Abstract
Serotonin is an important neurotransmitter that broadly participates in various biological processes. While serotonin deficiency has been associated with multiple pathological conditions such as depression, schizophrenia, Alzheimer’s disease and Parkinson’s disease, the serotonin-dependent mechanisms remain poorly understood. This study therefore aimed to identify novel biomarkers and metabolic pathways perturbed by serotonin deficiency using metabolomics approach in order to gain new metabolic insights into the serotonin deficiency-related molecular mechanisms. Serotonin deficiency was achieved through pharmacological inhibition of tryptophan hydroxylase (Tph) using p-chlorophenylalanine (pCPA) or genetic knockout of the neuronal specific Tph2 isoform. This dual approach improved specificity for the serotonin deficiency-associated biomarkers while minimizing nonspecific effects of pCPA treatment or Tph2 knockout (Tph2-/-). Non-targeted metabolic profiling and a targeted pCPA dose-response study identified 21 biomarkers in the pCPA-treated mice while 17 metabolites in the Tph2-/- mice were found to be significantly altered compared with the control mice. These newly identified biomarkers were associated with amino acid, energy, purine, lipid and gut microflora metabolisms. Oxidative stress was also found to be significantly increased in the serotonin deficient mice. These new biomarkers and the overall metabolic pathways may provide new understanding for the serotonin deficiency-associated mechanisms under multiple pathological states.
Serotonin is an important neurotransmitter that broadly functions in the regulation of multiple physiological systems, including the cardiovascular, pulmonary, gastrointestinal, genitourinary systems and the central nervous system (CNS)1. It participates in the modulation of various neurophysiological processes such as pain perception, energy balance, appetite, sleep, circadian rhythm and aging; neuropsychological processes such as perception, mood, learning, memory, stress and addiction; and behaviors such as play, social perception, aggression, cooperation, mating and sexuality1,2,3.
Efforts to understand serotonin functionality and signaling mechanisms have primarily focused on its 12 heterotrimeric guanine nucleotide binding protein-coupled receptors and one additional ligand-gated ion channel which have been grouped into seven distinct classes (5-HT1 to 5-HT7)4. This conventional approach to understand serotonin’s function in disease is reflected by the numerous drugs that have been developed to target serotonin receptors5,6,7. However, considerable challenges have emerged related to the non-specificity of serotonin receptors to individual biological processes. Consequently, many serotonergic drugs have wide-ranging side effects1. One such example is behavioral aggression which is regulated by 5-HT1A, 5-HT1B and 5-HT2A receptors8,9,10; however, the 5-HT1B receptor modulates not only aggression but also migraine, locomotion, drug abuse reinforcement, depression and anxiety11. These complicated, non-specific receptor-phenotype relationships represent a significant shortcoming in receptor-based approaches to understand serotonin pathophysiology. New approaches are therefore urgently needed to better understand serotonin mechanisms and manage the remarkably large number of pathological conditions.
Metabolomics, the downstream product of genomics, transcriptomics and proteomics, is an emerging ‘-omics’ approach of system biology that has provided often unexpected and unique insights into various biological processes. Unlike the genome or proteome, changes in the metabolome are rapid and represent the final response of an organism to both internal and external stimuli. Hence, metabolomics is particularly conducive to identifying pathophysiologically affected processes and moreover elucidating novel physiological and pathological mechanisms12. While metabolomics has been previously applied to several serotonin-related diseases, including depression13, schizophrenia14 and Parkinson’s disease15, its application to serotonin deficiency has not yet been explored and described.
This study therefore sought to identify novel serum metabolites that were significantly altered in serotonin deficient mice compared with control mice. Serotonin deficiency was achieved through two orthogonal routes which included (1) use of p-chlorophenylalanine (pCPA) to pharmacologically inhibit tryptophan hydroxylase (Tph), a rate-limiting enzyme in serotonin biosynthesis16,17 and (2) genetic knockout of the Tph2 isoform. This dual approach provided specificity for the serotonin deficiency-associated biomarkers from nonspecific effects of either pCPA treatment or Tph2 knockout (Tph2-/-). Ultra-performance liquid chromatography - mass spectrometry was used to pilot novel biomarkers and metabolic pathways in both non-targeted and targeted metabolomics manners and elucidate the fine serotonin deficiency-associated molecular mechanisms.
Results
Non-targeted metabolic profiling of pCPA-treated mice
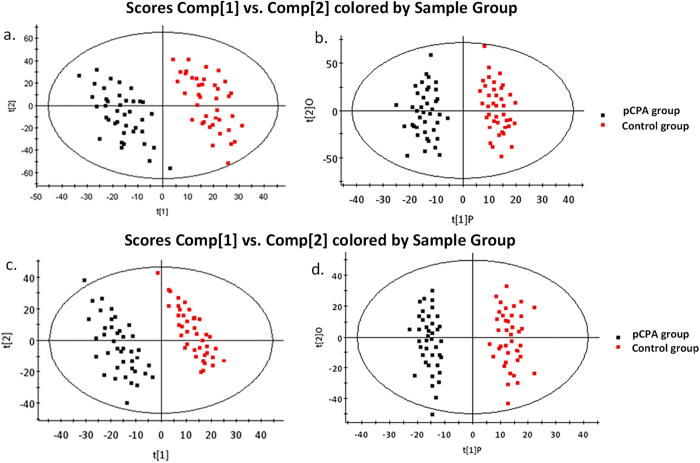
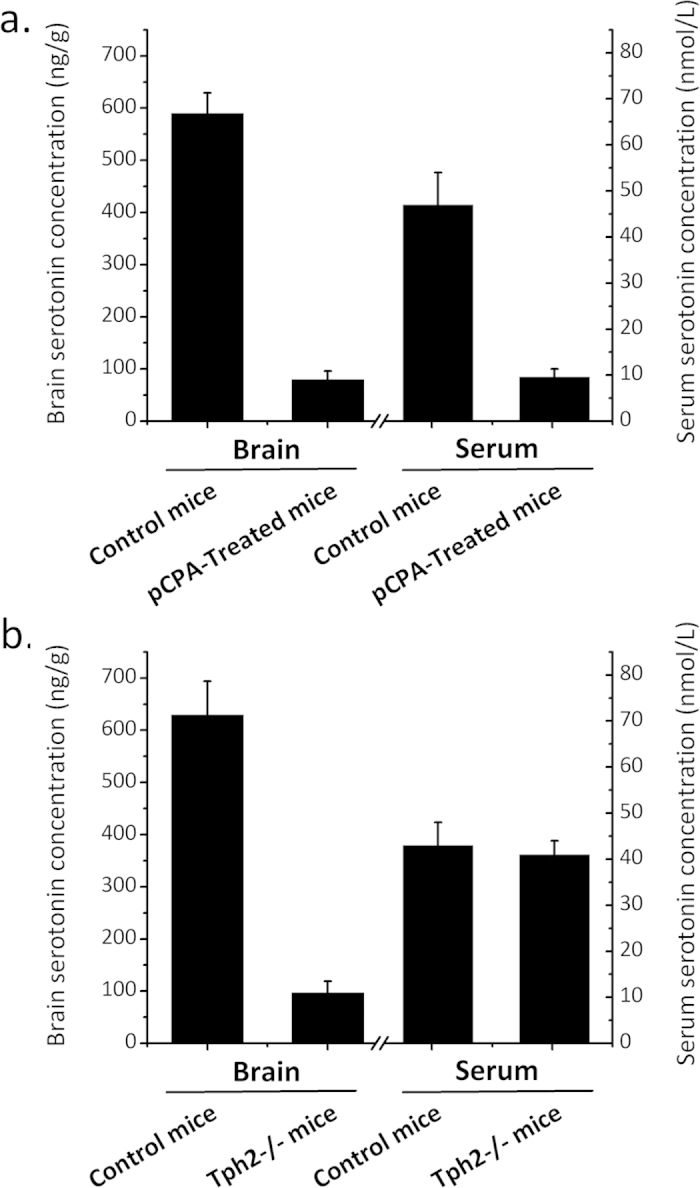
Mice were treated with 500 mg/kg pCPA or saline for three consecutive days. Serotonin levels were significantly reduced in both the brains and sera of the pCPA-treated mice compared with control mice (Fig. 1a). Non-targeted metabolomics approach using ultra-performance liquid chromatography - quadrupole time-of-flight mass spectrometry (UPLC-QTof-MS) was employed to identify potential biomarkers perturbed by the pharmacologically induced serotonin deficiency in the pCPA-treated mice. Principal component analysis (PCA) was performed to highlight key differences between the pCPA-treated and control mice (Fig. 2a,c). Orthogonal partial least squares discriminant analysis (OPLS-DA) further demonstrated a clear metabolic differentiation between the two sample groups (Fig. 2b,d). Variable importance for projection (VIP) scores were obtained for each metabolite based on its individual contribution to the statistical discrimination. In total, 33 preliminary metabolites were found to meet the selection criteria (VIP > 2.0, p-value < 0.05, fold change > 1.5) and selected for further characterization.
