Abstract
Urolithiasis is a common disease with increasing prevalence worldwide and a lifetime-estimated recurrence risk of over 50%. Imaging plays a critical role in the initial diagnosis, follow-up and urological management of urinary tract stone disease. Unenhanced helical computed tomography (CT) is highly sensitive (>95%) and specific (>96%) in the diagnosis of urolithiasis and is the imaging investigation of choice for the initial assessment of patients with suspected urolithiasis. The emergence of multi-detector CT (MDCT) and technological innovations in CT such as dual-energy CT (DECT) has widened the scope of MDCT in the stone disease management from initial diagnosis to encompass treatment planning and monitoring of treatment success. DECT has been shown to enhance pre-treatment characterization of stone composition in comparison with conventional MDCT and is being increasingly used. Although CT-related radiation dose exposure remains a valid concern, the use of low-dose MDCT protocols and integration of newer iterative reconstruction algorithms into routine CT practice has resulted in a substantial decrease in ionizing radiation exposure. In this review article, our intent is to discuss the role of MDCT in the diagnosis and post-treatment evaluation of urolithiasis and review the impact of emerging CT technologies such as dual energy in clinical practice.
Keywords: Advances, computed tomography, urolithiasis
INTRODUCTION
Urolithiasis is a common disease worldwide and affects a wide gamut of the patient population, irrespective of race, culture or geographic boundaries. In the past few decades, there has been an increasing incidence of urinary stone disease both in developed and developing nations due to changes in life style, particularly due to the rising prevalence of obesity.[1,2,3] Recent studies have also demonstrated a changing composition of urolithiasis as well as an appreciable increase in the incidence of stone disease in females and younger patients over the last decade.[1,4] Appropriate management of urolithiasis has important clinical implications due to their association with complications such as infection and chronic kidney disease and high rate of recurrence.[1,3]
Imaging has a critical role in the initial diagnosis, treatment planning and post-treatment surveillance of patients with urolithiasis. Unenhanced computed tomography (CT), first introduced for stone imaging in the 1990s, has since emerged as the reference gold standard for the initial and subsequent evaluation of patients with suspected kidney stones, superseding radiography and intravenous urography. Non-contrast CT offers several advantages compared with alternative imaging techniques such as plain radiography and ultrasound, including high sensitivity and specificity (>95% and >96%, respectively) for the detection of stones, easy availability, faster speed of acquisition and absence of need for administration of intravenous contrast.[5,6,7] With the emergence of multi-detector CT (MDCT) and advanced technologies like dual-energy CT (DECT), the scope of CT in urolithiasis management has further expanded. Besides aiding in accurate diagnosis, MDCT also plays an important role in treatment planning, follow-up and assessment of treatment success due to its ability to characterize stone composition and fragility.[8,9] In this review article, we will discuss the current and emerging role of CT in the management of patients with urinary stone disease and their impact on planning treatment strategies and patient follow-up.
MDCT
Unenhanced helical CT is highly sensitive (up to 98%) and specific (96–100%) in diagnosing urolithiasis, and is the imaging modality of choice for the initial evaluation of patients with suspected urinary stones.[5,6] CT is highly preferred due to easy availability, speed, ease of image acquisition, absence of need for oral or intravenous contrast media administration, and ability to detect extra-urinary pathologies such as appendicitis, diverticulitis or gynecological pathologies such as hemorrhagic cyst or ovarian torsion that may mimic the renal colic.[5,6,10,11] This is especially crucial in managing patients presenting with acute abdominal pain imitating renal colic in emergency departments (EDs) because, often times, these patients are not evaluated by urologists and therefore excluding other potential abdominal pathologies are also concurrent objectives of the ordering physicians.[5,12] In patients presenting to the emergency room with symptoms suggestive of renal colic, CT enables an alternative diagnosis in nearly 10–14%.[13,14,15,16,17] Furthermore, CT also permits the diagnosis of urinary abnormalities such as congenital conditions of the urinary tract and renal/urothelial neoplasms, recognition of which has immense clinical implications to patient management and prognosis.
MDCT, introduced in 1998, has opened new opportunities in the management of urolithiasis, such as the ability to perform multi-planar reformations and three-dimensional (3D) reconstructions that have enhanced the detection and quantification of urinary stone burden. Besides routine evaluation of the number of stones, location, size and presence or absence of hydronephrosis, the volumetric analysis of stone burden by MDCT has been a strong indicator for treatment planning and outcome. Furthermore, MDCT allows assessment of stone composition by measuring attenuation in Hounsfield Units (HU).[18,19,20,21]
MDCT technique
Stone protocol CT tailored for the diagnosis of urinary stone disease varies from a routine non-contrast abdomino–pelvic CT study and has different scan acquisition parameters. The coverage area for a stone protocol CT extends from the upper pole of both kidneys to the base of the urinary bladder.[14,22,23,24] Although thinner slices (1–3 mm) are desirable, acquiring CT images at a slice thickness of 5 mm complimented with 3 mm coronal/sagittal reformatted images improves stone detection while lowering radiation dose.[23,25] A tube potential of 100–120 kVp and automatic tube current modulation (ATCM) with an mA range of 80–500 mA is frequently used; however, it should be noted that the scan acquisition protocols are tailored to the patient body weight and CT scanner technology.[26] With the introduction of iterative reconstruction (IR) technology, stone protocol CT scans can be performed at lower mA and lower kVp, permitting substantial radiation dose reduction. The CT acquisition parameters for suspected renal stone evaluation in the emergency department scanners are slightly different from the routine stone protocol CTs to improve the ability of ED physicians in ruling out other potential non-urinary causes of patient symptoms. This includes acquiring thinner slices and adapting a scan coverage length similar to the abdomino–pelvic CT scan. Contrast administration is not required for routine stone diagnosis; however, it may be of value in identifying vascular calcifications or for differentiating distal ureteral stones from phleboliths.[27] The use of intravenous contrast should also be reserved for equivocal cases or in instances where possibilities of alternate diagnosis are high.
