Abstract
Diabetic nephropathy (DN) has become the leading cause of end-stage renal disease worldwide. Non-diabetic renal disease (NDRD), is known to occur in diabetic patients. The renal and retinal relationship in type 2 diabetes mellitus (T2DM) with nephropathy is not uniform. This study was carried to study the histological spectrum of nephropathy in type 2 diabetic patients with proteinuria and its relationship with diabetic retinopathy (DR). Total 31 (males - 26; females - 5) proteinuric type 2 diabetic patients were studied. Average age of patients was 50.7 years. Nephrotic syndrome was noted in 21 (67.7%) patients. Overall, isolated DN, NDRD and NDRD superimposed on DN (mixed lesion) were observed in 12 (38.7%), 13 (41.9%) and 6 (19.4%) cases, respectively. DR was absent in 21/31 (67.7%) cases. The spectrum of nephropathy in patients without DR included: DN in 6 (28.57%), NDRD in 12 (57.14%) and mixed lesion in 3 (14.29%). Kidney histology in patients with DR (n-10) revealed DN in 6 (60%), NDRD in 1 (10%) and mixed lesion in 3 (30%) patients. Thus, absence of DR favors NDRD but does not exclude DN because isolated DN was noted in 28.57% cases in absence of DR. Similarly biopsy proven NDRD (pure NDRD; 10% and mixed lesion; 30%) was noted in 40% of cases in presence of DR. In summary, patients with T2DM had higher incidence of NDRD. DR is less frequent (32.3%) in type 2 diabetes and is a poor predictor of type of nephropathy. Hence, renal biopsy is essential for precise diagnosis of nephropathy in patients with T2DM.
Keywords: Diabetic retinopathy, nephrotic syndrome, non-diabetic renal disease, type 2 diabetes mellitus
Introduction
The incidence and prevalence of diabetes mellitus (DM) are increasing.[1] As per USRDS reports, although the incidence of end-stage kidney disease (ESKD) from diabetes has declined by 1.8% in 2008 as compared with the preceding year, diabetes still remains the most common cause of ESKD in the United States and in many other countries.[2] Diabetic nephropathy (DN) is not the sole renal disease in diabetic patients. Kidney diseases other than diabetic nephropathy can occur in type 2 diabetic patients and such kidney diseases are known as non-diabetic renal disease (NDRD), either isolated or superimposed on DN.[3] The occurrence of NDRD in diabetic patients is reported in several studies from around the world.[4,5,6,7,8,9] DN is hard to reverse. However, certain NDRD, such as mesangial-proliferative glomerulonephritis, IgA nephropathy and membranous nephropathy, are often treatable, even remittable.[7,9] Thus, therapy and prognosis of DN and NDRD are quite different. Therefore, differential diagnosis of these two entities in diabetic patients is of considerable importance. Keeping in view the above knowledge, this study was performed to analyze spectrum of nephropathy in proteinuric type 2 diabetic patients and to study the relationship between nephropathy and retinopathy in patients with type 2 diabetes mellitus (T2DM) with proteinuria.
Materials and Methods
This study was carried in the Department of Nephrology, Sir Sunderlal Hospital, Institute of Medical Sciences, Banaras Hindu University, Varanasi, from October 2011 to September 2013. Diabetes was diagnosed as per WHO criteria.[10] All type 2 diabetic patients of both gender attending the nephrology services were screened for proteinuria. The urine was examined for detection of protein using dipstick method. The patients with dipstick proteinuria of 1+ or more were subjected to quantitative 24-h urinary protein. Type 2 diabetic patients with proteinuria of more than 1 g were included in this study. The duration between diagnosis of DM and onset of renal manifestations were recorded in individual patients. Patients with absence of voluntary consent, contraindication to kidney biopsy and severe renal failure (serum creatinine > 5 mg/dl) were excluded from the study. All patients were subjected to detailed history, physical examination and laboratory investigations. The kidney biopsy was done under ultrasound guidance. Biopsy sample was analyzed by light microscopy using Hematoxylin and Eosin stain, periodic acid Schiff stain, and acid Fuchsin orange G stains. In necessary cases, methyl violet and Congo red staining were also done. All patients included in this study underwent following investigations.
