Abstract
Oxidative stress has been identified as the root cause of the development and progression of several diseases. Supplementation of exogenous antioxidants or boosting endogenous antioxidant defenses of the body is a promising way of combating the undesirable effects of reactive oxygen species (ROS) induced oxidative damage. Plants have an innate ability to biosynthesize a wide range of non-enzymatic antioxidants capable of attenuating ROS- induced oxidative damage. Several in vitro methods have been used to screen plants for their antioxidant potential, and in most of these assays they revealed potent antioxidant activity. However, prior to confirming their in vivo therapeutic efficacy, plant antioxidants have to pass through several physiopharmacological processes. Consequently, the findings of in vitro and in vivo antioxidant potential assessment studies are not always the same. Nevertheless, the results of in vitro assays have been irrelevantly extrapolated to the therapeutic application of plant antioxidants without undertaking sufficient in vivo studies. Therefore, we have briefly reviewed the physiology and redox biology of both plants and humans to improve our understanding of plant antioxidants as therapeutic entities. The applications and limitations of antioxidant activity measurement assays were also highlighted to identify the precise path to be followed for future research in the area of plant antioxidants.
Keywords: Antioxidant activity, pharmacology, plants, prooxidants, secondary metabolites
Introduction
Antioxidants significantly delay or prevent oxidation of oxidizable substrates when present at lower concentrations than the substrate 1. Antioxidants can be synthesized in vivo (e.g., reduced glutathione (GSH), superoxide dismutase (SOD), etc.) or taken as dietary antioxidants 1,2. Plants have long been a source of exogenous (i.e., dietary) antioxidants. It is believed that two-thirds of the world's plant species have medicinal importance, and almost all of these have excellent antioxidant potential 3. The interest in the exogenous plant antioxidants was first evoked by the discovery and subsequent isolation of ascorbic acid from plants 4. Since then, the antioxidant potential of plants has received a great deal of attention because increased oxidative stress has been identified as a major causative factor in the development and progression of several life threatening diseases, including neurodegenerative and cardiovascular disease. In addition, supplementation with exogenous antioxidants or boosting of endogenous antioxidant defenses of the body has been found to be a promising method of countering the undesirable effects of oxidative stress 5.
There are currently approximately 19 in vitro and 10 in vivo methods of assessing antioxidant activity that are commonly applied for evaluation of the antioxidant activity of plant samples 6. In most of these in vitro assays plant samples showed potent antioxidant activity. This is likely due to their innate ability to synthesize non-enzymatic antioxidants such as ascorbic acid and glutathione, as well as secondary metabolites such as phenolic compounds.
Despite many plants being reported to have antioxidant potential by in vitro assays, only a few of these antioxidant activities have been confirmed or investigated in vivo 7. In vitro assays are generally used to confirm the antioxidant activity of plant samples within particular reaction systems; accordingly, the relevance of the findings of these assays to in vivo systems is uncertain 8. Moreover, several phytochemicals have been found to possess antioxidant activity within in vitro assays. However, only a few of these have been shown to be therapeutically useful under in vivo conditions due to their interference with physiopharmacological processes such as absorption, distribution, metabolism, storage and excretion. Nevertheless, phytochemicals are being screened for their in vitro antioxidant activity, and the results of these studies are then directly extrapolated to their therapeutic usefulness. This malpractice may raise fundamental questions about the significance of plants as exogenous sources of antioxidants and their therapeutic efficacies. Accordingly, in the present article, we briefly reviewed the physiology and redox biology of both plants and humans. In addition, the applications and limitations of antioxidant activity measurement assays are discussed 6,7. The information provided herein will enable correct interpretation of the findings of plant antioxidant potential assessment studies based on both in vitro and in vivo assays.
Why do all plants have antioxidant potential?
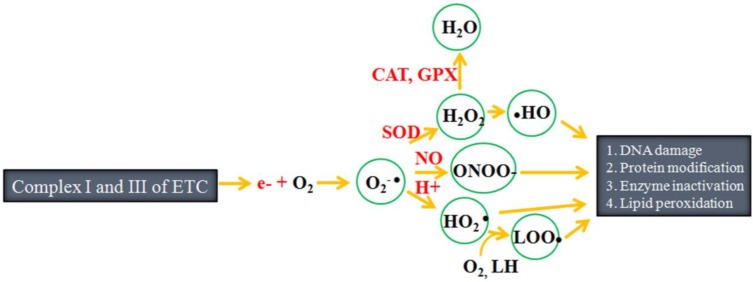
Chloroplasts and mitochondria are the two main powerhouses and sites of reactive oxygen species (ROS) generation within plant cells. These materials are also involved in maintenance of a fine balance between energy linked functions and control of ROS production. Peroxisomes, single membrane-bound subcellular organelles, are a third important site of production of ROS such as hydrogen peroxide (H2O2), superoxide (O2● -) and nitric oxide (NO●) within plant cells. Peroxisomes contain basic enzymatic constituents such as catalase (CAT), as well as hydrogen peroxide (H2O2)-producing flavin oxidases 9. Within the plant cell, ROS generation occurs at photosystem I and II (PS I and PS II) of the chloroplasts, membrane and matrix of the peroxisome, and complex I, ubiquinone and complex III of the mitochondrial electron transport chain (ETC) 10. Under normal physiological conditions, there is electron slippage from PS I and PS II of the chloroplasts, membrane of mitochondrial ETC and peroxisome. These electrons later react with molecular oxygen to produce superoxide radical (O2-●). The superoxide radical is subsequently converted to hydroperoxyl radical (HO2●) and finally to H2O2 11-13. Similar to ROS, reactive nitrogen species (RNS) such as the nitric oxide radical (NO•) and peroxinitrite (ONOO-) are also formed in various compartments of the cell including the chloroplasts, mitochondria and peroxisomes 14. The third type of free radical, reactive sulfur species (RSS), are reportedly formed from thiols by reaction with ROS 15. The overall process of free radicals generation is summarized in Fig. 1. These free radicals are constantly produced in the subcellular organelles of living cells. Most of the time, the production of free radicals is genetically planned, since they function as signaling molecules 12,16. However, overproduction of free radicals can also sometimes damage biomolecules such as DNA, proteins and lipids.
Figure 1.
Outline of free radical production: During electron transfer, approximately 1-2% of the electrons slip from complex I and III of the electron transfer chain (ETC), after which they react with molecular oxygen to form free radicals such as superoxide anion (O2-●), hydroperoxyl radical (HO2●), hydroxyl radical, hydrogen peroxide (H2O2), hydroperoxyl radical (●OH), peroxynitrite (ONOO-) and lipid peroxyl radical (LOO●). These free radicals target biomolecules such as DNA, protein and lipids, ultimately damaging them. SOD refers to superoxide dismutase, CAT refers to catalase and GPX refers to glutathione peroxidase. (Adapted from Carocho and Ferreira, 13 and Lü et al. 15).
