/fdp012. [DOI] [PubMed] [Google Scholar]
- 66.Shai I, et al. Ethnicity, obesity, and risk of type 2 diabetes in women: a 20-year follow-up study. Diabetes Care. 2006;29(7):1585–90. doi: 10.2337/dc06-0057. [DOI] [PubMed] [Google Scholar]
- 67.Hu FB, Willett WC. Optimal diets for prevention of coronary heart disease. Jama. 2002;288(20):2569–78. doi: 10.1001/jama.288.20.2569. [DOI] [PubMed] [Google Scholar]
- 68.Dall TM, et al. Potential health benefits and medical cost savings from calorie, sodium, and saturated fat reductions in the American diet. Am J Health Promot. 2009;23(6):412–22. doi: 10.4278/ajhp.080930-QUAN-226. [DOI] [PubMed] [Google Scholar]
- 69.Dall TM, et al. Predicted national productivity implications of calorie and sodium reductions in the American diet. Am J Health Promot. 2009;23(6):423–30. doi: 10.4278/ajhp.081010-QUAN-227. [DOI] [PubMed] [Google Scholar]
- 70.Kelly T, et al. Global burden of obesity in 2005 and projections to 2030. Int J Obes (Lond) 2008;32(9):1431–7. doi: 10.1038/ijo.2008.102. [DOI] [PubMed] [Google Scholar]
- 71.Beydoun MA, Wang Y. Gender-ethnic disparity in BMI and waist circumference distribution shifts in US adults. Obesity (Silver Spring) 2009;17(1):169–76. doi: 10.1038/oby.2008.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang Y, Beydoun MA. The obesity epidemic in the United States--gender, age, socioeconomic, racial/ethnic, and geographic characteristics: a systematic review and meta-regression analysis. Epidemiol Rev. 2007;29:6–28. doi: 10.1093/epirev/mxm007. [DOI] [PubMed] [Google Scholar]
- 73.Wang YC, Colditz GA, Kuntz KM. Forecasting the obesity epidemic in the aging U.S. population. Obesity (Silver Spring) 2007;15(11):2855–65. doi: 10.1038/oby.2007.339. [DOI] [PubMed] [Google Scholar]
- 74.Basu A. Forecasting Distribution of Body Mass Index in the United States: Is There More Room for Growth? Med Decis Making. 2009 doi: 10.1177/0272989X09351749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brown M, et al. Obesity Trend Analysis from the Health Survey for England, 1993–2007. National Heart Foundation; London: 2010. [Google Scholar]
- 76.Flanagan WM, et al. Potential impact of population-based colorectal cancer screening in Canada. Chronic Dis Can. 2003;24(4):81–8. [PubMed] [Google Scholar]
- 77.Wolfson MC. POHEM--a framework for understanding and modelling the health of human populations. World Health Stat Q. 1994;47(3–4):157–76. [PubMed] [Google Scholar]
- 78.Flanagan W, et al. International Microsimulation Association Conference. Ottawa: 2009. Population Health Model-Overview. [Google Scholar]
- 79.Ogden CL, Carroll MD, Flegal KM. Epidemiologic trends in overweight and obesity. Endocrinol Metab Clin North Am. 2003;32(4):741–60. vii. doi: 10.1016/s0889-8529(03)00074-4. [DOI] [PubMed] [Google Scholar]
- 80.Sassi F, et al. The obesity epidemic: analysis of past and projected future trends in Selected OECD countries. OECD; Paris: 2009. [Google Scholar]
- 81.Chinn S. Definitions of childhood obesity: current practice. Eur J Clin Nutr. 2006;60(10):1189–94. doi: 10.1038/sj.ejcn.1602436. [DOI] [PubMed] [Google Scholar]
- 82.Lobstein T, Jackson-Leach R. Child overweight and obesity in the USA: prevalence rates according to IOTF definitions. Int J Pediatr Obes. 2007;2(1):62–4. doi: 10.1080/17477160601103948. [DOI] [PubMed] [Google Scholar]
- 83.Wang Y, Lobstein T. Worldwide trends in childhood overweight and obesity. Int J Pediatr Obes. 2006;1(1):11–25. doi: 10.1080/17477160600586747. [DOI] [PubMed] [Google Scholar]