Urine was examined for red blood cell (RBC), white blood cell, urinary casts and crystals. 24-h protein excretion was estimated by sulfosalicylic acid (SSA reagent) precipitation method. Hematological investigations included Hb, total leukocyte count, differential leukocyte count and platelet count. Biochemical investigations included; serum creatinine, serum sodium and potassium, serum calcium, inorganic phosphate, serum glutamic pyruvic transaminase/serum glutamic oxaloacetic transaminase, bilirubin (total/direct), total protein, serum albumin, fasting blood glucose and 2-h postprandial and HbA1C. Immunological assays such as C3, C4, anti-Ds DNA antibody and anti-neutrophil cytoplasmic antibodies and viral markers (HBsAg and anti-hepatitis C virus) were carried in selected cases only, as and when required. Ultrasonography was carried to assess kidney size, cortical thickness and renal margin in each case. Chest X-ray and electrocardiogram were done as and when needed. Single ophthalmologist examined optic fundi for evidence of diabetic retinopathy (DR). Pupil was dilated using tropicamide eye drop and fundus was examined by direct ophthalmoscope, and binocular indirect ophthalmoscope in every individual. DR was classified into two groups, that is, non-proliferative DR (NPDR) and proliferative DR (PDR). NPDR was diagnosed in the presence of microaneurysm, dot blot or flame-shaped hemorrhage, hard exudates, or evidence of macular edema. The PDR was diagnosed in the presence of new vessels formation or pre-retinal or vitreous hemorrhage.
DN was diagnosed in the presence of nodular or diffuse glomerulosclerosis, glomerular hypertrophy, mesangial (diffuse or nodular) widening, glomerular capillary wall thickening, evidence of exudative lesions or fibrin caps (i.e. hyaline material heaped up on the inner side of the glomerular basement membrane) and the presence of microaneurysms of glomerular capillaries. The various NDRDs were diagnosed on the basis of their characteristic features on light microscopy. Membranous nephropathy was diagnosed by presence of diffuse and global capillary wall thickening in the absence of significant glomerular hypercellularity and increase in mesangial matrix. In few glomeruli, deposits and spikes were also seen at the outer surface of glomerular basement membrane. Focal segmental glomerulosclerosis was diagnosed on findings of focal areas of consolidation and otherwise normal pattern elsewhere with no evidence suggestive of DN. Diagnosis of amyloidosis was made by the presence of hyaline material deposits in glomerular basement membrane and mesangium and confirmed by positive staining of material with Congo red stain. Mesangio-proliferative glomerulonephritis was diagnosed by the presence of increase in number of mesangial cells and matrix without evidence of other features of DN such as nodule formation, thickening of glomerular basement membrane and intertubular arteriolar hyalinosis or arteriolar hyalinosis. Isolated NDRD was diagnosed in the absence of histological features of DN. Mixed lesions (NDRD superimposed above DN) were diagnosed in the presence of histological evidences of DN.
Statistical analysis
Based on the raw data collected, electronic data spread sheet was created in Excel-7 and exported to SPSS version-15 (IBM) for analysis. Demographic, background and baseline data were presented descriptively. All continuous variables like age and laboratory data were represented by mean and average. We have used the Fischer exact probability test and Pearson Chi-square test to study the statistical difference in different types of histological lesions between the patient with DR and without DR. P < 0.05 was taken as statistically significant. The sample size was calculated using the formula n = Z2PQ/L2; where Z = 1.96% at 95% confidence limit; P = 40%, Q = (1 − P), L = 17% (absolute permissible error) n = 31.
Results
Study design and clinical profile
Of 680 type 2 diabetic patients, 136 (20%) patients had proteinuria of >1 g/24 h. Total 105 of 136 were excluded from kidney biopsy for various reasons given in Figure 1. Finally, 31 (male 26; female 5) type 2 diabetic patients were included in the study between October 2011 and September 2013. The average age of the patients was 50.678 years with male to female ratio of 5.2:1. The mean Hb level was 12.787 g/dl and the mean serum creatinine level was 1.231 mg/dl. The mean serum albumin level was 2.738 g/dl. The 24-h urinary protein excretion ranged between 1.0 g and 16.4 g with mean 24-h proteinuria of 5.9 g. Twenty-one (67.7%) patients had nephrotic range proteinuria (>3.5 g/24 h). Urinary sediment was bland (normal) in 21 (67.7%). Five (16.1%) patients had microscopic hematuria and white blood cells were seen in 4 (12.9%) cases. RBC cast was seen in one patient.