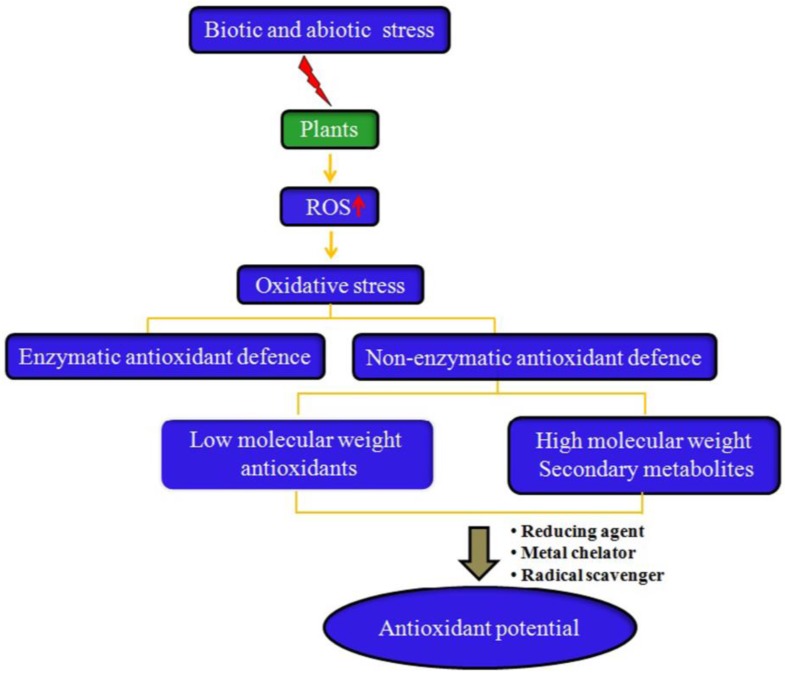
Plants have efficient complex enzymatic and non-enzymatic antioxidant defense systems to avoid the toxic effects of free radicals. Enzymatic systems include SOD, catalase (CAT), glutathione peroxidase (GPx), and glutathione reductase (GR) 7, while non-enzymatic systems consist of low molecular weight antioxidants (ascorbic acid, glutathione, proline, carotenoids, phenolic acids, flavonoids, etc.) and high molecular weight secondary metabolites such as tannins (Fig. 2).
Figure 2.
Why do all plants have antioxidant activity? Plants have an innate ability to synthesize non-enzymatic antioxidants. However, under biotic and abiotic stress conditions, the production of reactive oxygen species (ROS) increases in the plants, resulting in induction of oxidative stress. In response to increased oxidative stress, plants augment the production and accumulation of several low molecular weight antioxidants (e.g., vitamin C, vitamin E, phenolic acids, etc.) and high molecular antioxidant secondary metabolites such as tannins, which confer antioxidants to most plants under in vitro studies by functioning as free radical scavengers, reducing agents, and metal chelators.
There may be two main reasons for the synthesis and accumulation of these non-enzymatic antioxidants by plants. First, the genetic make-up of plants imparts them with an innate ability to synthesize a wide variety of phytochemicals to perform their normal physiological functions and/or protect themselves from microbial pathogens and animal herbivores. Another reason for the synthesis of reductant phytochemicals could be the natural tendency of plants to respond to environmental stress conditions.
Plants synthesize low molecular weight antioxidants such as glutathione and ascorbate within the chloroplast stroma and cytosol using NADPH as the ultimate electron donor 11. These low molecular weight antioxidants function as redox buffers that interact with numerous cellular components and influence plant growth and development by modulating processes from mitosis and cell elongation to senescence and death 17. In addition, these antioxidants may influence gene expression associated with biotic and abiotic stress responses to maximize defense. Vitamin C (ascorbic acid/ascorbate) is generated during aerobic metabolism, after which it reacts rapidly with O2-●, singlet oxygen and ozone (chemically), and H2O2 (enzymatically) through ascorbate peroxidase to neutralize their toxic effects. Vitamin C also helps regenerate antioxidant pigments, carotenoids (carotenes and xanthophylls), and vitamin E. Glutathione is a redoxactive molecule that can be present in a reduced form (GSH) or an oxidized disulfide form (GSSG) and plays important roles in biosynthetic pathways, detoxification, antioxidant biochemistry and redox homeostasis 18,19. GSSG is reduced to GSH by the enzyme glutathione reductase, which requires NADPH as the reducing power. GSH acts as an anti-oxidant by quenching reactive oxygen species and is involved in the ascorbate-glutathione cycle, which eliminates damaging peroxides 20. Plants also produce tocopherols (vitamin E) that act as important liposoluble redox buffer systems. Vitamin E, which is generally synthesized in chloroplasts and protoplastids, is located in the membranes of cells. This compound is a major singlet oxygen scavenger that provides protection against lipid peroxidation 17,21.
Plants also synthesize and accumulate a range of low and high molecular weight secondary metabolites that play important roles in ROS metabolism and avoidance of uncontrolled oxidation of essential biomolecules. These metabolites are also important to adaptation of plants to environmental fluctuations 22. Secondary metabolites provide passive and active resistance. In passive resistance, metabolites are continuously available, despite the presence of stressors, whereas in active resistance, metabolites are produced in response to specific stressors 23. These metabolites are synthesized through basic pathways, such as the glycolysis or shikimic acid pathways, which further branch out based on cell type, developmental stage and environmental cues. Secondary metabolites are generally derived from primary metabolites such as amino acids and carbohydrates via methylation, hydroxylation and glycosylation 24.
Higher plants survive in constantly fluctuating environments, due to their highly regulated and flexible metabolism 25. Under normal physiological conditions, the increase in free radical production is relatively small and housekeeping antioxidant capacity is sufficient to maintain redox homeostasis 26. The metabolic pathways of plants are sensitive to abiotic and biotic stress conditions such as high light intensity, heat, drought, anoxic conditions and pathogen attack, and it has been reported that there is an approximately 3 to 10 fold increase in free radicals production under stress conditions 14,25,27.
The ratio of GSH to GSSG has been shown to decrease due to the oxidation of reduced glutathione during detoxification of reactive oxygen species (ROS) in response to abiotic stresses 28. Moreover, plants increase the activity of GSH biosynthetic enzymes and glutathione levels in response to both abiotic and biotic stresses 29. Similar to glutathione, biosynthesis and recycling of ascorbic acid has been found to increase in response to various abiotic stresses within mutant and transgenic plant species 30,31. Vitamin E deficiency has also been shown to retard growth and change responses to abiotic stress conditions. In addition, increased vitamin E content has been shown to diminish detrimental effects of environmental stress in plants 32.
Some secondary antioxidant metabolites occur constitutively, while others are formed in response to biotic and abiotic stress conditions 33, 34. The accumulation of phenolic compounds along with enhancement of phenylopropanoid metabolism has been observed under different environmental stress conditions 35. In plants, phenolics can act as antioxidants by donating electrons to guaiacol-type peroxidases for the detoxification of H2O2 produced under stress conditions 36. Phenolics also provide protection against UV radiation through their potent radical scavenging ability. In addition, they function as enzyme inhibitors and feeding deterrents for herbivores while providing resistance against pathogens 37. Synthesis of flavonoids is known to be induced by UV stress, heavy metals toxicity, or low temperature and low nutrient conditions, which might attributed to their UV-absorbing, radical scavenging and metal cheating ability 35, 38,39. UV-B radiation was found to affect the production of various high molecular secondary metabolites such as tannins and lignin 40. Moreover, plants growing in tropical and high-altitude conditions have been shown to contain a higher proportion of flavonoids than those growing in temperate conditions owing to overexposure to light or UV radiation 41. Biotic stress like wounding has been found to induce phenolic metabolism such as increased synthesis of phenolic compounds 42. Tannins are reportedly useful for plant leaf defense against insect herbivores 43. Similar to phenolics, an increase in total indole alkaloid content in the shoots and roots of Catharanthus roseus has been observed under drought-induced stress 44. Alkaloids generally provide protection to plants against microbial or herbivore attack and UV-radiation 45,46. It has also been reported that monoterpenes and isoprenes are emitted at higher rates under high temperature 47.