Coronal and sagittal reformatted images of 3 mm thickness are routinely acquired and are an indispensable part of the stone CT protocol. Integration of multi-planar reformatted images with routine axial scans during image interpretation enables precise evaluation of the entire urinary tract and location of the impacted stones. They also improve the detection of small stones, particularly at the renal poles, and facilitate the differentiation of extrarenal calcifications from urinary stones.[13,25]
CT detection of urolithiasis
Evaluation of urolithiasis using MDCT should include interpretation of both axial and multi-planar reformatted images to improve accuracy. Unenhanced CT detects all types of urinary tract calculi, including stones such as uric acid, xanthine or cystine stones that are otherwise radiolucent on conventional radiographs.[5,26] The only exceptions are pure matrix stones and stone encountered in patients on indinavir treatment, which are usually missed on MDCT due to their soft tissue attenuation (15–30 HU).[5,14] In these situations, administration of intravenous contrast and delayed imaging might facilitate their diagnosis.[14]
The stone location and its site of impaction in the ureter are very important in stone management, with the treatment success rates being better for calculi located in the lower third of the ureter. Compared with conventional radiography, CT enables precise detection and localization of ureteral calculi.[15,26] Direct visualization of stone in the ureteric lumen with proximal ureteral dilatation and normal caliber of the distal ureter is a common finding.[7,22] The absence of ureteral dilatation has however been reported in a small number of cases, and presence or absence of ureteral dilatation does not indicate presence or absence of urinary tract obstruction. Certain secondary signs aid in the diagnosis of ureteral stones on CT, including reliable signs such as perinephric fat stranding, periureteral edema, hydroureter and hydronephrosis and less-consistent signs such as perinephric edema and lateral conal fascial thickening. The positive and negative predictive values of intra-renal collecting system dilatation and perinephric fat stranding in detecting ureterolithiasis nears 98% and 91%, respectively.[5,16] The failure to detect stone in patients with a high degree clinical suspicion necessitates a repeat meticulous evaluation for secondary signs. The presence of ureteric dilatation and perinephric stranding makes ureterolithiasis more likely, and the possible scenarios include either passage of a previously obstructing stone or presence of a stone with size or attenuation features, which limit detection on MDCT. On the contrary, the absence of these secondary signs allows one to reliably rule out urinary stone disease and necessitates evaluation of other potential causes of patient symptoms, including extra-urinary pathologies that mimic ureteric colic.[5]
CT in the assessment of stone burden
Stone size
A key element in the urological management of stone disease, which can consistently and significantly impact decision making, is the estimation of stone burden.[15,17,26] Stone size is a simpler metric for stone burden assessment and can be reliably obtained on CT. Urological decisions on the selection of treatment strategies, including need of medical expulsive therapy or endoscopic/percutaneous interventions, rely strongly on stone size determination. Furthermore, stone size also helps in deciding on the type of urological interventions, e.g., shock wave lithotripsy (SWL) versus ureteroscopy with lithotripsy versus percutaneous nephrolithotomy (PCNL). Stone size can be reliably estimated on CT by measuring the largest dimension. Measurements can be made on the soft tissue window (window width – 400 HU and window level – 30 HU) or on a bone window (window width – 1120 HU and window level – 300 HU).[28,29]
Stone volumetry
Despite the ease of using two-dimensional (2-D) measurements for estimation of stone burden, they are restricted in their ability to accurately quantify stone size for large stones with irregular contour such as stag horn calculi. Stone volumetry, a technique that allows estimation of stone volume, counteracts this limitation and allows accurate assessment of stone burden with MDCT. Different methods have been employed to determine the stone volume, including obtaining the product of three orthogonal measurements, generation of 3D volume measurements from the stone circumference data on various stone-bearing image sets and, more recently used, semi-automatic segmentation tools[18,19,20] [Figure 1]. Stone volume, besides being a valuable tool for pre-operative planning, has also been shown to be a reliable predictor of treatment success.[18,19] For example, it has been shown that using a stone volume cut-off of 700 mm3 can be used to successfully predict treatment outcome in patients undergoing SWL.[19]
Figure 1.
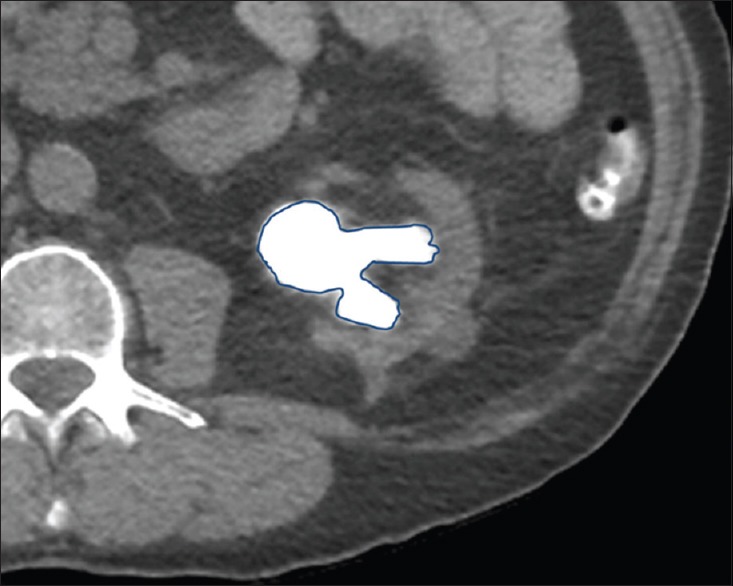
Volumetric assessment of renal stone burden. Axial non-contrast computed tomography image obtained prior to percutaneous nephrolithotomy demonstrates region of interest-based segmentation method for volume estimation
CT in the determination of stone fragility
Stone internal structure assessment is another imaging determinant that can impact the outcome following urological interventions, especially after SWL. High-resolution MDCT scanning with thin slices and reconstruction with bone algorithm allows visualization of the internal structure/architecture of the stones, particularly when seen on bone window setting. The internal architecture as seen on CT could either suggest internal homogeneity or heterogeneity. Stones with internal homogeneity have a uniform internal structure, are more rigid and are difficult to break with lithotripsy. On the other hand, stones with internal heterogeneity have areas of low attenuation or internal voids within the stone component. The internal heterogeneity is an indication of high stone fragility and the internal irregularities within-stone structures facilitate easy disintegration of stones on SWL.[30] Even for the inherently hard brushite, cystine and calcium oxalate monohydrate stones, presence of heterogeneity has been shown to increase the success of fragmentation with SWL.[30,31] On the contrary, stones with internal homogeneity are resistant to easy fragmentation and often necessitate multiple therapeutic sessions.
CT in the determination of stone composition
Urological management of urinary tract calculi relies on several different factors such as stone size, location, number, anatomy and chemical composition. Precise pre-treatment determination of urinary stone composition is essential and considerably impacts appropriate management.[15,21,24,32] For instance, uric acid stones are suitable for medical management with oral medications that assist in stone dissolution. While struvite stones are sensitive to SWL, cysteine stones and calcium oxalate monohydrate stones are comparatively resistant to treatment by SWL.[31,33,34,35] Knowledge of the stone composition is also useful for the prevention of recurrent disease. Traditionally, patient history, urine pH, urinary crystals, urease-positive organisms and plain radiographs were employed to predict stone composition.[35,36] More recently, CT is being increasingly used for the in vivo determination of stone composition and the emergence of new technological innovations such as DECT permits reliable determination of stone composition.[21,35,37,38]
Region of interest based methods
The most traditional method for estimation of stone composition on MDCT has been using placement of the ROI over the stone to obtain the attenuation value in HU. Bellin and colleagues showed that HU measurements based on ROI placement had an accuracy of 64–81% in predicting the composition of urinary stones in in vitro studies.[38] Similar studies have shown that HU measurements obtained using CT can be used to reliably identify uric acid, cysteine and calcium oxalate monohydrate stones with considerable accuracy (>85%) in in vitro studies.[38,39,40] The HU measurements of the various urinary stones at 120 kV usually fall under the following range: Uric acid, 200–450 HU; struvite, 600–900 HU; cysteine, 600–1100 HU; calcium phosphate, 1200–1600 HU; and calcium oxalate monohydrate and brushite, 1700–2800 HU.[26,37,38,41,42] Despite the substantial accuracy of CT in determining stone composition in in vitro studies, HU measurement for predicting stone composition in vivo is less reliable and challenging. Stone composition assessment using attenuation values (HU) is often dependent on the size of the ROI, slice thickness and accurate placement of ROI over the stone to eliminate partial volume averaging effects.[43] Most often, the stones encountered in vivo are of mixed composition, which makes precise determination complex, and furthermore the overlap in attenuation measurements of various stones have limited the role of ROI-based methods in distinguishing different type of stones.[44,45]
DECT
DECT technology introduced in the past decade holds great promise for the accurate determination of stone composition.[46,47,48,49] Dual-energy scanning involves simultaneous scanning using two different energies, which permits tissue characterization. Two different DECT systems are currently available in clinical practice: Dual-source DECT (dsDECT) and single-source DECT (ssDECT). DsDECT is assembled with two X-ray tubes (140 and 80 kVp) and two detectors disposed in a single gantry perpendicular to each other and ssDECT is assembled with one X-ray tube with rapid kV switching between high (140 kVp) and low energy (80 kVp).[50] Along with the distinct hardware configurations, these systems have different post-processing techniques that allow material separation and generation of images with different X-ray energies (keV).
