ABSTRACT
The soil-dwelling nematode Caenorhabditis elegans is a bacteriovorous animal, excreting the vast majority of its nitrogenous waste as ammonia (25.3±1.2 µmol gFW−1 day−1) and very little urea (0.21±0.004 µmol gFW−1 day−1). Although these roundworms have been used for decades as genetic model systems, very little is known about their strategy to eliminate the toxic waste product ammonia from their bodies into the environment. The current study provides evidence that ammonia is at least partially excreted via the hypodermis. Starvation reduced the ammonia excretion rates by more than half, whereas mRNA expression levels of the Rhesus protein CeRhr-2, V-type H+-ATPase (subunit A) and Na+/K+-ATPase (α-subunit) decreased correspondingly. Moreover, ammonia excretion rates were enhanced in media buffered to pH 5 and decreased at pH 9.5. Inhibitor experiments, combined with enzyme activity measurements and mRNA expression analyses, further suggested that the excretion mechanism involves the participation of the V-type H+-ATPase, carbonic anhydrase, Na+/K+-ATPase, and a functional microtubule network. These findings indicate that ammonia is excreted, not only by apical ammonia trapping, but also via vesicular transport and exocytosis. Exposure to 1 mmol l−1 NH4Cl caused a 10-fold increase in body ammonia and a tripling of ammonia excretion rates. Gene expression levels of CeRhr-1 and CeRhr-2, V-ATPase and Na+/K+-ATPase also increased significantly in response to 1 mmol l−1 NH4Cl. Importantly, a functional expression analysis showed, for the first time, ammonia transport capabilities for CeRhr-1 in a phylogenetically ancient invertebrate system, identifying these proteins as potential functional precursors to the vertebrate ammonia-transporting Rh-glycoproteins.
KEY WORDS: Vesicular transport, Na+/K+-ATPase, V-ATPase, Carbonic anhydrase
Highlighted Article: Hypodermal ammonia excretion in the soil nematode C. elegans involves Na+/K+-ATPase, V-ATPase, carbonic anhydrase, the microtuble network and a functional Rh protein.
INTRODUCTION
Ammonia is the primary end product of cellular amino acid metabolism occurring in tissues of all animals. In the present study, NH3 refers to gaseous ammonia, NH4+ to ammonium ions, and the term ‘ammonia’ is the sum of both molecules. In solutions, ammonia (pKa=9.2 to 9.8) occurs in a pH-dependent equilibrium of uncharged, membrane-permeable NH3 and ionic NH4+ (Cameron and Heisler, 1983). Because of its high pKa, the vast majority of ammonia exists in physiological solutions (i.e. body fluids), in the form of NH4+. As recently summarized in detail by Larsen et al. (2014), ammonia in either form, NH3 or NH4+, has multiple detrimental effects, which include disturbance of the energy metabolism (O'Donnell, 1997; Cooper and Plum, 1987; Wilkie, 1997), disruption of ionoregulatory capacity (Harris et al., 2001; Young-Lai et al., 1991) and immune competence in crustaceans (Le Moullac and Haffner, 2000) and has a number of severe negative effects on the central nervous system in mammals (Chan et al., 2000; Knecht et al., 1997; Marcaida et al., 1992; Norenberg et al., 1997; Palomero-Gallagher and Zilles, 2013). Because of its toxicity, ammonia is usually rapidly excreted either directly (as in most aquatic animals) or is detoxified into less harmful nitrogenous molecules such as urea (mammals, adult amphibians, elasmobranchs) and uric acid (other terrestrials, birds) (Larsen et al., 2014).
Currently, investigations on ammonia excretion/transport mechanisms in invertebrate systems are rather scarce and consequently the mechanisms involved are not fully understood. From studies of the past few decades, it has become clear that three proteins are key for the transcellular transport of ammonia in animals, the Na+/K+-ATPase, the V-type H+-ATPase (V-ATPase) and Rhesus (Rh) proteins. For the basolateral localized Na+/K+-ATPase, it was shown in 1960 by Jens Skou that this enzyme does accept NH4+ as a substrate by replacing K+ ions (Skou, 1960) and thereby is capable of actively pumping NH4+ from the body fluids into the respective ammonia-transporting epithelial cell. The direct participation of this pump in the ammonia transport mechanism has now been identified for numerous systems, including those in gills of crustaceans (Furriel et al., 2004; Masui et al., 2002; Weihrauch et al., 1998; Weihrauch et al., 1999) and fish (Mallery, 1983; Nawata et al., 2010a; Wood et al., 2013), frog skin (Cruz et al., 2013), mammalian kidney (Garvin et al., 1985; Wall and Koger, 1994) and intestine (Worrell et al., 2008). The second pump often, if not always, involved in the ammonia transport processes is the V-ATPase. This pump does not transport ammonia directly but generates, by acidifying one side of a given membrane, a partial pressure gradient for NH3, which can then diffuse along this gradient either directly through the lipid bilayer (Goldman and Rottenberg, 1973) or via Rh proteins, functioning as NH3 channels (Gruswitz et al., 2010). The participation of the V-ATPase in ammonia transport processes has been confirmed for many systems investigated so far, such as those in the gills of crustaceans (Weihrauch et al., 2002) and fish (Weihrauch et al., 2009; Wright and Wood, 2009), the epidermis of planarians (Weihrauch et al., 2012b), fish skin (Shih et al., 2008) and for the ammonia-uptake mechanism in the lepidopteran midgut (Weihrauch, 2006). Finally, in the year 2000, Marini and co-workers discovered that members of the Rhesus protein family, when expressed in yeast are able to mediate ammonia transport (Marini et al., 2000). Subsequent functional expression analysis showed that the glycosylated members of the Rh protein family (Rhag, Rhbg, Rhcg) from mammals and fish are functional ammonia transporters (Ludewig, 2004; Mak et al., 2006; Marini et al., 2000; Nakada et al., 2010; Nawata et al., 2010a,b; Zidi-Yahiaoui et al., 2005). For the human Rh protein RhCG, an X-ray crystallographic structure analysis revealed that this transporter forms a trimeric complex and each monomer promotes the passage of NH3 (Gruswitz et al., 2010). A role for Rh proteins in ammonia transport processes was also suggested in invertebrates. Indeed, in freshwater planarians (Weihrauch et al., 2012b), decapod crabs (Martin et al., 2011; Weihrauch et al., 2004b), insects (Weihrauch, 2006; Weihrauch et al., 2012a) and squid (Hu et al., 2013), an abundance of mRNA encoding Rh-like proteins was identified in the respective ammonia-transporting epithelia. However, although all invertebrates investigated so far were found to express Rh proteins (Huang and Peng, 2005), no evidence of their ability to transport ammonia has been published to date.
The animal of interest in this study is Caenorhabditis elegans, a nematode that lives in temperate regions within the water film of soil particles. These roundworms are bacterivorous, feeding on various types of bacteria and thereby having a predictably high protein intake (Nicholas, 1975). To date, very little is known regarding the nitrogen excretion strategy in this or other nematodal species, with the exception of a study conducted approximately 50 years ago on Caenorhabditis briggsae, a close relative of C. elegans, which indicated C. briggsae excretes predominantly ammonia and very small amounts of urea and uric acid (Rothstein, 1963). In nematodes, possible excretion routes include the gut, the nephridial system (excretory cell or H-cell) and the integument. In C. elegans, genes coding for two Rh proteins, CeRhr-1 and CeRhr-2, have been identified. The two proteins are highly conserved and similar to the mammalian Rh-glycoproteins. A transgenic analysis indicated that CeRhr-1 is predominantly expressed in the hypodermis, with lower levels of expression in other cell types (Ji et al., 2006). These findings suggest that the hypodermis possibly acts as a major site for ammonia excretion. The objective of this study is to further explore the ammonia excretion mechanism in terrestrial nematodes, employing C. elegans as a model system. Emphasis is given to the hypodermally expressed Rh protein CeRhr-1, which was investigated using a yeast complementation assay.
RESULTS
Feeding and starvation
In control (fed) worms, daily urea excretion rates (control medium, non buffered, pH 7) were about 10 times lower than ammonia excretion rates (Fig. 1).
Fig. 1.
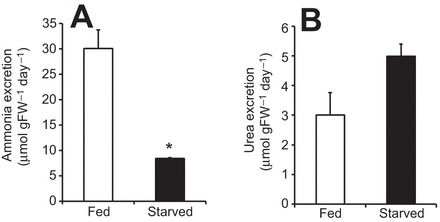
Ammonia and urea excretion rates of C. elegans under fed and starved (24 h) conditions. (A) Ammonia excretion. (B) Urea excretion. Data represent means±s.e.m. and were analyzed employing an unpaired, two-tailed Student's t-test (N=4–5); *P≤0.05.