Figure 1. Brain and serum serotonin concentrations of the a) pCPA-treated mice (n = 10) and b) Tph2-/- mice (n = 10).
Data are expressed as mean values ± standard deviation.
Figure 2. Multivariate statistical analysis results of serum metabolites in the pCPA-treated mice (n = 40) and the control mice (n = 40).
a) PCA scores plot (R2X = 0.447, Q2 = 0.77) and b) OPLS-DA scores plot (R2X = 0.416, R2Y = 0.713, Q2 = 0.57) in ESI+ mode, c) PCA scores plot (R2X = 0.455, Q2 = 0.82) and d) OPLS-DA scores plot (R2X = 0.417, R2Y = 0.498, Q2 = 0.66) in ESI- mode.
Several unidentified metabolites also met the selection criteria and have been outlined in Supplementary Table S1 which included retention times, precursor ions (m/z), fold changes, p-values and VIP scores. Limited by the current metabolomics identification techniques, they haven’t been identified. But we do believe they are valuable and helpful for relevant researchers. We will keep working on them in further research.
Targeted pCPA dose-response study
A pCPA dose-response study was conducted to confirm the relationships between the 33 preliminary identified metabolites and the pCPA-induced serotonin deficiency. Four groups of mice were treated with variable pCPA doses and the resulting candidate biomarker levels were semi-quantitatively compared. The 33 candidate biomarkers were analyzed using a triple quadrupole mass spectrometer (QQQ-MS) operating under multiple reaction monitoring (MRM) mode. Twenty-one of these candidates were found to be affected by the pCPA treatment dose (Supplementary Figure S1). The 21 biomarkers were therefore considered to be directly correlated with serotonin deficiency and selected as the reliable biomarkers of serotonin deficiency. Specifically, up-regulated metabolites included kynurenine, kynurenate, 3-hydroxykynurenine, phenylalanine, hippurate, guanosine, hypoxanthine and lysoPCs, while down-regulated metabolites included serotonin, 5-hydroxyindoleacetate, tyrosine, xanthine, uric acid, citrate, oxoglutarate, succinate, creatinine, 3-indolepropionic acid and indoxyl sulfate (Fig. 3). Statistics for each compound have been summarized in Table 1.
Figure 3. Heat map denoting fold changes (over normalized means) of the 21 biomarkers in mice injected with increasing dosages of pCPA and the control mice (n = 10).
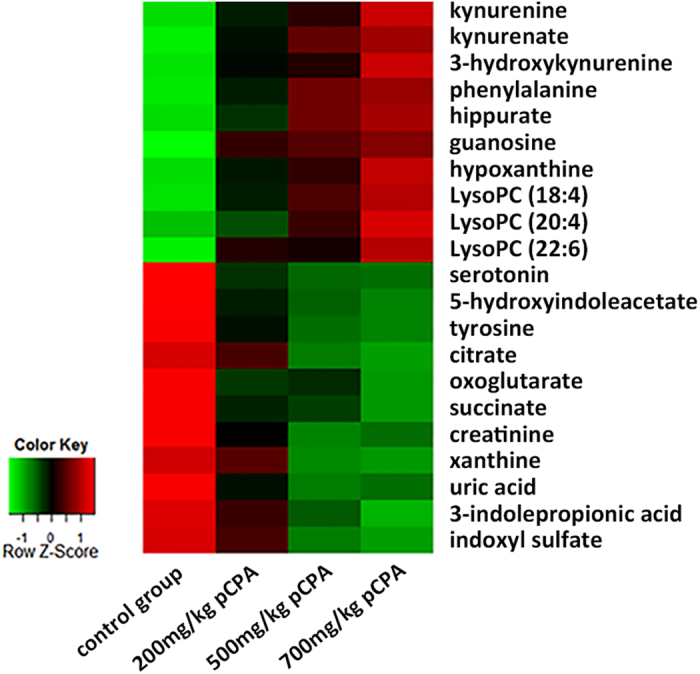
Columns correspond to different mice groups, and rows correspond to the altered metabolites. Shades of red represent elevated levels of metabolite, and shades of green represent reduced levels of the metabolites.
Table 1. Metabolites selected as biomarkers of pCPA-induced serotonin deficiency.
| Metabolite | HMDB ID | m/z | Representative MS/MS fragment ions (m/z)a | ESI+ |
ESI− |
||||
|---|---|---|---|---|---|---|---|---|---|
| Fold changeb | p valuec | VIPd | Fold changeb | p valuec | VIPd | ||||
| serotonin | HMDB00259 | 177.1009 | 160.0756, 132.0812, 115.0540* | −12.2 | 2.67 × 10−5 | 17.27 | |||
| 5-hydroxyindoleacetate | HMDB00763 | 192.0653 | 146.0595, 119.0484, 118.0656, 91.0566* | −4.5 | 9.24 × 10−6 | 12.76 | |||
| kynurenine | HMDB00684 | 209.0912 | 192.0653, 146.0596, 118.0650, 94.0656* | 2.8 | 1.28 × 10−5 | 9.21 | |||
| kynurenate | HMDB00715 | 190.0517 | 172.0407, 144.0455, 116.0511, 89.0402* | 1.8 | 4.98 × 10−5 | 8.34 | |||
| 3-hydroxykynurenine | HMDB00732 | 225.0866 | 190.0493, 162.0544, 110.0597* | 1.8 | 1.59 × 10−2 | 2.54 | |||
| phenylalanine | HMDB00159 | 166.0855 | 120.0806, 103.0549, 91.0542, 77.0396* | 2.3 | 6.29 × 10−6 | 19.22 | |||
| tyrosine | HMDB00158 | 182.0812 | 165.0548, 136.0753, 119.0491, 91.0541* | −1.6 | 5.22 × 10−4 | 6.52 | |||
| hippurate | HMDB00714 | 180.0650 | 105.0338, 77.0394, 51.0230* | 2.9 | 2.68 × 10−4 | 7.14 | 2.2 | 6.22 × 10−3 | 6.27 |
| creatinine | HMDB00562 | 114.0687 | 86.0741, 72.0480* | −3.7 | 9.22 × 10−3 | 4.87 | |||
| guanosine | HMDB00133 | 284.0983 | 152.0558, 135.0294, 110.0341* | 2.5 | 7.66 × 10−3 | 6.59 | |||
| hypoxanthine | HMDB00157 | 137.0457 | 119.0352, 110.0346, 94.0403, 55.0300* | 1.7 | 5.48 × 10−4 | 11.62 | |||
| 3-indolepropionic acid | HMDB02302 | 190.0857 | 130.0650, 103.0540, 77.0390, 55.0183* | −2.1 | 1.25 × 10−3 | 2.26 | |||
| lysoPC (18:4) | HMDB10389 | 516.3064 | 184.0730, 125.0001, 104.1067* | 2.1 | 3.64 × 10−4 | 6.77 | 1.5 | 4.32 × 10−5 | 5.79 |
| lysoPC (20:4) | HMDB10395/6 | 544.3387 | 184.0732, 104.1070, 86.0965* | 2.3 | 2.77 × 10−4 | 5.65 | 1.9 | 6.44 × 10−4 | 3.44 |
| lysoPC (22:6) | HMDB10404 | 568.3357 | 184.0730, 104.1068, 86.0966* | 1.8 | 6.43 × 10−5 | 3.27 | 1.7 | 1.42 × 10−3 | 5.67 |
| citrate | HMDB00094 | 191.0193 | 129.0187, 111.0088, 87.0089** | −2.0 | 5.12 × 10−5 | 7.27 | |||
| oxoglutarate | HMDB00208 | 145.0131 | 101.0235, 73.0293, 57.0347** | −1.7 | 8.22 × 10−3 | 6.63 | |||
| succinate | HMDB00254 | 117.0195 | 99.0086, 73.0298, 55.0191** | −1.6 | 1.29 × 10−2 | 6.94 | |||
| xanthine | HMDB00292 | 151.0253 | 108.0199, 80.0251, 65.9988** | −4.8 | 3.48 × 10−4 | 16.29 | |||
| uric acid | HMDB00289 | 167.0203 | 124.0147, 96.0204, 69.0100** | −6.3 | 6.22 × 10−5 | 23.64 | |||
| indoxyl sulfate | HMDB00682 | 212.0019 | 132.0451, 104.0502, 80.9653, 79.9576** | −3.3 | 7.22 × 10−3 | 9.54 | |||
aMetabolites labeled with * were confirmed by MS/MS in ESI+ mode, while metabolites labeled with ** were confirmed by MS/MS in ESI–mode.
