Abstract
TNF promotes a regulated form of necrosis, called necroptosis, upon inhibition of caspase activity in cells expressing RIPK3. Because necrosis is generally more pro-inflammatory than apoptosis, it is widely presumed that TNF-induced necroptosis may be detrimental in vivo due to excessive inflammation. However, because TNF is intrinsically highly pro-inflammatory, due to its ability to trigger the production of multiple cytokines and chemokines, rapid cell death via necroptosis may blunt rather than enhance TNF-induced inflammation. Here we show that TNF-induced necroptosis potently suppressed the production of multiple TNF-induced pro-inflammatory factors due to RIPK3-dependent cell death. Similarly, necroptosis also suppressed LPS-induced pro-inflammatory cytokine production. Consistent with these observations, supernatants from TNF-stimulated cells were more pro-inflammatory than those from TNF-induced necroptotic cells in vivo. Thus necroptosis attenuates TNF- and LPS-driven inflammation, which may benefit intracellular pathogens that evoke this mode of cell death by suppressing host immune responses.
Tumor necrosis factor (TNF) receptor (TNFR) stimulation can result in apoptosis via FADD-dependent recruitment of caspase-8 to the TNFR intracellular death domain.1, 2 Recent studies have also shown that TNFR engagement can result in a form of programmed necrosis, called necroptosis, where TNF-induced caspase-8 activation is frustrated either using pharmacological or viral-derived caspase inhibitors,3, 4, 5 or as a consequence of targeted disruption of the CASP-8 or FADD loci.6, 7, 8 Necroptosis is generally viewed as a pro-inflammatory mode of cell death, and many studies have concluded that this represents a host response to viral infection that limits viral replication.9, 10, 11 However, it is frequently overlooked that the majority of TNF-responsive cell types do not undergo apoptosis, or indeed necrosis, in response to TNFR engagement. Instead, most cells initiate highly robust nuclear factor kappa B (NFκB)-dependent pro-inflammatory responses upon stimulation with this cytokine.
TNF stimulation rapidly results in recruitment of ‘Complex I' components to the TNF receptor, which rapidly signal to NFκB activation.12 Complex I-mediated NFκB activation restrains activation of caspase-8 in a cytosolic ‘complex II'.12 To undergo apoptosis in response to TNF, cells typically require sensitization through the use of transcriptional/translational inhibitors or IAP-antagonists that block NFκB activation signals.13, 14, 15 Thus the primary consequence of TNFR stimulation in responsive cell types is the rapid synthesis and secretion of a diverse array of cytokines and chemokines that collectively trigger the inflammatory response.16, 17, 18 From this perspective, necroptosis could be interpreted as a strategy by viruses encoding caspase inhibitors to shut down the host pro-inflammatory programme by terminating synthesis of pro-inflammatory cytokines and chemokines.
Key biological outcomes of TNFR stimulation includes the recruitment of peripheral blood neutrophils through the generation of gradients of chemokines such as IL-8/MIP-2, recruitment of monocytes/macrophages (via MCP-1), activation of local macrophages and dendritic cells (via MCP-1 and GM-CSF), induction of monocyte/macrophage differentiation (GM-CSF), activation of local endothelium,16, 19, 20 as well as a range of other responses.16, 21 Therefore the central role of TNF as a trigger for pro-inflammatory cytokine/chemokine production, rather than apoptosis, is a key issue when evaluating the outcome of interventions, such as caspase inhibition, that impact on TNF-induced signal transduction pathways.
Because necrosis is generally pro-inflammatory, in comparison with apoptosis, it is widely presumed that the switching of TNF-induced signaling to necroptosis exacerbates inflammation.22 The latter view is predicated upon the idea that necrosis results in the release of intracellular constituents, called damage-associated molecular patterns (DAMPs), that promote inflammation.23, 24, 25, 26 However, the presumption that TNF-induced necroptosis represents a more inflammatory outcome than TNF stimulation alone fails to take account of the fact that TNF is intrinsically highly pro-inflammatory, as noted earlier. An alternative possibility is that necroptosis may suppress inflammation through abruptly terminating TNF-induced cytokine production. It is not known whether the release of intracellular DAMPs, due to necroptosis-associated cell rupture, compensates for inhibition of the production of TNF-induced pro-inflammatory factors. Therefore it is unclear whether necroptosis suppresses or enhances TNF-induced inflammatory responses.
Here we examine the inflammatory outcome of TNF- and lipopolysaccharide (LPS)-induced necroptosis and report that this mode of cell death resulted in a dramatic suppression of the production of multiple TNF-induced cytokines and chemokines, which was not compensated by the release of intracellular DAMPs. Knockdown of receptor-interacting protein kinase 3 (RIPK3) inhibited TNF-induced necroptosis and restored pro-inflammatory cytokine production. Thus intracellular pathogens encoding caspase inhibitors that skew TNF or LPS signaling towards necroptosis may do so as an adaptive strategy to suppress the host inflammatory response.
Results
TNF induces the production of multiple pro-inflammatory cytokines and chemokines
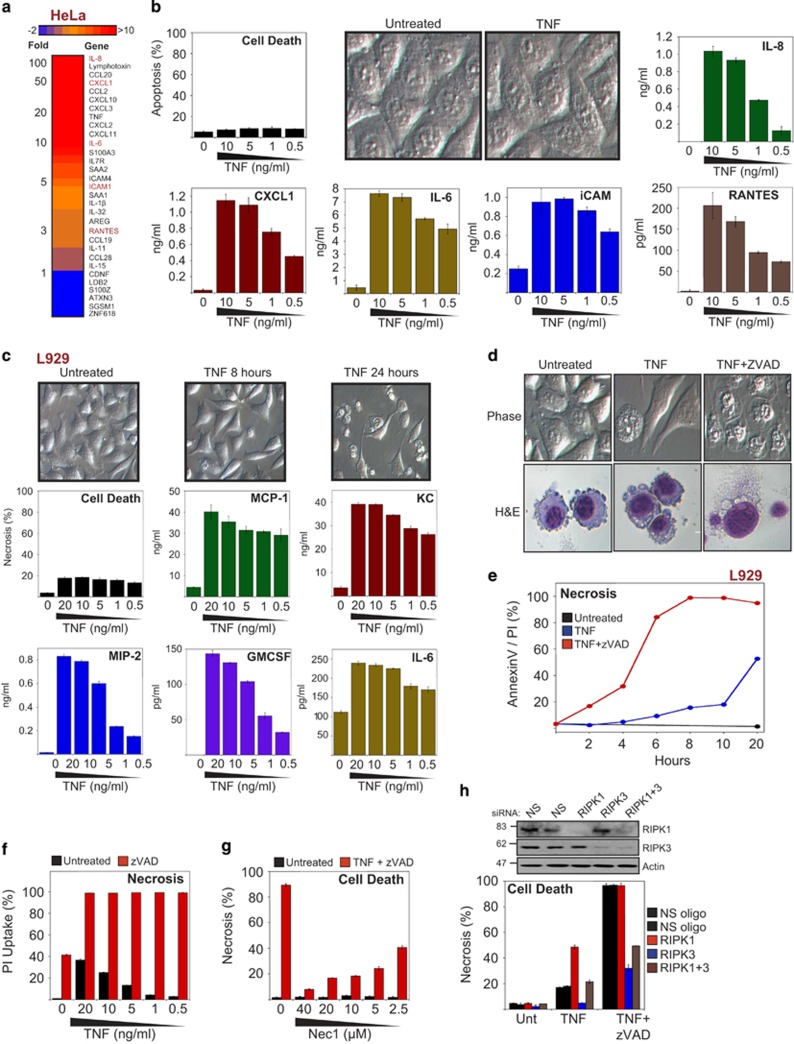
Although TNF is often considered a pro-apoptotic factor, this cytokine is predominantly an initiator of inflammation that is capable of transcriptionally upregulating a battery of inflammatory gene expression events, in the absence of cell death. Indeed, the majority of cell types typically do not undergo apoptosis in response to TNF unless transcription or translation is inhibited.13, 14 To illustrate this, we conducted gene expression array analyses on HeLa cells, which revealed dramatic upregulation of numerous pro-inflammatory cytokines and chemokines in response to TNF (Figure 1a), in the complete absence of cell death (Figure 1b). Thus TNF is highly pro-inflammatory whether cells die or not.
Figure 1.