Under fed conditions, high metabolic loads of ammonia, high excretion rates and high mRNA expression levels of involved transporters are assumed, which should alter when internal ammonia loads are lower during starvation. When animals were deprived of any food source for 24 h, daily ammonia excretion rates substantially decreased approximately by 72%, when compared with animals fed over the respective 24 h time period (Fig. 1A). In contrast, urea excretion rates did not change during starvation (Fig. 1B). Compared with control conditions, a mRNA expression analysis revealed that starvation caused downregulation of transcript levels encoding the CeRhr-2 protein, the subunit A of V-ATPase (hypodermal expressed isoform 8) and the α-subunit of the Na+/K+-ATPase, whereas expression levels of CeRhr-1 remained unchanged (Fig. 2). For comparison, quantitative PCR analyses revealed that for control worms, the highest mRNA expression levels were noted for the Na+/K+-ATPase (α-subunit), followed by the subunit A of the V-ATPase, CeRhr-1 and CeRhr-2 with, respectively, 10.57±1.64, 4.0±0.24, 0.39±0.019 and 0.03±0.0031 fg cDNA ng−1 total RNA (mean±s.d.).
Fig. 2.
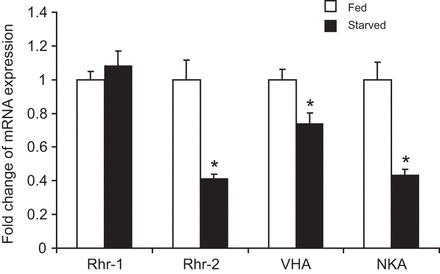
Changes of mRNA expression levels of CeRhr-1, CeRhr-2, V-ATPase (subunit A) and Na+/K+-ATPase (α-subunit) in C. elegans under fed and starved (24 h) conditions. CeRhr-1, N=4–5; CeRhr-2, N=5; V-ATPase (subunit A), N=4–5; Na+/K+-ATPase (α-subunit), N=4–5. Absolute mRNA expression levels of fed animals were set to 1 while values measured under starved conditions are given as ‘fold change’ of the respective control. *Significant differences between treatments (P≤0.05). Data represent means±s.e.m. and were analyzed using an unpaired, two-tailed Student's t-test prior to calculation for fold change values.
Ammonia excretion mechanism
In order to evaluate whether ammonia trapping was involved in the excretion mechanism, the ammonia excretion rates were monitored when animals were exposed in short-term experiments to various environmental pH regimes. When compared with control experiments (non-buffered medium, pH 7), the ammonia excretion rates remained unchanged in media buffered to pH 5.5, 7 (M9 medium) and 8, but increased when animals were placed in medium buffered to acidic pH 5 (Fig. 3). A decrease in the excretion rate by ∼33% and 45% was observed in an alkaline medium buffered to pH 9 and 9.5, respectively.
Fig. 3.
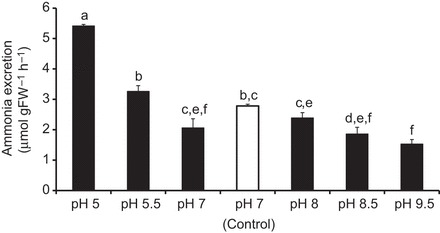
Ammonia excretion rates of C. elegans in media adjusted and buffered to various pH regimes. Control conditions are unbuffered control medium (pH=7). Data represent means±s.e.m. For statistical analysis, a Kruskal–Wallis test was applied with post hoc Mann–Whitney pairwise comparisons (N=4–6). Significant differences are indicated by different letters.
Pharmacological experiments were conducted to investigate the participation of enzymes and mechanisms, commonly found elsewhere to be involved with ammonia excretion, in invertebrates. Ammonia excretion rates in control worms were found to be very consistent between experiments with an average of 3.3±0.19 µmol gFW−1 h−1 (N=20). Rates were reduced by ∼28%, 61%, and 26% when the control medium was enriched by 5 µmol l−1 concanamycin C, 5 mmol l−1, acetazolamide, and 2 mmol l−1 colchicine targeting the V-type H+-ATPase, carbonic anhydrase and microtubule network, respectively. No inhibition was detected when 5 mmol l−1 ouabain was applied to target the Na+/K+-ATPase (Fig. 4).
Fig. 4.
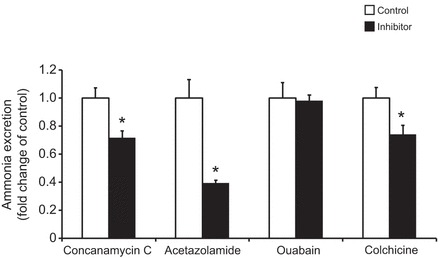
Effects of different inhibitors on ammonia excretion rates in C. elegans. Control values for each treatment were set to 1, with values measured under the influence of the inhibitors are given as ‘fold change’ of the respective control. The concentrations of the inhibitors were: concanamycin C, 5 µmol l−1 (N=5–6); acetazolamide, 5 mmol l−1 (N=5–6); ouabain, 5 mmol l−1 (N=6); colchicine, 2 mmol l−1 (N=5–6). Data represent means±s.e.m. and were analyzed employing an unpaired, two-tailed Student's t-test prior to calculation for fold change values. *P≤0.05.
The pharmacological experiments suggested that the Na+/K+-ATPase is not involved in the ammonia excretion process. In order to validate this result, enzyme activity assays were conducted, revealing that in control worms, the Na+/K+-ATPase does accept NH4+ as a substrate with a slightly lower but insignificant reduction in activity rate when K+ was replaced by NH4+ (Fig. 5).
Fig. 5.
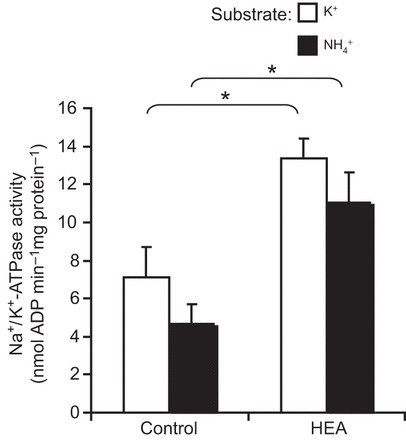
Specific activity of the Na+/K+-ATPase in C. elegans exposed to control conditions or acclimated for 48 h to 1 mmol l−1 NH4Cl (HEA). Either 10 mmol l−1 KCl or 10 mmol l−1 NH4Cl was used as substrate in the assay. Control worms, N=5–6; worms acclimated for 48 h to 1 mmol l−1 NH4Cl (HEA), N=5–6. *Significant differences between control worms and HEA acclimated worms (P≤0.05). Data represent means±s.e.m. and were analyzed employing an unpaired, two-tailed Student's t-test.
In addition, to determine whether the effects observed upon the application of colchicine were due to an overall reduction in metabolic rate, respiration rates were measured in control animals and in worms exposed to 2 mmol l−1 colchicine. The respiration rate decreased over 2 h, independent of the presence of colchicine; however, the rate of control worms (60±11 pmol O2 min−1 mg wet mass−1) was not different (P=0.29; N=4, paired Student's t-test) from the rate of worms exposed to colchicine (74±22 pmol O2 min−1 mg wet mass−1).
Functional expression of CeRhr-1
Sequence analysis of CeRhr-1 (GenBank accession number: NM_072035) showed that this 463 amino acid protein has 12 predicted transmembrane domains (Ji et al., 2006) with an N-glycolysation site at residue 276 (Prosearch in Biology WorkBench) (supplementary material Fig. S1). A sequence alignment of CeRhr-1 with Rh proteins from mammals, tunicates and other invertebrates including CeRhr-2 (the second Rh protein in C. elegans), revealed that all Rh proteins from these diverse animal groups contain the conserved AA residues (supplementary material Fig. S1) that are important for ammonia conductance (Zidi-Yahiaoui et al., 2009). In order to evaluate the role of CeRhr-1 in ammonia transport, functional expression analysis was performed using a yeast-complementation assay. Saccharomyces cerevisiae cells lacking their three ammonium transport systems (triple-mepΔ) are unable to grow on ammonium supplied as sole nitrogen source at a concentration below 5 mmol l−1 (Marini et al., 1997). As seen in Fig. 6, triple-mepΔ cells expressing CeRhr-1 were able to grow on ammonium after 4 days, in contrast to triple-mepΔ cells, which do not express CeRh-1. Growth of triple-mepΔ cells expressing CeRhr-1 on ammonium is similar to the growth of triple-mepΔ cells expressing HsRhCG, previously described as an ammonium transport protein (Marini et al., 2000). These results indicate that CeRhr-1 is also able to transport ammonium in yeast. Of note, similar growth was observed for all the strains in the presence of glutamate as a nitrogen source (the positive growth control).