Secondary metabolites as antioxidants
Plant metabolism is mainly classified as primary or secondary. Compounds produced through primary metabolism, which are generally referred to as primary metabolites; include sugars, fatty acids, amino acids and nucleic acids. Primary metabolites are required for maintenance of plant cells 48, while secondary metabolites are essential to the normal growth, development and defense of plants.
To date, thousands of different types of secondary metabolites have been identified in plants 23. Chemically, these compounds are either nitrogen-containing (alkaloids) or nitrogen-deficient (terpenoids and phenolics) 46. Nearly 20% of plant species accumulate alkaloids, which mainly include terpenoid indole alkaloids, tropane alkaloids, and purine alkaloids 49. However, under in vitro antioxidant measurement assay conditions, the radical scavenging potential of alkaloids is reportedly moderate to nonexistent. Terpenoids comprise another large family of secondary metabolites, consisting of over 40,000 different compounds 50. Monoterpenes, sesquiterpenes and diterpenes have been found to possess notable antioxidant activity in different in vitro assays. However, most of these activities have no physiological relevance 51. Tetraterpenes and carotenoids have been shown to possess potent antioxidant activity within both in vivo and in vitro studies 52; however, some valuable carotenoids such as beta-carotene showed prooxidant effects at high concentration and oxygen pressure 53. Among all secondary metabolites, phenolic antioxidants appear to be the most important since they have shown promising antioxidant activity in both in vivo and in vitro investigations. Plant phenolics are mainly classified into five major groups, phenolic acids, flavonoids, lignans, stilbenes and tannins 54-56. Phenolic compounds generally possess one or more aromatic rings with one or more hydroxyl groups. It has commonly been assumed that the antioxidant capacity of phenolics will increase with the number of free hydroxyls and conjugation of side chains to the aromatic rings 57. Flavonoids and phenolic acids, the largest classes of plant phenolics, are biosynthetically derived from the acetate and shikimate pathways, as well as the shikimate pathway from phenylalanine or tyrosine 58. Phytochemicals from these classes were found to have excellent antioxidant activity in both in vitro and in vivo investigations. Moreover, they are known to interact with other physiological antioxidants such as ascorbate or tocopherol and to synergistically amplify their biological effects 59. Flavonoids and phenylopropanoids are also oxidized by peroxidase, and act as H2O2 scavengers 35,60. Under experimental conditions, the antioxidant potential of plant phenolics is always linked to their electron donation, reducing power and metal ion chelating ability 61.
Methods used for assessment of antioxidant potential of plants
In vitro assays
In ethanopharmacological and nutraceutical investigations, in vitro antioxidant activity assessment methods are often used to screen and confer antioxidant potential to plants or their phytochemicals and sometimes to understand the probable mechanism of action of plant antioxidants 62. In the case of medicinal plants, these assays are used to confer free radical scavenging activity to plants, which in turn has great importance in understanding the role of plants in minimizing the oxidative stress linked pathophysiology of diseases. There are several in vitro assays used to measure and confer antioxidant activity to plants (Table 1); however, each of these has its own limitations regarding applicability. Therefore, multiple assay strategies have frequently been adapted to confer antioxidant potential. In these assays, plants are generally assessed for their function as reducing agents, hydrogen donors, singlet oxygen quenchers or metal chelators, after which they are classified as primary (chain-breaking) and secondary (preventive) antioxidants. Primary antioxidants act by donating a hydrogen atom, while secondary antioxidants function via binding of metal ions capable of catalyzing oxidative processes and scavenging oxygen, absorbing UV radiation, inhibiting enzymes or decomposing hydroperoxides 66.
Table 1.
In vitro assays commonly used to screen antioxidant activity of plants.
| Sr. No. | Assay | Mechanism | References |
|---|---|---|---|
| 1 | β-Carotene or crocin-bleaching assay | Hydrogen atom transfer | Karadag et al. 63 |
| 2 | ORAC (oxygen radical absorbance capacity) | Hydrogen atom transfer | Ou et al. 64 |
| 3 | IOU (inhibited oxygen uptake) | Hydrogen atom transfer | Karadag et al. 63 |
| 4 | LPIC (lipid peroxidation inhibition capacity) assay | Hydrogen atom transfer | Karadag et al. 63 |
| 5 | TRAP (total radical trapping antioxidant parameter) | Hydrogen atom transfer | Karadag et al. 63 |
| 6 | Copper reduction assay | Single electron transfer | Huang et al. 65 |
| 7 | FRAP (ferric reducing antioxidant power assay | Single electron transfer | Huang et al. 65 |
| 8 | Total phenolic content assay by Folin-Ciocalteu reagent | Single electron transfer | Karadag et al. 63 |
| 9 | ABTS ({2,2' - azinobis-(3-ethyl-benzothiazoline-6-sulphonic acid)}) assay | Both hydrogen atom and single electron transfer | Huang et al. 65 |
| 10 | DPPH (2,2-Diphenyl-1-picrylhydrazyl) assay | Both hydrogen atom and single electron transfer | Huang et al. 65 |
Based on the inactivation mechanism involved, antioxidant activity assessment methods are classified into hydrogen atom transfer (HAT) and electron transfer (ET) reaction-based methods. Bond dissociation energy and ionization potential are two major factors that determine the mechanism and efficiency of antioxidants 63. HAT-based methods measure the ability of an antioxidant to scavenge free radicals via hydrogen donation to form stable compounds. While these methods are more relevant to the radical chain-breaking antioxidant capacity, SET-based methods measure the ability of an antioxidant to transfer one electron to reduce any compound, including metals, carbonyls, and free radicals 63,67. Total radical trapping antioxidant parameter (TRAP), oxygen radical absorbance capacity (ORAC), lipid peroxidation inhibition capacity (LPIC) and carotene or crocin-bleaching assays are HAT-based methods. Other commonly used antioxidant activity assessing methods such as ferric reducing antioxidant power (FRAP) and copper reduction assay involve SET mechanisms 64. However, some methods, such as 2,2-Diphenyl-1-picrylhydrazyl (DPPH) and {2,2'-azinobis-(3-ethyl-benzothiazoline-6-sulphonic acid)} (ABTS), involve both HAT and SET mechanisms 67.
In vivo assays
Within in vivo assays, plant antioxidants are generally assessed for their effects on the activity of endogenous antioxidant enzymes or oxidative damage biomarkers before and after induction of oxidative stress in experimental animals. Some of these commonly used methods directly evaluate the enzymatic activity of endogenous antioxidants such as SOD, CAT, GPx and GR, while other methods involve quantification of oxidative damage biomarkers. The formation of specific end products resulting from interaction of ROS with biologically important macromolecules such as DNA, protein and lipids is measured by quantifying oxidative damage biomarker methods. DNA damage is determined by measuring the 8-hydroxydeoxyguanosine content. Carbonyl and aldehyde (e.g. malondialdehyde) contents are measured as markers of protein and lipid oxidation, respectively 6, 68.