- 84.Homer J, et al. Obesity Population Dynamics, Exploring Historical Growth and Plausible Futures in the U.S. International System Dynamics Conference; 2006; Nijmegen, Netherlands. [Google Scholar]
- 85.Swinburn BA, et al. Estimating the changes in energy flux that characterize the rise in obesity prevalence. Am J Clin Nutr. 2009;89(6):1723–8. doi: 10.3945/ajcn.2008.27061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hall KD, Jordan PN. Modeling weight-loss maintenance to help prevent body weight regain. Am J Clin Nutr. 2008;88(6):1495–503. doi: 10.3945/ajcn.2008.26333. [DOI] [PubMed] [Google Scholar]
- 87.Swinburn B, Sacks G, Ravussin E. Increased food energy supply is more than sufficient to explain the US epidemic of obesity. Am J Clin Nutr. 2009;90(6):1453–6. doi: 10.3945/ajcn.2009.28595. [DOI] [PubMed] [Google Scholar]
- 88.Hall KD, et al. The progressive increase of food waste in America and its environmental impact. PLoS One. 2009;4(11):e7940. doi: 10.1371/journal.pone.0007940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Swinburn B, Sacks G, Ravussin E. Estimating the quantitative relation between food energy intake and change in body weight (right of reply) Am J Clin Nutr. 2010;91(3):817. doi: 10.3945/ajcn.2009.28922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hall K, Chow C. Estimating the quantitative relation between food energy intake and changes in body weight. Am J Clin Nutr. 2010;91(3):816. doi: 10.3945/ajcn.2009.28922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Summerbell CD, et al. The association between diet and physical activity and subsequent excess weight gain and obesity assessed at 5 years of age or older: a systematic review of the epidemiological evidence. Int J Obes (Lond) 2009;33(Suppl 3):S1–92. doi: 10.1038/ijo.2009.80. [DOI] [PubMed] [Google Scholar]
- 92.Sorensen TI. Conference on “Multidisciplinary approaches to nutritional problems”. Symposium on “Diabetes and health”. Challenges in the study of causation of obesity. Proc Nutr Soc. 2009;68(1):43–54. doi: 10.1017/S0029665108008847. [DOI] [PubMed] [Google Scholar]
- 93.Flatt JP. Carbohydrate-fat interactions and obesity examined by a two-compartment computer model. Obes Res. 2004;12(12):2013–22. doi: 10.1038/oby.2004.252. [DOI] [PubMed] [Google Scholar]
- 94.Chow CC, Hall KD. The dynamics of human body weight change. PLoS Comput Biol. 2008;4(3):e1000045. doi: 10.1371/journal.pcbi.1000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hall KD. Mathematical modelling of energy expenditure during tissue deposition. Br J Nutr. 2010:1–4. doi: 10.1017/S0007114510000206. [DOI] [PubMed] [Google Scholar]
- 96.Hall KD. Computational model of in vivo human energy metabolism during semistarvation and refeeding. Am J Physiol Endocrinol Metab. 2006;291(1):E23–37. doi: 10.1152/ajpendo.00523.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Katan MB, Ludwig DS. Extra calories cause weight gain--but how much? Jama. 2010;303(1):65–6. doi: 10.1001/jama.2009.1912. [DOI] [PubMed] [Google Scholar]
- 98.Christiansen E, Swann A, Sorensen TI. Feedback models allowing estimation of thresholds for self-promoting body weight gain. J Theor Biol. 2008;254(4):731–6. doi: 10.1016/j.jtbi.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 99.Abdel-Hamid T. Modeling the dynamics of human energy regulation and its implications for obesity treatment. System Dynamics Review. 2002;18(4):431–471. [Google Scholar]
- 100.Abdel-Hamid T. Exercise and diet in obesity treatment: an integrative system dynamics perspective. Med Sci Sports Exerc. 2003;35(3):400–413. doi: 10.1249/01.MSS.0000053659.32126.2D. [DOI] [PubMed] [Google Scholar]