Fig. 6.
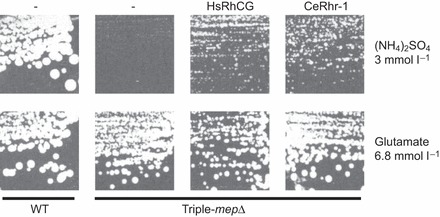
Growth tests of S. cerevisiae yeast cells on solid minimal medium. Medium contained 3 mmol l−1 (NH4)2SO4 or 6.8 mmol l−1 glutamate (positive growth control) as the sole nitrogen source. Wild-type cells (23344c) were transformed with the empty p426 vector (-) and, triple-mepΔ cells (31019b), deprived of the three endogenous Mep proteins, were transformed with the empty p426 vector (-) or, with a multi-copy plasmid (p426) bearing the HsRhCG, or CeRhr1 genes. Cells were incubated for 4 days at 29°C.
High environmental ammonia
In order to investigate the physiological response to elevated environmental ammonia levels, worms were exposed for 24 h to various high environmental ammonia (HEA) concentrations (with no food source supplemented during this time period). Compared with control levels, excretion rates decreased in the presence of 100 and 200 µmol l−1 NH4Cl, but roughly doubled and tripled when worms were exposed to 500 and 1000 µmol l−1 NH4Cl, respectively (Fig. 7). Moreover, when animals were exposed for 2 days to 1 mmol l−1 HEA (with no food source supplemented for the second day) body ammonia increased ∼10-fold, whereas body urea content only slightly increased (P=0.066) (Fig. 8). Furthermore, a 2 day exposure to 1 mmol l−1 HEA caused a significant increase of mRNA expression levels of CeRhr-1, CeRhr-2, V-ATPase (subunit A) and the α-subunit of the Na+/K+-ATPase (Fig. 9). Simultaneously, the activity of the Na+/K+-ATPase, increased in ammonia exposed animals ∼2-fold, regardless of whether K+ or NH4+ was provided in the assay as a substrate (Fig. 5).
Fig. 7.
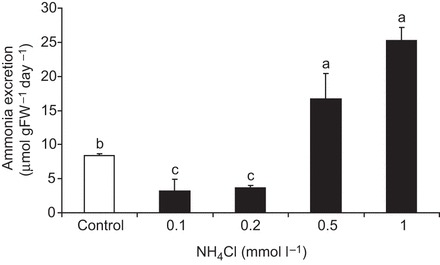
Ammonia excretion rates of C. elegans acclimated for 2 days to various NH4Cl concentrations. Excretion was measured in the corresponding NH4Cl concentration (N=4–5 for each condition). Control conditions were unbuffered control medium (pH=7, no NH4Cl added). Data represent means±s.e.m. For statistical analysis, a Kruskal–Wallis test was applied with post hoc Mann–Whitney pairwise comparisons. Significant differences are indicated by different letters.
Fig. 8.
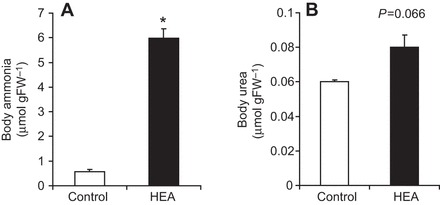
Ammonia and urea body content in C. elegans. (A) Ammonia body concentration and (B) urea body concentration in control worms (N=5–6) and worms acclimated for 2 days to 1 mmol l−1 NH4Cl (N=6). Data represent means±s.e.m. and were analyzed with an unpaired, two-tailed Student's t-test. *P≤0.05.
Fig. 9.
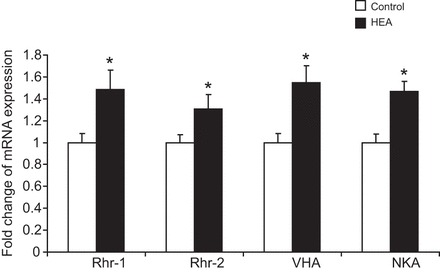
Changes in mRNA expression levels of CeRhr-1, CeRhr-2, V-ATPase (subunit A) and Na+/K+-ATPase (α-subunit) in C. elegans. CeRhr-1 (N=3–5), CeRhr-2 (N=4–5), V-ATPase (subunit A) (N=4–5) and Na+/K+-ATPase (α-subunit) (N=4–5) in control C. elegans and worms acclimated for 2 days to 1 mmol l−1 NH4Cl (HEA). Data represent means±s.e.m. and were analyzed with an unpaired, two-tailed Student's t-test prior to calculation of fold change values. *P≤0.05.
DISCUSSION
Feeding and starvation
Caenorhabditis elegans is a soil nematode that feeds continuously on bacteria (Abada et al., 2009). Therefore, in most experiments the fed state was considered as the control state. The obtained data confirmed that the worms are ammonotelic, as previously documented for C. briggsae (Rothstein, 1963), excreting roughly 10 times more ammonia than urea. Ammonia excretion rates were similar when compared with the relatively small freshwater planarian Schmidtea mediterrana (Weihrauch et al., 2012b) after feeding.
In the current study, the mRNA expression levels of Rhr-2, V-ATPase (subunit A) and Na+/K+-ATPase (α-subunit) in non-fed/starved animals were significantly lower in comparison to expression levels detected in feeding animals. A similar response was reported for V-ATPase and Rh protein mRNA expression levels in fed and starved planarians (Weihrauch et al., 2012b) and for V-ATPase and Rhcg2 in gills of fed and 48 h fasted freshwater trout (Zimmer et al., 2010). Since expression levels of Rhr-2, Na+/K+-ATPase (α-subunit) and V-ATPase (subunit A) (Fig. 2) are positively correlated with the ammonia excretion rates, this is a first indication, that these transporters at least are involved in the ammonia excretion and/or handling process in the worms. CeRhr-1, which is predominantly localized within the hypodermis but also in other tissues (Ji et al., 2006) did not respond to the putatively higher systemic ammonia load after feeding. Compared with CeRhr-2, CeRhr-1 showed, under control conditions, ∼10 times higher mRNA expression levels, suggesting that this protein serves as a housekeeping gene capable of dealing with normal physiological fluctuations of internal ammonia loads. In fact, CeRhr-1 is crucial in embryonic development (Ji et al., 2006), underlining the overall importance of this gene.
Ammonia excretion mechanism
Exposing C. elegans for 2 h to a low-pH environment (Fig. 3) caused a significant increase in ammonia excretion, suggesting ammonia excretion across the hypodermis via ammonia trapping as observed in the epidermis of freshwater planarians (Weihrauch et al., 2012b) and in the gill epithelia of fresh and seawater fish (Larsen et al., 2014; Weihrauch et al., 2009; Wright and Wood, 2009). The pharmacological experiments confirmed the participation of a V-ATPase and the carbonic anhydrase in the ammonia excretion mechanism. Further support for the ammonia trapping theory comes from a related study, which shows direct proton excretion via the hypodermis of C. elegans using the selective ion electrode technique (Adlimoghaddam et al., 2014). The multilayered cuticle could act here as an ‘unstirred apical boundary layer’ (Peixoto and De Souza, 1995). However, little change in the excretion rate within the pH range 8 to 5.5 also suggests that ammonia trapping in conjunction with an apical NH3 pathway plays only a partial, possibly rather minor, role in the overall ammonia excretion mechanism.
Interestingly, ammonia excretion also depended at least partially on a functional microtubule network, as evident from the application of colchicine, which caused a reduction in the excretion rates. The current study therefore suggests that ammonia is trapped in acidified vesicles, which are then transported along the microtubule network to the apical membrane, where NH4+ is released by exocytosis. Such a mechanism would keep cytoplasmic ammonia concentration low to avoid potentially toxic effects. This has been described so far only for invertebrate systems, including active branchial ammonia excretion in the green shore crab Carcinus maenas (Weihrauch et al., 2002), and for active ammonia uptake in the midgut of the tobacco hornworm Manduca sexta (Weihrauch, 2006). Of note, a vesicular microtubule-dependent mechanism for ammonia excretion would make this soil-dwelling organism rather independent of the quite variable environmental pH (Brady and Weil, 2008). This could explain the invariant ammonia excretion rates when the worms were placed in a buffered alkaline pH environment of 8.0 (Fig. 3), creating a situation where the partial pressure gradient for NH3 is reversed, assuming a physiological cytoplasmatic pH of ∼7.2–7.8. In organisms where no microtubule-dependent ammonia excretion mechanism is in place, ammonia excretion is hampered in alkaline environments, as observed in freshwater planarians (Weihrauch et al., 2012b) and leeches (A.R. Quijada-Rodriguez, personal communication). For basal ammonia uptake into the hypodermis, the obtained data indicate a participation of the Na+/K+-ATPase, which is supported by the finding that the enzyme does function unperturbed when NH4+ was given as a substrate instead of K+ along with Na+, Mg2+ and ATP. The lack of effect on whole animal ammonia excretion upon the application of ouabain could be explained by the basal ‘hard-to-reach’ localization of the pump. As mentioned in the Introduction, the Na+/K+-ATPase is usually a key player in transepithelial ammonia transport processes (Larsen et al., 2014).