Therapeutic relevance and in vivo behavior of plant antioxidants
Similar to plants, the human body is constantly exposed to oxidants and/or free radicals generated during physiological processes such as mitochondrial respiration. In plants, the production of free radicals increases during biotic and abiotic stresses, whereas the load of free radicals in humans increases under pathophysiological conditions such as inflammation, metabolism of foreign compounds, and radiation 69. In animal cells, free radicals are generated at the ETC of mitochondria, Ero1 and cytochrome P-450 enzymes of the endoplasmic reticulum (ER), and at the plasma membrane (at the NADPH oxidases) and inside (at the flavin oxidases) of peroxisomes 70. Mitochondria produce more than 90% of the cellular energy through oxidative phosphorylation, which involves the tricarboxylic acid cycle and ETC. However, about 1-2% of the electrons slip through complexes I and III of the ETC, after which they react with molecular oxygen to sequentially form ROS such as O2-●, HO2● and H2O2 5,11-13. The peroxisome is another major site of oxygen consumption within animal cells. In this site, electrons removed from various metabolites are used to reduce O2 to H2O2, which is then converted to H2O. The peroxisome plays a key role in both the production and scavenging of ROS in the cell, mainly in the form of H2O2. The respiratory pathway in peroxisomes is not coupled to oxidative phosphorylation; therefore, it does not produce ATP. However, free energy is released in the form of heat 71. It is well known that peroxisomes in plant cells contain a functional ascorbate-glutathione cycle; however, relatively little is known about the presence of this non-enzymatic antioxidant inside mammalian peroxisomes 72. While ER is responsible for much of a cell's protein synthesis and folding, it also produces ROS as a byproduct 73.
As in the case of the plants, free radicals also act as signaling molecules within animal cells. Specifically, they play an important role in apoptosis, gene expression and ion transportation 15. The human body also has an efficient antioxidant defense system that maintains a balance between free radical production and oxidative stress through enzymatic and non-enzymatic antioxidant defenses. Enzymatic antioxidant defenses include SOD, CAT, glutathione GPx, GR and glucose-6-phosphate dehydrogenase, while non-enzymatic antioxidant defenses primarily consist of vitamin A, coenzyme Q (Q10), uric acid and glutathione 13.
Peroxisomes and mitochondria are interconnected and in close contact with the ER to maintain various metabolic and signaling pathways 74. However, dysfunction in any of these organelles leads to overproduction of free radicals, which exerts a toxic effect on biomolecules such as DNA, proteins and lipids, which leads to deregulation of redox-sensitive metabolic and signaling pathways and pathological conditions 71.
It should be noted that environmental and complex genetic causes result in almost all cells overproducing life threatening free radicals in their early or late stages of life. The human body has efficient enzymatic antioxidant defense; however, its non-enzymatic antioxidant defense is less evolved than that of plants. This may be due to its low oxygen exposure physiology. Hence, it has been assumed that humans must constantly take in dietary antioxidants to keep the levels of free radicals in the body low.
Plants have always been a common source of food and medicines, either in the form of traditional preparations or as pure active principles 75. Most of the observed therapeutic effects of plants have been linked to their potent antioxidant activity. We previously proposed that antioxidant activity based healing of diseases or maintenance of a healthy lifestyle could be the scientific basis of traditional herbal medicines such as those used in Ayurveda 76. It has been suggested that free radicals are involved in the pathology of more than 50 human diseases, including aging 77. However, it is also important to consider that free radicals are not harmful at all times; rather, their toxicity depends on several factors including type of ROS/RNS, their concentration and localization, and the kinetics of production and elimination 70.
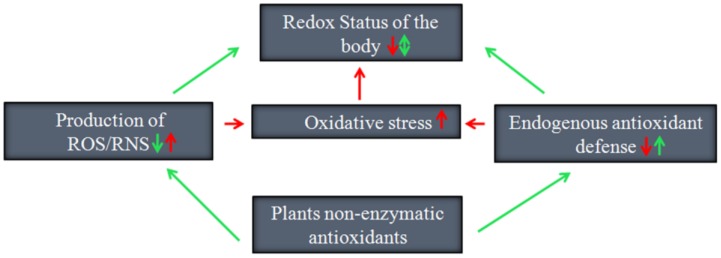
Despite the controversies regarding whether plant antioxidants are good or bad, supplementation of external antioxidants or boosting endogenous antioxidant defenses of the body is known to be a promising method to improve the free radical linked pathophysiology of diseases. Plant antioxidants such as ascorbic acid and flavonoids have been shown to be the best exogenous antioxidants. Indeed, these compounds not only restrain ROS production by scavenging free radicals, but also help boost endogenous antioxidant defenses of the body (Fig. 3). Nevertheless, the usefulness of endogenous antioxidants as therapeutic agents still remains an issue that must be carefully considered 78.
Figure 3.
Promising ways by which plant non-enzymatic antioxidants preserve the redox status of the human body.
While both plants and humans have somewhat similar redox biology, the physiology of humans is distinct from that of plants. It is important to consider the fact that plants produce antioxidants for their own requirements to perform specific functions. In the human body, plant antioxidants have to pass through several physiological processes including absorption, distribution, metabolism, storage and excretion before being able to achieve the expected therapeutic effect. As a result, most good plant antioxidants are unable to achieve the desired outcome because they generally have low bioavailability and some can exert pro-oxidant effects under in vivo conditions 79,80.
Similar to lower vertebrates, humans cannot synthesize Vitamin C due to the lack of gulonolactone oxidase 81. Accordingly, vitamin C is regarded as one of the most important dietary antioxidants that helps to decrease free-radical-mediated damage. Vitamin C is readily absorbed by active transport in the intestine and functions as an antioxidant by donating two of its electrons, which prevents other compounds from being oxidized 81,82. Vitamin C has been shown to be an excellent antioxidant under both in vitro and in vivo study conditions. However, in the presence of catalytic metal ions, vitamin C can also function as a pro-oxidant 83. Specifically, vitamin C has been found to be capable of converting Fe3+ into Fe2+, which subsequently reacts with oxygen or hydrogen peroxide to form superoxide and hydroxyl radicals that can subsequently damage biomolecules 84. However, the pro-oxidant properties of vitamin C are believed to be dependent on dose as well as the availability of catalytic metal ions 85. In addition, the antioxidant or pro-oxidant behavior of vitamin C has been shown to depend on the vitamin E status of the body 86. Vitamin E is another useful and powerful plant antioxidant that is generally present in lipid structures of cells. This compound reacts with peroxyl radicals to inhibit the propagation of lipid peroxidation 87. Similar to vitamin C, vitamin E also shows pro-oxidant effects at high concentration. Vitamin E has also been shown to react with free radicals to become a reactive radical, while it functions as a prooxidant in the absence of co-antioxidants 13.
Under in vivo conditions, the antioxidant potential of polyphenols is predominately dependent on their concentrations in the bloodstream after absorption from the gastrointestinal tract, as well as their modifications during metabolism. The chemical structures of polyphenols will also influence the in vivo antioxidant potential of polyphenols, as they determine the conjugation reactions with methyl, sulfate or glucuronide groups and the nature and amounts of metabolites formed by the gut microflora absorbed at the colon level 88.