TNF induces the secretion of multiple pro-inflammatory mediators irrespective of cell death. (a) HeLa cells were treated with TNF (10 ng/ml). After 6 h, cells were harvested and subjected to full genome microarray analysis. (b) HeLa cells were stimulated with TNF at the indicated concentrations. After 24 h, apoptosis was scored by morphology, and the cytokine/chemokine concentrations in the culture supernatants were determined by ELISA. (c) L929 cells were stimulated with TNF at the indicated concentrations. After 8 h, cell death was scored by morphology, and cytokine concentrations in the culture supernatants were determined by ELISA. (d) L929 cells were pretreated for 1 h in the presence or absence of zVAD (10 μM) followed by stimulation with TNF (10 ng/ml). After 8 h, cells were visualized by phase-contrast microscopy or H&E staining. (e) L929 cells were pretreated for 1 h in the presence or absence of zVAD (10 μM) followed by stimulation with TNF (10 ng/ml). At the indicated time points, cell death was analysed by AnnexinV/PI staining. (f) L929 cells were pretreated for 1 h in the presence or absence of zVAD (10 μM) followed by stimulation with TNF (10 ng/ml). After 24 h, cell death was analysed by PI staining. (g) L929 cells were pretreated for 1 h with the indicated concentrations of Nec1 and zVAD (10 μM), followed by addition of TNF (10 ng/ml). After 24 h, cell death was scored by morphology. (h) L929 cells were electroporated with either non-silencing siRNA or siRNA directed against RIPK1, RIPK3 or RIPK1 and RIPK3, as indicated. Forty-eight hours later, cells were pretreated in the presence or absence of zVAD (20 μM) for 1 h, followed by addition of TNF (10 ng/ml). After 5 h, cell death was scored by morphological assessment, and cell lysates were analysed by immunoblotting for levels of endogenous proteins. Error bars represent the mean±S.E.M. of triplicate determinations from a representative experiment. For cell death analyses, triplicate counts were performed on a minimum of 300 cells per treatment. All data are representative of at least three independent experiments
Murine L929 cells have been widely used as a model system to explore the mechanism of TNF-induced programmed necrosis (i.e., TNF-induced cell death that occurs where caspases are inhibited). However, the impact of necroptosis on TNF-induced inflammatory events has not been explored to date. This is surprising, because TNF-induced necroptosis is frequently considered to be more pro-inflammatory than TNF-initiated outcomes that occur in the presence of caspase activity.22, 27, 28, 29 This view appears to be predicated upon the notion that TNF promotes either apoptosis or necrosis, with the latter typically being pro-inflammatory and the former not. However, because TNF is itself highly pro-inflammatory as illustrated above (Figures 1a and b), the impact of necroptosis on TNF-induced inflammatory events remains unclear. As shown in Figure 1c, TNF-stimulation of L929 cells also resulted in the rapid synthesis of an array of cytokines and chemokines, including: MCP-1, KC/CXCL1, MIP-2, GMCSF, and IL-6. Similarly, primary murine embryonic fibroblasts (MEFs) also responded to TNFR stimulation by synthesizing potent pro-inflammatory factors in the absence of any appreciable cell death (Supplementary Figure S1a).
TNF induces RIPK3-dependent necroptosis in L929 cells and MEFs
As has been demonstrated by many groups, TNFR stimulation in RIPK3-epressing cells can be switched to necroptosis through inhibition of caspase activity.3, 30 As can be seen from Figures 1d–f and Supplementary Figure S1b, the poly-caspase inhibitor zVAD-fmk resulted in rapid TNF-induced necroptosis of L929 cells within 5–6 h of receptor stimulation. TNF/zVAD-induced necroptosis was inhibited by the addition of Necrostatin-1 (Figure 1g) or through knockdown of RIPK3 (Figure 1h). Similarly, MEFs also underwent necroptosis in response to TNF/zVAD treatment (Supplementary Figure S1c), which was also RIPK3-dependent and inhibited by necrostatin (Supplementary Figures S1c and d).
TNF-induced necroptosis inhibits the production of cytokines and chemokines
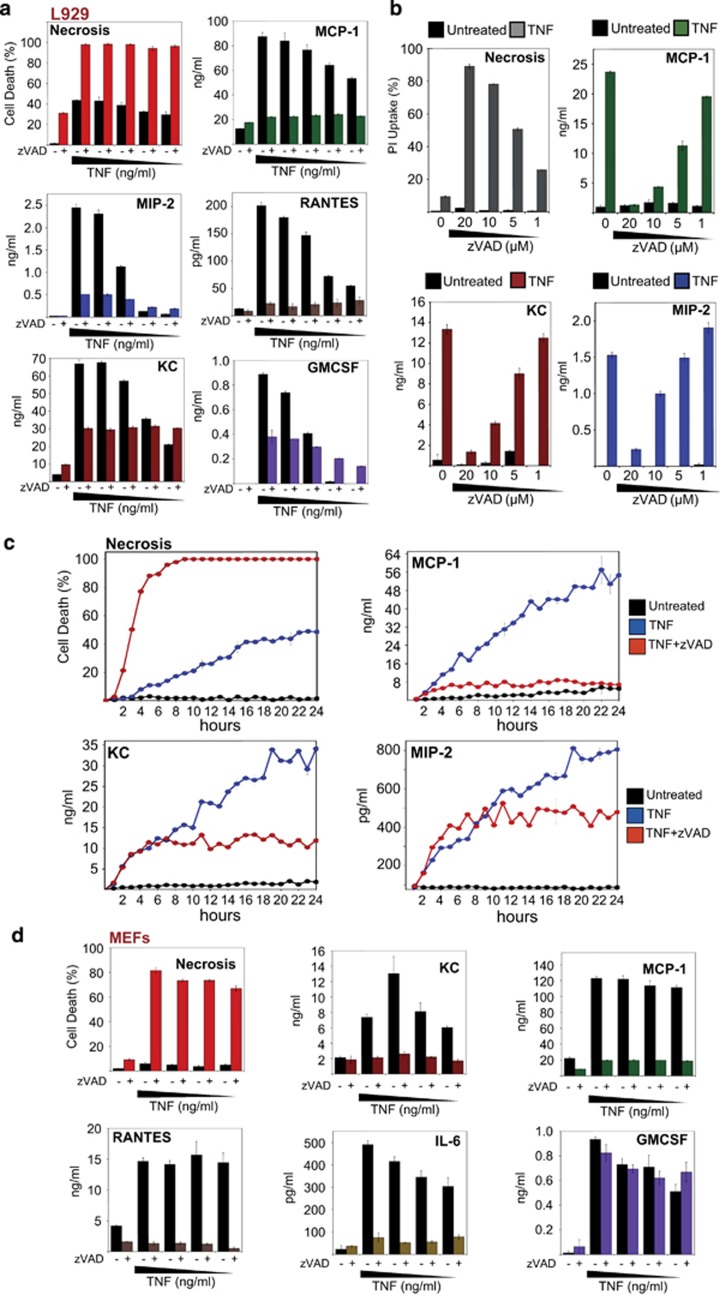
We next examined the impact of TNF-induced necroptosis on the release of TNF-induced pro-inflammatory mediators. As can be seen from Figure 2a, whereas TNF treatment of L929 cells resulted in a dose-dependent production of high concentrations of cytokines and chemokines over 24 h, addition of zVAD-fmk dramatically suppressed the production of MCP-1, KC, MIP-2, RANTES and GMCSF. Importantly, concentrations of zVAD that did not efficiently evoke necroptosis did not result in cytokine suppression (Figure 2b and Supplementary Figure S2a). Thus the production of multiple TNF-induced cytokines and chemokines was suppressed as a consequence of triggering necroptosis.
Figure 2.
Necroptosis suppresses TNF-induced cytokine and chemokine production. (a) L929 cells were pretreated for 1 h in the presence or absence of zVAD (20 μM), followed by addition of TNF to a final concentration of 20, 10, 5, 1 or 0.5 ng/ml (from left to right). After 24 h, cell death was scored by morphology, and cytokine/chemokine concentrations in the culture supernatants were determined by enzyme-linked immunosorbent assay (ELISA). (b) L929 cells were pretreated for 1 h with the indicated concentrations of zVAD, followed by addition of TNF (10 ng/ml). After 24 h, cell death was analysed by PI staining, and the culture supernatants were harvested to measure chemokines by ELISA. (c) L929 cells were pretreated for 1 h with zVAD (20 μM), followed by addition of TNF (10 ng/ml). At the indicated time points, cell death was scored by morphology, and the culture supernatants were harvested to measure chemokines by ELISA. (d) MEFs were pretreated for 1 h with zVAD (25 μM), followed by addition of TNF to 100, 50, 25 or 12 ng/ml (from left to right). After 24 h, cell death was scored by morphology, and cytokine/chemokine concentrations in the culture supernatants were determined by ELISA. Error bars represent the mean±S.E.M. of triplicate determinations from a representative experiment. For cell death analyses, triplicate counts were performed on a minimum of 300 cells per treatment. All data are representative of at least three independent experiments
A time course analysis of cytokine production in response to TNF or TNF/zVAD-fmk again revealed a dramatic suppression of cytokine production during necroptosis, which correlated with much earlier cell death under necroptotic conditions (Figure 2c). We also observed a similar dramatic reduction in TNF-induced pro-inflammatory cytokine production upon inducing necroptosis in MEFs (Figure 2d).
Some cells types are resistant to necroptosis under conditions of caspase inhibition alone but can be sensitized by neutralizing IAPs or inhibiting transcription/translation in tandem with caspase inhibition (Supplementary Figures S2b and c). In this context, we also found that inducing necroptosis of HT29 cells or MEFs using TNF/zVAD in the presence of IAP antagonist also dramatically suppressed cytokine production (Supplementary Figures S2b and c).