Functional expression of CeRhr-1
The results in the current study show that the hypodermally expressed CeRhr-1 protein is capable of mediating transport of ammonia when expressed in yeast: a finding that has not been published for any other invertebrate species to date. The fact that invertebrates are phylogenetically much older than vertebrates (Huang and Peng, 2005) underlines the general importance of this result, showing that the so-called primitive Rh proteins (Rh-Ps) could indeed be functional precursors of the vertebrate Rh-isoforms Rhag, Rhbg and Rhcg. As reported for many other Rh proteins, e.g. from the green crab Carcinus maenas (Weihrauch et al., 2004b), the Dungeness crab Metacarcinus magister (Martin et al., 2011), the rainbow trout Rhbg (Nawata et al., 2007) and the human RhCG (also called RhGK or PDRC2, GenBank accession number: AF081497), CeRhr-1 also has 12 predicted transmembrane domains in its amino acid sequence. An amino acid alignment (supplementary material Fig. S1) further confirmed that the amino acids presumably important for the ammonia conductance, as shown for the human RhCG (Zidi-Yahiaoui et al., 2009), are also conserved in CeRhr-1. One exception is that V231 of CeRhr-1 V231 showed more variability (supplementary material Fig. S1).
The high abundance of CeRhr-1 in the hypodermis (Ji et al., 2006), as well as the shown ammonia transport capacity of the protein strongly suggests that this transporter plays a significant role in the hypodermal ammonia excretion mechanism. Since considerably higher mRNA expression levels of CeRhr-1 were determined in comparison to those of CeRhr-2, the authors of the current study speculate a rather basal localization of the transporter, similar to the vertebrate Rhbg/RhBG. Indeed, higher mRNA expression levels were found for Rhbg/RhBG in the ammonia-transporting frog skin (Cruz et al., 2013) and also for the mammalian intestine (Handlogten et al., 2005) and fish skin (Nawata et al., 2007). Future studies must confirm both the cellular localization of CeRhr-1 and CeRhr-2 in the hypodermis and the ammonia transport capabilities of Rhr-2.
Effects of high environmental ammonia
When animals starved for 24 h were exposed to sublethal high concentrations of ammonia (0.5 and 1 mmol l−1), ammonia excretion rates increased significantly compared with controls (Fig. 7). This increase was accompanied by an augmentation of expression levels of genes important for ammonia excretion (Fig. 9), providing proper elimination of the ammonia load. Simultaneously, levels of body ammonia increased substantially by about 900%, whereas levels of body urea did not change (Fig. 8). Increased levels of body ammonia due to an extended exposure to 1 mmol l−1 ammonia was also observed in the freshwater planarian Schmidtea mediterranea (Weihrauch et al., 2012b), however, with only a doubling of the body ammonia content. A similar high increase in levels of body ammonia was detected in rainbow trout embryos when exposed for 2 days to ∼1.7 mmol l−1 ammonia (Sashaw et al., 2010); however, this increase was accompanied with an enhanced ammonia uptake, not ammonia excretion. The reduced ammonia excretion observed when worms were exposed to fairly low environmental ammonia concentrations (0.1 and 0.2 mmol l−1) could possibly be explained by a decrease or even a reversal of the ammonia gradient from the animal to the environment, assuming extracellular ammonia concentrations are somehow similar (100–200 µmol l−1) to values found other ammonotelic invertebrates (Weihrauch et al., 1999). Under such circumstances, active or secondarily active ammonia excretion could well be hampered, as shown for the gills of decapod crustaceans (Weihrauch et al., 1999).
Preliminary model for the hypodermal ammonia excretion mechanism in the soil nematode C. elegans
Because the functional CeRhr-1 ammonia transporter is expressed predominantly in the hypodermis (Ji et al., 2006) and environmental applied inhibitors have a direct effect on the ammonia excretion rates, it is assumed that at least a certain portion of the overall metabolic ammonia load is excreted via the hypodermis. The obtained data suggest that ammonia from the body fluids is actively transported via the Na+/K+-ATPase into the cytoplasm of the hypodermal syncytium where part of the ammonia is trapped into acidified vesicles, which are then transported along the microtubule network to the apical side for release of NH4+ by exocytotic processes. In addition, an apical V-ATPase is thought to generate a partial pressure gradient for NH3 by acidifying the unstirred space within the cuticle, driving NH3 out via an NH3-permeable pathway, possibly by CeRhr-2. The data further suggest that a cytoplasmic carbonic anhydrase provides protons for a V-ATPase, which is localized in the apical membrane of the hypodermis and also in the membrane of intracellular vesicles. Basal entrance of NH3 and, because of its assumed dual transport characteristics (Perry et al., 2010) possibly also CO2, is probably mediated by CeRhr-1. The cellular localization of the two Rh proteins in C. elegans is, however, speculative and requires further study. Involvement of a sodium–hydrogen exchanger (NHE), which could also participate in acidifying the unstirred layer over the apical membrane, has not been the subject of this study. In C. elegans, the hypodermal expressed sodium/proton exchanger NHX-3 is not localized in the apical membrane, but rather in intracellular vesicles (Nehrke and Melvin, 2002). In addition, in a parallel study, we gathered some evidence that NHEs are not likely to be involved in the ammonia excretion mechanism in C. elegans (Adlimoghaddam et al., 2014).
MATERIALS AND METHODS
Nematode cultivation
The hermaphrodite wild-type Caenorhabditis elegans Maupas 1900 strain (N2) used in this investigation was obtained from the Caenorhabditis Genetics Center (CGC, University of Minnesota, Minneapolis). C. elegans was maintained in the laboratory on Nematode Growth Medium (NGM) seeded with Escherichia coli OP50 as a food source at 16°C according to the standard methods described by Brenner (1974).
After 5 days of incubation, the chunking method was performed for transferring gravid worms from old to freshly seeded NGM plates for revitalization of the animals (Hope, 1999). After an incubation period of 2 days at 16°C, animals were washed from the plates with M9 buffer (22 mmol l−1 KH2PO4, 43.5 mmol l−1 Na2HPO4, 85.54 mmol l−1 NaCl and 3 mmol l−1 MgSO4, pH 7) and transferred aseptically into 250 ml of liquid medium (S-Basal: 43 mmol l−1 KH2PO4, 5.6 mmol l−1 K2HPO4 and 97 mmol l−1 NaCl, 0.92 mmol l−1 citric acid monohydrate, 8.81 mmol l−1 tri-potassium citrate monohydrate, 0.048 mmol l−1 disodium EDTA, 0.024 mmol l−1 FeSO4·7H2O, 0.015 mmol l−1 MnCl2·4H2O, 0.017 mmol l−1 ZnSO4·7H2O, 0.00097 mmol l−1 CuSO4·5H2O, 2.92 mmol l−1 CaCl2 and 2.92 mmol l−1 MgSO4). The liquid culture medium (250 ml) was enriched with ‘heat killed’ (100°C, 1 h) E. coli OP50 originating from a 1 liter overnight culture (∼13×109 cells ml−1) as a food source. The liquid culture was incubated at room temperature and agitated (Innova 2000 platform-shaker, New Brunswick, Canada) at 200 r.p.m. for 3 days. Worms from this liquid culture were then subjected to subsequent experiments. All experiments on living worms were performed at room temperature (RT, 22°C).
Nitrogen excretion experiments
Inhibitor and pH experiments
In this set of short-term experiments (2 h treatment), the effect of various pH regimes and inhibitors on the ammonia excretion rates were investigated. Here 0.1–0.15 g of worms from liquid culture were washed twice with 16 ml control medium (22 mmol l−1 KCl, 129 mmol l−1 NaCl, 1 mmol l−1 MgSO4, adjusted to pH 7 with HCl/NaOH) by centrifugation (880 g, 2 min) with subsequent discharging of the supernatant. After the washing steps, animals were exposed either to 8 ml control medium, phosphate buffered M9 medium (pH 7), low pH medium [control medium enriched with 5 mmol l−1 2-(N-orpholino)ethanesulfonic acid (MES) adjusted to pH 5 and 5.5] and high-pH medium (control medium enriched with 5 mmol l−1 Tris-HCl, pH 8.0, 8.5, 9.5). For all inhibitor experiments, the control medium was enriched with a particular inhibitor and the pH adjusted to 7. Inhibitors (targets are given in brackets) were employed at the following concentrations: 5 mmol l−1 acetazolamide (carbonic anhydrase); 2 mmol l−1 colchicine (microtubule network); 0.005 mmol l−1 concanamycin C (V-type H+-ATPase); 5 mmol l−1 ouabain (Na+/K+-ATPase). Concanamycin C was dissolved in dimethyl sulfoxide (DMSO) with a final concentration of 0.5%. In these experiments, 0.5% DMSO was also added to the control solutions. Inhibitor concentrations were chosen to be within the range applied in comparable studies on other invertebrates (Weihrauch et al., 1998, 2012b, 2004a). Comparatively high doses were needed for acetazolamide and colchicine, probably because of the need to penetrate the apical membrane of the hypodermal syncytium.