Besides its high sensitivity for the detection of urolithiasis, DECT offers enhanced ability to characterize stone composition and differentiate between various stone types. Determination of stone composition has been one of the most explored and recognized applications of DECT in the abdomen, and this technology has been validated in vivo and in vitro studies using both dsDECT and ssDECT, particularly for differentiation of uric acid and non–uric acid stones.[46,48] DECT allows determination of stone composition based on the principle of variation in attenuation characteristics of stones at different X-ray energies based on their composition. DECT allows differentiation of uric acid and non-uric acid (calcium-dominant) stones based on the fact that the uric acid stones are composed of elements with low atomic numbers (H, C, N, O) and their X-ray attenuation profile at multiple energies is different compared with that of non-uric acid stones, which are composed of elements with higher atomic numbers (P, Ca, S).[50,51,52] Based on this differential behavior, the dsDECT algorithm assumes that every voxel is a mixture of water, calcium and uric acid. Voxels with X-ray attenuation profile similar to calcium are colored distinctly from those with an attenuation profile similar to uric acid[50,51,52] [Figure 2]. In contrast, CT data acquired from an ssDECT scan is processed using a two-material (basis pair) decomposition algorithm on the scanner console generating two image series, generally iodine and water (high and low atomic number materials, respectively). Stones visualized on water images only are considered uric acid stones, whereas those stones visualized on both water and iodine images are characterized as non-uric acid calculi. Material decomposition images have shown 100% sensitivity and accuracy in distinguishing uric acid and non-uric acid stones, regardless of the size of the stones.[52] Additionally, effective Z (Zeff) images can be generated in vendor-specific workstations (ADW version 4.5; GE Healthcare, Milwaukee, WI, USA) for further analysis of renal stone composition. Zeff is a validated method for renal stone characterization and facilitates the identification of predominant material within mixed stones by taking into account the attenuation and atomic number of a specific material. Low Zeff is suggestive of uric acid stones, whereas high Zeff suggests non-uric acid stones. Accurate sub-categorization of renal stones is an evolving application of DECT and, with evolving DECT algorithms further subtype differentiation is becoming available. For instance, a tin filter installed to the high-energy tube of new-generation dsDECT scanners decreases the overlap between the two energy potentials, improving the spectral separation, which allows further characterization of renal stones with similar composition.[53]
Figure 2.
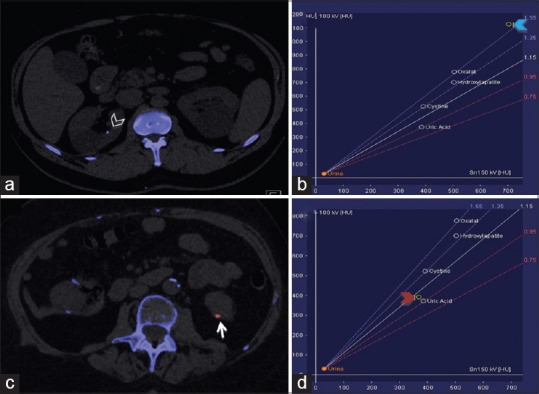
Characterization of kidney stones using dual-energy computed tomography (DECT). DECT helps to distinguish between uric acid and non-uric acid renal stones. a and c are axial images acquired from different patients who presented with flank pain. (a) Axial image showing a non-uric acid renal stone in the right kidney (arrowhead) colored in blue. (b) Graph showing the composition of this stone (blue arrowhead). (c) Axial image showing a uric acid renal calculus in the left kidney (arrow) colored in red. (d) Graph confirms the composition of the stone (red arrowhead)
The current DECT protocol for renal stones includes a low-dose MDCT acquisition using the single-energy mode, covering the abdomen and pelvis, to identify possible calculi in the urinary tract. Once the urinary stone is localized, a dual-energy acquisition targeted to the anatomical area of the stone is performed to minimize the total radiation dose.[26] After scan acquisition, the DECT data are processed on the CT console, generating different images series. Images with a specific virtual X-ray energy (50–70 keV) are generated for improved visualization of the renal stones by enhancing the contrast differences with the surrounding tissues. Because each DECT acquisition produces more datasets, the number of images and total reconstruction time for DECT studies is longer compared with single energy CT (SE-CT) studies. A vendor-specific workstation is required to generate Zeff images from DECT scans. The resultant DECT images from the reconstruction process can be viewed the same way as the SE-CT images.
Characterization of mixed renal stones using DECT can be challenging; however, the current technology allows recognition of the predominant stone type. Reported shortcomings of first-generation dsDECT for urolithiasis include a decrease in specificity for stones <3mm and in patients with large body habitus.[46] However, these small stones are spontaneously passed, having less clinical relevance.
CT in planning percutaneous interventions
In addition to contributing to treatment planning by determination of stone burden, location, composition and fragility, MDCT also plays an important role in the pre-surgical evaluation of patients who are candidates for interventional procedures like PCNL.[54] MDCT aids in the evaluation of the position of kidneys, the orientation of the pyelocalyceal system and the relationship of the kidney to various surrounding organs like spleen, liver and colon.[55]
Various parameters crucial for successful calyceal access during PCNL, including localization of posterior calyx and angle between the calyces, can be reliably evaluated using MDCT. The multi-planar reformations and 3-D post-processing have made visualization of the calyceal system precise and easy. Similarly, for patients who are candidates for SWL, stone-to-skin distance (SSD) is an important predictor for stone-free survival. SSD, which is the distance from the center of the stone to the skin surface, can be reliably determined on the axial MDCT scans[34,56,57] [Figure 3]. An SSD greater than 10 cm on CT has been shown to increase the failure rate of SWL and therefore PCNL or other ureteroscopic interventions may be advocated in these patients.[34,56,57]
Figure 3.