Caspase activity is not required for TNF-induced cytokine and chemokine synthesis
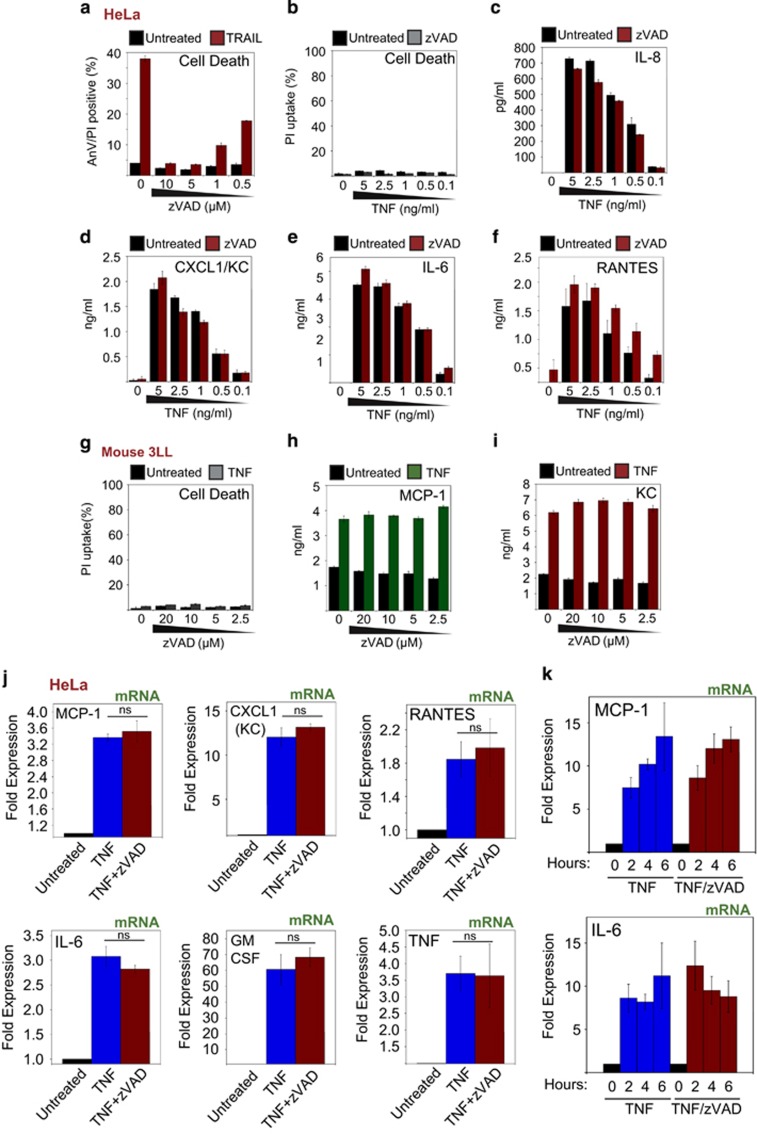
Although we only observed suppression of TNF-induced cytokine production when necroptosis occurred, it was formally possible that zVAD was having a direct inhibitory affect on TNF-induced cytokine production. To exclude the possibility that zVAD had a direct inhibitory effect on TNF-induced cytokine production, without recourse to necroptosis, we used HeLa cells as these cells lack RIPK3 and thus fail to undergo necroptosis in response to TNF/zVAD treatment. Treatment with zVAD efficiently blocked TRAIL-induced apoptosis in HeLa cells (Figure 3a) but failed to cause necroptosis when combined with TNF (Figure 3b). Under these conditions, zVAD failed to suppress TNF-induced cytokine production (Figures 3c–f and Supplementary Figures S3a and b), indicating that necroptosis is responsible for the suppression of cytokine production in RIPK3-expressing cells treated with TNF/zVAD. Furthermore, zVAD also potently inhibited Fas-induced apoptosis in HeLa cells (Supplementary Figure S3c), and consistent with this, Fas-induced IL-6 and KC were increased, rather than suppressed, in the presence of zVAD due to restoration of cell viability (Supplementary Figures S3d and e). Furthermore, zVAD failed to exert a suppressive affect on TNF-induced MCP-1 and KC in murine 3LL cells, which are also refractory to necroptosis (Figures 3g–i). We also confirmed that zVAD did not inhibit TNF-induced cytokine synthesis at the mRNA level, in the absence of cell death (Figures 3j and k).
Figure 3.
Caspase activity is not required for TNF-induced cytokine production. (a) HeLa cells were pretreated for 1 h with the indicated concentrations of zVAD, followed by addition of TNF-related apoptosis-inducing ligand (TRAIL; 100 ng/ml). After 8 h, cell death was analysed by AnnexinV/PI staining. (b–f) HeLa cells were pretreated for 1 h in the presence or absence of zVAD (10 μM), followed by addition of TNF (10 ng/ml). After 24 h, cell death was analysed by AnnexinV/PI staining, and cytokine/chemokine concentrations in the culture supernatants were determined by ELISA. (g–i) Murine 3LL cells were pretreated for 1 h with the indicated concentrations of zVAD, followed by addition of TNF (10 ng/ml). After 24 h, cell death was analysed by PI staining, and chemokine concentrations in the culture supernatants were determined by ELISA. Error bars represent the mean±S.E.M. of triplicate cell cultures. (j) HeLa cells were seeded at 5 × 105 in 6-cm dishes. The following day, cells were pretreated for 1 h with zVAD (10 μM), followed by addition of TNF (10 ng/ml). After 4 h, or at the indicated time points (k), total RNA was isolated, reverse transcribed and used to seed real-time PCR reactions. Error bars represent the mean±S.E.M. of triplicate cell cultures
Knockdown of RIPK3 or MLKL restores TNF-induced cytokine production
As noted above, a straightforward reason for necroptosis-mediated suppression of TNF-induced cytokine production was simply because cells underwent rapid cell lysis, thereby terminating cytokine production. However, an alternative reason could be that excessive recruitment of RIPK3 onto RIPK1, as a consequence of caspase inhibition, interfered with RIPK1-dependent recruitment of NEMO/IKKγ and NFκB activation downstream. Indeed, Dixit and colleagues have previously reported that RIPK3 suppresses RIPK1-dependent NFκB activation upon transient overexpression.31 Thus it was formally possible that other effects of caspase inhibition (such as stabilization of the deubiquitinating enzyme cylindromatosis), as opposed to RIPK3-dependent cell death, were responsible for suppressing TNF-induced inflammatory cytokine production.
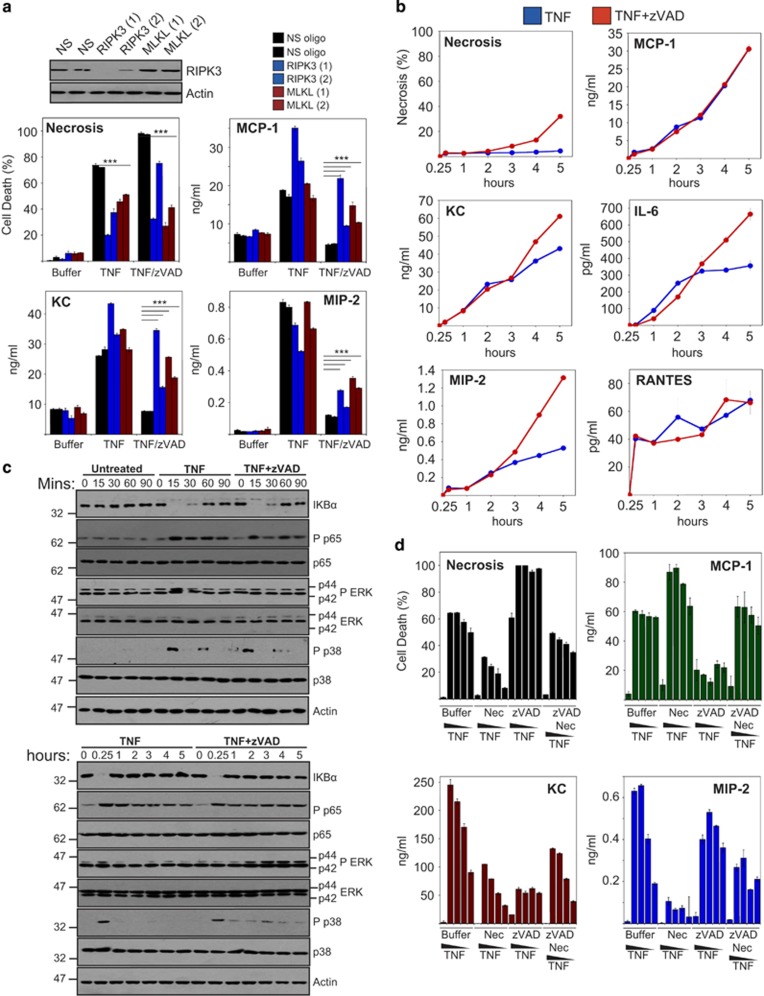
To explore whether RIPK3-dependent cell death was the primary reason for the suppression of TNF-induced cytokine production under necroptosis-inducing conditions, we silenced RIPK3 expression, followed by stimulation with TNF/zVAD to assess the effects on TNF-induced necroptosis and cytokine production. As shown in Figure 4a, knockdown of RIPK3 blocked TNF-induced necroptosis and also partly restored TNF-induced cytokine production in the presence of zVAD. Similarly, knockdown of the downstream target of RIPK3, MLKL, also blocked TNF/zVAD-induced necroptosis and restored cytokine production, suggesting that termination of cell viability via RIPK3 and MLKL activation was responsible for the decline in cytokine synthesis.