At the end of all experiments, worms were evaluated under the microscope for performance and survival under the influence of the respective inhibitor or pH treatments for at least 2 h.
A first sampling was carried out following a 30 min pre-incubation period (200 r.p.m., RT). After the pre-incubation phase, worms were pelleted (880 g, 1 min) and a 4 ml sample (out of 8 ml total) was taken to determine background ammonia. After re-suspension, the experiment continued with the remaining 4 ml for 1.5 h (200 r.p.m., RT) with subsequent sampling. All samples were frozen immediately at −80°C for further ammonia analysis. Background ammonia values measured after the pre-incubation phase were subtracted from values gained at the final sampling step. At the end of each experiment, fresh weights (FW) of pelleted worms (1700 g, 3 min) were determined to the nearest mg.
Starvation and HEA
In this series of experiments, 8 ml of the liquid culture (equals a total fresh weight of worms of ∼0.1–0.15 g) were transferred to a 15 ml tube and subsequently washed twice with 15 ml control medium (880 g, 2 min). After the second wash, worms were cultivated (250 g, RT) in 8 ml of sterilized control medium, enriched with ‘heat-killed’ E. coli OP50 for 24 h. After a washing step (2×880 g, 2 min) the medium was replaced, with either control medium (starved animals) or control medium enriched with ‘heat-killed’ E. coli OP50 (fed animals). Under these different conditions, worms were cultured (250 g, RT) for another 24 h. For HEA experiments, animals were starved as described above, but over the entire time period (2×24 h) the media was enriched with 1 mmol l−1 NH4Cl (HEA). At the end of the second 24 h period, worms were pelleted (1700 g, 3 min) and the supernatant frozen immediately at −80°C for further ammonia and urea analysis. After determination of the FW, worms were subjected to the RNA isolation procedure.
Determination of ammonia and urea
Body ammonia concentration
Determination of body ammonia was modified from Veauvy et al. (2009) with the entire procedure performed on ice or in a refrigerated centrifuge. Frozen (−80°C) worms (∼0.1–0.15 g) were homogenized (Powermax AHS 200, VWR international, Radnor, PA, USA) in 10 volumes of ice-cold 6% perchloric acid (assuming a 1 g ml−1 density of tissue). Homogenates were left on ice for 10 min to allow for deproteinization of the samples to occur. Homogenates were then centrifuged (5 min, 4°C, 5000 g). One milliliter of supernatant was then removed and stored at −80°C for further urea analysis. The rest of the supernatant was neutralized with 0.6 volumes of 2.5 mmol l−1 KHCO3. Neutralized samples were centrifuged (5 min, 4°C, 16,000 g) and immediately analyzed for ammonia using a NH3-selective electrode (see below).
Determination of ammonia and urea in excretion experiments using an NH3-selective electrode
Ammonia samples from excretion experiments were analyzed using a gas-sensitive NH3 electrode (Thermo Orion, Beverly, MA, USA) connected to a digital pH meter as described in detail by Weihrauch et al. (1998) For the parallel determination of ammonia (A) and urea (U), samples were split in half, SA and SU, as also described by Cruz et al. (2013). Sample A was analyzed for background ammonia concentration, whereas sample SU was treated with urease type III, employed at a final concentration of 10 units ml−1 (Sigma, St Louis, MO, USA) for 30 min at room temperature to convert all urea into ammonia and CO2. The actual urea concentration in the sample was then calculated according to the following equation:
| 1 |
Standard curves were prepared from the corresponding experimental solutions and all r2 values were greater than 0.98. Samples with high ammonia background (HEA experiments) were diluted 1:20 in control medium (pH 7).
Determination of body urea
After deproteinization (see body ammonia measurements), body urea was analyzed utilizing a colorimetric assay established by Rahmatullah and Boyde (1980). In brief, two parts sample or standard were mixed with one part assay reagents and incubated in a 100°C water bath for 10 min. Samples were cooled and absorbance at 540 nm was spectrophotometrically analyzed on a microplate reader (Powerwave, BioTek, Winooski, VT, USA). Assay reagent concentrations were as follows: 1.2 mmol l−1 H2SO4, 0.33 mmol l−1 H3PO4, 13.7 μmol l−1 FeCl3, 0.61 nmol l−1 thiosemicarbazide and 2.74 μmol l−1 diacetylmonoxime. Urea standards (0, 20, 40, 80, 100 and 120 µmol l−1 urea) were always prepared fresh in deionized water.
Na+/K+-ATPase activity
Control worms and worms acclimated for 48 h to 1 mmol l−1 NH4Cl were collected and frozen in −80°C until processing for the respective enzyme activity assay. Frozen samples (0.6–0.8 g FW) were added to approximately 2–3 volumes of ice-cold homogenization buffer [150 mmol l−1 sucrose, 50 mmol l−1 imidazole, 10 mmol l−1 EDTA and 0.1% (w/v) sodium deoxycholate] and homogenized at 4°C using three bursts (10 s each) using an Ultra-Turrax homogenizer. Samples were left to cool on ice between bursts. The homogenates were centrifuged at 5000 g for 1 min at 4°C and the supernatant was used then directly for the enzyme activity assays. The protein concentration of the supernatants was determined using the Biuret assay with bovine serum albumin as a standard.
The activity of the Na+/K+-ATPase was assessed spectrophotometrically using an assay based on previous assays linking ADP formation to NADH oxidation (Gibbs and Somero, 1989; McCormick, 1993). Samples were added to a reaction buffer (pH 7.5 at 20°C) containing 100 mmol l−1 NaCl, 5 mmol l−1 MgCl2, 50 mmol l−1 imidazole, 3 mmol l−1 ATP, 2 mmol l−1 phosphoenolpyruvate, 0.2 mmol l−1 NADH, 5 IU ml−1 pyruvate kinase and 4 IU ml−1 lactate dehydrogenase using either 10 mmol l−1 KCl or 10 mmol l−1 NH4Cl as the substrate. All assays were performed at 20°C and the Na+/K+-ATPase activity was determined as the difference in the rate of absorbance change at 340 nm in the presence (Na+/K+-ATPase dependent activity) or absence (total ATPase activity) of 1 mmol l−1 ouabain in parallel cuvettes using a mmol l−1 extinction coefficient of 6.2 for NADH. See also Cruz et al. (2013) for further details.
Respiration
Worms were cultured under control conditions, pelleted and washed as described above, split into two and resuspended at 25–50 mg fresh mass per ml in either control medium or control medium supplemented with 2 mmol l−1 of colchicine in a total volume of 2 ml. Respiration was measured at 20°C using an OxygraphY2K (Oroboros Instruments Corp., Innsbruck, Austria) under conditions of constant stirring. Oxygen consumption was monitored over 2 h, with periodic opening of the chambers to reoxygenate medium and prevent unwanted effects of hypoxia. Oxygen concentrations were determined assuming an oxygen solubility of 0.9 for the medium relative to distilled water, to account for the effects of solutes on oxygen solubility, and automatically corrected for barometric pressure and assay temperature by the Oroboros software. The average for at least 10 min of linear oxygen consumption, between approximately 90 and 120 min after addition of worms to the chamber, was taken for each measurement to determine the rate of oxygen consumption per mg of fresh mass of worms.