bFold change was calculated from the arithmetic mean values of the pCPA group and the control group. Fold change with a positive value indicates a relatively higher concentration in the pCPA-treated mice, while a negative value indicates a relatively lower concentration compared with the control mice.
cP-values were determined by the Mann-Whitney U test.
dVIP denotes variable importance for projection where values larger than 2.00 reflects high contribution to the distinction between the pCPA group and the control group.
pCPA-induced pathway analysis
For the 21 biomarkers found to be associated with pCPA-induced serotonin deficiency, a metabolic pathway analysis was conducted using MetaboAnalyst 2.0. Using this approach, 13 specific metabolic pathways were found to be perturbed (Supplementary Figure S2, Supplementary Table S2). The individual contribution from each perturbed pathway was visualized by plotting the log p-value from the pathway enrichment analysis against the pathway impact valued obtained from the pathway topological analysis. Among them, the most relevant and important ones were phenylalanine, tyrosine and tryptophan biosynthesis, phenylalanine metabolism, tryptophan metabolism, citric acid cycle and purine metabolism. In addition to the Kyoto Encyclopedia of Genes and Genomes (KEGG) database-based pathway correlation results, changes in the citric acid cycle and serum creatinine may suggest possible disruption of energy metabolism. The up-regulated lysoPCs indicated the perturbation of the lipid metabolism while the changes of the gut microflora products indicated the gut microflora perturbation (Supplementary Table S3).
Non-targeted metabolic profiling of Tph2-/- mice
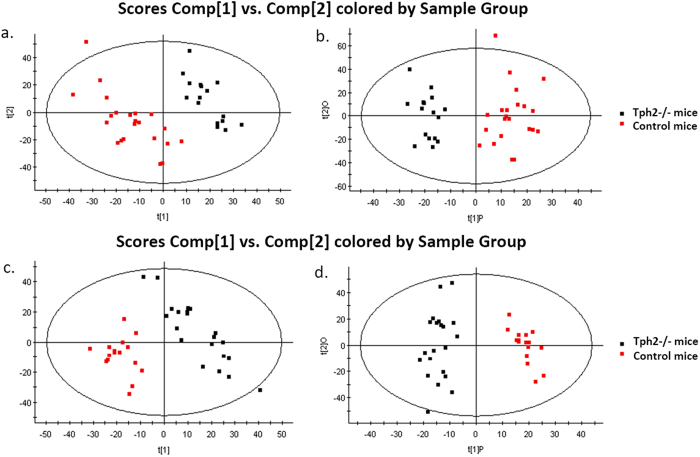
Non-targeted metabolic profiling using UPLC-QTof-MS was used to identify potential metabolites perturbed by the genetic serotonin deficiency in the Tph2-/- mice. The genetic knockout of the Tph2 isoform caused a significant decrease of brain serotonin levels although the serum serotonin concentrations remained unchanged (Fig. 1b). PCA and OPLS-DA demonstrated clear differences between the Tph2-/- mice and control mice (Fig. 4). A total of 17 identifiable metabolites were found to meet the selection criteria (VIP > 2.0, p-value < 0.05, fold change > 1.5). Multiple unidentified candidate biomarkers also met the selection criteria and have been described in Supplementary Table S4. Specifically, up-regulated metabolites included kynurenine, 3-hydroxykynurenine, xanthurenate, phenylalanine, hippurate, guanosine, hypoxanthine and lysoPCs, while down-regulated metabolites included 5-hydroxyindoleacetate, tyrosine, creatine, xanthosine, citrate, pyruvate, succinate and uric acid (Table 2).
Figure 4. Multivariate statistical analysis results of serum metabolites in the Tph2-/- mice (n = 20) and the control mice (n = 20).
a) PCA scores plot (R2X = 0.536, Q2 = 0.84) and b) OPLS-DA scores plot (R2X = 0.518, R2Y = 0.453, Q2 = 0.61) in ESI+ mode, c) PCA scores plot (R2X = 0.533, Q2 = 0.86) and d) OPLS-DA scores plot (R2X = 0.493, R2Y = 0.467, Q2 = 0.69) in ESI− mode.
Table 2. Metabolites selected as biomarkers in Tph2-/- mice.
| Metabolite | HMDB ID | m/z | Representative MS/MS fragment ions (m/z)a | ESI+ |
ESI− |
||||
|---|---|---|---|---|---|---|---|---|---|
| Fold changeb | p valuec | VIPd | Fold changeb | p valuec | VIPd | ||||
| 5-hydroxyindoleacetate | HMDB00763 | 192.0650 | 146.0594, 119.0482,118.0650, 91.0564* | −4.7 | 4.29 × 10−3 | 7.29 | |||
| kynurenine | HMDB00684 | 209.0914 | 146.0599, 118.0650, 94.0655* | 2.1 | 8.36 × 10−5 | 10.23 | |||
| 3-hydroxykynurenine | HMDB00732 | 225.0861 | 208.0591, 190.0493, 162.0544, 110.0597* | 2.3 | 9.37 × 10−4 | 4.12 | |||
| xanthurenate | HMDB00881 | 206.0442 | 188.0336, 160.0382, 132.0435* | 1.9 | 4.85 × 10−3 | 5.24 | |||
| phenylalanine | HMDB00159 | 166.0852 | 120.0806, 103.0537, 91.0542, 77.0396* | 3.1 | 3.68 × 10−4 | 11.86 | |||
| tyrosine | HMDB00158 | 182.0811 | 165.0548, 136.0753, 119.0494, 91.0541* | −2.4 | 2.41 × 10−2 | 3.29 | |||
| hippurate | HMDB00714 | 180.0650 | 105.0339, 77.0392, 51.0230* | 2.7 | 2.68 × 10−3 | 7.44 | 2.5 | 2.71 × 10−2 | 9.43 |
| creatine | HMDB00064 | 132.0774 | 90.0555, 87.0607, 72.0554* | −1.9 | 6.37 × 10−4 | 2.97 | |||
| xanthosine | HMDB00299 | 285.0834 | 153.0398, 136.0130, 110.0338* | −2.2 | 3.11 × 10−2 | 7.49 | – | ||
| guanosine | HMDB00133 | 284.0983 | 152.0558, 135.0294, 110.0341* | 3.1 | 4.97 × 10−3 | 6.50 | |||
| hypoxanthine | HMDB00157 | 137.0457 | 119.0351, 110.0348, 94.0403, 55.0297* | 2.8 | 7.24 × 10−3 | 9.21 | |||
| lysoPC (20:4) | HMDB10395/6 | 544.3387 | 184.0733, 104.1072, 86.0965* | 2.7 | 5.29 × 10−4 | 4.92 | |||
| lysoPC (22:4) | HMDB10401 | 572.3711 | 184.0730, 104.1071, 86.0964* | 1.9 | 8.95 × 10−3 | 2.38 | 2.0 | 3.14 × 10−2 | 4.28 |
| citrate | HMDB00094 | 191.0193 | 129.0184, 111.0088, 87.0090** | – | −2.6 | 5.46 × 10−3 | 8.73 | ||
| pyruvate | HMDB00243 | 87.0081 | 43.0185** | – | −1.7 | 1.28 × 10−2 | 3.66 | ||
| succinate | HMDB00254 | 117.0195 | 99.0086, 73.0300, 55.0190** | – | −2.4 | 8.34 × 10−4 | 9.24 | ||
| uric acid | HMDB00289 | 167.0203 | 124.0147, 96.0203, 69.0097** | – | −4.3 | 6.41 × 10−3 | 19.17 | ||
aMetabolites labeled with * were confirmed by MS/MS in ESI+ mode, while metabolites labeled with ** were confirmed by MS/MS in ESI− mode.
bFold change was calculated from the arithmetic mean values of the Tph2-/- mice and the control mice. Fold change with a positive value indicates a relatively higher concentration in the Tph2-/- mice, while a negative value indicates a relatively lower concentration compared with the control mice.
cP-values were determined by the Mann-Whitney U test.
dVIP denotes variable importance for projection where values larger than 2.00 reflects high contribution to the distinction between the Tph2-/- group and the control group.