Figure 4.
RIPK3- and MLKL-dependant termination of cell viability attenuates TNF-induced cytokine and chemokine production. (a) L929 cells were electroporated with either non-silencing siRNA or siRNA directed against RIPK3 and MLKL, as indicated. Forty-eight hours later, cells were pretreated in the presence or absence of zVAD (10 μM) for 1 h, followed by addition of TNF (10 ng/ml). After 24 h, cell death was scored by morphology, and cytokine/chemokine concentrations in the culture supernatants were determined by enzyme-linked immunosorbent assay (ELISA). (b) L929 cells were pretreated for 1 h in the presence or absence of zVAD (10 μM), followed by addition of TNF (1 ng/ml). At the indicated time points, cell death was scored by morphology, and cytokine/chemokine concentrations in the culture supernatants were determined by ELISA. (c) L929 cells were pretreated for 1 h in the presence or absence of zVAD (10 μM), followed by addition of TNF (10 ng/ml). Cell lysates were prepared at the indicated time points and analysed by immunoblotting for levels of endogenous proteins. (d) L929 cells were pretreated in the presence or absence of Nec1 (5 μM) and/or zVAD (10 μM), followed by addition of TNF to 20, 10, 5 or 2.5 ng/ml (from left to right). After 24 h, cell death was scored by morphology, and cytokine/chemokine concentrations in the culture supernatants were determined by ELISA. Error bars represent the mean±S.E.M. of triplicate determinations from a representative experiment. For cell death analyses, triplicate counts were performed on a minimum of 300 cells per treatment. All data are representative of at least three independent experiments
We also examined cytokine and chemokine production within 5 h of stimulation of TNF alone, or TNF/zVAD, to ask whether cytokine production or NFκB activation upstream were attenuated prior to significant cell death (Figures 4b and c). These experiments suggested that early TNF-induced cytokine production (Figure 4b), as well as NFκB and MAPK activation (Figure 4c), were highly similar in response to TNF and TNF/zVAD treatment, again suggesting that the decline in cytokine synthesis seen under necroptosis-inducing conditions was due to cell death rather than impaired signal transduction.
Because the RIPK1 inhibitor, necrostatin-1, can block TNF/zVAD-induced necroptosis, we also used this approach to ask whether blocking cell death under necroptotic-inducing conditions restored cytokine production. Necrostatin-1 efficiently blocked TNF/zVAD-induced necroptosis, as expected (Figure 4d). However, we also observed that necrostatin had robust inhibitory effects on TNF-induced KC and MIP-2 production, in the absence of zVAD (Figure 4d). This suggests that at least some of the anti-inflammatory effects that have been attributed to necrostatin-1 in previous studies could be due to direct effects on TNF-induced cytokine production, rather than due to inhibition of necroptosis. However, this aside, we also observed that necrostatin treatment did restore MCP-1 and KC production in response to TNF/zVAD treatment to some degree (Figure 4d). To investigate the effects of necrostatin-1 on TNF-induced cytokines further, we also used MEFs, which do not die in response to TNF alone (Supplementary Figure S2). Once again, we observed that necrostatin-1 significantly inhibited secretion of KC, RANTES and IL-6 in the absence of cell death or caspase inhibition (Supplementary Figure S4).
Necroptosis suppresses LPS/TLR4-induced cytokine production
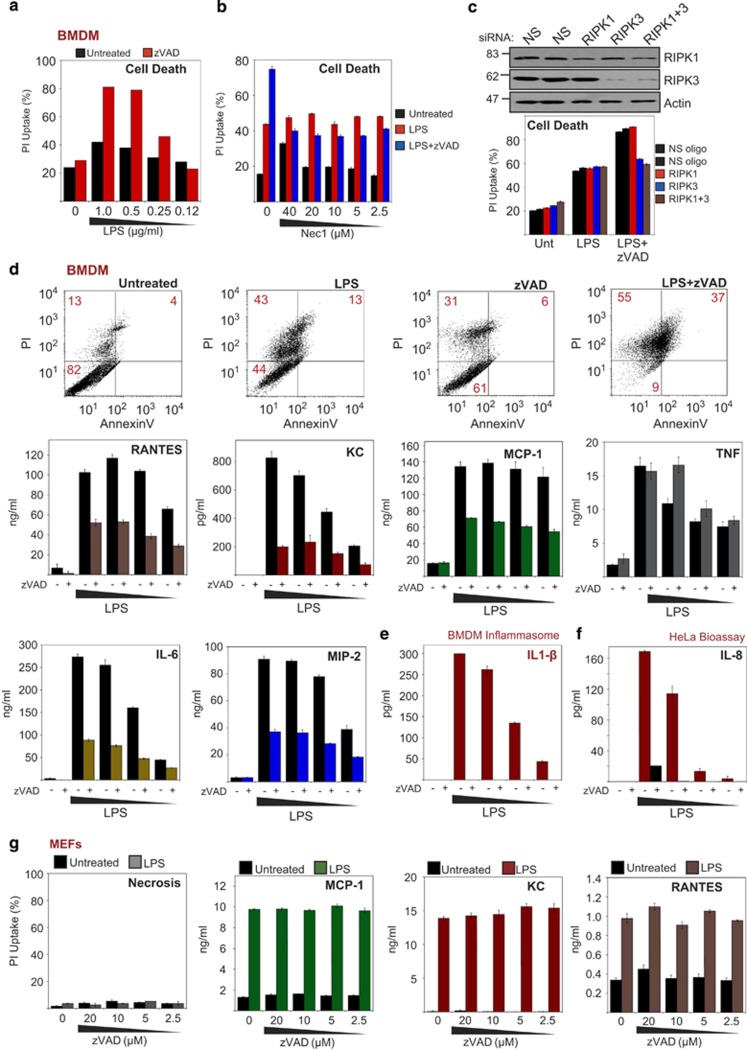
Because necroptosis can also be invoked in response to LPS stimulation, we next explored whether this also blunted pro-inflammatory cytokine and chemokine production in this context. As shown in Figure 5a, LPS induced robust necrosis in primary bone marrow-derived macrophages (BMDMs) in the presence of zVADfmk. LPS/zVAD-induced necroptosis was partly inhibited by addition of necrostatin-1 (Figure 5b) or through knockdown of RIPK3 (Figure 5c). However, a component of the LPS-induced necrosis observed was not necrostatin-1-inhibitable or blocked by RIPK3 knockdown. Moreover, similar to what we observed with TNF, LPS-induced IL-6, MIP-2, RANTES, KC and MCP-1 production were also considerably blunted under necroptosis-inducing conditions (Figure 5d).
Figure 5.
TLR4-dependent necroptosis suppresses pro-inflammatory cytokine production. (a) BMDMs were pretreated for 1 h with zVAD (15 μM) or not, followed by addition of LPS to the indicated concentrations. Forty-eight hours later, cell death was scored by PI staining. (b) BMDMs were pretreated for 1 h with the indicated concentrations of Nec1 in the presence or absence of zVAD (15 μM), followed by addition of LPS (1 μg/ml). After 24 h, cell death was scored by PI staining. (c) BMDMs were electroporated with either non-silencing siRNA or siRNA directed against RIPK1, RIPK3 or RIPK1 and RIPK3, as indicated. Forty-eight hours later, cells were pretreated for 1 h in the presence or absence of zVAD (15 μM), followed by addition of LPS (1 μg/ml). After 24 h, cell death was scored by PI staining. (d) BMDMs were pretreated for 1 h in the presence or absence of zVAD (15 μM), followed by addition of LPS to 2, 1, 0.5 and 0.25 μg/ml (left to right). Forty-eight hours later, cell death was scored by PI staining, and cytokine/chemokine concentrations in the culture supernatants were determined by enzyme-linked immunosorbent assay (ELISA). (e) BMDMs were pretreated for 1 h in the presence or absence of zVAD (15 μM), followed by addition of LPS to the indicated concentrations. Forty-eight hours later, culture supernatants were analysed for mature IL-1β by ELISA. (f) HeLa cell cultures were treated with 100 μl of the BMDM supernatent from panel (e). After 24 h, HeLa supernatents were analysed for IL-8 by ELISA (HeLa bioassay). (g) MEFs were pretreated for 1 h with the indicated concentrations of zVAD, followed by addition of LPS (5 μg/ml). After 24 h, cell death was analysed by PI staining, and chemokine concentrations in the culture supernatants were determined by ELISA
It is important to note that IL-1β, a key LPS-induced cytokine, requires caspase-1 activity, which is blocked under necroptosis-inducing conditions. Thus LPS/zVAD treatment greatly reduced the production of mature IL-1β as expected (Figure 5e). Consistent with this, supernatants from LPS/zVAD-treated BMDMs had greatly reduced pro-inflammatory activity when transferred onto HeLa cells (as measured by the production of IL-8 from the latter), when compared with supernatants from BMDMs treated with LPS alone (Figure 5f). Using MEFs, which do not engage RIPK3 or undergo necroptosis upon LPS/zVAD treatment, we also confirmed that zVAD did not suppress LPS-induced chemokines independently of necroptosis (Figure 5g). Similar results were also observed using THP-1 cells, which failed to under necroptosis in response to LPS/zVAD treatment (Supplementary Figure S5b).