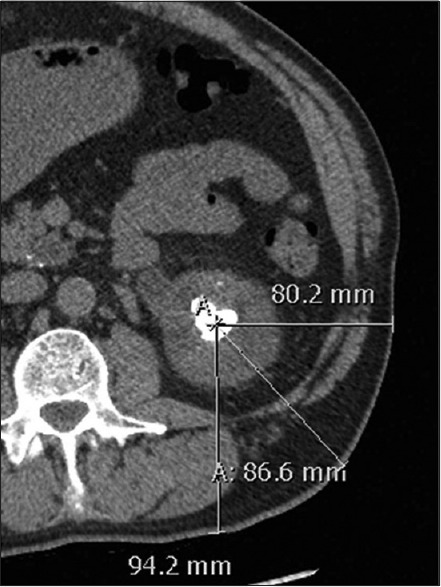
Measurement of stone-to-skin distance (SSD) in a 54-year-old man. On an axial non-contrast computed tomography scan, the distance from the center of the stone to the surface of the skin at 0°, 45° and 90° is 8.0, 8.7 and 9.4 cm, respectively. The mean of these three measurements is used to represent the average SSD, which is 8.7 cm
CT in post-treatment follow-up
Following urological treatment of patients with stone disease, the main objectives of CT imaging are to (i) ascertain stone-free status, (ii) identify the presence of residual stones, (iii) rule out stricture in the urinary system and (iv) detect any complications related to urological interventions.[55,58] MDCT is a modality of choice for identifying residual stone burden after interventional procedures like PCNL and SWL.[58,59,60] CT aids in accurately localizing the residual fragments in the kidney/ureters and thereby facilitates their removal. This is essential because recurrence rates are higher in patients (50–80%) with persistent residual stones compared with those with stone-free status.[61,62] CT has a definitive role in the follow-up of stones that are lucent on conventional imaging; however, its additional value in stones that are radioopaque on KUB or scout images remains debatable.[63]
Stone versus stent
Following urologic interventions, ureteral stents or nephrostomy tubes are often placed during the immediate post-treatment period to facilitate urine drainage. In these patients, it is essential to differentiate residual stone fragments in the renal collecting system or ureters from in situ stents for appropriate post-treatment follow-up. The use of bone window settings is helpful in these instances to better differentiate between the stones and stents, which can appear to have similar density on soft tissue window settings [Figure 4].
Figure 4.
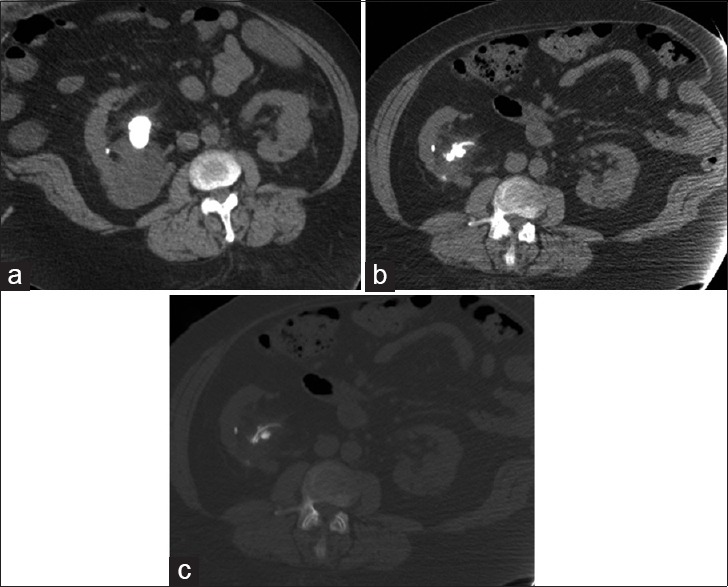
Staghorn calculus in a 47-year-old man with treated lithotripsy. (a) Axial non-contrast computed tomography (CT) obtained prior to lithotripsy shows large right staghorn calculus with hydronephrosis of the upper pole renal calyces. (b) Post-treatment CT scan in soft tissue window settings (400/30) shows reduction in stone burden with residual stone fragments in the renal pelvis. (c) CT image in the bone window settings (1100/300) allows improved distinction of the stone from the ureteral stent
CT in urolithiasis and ionizing radiation
Ionizing radiation exposure from CT scans and its harmful effects remains a concern for healthcare professionals and patients alike. In patients with urolithiasis, it is particularly concerning for young patients with recurrent disease who need to undergo repeated CT scans and are therefore at increased risk of cumulative radiation exposure.[64,65,66,67] The reported cumulative radiation dose associated with repeated CT exams may range from 8.5 to as high as 154 mSv.[68] A crucial step in limiting radiation exposure is through judicious use of CT in evaluation of patients with stone disease. For example, in pregnant patients and children, ultrasound could be favored for the diagnosis of stone disease.[69]
In those patients who do undergo a CT scan, an important measure to decrease radiation exposure is by protocol optimization. Multiple strategies for radiation dose reduction in stone protocol CT scans have been implemented at every step of the CT protocol. An initial measure is a decrease in the z-axis coverage, particularly for follow-up studies. Use of 5 mm slice thickness, accompanied with coronal reformats of 2.5–3 mm, also reduces radiation dose as discussed previously. Appropriate patient centering on the CT scanner gantry has also been reported to decrease the dose by 20%.[70,71,72,73] Tube current reduction through fixed, low values customized for patient's weight (50–100mAs), or ATCM helps to decrease the radiation exposure while showing similar performance on renal stone detection.[70,73] Automated techniques modulate the mA based on the body attenuation on different axis, obtained from the scout radiographs. Different approaches are available, each with unique, user-selectable parameters for tube current modulation, including noise index (NI) and reference mAs. NIs for urolithiasis CT protocols are slightly higher (20–35) compared with those used in other abdomino–pelvic CT scans.[10,11,12,18,19,20] CT scans for the initial assessment of renal stones are usually acquired at 20 NI, and it is subsequently increased up to 35 for follow-up scans, allowing preservation of diagnostic performance while limiting radiation dose.[73] Tube voltage reduction has a recognized dual benefit of improved image contrast and reduced radiation dose for high-contrast CT examinations, such as stone protocol CT, because the high contrast between renal stones and surrounding tissues permits accurate detection.[70,71,72] The implementation of these protocols in practice has been shown to detect calcium oxalate stones with high sensitivity and specificity, while substantially reducing radiation exposure (70–95%).[74] In a study by Huang et al., low-dose protocols were equally sensitive to conventional-dose CT in detecting uric acid stones at very low radiation doses.[75] Recent studies using ultralow-dose protocols have demonstrated a high sensitivity and specificity (97% and 95%, respectively) in detecting calculi while reducing radiation doses to conventional radiographic equivalents (0.6 mSv).[76]
Introduction of iterative reconstructions such as adaptive statistical iterative reconstruction and sonogram affirmed iterative reconstruction allows substantial reduction in radiation exposure while preserving image quality. Several studies have shown the benefit of statistical-based IR reconstructions in CT for urolithiasis, allowing a considerable reduction in radiation dose [Figures 5 and 6].[49,77,78,79,80,81]
Figure 5.