Quantitative PCR
Total RNA was extracted under RNase-free conditions using the RNeasy plus Mini Kit (Qiagen Inc, Mississauga, Ontario, Canada). For cDNA synthesis, 0.3 µg total RNA was treated with DNase I (Invitrogen, Carlsbad, CA, USA) to eliminate any genomic DNA contamination. Total RNA concentration of the samples were quantified and tested for quality by means of a NanoDrop 2000C spectrophotometer (Nanodrop Technologies, Wilmington, DE, USA). The DNase treated RNA was further checked for DNA contamination by a high-cycle (40 cycles) polymerase chain reaction (PCR) using the primer pair CeActin-F/CeActin-R (supplementary material Table S1). Treated RNA (0.3 µg) was reverse transcribed into cDNA (iScript™ cDNA Synthesis Kit, Bio-Rad, Mississauga, Ontario, Canada). All primers useed in qPCR were designed based on published sequences as indicated in supplementary material Table S1. Optimized PCR protocols for each primer pair gained a single amplicon of the predicted size (data not shown). In addition, PCR products were evaluated for accuracy by sequencing (Robarts Research Institute, London, Ontario, Canada). For qPCR (Miniopticon, Biorad, Mississauga, Ontario, Canada), standard curves were generated using a dilution series of known quantities (104, 103, 102, 101, 100, 10−1 fg DNA) of the respective purified PCR product (QIAquick Gel Extraction Kit, Qiagen, Mississauga, Ontario, Canada) of the target gene. A minimum R2 value of 0.98 was required for the standard curve. Real-time PCR assays were performed employing SSoFast™ EvaGreen supermix (Bio-Rad, Mississauga, Ontario, Canada) in a 15 μl assay. Single-product PCR was verified performing a melting curve analysis.
Yeast complementation assay
Specific primers (supplementary material Table S1) were designed to target the entire open reading frame (ORF) for C. elegans Rhr-1. These primers contained restriction sites for SpeI on the 5′ end and SmaI on the 3′ end, facilitating directional cloning into the yeast expression vector (pRS426-MET25). The ORF were amplified by Phusion high-fidelity DNA polymerase (Thermo Scientific, Ottawa, ON, Canada) and sequenced to confirm accuracy (Robarts Research Institute, London, Ontario, Canada). After digestion with SpeI and SmaI using the FastDigest kit (Thermo Scientific, Ottawa, ON, Canada), the PCR product and pRS426-MET25 vector were gel purified (QIAquick Gel Extraction Kit, Qiagen Inc, Mississauga, ON, Canada) and ligated with the DNA ligase kit (Promega, Madison, WI, USA). The ligation reaction was incubated overnight at 4°C und used for transformation of competent E. coli DH5α cells. Vectors containing the Rhr-1 ORF were isolated from the overnight culture using the Qiaprep Spin Miniprep kit (Qiagen, Mississauga, ON, Canada). Accuracy and orientation of the insert was confirmed by sequencing (Robarts Research Institute, London, Ontario, Canada). Saccharomyces cerevisiae strains used in this study are 23344c, ura3 (M. Grenson, personal communication) and 31019b, mep1Δ mep2Δ mep3Δ ura3 (Marini et al., 1997). They are isogenic with the wild-type Σ1278b (Bechet et al., 1970). Cells were transformed by heat shock (42°C) after permeabilization with acetate lithium (100 mmol l−1) treatment (Gietz et al., 1992). Cells were grown in a minimal buffered (pH 6.1) medium with 3% glucose as the carbon source (Jacobs et al., 1980). To this medium, nitrogen sources, such as 6.8 mmol l−1 or 3 mmol l−1 ammonium [(NH4)2SO4] were added as specified in the text.
Chemicals
Ammonium chloride, Tris hydrochloride and MES were purchased from Fisher Scientific (Ottawa, Ontario, Canada). If not stated otherwise, all other chemicals were purchased at analytical grade from Sigma-Aldrich (St Louis, MO, USA).
Statistics
In this study, each N value represents the combined pooled worms with a mass of ∼0.1 g. Values from all experiments specified as the mean±s.e.m. All statistical analyses were performed using PAST3 (http://palaeo-electronica.org/2001_1/past/issue1_01.htm; Hammer et al., 2001). All data sets for comparison of multiple means were first tested for normal distribution with the Shapiro–Wilk test (α=5%), followed by log-transformation in case the null-hypothesis (H0=data is normally distributed) had to be rejected. Levene's test was performed to ensure homogeneity of variance. Because data for Figs 3 and 7 were not normally distributed (before and after log transformation) and/or homogeneity of variance was not given, the Kruskal–Wallis test was applied with post hoc Mann–Whitney pairwise comparisons. For all other experiments, an unpaired Student's t-test was applied. All results were considered significant at P≤0.05. The statistical method used in each particular experiment is given in the respective figure legends.
Supplementary Material
Acknowledgements
The authors would like to thank Sandra Fehsenfeld for re-evaluating the statistical analysis of this study.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
A.A. designed and executed the majority of the experiments, analyzed the data and wrote the manuscript. M.B. and A.-M.M. conducted the yeast expression studies. A.A. and J.R.T. designed and conducted experiments to determine respiration rates and measure enzyme activity. A.A. and A.-K.C.B. cultured the worms. D.W. (project leader) provided intellectual input and revised the manuscript. All authors reviewed and corrected the manuscript.
Funding
This work was supported by NSERC Canada Discovery Grants to D.W., A.-K.C.B. M.B., J.R.T. and A.-M.M. were supported by F.R.S.M. (3.4546.04 and 3.4633.09) and M.I.S. (F.4.521.10.F) grants from the F.R.S.-FNRS, an ARC convention (AUWB-2012-12/17), and the Jean Brachet and the Alice et David Van Buuren Foundations. D.W., J.R.T. and A.-K.C.B. are also supported by Canada Foundation for Innovation. J.R.T. is also supported by the Canada Research Chairs program. C. elegans strains were provided by the CGC, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440).
Supplementary material
Supplementary material available online at http://jeb.biologists.org/lookup/suppl/doi:10.1242/jeb.111856/-/DC1
References
- Abada E. A.-e., Sung H., Dwivedi M., Park B.-J., Lee S.-K. and Ahnn J. (2009). C. elegans behavior of preference choice on bacterial food. Mol. Cells 28, 209-213. 10.1007/s10059-009-0124-x [DOI] [PubMed] [Google Scholar]
- Adlimoghaddam A., Weihrauch D. and O'Donnell M. J. (2014). Localization of K+, H+, Na+ and Ca2+ fluxes to the excretory pore in Caenorhabditis elegans: application of scanning ion-selective microelectrodes. J. Exp. Biol. 217, 4119-4122. 10.1242/jeb.112441 [DOI] [PubMed] [Google Scholar]
- Bechet J., Grenson M. and Wiame J. M. (1970). Mutations affecting the repressibility of arginine biosynthetic enzymes in Sacchromyces cerevisiae. Eur. J. Biochem. 12, 31-39. 10.1111/j.1432-1033.1970.tb00817.x [DOI] [PubMed] [Google Scholar]
- Brady N. C. and Weil R. R. (2008). The Nature and Properties of Soils. Columbus, OH, USA: Prentice Hall Publishing. [Google Scholar]
- Brenner S. (1974). The genetics of Caenorhabditis elegans. Genetics 77, 71-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron J. N. and Heisler N. (1983). Studies of ammonia in the rainbow trout: physicochemical parameters, acid-base behaviour and respiratory clearance. J. Exp. Biol. 105, 107-125. [Google Scholar]
- Chan H., Hazell A. S., Desjardins P. and Butterworth R. F. (2000). Effects of ammonia on glutamate transporter (GLAST) protein and mRNA in cultured rat cortical astrocytes. Neurochem. Int. 37, 243-248. 10.1016/S0197-0186(00)00026-7 [DOI] [PubMed] [Google Scholar]
- Cooper A. J. L. and Plum F. (1987). Biochemistry and physiology of brain ammonia. Physiol. Rev. 67, 440-519. [DOI] [PubMed] [Google Scholar]