- 101.Carter R, et al. Assessing cost-effectiveness in obesity (ACE-obesity): an overview of the ACE approach, economic methods and cost results. BMC Public Health. 2009;9:419. doi: 10.1186/1471-2458-9-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Butte NF, Ellis KJ. Comment on “Obesity and the environment: where do we go from here?”. Science. 2003;301(5633):598. doi: 10.1126/science.1085985. author reply 598. [DOI] [PubMed] [Google Scholar]
- 103.Jordan PN, Hall KD. Dynamic coordination of macronutrient balance during infant growth: insights from a mathematical model. Am J Clin Nutr. 2008;87(3):692–703. doi: 10.1093/ajcn/87.3.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Plachta-Danielzik S, et al. Energy gain and energy gap in normal-weight children: longitudinal data of the KOPS. Obesity (Silver Spring) 2008;16(4):777–83. doi: 10.1038/oby.2008.5. [DOI] [PubMed] [Google Scholar]
- 105.Swinburn BA, et al. Estimating the effects of energy imbalance on changes in body weight in children. Am J Clin Nutr. 2006;83(4):859–63. doi: 10.1093/ajcn/83.4.859. [DOI] [PubMed] [Google Scholar]
- 106.Edwards KL, et al. The neighbourhood matters: studying exposures relevant to childhood obesity and the policy implications in Leeds, UK. J Epidemiol Community Health. 2009 doi: 10.1136/jech.2009.088906. [DOI] [PubMed] [Google Scholar]
- 107.Edwards KL, Clarke GP. The design and validation of a spatial microsimulation model of obesogenic environments for children in Leeds, UK: SimObesity. Soc Sci Med. 2009;69(7):1127–34. doi: 10.1016/j.socscimed.2009.07.037. [DOI] [PubMed] [Google Scholar]
- 108.Bahr DB, et al. Exploiting social networks to mitigate the obesity epidemic. Obesity (Silver Spring) 2009;17(4):723–8. doi: 10.1038/oby.2008.615. [DOI] [PubMed] [Google Scholar]
- 109.Trogden J, Nonnemkaer J. Peer Effects in Adolescent Overweight. Journal of Health Economics. 2008;27(5):1378–1387. doi: 10.1016/j.jhealeco.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 110.Cohen-Cole E, Fletcher JM. Is obesity contagious? Social networks vs. environmental factors in the obesity epidemic. J Health Econ. 2008;27(5):1382–7. doi: 10.1016/j.jhealeco.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 111.Halliday J, Kwak M. Weight Gain in Adolescent Peers. Economics & Human Biology. 2009;7(2):181–90. doi: 10.1016/j.ehb.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 112.Fowler JH, Christakis NA. Estimating peer effects on health in social networks: a response to Cohen-Cole and Fletcher; and Trogdon, Nonnemaker, and Pais. J Health Econ. 2008;27(5):1400–5. doi: 10.1016/j.jhealeco.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Burke M, Heiland F. Social Dynamics of Obesity. Economic Inquiry. 2007:45–3. 571–591. [Google Scholar]
- 114.Hammond RA, EJM . Exploring price-independent mechanisms in the obesity epidemic (paper no. 48) The Brookings Institution; Washington (DC): 2007. [Google Scholar]
- 115.Bouchard C. Gene-environment interactions in the etiology of obesity: defining the fundamentals. Obesity (Silver Spring) 2008;16(Suppl 3):S5–S10. doi: 10.1038/oby.2008.528. [DOI] [PubMed] [Google Scholar]
- 116.Veerman JL, et al. By how much would limiting TV food advertising reduce childhood obesity? Eur J Public Health. 2009;19(4):365–9. doi: 10.1093/eurpub/ckp039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Magnus A, et al. The cost-effectiveness of removing television advertising of high-fat and/or high-sugar food and beverages to Australian children. Int J Obes (Lond) 2009;33(10):1094–102. doi: 10.1038/ijo.2009.156. [DOI] [PubMed] [Google Scholar]