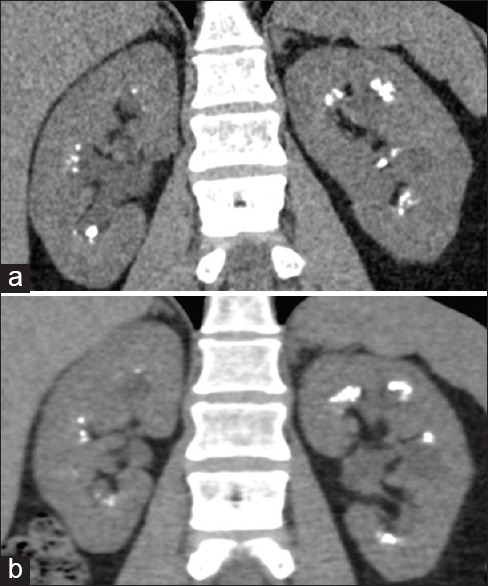
Iterative reconstruction techniques in computed tomography (CT) for urolithiasis. Iterative reconstruction (IR) techniques reduce image noise and allow acquisition of CT scans with low radiation dose, preserving diagnostic accuracy. Coronal images from a patient with medullary sponge kidney acquired in different dates. (a) Image reconstructed with Filter back projection (CTDIvol: 11.45 mGy) and (b) image reconstructed with an IR technique (adaptive statistical iterative reconstruction 60%) (CTDIvol: 7.01 mGy). Note the decreased image noise, preserved image quality and visualization of the calculi in the image obtained with the IR technique compared that obtained with Filtered Back Projection (FBP). Also note the radiation dose reduction obtained using the IR technique and different image texture on the images obtained with the IR technique
Figure 6.
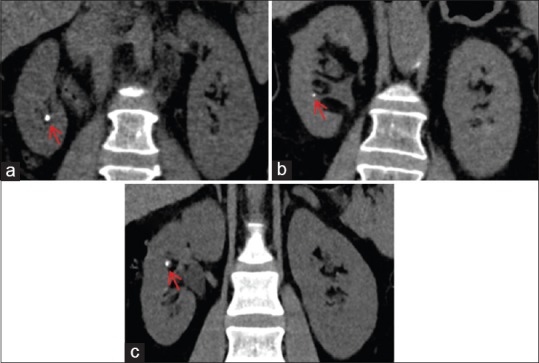
Iterative reconstruction (IR) levels in computed tomography (CT) scans performed for renal stones. IR techniques decrease image noise, allowing radiation dose reduction, while image quality is preserved or even improved. High levels of IR techniques are desired for CT scans performed for renal stones. (a–c) Coronal images from different patients generated with high levels of different IR techniques, showing renal stones in the right kidney (red arrows). (a) Image reconstructed with adaptive statistical iterative reconstruction 80%; patient's weight 70 kg and CTDIvol: 3.84 mGy. (b) iDose4 L5; patient's weight 71 kg and CTDIvol: 5.66 mGy. (c) Sonogram affirmed iterative reconstruction 4; patient's weight 81 kg and CTDIvol: 8.74 mGy. Note the comparable image quality and contrast preservation among the different images, allowing the diagnosis of urolithiasis
There is a common perception that DECT scanning leads to increased ionizing radiation exposure as compared with conventional CT, which limits the wide implementation of DECT. However, radiation dose considerations with DECT are comparable to that achieved with conventional CT. Indeed, DECT exams for urolithiasis can be performed under 5 mSv in ssDECT and dsDECT scanners. Moreover, the use of IR techniques in DECT scanners has allowed reduction in radiation dose while image quality and capability of stone characterization are maintained.
CONCLUSION
MDCT plays a critical role in the management of patients with urolithiasis. Their applicability ranges from the initial diagnosis to planning treatment strategies and in post-treatment follow-up. The emerging technological innovations in CT are bridging the gap between radiological interpretations and urologists’ expectations. With the widespread implementation of low-dose protocols and iterative reconstruction algorithms in routine clinical practice, the radiation dose concerns can be minimized without affecting the diagnostic yield of the CT exams.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Neisius A, Preminger GM. Stones in 2012: Epidemiology, prevention and redefining therapeutic standards. Nat Rev Urol. 2013;10:75–7. doi: 10.1038/nrurol.2012.253. [DOI] [PubMed] [Google Scholar]
- 2.Bartoletti R, Cai T, Mondaini N, Melone F, Travaglini F, Carini M, et al. Epidemiology and risk factors in urolithiasis. Urol Int. 2007;79(Suppl 1):3–7. doi: 10.1159/000104434. [DOI] [PubMed] [Google Scholar]
- 3.Scales CD, Jr, Smith AC, Hanley JM, Saigal CS. Urologic Diseases in America Project. Prevalence of kidney stones in the United States. Eur Urol. 2012;62:160–5. doi: 10.1016/j.eururo.2012.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seitz C, Fajkovic H. Epidemiological gender-specific aspects in urolithiasis. World J Urol. 2013;31:1087–92. doi: 10.1007/s00345-013-1140-1. [DOI] [PubMed] [Google Scholar]
- 5.Smith RC, Verga M, McCarthy S, Rosenfield AT. Diagnosis of acute flank pain: Value of unenhanced helical CT. AJR Am J Roentgenol. 1996;166:97–101. doi: 10.2214/ajr.166.1.8571915. [DOI] [PubMed] [Google Scholar]
- 6.Dhar M, Denstedt JD. Imaging in diagnosis, treatment, and follow-up of stone patients. Adv Chronic Kidney Dis. 2009;16:39–47. doi: 10.1053/j.ackd.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Pfister SA, Deckart A, Laschke S, Dellas S, Otto U, Buitrago C, et al. Unenhanced helical computed tomography vs intravenous urography in patients with acute flank pain: Accuracy and economic impact in a randomized prospective trial. Eur Radiol. 2003;13:2513–20. doi: 10.1007/s00330-003-1937-1. [DOI] [PubMed] [Google Scholar]
- 8.Rosen MP, Siewert B, Sands DZ, Bromberg R, Edlow J, Raptopoulos V. Value of abdominal CT in the emergency department for patients with abdominal pain. Eur Radiol. 2003;13:418–24. doi: 10.1007/s00330-002-1715-5. [DOI] [PubMed] [Google Scholar]
- 9.Kaza RK, Platt JF, Cohan RH, Caoili EM, Al-Hawary MM, Wasnik A. Dual-energy CT with single- and dual-source scanners: Current applications in evaluating the genitourinary tract. Radiographics. 2012;32:353–69. doi: 10.1148/rg.322115065. [DOI] [PubMed] [Google Scholar]
- 10.Boulay I, Holtz P, Foley WD, White B, Begun FP. Ureteral calculi: Diagnostic efficacy of helical CT and implications for treatment of patients. AJR Am J Roentgenol. 1999;172:1485–90. doi: 10.2214/ajr.172.6.10350277. [DOI] [PubMed] [Google Scholar]
- 11.Katz DS, Lane MJ, Sommer FG. Unenhanced helical CT of ureteral stones: Incidence of associated urinary tract findings. AJR Am J Roentgenol. 1996;166:1319–22. doi: 10.2214/ajr.166.6.8633440. [DOI] [PubMed] [Google Scholar]
- 12.Carter MR, Green BR. Renal calculi: Emergency department diagnosis and treatment. Emerg Med Pract. 2011;13:1–17. quiz 8. [PubMed] [Google Scholar]