- Cruz M. J., Sourial M. M., Treberg J. R., Fehsenfeld S., Adlimoghaddam A. and Weihrauch D. (2013). Cutaneous nitrogen excretion in the African clawed frog Xenopus laevis: effects of high environmental ammonia (HEA). Aquat. Toxicol. 136-137, 1-12. 10.1016/j.aquatox.2013.03.002 [DOI] [PubMed] [Google Scholar]
- Furriel R. P. M., Masui D. C., McNamara J. C. and Leone F. A. (2004). Modulation of gill Na+,K+-ATPase activity by ammonium ions: putative coupling of nitrogen excretion and ion uptake in the freshwater shrimp Macrobrachium olfersii. J. Exp. Zool. A Comp. Exp. Biol. 301A, 63-74. 10.1002/jez.a.20008 [DOI] [PubMed] [Google Scholar]
- Garvin J. L., Burg M. B. and Knepper M. A. (1985). Ammonium replaces potassium in supporting sodium transport by the Na-K-ATPase of renal proximal straight tubules. Am. J. Physiol. 249, F785-F788. [DOI] [PubMed] [Google Scholar]
- Gibbs A. and Somero G. N. (1989). Pressure adaptation of Na+/K+-ATPase in gills of marine teleosts. J. Exp. Biol. 143, 475-492. [DOI] [PubMed] [Google Scholar]
- Gietz D., St Jean A., Woods R. A. and Schiestl R. H. (1992). Improved method for high efficiency transformation of intact yeast cells. Nucleic Acids Res. 20, 1425 10.1093/nar/20.6.1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman R. and Rottenberg H. (1973). Ion distribution in lysosomal suspensions. FEBS Lett. 33, 233-238. 10.1016/0014-5793(73)80200-5 [DOI] [PubMed] [Google Scholar]
- Gruswitz F., Chaudharya S., Ho J. D., Schlessinger A., Pezeshki B., Ho C.-M., Sali A., Westhoff C. M. and Stroud R. M. (2010). Function of human Rh based on structure of RhCG at 2.1Å. Proc. Natl. Acad. Sci. USA 107, 9638-9643. 10.1073/pnas.1003587107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer Ø., Harper D. A. T. and Ryan P. D. (2001). PAST: paleontological statistics software package for education and data analysis Palaeontol. Electron. 4, (9 pp.). [Google Scholar]
- Handlogten M. E., Hong S.-P., Zhang L., Vander A. W., Steinbaum M. L., Campbell-Thompson M. and Weiner I. D. (2005). Expression of the ammonia transporter proteins Rh B glycoprotein and Rh C glycoprotein in the intestinal tract. Am. J. Physiol. Gastrointest. Liver Physiol. 288, G1036-G1047. 10.1152/ajpgi.00418.2004 [DOI] [PubMed] [Google Scholar]
- Harris R., Coley S., Collins S. and McCabe R. (2001). Ammonia uptake and its effects on ionoregulation in the freshwater crayfish Pacifastacus leniusculus (Dana). J. Comp. Physiol. B 171, 681-693. 10.1007/s003600100219 [DOI] [PubMed] [Google Scholar]
- Hope I. A. (1999). C. elegans: A Practical Approach. Oxford: Oxford University Press. [Google Scholar]
- Hu M. Y., Lee J.-R., Lin L.-Y., Shih T.-H., Stumpp M., Lee M.-F., Hwang P.-P. and Tseng Y.-C. (2013). Development in a naturally acidified environment: Na+/H+-exchanger 3-based proton secretion leads to CO2 tolerance in cephalopod embryos. Front. Zool. 10, 51 10.1186/1742-9994-10-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C.-H. and Peng J. (2005). Evolutionary conservation and diversification of Rh family genes and proteins. Proc. Natl. Acad. Sci. USA 102, 15512-15517. 10.1073/pnas.0507886102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs P., Jauniaux J.-C. and Grenson M. (1980). A cis-dominant regulatory mutation linked to the argB-argC gene cluster in Saccharomyces cerevisiae. J. Mol. Biol. 139, 691-704. 10.1016/0022-2836(80)90055-8 [DOI] [PubMed] [Google Scholar]
- Ji Q., Hashmi S., Liu Z., Zhang J., Chen Y. and Huang C.-H. (2006). CeRh1 (rhr-1) is a dominant Rhesus gene essential for embryonic development and hypodermal function in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 103, 5881-5886. 10.1073/pnas.0600901103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht K., Michalak A., Rose C., Rothstein J. D. and Butterworth R. F. (1997). Decreased glutamate transporter (GLT-1) expression in frontal cortex of rats with acute liver failure. Neurosci. Lett. 229, 201-203. 10.1016/S0304-3940(97)00444-8 [DOI] [PubMed] [Google Scholar]
- Larsen E. H., Deaton L. E., Onken H., O'Donnell M., Grosell M., Dantzler W. H. and Weihrauch D. (2014). Osmoregulation and excretion. Comp. Physiol. 4, 405-573. 10.1002/cphy.c130004 [DOI] [PubMed] [Google Scholar]
- Le Moullac G. and Haffner P. (2000). Environmental factors affecting immune responses in Crustacea. Aquaculture 191, 121-131. 10.1016/S0044-8486(00)00422-1 [DOI] [Google Scholar]
- Ludewig U. (2004). Electroneutral ammonium transport by basolateral rhesus B glycoprotein. J. Physiol. 559, 751-759. 10.1113/jphysiol.2004.067728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak D.-O. D., Dang B., Weiner I. D., Foskett J. K. and Westhoff C. M. (2006). Characterization of ammonia transport by the kidney Rh glycoproteins RhBG and RhCG. Am. J. Physiol. Renal Physiol. 290, F297-F305. 10.1152/ajprenal.00147.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallery C. H. (1983). A carrier enzyme basis for ammonium excretion in teleost gill. NH+4-stimulated Na-dependent ATPase activity in Opsanus beta. Comp. Biochem. Physiol. A Comp. Physiol. 74, 889-897. 10.1016/0300-9629(83)90364-X [DOI] [PubMed] [Google Scholar]
- Marcaida G., Felipo V., Hermenegildo C., Miñana M.-D. and Grisolia S. (1992). Acute ammonia toxicity is mediated by the NMDA type of glutamate receptors. FEBS Lett. 296, 67-68. 10.1016/0014-5793(92)80404-5 [DOI] [PubMed] [Google Scholar]
- Marini A. M., Soussi-Boudekou S., Vissers S. and Andre B. (1997). A family of ammonium transporters in Saccharomyces cerevisiae. Mol. Cell. Biol. 17, 4282-4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marini A. M., Matassi G., Raynal V., Andre B., Cartron J. P. and Cherif-Zahar B. (2000). The human Rhesus-associated RhAG protein and a kidney homologue promote ammonium transport in yeast. Nat. Genet. 26, 341-344. 10.1038/81656 [DOI] [PubMed] [Google Scholar]
- Martin M., Fehsenfeld S., Sourial M. M. and Weihrauch D. (2011). Effects of high environmental ammonia on branchial ammonia excretion rates and tissue Rh-protein mRNA expression levels in seawater acclimated Dungeness crab Metacarcinus magister. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 160, 267-277. 10.1016/j.cbpa.2011.06.012 [DOI] [PubMed] [Google Scholar]
- Masui D. C., Furriel R. P. M., McNamara J. C., Mantelatto F. L. M. and Leone F. A. (2002). Modulation by ammonium ions of gill microsomal (Na+,K+)-ATPase in the swimming crab Callinectes danae: a possible mechanism for regulation of ammonia excretion. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 132, 471-482. 10.1016/S1532-0456(02)00110-2 [DOI] [PubMed] [Google Scholar]
- McCormick S. D. (1993). Methods for nonlethal gill biopsy and measurement of Na+, K+-ATPase activity. Can. J. Fish. Aquat. Sci. 50, 656-658. 10.1139/f93-075 [DOI] [Google Scholar]
- Nakada T., Westhoff C. M., Yamaguchi Y., Hyodo S., Li X., Muro T., Kato A., Nakamura N. and Hirose S. (2010). Rhesus glycoprotein p2 (Rhp2) is a novel member of the Rh family of ammonia transporters highly expressed in shark kidney. J. Biol. Chem. 285, 2653-2664. 10.1074/jbc.M109.052068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawata C. M., Hung C. C. Y., Tsui T. K. N., Wilson J. M., Wright P. A. and Wood C. M. (2007). Ammonia excretion in rainbow trout (Oncorhynchus mykiss): evidence for Rh glycoprotein and H+-ATPase involvement. Physiol. Genomics 31, 463-474. 10.1152/physiolgenomics.00061.2007 [DOI] [PubMed] [Google Scholar]
- Nawata C. M., Hirose S., Nakada T., Wood C. M. and Kato A. (2010a). Rh glycoprotein expression is modulated in pufferfish (Takifugu rubripes) during high environmental ammonia exposure. J. Exp. Biol. 213, 3150-3160. 10.1242/jeb.044719 [DOI] [PubMed] [Google Scholar]
- Nawata C. M., Wood C. M. and O'Donnell M. J. (2010b). Functional characterization of Rhesus glycoproteins from an ammoniotelic teleost, the rainbow trout, using oocyte expression and SIET analysis. J. Exp. Biol. 213, 1049-1059. 10.1242/jeb.038752 [DOI] [PubMed] [Google Scholar]