Metabolic pathway analysis of Tph2-/- mice
Metabolic pathway analysis of the biomarkers of Tph2-/- mice identified 18 perturbed metabolic pathways (Supplementary Figure S3, Supplementary Table S5). Among them, the most relevant and important ones were phenylalanine, tyrosine and tryptophan biosynthesis, phenylalanine metabolism, citrate cycle, purine metabolism and tryptophan metabolism, which have also been perturbed in the pCPA-treated mice. Changes in the citric acid cycle and serum creatine also suggest possible disruption of energy metabolism while the up-regulated lysoPCs indicated the perturbation of the lipid metabolism (Supplementary Table S6).
Metabolic pathway network construction
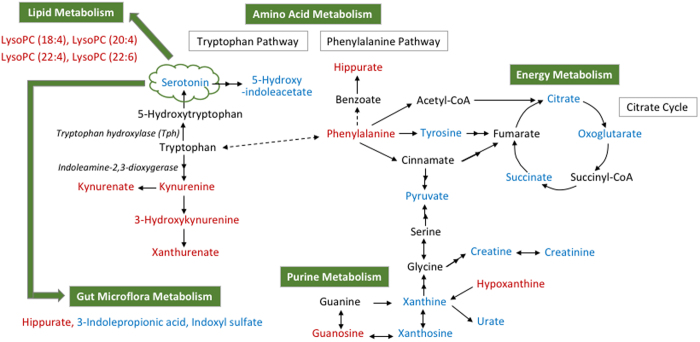
Based on the overall 26 biomarkers identified in the pCPA-treated and Tph2-/- mice, metabolic pathway analysis suggested the perturbation of phenylalanine, tyrosine and tryptophan biosynthesis, phenylalanine metabolism, citrate cycle, tryptophan metabolism and purine metabolism, among others (Supplementary Table S7). Visualized pathway analysis has been summarized in Supplementary Figure S4. Overall, the serotonin deficiency-associated biomarkers and perturbed pathways were integrated and broadly categorized to demonstrate the perturbation of amino acid, energy, purine, lipid, and gut microflora metabolisms (Supplementary Table S8, Fig. 5).
Figure 5. An overview of the integrated metabolic pathway network in response to serotonin deficiency.
Red-labeled metabolites indicate up-regulation in mice with serotonin deficiency, while blue-labeled metabolites indicate the down-regulation compared with the control mice. Metabolite relationships were derived from HMDB and KEGG databases. Solid arrows represent direct metabolic reactions, and dashed arrows represent multiple reactions and indirect connections between two metabolites.
ROS, MDA, T-AOC, SOD, CAT and GPx activity alterations
Reactive oxygen species (ROS) production and malondialdehyde (MDA) levels were significantly increased in both the pCPA-treated mice and Tph2-/- mice compared with control mice (p-value < 0.05) (Table 3). To further characterize the antioxidant status in the serotonin deficient mice, the total antioxidant capacity (T-AOC) levels were measured and found to be decreased (p-value < 0.05) (Table 3). A decrease in the activities of all measured antioxidant enzymes, including superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx) was also obseved (p-value < 0.05) (Table 3).
Table 3. Antioxidant activities in serotonin deficient mice.
| Oxidants | pCPA-treated mice |
Tph2-/- mice |
||
|---|---|---|---|---|
| Control group (n = 20) | pCPA-treated group (n = 20) | Control group (n = 20) | Tph2-/- group (n = 20) | |
| ROS (1/mgpr) | 17.61 ± 1.99 | 29.84 ± 3.21* | 20.46 ± 2.21 | 34.62 ± 2.11* |
| MDA (μmol/L) | 2.24 ± 0.44 | 3.16 ± 0.88* | 2.03 ± 0.36 | 3.38 ± 1.12* |
| T-AOC (mmol/L FeSO4) | 1.32 ± 0.14 | 0.94 ± 0.07* | 1.62 ± 0.21 | 0.72 ± 0.11* |
| SOD (U/mL) | 1.14 ± 0.10 | 0.72 ± 0.04* | 1.44 ± 0.11 | 0.62 ± 0.07* |
| CAT (U/mL) | 0.16 ± 0.024 | 0.13 ± 0.0088* | 0.14 ± 0.011 | 0.079 ± 0.0084* |
| GPx (U/mL) | 2.72 ± 0.33 | 1.82 ± 0.094* | 2.46 ± 0.19 | 1.64 ± 0.19* |
ROS, reactive oxygen species; MDA, malondialdehyde; T-AOC, total antioxidant capacity; SOD, superoxide dismutase; CAT, catalase; GPx, glutathione peroxidase.
Data are expressed as mean values ± standard deviation.
*p-value < 0.05 compared with control group. P-values were determined by the Mann-Whitney U test.
Discussion
The wide-ranging importance of serotonin to physiological, neuropsychological and behavioral processes underscores the critical need to understand the molecular mechanisms of serotonin deficiency. Indeed, primates with low serotonergic activity were reported to exhibit behaviors indicative of impaired impulse control, unrestrained aggression, social isolation and low social dominance18. Serotonin deficiency is also seen in a wide range of disease, including depression19,20, Alzheimer’s disease21,22, Parkinson’s disease23,24 and schizophrenia12,25, among others. The current study therefore sought to identify novel biomarkers associated with serotonin deficiency to better understand how serotonin deficiency may broadly impact various metabolic pathways under pathological conditions.
Serotonin deficiency was achieved either by the pharmacological inhibition of Tph or genetic knockout of the Tph2 isoform. Notably, Tph has two isoforms that exhibit non-overlapping distribution pattern26. Tph1 is expressed in the periphery and pineal gland while Tph2 is expressed exclusively in the CNS19. Hence the pCPA treatment would inhibit both Tph2 and Tph1 activity thus affecting both the periphery and CNS serotonin levels. Meanwhile, the knockout of Tph2 isoform would specifically affect CNS serotonin synthesis in the Tph2-/- mice. Indeed, the pCPA treatment caused a significant decrease in both the brain and serum serotonin levels while the knockout of Tph2 only caused a significant decrease of brain serotonin levels in the Tph2-/- mice. Perturbed metabolites observed in both mice models would indicate metabolic dysregulation that originated from CNS serotonin deficiency. This dual model approach minimizes the risk of incorrectly assigning statistically significant metabolites as perturbed serotonin deficiency-associated biomarkers that may actually arise from nonspecific effects of either model.
Non-targeted metabolic profiling of pCPA-treated mice revealed 33 preliminary biomarkers of serotonin deficiency following rigorous multivariate statistical data analyses. By means of a quantitatively targeted pCPA dose-response study, 21 of the above preliminary biomarkers were confirmed having response to pCPA dose, indicating a causative relationship between the 21 biomarkers and serotonin deficiency. Metabolic pathway analysis suggested that these metabolites were associated with 13 specific pathways and most notably the phenylalanine, tyrosine and tryptophan biosynthesis, phenylalanine metabolism, tryptophan metabolism, citric acid cycle and purine metabolism. Pathway correlation also suggested disruption of energy metabolism, lipid metabolism and gut microflora metabolism. Furthermore, the non-targeted metabolomics of the Tph2-/- mice yielded 17 dysregulated metabolites which similarly corresponded with the phenylalanine, tyrosine and tryptophan biosynthesis, phenylalanine metabolism, tryptophan metabolism, citric acid cycle, purine metabolism as well as energy metabolism and lipid metabolism. In total, 26 unique metabolites were found altered in the pCPA-treated and Tph2-/- serotonin deficient mice. These newly identified biomarkers and their associated metabolic pathways constructed the novel serotonin deficiency-affected metabolic pathway network that described the metabolic implications of serotonin deficiency. The metabolic pathway network has been summarized in Fig. 5.