Figure 1.
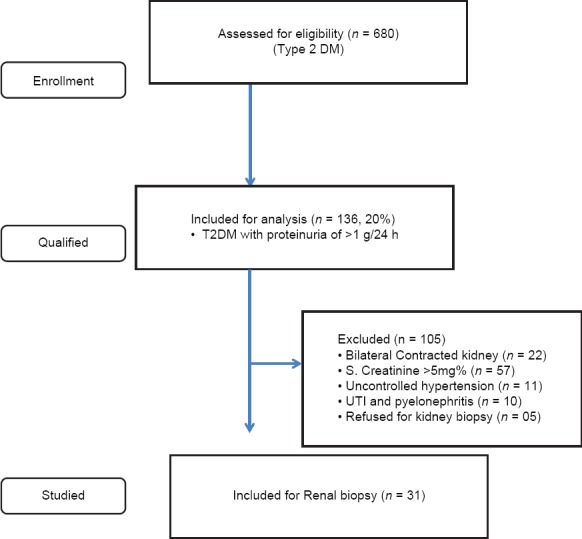
Flow chart showing plan of study (study period; Oct 2011- Sept 2013)
Pattern of nephropathy and renal–retinal relationship
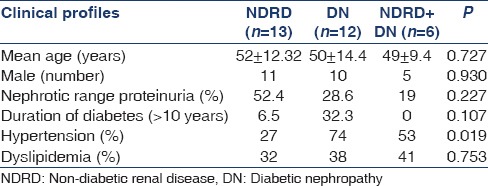
Kidney histology of 31 biopsied patients revealed; isolated DN in 38.7% (n-12), pure NDRD in 41.9% (n-13) and mixed lesions in remaining 19.4% (n-6) cases [Table 1]. Idiopathic membranous nephropathy was the most common NDRD lesion noted in 21% (n-4) cases. Amyloidosis and focal segmental glomerulosclerosis were observed in two patients each, respectively. Focal mesangio-proliferative, proliferative glomerulonephritis with vasculitis, myeloma cast nephropathy, chronic tubulo-interstitial nephritis and minimal change disease were noted in one case each. The most common NDRD superimposed on DN was diffuse proliferative glomerulonephritis (n-2). The other NDRD in this group included chronic tubulo-interstitial nephritis, amyloidosis, minimal change disease and crescentic GN, all consisting of one case each. The statistical analysis using Pearson Chi-square test showed that the pattern of distribution of different histological lesions namely DN alone, NDRD alone and mixed lesions (NDRD + DN) is not uniform in diabetic patients and difference between the histological variant was statistically significant (P < 0.05) as shown in Table 2. Based on the presence or absence of retinopathy, patients were divided into two groups: Group A - with DR (n-10) and Group B - without DR (n-21). In group A, 60% (n-6) had pure DN. Only one patient had NDRD (10%) and mixed lesion (NDRD superimposed on DN) was noted in 3 (30%) cases. We observed NDRD in 40% of our patients, occurring either alone (n-1) or superimposed on DN (n-3) in presence of DR. In Group B (n-21), isolated NDRD was noted in 12 (57.14%) cases. DN was seen in 42.86% (isolated 28.57% and as mixed lesion 14.29%) cases in absence of DR. We also observed that the NDRD was more frequent in patient without DR in comparison to patient with DR and the difference was statistically significant (p < 0.05). However, there was no statistically significant difference with respect to DN alone and mixed lesions in diabetic patient with or without DR [Table 2]. It was noted that there was no difference with respect to mean age, male gender and dyslipidemia in diabetic patients with NDRD, DN and mixed lesions. However, hypertension was noted in 74% of patients with DN as compared to 27% of cases with NDRD and difference was statistically significant (p = 0.019). Nephrotic syndrome was noted in higher proportion of patients (52.4%) with NDRD in comparison to DN (28.6%), but difference was statistically not significant. The longer duration of diabetes (more than 10 years) was positively correlated with development of DN (32.3% vs. 6.5%) [Table 3].
Table 1.