- 13.Metser U, Ghai S, Ong YY, Lockwood G, Radomski SB. Assessment of urinary tract calculi with 64-MDCT: The axial versus coronal plane. AJR Am J Roentgenol. 2009;192:1509–13. doi: 10.2214/AJR.08.1545. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz BF, Schenkman N, Armenakas NA, Stoller ML. Imaging characteristics of indinavir calculi. J Urol. 1999;161:1085–7. [PubMed] [Google Scholar]
- 15.Eisner BH, McQuaid JW, Hyams E, Matlaga BR. Nephrolithiasis: What surgeons need to know. AJR Am J Roentgenol. 2011;196:1274–8. doi: 10.2214/AJR.11.6434. [DOI] [PubMed] [Google Scholar]
- 16.Ege G, Akman H, Kuzucu K, Yildiz S. Acute ureterolithiasis: Incidence of secondary signs on unenhanced helical CT and influence on patient management. Clin Radiol. 2003;58:990–4. doi: 10.1016/s0009-9260(03)00294-0. [DOI] [PubMed] [Google Scholar]
- 17.Preminger GM, Assimos DG, Lingeman JE, Nakada SY, Pearle MS, Wolf JS., Jr Chapter 1: AUA guideline on management of staghorn calculi: Diagnosis and treatment recommendations. J Urol. 2005;173:1991–2000. doi: 10.1097/01.ju.0000161171.67806.2a. [DOI] [PubMed] [Google Scholar]
- 18.Bandi G, Meiners RJ, Pickhardt PJ, Nakada SY. Stone measurement by volumetric three-dimensional computed tomography for predicting the outcome after extracorporeal shock wave lithotripsy. BJU Int. 2009;103:524–8. doi: 10.1111/j.1464-410X.2008.08069.x. [DOI] [PubMed] [Google Scholar]
- 19.Wang LJ, Wong YC, Chuang CK, Chu SH, Chen CS, See LC, et al. Predictions of outcomes of renal stones after extracorporeal shock wave lithotripsy from stone characteristics determined by unenhanced helical computed tomography: A multivariate analysis. Eur Radiol. 2005;15:2238–43. doi: 10.1007/s00330-005-2742-9. [DOI] [PubMed] [Google Scholar]
- 20.Yoshida S, Hayashi T, Morozumi M, Osada H, Honda N, Yamada T. Three-dimensional assessment of urinary stone on non-contrast helical computed tomography as the predictor of stonestreet formation after extracorporeal shock wave lithotripsy for stones smaller than 20 mm. Int J Urol. 2007;14:665–7. doi: 10.1111/j.1442-2042.2007.01767.x. [DOI] [PubMed] [Google Scholar]
- 21.Gücük A, Uyetürk U. Usefulness of hounsfield unit and density in the assessment and treatment of urinary stones. World J Nephrol. 2014;3:282–6. doi: 10.5527/wjn.v3.i4.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dalla Palma L, Pozzi-Mucelli R, Stacul F. Present-day imaging of patients with renal colic. Eur Radiol. 2001;11:4–17. doi: 10.1007/s003300000589. [DOI] [PubMed] [Google Scholar]
- 23.Memarsadeghi M, Heinz-Peer G, Helbich TH, Schaefer-Prokop C, Kramer G, Scharitzer M, et al. Unenhanced multi-detector row CT in patients suspected of having urinary stone disease: Effect of section width on diagnosis. Radiology. 2005;235:530–6. doi: 10.1148/radiol.2352040448. [DOI] [PubMed] [Google Scholar]
- 24.Kijvikai K, de la Rosette JJ. Assessment of stone composition in the management of urinary stones. Nat Rev Urol. 2011;8:81–5. doi: 10.1038/nrurol.2010.209. [DOI] [PubMed] [Google Scholar]
- 25.Lin WC, Uppot RN, Li CS, Hahn PF, Sahani DV. Value of automated coronal reformations from 64-section multidetector row computerized tomography in the diagnosis of urinary stone disease. J Urol. 2007;178:907–11. doi: 10.1016/j.juro.2007.05.042. [DOI] [PubMed] [Google Scholar]
- 26.Kambadakone AR, Eisner BH, Catalano OA, Sahani DV. New and evolving concepts in the imaging and management of urolithiasis: Urologists’ perspective. Radiographics. 2010;30:603–23. doi: 10.1148/rg.303095146. [DOI] [PubMed] [Google Scholar]
- 27.Spencer BA, Wood BJ, Dretler SP. Helical CT and ureteral colic. Urol Clin North Am. 2000;27:231–41. doi: 10.1016/s0094-0143(05)70253-6. [DOI] [PubMed] [Google Scholar]
- 28.Coll DM, Varanelli MJ, Smith RC. Relationship of spontaneous passage of ureteral calculi to stone size and location as revealed by unenhanced helical CT. AJR Am J Roentgenol. 2002;178:101–3. doi: 10.2214/ajr.178.1.1780101. [DOI] [PubMed] [Google Scholar]
- 29.Preminger GM, Tiselius HG, Assimos DG, Alken P, Buck AC, Gallucci M, et al. 2007 Guideline for the management of ureteral calculi. Eur Urol. 2007;52:1610–31. doi: 10.1016/j.eururo.2007.09.039. [DOI] [PubMed] [Google Scholar]
- 30.Zarse CA, Hameed TA, Jackson ME, Pishchalnikov YA, Lingeman JE, McAteer JA, et al. CT visible internal stone structure, but not Hounsfield unit value, of calcium oxalate monohydrate (COM) calculi predicts lithotripsy fragility in vitro. Urol Res. 2007;35:201–6. doi: 10.1007/s00240-007-0104-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim SC, Burns EK, Lingeman JE, Paterson RF, McAteer JA, Williams JC., Jr Cystine calculi: Correlation of CT-visible structure, CT number, and stone morphology with fragmentation by shock wave lithotripsy. Urol Res. 2007;35:319–24. doi: 10.1007/s00240-007-0117-1. [DOI] [PubMed] [Google Scholar]
- 32.Magrill D, Patel U, Anson K. Impact of imaging in urolithiasis treatment planning. Curr Opin Urol. 2013;23:158–63. doi: 10.1097/MOU.0b013e32835d8e40. [DOI] [PubMed] [Google Scholar]
- 33.Ngo TC, Assimos DG. Uric Acid nephrolithiasis: Recent progress and future directions. Rev Urol. 2007;9:17–27. [PMC free article] [PubMed] [Google Scholar]
- 34.Perks AE, Schuler TD, Lee J, Ghiculete D, Chung DG, D’A Honey RJ, et al. Stone attenuation and skin-to-stone distance on computed tomography predicts for stone fragmentation by shock wave lithotripsy. Urology. 2008;72:765–9. doi: 10.1016/j.urology.2008.05.046. [DOI] [PubMed] [Google Scholar]
- 35.Sheir KZ, Mansour O, Madbouly K, Elsobky E, Abdel-Khalek M. Determination of the chemical composition of urinary calculi by noncontrast spiral computerized tomography. Urol Res. 2005;33:99–104. doi: 10.1007/s00240-004-0454-2. [DOI] [PubMed] [Google Scholar]