Briefly, tryptophan is an essential amino acid which is metabolized by the serotonin and kynurenine pathways. Importantly, serotonin biosynthesis involves the hydroxylation of tryptophan to 5-hydroxytryptophan (5-HTP) via Tph followed by decarboxylation of 5-HTP to serotonin via aromatic L-amino acid decarboxylase. The Tph-mediated hydroxylation reaction is the rate-limiting step of the serotonin biosynthesis process. Serotonin is then further metabolized to 5-hydroxyindoleacetate and melatonin, among others. In this study where Tph was either pharmacologically inhibited or genetically lacking, serotonin levels significantly decreased. This in turn led to the decrease of 5-hydroxyindoleacetate, one of the downstream serotonin metabolites.
In the kynurenine pathway, kynurenine is synthesized from tryptophan via indoleamine 2, 3-dioxygenase and further metabolized to kynurenate, 3-hydroxykynurenine, 3-hydroxykynurenamine, xanthurenate and others27. The elevated levels of kynurenine, kynurenate, 3-hydroxykynurenine and xanthurenate observed in this study indicated activation of the kynurenine pathway under serotonin deficient conditions. This observation may be the result of two competitive pathways for tryptophan where inhibition of one causes the other to experience an apparent activation. Indeed, enhanced indoleamine 2, 3-dioxygenase activity have been observed in patients with depression and anxiety28,29. Similarly, increased kynurenine levels have been reported in highly depressed patients30, while elevated kynurenate levels were found in the cerebrospinal fluid31 and cortical32 of patients with schizophrenia. In addition, the downstream metabolite 3-hydroxykynurenine has also been widely reported to be increased in multiple serotonin deficiency-related neurodegenerative disorders including Alzheimer’s disease, Parkinson’s disease and Huntington’s disease33.
The increased levels of phenylalanine and hippuric acid coupled with decreased tyrosine concentrations observed in this study indicated perturbed phenylalanine metabolism. Specifically, tyrosine is synthesized from phenylalanine via phenylalanine hydroxylase. The simultaneous increase in phenylalanine and decrease in tyrosine suggested decreased phenylalanine hydroxylase activity. This conclusion appeared to agree with previously reported elevation of plasma phenylalanine-tyrosine ratios in depressed patients34 as well as decreased levels of tetrahydrobiopterin, which is an essential cofactor of phenylalanine hydroxylase, in patients with depression35, schizophrenia and schizoaffective disorder36.
Decreased levels of citrate, oxoglutarate, succinate and pyruvate in serotonin deficient mice suggested deactivation of the citrate cycle, which represents a critical energy metabolism pathway that involves the oxidation of carbohydrates, fats and proteins. Notably, the citrate cycle occurs in the mitochondrial matrix, which is particularly sensitive to free radical damage based on its role as the primary source for intracellular free radicals37. Our oxidative stress data indicated a significantly 70% ROS increase as well as a 40%–60% MDA increase in the pCPA-treated and Tph2-/- mice. Considerably decreased antioxidant capacities measured from T-AOC, SOD, CAT and GPx assays were also noted. These results implied that serotonin deficiency was related to systemically increased oxidative stress that may extend to deregulated citrate cycle metabolism. Increased cellular oxidative stress and mitochondrial dysfunctions have been similarly reported in several diseases associated with serotonin deficiency, including schizophrenia38, Parkinson’s disease and Alzheimer’s disease39. Decreased levels of creatine and creatinine in the serotonin deficient mice may also indicate altered energy metabolism. Specifically, these two compounds are the downstream products of creatine phosphate, an important cellular energy carrier. These findings were in accordance with previous reports that serotonin is an important energy regulator within the body40,41. Serotonin deficiency induced energy metabolism imbalances were also observed in depression42.
Purine metabolism deactivation was noted by decreased xanthine, xanthosine and uric acid concentrations coupled with increased guanosine and hypoxanthine levels. Given that purine metabolism is a major metabolism of a homeostatic response of mitochondria to oxidative stress, the disturbance of the mitochondria may lead to the perturbation of purine metabolism, which is also reported in schizophrenia43. Specifically, uric acid is an end product of purine metabolism, mainly synthesized from adenine- and guanine-based purines by the enzyme xanthine oxidase. Low levels of uric acid have been associated with a wide range of diseases including depression44, schizophrenia45, Alzheimer’s disease46 and Parkinson’s disease15,47,48.
In addition, increased levels of lysoPC (18:4), lysoPC (20:4), lysoPC (22:4) and lysoPC (22:6) were observed in mice with serotonin deficiency. Lysophosphatidylcholines (LysoPCs) are the products of phosphatidylcholines (PCs) via enzymatic action of phospholipase A2 (PLA2). Under oxidative stress, lipid peroxidation occurs, evidenced by increased MDA levels observed in both pCPA-treated mice and Tph2-/- mice. As a response, the PLA2 activity is enhanced, giving rise to the increase of the lysoPC levels49.
Finally, the gut microflora in mice treated with pCPA were found to be perturbed, evidenced by an increase in hippuric acid and a decrease in 3-indolepropionic acid and indoxyl sulfate. Hippuric acid is metabolized from benzoic acid, which is metabolized from the dietary polyphenol 3-hydroxyphenyl propionic acid by the gut microflora50. Because the mice were provided a constant diet, changes to hippuric acid were likely the result of gut microbiome perturbation. 3-Indolepropionic and indoxyl sulfate are both metabolites of tryptophan by gut microflora. While peripheral serotonin is an important gastrointestinal signaling molecule that specifically functions as a sensory transducer and a paracrine messenger in the gut5, the perturbed gut microflora metabolism may be the result of the peripheral serotonin deficiency in the pCPA-treated mice.
In summary, we used both the non-targeted and targeted metabolomic approach to identify novel biomarkers perturbed by serotonin deficiency and to elucidate the affected metabolic pathways. Serotonin deficiency was achieved either by the pharmacological inhibition of Tph or genetic knockout of the Tph2 isoform. A total of 26 unique metabolites were observed to be dysregulated in the serotonin deficient mice. These findings indicated serotonin deficiency affected tryptophan metabolism, phenylalanine metabolism, energy metabolism, purine metabolism, lipid metabolism and gut microflora metabolism. Further evidence of oxidative stress may provide a mechanistic basis for some of the dysregulated pathways. In-depth study should focus on using these findings as a platform to elucidate specific pathophysiological mechanisms of serotonin deficiency in relation to these metabolic pathway perturbations.
Methods
Chemicals and reagents
Formic acid (HPLC grade) was purchased from Dikma Technologies Inc. (Lake Forest, CA, USA). Ultra-pure water was obtained from Hangzhou Wahaha Group Co., Ltd. (Zhejiang, China). Methanol (HPLC grade), acetonitrile (HPLC grade), chloroform (HPLC grade), isopropanol (HPLC grade), Tween, pCPA, serotonin hydrochloride, 5-hydroxyindoleacetate, kynurenine, kynurenate, 3-hydroxykynurenine, phenylalanine, tyrosine, sodium hippurate hydrate, creatinine, guanosine, hypoxanthine, 3-indolepropionic acid, citrate, oxoglutarate, succinate, xanthine, uric acid and indoxyl sulfate potassium salt were purchased from Sigma-Aldrich (St. Louis, MO, USA). T-AOC assay kit with Ferric Reducing Ability of Plasma (FRAP) method, SOD assay kit, total GPx assay Kit and lipid peroxidantion MDA assay kit were purchased from Beyotime Institute of Biotechnology (Jiangsu, China). CAT assay kit and reactive oxygen species assay kit were obtained from Nanjing Jiancheng Bioengineering Institute (Jiangsu, China).
pCPA treatment
Vertebrate experiments were approved by the institutional review board, the Institutional Animal Care and Use Committee of Peking University and all experiments were carried out in accordance with the approved guidelines and regulations. Animal studies were conducted at a AAA-certified animal facility at the Laboratory Animal Center of Peking University (LAC-PKU). Adult C57BL/6 J male mice between 11 and 13 weeks old were acquired from the Vital River Laboratories (Beijing, China) and housed in the LAC-PKU. The mice were housed in a controlled environment at 22–24 °C and a relative humidity of 40%–60%, and supplemented with 12 h light/dark cycles. Food and tap water were given ad libitum. Mice were housed individually for one week prior to the experiment to ensure adequate adjustment time.