Flavonoids are the most abundant dietary polyphenols, with over 5000 reported to date 89,90. Flavonoids are classified into six major subclasses, flavones, flavonols, flavanones, catechins or flavanols, anthocyanidins and isoflavones. In plants, most flavonoids are attached to sugars (glycosides), although they are occasionally found as aglycones 89. Expect for some flavan-3-ols and proanthocyanidins, most flavonoids are not completely absorbed and reach the circulatory system owing to their glycoside conjugates 91. Occasionally, major fractions of absorbed flavonoids are metabolized to conjugates or further metabolized in the colon, where they produce a wide array of low molecular weight aromatic acids such as phenylvaleric, phenylpropionic, phenylacetic and benzoic acids as a result of their antioxidant potency being reduced 92-94. Moreover, flavonoids and their in vivo metabolites have been reported to exert other potential biological activities than conventional hydrogen-donating antioxidants, such as the ability to act as signaling molecules in cells through actions in the protein kinase and lipid kinase signaling pathways 94,95. In addition to their notable antioxidant behavior, polyphenols have pro-oxidant properties. Indeed, it has been reported that plant polyphenols may increase oxidative damage in vivo via interactions with transition metal ions that increase their ability to form free radicals from peroxides 96.
Carotenoids, which are abundant in fruits and vegetables, scavenge peroxyl radicals and act predominantly as antioxidants 97. The antioxidant potential of dietary carotenoids such as beta-carotene and lycopene in biological systems is dependent on a number of factors, including the presence of other co-antioxidants. It has also been reported that carotenoids may lose their effectiveness as antioxidants at high concentrations or at high partial pressures of oxygen 98.
Strategy for plant antioxidant potential measurement
In vitro antioxidant potential assessment methods do not provide exact therapeutic implications of plant antioxidants. Moreover, the antioxidant potential of plants or their phytochemicals is influenced by several factors under in vivo conditions, including gut absorption, metabolism, bioavailability, and presence of co-antioxidants and transition metal ions. Consequently the results of in vivo antioxidant assessment studies of plant antioxidants are not consistent 62. Hence, there is a need to develop an expansive study strategy that will include a set of in vitro and in vivo experiments to provide more accurate therapeutic values to plant antioxidants.
One commonly suggested strategy is that both in vitro and in vivo antioxidant assessment studies be conducted simultaneously to confer therapeutic antioxidant potential to plants or their components. Holst and Williamson 99 proposed that in vitro plant antioxidant assessment studies be driven by in vivo results, and not vice versa. They further suggested that once a phytochemical is shown to exert an effect in vivo, their mechanisms can be tested in vitro to avoid disappointments when testing in vitro concepts in vivo. It is believed that the proposed antioxidant activity assessment studies would be more suitable for investigation of antioxidant activity of flavonoids and lignans, as these phytochemicals are generally metabolized to low molecular antioxidants in the body. Most ingested flavonoids have been shown to be extensively degraded to various phenolic acids, which could have radical scavenging ability 100. Similarly, the lignan secoisolariciresinol diglucoside is metabolized to more powerful antioxidants such as secoisolariciresinol, enterodiol and enterolactone within the body 101.
In line with the above plant antioxidant activity assessment study strategies, it is advisable disease pathophysiology targeted combined in vitro and in vivo antioxidant activity assessment study strategy to attribute more precise therapeutic value to individual or combined plant antioxidant entities. In this strategy, there is a need to first identify the major target of free radical linked disease pathophysiology such as mitochondrial dysfunction, which is an underlying cause of several degenerative diseases. Thereafter, plant extracts or antioxidants should be screened for low molecular antioxidants that could have potent radical scavenging activity in vitro and are able to cross blood brain barriers and reach the target by comparing them with proposed or known structural analogs of mitochondria-targeted antioxidants through in silico methods. Finally, these results should be validated by in vivo studies.
Conclusions
Over the past few decades, significant scientific information has been accumulated regarding plant redox biology and its antioxidant defense. However, this information has not been collectively discussed together with human redox biology and exogenous antioxidants metabolism, which is essential in understanding the therapeutic utility of plant antioxidants. Because of their high oxygen exposure physiology, plants may have more sites of ROS generation; therefore, they could evolve more efficient non-enzymatic antioxidant systems than humans. Plants synthesize and accumulate several non-enzymatic antioxidants such as ascorbic acid, glutathione and phenolics. Some of these antioxidants occur constitutively, while others are formed in response to abiotic and biotic stress conditions. Almost all plants or their phytochemicals exhibit some antioxidant activity under in vitro assays conditions. However, for in vivo studies, plant antioxidants have to pass through several physiopharmacological processes including absorption, distribution, metabolism, storage and excretion. Consequently, the antioxidant potential of plants or their phytochemicals is influenced by several factors in vivo, including gut absorption, metabolism, bioavailability, and the presence or absence of co-antioxidants and transition metal ions. Therefore, the results of the in vitro antioxidant potential assessment studies are often contradictory to those of in vivo studies. Nevertheless, without undertaking sufficient in vivo studies, the results of in vitro assays have been irrelevantly linked to the therapeutic applications of plant antioxidants. Hence, we proposed disease pathophysiology targeting combined in vitro and in vivo antioxidant activity to attribute more precise therapeutic value to individual or combined plant antioxidant entities.
Acknowledgments
This research was supported by a Yeungnam University Research Grant (214A345035).
Abbreviations
- ABTS
2,2'-Azinobis-(3-ethyl-benzothiazoline-6-sulphonic acid)
- CAT
Catalase
- DPPH
2,2-Diphenyl-1-picrylhydrazyl
- ETC
Electron Transport Chain
- ET
Transfer
- FRAP
Ferric Reducing Antioxidant Power
- GPx
Glutathione Peroxidase
- GR
Glutathione Reductase
- GSH
Reduced Glutathione
- GSSG
Oxidized Glutathione
- H2O2
Hydrogen Peroxide
- HAT
Hydrogen Atom Transfer
- HO2●
Hydroperoxyl Radical
- LPIC
Lipid Peroxidation Inhibition Capacity
- NADPH
Nicotinamide Adenine Dinucleotide Phosphate
- NO
Nitric Oxide
- O2-●
Superoxide Anion
- ONOO
Peroxynitrile
- ORAC
Oxygen Radical Absorbance Capacity
- PS I
Photosystem I
- PS II
Photosystem II
- RNS
Reactive Nitrogen Species
- ROS
Reactive Oxygen Species
- RSS
Reactive Sulfur Species
- SOD
Superoxide Dismutase
- TCA
Tricarboxylic Acid Cycle
- TRAP
Total Radical Trapping Antioxidant Parameter.