Pattern of nephropathy in type 2 diabetes proteinuric patients (n=31)

Table 2.
Renal retinal relationship in type 2 diabetic patients (n=31)
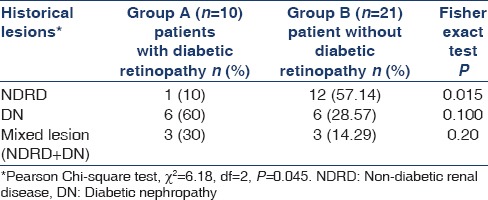
Table 3.
Comparison of various clinical profile between different types of nephropathies
Discussions
Diabetic nephropathy is the leading cause of chronic kidney disease in patients starting renal replacement therapy[11] and is associated with increased cardiovascular mortality.[12] Although patients with T2DM often experience DN, but they can also develop other renal diseases, pathologically unrelated to diabetes and known as NDRD.[8,13] A wide spectrum of NDRD, including both glomerular and tubulo-interstitial lesions are reported in patients with T2DM.[4,5,6,7,8,9,14] In the present study, the average age of patients was 50.678 years. Unnikrishnan et al., covering south Indian population reported the average age of patients as 51 ± 12 years.[15] Mak et al., reported average age was 57 ± 1.8 years in patients having DN and 50 ± 1.9 years in patients having mixed lesions in their study.[7] Similarly Viswanathan et al., observed average age of patients in progressors (persistently proteinuric) was 51 ± 7.8 years while 46.6 ± 10.1 years in non-progressors (normoalbuminuric) patients.[16] Gall et al., in a prospective observational study involving 176 patients with type 2 diabetes reported that males had a 2.6 times greater risk of developing incipient or overt nephropathy.[17] We noted male to female ratio was 5.2:1. Thus, our observation was similar to other studies with regard to age and gender. We observed urinary sediment was normal in majority (67.7%) of diabetic proteinuric patients. Micro-hematuria was seen in 5 (16.1%) patients, of which 4/5 (80%) patients had lesion of NDRD superimposed on DN. Mixed lesions has been reported in 59% of patients with micro-hematuria similar to us.[7] Average 24-h urinary protein excretion was 5.9 g in our patients and 21 (67.7%) patients had nephrotic range proteinuria (>3.5 g/24 h). Nephrotic syndrome was reported in 60.9% of type 2 diabetic patients from this center in a previous study.[3] There was significant difference in degree of 24-h proteinuria between DN and NDRD group. Among 21 patients with nephrotic range proteinuria; NDRD, DN and mixed lesion were noted in 52.4%, 28.6% and 19% of cases, respectively. Thus, we observed nephrotic range proteinuria was more common in NDRD in comparison to patients with DN. However, Mak et al., found that the mean level of proteinuria was higher in DN as compared with NDRD (6.3 ± 0.7 vs. 3.2 ± 0.4 g/24 h, p = 0.009) in their study.[7] It is important to mention that all patients were Chinese in Mak et al., study. The racial difference may be a possible explanation for such variation in proteinuria.
The reported frequency of NDRD is widely variable ranging between 13% and 53% of the total renal biopsies of diabetic patients.[4,5,6,7,8,9] The wide variation in frequency of NDRD in various studies are due to policy of renal biopsy criteria, regional and/or racial variations of different study population [Table 4].[18,19,20,21,22,23,24,25,26,27]
Table 4.