The pCPA treatment procedures used in this study have been described elsewhere51, but a few minor modifications were made in the present study. Specifically, mice were randomly divided into pCPA and saline control groups, each comprising 40 samples. Mice of the pCPA group were injected intraperitoneally with 500 mg/kg pCPA per day for three consecutive days, while the control group received saline solution instead. In the pCPA dose-response study, a new batch of 40 mice were randomly divided into four groups and injected intraperitoneally with either 1) saline, 2) 200 mg/kg pCPA, 3) 500 mg/kg pCPA or 4) 700 mg/kg pCPA. pCPA was suspended in 1% Tween saline.
Tph2-/- mice
Tph2-/- mice were provided by Prof. Yi Rao, generated and genotyped as previously described51,52,53. Mice were housed individually in a controlled environment at 22–24 °C and a relative humidity of 40%–60%, and supplemented with 12 h light/dark cycles. Food and tap water were given ad libitum. All mice used were between 12 and 16 weeks old.
Sample collection
Mice were anesthetized with pentobarbital sodium followed by blood collection from the orbital sinus at 9 am to 10 am. Upon one hour of sitting at room temperature, the blood was centrifuged at 3000 xg to extract sera. All serum samples were then immediately stored at −80 °C. After blood collection, mice were euthanized immediately and the brains were obtained on ice.
Determination of brain and serum serotonin concentrations in pCPA-treated and Tph2-/- mice
Brain and serum serotonin concentrations of both the pCPA-treated mice and Tph2-/- mice were determined by UPLC-QTof-MS. Mice brain were weighed and homogenized in cold methanol (−20 °C) (4 mL methanol per gram of brain tissue) while 100 μL serum samples were mixed with 400 μL cold methanol. The homogenate or the mixture was then centrifuged at 10,000 xg for 15 min. The supernatant was dried under nitrogen and resuspended with 150 μL pure water, 120 μL chloroform and 30 μL isopropanol. After centrifugation, the upper aqueous layer was injected into the UPLC-QTof-MS system for analysis. UPLC-QTof-MS analysis was performed under the same conditions used in the non-targeted metabolic profiling. The serotonin concentrations were calculated using the Waters Masslynx software (version 4.1) based on the standard sample.
UPLC-QTof-MS analysis
Prior to analysis, the serum samples were naturally thawed at 4 °C. A total of 100 μL serum was added into 400 μL methanol (−20 °C) and the mixture was vortexed vigorously to precipitate protein. After centrifugation, the supernatant was transferred to a 1.5 mL auto-sampler vial for analysis.
A quality control (QC) sample was prepared by mixing equal volumes (10 μL) from each serum sample. This “pooled” sample was used to estimate a “mean” profile representing all the analytes encountered during analysis54.
UPLC analysis was performed on an ACQUITY UPLC HSS T3 column (100 mm × 2.1 mm, 1.8 μm; Waters Corp., Dublin, Ireland) using an ACQUITY UPLC (Waters Corp., Milford, USA). A 5 μL injection was made onto the column, which was maintained at 30 °C and eluted with A) water (0.1% (v/v) formic acid, 2% (v/v) acetonitrile) and B) acetonitrile (0.1% (v/v) formic acid) at a flow rate of 0.3 mL/min for 17 min. The gradient duration program was as follows: 0–3 min, 0% B; 3–5 min, 0–30% B; 5–12 min, 30–100% B; 12–17 min, 100% B and re-equilibrated with 0% B for 3 min.
MS analysis was conducted using a Xevo QTof mass spectrometer (Waters, Manchester, UK), operating in both positive (ESI +) and negative (ESI–) electrospray ionization modes. The parameters were as follows: mass range, 50–1,000 Da; scan time, 0.3 s; capillary voltage, 3.0 kV (ESI+)/2.6 kV (ESI–); sample cone voltage, 30 V; extraction cone voltage, 4.0 V; source temperature, 120 °C; desolvation temperature, 450 °C; cone gas flow, 40 L/h; desolvation gas flow, 700 L/h. The data were acquired in MSE mode, in which the centroid MS spectra and the auto MS/MS spectra with collision energies ramping from 10–40 eV were acquired simultaneously. Leucine-enkephalin (278.1141 Da, 556.2771 Da in ESI+ mode and 236.1035 Da, 554.2615 Da in ESI− mode) was used as a lock mass standard to ensure real-time accuracy.
The pooled “QC” sample was injected six times at the beginning of the analysis batch to ensure system equilibrium and then every 10 samples to further monitor the analysis stability55,56. All samples were injected randomly in the batch. Six metabolites with different m/z values and polarities were selected for quality control/quality assurance purposes under ESI+ and ESI− modes separately. The metabolite retention times and selected mass to charge ratios have been tabulated in Supplementary Table S9, indicating good system stability and repeatability.
UPLC-QTof-MS data processing
The data were analyzed using Waters Masslynx and Makerlynx XS software (version 4.1). Raw data were deconvoluted, aligned, normalized and assembled into a data matrix. Data were aligned with a 0.01 Da mass tolerance and a retention time window tolerance of 0.1 min, followed by filtering using the “80% rule”57. Then the data matrix was mean-centered, pareto-scaled and analyzed by PCA and OPLS-DA. Metabolites with VIP scores larger than 2 were considered to be statistically significant. In addition to VIP scores, Mann-Whitney U tests were used to compare metabolite levels between the pCPA-treated/Tph2-/- mice and control mice using SPSS 20.0. Finally, Fold changes were calculated from the arithmetic mean values of the pCPA-treated/Tph2-/- and control groups.
UHPLC-QQQ-MS based dose-response study
The pCPA dose-response study was performed using an Agilent 1290 Infinity LC system (Agilent Technologies, Santa Clara, CA) coupled with an Agilent 6460 Triple Quadrupole mass spectrometer (Agilent Technologies, Santa Clara, CA). UHPLC analysis was also performed with the ACQUITY UPLC HSS T3 column (100 mm × 2.1 mm, 1.8 μm; Waters Corp., Dublin, Ireland) at 30 °C. The gradient duration program was the same as that used in the UPLC-QTof-MS analysis.
The ultra-high performance liquid chromatography-triple quadrupole mass spectrometry (UHPLC-QQQ-MS) analysis was performed under MRM mode. The parameters were as follows: dry gas temperature, 350 °C; dry gas flow, 10 L/min; sheath gas temperature, 350 °C; sheath gas flow, 10 L/min, nebulizer pressure, 40 psi; capillary entrance voltage, 4000 V. Optimized precursor/fragment ions and collision energies were as follows, positive ion mode: 114.1 → 86.1, 10 eV; 137.0 → 110.0, 30 eV; 160.1 → 117.1, 40 eV; 166.1 → 120.1, 20 eV; 177.1 → 160.1, 20 eV; 180.1 → 105.0, 10 eV; 182.1 → 123.0, 30 eV; 182.1 → 136.1, 10 eV; 190.1 → 130.1, 40 eV; 190.1 → 144.0, 20 eV; 192.1 → 146.1, 30 eV; 209.1 → 146.1, 20 eV; 225.1 → 110.1, 10 eV; 284.1 → 152.1, 30 eV; 325.0 → 97.0, 30 eV; 364.1 → 152.1, 40 eV; 514.3 → 184.1, 30 eV; 516.3 → 462.3, 20 eV; 542.3 → 184.1, 30 eV; 566.3 → 184.1, 30 eV; negative ion mode: 117.0 → 73.0, 20 eV; 145.0 → 101.0, 10 eV; 151.0 → 108.0, 20 eV; 152.1 → 122.0, 20 eV; 167.0 → 124.0, 10 eV; 180.1 → 119.1, 30 eV; 191.0 → 87.0, 20 eV; 199.2 → 176.6, 30 eV; 199.0 → 91.1, 20 eV; 212.0 → 80.0, 30 eV; 355.1 → 191.1, 20 eV; 391.3 → 283.3, 40 eV; 407.3 → 343.3, 40 eV. Data were analyzed using the MassHunter Workstation Software (version B.06.00).