Collectively, the above data indicate that necroptosis attenuates the production of numerous LPS-induced pro-inflammatory cytokines in RIPK3-expressing cells via termination of cell viability and through inhibition of caspase activity, which is required for IL-1β maturation.
TNF-induced necroptosis results in reduced inflammation in vivo
The preceding experiments demonstrated that TNF or LPS-induced cytokine and chemokine production was dramatically suppressed under necroptosis-inducing conditions. However, a caveat is that necroptosis liberates intracellular constituents, some of which can function as DAMPs, that could potentially compensate for the loss of cytokine and chemokine production. Thus whether necroptosis enhances or suppresses TNF-induced inflammation depends on the relative potency of endogenous DAMPs, which are liberated via necroptosis, versus TNF-induced pro-inflammatory cytokines and chemokines in driving inflammatory processes in vivo.
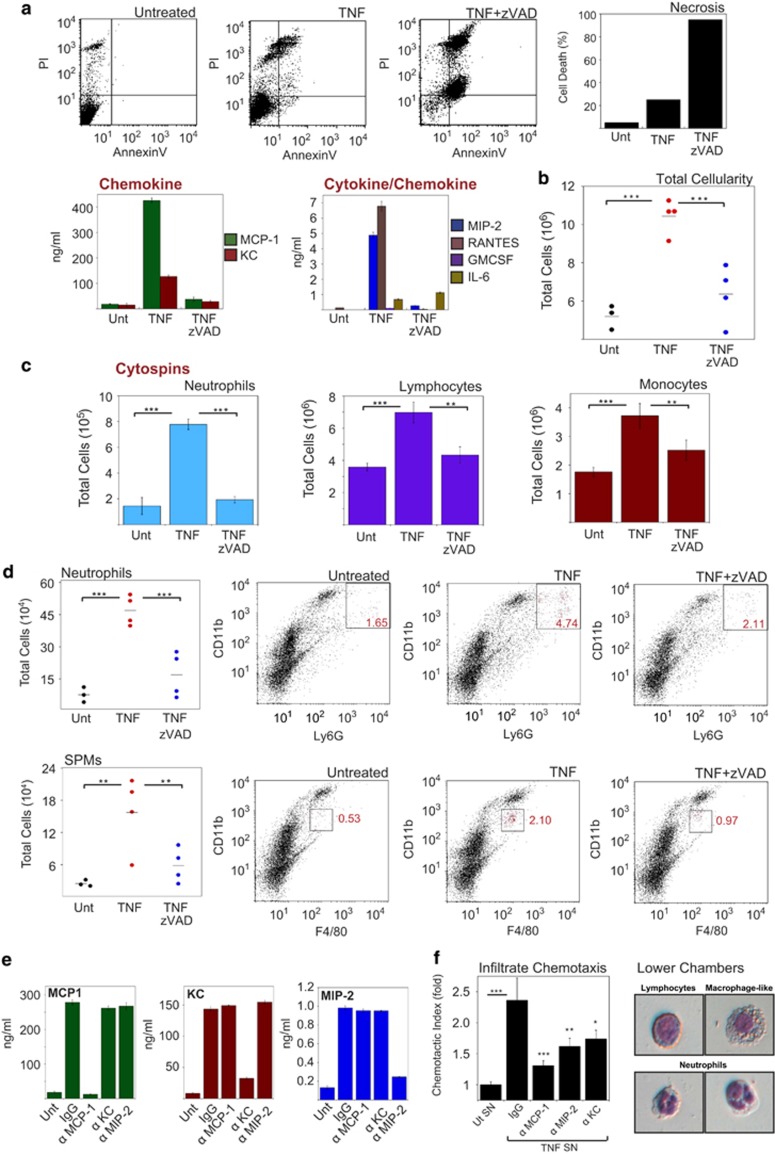
To explore this issue, we introduced supernatants from L929 cells treated with TNF alone or TNF/zVAD (necroptotic) into the peritoneal cavities of immunocompetent BALB/c mice and monitored the peritoneal exudates after 24 h to explore whether supernatants from necroptotic cells were more or less inflammatory than those from cells treated with TNF alone. As shown earlier, supernatants from TNF-treated cells contained much higher concentrations of pro-inflammatory cytokines than those from cells treated with TNF/zVAD (Figure 6a). Consistent with this, supernatants from TNF-treated cells induced robust infiltration of cells into the peritoneal cavities of mice (Figure 6b), with increases in the percentages of neutrophils, lymphocytes, monocytes and small peritoneal macrophages (Figures 6c and d). In contrast, supernatants from TNF/zVAD-treated (necroptotic) cells exhibited little increase in peritoneal cellularity (Figure 6b), or increases in neutrophils, lymphocytes or monocytes, consistent with our in vitro observations. Thus necroptosis attenuates rather than exacerbates TNF-induced inflammation.
Figure 6.
Necroptosis attenuates the inflammatory properties of TNF-stimulated cells in vivo. (a) L929 cells at a density of 2 × 106/ml in RPMI supplemented with 1% FCS were pretreated in the presence or absence of zVAD (50 μM), followed by addition of TNF (10 ng/ml). After 24 h, cell death was scored by PI staining, and cytokine/chemokine concentrations in the culture supernatants were determined by enzyme-linked immunosorbent assay (ELISA). Supernatants were centrifugated to remove cells or debris. (b) Female Balb/c mice (four per group) were injected intraperitoneally with 300 μl of the indicated L929 cell supernatants from panel (a). After 24 h, mice were killed, and the peritoneal cavity was washed with 4 ml PBS. Total cellularity was scored by microscopy. (c) Cytospins were made from peritoneal-derived cells, stained with hematoxylin/eosin and used to score the percentage of immune cell types by morphology. Population numbers were then generated from total cellularity. (d) Peritoneal infiltrates from panel (b) were immunostained and quantified by flow cytometry as indicated. (e) Supernatants from panel (a) were immunodepleted of the indicated chemokines, and the depletion efficiency was analysed by ELISA. (f) Depleted supernatants from panel (e) were used in a chemotaxis assay using peritoneal infiltrates from mice that received supernatants from TNF-treated L929 cells. Cells from each individual mouse were used as experimental replicates (n=4). Cytospins were made from cells that successfully migrated, stained with H&E and viewed by phase-contrast microscopy. Error bars represent the mean±S.E.M. *P<0.1; **P<0.05; ***P<0.01 by Student's t-test
To explore which chemokines were having the dominant role in recruiting cells to the peritoneum in response to TNF treatment (Figure 6b), we also performed chemotaxis assays ex vivo using peritoneal exudate cells from TNF-treated mice. Chemotaxis of peritoneal exudate cells was measured in response to supernatants from TNF-treated cells that were either mock depleted (IgG) or depleted with anti-MCP-1, anti-KC or anti-MIP-2 monoclonal antibodies (Figure 6e). As shown in Figure 6f, chemotaxis of peritoneal exudate cells was largely abolished upon depletion of MCP-1 from the supernatants, consistent with the very high concentrations of this chemokine produced in response to TNF (Figure 6a).