DN versus NDRD in T2DM: Literature summary
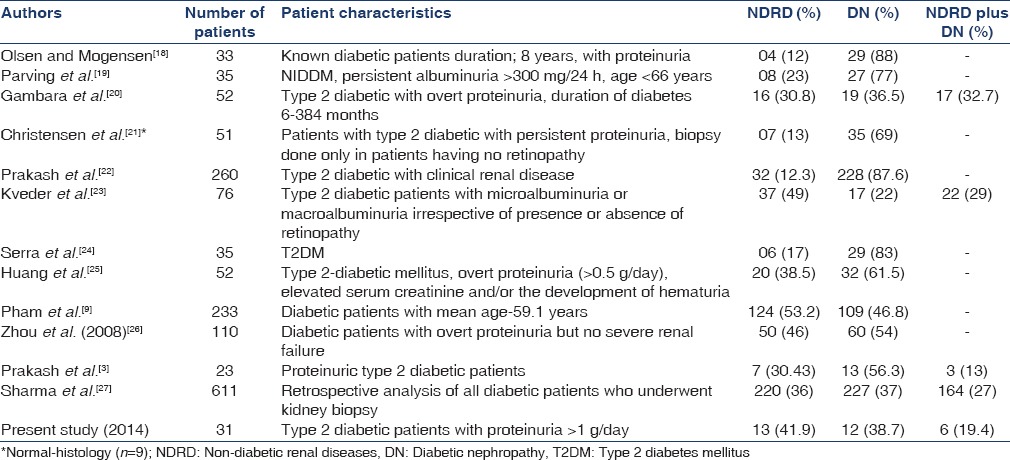
In a Saudi Arabian study, NDRD was seen in 50% of all the diabetic renal biopsies.[28] The most commonly diagnosed NDRD in these biopsies was membranous GN, which represents 18.75% of diabetic renal biopsies.[28] Our study revealed isolated DN in 38.7% (n-12), pure NDRD in 41.9% (n-13) and mixed lesions in remaining 19.4% (n-6) cases. Idiopathic membranous nephropathy was the most common NDRD lesion noted in 21% (n-4) cases similar to other studies.[28,29] Castellano et al., in their study of 20 patients with T2DM reported DN in 9 (45%), and NDRD in 11 (55%) patients and membranous nephropathy was the commonest NDRD lesion.[29] Soni et al., in their study observed that the most common NDRD were acute interstitial nephritis (18.1%), followed by post-infectious glomerulonephritis (17.24%), membranous nephropathy (11.20%) and focal segmental glomerulosclerosis (7.75%).[4] Previous renal biopsy (n-23) study from our center revealed: isolated DN, 13 (56.3%); NDRD, 7 (30.43%); and 3 (13%) non-DN (NDN) superimposed on DN.[3] Another study from our center reported that non-diabetic kidney disease was noted in 32/260 (12.3%) patients on the basis of clinical screening.[22] A study from South India reported that eight cases (50%) had pathological changes suggestive of diabetic etiology, 5 (33.3%) had classical membranous nephropathy, 1 (6.2%) had tubulo-interstitial disease and 2 (12.3%) had minimal changes in 16 patients with T2DM subjected to kidney biopsy.[30] Thus, idiopathic membranous nephropathy is the most common NDRD lesion in diabetic patients similar to our study.[28,29,30] Das et al., in their biopsy study of 75 cases noted that 64% cases had NDRD and 36% had DGS.[31] The commonest NDRD was MCD (12.5%).[31] In a retrospective biopsy study involving 611 diabetic patients, showed DN alone, NDRD alone and mixed lesion (NDRD on DN) in 37%, 36% and 27% patients, respectively.[27] They reported that acute tubular necrosis was the most frequent (17.3% in NDRD alone and 43.4% in NDRD on DN) lesion among patients with NDRD.
We have demonstrated in our study that development of DN takes several years from the diagnosis and/or onset of DM. NDRD versus DN was seen in 12.9% versus 3.2% of cases, respectively with duration of diabetes <4 years. NDRD and DN were observed in 22.6% and 3.2% of patients, respectively with duration of diabetes between 5 and 10 years. We noted DN versus NDRD in 32.2% versus 6.5% of the cases with the duration of diabetes more than 10 years [Figure 2]. Similar observations were made by Sharma et al., in which median duration of DM in patients with NDRD alone was 5 years, which was significantly shorter than in patients with DN alone (13 years) and DN + NDRD (10 years).[27] In multivariate analysis, longer duration of diabetes was associated with a greater likelihood of DN and lower likelihood of NDRD.[27]
Figure 2.