References
- 1.Halliwell B. Biochemistry of oxidative stress. Biochem Soc Trans. 2007;35:1147–50. doi: 10.1042/BST0351147. [DOI] [PubMed] [Google Scholar]
- 2.Sies H. Oxidative stress: oxidants, antioxidants. Exp Physiol. 1997;82:291–95. doi: 10.1113/expphysiol.1997.sp004024. [DOI] [PubMed] [Google Scholar]
- 3.Krishnaiah D, Sarbatly R, Nithyanandam R. A review of the antioxidant potential of medicinal plant species. Food Bioprod Process. 2011;89:217–33. [Google Scholar]
- 4.Szent-Giörgyi A. Lost in the twentieth century. Annu Rev Biochem. 1963;36:1–15. doi: 10.1146/annurev.bi.32.070163.000245. [DOI] [PubMed] [Google Scholar]
- 5.Kasote DM, Hegde MV, Katyare SS. Mitochondrial dysfunction in psychiatric and neurological diseases: cause(s), consequence(s), and implications of antioxidant therapy. Biofactors. 2013;39:392–06. doi: 10.1002/biof.1093. [DOI] [PubMed] [Google Scholar]
- 6.Alam MN, Bristi NJ, Rafiquzzaman M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm J. 2013;21:143–52. doi: 10.1016/j.jsps.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chand S, Dave R. In vitro models for antioxidant activity evaluation and some medicinal plants possessing antioxidant properties: an overview. Afr J Microbiol Res. 2009;3:981–96. [Google Scholar]
- 8.Badarinath AV, Rao KM, Chetty CMS, Ramkanth V, Rajan TVS, Gnanaprakash K. A review on in-vitro antioxidant methods: comparisons, correlations and considerations. Int J PharmTech Res. 2010;2:1276–85. [Google Scholar]
- 9.del Río LA, Sandalio LM, Corpas FJ, Palma JM, Barroso JB. Reactive oxygen species, reactive nitrogen species in peroxisomes. Production, scavenging, and, role in cell signaling. Plant Physiol. 2006;141:330–35. doi: 10.1104/pp.106.078204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gill SS, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem. 2010;48:909–30. doi: 10.1016/j.plaphy.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 11.Alscher RG, Donahue JL, Cramer CL. Reactive oxygen species and antioxidants: Relationships in green cells. Physiol Plant. 1997;100:224–33. [Google Scholar]
- 12.Zhao J, Davis LC, Verpoorte R. Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol Adv. 2005;23:283–333. doi: 10.1016/j.biotechadv.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Carocho M, Ferreira IC. A review on antioxidants, prooxidants and related controversy: natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem Toxicol. 2013;51:15–25. doi: 10.1016/j.fct.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 14.Møller IM, Jensen PE, Hansson A. Oxidative modifications to cellular components in plants. Annu Rev Plant Biol. 2007;58:459–81. doi: 10.1146/annurev.arplant.58.032806.103946. [DOI] [PubMed] [Google Scholar]
- 15.Lü JM, Lin PH, Yao Q, Chen C. Chemical and molecular mechanisms of antioxidants: experimental approaches and model systems. J Cell Mol Med. 2010;14:840–60. doi: 10.1111/j.1582-4934.2009.00897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002;7:405–10. doi: 10.1016/s1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- 17.Foyer CH. Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell. 2005;17:1866–75. doi: 10.1105/tpc.105.033589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meyer AJ, Hell R. Glutathione homeostasis and redox-regulation by sulfhydryl groups. Photosynth Res. 2005;86:435–457. doi: 10.1007/s11120-005-8425-1. [DOI] [PubMed] [Google Scholar]
- 19.Noctor G, Mhamdi A, Chaouch S, Han Y, Neukermans J, Marquez-Garcia B. et al. Glutathione in plants: an integrated overview. Plant Cell Environ. 2012;35:454–84. doi: 10.1111/j.1365-3040.2011.02400.x. [DOI] [PubMed] [Google Scholar]
- 20.Galant A, Preussm ML, Cameron JC, Jez JM. Plant glutathione biosynthesis: diversity in biochemical regulation and reaction products. Front. Plant Sci. 2011;2:45. doi: 10.3389/fpls.2011.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jaleel CA, Riadh K, Gopi R, Manivannan P, Inès J, Al-Juburi HJ. et al. ZhaoAntioxidant defense response: physiological plasticity in higher plants under abiotic constraints. Acta Physiol Plant. 2009;31:427–36. [Google Scholar]
- 22.Baier M, Dietz KJ. Chloroplasts as source and target of cellular redox regulation: a discussion on chloroplast redox signals in the context of plant physiology. J Exp Bot. 2005;56:1449–62. doi: 10.1093/jxb/eri161. [DOI] [PubMed] [Google Scholar]
- 23.Korkina LG. Phenylpropanoids as naturally occurring antioxidants: from plant defense to human health. Cell Mol Biol. 2007;53:15–25. [PubMed] [Google Scholar]
- 24.Aharoni A, Dixit S, Jetter R, Thoenes E, van Arkel G, Pereira A. The SHINE clade of AP2 domain transcription factors activates wax biosynthesis, alters cuticle properties, and confers drought tolerance when overexpressed in Arabidopsis. Plant Cell. 2004;16:2463–2480. doi: 10.1105/tpc.104.022897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shao HB, Chu LY, Shao MA, Jaleel CA, Mi HM. Higher plant antioxidants and redox signaling under environmental stresses. C R Biol. 2008;331:433–41. doi: 10.1016/j.crvi.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 26.Van Breusegem F, Dat J. Reactive oxygen species in plant cell death. Plant Physiol. 2006;141:384–90. doi: 10.1104/pp.106.078295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suzuki N, Koussevitzky S, Mittler R, Miller G. ROS and redox signalling in the response of plants to abiotic stress. Plant Cell Environ. 2012;35:259–70. doi: 10.1111/j.1365-3040.2011.02336.x. [DOI] [PubMed] [Google Scholar]
- 28.Szalai G, Kellos T, Galiba G, Kocsy G. Glutathione as an antioxidant and regulatory molecule in plants under abiotic stress conditions. J Plant Growth Regul. 2009;28:66–80. [Google Scholar]
- 29.Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol. 2004;55:373–99. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- 30.Gao Q, Zhang L. Ultraviolet-B-induced oxidative stress and antioxidant defense system responses in ascorbate-deficient vtc1 mutants of Arabidopsis thaliana. J Plant Physiol. 2008;165:138–148. doi: 10.1016/j.jplph.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 31.Wang Z, Xiao Y, Chen W, Tang K, Zhang L. Increased vitamin C content accompanied by an enhanced recycling pathway confers oxidative stress tolerance in Arabidopsis. J Integr Plant Biol. 2010;52:400–09. doi: 10.1111/j.1744-7909.2010.00921.x. [DOI] [PubMed] [Google Scholar]
- 32.Espinoza A, San Martín A, López-Climent M, Ruiz-Lara S, Gómez-Cadenas A, Casaretto JA. Engineered drought-induced biosynthesis of α-tocopherol alleviates stress-induced leaf damage in tobacco. J Plant Physiol. 2013;170:1285–94. doi: 10.1016/j.jplph.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 33.Nicholson RL, Hammerschmidt R. Phenolic compounds and their role in disease resistance. Annu Rev Phytopathol. 1992;30:369–89. [Google Scholar]