- 36.Ramakumar S, Patterson DE, LeRoy AJ, Bender CE, Erickson SB, Wilson DM, et al. Prediction of stone composition from plain radiographs: A prospective study. J Endourol. 1999;13:397–401. doi: 10.1089/end.1999.13.397. [DOI] [PubMed] [Google Scholar]
- 37.Deveci S, Coskun M, Tekin MI, Peskircioglu L, Tarhan NC, Ozkardes H. Spiral computed tomography: Role in determination of chemical compositions of pure and mixed urinary stones-an in vitro study. Urology. 2004;64:237–40. doi: 10.1016/j.urology.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 38.Bellin MF, Renard-Penna R, Conort P, Bissery A, Meric JB, Daudon M, et al. Helical CT evaluation of the chemical composition of urinary tract calculi with a discriminant analysis of CT-attenuation values and density. Eur Radiol. 2004;14:2134–40. doi: 10.1007/s00330-004-2365-6. [DOI] [PubMed] [Google Scholar]
- 39.Dretler SP. Special article: Calculus breakability--fragility and durility. J Endourol. 1994;8:1–3. doi: 10.1089/end.1994.8.1. [DOI] [PubMed] [Google Scholar]
- 40.Rassweiler JJ, Renner C, Chaussy C, Thuroff S. Treatment of renal stones by extracorporeal shockwave lithotripsy: An update. Eur Urol. 2001;39:187–99. doi: 10.1159/000052435. [DOI] [PubMed] [Google Scholar]
- 41.Mostafavi MR, Ernst RD, Saltzman B. Accurate determination of chemical composition of urinary calculi by spiral computerized tomography. J Urol. 1998;159:673–5. [PubMed] [Google Scholar]
- 42.Saw KC, McAteer JA, Monga AG, Chua GT, Lingeman JE, Williams JC., Jr Helical CT of urinary calculi: Effect of stone composition, stone size, and scan collimation. AJR Am J Roentgenol. 2000;175:329–32. doi: 10.2214/ajr.175.2.1750329. [DOI] [PubMed] [Google Scholar]
- 43.Ketelslegers E, Van Beers BE. Urinary calculi: Improved detection and characterization with thin-slice multidetector CT. Eur Radiol. 2006;16:161–5. doi: 10.1007/s00330-005-2813-y. [DOI] [PubMed] [Google Scholar]
- 44.da Silva SF, Silva SL, Daher EF, Silva GB, Junior, Mota RM, Bruno da Silva CA. Determination of urinary stone composition based on stone morphology: A prospective study of 325 consecutive patients in an emerging country. Clin Chem Lab Med. 2009;47:561–4. doi: 10.1515/CCLM.2009.124. [DOI] [PubMed] [Google Scholar]
- 45.Moe OW. Kidney stones: Pathophysiology and medical management. Lancet. 2006;367:333–44. doi: 10.1016/S0140-6736(06)68071-9. [DOI] [PubMed] [Google Scholar]
- 46.Jepperson MA, Cernigliaro JG, Sella D, Ibrahim E, Thiel DD, Leng S, et al. Dual-energy CT for the evaluation of urinary calculi: Image interpretation, pitfalls and stone mimics. Clin Radiol. 2013;68:e707–14. doi: 10.1016/j.crad.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 47.Fletcher JG, Takahashi N, Hartman R, Guimaraes L, Huprich JE, Hough DM, et al. Dual-energy and dual-source CT: Is there a role in the abdomen and pelvis? Radiol Clin North Am. 2009;47:41–57. doi: 10.1016/j.rcl.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 48.Hartman R, Kawashima A, Takahashi N, Silva A, Vrtiska T, Leng S, et al. Applications of dual-energy CT in urologic imaging: An update. Radiol Clin North Am. 2012;50:191–205, v. doi: 10.1016/j.rcl.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 49.Kulkarni NM, Pinho DF, Kambadakone AR, Sahani DV. Emerging technologies in CT- radiation dose reduction and dual-energy CT. Semin Roentgenol. 2013;48:192–202. doi: 10.1053/j.ro.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 50.Johnson TR, Krauss B, Sedlmair M, Grasruck M, Bruder H, Morhard D, et al. Material differentiation by dual energy CT: Initial experience. Eur Radiol. 2007;17:1510–7. doi: 10.1007/s00330-006-0517-6. [DOI] [PubMed] [Google Scholar]
- 51.Graser A, Johnson TR, Bader M, Staehler M, Haseke N, Nikolaou K, et al. Dual energy CT characterization of urinary calculi: Initial in vitro and clinical experience. Invest Radiol. 2008;43:112–9. doi: 10.1097/RLI.0b013e318157a144. [DOI] [PubMed] [Google Scholar]
- 52.Primak AN, Fletcher JG, Vrtiska TJ, Dzyubak OP, Lieske JC, Jackson ME, et al. Noninvasive Differentiation of Uric Acid versus Non-Uric Acid Kidney Stones Using Dual-Energy CT. Acad Radiol. 2007;14:1441–7. doi: 10.1016/j.acra.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Assimos D. Re: Differentiation of kidney stones using dual-energy CT with and without a tin filter. J Urol. 2013;189:172–3. doi: 10.1016/j.juro.2012.09.125. [DOI] [PubMed] [Google Scholar]
- 54.Bilen CY, Kocak B, Kitirci G, Danaci M, Sarikaya S. Simple trigonometry on computed tomography helps in planning renal access. Urology. 2007;70:242–5. doi: 10.1016/j.urology.2007.03.079. [DOI] [PubMed] [Google Scholar]
- 55.Park S, Pearle MS. Imaging for percutaneous renal access and management of renal calculi. Urol Clin North Am. 2006;33:353–64. doi: 10.1016/j.ucl.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 56.El-Nahas AR, El-Assmy AM, Mansour O, Sheir KZ. A prospective multivariate analysis of factors predicting stone disintegration by extracorporeal shock wave lithotripsy: The value of high-resolution noncontrast computed tomography. Eur Urol. 2007;51:1688–94. doi: 10.1016/j.eururo.2006.11.048. [DOI] [PubMed] [Google Scholar]
- 57.Pareek G, Hedican SP, Lee FT, Jr, Nakada SY. Shock wave lithotripsy success determined by skin-to-stone distance on computed tomography. Urology. 2005;66:941–4. doi: 10.1016/j.urology.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 58.Kupeli B, Gurocak S, Tunc L, Senocak C, Karaoglan U, Bozkirli I. Value of ultrasonography and helical computed tomography in the diagnosis of stone-free patients after extracorporeal shock wave lithotripsy (USG and helical CT after SWL) Int Urol Nephrol. 2005;37:225–30. doi: 10.1007/s11255-004-7975-z. [DOI] [PubMed] [Google Scholar]
- 59.Delvecchio FC, Preminger GM. Management of residual stones. Urol Clin North Am. 2000;27:347–54. doi: 10.1016/s0094-0143(05)70263-9. [DOI] [PubMed] [Google Scholar]