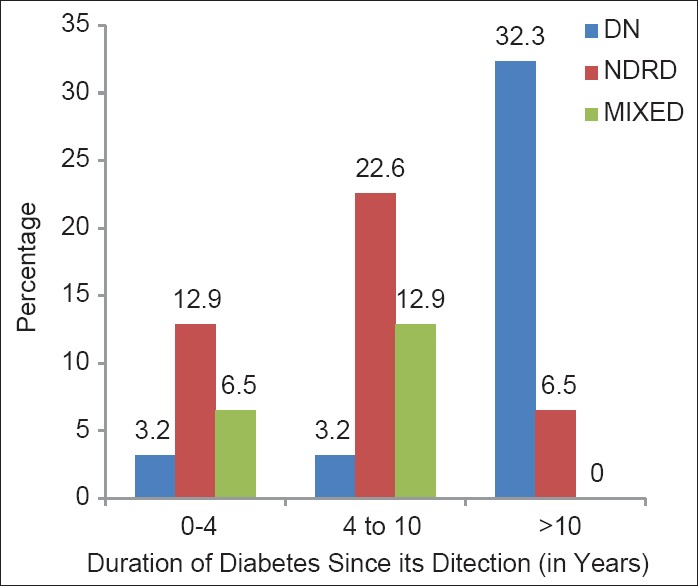
Type of nephropathy in relation to duration of diabetes (n = 31)
DR is present in virtually all type 1 diabetic patients with DN, whereas only 50–60% of proteinuric type 2 diabetic patients have retinopathy.[32] Absence of retinopathy should prompt further investigation for non-diabetic glomerulopathies.[19] We had previously reported, 40% of proteinuric type 2 diabetic patients had NDN even in presence of DR and chances of developing diabetic and NDN is nearly equal in patients without DR.[3] However, a meta-analysis involving 2012 patients from 26 studies concluded that DR is useful in diagnosing or screening for DN in patients with type 2 diabetes and renal disease.[33] PDR may be a highly specific indicator for DN.[33] In our study we observed, in patients without DR, 71.41% cases had evidence of NDRD (either isolated NDRD or NDRD superimposed on DN) and 42.86% cases had evidence of DN (either isolated DN or NDRD superimposed on DN). It means absence of DR predicts NDRD in vast majority of cases (71.43%); but cannot exclude the lesion of DN. DN still can occur in nearly 42.86% of cases in absence of DR. Similarly presence of DR predicts lesion of DN (either isolated DN or NDRD superimposed on DN) in most (90%) patients but, does not rule out the occurrence of NDRD. We noted NDRD in 40% (NDRD alone 10%, NDRD with DN 30%) in presence of DR. Thus, presence or absence of DR is not helpful in predicting the nature of nephropathy in type 2 diabetic patients and it is difficult to differentiate between DN and NDRD without the aid of renal biopsy. Further, diagnosing NDRD is especially important when it leads to a specific change in therapy. Small sample size, lack of immunofluorescence and electron microscopy study of kidney tissue were the possible limitations of this study.
In summary, all type 2 diabetic patients with clinical renal disease do not have classical DN. Non-diabetic kidney disease either alone or superimposed on DN were seen in 61.3% of diabetic patients. We observed that longer duration (>10 years) of diabetes was the strongest predictor of DN and diabetic patients having NDRD alone had shorter duration of DM. DR was absent in 21/31 (67.7%) patients. Thus, retinal lesion is less frequent in type 2 diabetes in comparison to type 1 diabetes. Non-diabetic kidney disease was noted in 40% of the cases in presence of DR and 43% of the cases had biopsy proven DN in absence of DR. However, we observed that the absence of retinopathy favors NDRD but does not exclude occurrence of DN. Therefore, DR does not predict nature of nephropathy in patients with type 2 diabetes. Renal biopsy is necessary for precise diagnosis of diabetic and non-diabetic kidney disease in type 2 diabetic patients with proteinuria.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1. [Last accessed on 2013 Oct 28]. Available from: http://www.idf.org/diabetesatlas/internationaldiabetes federation .