- 34.Bailey BA, Strem MD, Bae H, Antunez de Mayolo G, Guiltinan MJ. Gene expression in leaves of Theobroma cacao in response to mechanical wounding, ethylene, and/or methyl jasmonate. Plant Sci. 2005;168:1247–58. [Google Scholar]
- 35.Michalak A. Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Polish J of Environ Stud. 2006;15:523–30. [Google Scholar]
- 36.Sakihama Y, Cohen MF, Grace SC, Yamasaki H. Plant phenolic antioxidant and prooxidant activities: phenolics-induced oxidative damage mediated by metals in plants. Toxicology. 2002;177:67–80. doi: 10.1016/s0300-483x(02)00196-8. [DOI] [PubMed] [Google Scholar]
- 37.Bennett RN, Wallsgrove RM. Secondary metabolites in plant defence mechanisms. New Phytol. 1994;127:617–633. doi: 10.1111/j.1469-8137.1994.tb02968.x. [DOI] [PubMed] [Google Scholar]
- 38.Winkel-Shirley B. Biosynthesis of flavonoids and effects of stress. Curr Opin Plant Biol. 2002;5:218–23. doi: 10.1016/s1369-5266(02)00256-x. [DOI] [PubMed] [Google Scholar]
- 39.Rivero RM, Ruiz JM, García PC, López-Lefebre LR, Sánchez E, Romero L. Resistance to cold and heat stress: accumulation of phenolic compounds in tomato and watermelon plants. Plant Sci. 2001;160:315–21. doi: 10.1016/s0168-9452(00)00395-2. [DOI] [PubMed] [Google Scholar]
- 40.Rozema J, van de Staaij J, Björn LO, Caldwell M. UV-B as an environmental factor in plant life: stress and regulation. Trends Ecol Evol. 1997;12:22–28. doi: 10.1016/s0169-5347(96)10062-8. [DOI] [PubMed] [Google Scholar]
- 41.Chappell J, Hahlbrock K. Transcription of plant defence genes in response to UV light and fungal elicitor. Nature. 1984;311:76–78. [Google Scholar]
- 42.Saltveit ME. Wound-induced changes in phenolic metabolism and tissue browning are altered by heat shock. Postharvest Biol Tec. 2000;21:61–69. [Google Scholar]
- 43.Barbehenn RV, Peter Constabel C. Tannins in plant-herbivore interactions. Phytochemistry. 2011;72:1551–65. doi: 10.1016/j.phytochem.2011.01.040. [DOI] [PubMed] [Google Scholar]
- 44.Jaleel CA, Manivannan P, Sankar B, Kishorekumar A, Gopi R, Somasundaram R. et al. Water deficit stress mitigation by calcium chloride in Catharanthus roseus: effects on oxidative stress, proline metabolism and indole alkaloid accumulation. Colloids Surf B Biointerfaces. 2007;60:110–116. doi: 10.1016/j.colsurfb.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 45.De Luca V, St Pierre B. The cell and developmental biology of alkaloid biosynthesis. Trends Plant Sci. 2000;5:168–173. doi: 10.1016/s1360-1385(00)01575-2. [DOI] [PubMed] [Google Scholar]
- 46.Patra B, Schluttenhofer C, Wu Y, Pattanaik S, Yuan L. Transcriptional regulation of secondary metabolite biosynthesis in plants. Biochim Biophys Acta. 2013;1829:1236–47. doi: 10.1016/j.bbagrm.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 47.Loreto F, Förster A, Dürr M, Csiky O, Seufert G. On the monoterpene emission under heat stress and on the increased thermotolerance of leaves of Quercus ilex L. fumigated with selected monoterpenes. Plant Cell Environ. 1998;21:101–07. [Google Scholar]
- 48.Kliebenstein DJ, Osbourn A. Making new molecules - evolution of pathways for novel metabolites in plants. Curr Opin Plant Biol. 2012;15:415–23. doi: 10.1016/j.pbi.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 49.Ziegler J, Facchini PJ. Alkaloid biosynthesis: metabolism and trafficking. Annu. Rev. Plant Biol. 2008;59:735–69. doi: 10.1146/annurev.arplant.59.032607.092730. [DOI] [PubMed] [Google Scholar]
- 50.Aharoni A, Jongsma MA, Bouwmeester HJ. Volatile science? Metabolic engineering of terpenoids in plants. Trends Plant Sci. 2005;10:594–02. doi: 10.1016/j.tplants.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 51.Baratta MT, Dorman HJD, Deans SG. Chemical composition, antimicrobial and antioxidative activity of laure, sage, rosemary, oregano and coriander essential oils. J Essent Oil Res. 1998;10:618–27. [Google Scholar]
- 52.Palozza P, Krinsky NI. Antioxidant effects of carotenoids in Vivo and in Vitro: An overview. Methods Enzymol. 1992;213:403–20. doi: 10.1016/0076-6879(92)13142-k. [DOI] [PubMed] [Google Scholar]
- 53.Burton GW, Ingold KU. Beta-Carotene: an unusual type of lipid antioxidant. Science. 1984;224:569–73. doi: 10.1126/science.6710156. [DOI] [PubMed] [Google Scholar]
- 54.Duthie GG, Duthie SJ, Kyle JA. Plant polyphenols in cancer and heart disease: implications as nutritional antioxidants. Nutr Res Rev. 2000;13:79–106. doi: 10.1079/095442200108729016. [DOI] [PubMed] [Google Scholar]
- 55.Myburgh KH. Polyphenol supplementation: benefits for exercise performance or oxidative stress? Sports Med. 2014;1:S57–70. doi: 10.1007/s40279-014-0151-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Blokhina O, Virolainen E, Fagerstedt KV. Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann Bot. 2003;91:179–94. doi: 10.1093/aob/mcf118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morgan JF, Klucas RV, Grayer RJ, Abian J, Becana M. Complexes of iron with phenolic compounds from soybean nodules and other legume tissues: prooxidant and antioxidant properties. Free Radic Biol Med. 1997;22:861–70. doi: 10.1016/s0891-5849(96)00426-1. [DOI] [PubMed] [Google Scholar]
- 58.Dewick PM. The Shikimate Pathway: Aromatic Amino Acids and Phenylpropanoids, in Medicinal Natural Products: A Biosynthetic Approach, 3rd Edition. Chichester, UK: John Wiley & Sons, Ltd; 2009. [Google Scholar]
- 59.Croft KD. The chemistry and biological effects of flavonoids and phenolic acids. Ann N Y Acad Sci. 1998;854:435–42. doi: 10.1111/j.1749-6632.1998.tb09922.x. [DOI] [PubMed] [Google Scholar]
- 60.Sakihama Y, Cohen MF, Grace SC, Yamasaki H. Plant phenolic antioxidant and prooxidant activities: phenolics-induced oxidative damage mediated by metals in plants. Toxicology. 2002;177:67–80. doi: 10.1016/s0300-483x(02)00196-8. [DOI] [PubMed] [Google Scholar]
- 61.Rice-Evans C, Miller N, Paganga G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997;2:152–59. [Google Scholar]
- 62.Antolovich M, Prenzler PD, Patsalides E, McDonald S, Robards K. Methods for testing antioxidant activity. Analyst. 2002;127:183–98. doi: 10.1039/b009171p. [DOI] [PubMed] [Google Scholar]
- 63.Karadag A, Ozcelik B, Saner S. Review of methods to determine antioxidant capacities. Food Anal. Methods. 2009;2:41–60. [Google Scholar]
- 64.Ou B, Woodill-Hampsch M, Prior RL. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J Agric Food Chem. 2001;49:4619–26. doi: 10.1021/jf010586o. [DOI] [PubMed] [Google Scholar]
- 65.Huang D, Ou B, Prior R L. The chemistry behind antioxidant capacity assays. J Agric Food Chem. 2005;53:1841–56. doi: 10.1021/jf030723c. [DOI] [PubMed] [Google Scholar]
- 66.Kasote DM. Flaxseed phenolics as natural antioxidants. Int Food Res J. 2013;20:1797–04. [Google Scholar]
- 67.Prior R, L Wu X, Schaich K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J Agric Food Chem. 2005;53:4290–02. doi: 10.1021/jf0502698. [DOI] [PubMed] [Google Scholar]