- 60.Nakamoto T, Sagami K, Yamasaki A, Ueda M, Fujiwara S, Igawa M, et al. Long-term results of endourologic treatment of urinary calculi: Investigation of risk factors for recurrence or regrowth. J Endourol. 1993;7:297–301. doi: 10.1089/end.1993.7.297. [DOI] [PubMed] [Google Scholar]
- 61.Pearle MS, Watamull LM, Mullican MA. Sensitivity of noncontrast helical computerized tomography and plain film radiography compared to flexible nephroscopy for detecting residual fragments after percutaneous nephrostolithotomy. J Urol. 1999;162:23–6. doi: 10.1097/00005392-199907000-00006. [DOI] [PubMed] [Google Scholar]
- 62.Waldmann TB, Lashley DB, Fuchs EF. Unenhanced computerized axial tomography to detect retained calculi after percutaneous ultrasonic lithotripsy. J Urol. 1999;162:312–4. [PubMed] [Google Scholar]
- 63.Osman Y, El-Tabey N, Refai H, Elnahas A, Shoma A, Eraky I, et al. Detection of residual stones after percutaneous nephrolithotomy: Role of nonenhanced spiral computerized tomography. J Urol. 2008;179:198–200. doi: 10.1016/j.juro.2007.08.175. [DOI] [PubMed] [Google Scholar]
- 64.Brenner DJ, Hall EJ. Computed tomography-an increasing source of radiation exposure. N Engl J Med. 2007;357:2277–84. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 65.Astroza GM, Neisius A, Wang AJ, Nguyen G, Toncheva G, Wang C, et al. Radiation Exposure in the Follow-Up of Patients with Urolithiasis Comparing Digital Tomosynthesis, Non-Contrast CT, Standard KUB, and IVU. J Endourol. 2013;27:1187–91. doi: 10.1089/end.2013.0255. [DOI] [PubMed] [Google Scholar]
- 66.Neisius A, Wang AJ, Wang C, Nguyen G, Tsivian M, Kuntz NJ, et al. Radiation Exposure in Urology: A Genitourinary Catalogue for Diagnostic Imaging. J Urol. 2013 doi: 10.1016/j.juro.2013.06.013. In Press. [DOI] [PubMed] [Google Scholar]
- 67.Fahmy NM, Elkoushy MA, Andonian S. Effective radiation exposure in evaluation and follow-up of patients with urolithiasis. Urology. 2012;79:43–7. doi: 10.1016/j.urology.2011.07.1387. [DOI] [PubMed] [Google Scholar]
- 68.Katz SI, Saluja S, Brink JA, Forman HP. Radiation dose associated with unenhanced CT for suspected renal colic: Impact of repetitive studies. AJR Am J Roentgenol. 2006;186:1120–4. doi: 10.2214/AJR.04.1838. [DOI] [PubMed] [Google Scholar]
- 69.Semins MJ, Matlaga BR. Kidney stones during pregnancy. Nat Rev Urol. 2014;11:163–8. doi: 10.1038/nrurol.2014.17. [DOI] [PubMed] [Google Scholar]
- 70.Mulkens TH, Daineffe S, De Wijngaert R, Bellinck P, Leonard A, Smet G, et al. Urinary stone disease: Comparison of standard-dose and low-dose with 4D MDCT tube current modulation. AJR Am J Roentgenol. 2007;188:553–62. doi: 10.2214/AJR.05.1863. [DOI] [PubMed] [Google Scholar]
- 71.Spielmann AL, Heneghan JP, Lee LJ, Yoshizumi T, Nelson RC. Decreasing the radiation dose for renal stone CT: A feasibility study of single- and multidetector CT. AJR Am J Roentgenol. 2002;178:1058–62. doi: 10.2214/ajr.178.5.1781058. [DOI] [PubMed] [Google Scholar]
- 72.Hamm M, Knopfle E, Wartenberg S, Wawroschek F, Weckermann D, Harzmann R. Low dose unenhanced helical computerized tomography for the evaluation of acute flank pain. J Urol. 2002;167:1687–91. [PubMed] [Google Scholar]
- 73.Kalra MK, Maher MM, Toth TL, Schmidt B, Westerman BL, Morgan HT, et al. Techniques and applications of automatic tube current modulation for CT. Radiology. 2004;233:649–57. doi: 10.1148/radiol.2333031150. [DOI] [PubMed] [Google Scholar]
- 74.Sodickson A. Strategies for reducing radiation exposure from multidetector computed tomography in the acute care setting. Can Assoc Radiol J. 2013;64:119–29. doi: 10.1016/j.carj.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 75.Huang GO, Engebretsen SR, Smith JC, Wallner CL, Culpepper DJ, Creech JD, et al. Detection of uric acid stones in the ureter using low- and conventional-dose computed tomography. Urology. 2014;84:571–4. doi: 10.1016/j.urology.2014.02.021. [DOI] [PubMed] [Google Scholar]
- 76.Kluner C, Hein PA, Gralla O, Hein E, Hamm B, Romano V, et al. Does ultra-low-dose CT with a radiation dose equivalent to that of KUB suffice to detect renal and ureteral calculi? J Comput Assist Tomogr. 2006;30:44–50. doi: 10.1097/01.rct.0000191685.58838.ef. [DOI] [PubMed] [Google Scholar]
- 77.Sagara Y, Hara AK, Pavlicek W, Silva AC, Paden RG, Wu Q. Abdominal CT: Comparison of low-dose CT with adaptive statistical iterative reconstruction and routine-dose CT with filtered back projection in 53 patients. AJR Am J Roentgenol. 2010;195:713–9. doi: 10.2214/AJR.09.2989. [DOI] [PubMed] [Google Scholar]
- 78.Noël PB, Renger B, Fiebich M, Münzel D, Fingerle AA, Rummeny EJ, et al. Does iterative reconstruction lower CT radiation dose: Evaluation of 15,000 examinations. PLoS One. 2013;8:e81141. doi: 10.1371/journal.pone.0081141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kulkarni NM, Uppot RN, Eisner BH, Sahani DV. Radiation dose reduction at multidetector CT with adaptive statistical iterative reconstruction for evaluation of urolithiasis: How low can we go? Radiology. 2012;265:158–66. doi: 10.1148/radiol.12112470. [DOI] [PubMed] [Google Scholar]
- 80.Schindera ST, Odedra D, Mercer D, Thipphavong S, Chou P, Szucs-Farkas Z, et al. Hybrid Iterative Reconstruction Technique for Abdominal CT Protocols in Obese Patients: Assessment of Image Quality, Radiation Dose, and Low-Contrast Detectability in a Phantom. AJR Am J Roentgenol. 2014;202:W146–52. doi: 10.2214/AJR.12.10513. [DOI] [PubMed] [Google Scholar]
- 81.Scott Kriegshauser J, Naidu SG, Paden RG, He M, Wu Q, Hara AK. Feasibility of ultra-low radiation dose reduction for renal stone CT using model-based iterative reconstruction: Prospective pilot study. Clin Imaging. 2015;39:99–103. doi: 10.1016/j.clinimag.2014.10.013. [DOI] [PubMed] [Google Scholar]