LPS-induced inflammation is suppressed through caspase inhibition
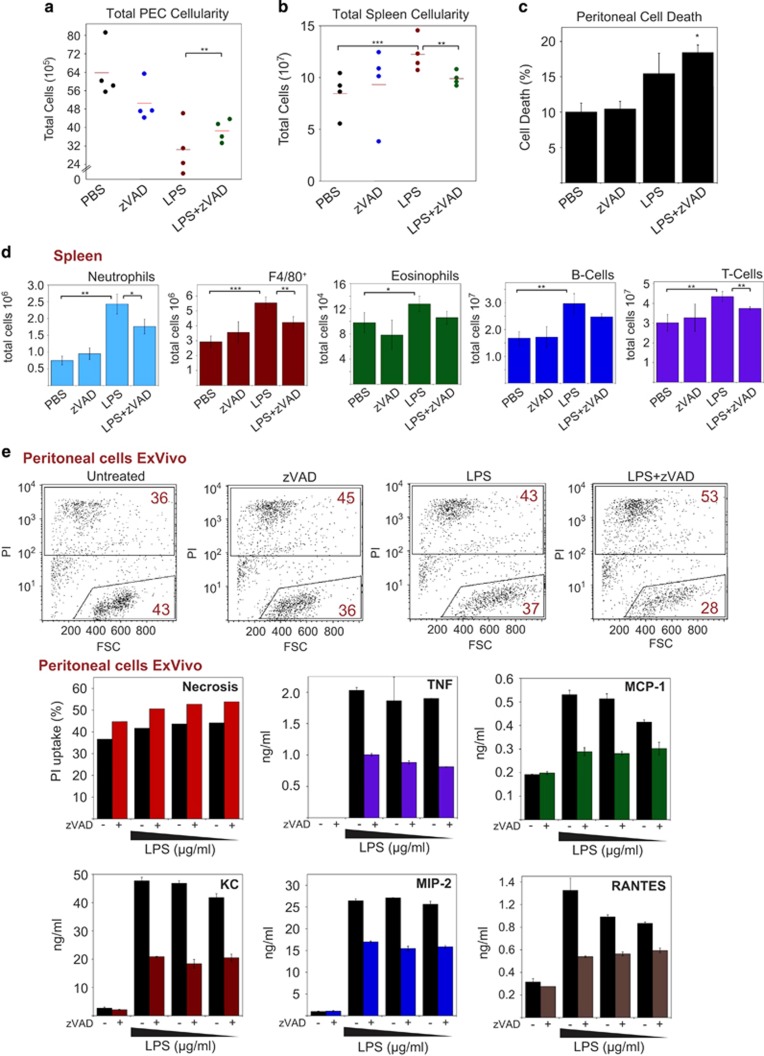
As demonstrated earlier, LPS also promotes necroptosis in the presence of caspase inhibition (Figure 5a), which led to suppression of the production of LPS-induced cytokines in vitro (Figures 5d and e). To explore whether caspase inhibition also attenuated LPS-driven inflammation in vivo, we treated balb/c mice with LPS intraperitoneally in the presenece or absence of zVAD-fmk, to promote necroptosis. Previous studies have shown that LPS induces cell death in the peritoneal cavity, as well as activation and efflux of resident macrophages and lymphocytes.32, 33 The latter events are mirrored by an increase in splenic cellularity, composed of migrating neutrophils, macrophages, monocytes and clonally expanding lymphocytes.33, 34
As shown in Figure 7a, LPS administration led to a dramatic reduction in peritoneal cellularity, which was significantly inhibited in LPS/zVAD-treated animals. However, it should also be noted that zVAD alone also led to a reduction in peritoneal cellularity. Consistent with this, we observed an increase in splenic cellularity in response to LPS, which was attenuated in the presence of zVAD Figure 7b. We also confirmed that LPS/zVAD treatment caused a significant increase in peritoneal cell death, compared with LPS alone Figure 7c.
Figure 7.
Necroptosis attenuates LPS-induced inflammation in vivo. (a) Female balb/c mice (4 per group) were injected (intraperitoneally, 200 μl) with PBS or zVAD (250 μg per mouse). After 1 h, mice were injected again with either PBS or LPS (10 μg per mouse). After a further 24 h, mice were killed, and the peritoneal cavity was washed with 4 ml PBS. Total cellularity was scored by microscopy. (b) Spleens were removed, and splenocytes were collected through a strainer. Erythrocytes were removed using RBC lysis buffer and then total cellularity was analysed by microscopy. (c) Peritoneal cell death was analysed by Aqua positivity. (d) Splenocytes were immunostained and analysed by flow cytometry. Population numbers were then generated by multiplying cell percentages by total splenic cellularity counts. Neutrophils (CD11b+, Gr1hi, F4/80med, SiglecF-), eosinophils (SiglecF+), B-cells (CD19+), T-cells (CD3+). (e) Peritoneal-derived cells from control mice were seeded at 1 × 106/ml in 24-well plates. Cells were pretreated with zVAD (20 μM) or not, followed by addition of LPS to 10, 5 and 1 μg/ml. After 48 h, cell death was analysed by forward scatter/PI staining, and cytokine/chemokine concentrations in the culture supernatants were determined by ELISA. Error bars represent the mean±S.E.M. *P<0.1; **P<0.05; ***P<0.01 by Student's t-test
As shown in Figure 7d, LPS treatment induced robust increases in splenic neutrophils, F4/80+ macrophages, eosinophils and B- and T-lymphocytes, which were all diminished in the presence of LPS/zVAD. This is consistent with a suppression rather than enhancement of LPS-induced inflammatory responses under necroptotic (caspase inhibition) conditions. Once again, these data argue that necroptosis suppresses rather than enhances inflammatory responses, most likely through killing responding cells and reducing inflammatory cytokine production, as well as through blunting the production of IL-1β and IL-18 in a caspase-dependent manner.
To explore this further, we recovered peritoneal cells from PBS-treated mice and stimulated with either LPS or LPS/zVAD to evaluate cytokine production ex vivo. LPS/zVAD-treated peritoneal exudates exhibited increases in cell death as compared with cells treated with LPS or zVAD alone (Figure 7e). Furthermore, we again observed dramatic reductions in LPS-induced TNF, MCP-1, KC, MIP-2 and RANTES in the LPS/zVAD (necroptotic) condition (Figure 7e).
Taken together, the above data argue that TNF- or LPS-induced necroptosis is significantly less pro-inflammatory than unimpeded TNF or LPS stimulation. Our data also argue that conventional TNF- or LPS-induced cytokines and chemokines exert more potent pro-inflammatory effects than endogenous DAMPs that are released during necrosis or necroptosis.
Discussion
Here we have shown that, in contrast to current interpretations of the biological consequences of necroptosis, RIPK3-dependent cell death resulted in a dampening of TNF-induced pro-inflammatory cytokine/chemokine secretion, thereby leading to a less inflammatory outcome. Similarly, we also found that LPS-initiated necroptosis was less inflammatory than LPS stimulation involving caspase participation. The necroptosis effectors RIPK3 and MLKL, in addition to being able to promote necroptosis under conditions where caspase activity is blocked, are very likely to have other cellular functions, which remain unknown at present. However, we propose that these molecules could also be regarded as negative regulators of TNF- and LPS-induced inflammation, as their activation rapidly shuts down cytokine synthesis through cell lysis. From this perspective, our findings also have major implications for the interpretation of the pathology of RIPK3 null animals in response to pathogen challenge as well as sterile injury. RIPK3 null animals are often protected from pathogen challenge or injury-induced inflammation, and this is frequently attributed to blocking necroptosis. However, our data suggest that RIPK3 null animals would make more effective and prolonged immune responses through preventing the shutdown of cytokine/chemokine synthesis that would otherwise occur via necroptosis. Viewed in this light, our data also cast doubt upon the view that necroptosis is invariably a host response to pathogens encoding caspase inhibitory proteins. Instead, it is possible that necroptosis could also serve as a pathogen-driven mechanism to limit the host inflammatory response in at least some contexts. Thus infectious agents that promote necroptosis may do so as a mechanism to neutralize host immune responses by rapidly terminating conventional cytokine and chemokine production. In this situation, the liberation of endogenous DAMPs as a consequence of necroptosis may be insufficient to compensate for the loss of cytokine and chemokine synthesis.
Support for our observations come from a study by Linkermann et al.35 who reported that the necroptosis inhibitor, necrostatin-1, failed to protect from TNF/zVAD-induced shock and lethality but rather accelerated time to death compared with TNF/zVAD treatment alone. This is consistent with our observations that necroptosis suppresses the inflammatory effects of TNF, which can lead to lethality, rather than enhancing these effects as is frequently speculated. Suppressing necroptosis in the context of TNF stimulation is likely to enhance the lethal effects of this cytokine through prolonging and exacerbating the cytokine storm. Moreover, Linkerman et al.35 also observed similar effects with cerulein-induced pancreatitis, where necrostatin-1 worsened the inflammatory symptoms, again most likely by restoring production of a plethora of TNF-inducible inflammatory mediators. However, it should also be noted that interpreting effects seen with necrostatin-1 in vivo is very problematic, as necrostatin may have direct inhibitory effects on the production of some TNF-induced cytokines as we have shown (Figure 4 and Supplementary Figure S4). In particular, we have found that TNF-induced production of IL-6, which is a key player in models of severe systemic inflammation (SIRS) induced by TNF, is dramatically reduced in the presence of necrostatin (Supplementary Figure S4). Indeed, direct effects of necrostatin-1 on TNF-induced cytokine production might well explain the protection afforded by this kinase inhibitor during TNF-induced shock in vivo,27 especially as the latter effects were seen in the absence of caspase inhibition where necroptosis would not be expected.
Whether necroptosis is beneficial to the host, or to pathogens, may be context-dependent. RIPK3 null mice display increased susceptibility to replication of vaccinia virus.9 Thus, in this instance, necroptosis may be beneficial to the host by depriving the virus time to replicate. Furthermore, cytomegalovirus encodes caspase inhibitors and vIRA, which disrupts the host RHIM-dependent RIPK1 and RIPK3 interaction to promote pathogenesis.10 However, the role of necroptosis as an anti-viral strategy appears to be more complex and context dependent than initially presumed. Previous studies using poxviruses that encode caspase inhibitors have shown that virus deficient in cytokine response modifier A (CrmA), a potent Caspase-1 and -8 inhibitor, is less pathogenic than wild-type virus capable of inducing necroptosis and processing IL-1β.36 Although it is unclear whether CrmA evolved to suppress caspase-1-mediated IL-1β maturation or to promote necroptosis through caspase-8 inhibition, it suggests that the ability to inhibit caspase activity, thus promoting necroptosis-inducing conditions, can be beneficial to the virus rather than the host in at least some situations. Similarly, rabbits infected with myxoma virus lacking the CrmA homolog Serp2 display increased viral control.37
The preceding observations suggest that promoting necroptosis can be beneficial to viruses in certain contexts. This fits well with the implications of our observations and suggests that some viruses may deliberately shut down the host inflammatory response by invoking necroptosis in a cell that receives TNF stimulation. This might also have the consequence of facilitating viral spread by killing the very cells that act as sentinels in tissues vulnerable to viral infection. It is also relevant to note that were necroptosis always an effective host-response to viral infection, designed to terminate viral replication by depriving the virus of a host, then it would be expected that viruses would rapidly emerge that have lost their caspase inhibitory protein(s). However, the fact that multiple viruses do encode caspase inhibitors strongly suggests that these are beneficial to viral replication or spread. Thus viral initiation of necroptosis could also be viewed a strategy adopted by certain viruses to suppress the host inflammatory response, rather than a host response designed to terminate viral replication.