- Nehrke K. and Melvin J. E. (2002). The NHX family of Na+-H+ exchangers in Caenorhabditis elegans. J. Biol. Chem. 277, 29036-29044. 10.1074/jbc.M203200200 [DOI] [PubMed] [Google Scholar]
- Nicholas W. L. (1975). The Biology of Free-Living Nematodes. University of California: Oxford University Press. [Google Scholar]
- Norenberg M. D., Huo Z., Neary J. T. and Roig-Cantesano A. (1997). The glial glutamate transporter in hyperammonemia and hepatic encephalopathy: relation to energy metabolism and glutamatergic neurotransmission. Glia 21, 124-133. [DOI] [PubMed] [Google Scholar]
- O'Donnell M. J. (1997). Mechanisms of excretion and ion transport in invertebrates. In Comparative Physiology (ed. Dantzler W. H.), pp. 1207-1289. New York: Oxford University Press. [Google Scholar]
- Palomero-Gallagher N. and Zilles K. (2013). Neurotransmitter receptor alterations in hepatic encephalopathy: a review. Arch. Biochem. Biophys. 536, 109-121. 10.1016/j.abb.2013.02.010 [DOI] [PubMed] [Google Scholar]
- Peixoto C. A. and De Souza W. (1995). Freeze-fracture and deep-etched view of the cuticle of Caenorhabditis elegans. Tissue Cell 27, 561-568. 10.1016/S0040-8166(05)80065-5 [DOI] [PubMed] [Google Scholar]
- Perry S. F., Braun M. H., Noland M., Dawdy J. and Walsh P. J. (2010). Do zebrafish Rh proteins act as dual ammonia-CO2 channels? J. Exp. Zool. A Ecol. Genet. Physiol. 313A, 618-621. 10.1002/jez.631 [DOI] [PubMed] [Google Scholar]
- Rahmatullah M. and Boyde T. R. C. (1980). Improvements in the determination of urea using diacetyl monoxime; methods with and without deproteinisation. Clin. Chim. Acta 107, 3-9. 10.1016/0009-8981(80)90407-6 [DOI] [PubMed] [Google Scholar]
- Rothstein M. (1963). Nematode biochemistry-III. Excretion products. Comp. Biochem. Physiol. 9, 51-59. 10.1016/0010-406X(63)90028-8 [DOI] [PubMed] [Google Scholar]
- Sashaw J., Nawata M., Thompson S., Wood C. M. and Wright P. A. (2010). Rhesus glycoprotein and urea transporter genes in rainbow trout embryos are upregulated in response to alkaline water (pH 9.7) but not elevated water ammonia. Aquat. Toxicol. 96, 308-313. 10.1016/j.aquatox.2009.11.012 [DOI] [PubMed] [Google Scholar]
- Shih T.-H., Horng J.-L., Hwang P.-P. and Lin L.-Y. (2008). Ammonia excretion by the skin of zebrafish (Danio rerio) larvae. Am. J. Physiol. Cell Physiol. 295, C1625-C1632. 10.1152/ajpcell.00255.2008 [DOI] [PubMed] [Google Scholar]
- Skou J. C. (1960). Further investigations on a Mg+++Na+-activated adenosintriphosphatase, possibly related to the active, linked transport of Na+ and K+ across the nerve membrane. Biochim. Biophys. Acta 42, 6-23. 10.1016/0006-3002(60)90746-0 [DOI] [Google Scholar]
- Veauvy C. M., Walsh P. J. and McDonald M. D. (2009). Effect of elevated ammonia on tissue nitrogen metabolites in the ureotelic gulf toadfish (Opsanus beta) and the ammoniotelic midshipman (Porichthys notatus). Physiol. Biochem. Zool. 82, 345-352. 10.1086/588829 [DOI] [PubMed] [Google Scholar]
- Wall S. M. and Koger L. M. (1994). NH+4 transport mediated by Na(+)-K(+)-ATPase in rat inner medullary collecting duct. Am. J. Physiol. 267, F660-F670. [DOI] [PubMed] [Google Scholar]
- Weihrauch D. (2006). Active ammonia absorption in the midgut of the Tobacco hornworm Manduca sexta L.: iransport studies and mRNA expression analysis of a Rhesus-like ammonia transporter. Insect Biochem. Mol. Biol. 36, 808-821. 10.1016/j.ibmb.2006.08.002 [DOI] [PubMed] [Google Scholar]
- Weihrauch D., Becker W., Postel U., Riestenpatt S. and Siebers D. (1998). Active excretion of ammonia across the gills of the shore crab Carcinus maenas and its relation to osmoregulatory ion uptake. J. Comp. Physiol. B 168, 364-376. 10.1007/s003600050156 [DOI] [Google Scholar]
- Weihrauch D., Becker W., Postel U., Luck-Kopp S. and Siebers D. (1999). Potential of active excretion of ammonia in three different haline species of crabs. J. Comp. Physiol. B 169, 25-37. 10.1007/s003600050190 [DOI] [Google Scholar]
- Weihrauch D., Ziegler A., Siebers D. and Towle D. W. (2002). Active ammonia excretion across the gills of the green shore crab Carcinus maenas: participation of Na(+)/K(+)-ATPase, V-type H(+)-ATPase and functional microtubules. J. Exp. Biol. 205, 2765-2775. [DOI] [PubMed] [Google Scholar]
- Weihrauch D., McNamara J. C., Towle D. W. and Onken H. (2004a). Ion-motive ATPases and active, transbranchial NaCl uptake in the red freshwater crab, Dilocarcinus pagei (Decapoda, Trichodactylidae). J. Exp. Biol. 207, 4623-4631. 10.1242/jeb.01333 [DOI] [PubMed] [Google Scholar]
- Weihrauch D., Morris S. and Towle D. W. (2004b). Ammonia excretion in aquatic and terrestrial crabs. J. Exp. Biol. 207, 4491-4504. 10.1242/jeb.01308 [DOI] [PubMed] [Google Scholar]
- Weihrauch D., Wilkie M. P. and Walsh P. J. (2009). Ammonia and urea transporters in gills of fish and aquatic crustaceans. J. Exp. Biol. 212, 1716-1730. 10.1242/jeb.024851 [DOI] [PubMed] [Google Scholar]
- Weihrauch D., Donini A. and O'Donnell M. J. (2012a). Ammonia transport by terrestrial and aquatic insects. J. Insect. Physiol. 58, 473-487. 10.1016/j.jinsphys.2011.11.005 [DOI] [PubMed] [Google Scholar]
- Weihrauch D., Chan A. C., Meyer H., Doring C., Sourial M. and O'Donnell M. J. (2012b). Ammonia excretion in the freshwater planarian Schmidtea mediterranea. J. Exp. Biol. 215, 3242-3253. 10.1242/jeb.067942 [DOI] [PubMed] [Google Scholar]
- Wilkie M. P. (1997). Mechanisms of ammonia excretion across fish gills. Comp. Biochem. Physiol. A 118, 39-50. 10.1016/S0300-9629(96)00407-0 [DOI] [Google Scholar]
- Wood C. M., Nawata C. M., Wilson J. M., Laurent P., Chevalier C., Bergman H. L., Bianchini A., Maina J. N., Johannsson O. E., Bianchini L. F. et al. (2013). Rh proteins and NH4(+)-activated Na+-ATPase in the Magadi tilapia (Alcolapia grahami), a 100% ureotelic teleost fish. J. Exp. Biol. 216, 2998-3007. 10.1242/jeb.078634 [DOI] [PubMed] [Google Scholar]
- Worrell R. T., Merk L. and Matthews J. B. (2008). Ammonium transport in the colonic crypt cell line, T84: role for Rhesus glycoproteins and NKCC1. Am. J. Physiol. Gastrointest. Liver Physiol. 294, G429-G440. 10.1152/ajpgi.00251.2006 [DOI] [PubMed] [Google Scholar]
- Wright P. A. and Wood C. M. (2009). A new paradigm for ammonia excretion in aquatic animals: role of Rhesus (Rh) glycoproteins. J. Exp. Biol. 212, 2303-2312. 10.1242/jeb.023085 [DOI] [PubMed] [Google Scholar]
- Young-Lai W. W., Charmantier-Daures M. and Charmantier G. (1991). Effect of ammonia on survival and osmoregulation in different life stages of the lobster Homarus americanus. Mar. Biol. 110, 293-300. 10.1007/BF01313716 [DOI] [Google Scholar]
- Zidi-Yahiaoui N., Mouro-Chanteloup I., D'Ambrosio A.-M., Lopez C., Gane P., Le van Kim C., Cartron J.-P., Colin Y. and Ripoche P. (2005). Human Rhesus B and Rhesus C glycoproteins: properties of facilitated ammonium transport in recombinant kidney cells. Biochem. J. 391, 33-40. 10.1042/BJ20050657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zidi-Yahiaoui N., Callebaut I., Genetet S., Le Van Kim C., Cartron J. P., Colin Y., Ripoche P. and Mouro-Chanteloup I. (2009). Functional analysis of human RhCG: comparison with E. coli ammonium transporter reveals similarities in the pore and differences in the vestibule. Am. J. Physiol. Cell Physiol. 297, C537-C547. 10.1152/ajpcell.00137.2009 [DOI] [PubMed] [Google Scholar]
- Zimmer A. M., Nawata C. M. and Wood C. M. (2010). Physiological and molecular analysis of the interactive effects of feeding and high environmental ammonia on branchial ammonia excretion and Na(+) uptake in freshwater rainbow trout. J. Comp. Physiol. B 180, 1191-1204. 10.1007/s00360-010-0488-4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