- 118.Sassi F, et al. Improving lifestyles, tackling obesity: The health and economic impact of prevention strategies. Organisation for Economic Co-operation and Development; Paris: 2009. [Google Scholar]
- 119.Schroeter C, Lusk J, Tyner W. Determining the impact of food price and income changes on body weight. Journal of Health Economics. 2008;27:45–68. doi: 10.1016/j.jhealeco.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 120.Ahmad S. The cost-effectiveness of raising the legal smoking age in California. Med Decis Making. 2005;25(3):330–40. doi: 10.1177/0272989X05276859. [DOI] [PubMed] [Google Scholar]
- 121.Levy DT, Friend KB. A simulation model of tobacco youth access policies. J Health Polit Policy Law. 2000;25(6):1023–50. doi: 10.1215/03616878-25-6-1023. [DOI] [PubMed] [Google Scholar]
- 122.Levy DT, Friend K. A computer simulation model of mass media interventions directed at tobacco use. Prev Med. 2001;32(3):284–294. doi: 10.1006/pmed.2000.0808. [DOI] [PubMed] [Google Scholar]
- 123.Farrelly M, Bray J. Responses to increases in cigarette prices by race/ethnicity, income, and age groups--United States, 1976–1993. Morbidity and Mortality Weekly Report. 1998;47(29):605–609. [PubMed] [Google Scholar]
- 124.Levy DT, Cummings KM, Hyland A. Increasing taxes as a strategy to reduce cigarette use and deaths: results of a simulation model. Prev Med. 2000;31(3):279–86. doi: 10.1006/pmed.2000.0696. [DOI] [PubMed] [Google Scholar]
- 125.Sturm R, Datar A. Body mass index in elementary school children, metropolitan area food prices and food outlet density. Public Health. 2005;119(12):1059–68. doi: 10.1016/j.puhe.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 126.Sturm R, Datar A. Food prices and weight gain during elementary school: 5-year update. Public Health. 2008;122(11):1140–3. doi: 10.1016/j.puhe.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Auld M, Powell L. Economics of Food Energy Density and Adolescent Body Weight. Economica. 2009 in press. [Google Scholar]
- 128.Powell L, et al. Access to Fast Food and Food Prices: Relationship with Fruit and Vegetable Consumption and Overweight among Adolescents. Advances in Health Economics and Health Services Research. 2007;17:23–48. [PubMed] [Google Scholar]
- 129.Levy DT, Friend K. A simulation model of policies directed at treating tobacco use and dependence. Med Decis Making. 2002;22(1):6–17. doi: 10.1177/0272989X0202200101. [DOI] [PubMed] [Google Scholar]
- 130.Fendrick A. The future of health economic modeling: have we gone too far or not far enough? Value Health. 2006;9(3):179–180. doi: 10.1111/j.1524-4733.2006.00115.x. [DOI] [PubMed] [Google Scholar]
- 131.Eddy D. Accuracy versus transparency in harmacoeconomic modelling: finding the right balance. Pharmacoeconomics. 2006;24(9):837–844. doi: 10.2165/00019053-200624090-00002. [DOI] [PubMed] [Google Scholar]
- 132.Berry DA, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353(17):1784–92. doi: 10.1056/NEJMoa050518. [DOI] [PubMed] [Google Scholar]
- 133.Cronin KA, et al. Impact of adjuvant therapy and mammography on U.S. mortality from 1975 to 2000: comparison of mortality results from the cisnet breast cancer base case analysis. J Natl Cancer Inst Monogr. 2006;(36):112–21. doi: 10.1093/jncimonographs/lgj015. [DOI] [PubMed] [Google Scholar]
- 134.Group TMHM. Computer modeling of diabetes and its complications: a report on the Fourth Mount Hood Challenge Meeting. Diabetes Care. 2007;30(6):1638–46. doi: 10.2337/dc07-9919. [DOI] [PubMed] [Google Scholar]