Infection with the intracellular bacterium Salmonella typhimurium has also been shown to lead to RIPK3-dependent necroptosis.38 However, infection of RIPK3 null macrophages (incapable of undergoing necroptosis) led to decreased bacterial burdens, suggesting that rapid RIPK3-dependent necroptosis of macrophages is beneficial to the pathogen and helps to attenuate the host immune response.
Neutrophils are rapidly recruited from the peripheral circulation to sites of infection via chemokine gradients generated by tissue-resident innate immune cells, where they deploy aggressive anti-microbial mechanisms. Local macrophages, which provide a rich source of neutrophil chemoattractants KC and MIP-2, are required for neutrophil migration into skin infected with Staphyloccus aureus and subsequent bacterial clearance.39 However, S. aureus utilizes virulence factor hemolysin to promote rapid macrophage necrosis at the infection site, thereby dampening chemokine generation and neutrophil recruitment.39 This demonstrates the importance of the initial chemokine wave that is released in response to pathogen infection and highlights responder cell necrosis as a pathogen strategy to terminate it. Indeed, we also found that synthesis of chemokines KC and MIP-2 was rapidly terminated as a consequence of TNF- and LPS-induced necroptosis (Figures 3b and 5d) and translated into impeded neutrophil recruitment to the peritoneal cavity (Figure 6c) and spleen (Figure 7d) in vivo.
In conclusion, here we have provided evidence to argue that necroptosis may be less inflammatory than the alternative outcomes (cell survival or apoptosis) due to rapid cessation of the transcriptional pro-inflammatory programme induced by TNF or LPS. Thus viruses and other microorganisms that evoke this mode of cell death may well do so as an adaptive strategy to minimize the host inflammatory response.
Materials and Methods
Materials
The following antibodies were used: anti-RIPK1, anti-IκB (BD, Oxford, UK), RIPK3 (ProSci, Poway, CA, USA), anti-phospho-p65, anti-phospho ERK, anti-ERK, anti-phospho-p38antip38 (Cell Signaling, Hitchin, UK), and anti p65 (Santa Cruz, Dallas, TX, USA). Recombinant TNFα was purchased from Roche (Dublin, Ireland). zVAD-fmk was from Bachem (Bubendorf, Switzerland); Nec1 and LPS were from Sigma (Arklow, Ireland).
Cell culture
HeLa and L929 cells were cultured in RPMI media (Gibco, Renfrew, UK), supplemented with 5% fetal calf serum (FCS). MEFs, BMDMs and HT-29 cells were cultured in DMEM (Gibco) supplemented with 10% FCS. Cells were cultured at 37 °C in humidified atmosphere with 5% CO2.
Cell death assays
Cells were plated at 2 × 105 cells/well in six-well plates and treated as indicated 24 h later. Necroptosis was enumerated based on cell morphology (cell swelling, detachment from the plate, membrane rupture) and by flow cytometry-based Annexin V/propidium iodide (PI) staining). A minimum of 300 cells was counted in each treatment. For flow cytometry-based cell death analysis, cells were harvested by trypsinization and then added to dead cells that were detached from the culture dish. Cells were then incubated with AnnexinV (2 μg/ml) and PI (10 μg/ml), followed by aquisition of at least 2500 cells. Necroptotic cell death was confirmed by AnnexinV/PI double positivity, in the absence of cells with annexinV single positivity. Each assay was repeated a minimum of three times.
Measurement of cytokines and chemokines
Cells were plated at 2 × 105 cells/well in six-well plates and treated as indicated 24 h later in 2 ml of fresh media. Cytokines and chemokines were measured from cell culture supernatants using specific paired antibody ELISA kits obtained from R&D Systems (Abingdon, UK) (human IL-8, human sICAM-1, human RANTES, human IL-6, human CXCL1, mouse KC, mouse MIP-2, mouse IL1β, mouse RANTES) and eBiosciences (Wycombe, UK) (mouse MCP-1, mouse TNF, mouse IL-6, mouse GMCSF). Generally, antibodies were coated on ELISA plates overnight and diluted as per the manufacturer's instructions in PBS. Samples were then incubated on the ELISA plate for 2 h, followed by incubation with biotin-labelled detection antibody for 2 h. Samples were then incubated with streptavidin-HRP for 30 min prior to detection with TMB substrate. The reaction was stopped with 3 N sulphuric acid, and the absorbance was read on an ELISA plate reader (Tecan, Maennedorf, Switzerland). Each assay was repeated a minimum of three times, and all cytokine assays were carried out using triplicate samples from each culture.
Gene expression analysis by microarray
HeLa cells were seeded at 1 × 106 in 6-cm plates and treated with TNF (10 ng/ml) 24 h later. After 6 h, cells were harvested using ‘RNA protect' (Qiagen, Manchester, UK). RNA extraction, cDNA synthesis and chip hybridization was carried out by IMGM Laboratories (Martinsried, Germany).
Western immunoblotting
To analyse proteins, sodium dodecyl sulphate polyacrylamide gel electrophoresis was utilized. Protein samples were initially prepared in sample buffer (2% SDS, 50 mM Tris-HCl, pH 6.8, 10% glycerol, 2.5% β-mercaptoethanol) and then boiled for 7 min to denature proteins. Samples were then loaded into 8–12% polyacrylamide gels and electrophoresed at 75 V. Resolved proteins were then transferred on to nitrocellulose membranes at 40 mA overnight. Membranes were blocked for 1 h (5% NFDM, 0.05% NaN3 in Tris-buffered saline, Tween-20 (TBST)), then incubated with primary antibody and typically diluted at 1 : 1000 for 2 h. Membranes were washed for 3 × 10 min with TBST and then incubated with HRP-conjugated secondary antibody in TBST containing 5% NFDM. After a final 3 × 10 min wash, proteins were detected using West Coast Super Signal (Thermo, Renfrew, UK).
RNA interference
To ablate mouse RIPK-1, mouse RIPK3 and mouse MLKL expression in L929, MEFs and BMDMs, 5 × 105 cells were transfected with 100 nM siRNA using nucleofection (Amaxa, Basel, Switzerland) on programme T-020, as per the manufacturer's instructions. Cells were then seeded at 1 × 105 in six-well plates and incubated with siRNA for 48 h followed by the indicated treatment. siRNA sequences were as follows: mouse RIPK1: sense: 5′-CCACUAGUCUGACUGAUGA-3′, mouse RIPK3#1: sense: 5′-CCCGACGAUGUCUUCUGUCAA-3′, mouse RIPK3#2: sense: 5′-AAGAUUAACCAUAGCCUUCACCUCCCA-3′, mouse MLKL#1: sense: 5′-GAGAUCCAGUUCAACGAUA-3′, mouse MLKL#2: sense: 5′-GGAAUACCGUUUCAGAUGU-3′.
Real-time PCR
HeLa cells were seeded at 5 × 105 in 6-cm dishes. The following day, cells were treated as described, and then total RNA was isolated using an ‘RNeasy' kit (Qiagen). Normalized RNA (1 μg) was then reverse transcribed using an ‘omniscript' RT kit (Qiagen). Resulting cDNA was again normalized and used to seed real-time PCR reactions on a ROCHE lightcycler using ‘Fast Start' (Roche). Actin was used as the control for fold change in expression. Cytokine and chemokine primers for real-time PCR are included in the Supplementary Information.
Chemotaxis assays
Chemotaxis assays were performed in Neuro Probe (Gaithersburg, MD, USA) 10-Well Chemotaxis Chambers as per the manufacturer's instructions. Briefly, Supernatants (150 μl) were added to the bottom well of a chemotaxis chamber, and then 8-μM nitrocellulose filters were placed on top and secured by application of the top chamber, to which 200 μl of peritoneal exudate cells were added at 106/ml. Total cells entering the bottom chamber were counted after 6 h. For antibody-mediated depletion of chemokines, supernatants were incubated with protein A/G agarose coupled to specific antibodies (1–2 μg/ml) for 2 h, followed by depletion of the agarose–antibody–chemokine complexes from the supernatants by centrifugation.
Animals
Balb/C mice were purchased from Harlan (Blackthorn, UK). Animal experiments were in accordance with the regulations of the Trinity College Dublin ethics committee and the Irish Department of Health.
Statistical analysis
Statistical significance was assessed by unpaired, one-tailed Student's t-test, in which equal variance was assumed. Error bars represent the mean±S.E.M. *P<0.1; **P<0.05; ***P<0.01 by Student's t-test. For cytokine/chemokine measurments, triplicate sample denominations were assayed.