- 68.Kohen R, Nyska A. Oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol Pathol. 2002;30:620–50. doi: 10.1080/01926230290166724. [DOI] [PubMed] [Google Scholar]
- 69.Rodrigo R, Guichard C, Charles R. Clinical pharmacology and therapeutic use of antioxidant vitamins. Fundam Clin Pharmacol. 2007;21:111–27. doi: 10.1111/j.1472-8206.2006.00466.x. [DOI] [PubMed] [Google Scholar]
- 70.Nordgren M, Fransen M. Peroxisomal metabolism and oxidative stress. Biochimie. 2014;98:56–62. doi: 10.1016/j.biochi.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 71.Schrader M, Fahimi HD. Peroxisomes and oxidative stress. Biochim Biophys Acta. 2006;63:1755–66. doi: 10.1016/j.bbamcr.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 72.Fransen M, Nordgren M, Wang B, Apanasets O. Role of peroxisomes in ROS/RNS-metabolism: implications for human disease. Biochim Biophys Acta. 2012;1822:1363–73. doi: 10.1016/j.bbadis.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 73.Zhang K, Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature. 2008;454:455–62. doi: 10.1038/nature07203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thoms S, Grønborg S, Gärtner J. Organelle interplay in peroxisomal disorders. Trends Mol Med. 2009;15:293–02. doi: 10.1016/j.molmed.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 75.Farnsworth NR, Akerele O, Bingel AS, Soejarto DD, Guo Z. Medicinal plants in therapy. Bull World Health Organ. 1985;63:965–81. [PMC free article] [PubMed] [Google Scholar]
- 76.Hegde MV, Patil S, Bhalerao S. A philosophy for integration of ayurveda with modern medicine: A biochemist's perspective. Curr Sci. 2008;95:721–22. [Google Scholar]
- 77.Halliwell B. Drug antioxidant effects. Drugs. 1991;42:569–05. doi: 10.2165/00003495-199142040-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Halliwell B. Reactive species and antioxidants. redox biology is a fundamental theme of aerobic life. Plant Physiol. 2006;141:312–22. doi: 10.1104/pp.106.077073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Halliwell B. Commentary: Vitamin C: Antioxidant or Pro-Oxidant In Vivo? Free Rad Res. 1996;25:439–54. doi: 10.3109/10715769609149066. [DOI] [PubMed] [Google Scholar]
- 80.Bast A, Haenen GR. Ten misconceptions about antioxidants. Trends Pharmacol Sci. 2013;34:430–36. doi: 10.1016/j.tips.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 81.Putchala MC, Ramani P, Sherlin HJ, Premkumar P, Natesan A. Ascorbic acid and its pro-oxidant activity as a therapy for tumours of oral cavity -- a systematic review. Arch. Oral Biol. 2013;58:563–74. doi: 10.1016/j.archoralbio.2013.01.016. [DOI] [PubMed] [Google Scholar]
- 82.Heo JH. et al. The possible role of antioxidant vitamin C in Alzheimer's disease treatment and prevention. Am J Alzheimers Dis Other Demen. 2013;28:120–25. doi: 10.1177/1533317512473193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kang MJ, Lee SS, Koh HC. Prooxidant properties of ascorbic acid in the nigrostriatal dopaminergic system of C57BL/6 mice. Toxicology. 2012;294:1–8. doi: 10.1016/j.tox.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 84.Rietjens IM, Boersma MG, Haanm Ld, Spenkelink B, Awad HM, Cnubben NH. et al. The pro-oxidant chemistry of the natural antioxidants vitamin C, vitamin E, carotenoids and flavonoids. Environ Toxicol Pharmacol. 2002;11:321–33. doi: 10.1016/s1382-6689(02)00003-0. [DOI] [PubMed] [Google Scholar]
- 85.Du J, Cullen JJ, Buettner GR. Ascorbic acid: chemistry, biology and the treatment of cancer. Biochim Biophys Acta. 2012;1826:443–57. doi: 10.1016/j.bbcan.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen LH. Interaction of vitamin E and ascorbic acid (review) In Vivo. 1989;3:199–09. [PubMed] [Google Scholar]
- 87.Wagner BA, Buettner GR, Burns CP. Vitamin E slows the rate of free radical-mediated lipid peroxidation in cells. Arch Biochem Biophys. 1996;334:261–67. doi: 10.1006/abbi.1996.0454. [DOI] [PubMed] [Google Scholar]
- 88.Scalbert A, Williamson G. Dietary intake and bioavailability of polyphenols. J Nutr. 2000;130:2073S–2085S. doi: 10.1093/jn/130.8.2073S. [DOI] [PubMed] [Google Scholar]
- 89.Ross JA, Kasum CM. Dietary flavonoids: bioavailability, metabolic effects, and safety. Annu Rev Nutr. 2002;22:19–34. doi: 10.1146/annurev.nutr.22.111401.144957. [DOI] [PubMed] [Google Scholar]
- 90.Dai J, Mumper RJ. Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. Molecules. 2010;15:7313–52. doi: 10.3390/molecules15107313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Donovan JL, Manach C, Faulks RM, Kroon PA. Eds. absorption and metabolism of dietary plant secondary metabolites, in plant secondary metabolites: occurrence, structure and role in the human diet, Blackwell Publishing Ltd, Oxford, UK; 2006. [Google Scholar]
- 92.Scalbert A, Morand C, Manach C, Rémésy C. Absorption and metabolism of polyphenols in the gut and impact on health. Biomed. Pharmacother. 2002;56:76–82. doi: 10.1016/s0753-3322(02)00205-6. [DOI] [PubMed] [Google Scholar]
- 93.Williamson G, Barron D, Shimoi K, Terao J. In vitro biological properties of flavonoid conjugates found in vivo. Free Radic Res. 2005;39:457–69. doi: 10.1080/10715760500053610. [DOI] [PubMed] [Google Scholar]
- 94.Halliwell B, Rafter J, Jenner A. Health promotion by flavonoids, tocopherols, tocotrienols, and other phenols: direct or indirect effects? Antioxidant or not? Am J Clin Nutr. 2005;81:268S–76S. doi: 10.1093/ajcn/81.1.268S. [DOI] [PubMed] [Google Scholar]
- 95.Williams RJ, Spencer JP, Rice-Evans C. Flavonoids: antioxidants or signalling molecules? Free Radic Biol Med. 2004;36:838–49. doi: 10.1016/j.freeradbiomed.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 96.Decker EA. Phenolics: prooxidants or antioxidants? Nutr Rev. 1997;55:396–98. doi: 10.1111/j.1753-4887.1997.tb01580.x. [DOI] [PubMed] [Google Scholar]
- 97.Jomova K, Valko M. Health protective effects of carotenoids and their interactions with other biological antioxidants. Eur J Med Chem. 2013;70:102–10. doi: 10.1016/j.ejmech.2013.09.054. [DOI] [PubMed] [Google Scholar]
- 98.Young AJ, Lowe GM. Antioxidant and prooxidant properties of carotenoids. Arch Biochem Biophys. 2001;385:20–27. doi: 10.1006/abbi.2000.2149. [DOI] [PubMed] [Google Scholar]
- 99.Holst B, Williamson G. Nutrients and phytochemicals: from bioavailability to bioefficacy beyond antioxidants. Curr Opin Biotechnol. 2008;19:73–82. doi: 10.1016/j.copbio.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 100.Pietta PG. Flavonoids as antioxidants. J Nat Prod. 2000;63:1035–42. doi: 10.1021/np9904509. [DOI] [PubMed] [Google Scholar]
- 101.Prasad K. Antioxidant Activity of Secoisolariciresinol Diglucoside-derived Metabolites, Secoisolariciresinol, Enterodiol, and Enterolactone. Int J Angiol. 2000;9:220–25. doi: 10.1007/BF01623898. [DOI] [PubMed] [Google Scholar]