- 2.US Renal Data System, USRDS 2010 Annual Data Report. Atlas of Chronic Kidney Disease and End. Stage Renal Disease in the United States. Annual Data Report. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. 2010 [Google Scholar]
- 3.Prakash J, Lodha M, Singh SK, Vohra R, Raja R, Usha Diabetic retinopathy is a poor predictor of type of nephropathy in proteinuric type 2 diabetic patients. J Assoc Physicians India. 2007;55:412–6. [PubMed] [Google Scholar]
- 4.Soni SS, Gowrishankar S, Kishan AG, Raman A. Non diabetic renal disease in type 2 diabetes mellitus. Nephrology (Carlton) 2006;11:533–7. doi: 10.1111/j.1440-1797.2006.00681.x. [DOI] [PubMed] [Google Scholar]
- 5.Yoshikawa Y, Truong LD, Mattioli CA, Ordonez NG, Balsaver AM. Membranous glomerulonephritis in diabetic patients: A study of 15 cases and review of the literature. Mod Pathol. 1990;3:36–42. [PubMed] [Google Scholar]
- 6.Kobayashi K, Harada A, Onoyama K, Shimamatsu K, Maeda T, Fujimi S, et al. Idiopathic membranous glomerulonephritis associated with diabetes mellitus: Light, immunofluorescence and electron microscopic study. Nephron. 1981;28:163–8. doi: 10.1159/000182163. [DOI] [PubMed] [Google Scholar]
- 7.Mak SK, Gwi E, Chan KW, Wong PN, Lo KY, Lee KF, et al. Clinical predictors of non-diabetic renal disease in patients with non-insulin dependent diabetes mellitus. Nephrol Dial Transplant. 1997;12:2588–91. doi: 10.1093/ndt/12.12.2588. [DOI] [PubMed] [Google Scholar]
- 8.Lee EY, Chung CH, Choi SO. Non-diabetic renal disease in patients with non-insulin dependent diabetes mellitus. Yonsei Med J. 1999;40:321–6. doi: 10.3349/ymj.1999.40.4.321. [DOI] [PubMed] [Google Scholar]
- 9.Pham TT, Sim JJ, Kujubu DA, Liu IL, Kumar VA. Prevalence of nondiabetic renal disease in diabetic patients. Am J Nephrol. 2007;27:322–8. doi: 10.1159/000102598. [DOI] [PubMed] [Google Scholar]
- 10.American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 2010 Jan;33(Supplement 1):S62–9. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bethesda, MD: National Institute of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2003. US Renal Data System: USRDS 2003 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. [Google Scholar]
- 12.Valmadrid CT, Klein R, Moss SE, Klein BE. The risk of cardiovascular disease mortality associated with microalbuminuria and gross proteinuria in persons with older-onset diabetes mellitus. Arch Intern Med. 2000;160:1093–100. doi: 10.1001/archinte.160.8.1093. [DOI] [PubMed] [Google Scholar]
- 13.John GT, Date A, Korula A, Jeyaseelan L, Shastry JC, Jacob CK. Nondiabetic renal disease in noninsulin-dependent diabetics in a south Indian Hospital. Nephron. 1994;67:441–3. doi: 10.1159/000188019. [DOI] [PubMed] [Google Scholar]
- 14.Prakash J. Non-diabetic renal disease (NDRD) in patients with type 2 diabetes mellitus (type 2 DM) J Assoc Physicians India. 2013;61:194–9. [PubMed] [Google Scholar]
- 15.Unnikrishnan RI, Rema M, Pradeep R, Deepa M, Shanthirani CS, Deepa R, et al. Prevalence and risk factor of diabetic nephropathy in an urban south Indian population; The Chennai Urban Rural Epidemiology study (CURES-45) Diabetes Care. 2007;30:2019–24. doi: 10.2337/dc06-2554. [DOI] [PubMed] [Google Scholar]
- 16.Viswanathan V, Tilak P, Kumpatla S. Risk factors associated with the development of overt nephropathy in type 2 diabetes patients: A 12 years observational study. Indian J Med Res. 2012;136:46–53. [PMC free article] [PubMed] [Google Scholar]
- 17.Gall MA, Hougaard P, Borch-Johnsen K, Parving HH. Risk factors for development of incipient and overt diabetic nephropathy in patients with non-insulin dependent diabetes mellitus: Prospective, observational study. BMJ. 1997;314:783–8. doi: 10.1136/bmj.314.7083.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olsen S, Mogensen CE. How often is NIDDM complicated with non-diabetic renal disease? An analysis of renal biopsies and the literature. Diabetologia. 1996;39:1638–45. doi: 10.1007/s001250050628. [DOI] [PubMed] [Google Scholar]