Metabolite identification
Selected biomarkers were initially compared with comprehensive online databases (METLIN: http://metlin.scripps.edu/, HMDB: http://www.hmdb.ca/, KEGG: http://www.kegg.com/, LIPIDMAPS: http://www.lipidmaps.org/ and MassBank: http://www.massbank.jp/) using exact m/z values and MS/MS fragments. The identification information was then confirmed by chemical standards with the exact m/z values, MS/MS fragments and retention times. MS/MS spectra were acquired with collision energies of 10, 20 and 40 eV.
Metabolic pathway analysis (MetPA) by MetaboAnalyst
Metabolic Pathway Analysis (MetPA) by MetaboAnalyst 2.0 (www.metaboanalyst.ca/) was used to sort the significantly altered metabolites associated with serotonin deficiency into biologically relevant metabolic pathways. MetPA is a web-based application that combines pathway enrichment analysis and network topological analysis, to aid in the visualization of metabolomics data within the biological context of metabolic pathways58,59. Notably, the MetPA database includes metabolic pathways encompassing 21 model organisms including the mice used in this study.
Determination of T-AOC, SOD, CAT, GPx, ROS and MDA levels and activities
The experimental procedures for determining the T-AOC, SOD, CAT, GPx, ROS and MDA activities were based on the protocols provided by Beyotime Institute of Biotechnology and Nanjing Jiancheng Bioengineering Institute. The T-AOC levels, MDA levels, SOD, CAT and GPx activities were determined using mice serum while ROS production was measured using brain. T-AOC was measured by reduction of Fe3+ -TPTZ complex to the ferrous form Fe2+ -TPTZ. SOD activity was determined using the xanthine/xanthine oxidase method. MDA activity was measured by analyzing the reaction of MDA with thiobarbituric acid (TBA), which forms an MDA-TBA adduct that absorbs strongly at 535 nm. Protein content was measured by the Braford method60 using bovine serum albumin (BSA) as a standard protein. All data were presented as mean ± standard deviation (SD). Statistical analysis was performed with a one-way analysis of variance (ANOVA) using SPSS 20.0. Results with p-values less than 0.05 were considered statistically significant. All experiments were repeated at least three times.
Additional Information
How to cite this article: Weng, R. et al. Metabolomics Approach Reveals Integrated Metabolic Network Associated with Serotonin Deficiency. Sci. Rep. 5, 11864; doi: 10.1038/srep11864 (2015).
Supplementary Material
Acknowledgments
We thank Prof. Yi Rao and Dr. Yan Liu for providing the Tph2-/- mice; Dr. Rui Wu for discussions on data processing; Prof. Yan Zhang and Dr. Xiaqin Sun for the assistance of the pCPA treatment. This research is supported by the National Natural Science Foundation of China (No. 21322505 and 21175008) and the Ministry of Science and Technology of China (No. 2012YQ09019409 and 2013YQ510391). Casey Burton received funding through a National Science Foundation Graduate Research Fellowship Award No. #DGE-1011744.
Footnotes
Author Contributions R.W., Y.B. and H.L. designed the research; R.W., X.X. and Y.L. collected the data; Y.T. conducted the vertebrate experiments; R.W. and S.S. analyzed the data and wrote the manuscript; C.B., C.C. and Y.B. contributed to the discussion and reviewed the manuscript.
References
- Berger M., Gray J. A. & Roth B. L. The Expanded Biology of Serotonin. Annu. Rev. Med. 60, 355–366 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegebaum C., Gutknecht L., Schmitt A., Lesch K. P. & Reif A. Serotonin now: Part 1. Neurobiology and developmental genetics. Fortschr. Neurol. Psychiatr. 78, 319–331 (2010). [DOI] [PubMed] [Google Scholar]
- Kiser D., SteemerS B., Branchi I. & Homberg J. R. The reciprocal interaction between serotonin and social behaviour. Neurosci. Biobehav. Rev. 36, 786–798 (2012). [DOI] [PubMed] [Google Scholar]
- Nichols D. E. & Nichols C. D. Serotonin receptors. Chem. Rev. 108, 1614–1641 (2008). [DOI] [PubMed] [Google Scholar]
- Gershon M. D. & Tack J. The serotonin signaling system: From basic understanding to drug development-for functional GI disorders. Gastroenterology 132, 397–414 (2007). [DOI] [PubMed] [Google Scholar]
- Hayes D. J. & Greenshaw A. J. 5-HT receptors and reward-related behaviour: A review. Neurosci. Biobehav. Rev. 35, 1419–1449 (2011). [DOI] [PubMed] [Google Scholar]
- Gray J. A. & Roth B. L. The pipeline and future of drug development in schizophrenia. Mol. Psychiatr. 12, 904–922 (2007). [DOI] [PubMed] [Google Scholar]
- Rosell D. R. et al. Increased serotonin 2A receptor availability in the orbitofrontal cortex of physically aggressive personality disordered patients. Biol. Psychiatry 67, 1154–1162 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte A. V. et al. Aggression is related to frontal serotonin-1A receptor distribution as revealed by PET in healthy subjects. Hum. Brain Mapp. 30, 2558–2570 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivier B. & van Oorschot R. 5-HT(1B) receptors and aggression: A review. Eur. J. Pharmacol. 526, 207–217 (2005). [DOI] [PubMed] [Google Scholar]
- Sari Y. Serotonin(1B) receptors: from protein to physiological function and behavior. Neurosci. Biobehav. Rev. 28, 565–582 (2004). [DOI] [PubMed] [Google Scholar]
- Johnson C. H. & Gonzalez F. J. Challenges and opportunities of metabolomics. J. Cell. Physiol. 227, 2975–2981 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia H. M. et al. Integration of H-1 NMR and UPLC-Q-TOF/MS for a comprehensive urinary metabonomics study on a rat model of depression induced by chronic unpredictable mild stress. Plos One 8, e63624 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J. et al. Potential metabolite markers of schizophrenia. Mol. Psychiatr. 18, 67–78 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanov M. et al. Metabolomic profiling to develop blood biomarkers for Parkinson’s disease. Brain 131, 389–396 (2008). [DOI] [PubMed] [Google Scholar]
- Koe B. K. & Weissman A. p-Chlorophenylalanine - a specific depletor of brain serotonin. J. Pharmacol. Exp. Ther. 154, 499–516 (1966). [PubMed] [Google Scholar]
- Jequier E., Lovenber. W. & Sjoerdsm. A. Tryptophan hydroxylase inhibition - mechanism by which p-chlorophenylalanine depletes rat brain serotonin. Mol. Pharmacol. 3, 274–278 (1967). [PubMed] [Google Scholar]
- Higley J. D. & Linnoila M. Low central nervous system serotonergic activity is traitlike and correlates with impulsive behavior - A nonhuman primate model investigating genetic and environmental influences on neurotransmission. Ann. N.Y. Acad. Sci. 836, 39–56 (1997). [DOI] [PubMed] [Google Scholar]
- Jacobsen J. P. R., Medvedev I. O. & Caron M. G. The 5-HT deficiency theory of depression: Perspectives from a naturalistic 5-HT deficiency model, the tryptophan hydroxylase 2(Arg)439(His) knockin mouse. Philos. Trans. R. Soc. B-Biol. Sci. 367, 2444–2459 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badawy A. A. B. Tryptophan: The key to boosting brain serotonin synthesis in depressive illness. J. Psychopharmacol. 27, 878–893 (2013). [DOI] [PubMed] [Google Scholar]
- Rodriguez J. J., Noristani H. N. & Verkhratsky A. The serotonergic system in ageing and Alzheimer’s disease. Prog. Neurobiol. 99, 15–41 (2012). [DOI] [PubMed] [Google Scholar]
- Meltzer C. C. et al. Serotonin in aging, late-life depression, and Alzheimer’s disease: The emerging role of functional imaging. Neuropsychopharmacology 18, 407–430 (1998). [DOI] [PubMed] [Google Scholar]