Acknowledgments
The Martin's laboratory is supported by SRC (07/SRC/B1144) and PI (08/IN.1/B2031) grants from Science Foundation Ireland. The Lavelle's laboratory is supported by SRC (07/SRC/B1144) and PI grants from Science Foundation Ireland. SJM is a Science Foundation Ireland Principal Investigator.
Glossary
- TNF
tumor necrosis factor
- LPS
lipopolysaccharide
- RIPK3
receptor-interacting protein kinase 3
- FADD
fas-associating death domain-containing protein
- TNFR
TNF receptor
- NFκB
nuclear factor kappa B
- MCP-1
monocyte chemotactic protein 1
- RANTES
regulated upon activation normal t cell expressed and presumably secreted
- MIP-2
macrophage inflammatory protein 2
- KC
keratinocyte chemoattractant
- GMCSF
granulocyte-macrophage colony-stimulating factor
- IL-8
interleukin 8
- MEF
murine embryonic fibroblast
- MLKL
mixed lineage kinase domain-like
- Nec1
Necrostatin 1
- CrmA
cytokine response modifier A
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on Cell Death and Differentiation website (http://www.nature.com/cdd)
Edited by G Melino
Supplementary Material
References
- Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- Wang L, Du F, Wang X. TNF-alpha induces two distinct caspase-8 activation pathways. Cell. 2008;133:693–703. doi: 10.1016/j.cell.2008.03.036. [DOI] [PubMed] [Google Scholar]
- Lin Y, Choksi S, Shen HM, Yang QF, Hur GM, Kim YS, et al. Tumor necrosis factor-induced nonapoptotic cell death requires receptor-interacting protein-mediated cellular reactive oxygen species accumulation. J Biol Chem. 2004;279:10822–10828. doi: 10.1074/jbc.M313141200. [DOI] [PubMed] [Google Scholar]
- Mocarski ES, Upton JW, Kaiser WJ. Viral infection and the evolution of caspase 8-regulated apoptotic and necrotic death pathways. Nat Rev Immunol. 2012;12:79–88. doi: 10.1038/nri3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, Wang L, Miao L, Wang T, Du F, Zhao L, et al. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-α. Cell. 2009;137:1100–1111. doi: 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]
- Oberst A, Dillon CP, Weinlich R, McCormick LL, Fitzgerald P, Pop C, et al. Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature. 2011;471:363–367. doi: 10.1038/nature09852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon CP, Oberst A, Weinlich R, Janke LJ, Kang TB, Ben-Moshe T, et al. Survival function of the FADD-Caspase-8-cFLIP(L) complex. Cell Rep. 2012;1:401–407. doi: 10.1016/j.celrep.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser WJ, Upton JW, Long AB, Livingston-Rosanoff D, Daley-Bauer LP, Hakem R, et al. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature. 2011;471:368–372. doi: 10.1038/nature09857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildford M, et al. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137:1112–1123. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upton JW, Kaiser WJ, Mocarski ES. Virus inhibition of RIP3-dependent necrosis. Cell Host Microbe. 2010;7:302–313. doi: 10.1016/j.chom.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Paolo NC, Doronin K, Baldwin LK, Papayannopoulou T, Shayakhmetov DM. The transcription factor IRF3 triggers ‘defensive suicide' necrosis in response to viral and bacterial pathogens. Cell Rep. 2013;3:1480–1486. doi: 10.1016/j.celrep.2013.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181–190. doi: 10.1016/s0092-8674(03)00521-x. [DOI] [PubMed] [Google Scholar]
- Van Antwerp DJ, Martin SJ, Kafri T, Green DR, Verma IM. Suppression of TNF-alpha-induced apoptosis by NF-kappaB. Science. 1996;274:787–789. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- Beg AA, Baltimore D. An essential role for NF-kappaB in preventing TNF-alpha-induced cell death. Science. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- Vince JE, Wong WW, Khan N, Feltham R, Chau D, Ahmed AU, et al. IAP antagonists target cIAP1 to induce TNFalpha-dependent apoptosis. Cell. 2007;131:682–693. doi: 10.1016/j.cell.2007.10.037. [DOI] [PubMed] [Google Scholar]
- Esche C, Stellato C, Beck LA. Chemokines: key players in innate and adaptive immunity. J Invest Dermatol. 2005;125:615–628. doi: 10.1111/j.0022-202X.2005.23841.x. [DOI] [PubMed] [Google Scholar]
- Rot A, Von Andrian UH. Chemokines in innate and adaptive host defense: basic chemokinese grammar for immune cells. Ann Rev Immunol. 2004;22:891–928. doi: 10.1146/annurev.immunol.22.012703.104543. [DOI] [PubMed] [Google Scholar]
- Rollins BJ. Chemokines. Blood. 1997;90:909–928. [PubMed] [Google Scholar]
- Janes KA, Gaudet S, Albeck JG, Nielsen UB, Lauffenburger DA, Sorger PK. The response of human epithelial cells to TNF involves an inducible autocrine cascade. Cell. 2006;124:1225–1239. doi: 10.1016/j.cell.2006.01.041. [DOI] [PubMed] [Google Scholar]
- Burgess AW, Metcalf D. The nature and action of granulocyte-macrophage colony stimulating factors. Blood. 1980;56:947–958. [PubMed] [Google Scholar]
- Brennan FM, McInnes IB. Evidence that cytokines play a role in rheumatoid arthritis. J Clin Invest. 2008;118:3537–3545. doi: 10.1172/JCI36389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczmarek A, Vandenabeele P, Krysko DV. Necroptosis: the release of damage-associated molecular patterns and its physiological relevance. Immunity. 2013;38:209–223. doi: 10.1016/j.immuni.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GY, Nuñez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10:826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol. 2008;8:279–289. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duprez L, Takahashi N, Van Hauwermeiren F, Vandendriessche B, Goossens V, Vanden Berghe T, et al. RIP kinase-dependent necrosis drives lethal systemic inflammatory response syndrome. Immunity. 2011;35:908–918. doi: 10.1016/j.immuni.2011.09.020. [DOI] [PubMed] [Google Scholar]
- Vanlangenakker N, Vanden Berghe T, Vandenabeele P. Many stimuli pull the necrotic trigger, an overview. Cell Death Differ. 2012;19:75–86. doi: 10.1038/cdd.2011.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriwaki K, Ka-Ming Chan F. RIP3: a molecular switch for necrosis and inflammation. Genes Dev. 2013;27:1640–1649. doi: 10.1101/gad.223321.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercammen D, Beyaert R, Denecker G, Goossens V, Van Loo G, Declercq W, et al. Inhibition of caspases increases the sensitivity of L929 cells to necrosis mediated by tumor necrosis factor. J Exp Med. 1998;9:1477–1485. doi: 10.1084/jem.187.9.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Lee J, Navas T, Baldwin DT, Stewart TA, Dixit VM. RIP3, a novel apoptosis-inducing kinase. J Biol Chem. 1999;274:16871–16875. doi: 10.1074/jbc.274.24.16871. [DOI] [PubMed] [Google Scholar]
- He S, Liang Y, Shao F, Wang X. Toll-like receptors activate programmed necrosis in macrophages through a receptor-interacting kinase-3-mediated pathway. PNAS. 2011;108:20054–20059. doi: 10.1073/pnas.1116302108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Tung JW, Ghosn EEB, Herzenberg LA, Herzenberg LA. Division and differentiation of natural antibody-producing cells in mouse spleen. PNAS. 2007;104:4542–4546. doi: 10.1073/pnas.0700001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao C, Lawrence DA, Strickland DK, Zhang L. A specific role of integrin Mac-1 in accelerated macrophage efflux to the lymphatics. Blood. 2005;106:3234–3241. doi: 10.1182/blood-2005-03-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linkermann A, Bräsen JH, De Zen F, Weinlich R, Schwendener RA, Green DR, et al. Dichotomy between RIP1- and RIP3-mediated necroptosis in tumor necrosis factor-α–induced shock. Mol Med. 2012;18:577–586. doi: 10.2119/molmed.2011.00423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNeill AL, Moldawer LL, Moyer RW. The role of the cowpox virus crmA gene during intratracheal and intradermal infection of C57BL/6 mice. Virology. 2009;384:151–160. doi: 10.1016/j.virol.2008.10.041. [DOI] [PubMed] [Google Scholar]
- Messud-Petit F, Gelfi J, Delverdier M, Amardeilh MF, Py R, Sutter G, et al. Serp2, an inhibitor of the interleukin-1b-converting enzyme, is critical in the pathobiology of myxoma virus. J Virol. 1998;72:7830–7839. doi: 10.1128/jvi.72.10.7830-7839.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson N, McComb S, Mulligan R, Dudani R, Krishnan L, Sad S. Type I interferon induces necroptosis in macrophages during infection with Salmonella enterica serovar Typhimurium. Nat Immunol. 2012;13:954–962. doi: 10.1038/ni.2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abtin A, Jain R, Mitchell AJ, Roediger B, Brzoska AJ, Tikoo S, et al. Perivascular macrophages mediate neutrophil recruitment during bacterial skin infection. Nat Immunol. 2014;15:45–53. doi: 10.1038/ni.2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.