- 19.Parving HH, Gall MA, Skøtt P, Jørgensen HE, Løkkegaard H, Jørgensen F, et al. Prevalence and causes of albuminuria in non-insulin-dependent diabetic patients. Kidney Int. 1992;41:758–62. doi: 10.1038/ki.1992.118. [DOI] [PubMed] [Google Scholar]
- 20.Gambara V, Mecca G, Remuzzi G, Bertani T. Heterogeneous nature of renal lesions in type II diabetes. J Am Soc Nephrol. 1993;3:1458–66. doi: 10.1681/ASN.V381458. [DOI] [PubMed] [Google Scholar]
- 21.Christensen PK, Larsen S, Horn T, Olsen S, Parving HH. Causes of albuminuria in patients with type 2 diabetes without diabetic retinopathy. Kidney Int. 2000;58:1719–31. doi: 10.1046/j.1523-1755.2000.00333.x. [DOI] [PubMed] [Google Scholar]
- 22.Prakash J, Sen D, Usha, Kumar NS. Non-diabetic renal disease in patients with type 2 diabetes mellitus. J Assoc Physicians India. 2001;49:415–20. [PubMed] [Google Scholar]
- 23.Kveder R, Kajtna-Koselj M, Rott T, Bren AF. Nephrotic syndrome in patients with diabetes mellitus is not always associated with diabetic nephropathy. Nephrol Dial Transplant. 2001;16(Suppl 6):86–7. doi: 10.1093/ndt/16.suppl_6.86. [DOI] [PubMed] [Google Scholar]
- 24.Serra A, Romero R, Bayés B, Lopez D, Bonet J. Is there a need for changes in renal biopsy criteria in proteinuria in type 2 diabetes? Diabetes Res Clin Pract. 2002;58:149–53. doi: 10.1016/s0168-8227(02)00131-6. [DOI] [PubMed] [Google Scholar]
- 25.Huang F, Yang Q, Chen L, Tang S, Liu W, Yu X. Renal pathological change in patients with type 2 diabetes is not always diabetic nephropathy: A report of 52 cases. Clin Nephrol. 2007;67:293–7. doi: 10.5414/cnp67293. [DOI] [PubMed] [Google Scholar]
- 26.Zhou J, Chen X, Xie Y, Li J, Yamanaka N, Tong X. A differential diagnostic model of diabetic nephropathy and non-diabetic renal diseases. Nephrol Dial Transplant. 2008;23:1940–5. doi: 10.1093/ndt/gfm897. [DOI] [PubMed] [Google Scholar]
- 27.Sharma SG, Bomback AS, Radhakrishnan J, Herlitz LC, Stokes MB, Markowitz GS, et al. The modern spectrum of renal biopsy findings in patients with diabetes. Clin J Am Soc Nephrol. 2013;8:1718–24. doi: 10.2215/CJN.02510213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jalalah SM. Non-diabetic renal disease in diabetic patients. Saudi J Kidney Dis Transpl. 2008;19:813–6. [PubMed] [Google Scholar]
- 29.Castellano I, Covarsí A, Novillo R, Gómez-Martino JR, Ferrando L. Renal histological lesions in patients with type II diabetes mellitus. Nefrologia. 2002;22:162–9. [PubMed] [Google Scholar]
- 30.Premalatha G, Vidhya K, Deepa R, Ravikumar R, Rema M, Mohan V. Prevalence of non-diabetic renal disease in type 2 diabetic patients in a diabetes centre in Southern India. J Assoc Physicians India. 2002;50:1135–9. [PubMed] [Google Scholar]
- 31.Das U, Dakshinamurty KV, Prayaga A, Uppin MS. Nondiabetic kidney disease in type 2 diabetic patients: A single center experience. Indian J Nephrol. 2012;22:358–62. doi: 10.4103/0971-4065.103912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gall MA, Rossing P, Skøtt P, Damsbo P, Vaag A, Bech K, et al. Prevalence of micro- and macroalbuminuria, arterial hypertension, retinopathy and large vessel disease in European type 2 (non-insulin-dependent) diabetic patients. Diabetologia. 1991;34:655–61. doi: 10.1007/BF00400995. [DOI] [PubMed] [Google Scholar]
- 33.He F, Xia X, Wu XF, Yu XQ, Huang Fx. Diabetic retinopathy in predicting diabetic nephropathy in patients with type 2 diabetes and renal disease: A meta-analysis. Diabetologia. 2013;56:457–66. doi: 10.1007/s00125-012-2796-6. [DOI] [PubMed] [Google Scholar]