- Huot P. & Fox S. H. The serotonergic system in motor and non-motor manifestations of Parkinson’s disease. Exp. Brain Res. 230, 463–476 (2013). [DOI] [PubMed] [Google Scholar]
- Tan S. K. H., Hartung H., Sharp T. & Temel Y. Serotonin-dependent depression in Parkinson’s disease: A role for the subthalamic nucleus? Neuropharmacology 61, 387–399 (2011). [DOI] [PubMed] [Google Scholar]
- Craven R. M., Priddle T. H., Cooper S. J., Crow T. J. & Esiri M. M. The dorsal raphe nucleus in schizophrenia: a post mortem study of 5-hydroxytryptamine neurones. Neuropathol. Appl. Neurobiol. 31, 258–269 (2005). [DOI] [PubMed] [Google Scholar]
- Gutknecht L., Kriegebaum C., Waider J., Schmitt A. & Lesch K. P. Spatio-temporal expression of tryptophan hydroxylase isoforms in murine and human brain: convergent data from Tph2 knockout mice. Eur Neuropsychopharmacol 19, 266–282 (2009). [DOI] [PubMed] [Google Scholar]
- Schwarcz R. & Pellicciari R. Manipulation of brain kynurenines: Glial targets, neuronal effects, and clinical opportunities. J. Pharmacol. Exp. Ther. 303, 1–10 (2002). [DOI] [PubMed] [Google Scholar]
- Schiepers O. J. G., Wichers M. C. & Maes M. Cytokines and major depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 29, 201–217 (2005). [DOI] [PubMed] [Google Scholar]
- Takikawa O. Biochemical and medical aspects of the indoleamine 2,3-dioxygenase-initiated L-tryptophan metabolism. Biochem. Biophys. Res. Commun. 338, 12–19 (2005). [DOI] [PubMed] [Google Scholar]
- Capuron L. et al. Interferon-alpha-induced changes in tryptophan metabolism: Relationship to depression and paroxetine treatment. Biol. Psychiatry 54, 906–914 (2003). [DOI] [PubMed] [Google Scholar]
- Erhardt S. et al. Kynurenic acid levels are elevated in the cerebrospinal fluid of patients with schizophrenia. Neurosci. Lett. 313, 96–98 (2001). [DOI] [PubMed] [Google Scholar]
- Schwarcz R. et al. Increased cortical kynurenate content in schizophrenia. Biol. Psychiatry 50, 521–530 (2001). [DOI] [PubMed] [Google Scholar]
- Okuda S., Nishiyama N., Saito H. & Katsuki H. 3-hydroxykynurenine, an endogenous oxidative stress generator, causes neuronal cell death with apoptotic features and region selectivity. J. Neurochem. 70, 299–307 (1998). [DOI] [PubMed] [Google Scholar]
- Hoekstra R. et al. Effect of electroconvulsive therapy on biopterin and large neutral amino acids in severe, medication-resistant depression. Psychiatry Res. 103, 115–123 (2001). [DOI] [PubMed] [Google Scholar]
- Hashimoto R. et al. Plasma tetrahydrobiopterin levels in patients with psychiatric disorders. Neuropsychobiology 23, 140–143 (1990). [DOI] [PubMed] [Google Scholar]
- Richardson M. A., Read L. L., Reilly M. A., Clelland J. D. & Clelland C. L. T. Analysis of plasma biopterin levels in psychiatric disorders suggests a common BH4 deficit in schizophrenia and schizoaffective disorder. Neurochem. Res. 32, 107–113 (2007). [DOI] [PubMed] [Google Scholar]
- Xu J. et al. Global and targeted metabolomics of esophageal squamous cell carcinoma discovers potential diagnostic and therapeutic biomarkers. Mol. Cell. Proteomics 12, 1306–1318 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X. P. et al. Pathogenesis of neural tube defects: the story beyond methylation or one-carbon unit metabolism. Metabolomics 8, 919–929 (2012). [Google Scholar]
- Lim Y. A. et al. A beta and human amylin share a common toxicity pathway via mitochondrial dysfunction. Proteomics 10, 1621–1633 (2010). [DOI] [PubMed] [Google Scholar]
- Tecott L. H. Serotonin and the orchestration of energy balance. Cell Metab. 6, 352–361 (2007). [DOI] [PubMed] [Google Scholar]
- Tecott L. H. & Abdallah L. Mouse genetic approaches to feeding regulation: Serotonin 5-HT2C receptor mutant mice. CNS Spectr. 8, 584–588 (2003). [PubMed] [Google Scholar]
- Brunelli L. et al. A combination of untargeted and targeted metabolomics approaches unveils changes in the kynurenine pathway following cardiopulmonary resuscitation. Metabolomics 9, 839–852 (2013). [Google Scholar]
- Yao J. K. et al. Homeostatic imbalance of purine catabolism in first-episode neuroleptic-naive patients with schizophrenia. Plos One 5, e9508 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhari K. et al. Clinical correlation of alteration of endogenous antioxidant-uric acid level in major depressive disorder. Indian J. Clin. Biochem. 25, 77–81 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J. K., Reddy R. & van Kammen D. P. Reduced level of plasma antioxidant uric acid in schizophrenia. Psychiatry Res. 80, 29–39 (1998). [DOI] [PubMed] [Google Scholar]
- Kim T. S. et al. Decreased plasma antioxidants in patients with Alzheimer’s disease. Int. J. Geriatr. Psychiatr. 21, 344–348 (2006). [DOI] [PubMed] [Google Scholar]
- de Lau L. M. L., Koudstaal P. J., Hofman A. & Breteler M. M. B. Serum uric acid levels and the risk of Parkinson disease. Ann. Neurol. 58, 797–800 (2005). [DOI] [PubMed] [Google Scholar]
- Ascherio A. et al. Urate as a predictor of the rate of clinical decline in parkinson disease. Arch. Neurol. 66, 1460–1468 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapirstein A. & Bonventre J. V. Phospholipases A(2) in ischemic and toxic brain injury. Neurochem. Res. 25, 745–753 (2000). [DOI] [PubMed] [Google Scholar]
- Nicholson J. K., Holmes E. & Wilson I. D. Gut microorganisms, mammalian metabolism and personalized health care. Nat. Rev. Microbiol. 3, 431–438 (2005). [DOI] [PubMed] [Google Scholar]
- Liu Y. et al. Molecular regulation of sexual preference revealed by genetic studies of 5-HT in the brains of male mice. Nature 472, 95–125 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. Y., Kim A., Zhao Z. Q., Liu X. Y. & Chen Z. F. Postnatal maintenance of the 5-Ht1a-Pet1 autoregulatory loop by serotonin in the raphe nuclei of the brainstem. Mol. Brain 7, 48–58 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z. et al. Dorsal raphe neurons signal reward through 5-HT and glutamate. Neuron 81, 1360–1374 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gika H. G., Theodoridis G. A., Wingate J. E. & Wilson I. D. Within-day reproducibility of an HPLC-MS-Based method for metabonomic analysis: Application to human urine. J. Proteome Res. 6, 3291–3303 (2007). [DOI] [PubMed] [Google Scholar]
- Nugent J. L. et al. Altered Tissue Metabolites Correlate with Microbial Dysbiosis in Colorectal Adenomas. J. Proteome Res. 13, 1921–1929 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Want E. J. et al. Global metabolic profiling procedures for urine using UPLC-MS. Nature 5, 1005–1018 (2010). [DOI] [PubMed] [Google Scholar]
- Bijlsma S. et al. Large-scale human metabolomics studies: A strategy for data (pre-) processing and validation. Anal. Chem. 78, 567–574 (2006). [DOI] [PubMed] [Google Scholar]
- Xia J. G., Mandal R., Sinelnikov I. V., Broadhurst D. & Wishart D. S. MetaboAnalyst 2.0-a comprehensive server for metabolomic data analysis. Nucleic Acids Res. 40, W127–W133 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia J. G. & Wishart D. S. MetPA: A web-based metabolomics tool for pathway analysis and visualization. Bioinformatics 26, 2342–2344 (2010). [DOI] [PubMed] [Google Scholar]
- Bradford M. M. Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein-dye binding. Anal. Biochem. 72, 248–254 (1976). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.